Abstract
Insulin secretion must be tightly coupled to nutritional state to maintain blood glucose homeostasis. To this end, pancreatic β-cells sense and respond to changes in metabolic conditions, thereby anticipating insulin demands for a given physiological context. This is achieved in part through adjustments of nutrient metabolism, which is controlled at several levels including allosteric regulation, posttranslational modifications, and altered expression of metabolic enzymes. In this review, we discuss mechanisms of β-cell metabolic and functional adaptation in the context of two physiological states that alter glucose-stimulated insulin secretion: fasting and insulin resistance. We review current knowledge of metabolic changes that occur in the β-cell during adaptation and specifically discuss transcriptional mechanisms that underlie β-cell adaptation. A more comprehensive understanding of how β-cells adapt to changes in nutrient state could identify mechanisms to be co-opted for therapeutically modulating insulin secretion in metabolic disease.
Keywords: adaptation, β-cell, fasting, insulin resistance, insulin secretion, metabolism, transcription
Introduction
Insulin plays a key role in the regulation of blood glucose levels in response to nutrient state. Insulin is produced by pancreatic β-cells, which must adapt the rate of insulin secretion to myriad physiological conditions. Although insulin demand varies with regard to nutrition, exercise, age, and reproductive state, β-cells can compensate for these altered demands with a proportional insulin secretory response [1-4]. The β-cell’s ability to adapt to insulin demand is the key determinant of blood glucose control during metabolic challenges. For example, longitudinal studies of progression to type 2 diabetes mellitus (T2D) indicate that failed compensatory insulin secretion during insulin resistance predicts diabetes susceptibility [5,6]. Conversely, attenuation of insulin secretion under conditions of fasting or exercise is critical to prevent hypoglycemia and the ensuing neuroglycopenia [7]. Thus, the adjustment of insulin secretion commensurate with insulin demand is critical for the maintenance of blood glucose control during fluctuations of energy supply.
Insulin secretion is tightly coupled to nutrient state through the sensing of circulating nutrients [8]. Glucose is the key stimulus for insulin secretion, with the rate of its metabolism by the β-cell determining the insulin secretory response (Fig. 1, and discussed in detail in the next section). β-cells additionally metabolize fatty acids and amino acids, which modulate insulin secretion during glucose stimulation [8]. Thus, the β-cell serves as a nutrient sensor, with a predominate role for glucose and secondary roles for fatty acids and amino acids.
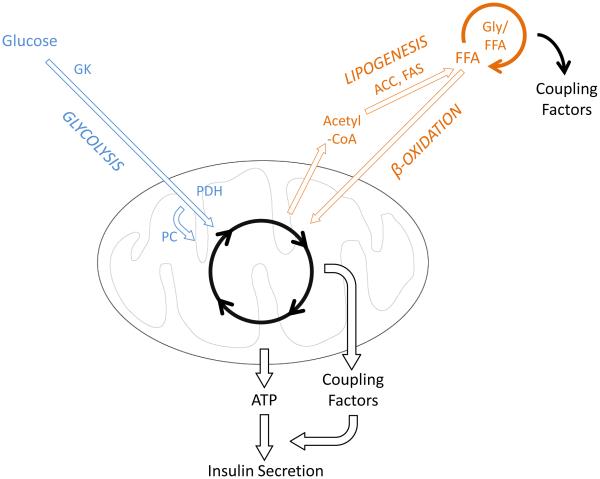
Figure 1. β-cell metabolism under normal conditions.
Glucose metabolism is the key determinant of ATP generation and the triggering of insulin secretion, whereas fatty acids modulate insulin secretion via Gly/FFA cycling. Arrow thickness indicates rate of metabolism. Abbreviations: FFA, free fatty acid; Gly/FFA, glycerolipid-free fatty acid cycle; ACC, acetyl-coA carboxylase; FAS, fatty acid synthase; GK, glucokinase; PDH, pyruvate dehydrogenase; PC, pyruvate carboxylase.
To accommodate changes of insulin demand, β-cells adjust nutrient metabolism to sensitize or desensitize the insulin secretory response [1, 9]. These adaptations occur through altered activities of metabolic enzymes via several regulatory mechanisms, including allosteric control, covalent modifications, and alterations of protein abundance through transcription and translation [2,8-16]. In this way, all relevant inputs from endocrine, neuronal, and nutritional sources influence β-cell function according to physiological state.
In this review, we discuss how β-cells metabolically adjust to nutritional challenges and specifically emphasize the role of transcription factors in this process. By controlling expression levels of metabolic enzymes in the β-cell, transcriptional changes establish the context for acute regulation through substrate availability, posttranslational modifications, and allosteric control. Altered expression of metabolic enzymes through transcriptional and subsequent translational changes likely mediate adaptive responses over a time course of hours to days. For example, extended fasting (>24 hours) dampens the insulin secretory response to glucose, and recovery of this response requires transcriptional changes [13]. Furthermore, culturing islets in high glucose enhances insulin secretion during subsequent glucose challenges, and this effect requires ongoing translation [17,18]. Finally, there is emerging evidence that diurnal rhythms of gene expression in the β-cell influence insulin secretion [19], indicating that transcriptional changes are relevant to daily adjustments of β-cell function. Thus, modulation of protein abundance through transcription and translation is a key regulatory mechanism for adapting β-cell function to the nutritional state of the organism.
The role of metabolism in the regulation of insulin secretion
The triggering pathway of insulin secretion
β-cells sense feeding/fasting states through the metabolism of various nutrients, primarily glucose, amino acids, and fatty acids (Fig. 1) [8]. However, glucose is the only nutrient that is both necessary and sufficient to stimulate insulin secretion [20]. As such, the rate-limiting steps of glycolysis are the key determinants of the initiation of insulin release. The predominant control step of glycolysis in β-cells is glucose phosphorylation, which is catalyzed by the low-affinity enzyme glucokinase (GK) [8,20]. The kinetics of GK, with a Km of 8 mM, sets the threshold for the rapid acceleration of glucose metabolism responsible for stimulating insulin release [20]. Following completion of glycolysis, glucose enters the tricarboxylic acid (TCA) cycle via pyruvate, thereby increasing cellular ATP. As ATP levels rise in response to glucose, KATP channels in the plasma membrane are inactivated, resulting in membrane depolarization. Subsequent opening of voltage-gated Ca2+ channels stimulates exocytosis of insulin vesicles. This signaling cascade comprises the triggering pathway of glucose-stimulated insulin secretion (GSIS) [8, 20].
The amplifying pathway of insulin secretion
While the triggering pathway is responsible for initiating insulin secretion in response to a threshold level of glucose, the quantity of insulin released is further adjusted by diverse nutritional signals via the amplifying pathway [8,20]. The amplifying pathway serves to potentiate the effect of the triggering pathway upon GSIS, and is thus dependent upon concurrent activation of the triggering pathway [20]. The amplifying pathway integrates diverse metabolic cues as well as endocrine and neuronal signals to adjust insulin secretion according to specific physiological states [8]. The dependence upon the triggering pathway ensures that amplification of insulin secretion occurs exclusively in the presence of stimulatory glucose levels. This control step is a safeguard that prevents aberrant stimulation of insulin secretion, which could lead to hypoglycemia.
Nutrient-derived metabolites that participate in the amplifying pathway, termed metabolic coupling factors, arise from diverse metabolic inputs and signal through a variety of target molecules [8]. Metabolic coupling factors serve to increase the effect of Ca2+ upon insulin exocytosis, which explains the requirement for concomitant activation of the triggering pathway. Mechanistically, signals derived from coupling factors typically converge at the level of the vesicle exocytosis machinery. The main sources of metabolic coupling factors are the mitochondria [21,22] and the glycerolipid-free fatty acid cycle [23,24]. In this way, metabolic coupling factor production occurs at sites in which nutritional inputs are integrated [8] , thereby providing responsiveness to diverse metabolic cues indicative of physiological state.
Mitochondrial metabolism gives rise to a robust postprandial insulin secretory response owing to simultaneous activation of both triggering and amplifying pathways of GSIS [8,20]. Rapid glucose oxidation by the mitochondria elevates cellular ATP, resulting in triggering pathway activation. During nutrient stimulation, intermediates within the TCA cycle such as citrate and malate also accumulate in the β-cell [25]. These metabolites serve as precursors to metabolic coupling factors [8], thereby stimulating the amplifying pathway of GSIS. Accumulation of mitochondrial-derived metabolic coupling factors during oxidative metabolism requires ongoing replenishment of TCA cycle metabolites, a process termed anaplerosis [21,26]. The key contributors to anaplerosis in the β-cell are glucose [25] and amino acids [27], which are the major dietary stimuli (e.g. from carbohydrates and proteins) for postprandial insulin secretion. During glucose stimulation, a large proportion of glucose-derived pyruvate enters the mitochondria via the pyruvate carboxylase reaction (Fig. 1), thereby replenishing TCA cycle metabolites. Anaperotic effects also account for amino acid potentiation of insulin secretion. For example, glutamine can be converted to α-ketoglutarate via glutamate, and this reaction is accelerated in the presence of additional amino acids, particularly leucine [27]. The convergence of glucose and dietary amino acids at the level of the mitochondria permits the β-cell to sense food intake and mount a proportional insulin secretory response.
The glycerolipid-free fatty acid cycle integrates glucose and fatty acid metabolism to adjust GSIS relative to islet lipid stores, which reflect long-term fuel availability [24]. Within the β-cell, fatty acids can be derived from the circulation or synthesized through de novo lipogenesis (Fig. 1). As fatty acids are esterified into glycerolipids, they enter the glycerolipid-free fatty acid cycle [24]. Following glycerolipid synthesis, acyl chains are progressively esterified to form triacylglycerol (via mono- and diacylglycerol). The cycle is completed by subsequent lipolysis, culminating in the release of free fatty acids and glycerol [24]. Enhanced glycerolipid-fatty acid cycling results in the accumulation of signaling lipids, particularly monoacylglycerols, which serve as key metabolic coupling factors [23]. Within islets, the levels of glycerolipid-free fatty acid cycle intermediates fluctuate relative to fuel availability [24]. During fasting, when islet energy stores are low, fatty acids are oxidized to generate ATP, thereby reducing fatty acid availability for the glycerolipid-free fatty acid cycle [28]. In the fed state, de novo lipogenesis increases and fatty acids accumulate [28]. Furthermore, cross-talk between lipid and glucose metabolism links postprandial increases of blood glucose levels to accelerated glycerolipid-free fatty acid cycling [23,25]. Rapid glucose metabolism leads to the accumulation of cytosolic acetyl-coA and subsequently malonyl-coA, which is a potent inhibitor of fatty acid oxidation [25]. As fatty acids accumulate during glucose stimulation, glycerolipid-free fatty acid cycling is accelerated, resulting in metabolic coupling factor generation and stimulation of the amplifying pathway [23]. Thus, physiological conditions influencing lipid availability and metabolism profoundly influence the insulin secretory response through glycerolipid-free fatty acid cycling.
β-cell adaptation to fasting
The ability of an organism to store and utilize energy during feeding and fasting requires appropriate insulin signaling at target tissues. The activity of insulin ensures that glucose is the major energy source in the fed state, while fatty acid utilization dominates in the fasted state [29]. Specifically, insulin serves to promote systemic glucose utilization, to suppress the release of fatty acids from adipose tissue (lipolysis), and to reduce hepatic glucose production in the fed state. During fasting, the suppression of insulin release is necessary to increase lipolysis by adipocytes, promote hepatic glucose production, and reduce glucose disposal by insulin target tissues [29]. These effects are critical for the switch to fatty acid utilization by peripheral tissues during fasting, thereby conserving glucose for use by the brain.
In the fasted state, GSIS is attenuated [30], likely to preserve systemic glucose levels under conditions of sustained nutrient scarcity [29]. The potentiation of GSIS by amino acids is also suppressed during fasting [31], which may be secondary to reduced activation of the triggering pathway by glucose. However, the stimulatory effect of fatty acids upon insulin secretion is maintained in the fasted state [32]. Thus, the reduced contribution of glucose and amino acids to insulin secretion during fasting results in an expanded role for fatty acids in controlling insulin release [32].
Adaptations of glucose metabolism during fasting
The suppression of GSIS during fasting has been attributed in part to reduced glucose metabolism by the β-cell (Fig. 2) [4]. Glucose oxidation is slowed, accompanied by reduced activities of glycolytic enzymes such as GK, hexokinase (HK), and phosphofructokinase (PFK) [10,33]. Rescue experiments with cell-permeable metabolites indicate that decelerated metabolism occurs in upper glycolysis (e.g. reactions catalyzed by GK, HK, and PFK), supporting a role for these enzymes in the adaptive response to fasting [4]. Finally, activities of GK, HK, and PFK recover with the same kinetics as the recovery of GSIS during refeeding [10]. As the phosphorylation steps carried out by these enzymes are key regulatory sites for hormonal and allosteric control of glycolysis, changes in their abundance likely contribute to the control of glucose metabolism during fasting.
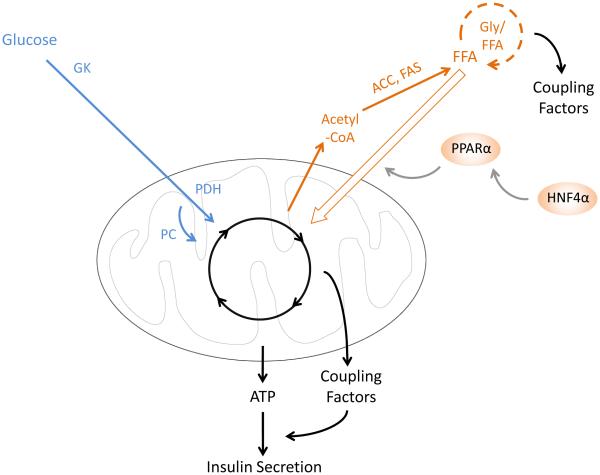
Figure 2. β-cell metabolism during extended fasting.
Glucose metabolism is slowed and fatty acid oxidation is increased, leading to depletion of Gly-FFA cycle-derived coupling factors. Arrow thickness indicates rate of metabolism. Abbreviations: FFA, free fatty acid; Gly/FFA, glycerolipid-free fatty acid cycle; ACC, acetyl-coA carboxylase; FAS, fatty acid synthase; GK, glucokinase; PDH, pyruvate dehydrogenase; PC, pyruvate carboxylase.
Adaptations of lipid metabolism during fasting
In the fasted state, circulating fatty acid levels are increased, and β-cell fatty acid oxidation is accelerated (Fig. 2) [34]. Importantly, increased fatty acid catabolism reduces the secretory response to glucose [28] through cross-talk between the metabolism of these nutrients. As discussed in Section II, glucose and lipid metabolism converge upon the glycerolipid-free fatty acid cycle to gauge metabolic coupling factor generation to levels of lipid stores in the β-cell. Accelerated β-oxidation during fasting depletes glycerolipids [28,34], thereby reducing the ability of glucose metabolism to promote metabolic coupling factor generation through this cycle. Pharmacological inhibition of fatty acid oxidation in fasted islets restores glucose responsiveness [28], emphasizing the importance of accelerated lipid metabolism to β-cell functional adaptations to fasting.
Transcriptional regulation of β-cell metabolism during fasting
Transcriptional regulation plays a key role in the β-cell response to fasting. The primary role for transcriptional changes in attenuated GSIS during fasting can be demonstrated by pharmacological inhibition of transcription during the re-feeding phase [13]. Normally, glucose responsiveness recovers over the course of 1-2 days of refeeding; however, blocking transcription completely prevents the recovery of GSIS [13]. While transcriptional changes are critical for this response, relatively few genes have been directly implicated. The best-characterized transcriptional change associated with reduced GSIS during fasting is the repression of GK [35,36], which is known to be rate-limiting for GSIS at intermediate glucose concentrations. While several other gene expression changes have been described, for example in the glucose transporter, preproinsulin, and the voltage-gated calcium channel [35], their relative roles in altered GSIS during fasting are unknown.
Knockout mouse models exhibiting fasting hyperinsulinemia have revealed key transcriptional mediators of β-cell adaptation to fasting. Peroxisome proliferation activated receptor α (Pparα) is a fasting-inducible transcription factor that promotes mitochondrial function and fatty acid oxidation [37]. Animals deficient for Pparα exhibit fasting hypoglycemia and hyperlipidemia associated with reduced fatty acid oxidation in several metabolic tissues, indicating that Pparα is critical for the switch to systemic fatty acid metabolism during nutrient deprivation. Islets isolated from Pparα-deficient mice hypersecrete insulin and fail to increase fatty acid oxidation in the fasted state [38]. Thus, increased fatty acid oxidation mediated by Pparα is required for reduced GSIS during fasting. Pparα itself is transcriptionally regulated by Hepatic nuclear factor 4α (Hnf4α) [39] (Fig. 2). β-cell-specific inactivation of Hnf4α causes hyperinsulinemic hypoglycemia associated with a failure of islets to suppress insulin secretion when glucose levels are reduced. Hnf4α-deficient β-cells exhibit reduced expression of Pparα as well as Kir6.2, which encodes a subunit of the KATP channel. Thus, Hnf4α transcriptionally regulates genes involved in fatty acid metabolism (Pparα) as well as those controlling the triggering pathway of insulin secretion (Kir6.2) [39].
An additional level of regulation of the Hnf4α and Pparα gene regulatory network was revealed by β-cell-specific inactivation of Forkhead Box O (FoxO) family transcription factors. FoxOs are nutritionally- and hormonally-regulated transcription factors that regulate glucose and lipid metabolism in several metabolic tissues. Posttranslational modifications of FoxOs modulate their activity in response to nutritional state [40,41]. For example, in β-cells, FoxO1 is activated in response to metabolic stress such as hyperglycemia [41]. β-cell-specific deletion of the major FoxO family members (FoxO1, FoxO3a, FoxO4) revealed similarities between FoxO deficiency and fasting with respect to β-cell metabolism and physiology [42]. Mice lacking all three FoxOs in β-cells develop glucose intolerance associated with reduced GSIS. Islets from mutant mice exhibit slower glucose oxidation and augmented fatty acid oxidation. Together, these physiological features closely resemble those of islets purified from fasted mice [34]. Transcriptomes of the mutant islets revealed upregulation of Hnf4α and Pparα gene regulatory networks [42], which are the key regulators of the β-cell response to fasting as discussed above. It remains to be determined whether FoxO inhibition normally plays a role in Pparα and Hnf4α function during the β-cell response to fasting.
Future perspectives of β-cell metabolism during fasting
The ability of the β-cell to attenuate glucose metabolism and insulin secretion in the fasted state is critical for sparing systemic glucose for use by the nervous system [29]. However, several aspects of this adaptive β-cell response remain enigmatic. The transcriptional regulators controlling the expression of genes encoding glycolytic enzymes, such as GK, during fasting are not well-defined. Furthermore, the mechanisms that underlie reduction of amino acid-potentiated insulin secretion in the fasted state remain unexplored. Considering the critical role for transcription in these functional adaptations [13], assessment of the transcriptome and cistrome of islets during fasting would begin to clarify the underlying mechanisms. A more complete understanding of such processes could improve current models of the pathogenesis of hyperinsulinemic disorders and additionally reveal adaptive mechanisms that could be co-opted for therapeutic modulation of insulin secretion.
The role of metabolism in β-cell adaptation to increased workload
Nutrient overload is associated with the development of insulin resistance, wherein increased insulin levels are required to reduce blood glucose concentration. To maintain blood glucose homeostasis, the insulin demand must be met by compensatory increases of insulin release by pancreatic β-cells. During adaptation to insulin resistance, the insulin secretory response to glucose is sensitized [3,9]. In this way, β-cells anticipate postprandial insulin requirements to enable timely normalization of blood glucose levels. Additionally, increased β-cell proliferation expands the functional mass of β-cells, thereby increasing total insulin release, as reviewed elsewhere [43]. Failure of β-cell compensation is the defining event of T2D pathogenesis [5,6,44,45]. Conversely, sustained β-cell adaptation is capable of preventing T2D, even in the face of decades of severe insulin resistance.
Much of what is known about β-cell compensation has been learned from rodent models of increased β-cell workload, which have enabled the study of islet-intrinsic adaptation mechanisms. Universally, these models exhibit increased insulin secretion from β-cells. An increase in β-cell workload can be achieved by either reducing the total number of β-cells or by inducing insulin resistance. The predominant experimental models are the partial pancreatectomized rat [14,16] as well as several models of obesity [46]. In partial pancreatectomy a fraction of the pancreas (60-90%) is removed, thereby causing a requirement for enhanced insulin secretion from the remaining β-cells [14,16]. Obesity induced by genetic (ob/ob mice, Zucker Fatty rats) or dietary (high fat diet) manipulations [46] increases β-cell workload due to insulin resistance, and expose the β-cell to additional systemic insults such as dyslipidaemia and inflammation [47].
Glucose metabolism in the β-cell during compensation
One of the defining characteristics of compensating β-cells is a sensitized insulin secretory response to glucose (Fig. 3) [9,14,16], wherein more insulin is secreted relative to glucose concentration. The sensitized response to glucose is associated with accelerated glucose oxidation [12,48] and upregulation of several glycolytic enzymes [9,14,16]. Increased transcription and activity of GK and low Km hexokinases has been observed in animal models of β-cell adaptation [2,14,16], although some variability exists among models. Inhibition of glycolysis reduces basal insulin secretion by compensating islets [49], indicating that accelerated glucose metabolism indeed drives sensitized GSIS during adaptation to insulin resistance. Several metabolic adaptations relating to the amplifying pathway of GSIS have also been described; for example, pyruvate carboxylase activity is increased and several enzymatic reactions of the pyruvate cycling pathway (malic enzyme, malate dehydrogenase) are accelerated during compensation [9]. This leads to accumulation of mitochondrial metabolites implicated in the production of metabolic coupling factors (Fig. 3). Treating compensated islets with an inhibitor of pyruvate carboxylase reduces insulin secretion specifically in high glucose [9], indicating that increased pyruvate carboxylase activity and subsequent activation of the amplifying pathway contributes to enhanced maximal GSIS during adaptation.
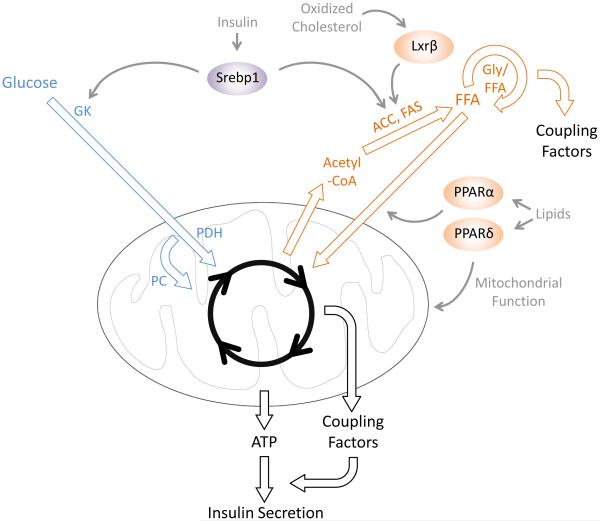
Figure 3. β-cell metabolism during compensation for increased workload.
Both glucose and fatty acid oxidation are accelerated, and metabolic coupling factor production from both the mitochondria and the Gly-FFA cycle is increased. Arrow thickness indicates rate of metabolism. Abbreviations: FFA, free fatty acid; Gly/FFA, glycerolipid-free fatty acid cycle; ACC, acetyl-coA carboxylase; FAS, fatty acid synthase; GK, glucokinase; PDH, pyruvate dehydrogenase; PC, pyruvate carboxylase.
Lipid metabolism during compensation
In obesity, the β-cell must cope with elevated levels of systemic fatty acids and triglycerides [47]. Increased lipid delivery has dual effects on β-cell function, as lipids can stimulate GSIS as well as exert toxic effects upon the β-cell [50]. Thus, increasing insulin secretion during adaptation requires production of lipid-derived metabolic coupling factors while avoiding lipid toxicity.
Lipid toxicity occurs when free fatty acid supply outstrips the capacity for fatty acid oxidation and esterification into triglycerides [50,51]. Excess free fatty acids can be aberrantly metabolized into signaling molecules that cause β-cell apoptosis [50]. Conversely, esterification into triglycerides diverts fatty acids away from metabolic fates that are toxic to the β-cell. To avoid such toxicity, compensating islets increase fatty acid esterification into triglycerides [1]. Furthermore, increased islet fatty acid oxidation serves to limit lipid accumulation in the obese state (Fig. 3) [1]. These metabolic adaptations prevent lipid toxicity to preserve β-cell function during obesity.
Excess lipid supply during obesity also contributes to enhanced production of metabolic coupling factors and increased GSIS [9]. Increased triglyceride synthesis in compensating islets provides substrates for glycerolipid-free fatty acid cycling (Fig. 3) [1]. Subsequent lipolysis of the resulting triglycerides produces monoacylglycerols, which potentiate GSIS via the amplifying pathway [23]. Accordingly, both triglyceride production and lipolysis are accelerated in compensating islets [1]. Thus, obesity-associated adaptations of lipid metabolism in β-cells permit simultaneous increases of insulin secretion and fatty acid detoxification.
Transcriptional control of metabolism during β-cell compensation
The adaptation of glucose and lipid metabolism during increased β-cell workload is critical for enhanced GSIS as well as the avoidance of lipid toxicity. An important mechanism of metabolic adaptation is a change in the expression level of genes encoding metabolic enzymes. The role of transcription factors in β-cell adaptation has been studied using gene knockouts and pharmacological inhibitors. To distinguish between roles of a transcription factor in β-cells and other tissues, ex vivo assays on isolated islets have been used to ascertain β-cell-specific effects. The predominant ex vivo assay for adaptive insulin secretion involves culture of islets for at least 8 hours in high glucose (hereafter referred to as glucose conditioning) [18,52], which increases GSIS as observed in vivo during insulin resistance. Glucose conditioning mimics the metabolic changes observed in animal models of obesity [15,53]. Similarly, lipid conditioning, wherein islets are incubated for at least 24 hours in elevated fatty acids, also mimics some of the adaptations found in islets of obese mice such as sensitized GSIS [48,54]. Ex vivo lipid culture additionally tests the ability of islets to detoxify excess lipids.
The transcription factor sterol regulatory element binding protein 1c (Srebp1c) has been identified as the master regulator of lipogenesis during adaptation to glucose conditioning. In islets, Srebp1c is activated in response to autocrine insulin signaling (Fig. 3) [55]. Srebp1c activates target genes encoding lipogenic enzymes, such as fatty acid synthetase (FAS) and acetyl coA carboxylase (ACC), which together are responsible for converting nutrient-derived acetyl-CoA into long chain fatty acids and eventually triglycerides. In this way, Srebp1c links autocrine insulin signaling to islet lipogenesis. Islets from Srebp1c mutant mice exhibit a blunted adaptive response to glucose conditioning [55], which is associated with a failure to upregulate lipogenic genes and reduced triglyceride synthesis. It is likely that impaired lipogenesis in the absence of Srebp1c affects the generation of lipid-derived coupling factors, which are critical for enhancing GSIS during compensation. Interestingly, Srebp1c is also necessary for the increase in GK expression during adaptation [55], indicating that Srebp1c has an additional role in regulating β-cell glucose metabolism.
In addition to its key function of repressing insulin secretion during fasting [38], Pparα is also critical for β-cell adaptation to obesity (Fig. 3) [56]. Pparα deficiency in ob/ob mice causes β-cell decompensation and hyperglycemia. Interestingly, Ppar-deficient ob/ob mice exhibit normal insulin sensitivity but insufficient insulin secretion, indicating a specific role for Pparα in β-cell adaptation. Thus, Pparα has context-specific functions in the β-cell: the suppression of insulin release during fasting [38], and appropriate compensation during insulin resistance [56]. Although the exact mechanism of failed compensation in Pparα-deficient mice is unknown, it is possible that the ability of β-cells to detoxify lipids via β-oxidation is perturbed. Supporting such a mechanism, Pparα agonists have been shown to rescue insulin secretion defects of islets challenged ex vivo with high glucose and elevated lipids [56].
Pparδ functions as a transcriptional activator of β-oxidation genes in several tissues in response to fatty acids. A role for Pparδ has been demonstrated in the adaptive response of β-cells to elevated fatty acids. Fatty-acid induced enhancement of β-oxidation and GSIS in insulinoma cells is dependent upon Pparδ (Fig. 3) [57]. Furthermore, Pparδ agonists enhance fatty acid oxidation and improve GSIS in islets of diabetic mice [58]. It has also been proposed that endogenous lipids that normally bind Pparδ are depleted in dysfunctional islets [59]. Together, these observations suggest that activation of Pparδ by endogenous lipids promotes compensatory fatty acid oxidation in β-cells during obesity.
Lxr transcription factors are activated by oxidized cholesterol and, in some tissues, by glucose. These transcription factors are known to promote lipogenesis and cholesterol efflux, likely to prevent excessive cholesterol accumulation. Pharmacological Lxr activation in β-cells mimics several physiological and metabolic characteristics of compensation [60, 61]. Lxr agonists increase GSIS associated with increased lipogenesis, fatty acid oxidation, and pyruvate carboxylation (which drives anaplerosis) (Fig. 3). Mice deficient for Lxrβ exhibit insulin secretion defects associated with severe lipid accumulation in islets [62], consistent with the general role for Lxr in cholesterol efflux. The role of cholesterol and its oxidized derivatives in β-cell adaptation and decompensation are still unclear; however, several studies [63,64] suggest that stimulation of cholesterol metabolism may be critical to avoid lipid toxicity during obesity.
Future perspectives of β-cell metabolism during compensation
Successful β-cell compensation enables maintenance of normoglycemia under conditions of insulin resistance. The functional adaptations underlying compensatory insulin secretion can be explained in part by altered abundance of metabolic enzymes, as mediated by transcription factors sensing the nutritional environment. However, several metabolic adaptations have yet to be explained mechanistically. For example, increased glycerolipid-free fatty acid cycling during obesity associates with increased activities of several lipases [1], yet the mediators of these metabolic adaptations are unknown. Similarly, it is unclear how pyruvate cycling reactions (malic enzyme, malate dehydrogenase) are accelerated during compensation [9]. β-cells that have failed to compensate for insulin resistance exhibit defects in metabolic coupling factor generation via both pyruvate cycling [65,66] and glycerolipid-free fatty acid cycling [12,64] (Fig. 4), underscoring the importance of understanding these pathways in the insulin-resistant state.
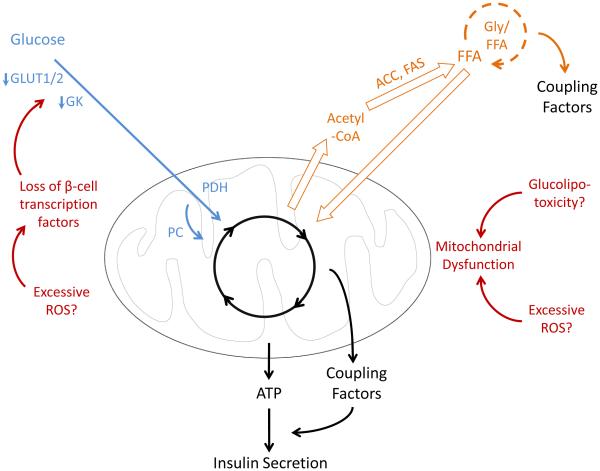
Figure 4. Metabolic defects of decompensating β-cells in T2D.
Defective glucose metabolism and reduced Gly-FFA cycling leads to a loss of GSIS. Several proposed causes of metabolic defects observed in T2D are indicated. Of note, insults predominately affecting β-cell survival have been excluded. Proposed mechanisms are based on the following references: Prentki and Nolan [51], Del Guerra et al. [67], Prentki and Madiraju [24], Weir et al. [2], Supale et al. [70], and Guo et al. [71]. Arrow thickness indicates rate of metabolism. Abbreviations: FFA, free fatty acid; Gly/FFA, glycerolipid-free fatty acid cycle; ACC, acetyl-coA carboxylase; FAS, fatty acid synthase; GLUT1/2, glucose transporters 1 and 2; GK, glucokinase; PDH, pyruvate dehydrogenase; PC, pyruvate carboxylase; ROS, reactive oxygen species.
Perspectives on metabolic defects of decompensating β-cells
T2D is the end result of insulin demand exceeding the insulin secretory capacity of the β-cell. Longitudinal studies of T2D pathogenesis indicate that insufficient β-cell compensation for insulin resistance is the precipitating event in T2D [5,6]. The etiology of β-cell dysfunction and failure in the progression to T2D involves diverse cellular insults and has been reviewed in detail elsewhere [2,51]. Of note, several metabolic defects contributing to dysfunctional GSIS have been described in islets purified from overtly diabetic donors. Glucose oxidation is slowed and associated with reduced expression of glucose metabolism genes and structural abnormalities of mitochondria (Fig. 4) [67,68]. Furthermore, metabolic coupling factor generation is reduced in decompensated islets, as described in Section IV (Fig. 4) [64,65]. An unresolved question is whether the metabolic abnormalities of β-cells observed in T2D are the cause or effect of poorly-controlled hyperglycemia. Although insufficient insulin secretion precipitates the development of T2D, the resulting systemic defects perpetuate β-cell dysfunction [44,45,50,51]. Thus, distinguishing islet abnormalities that are causative of defective GSIS from those that occur secondary to hyperglycemia is challenging, and little is known regarding the metabolic defects of β-cells at the onset of failed compensation [3]. A better understanding of the mechanisms of β-cell adaptation to insulin resistance, and the causes of its failure during T2D progression, would provide insights regarding possible interventions to improve compensation and prevent T2D.
Summary and Outlook
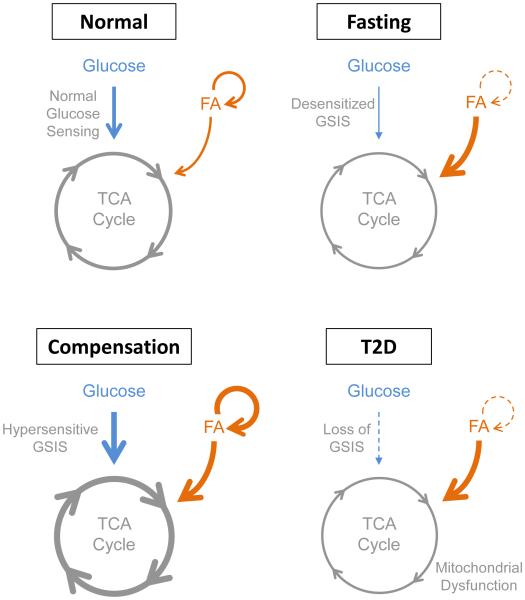
Under conditions of fasting or insulin resistance, β-cells undergo characteristic adaptations to adjust insulin secretion commensurate with altered insulin demand (Fig. 5). Nutrient-sensing transcription factors detect altered supply of glucose and lipids from the circulation and adjust β-cell metabolism accordingly. Extended fasting is associated with reduced glucose oxidation and accelerated fatty acid oxidation in the β-cell, mediated in part by the lipid-sensing transcription factor Pparα (Fig 2). Compensation for insulin resistance requires accelerated glucose and fatty acid oxidation and increased glycerolipid-free fatty acid cycling, coordinated by transcription factors responsive to insulin (Srebp1c), lipids (Pparα and Pparδ), and cholesterol derivatives (Lxrβ) (Fig. 3).
Figure 5. Overview of β-cell metabolism and glucose-stimulated insulin secretion under different physiological and pathological conditions.
Arrow thickness indicates rate of metabolism. Abbreviations: GSIS, glucose-stimulated insulin secretion; FA, fatty acid; TCA, tricarboxylic acid; T2D, type 2 diabetes mellitus.
Although we have highlighted several mechanisms of metabolic sensing by the β-cell, much remains to be understood of how the β-cell detects and responds to changes of nutritional state. A few transcription factors that mediate the β-cell response to glucose and lipids have been identified, yet little is known about how responses to other nutrients and metabolites are sensed by β-cells and converted into transcriptional changes. Furthermore, nutrient-responsive transcriptional regulation may extend to the epigenome, through dependence of some chromatin modifying enzymes upon metabolically-linked substrates and cofactors [69]. Finally, the discovery of circadian oscillations of gene expression in the islet [19] raises the possibility that nutritional cues could interact with and possibly entrain circadian rhythms in the β-cell. A more comprehensive understanding of β-cell nutrient sensing could improve our understanding of T2D pathogenesis and identify mechanisms to be co-opted for therapeutically modulating insulin secretion in metabolic disease.
Acknowledgements
The authors would like to thank Andrea C. Carrano for providing helpful comments on the manuscript. We also thank Johanna Fleischman for assistance with figure preparation. Work in the Sander laboratory is supported by grants from the National Institutes of Health, the Juvenile Diabetes Research Foundation, the Helmsley Charitable Trust, and the California Institute of Regenerative Medicine. M.W. was supported by a postdoctoral fellowship from the Juvenile Diabetes Research Foundation (3-PDF-2014-193-A-N), and was additionally supported by the NIH training program in diabetes research T32 DK007494-30.
Footnotes
Conflict of interest statement The authors declare no conflicts of interest.
Callout Sentences
To appear in Section I or II: “By controlling expression levels of metabolic enzymes in the β-cell, transcriptional changes establish the context for acute regulation through substrate availability, posttranslational modifications, and allosteric control.”
To appear in Section III: “Extended fasting is associated with reduced glucose oxidation and accelerated fatty acid oxidation in the β-cell, mediated in part by the lipid-sensing transcription factor Pparα.”
To appear in Section IV: “Compensation for insulin resistance requires accelerated glucose and fatty acid oxidation and increased glycerolipid-free fatty acid cycling, as mediated by transcription factors responsive to insulin (Srebp1c), lipids (Pparα and Pparδ), and cholesterol derivatives (Lxrβ).”
References
- 1.Nolan CJ, Leahy JL, Delghingaro-Augusto V, et al. Beta cell compensation for insulin resistance in Zucker fatty rats: increased lipolysis and fatty acid signalling. Diabetologia. 2006;49:2120–2120. doi: 10.1007/s00125-006-0305-5. [DOI] [PubMed] [Google Scholar]
- 2.Weir GC, Laybutt DR, Kaneto H, Bonner-Weir S, Sharma A. Beta-cell adaptation and decompensation during the progression of diabetes. Diabetes. 2001;50(Suppl 1):S154–154. doi: 10.2337/diabetes.50.2007.s154. [DOI] [PubMed] [Google Scholar]
- 3.Ferrannini E. The stunned beta cell: a brief history. Cell Metab. 2010;11:349–349. doi: 10.1016/j.cmet.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Levy J, Herchuelz A, Sener A, Malaisse WJ. The stimulus-secretion coupling of glucose-induced insulin release. XX. fasting: a model for altered glucose recognition by the B-cell. Metabolism. 1976;25:583–583. doi: 10.1016/0026-0495(76)90012-3. [DOI] [PubMed] [Google Scholar]
- 5.Cnop M, Vidal J, Hull RL, et al. Progressive loss of beta-cell function leads to worsening glucose tolerance in first-degree relatives of subjects with type 2 diabetes. Diabetes Care. 2007;30:677–677. doi: 10.2337/dc06-1834. [DOI] [PubMed] [Google Scholar]
- 6.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–787. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Service FJ. Hypoglycemic disorders. N Engl J Med. 1995;332:1144–1144. doi: 10.1056/NEJM199504273321707. [DOI] [PubMed] [Google Scholar]
- 8.Prentki M, Matschinsky FM, Madiraju SR. Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 2013;18:162–162. doi: 10.1016/j.cmet.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Liu YQ, Jetton TL, Leahy JL. beta-Cell adaptation to insulin resistance. Increased pyruvate carboxylase and malate-pyruvate shuttle activity in islets of nondiabetic Zucker fatty rats. J Biol Chem. 2002;277:39163–39163. doi: 10.1074/jbc.M207157200. [DOI] [PubMed] [Google Scholar]
- 10.Burch PT, Trus MD, Berner DK, Leontire A, Zawalich KC, Matschinsky FM. Adaptation of glycolytic enzymes: glucose use and insulin release in rat pancreatic islets during fasting and refeeding. Diabetes. 1981;30:923–923. doi: 10.2337/diab.30.11.923. [DOI] [PubMed] [Google Scholar]
- 11.Cockburn BN, Ostrega DM, Sturis J, Kubstrup C, Polonsky KS, Bell GI. Changes in pancreatic islet glucokinase and hexokinase activities with increasing age, obesity, and the onset of diabetes. Diabetes. 1997;46:1434–1434. doi: 10.2337/diab.46.9.1434. [DOI] [PubMed] [Google Scholar]
- 12.Delghingaro-Augusto V, Nolan CJ, Gupta D, et al. Islet beta cell failure in the 60% pancreatectomised obese hyperlipidaemic Zucker fatty rat: severe dysfunction with altered glycerolipid metabolism without steatosis or a falling beta cell mass. Diabetologia. 2009;52:1122–1122. doi: 10.1007/s00125-009-1317-8. [DOI] [PubMed] [Google Scholar]
- 13.Grey NJ, Goldring S, Kipnis DM. The effect of fasting, diet, and actinomycin D on insulin secretion in the rat. J Clin Invest. 1970;49:881–881. doi: 10.1172/JCI106307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosokawa H, Hosokawa YA, Leahy JL. Upregulated hexokinase activity in isolated islets from diabetic 90% pancreatectomized rats. Diabetes. 1995;44:1328–1328. doi: 10.2337/diab.44.11.1328. [DOI] [PubMed] [Google Scholar]
- 15.Liu YQ, Moibi JA, Leahy JL. Chronic high glucose lowers pyruvate dehydrogenase activity in islets through enhanced production of long chain acyl-CoA: prevention of impaired glucose oxidation by enhanced pyruvate recycling through the malate-pyruvate shuttle. J Biol Chem. 2004;279:7470–7470. doi: 10.1074/jbc.M307921200. [DOI] [PubMed] [Google Scholar]
- 16.Liu YQ, Nevin PW, Leahy JL. beta-cell adaptation in 60% pancreatectomy rats that preserves normoinsulinemia and normoglycemia. Am J Physiol Endocrinol Metab. 2000;279:E68–E73. doi: 10.1152/ajpendo.2000.279.1.E68. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Barrado MJ, Ravier MA, Rolland JF, Gilon P, Nenquin M, Henquin JC. Inhibition of protein synthesis sequentially impairs distinct steps of stimulus-secretion coupling in pancreatic beta cells. Endocrinology. 2001;142:299–299. doi: 10.1210/endo.142.1.7910. [DOI] [PubMed] [Google Scholar]
- 18.Schuit F, Flamez D, De Vos A, Pipeleers D. Glucose-regulated gene expression maintaining the glucose-responsive state of beta-cells. Diabetes. 2002;51(Suppl 3):S326–326. doi: 10.2337/diabetes.51.2007.s326. [DOI] [PubMed] [Google Scholar]
- 19.Perelis M, Marcheva B, Ramsey KM, et al. Pancreatic beta cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science. 2015;350:aac4250. doi: 10.1126/science.aac4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henquin JC. Regulation of insulin secretion: a matter of phase control and amplitude modulation. Diabetologia. 2009;52:739–739. doi: 10.1007/s00125-009-1314-y. [DOI] [PubMed] [Google Scholar]
- 21.Flamez D, Berger V, Kruhoffer M, Orntoft T, Pipeleers D, Schuit FC. Critical role for cataplerosis via citrate in glucose-regulated insulin release. Diabetes. 2002;51:2018–2018. doi: 10.2337/diabetes.51.7.2018. [DOI] [PubMed] [Google Scholar]
- 22.Odegaard ML, Joseph JW, Jensen MV, et al. The mitochondrial 2-oxoglutarate carrier is part of a metabolic pathway that mediates glucose- and glutamine-stimulated insulin secretion. J Biol Chem. 2010;285:16530–16530. doi: 10.1074/jbc.M109.092593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao S, Mugabo Y, Iglesias J, et al. alpha/beta-Hydrolase domain-6-accessible monoacylglycerol controls glucose-stimulated insulin secretion. Cell Metab. 2014;19:993–993. doi: 10.1016/j.cmet.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Prentki M, Madiraju SR. Glycerolipid/free fatty acid cycle and islet beta-cell function in health, obesity and diabetes. Mol Cell Endocrinol. 2012;353:88–88. doi: 10.1016/j.mce.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Schuit F, De Vos A, Farfari S, et al. Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in beta cells. J Biol Chem. 1997;272:18572–18572. doi: 10.1074/jbc.272.30.18572. [DOI] [PubMed] [Google Scholar]
- 26.Lu D, Mulder H, Zhao P, et al. 13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS) Proc Natl Acad Sci U S A. 2002;99:2708–2708. doi: 10.1073/pnas.052005699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malaisse-Lagae F, Sener A, Garcia-Morales P, Valverde I, Malaisse WJ. The stimulus-secretion coupling of amino acid-induced insulin release. Influence of a nonmetabolized analog of leucine on the metabolism of glutamine in pancreatic islets. J Biol Chem. 1982;257:3754–3754. [PubMed] [Google Scholar]
- 28.Tamarit-Rodriguez J, Vara E, Tamarit J. Starvation-induced changes of palmitate metabolism and insulin secretion in isolated rat islets stimulated by glucose. Biochem J. 1984;221:317–317. doi: 10.1042/bj2210317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cahill GF., Jr Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–1. doi: 10.1146/annurev.nutr.26.061505.111258. [DOI] [PubMed] [Google Scholar]
- 30.Cahill GF, Jr., Herrera MG, Morgan AP, et al. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966;45:1751–1751. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boden G, Baile CA, McLaughlin CL, Matschinsky FM. Effects of starvation and obesity on somatostatin, insulin, and glucagon release from an isolated perfused organ system. Am J Physiol. 1981;241:E215–E220. doi: 10.1152/ajpendo.1981.241.3.E215. [DOI] [PubMed] [Google Scholar]
- 32.Stein DT, Esser V, Stevenson BE, et al. Essentiality of circulating fatty acids for glucose-stimulated insulin secretion in the fasted rat. J Clin Invest. 1996;97:2728–2728. doi: 10.1172/JCI118727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malaisse WJ, Sener A, Levy J. The stimulus-secretion coupling of glucose-induced insulin release. Fasting-induced adaptation of key glycolytic enzymes in isolated islets. J Biol Chem. 1976;251:1731–1731. [PubMed] [Google Scholar]
- 34.Vara E, Tamarit-Rodriguez J. Glucose stimulation of insulin secretion in islets of fed and starved rats and its dependence on lipid metabolism. Metabolism. 1986;35:266–266. doi: 10.1016/0026-0495(86)90212-x. [DOI] [PubMed] [Google Scholar]
- 35.Iwashima Y, Kondoh-Abiko A, Seino S, et al. Reduced levels of messenger ribonucleic acid for calcium channel, glucose transporter-2, and glucokinase are associated with alterations in insulin secretion in fasted rats. Endocrinology. 1994;135:1010–1010. doi: 10.1210/endo.135.3.8070343. [DOI] [PubMed] [Google Scholar]
- 36.Tiedge M, Lenzen S. Effects of glucose refeeding and glibenclamide treatment on glucokinase and GLUT2 gene expression in pancreatic B-cells and liver from rats. Biochem J. 1995;308:139–139. doi: 10.1042/bj3080139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J Clin Invest. 1999;103:1489–1489. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gremlich S, Nolan C, Roduit R, et al. Pancreatic islet adaptation to fasting is dependent on peroxisome proliferator-activated receptor alpha transcriptional up-regulation of fatty acid oxidation. Endocrinology. 2005;146:375–375. doi: 10.1210/en.2004-0667. [DOI] [PubMed] [Google Scholar]
- 39.Gupta RK, Vatamaniuk MZ, Lee CS, et al. The MODY1 gene HNF-4alpha regulates selected genes involved in insulin secretion. J Clin Invest. 2005;115:1006–1006. doi: 10.1172/JCI200522365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim-Muller JY, Kim YJ, Fan J, et al. FoxO1 deacetylation decreases fatty acid oxidation in beta-cells and sustains insulin secretion in diabetes. J Biol Chem. 2016;291:9648–9648. doi: 10.1074/jbc.M115.705608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kitamura YI, Kitamura T, Kruse JP, et al. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2005;2:153–153. doi: 10.1016/j.cmet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Kim-Muller JY, Zhao S, Srivastava S, et al. Metabolic inflexibility impairs insulin secretion and results in MODY-like diabetes in triple FoxO-deficient mice. Cell Metab. 2014;20:593–593. doi: 10.1016/j.cmet.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linnemann AK, Baan M, Davis DB. Pancreatic beta-cell proliferation in obesity. Adv Nutr. 2014;5:278–278. doi: 10.3945/an.113.005488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–773. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leahy JL. Pathogenesis of type 2 diabetes mellitus. Arch Med Res. 2005;36:197–197. doi: 10.1016/j.arcmed.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Fellmann L, Nascimento AR, Tibirica E, Bousquet P. Murine models for pharmacological studies of the metabolic syndrome. Pharmacol Ther. 2013;137:331–331. doi: 10.1016/j.pharmthera.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–193. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- 48.Milburn JL, Jr, Hirose H, Lee YH, et al. Pancreatic beta-cells in obesity. Evidence for induction of functional, morphologic, and metabolic abnormalities by increased long chain fatty acids. J Biol Chem. 1995;270:1295–1295. doi: 10.1074/jbc.270.3.1295. [DOI] [PubMed] [Google Scholar]
- 49.Zhou YP, Cockburn BN, Pugh W, Polonsky KS. Basal insulin hypersecretion in insulin-resistant Zucker diabetic and Zucker fatty rats: role of enhanced fuel metabolism. Metabolism. 1999;48:857–857. doi: 10.1016/s0026-0495(99)90219-6. [DOI] [PubMed] [Google Scholar]
- 50.Poitout V, Amyot J, Semache M, Zarrouki B, Hagman D, Fontes G. Glucolipotoxicity of the pancreatic beta cell. Biochim Biophys Acta. 2010;1801:289–289. doi: 10.1016/j.bbalip.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1802. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dai C, Brissova M, Hang Y, et al. Islet-enriched gene expression and glucose-induced insulin secretion in human and mouse islets. Diabetologia. 2012;55:707–707. doi: 10.1007/s00125-011-2369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khaldi MZ, Guiot Y, Gilon P, Henquin JC, Jonas JC. Increased glucose sensitivity of both triggering and amplifying pathways of insulin secretion in rat islets cultured for 1 wk in high glucose. Am J Physiol Endocrinol Metab. 2004;287:E207–E217. doi: 10.1152/ajpendo.00426.2003. [DOI] [PubMed] [Google Scholar]
- 54.Hirose H, Lee YH, Inman LR, Nagasawa Y, Johnson JH, Unger RH. Defective fatty acid-mediated beta-cell compensation in Zucker diabetic fatty rats. Pathogenic implications for obesity-dependent diabetes. J Biol Chem. 1996;271:5633–5633. doi: 10.1074/jbc.271.10.5633. [DOI] [PubMed] [Google Scholar]
- 55.Diraison F, Ravier MA, Richards SK, Smith RM, Shimano H, Rutter GA. SREBP1 is required for the induction by glucose of pancreatic beta-cell genes involved in glucose sensing. J Lipid Res. 2008;49:814–814. doi: 10.1194/jlr.M700533-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lalloyer F, Vandewalle B, Percevault F, et al. Peroxisome proliferator-activated receptor alpha improves pancreatic adaptation to insulin resistance in obese mice and reduces lipotoxicity in human islets. Diabetes. 2006;55:1605–1605. doi: 10.2337/db06-0016. [DOI] [PubMed] [Google Scholar]
- 57.Ravnskjaer K, Frigerio F, Boergesen M, Nielsen T, Maechler P, Mandrup S. PPARdelta is a fatty acid sensor that enhances mitochondrial oxidation in insulin-secreting cells and protects against fatty acid-induced dysfunction. J Lipid Res. 2010;51:1370–1370. doi: 10.1194/jlr.M001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winzell MS, Wulff EM, Olsen GS, Sauerberg P, Gotfredsen CF, Ahren B. Improved insulin sensitivity and islet function after PPARdelta activation in diabetic db/db mice. Eur J Pharmacol. 2010;626:297–297. doi: 10.1016/j.ejphar.2009.09.053. [DOI] [PubMed] [Google Scholar]
- 59.Tang T, Abbott MJ, Ahmadian M, Lopes AB, Wang Y, Sul HS. Desnutrin/ATGL activates PPARdelta to promote mitochondrial function for insulin secretion in islet beta cells. Cell Metab. 2013;18:883–883. doi: 10.1016/j.cmet.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Efanov AM, Sewing S, Bokvist K, Gromada J. Liver X receptor activation stimulates insulin secretion via modulation of glucose and lipid metabolism in pancreatic beta-cells. Diabetes. 2004;53(Suppl 3):S75–75. doi: 10.2337/diabetes.53.suppl_3.s75. [DOI] [PubMed] [Google Scholar]
- 61.Green CD, Jump DB, Olson LK. Elevated insulin secretion from liver X receptor-activated pancreatic beta-cells involves increased de novo lipid synthesis and triacylglyceride turnover. Endocrinology. 2009;150:2637–2637. doi: 10.1210/en.2008-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gerin I, Dolinsky VW, Shackman JG, et al. LXRbeta is required for adipocyte growth, glucose homeostasis, and beta cell function. J Biol Chem. 2005;280:23024–23024. doi: 10.1074/jbc.M412564200. [DOI] [PubMed] [Google Scholar]
- 63.Busch AK, Gurisik E, Cordery DV, et al. Increased fatty acid desaturation and enhanced expression of stearoyl coenzyme A desaturase protects pancreatic beta-cells from lipoapoptosis. Diabetes. 2005;54:2917–2917. doi: 10.2337/diabetes.54.10.2917. [DOI] [PubMed] [Google Scholar]
- 64.Peyot ML, Pepin E, Lamontagne J, et al. Beta-cell failure in diet-induced obese mice stratified according to body weight gain: secretory dysfunction and altered islet lipid metabolism without steatosis or reduced beta-cell mass. Diabetes. 2010;59:2178–2178. doi: 10.2337/db09-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferdaoussi M, Dai X, Jensen MV, et al. Isocitrate-to-SENP1 signaling amplifies insulin secretion and rescues dysfunctional beta cells. J Clin Invest. 2015;125:3847–3847. doi: 10.1172/JCI82498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boucher A, Lu D, Burgess SC, et al. Biochemical mechanism of lipid-induced impairment of glucose-stimulated insulin secretion and reversal with a malate analogue. J Biol Chem. 2004;279:27263–27263. doi: 10.1074/jbc.M401167200. [DOI] [PubMed] [Google Scholar]
- 67.Del Guerra S, Lupi R, Marselli L, et al. Functional and molecular defects of pancreatic islets in human type 2 diabetes. Diabetes. 2005;54:727–727. doi: 10.2337/diabetes.54.3.727. [DOI] [PubMed] [Google Scholar]
- 68.Doliba NM, Qin W, Najafi H, et al. Glucokinase activation repairs defective bioenergetics of islets of Langerhans isolated from type 2 diabetics. Am J Physiol Endocrinol Metab. 2012;302:E87–E102. doi: 10.1152/ajpendo.00218.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1076. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Supale S, Li N, Brun T, Maechler P. Mitochondrial dysfunction in pancreatic beta cells. Trends Endocrinol Metab. 2012;23:477–477. doi: 10.1016/j.tem.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 71.Guo S, Dai C, Guo M, et al. Inactivation of specific beta cell transcription factors in type 2 diabetes. J Clin Invest. 2013;123:3305–3305. doi: 10.1172/JCI65390. [DOI] [PMC free article] [PubMed] [Google Scholar]