Abstract
AIMS--To determine whether aortic adventitial chronic inflammation associated with advanced atherosclerosis ("chronic periaortitis") is associated with any detectable cytokine gene expression. METHODS--RNA was extracted from six fresh surgical specimens of atherosclertic aortic aneurysm wall showing a spectrum of chronic periaortitis. Controls included four normal aortas and an HUT 78 T cell line. Reverse transcriptase and the polymerase chain reaction (PCR) were used to amplify mRNA for interleukins-1 alpha (IL-1 alpha), -2 (IL-2), -4 (IL-4), IL-2 receptor-alpha (IL-2R-alpha), tumour necrosis factor alpha (TNF-alpha) and gamma interferon (IFN-gamma) with beta-actin as an internal control. RESULTS--No TNF-alpha mRNA was detected in any of the inflamed aortic tissue samples, in contrast to the aortic T lymphocytes propagated in culture in IL-2 conditioned medium (aortic cultured T cells) and peripheral blood mononuclear cells from these patients. In contrast, IFN-gamma, IL-1 alpha, IL-2, IL-2 receptor and IL-4 PCR products were detected for each inflamed aortic tissue RNA sample with IFN-gamma mRNA expression increasing with increasing degrees of adventitial inflammation. Only beta-actin mRNA was present in the normal aorta. CONCLUSIONS--These findings indicate the active nature of aortic adventitial chronic inflammation associated with human advanced atherosclerosis ("chronic periaortitis") and show its possible progressive potential to the clinically important diseases termed "idiopathic retroperitoneal fibrosis" and "inflammatory aneurysm".
Full text
PDF



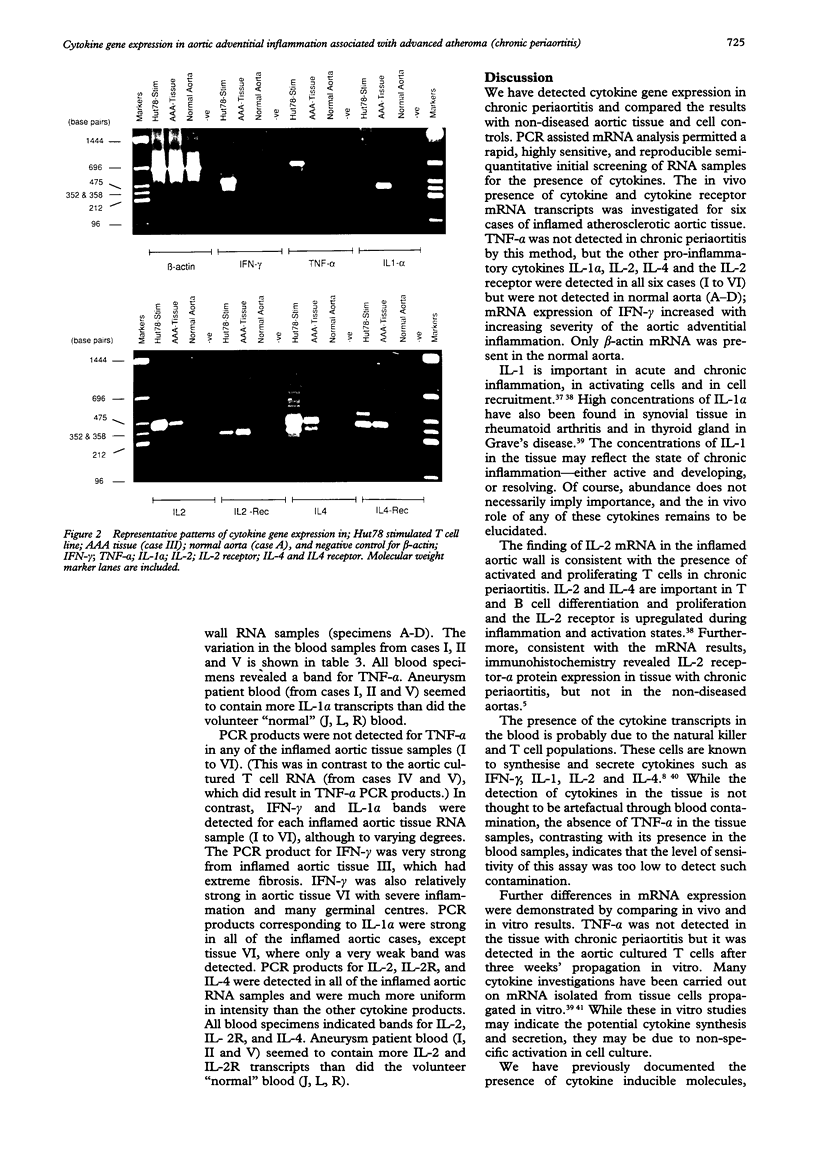


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K. I., Lee F., Miyajima A., Miyatake S., Arai N., Yokota T. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- Brennan F. M., Field M., Chu C. Q., Feldmann M., Maini R. N. Cytokine expression in rheumatoid arthritis. Br J Rheumatol. 1991;30 (Suppl 1):76–80. [PubMed] [Google Scholar]
- Brenner C. A., Tam A. W., Nelson P. A., Engleman E. G., Suzuki N., Fry K. E., Larrick J. W. Message amplification phenotyping (MAPPing): a technique to simultaneously measure multiple mRNAs from small numbers of cells. Biotechniques. 1989 Nov-Dec;7(10):1096–1103. [PubMed] [Google Scholar]
- Brown K. A. The polymorphonuclear cell in rheumatoid arthritis. Br J Rheumatol. 1988 Apr;27(2):150–155. doi: 10.1093/rheumatology/27.2.150. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clinton S. K., Libby P. Cytokines and growth factors in atherogenesis. Arch Pathol Lab Med. 1992 Dec;116(12):1292–1300. [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Ehlers S., Smith K. A. Differentiation of T cell lymphokine gene expression: the in vitro acquisition of T cell memory. J Exp Med. 1991 Jan 1;173(1):25–36. doi: 10.1084/jem.173.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erber W. N., Pinching A. J., Mason D. Y. Immunocytochemical detection of T and B cell populations in routine blood smears. Lancet. 1984 May 12;1(8385):1042–1046. doi: 10.1016/s0140-6736(84)91451-x. [DOI] [PubMed] [Google Scholar]
- Feldmann M., Brennan F. M., Chantry D., Haworth C., Turner M., Abney E., Buchan G., Barrett K., Barkley D., Chu A. Cytokine production in the rheumatoid joint: implications for treatment. Ann Rheum Dis. 1990 Jun;49 (Suppl 1):480–486. [PubMed] [Google Scholar]
- Feldmann M., Brennan F. M., Chantry D., Haworth C., Turner M., Katsikis P., Londei M., Abney E., Buchan G., Barrett K. Cytokine assays: role in evaluation of the pathogenesis of autoimmunity. Immunol Rev. 1991 Feb;119:105–123. doi: 10.1111/j.1600-065x.1991.tb00580.x. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Leung D. W., Pennica D., Yelverton E., Najarian R., Simonsen C. C., Derynck R., Sherwood P. J., Wallace D. M., Berger S. L. Expression of human immune interferon cDNA in E. coli and monkey cells. Nature. 1982 Feb 11;295(5849):503–508. doi: 10.1038/295503a0. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. A., Ma G. P., Chisolm G. M. Oxidized low density lipoprotein suppresses the expression of tumor necrosis factor-alpha mRNA in stimulated murine peritoneal macrophages. J Immunol. 1990 Mar 15;144(6):2343–2350. [PubMed] [Google Scholar]
- Holbrook N. J., Smith K. A., Fornace A. J., Jr, Comeau C. M., Wiskocil R. L., Crabtree G. R. T-cell growth factor: complete nucleotide sequence and organization of the gene in normal and malignant cells. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1634–1638. doi: 10.1073/pnas.81.6.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P. M., Bliss E., Morton J. A., Burns J., McGee J. O. Monoclonal antibody EBM/11: high cellular specificity for human macrophages. J Clin Pathol. 1988 May;41(5):510–515. doi: 10.1136/jcp.41.5.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. E., Kunkel S. L., Pearce W. H., Shah M. R., Parikh D., Evanoff H. L., Haines G. K., Burdick M. D., Strieter R. M. Enhanced production of the chemotactic cytokines interleukin-8 and monocyte chemoattractant protein-1 in human abdominal aortic aneurysms. Am J Pathol. 1993 May;142(5):1423–1431. [PMC free article] [PubMed] [Google Scholar]
- Kung P., Goldstein G., Reinherz E. L., Schlossman S. F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979 Oct 19;206(4416):347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- Kurnick J. T., Grönvik K. O., Kimura A. K., Lindblom J. B., Skoog V. T., Sjöberg O., Wigzell H. Long term growth in vitro of human T cell blasts with maintenance of specificity and function. J Immunol. 1979 Apr;122(4):1255–1260. [PubMed] [Google Scholar]
- March C. J., Mosley B., Larsen A., Cerretti D. P., Braedt G., Price V., Gillis S., Henney C. S., Kronheim S. R., Grabstein K. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985 Jun 20;315(6021):641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- Mitchinson M. J. Chronic periaortitis and periarteritis. Histopathology. 1984 Jul;8(4):589–600. doi: 10.1111/j.1365-2559.1984.tb02371.x. [DOI] [PubMed] [Google Scholar]
- Nathan C., Sporn M. Cytokines in context. J Cell Biol. 1991 Jun;113(5):981–986. doi: 10.1083/jcb.113.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelken N. A., Coughlin S. R., Gordon D., Wilcox J. N. Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Invest. 1991 Oct;88(4):1121–1127. doi: 10.1172/JCI115411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido T., Shimizu A., Ishida N., Sabe H., Teshigawara K., Maeda M., Uchiyama T., Yodoi J., Honjo T. Molecular cloning of cDNA encoding human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):631–635. doi: 10.1038/311631a0. [DOI] [PubMed] [Google Scholar]
- Parums D. V., Brown D. L., Mitchinson M. J. Serum antibodies to oxidized low-density lipoprotein and ceroid in chronic periaortitis. Arch Pathol Lab Med. 1990 Apr;114(4):383–387. [PubMed] [Google Scholar]
- Parums D. V. The spectrum of chronic periaortitis. Histopathology. 1990 May;16(5):423–431. doi: 10.1111/j.1365-2559.1990.tb01541.x. [DOI] [PubMed] [Google Scholar]
- Paul W. E. Pleiotropy and redundancy: T cell-derived lymphokines in the immune response. Cell. 1989 May 19;57(4):521–524. doi: 10.1016/0092-8674(89)90121-9. [DOI] [PubMed] [Google Scholar]
- Pearce W. H., Sweis I., Yao J. S., McCarthy W. J., Koch A. E. Interleukin-1 beta and tumor necrosis factor-alpha release in normal and diseased human infrarenal aortas. J Vasc Surg. 1992 Nov;16(5):784–789. [PubMed] [Google Scholar]
- Pennica D., Nedwin G. E., Hayflick J. S., Seeburg P. H., Derynck R., Palladino M. A., Kohr W. J., Aggarwal B. B., Goeddel D. V. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984 Dec 20;312(5996):724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- Ramshaw A. L., Parums D. V. Immunohistochemical characterization of inflammatory cells associated with advanced atherosclerosis. Histopathology. 1990 Dec;17(6):543–552. doi: 10.1111/j.1365-2559.1990.tb00794.x. [DOI] [PubMed] [Google Scholar]
- Ramshaw A. L., Parums D. V. The distribution of adhesion molecules in chronic periaortitis. Histopathology. 1994 Jan;24(1):23–32. doi: 10.1111/j.1365-2559.1994.tb01267.x. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988 Aug 5;241(4866):708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Rees R. C. Cytokines as biological response modifiers. J Clin Pathol. 1992 Feb;45(2):93–98. doi: 10.1136/jcp.45.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Masuda J., Raines E. W., Gown A. M., Katsuda S., Sasahara M., Malden L. T., Masuko H., Sato H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science. 1990 May 25;248(4958):1009–1012. doi: 10.1126/science.2343305. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Stein H., Gerdes J., Mason D. Y. The normal and malignant germinal centre. Clin Haematol. 1982 Oct;11(3):531–559. [PubMed] [Google Scholar]
- Takeya M., Yoshimura T., Leonard E. J., Takahashi K. Detection of monocyte chemoattractant protein-1 in human atherosclerotic lesions by an anti-monocyte chemoattractant protein-1 monoclonal antibody. Hum Pathol. 1993 May;24(5):534–539. doi: 10.1016/0046-8177(93)90166-e. [DOI] [PubMed] [Google Scholar]
- Thornhill M. H., Wellicome S. M., Mahiouz D. L., Lanchbury J. S., Kyan-Aung U., Haskard D. O. Tumor necrosis factor combines with IL-4 or IFN-gamma to selectively enhance endothelial cell adhesiveness for T cells. The contribution of vascular cell adhesion molecule-1-dependent and -independent binding mechanisms. J Immunol. 1991 Jan 15;146(2):592–598. [PubMed] [Google Scholar]
- Tipping P. G., Hancock W. W. Production of tumor necrosis factor and interleukin-1 by macrophages from human atheromatous plaques. Am J Pathol. 1993 Jun;142(6):1721–1728. [PMC free article] [PubMed] [Google Scholar]
- Veres G., Gibbs R. A., Scherer S. E., Caskey C. T. The molecular basis of the sparse fur mouse mutation. Science. 1987 Jul 24;237(4813):415–417. doi: 10.1126/science.3603027. [DOI] [PubMed] [Google Scholar]
- Wood K. M., Cadogan M. D., Ramshaw A. L., Parums D. V. The distribution of adhesion molecules in human atherosclerosis. Histopathology. 1993 May;22(5):437–444. doi: 10.1111/j.1365-2559.1993.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Yokota T., Otsuka T., Mosmann T., Banchereau J., DeFrance T., Blanchard D., De Vries J. E., Lee F., Arai K. Isolation and characterization of a human interleukin cDNA clone, homologous to mouse B-cell stimulatory factor 1, that expresses B-cell- and T-cell-stimulating activities. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5894–5898. doi: 10.1073/pnas.83.16.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]