Abstract
Sepsis is often characterized by an acute brain inflammation and dysfunction, which is associated with increased morbidity and mortality worldwide. Preventing cerebral leukocyte recruitment may provide the key to halt progression of systemic inflammation to the brain. Here we investigated the influence of the anti-inflammatory and anti-oxidant compound, sulforaphane (SFN) on lipopolysachharide (LPS)-induced cellular interactions in the brain.
The inflammatory response elicited by LPS was blunted by SFN administration (5 and 50 mg/Kg i.p.) 24 h prior to LPS treatment in WT animals, as visualized and quantified using intravital microscopy. This protective effect of SFN was lost in Nrf2-KO mice at the lower dose tested, however 50 mg/Kg SFN revealed a partial effect, suggesting SFN works in part independently of Nrf2 activity. In vitro, SFN reduced neutrophil recruitment to human brain endothelial cells via a down regulation of E-selectin and vascular cell adhesion molecule 1 (VCAM-1). Our data confirm a fundamental dose-dependent role of SFN in limiting cerebral inflammation. Furthermore, our data demonstrate that not only is Nrf2 in part essential in mediating these neuroprotective effects, but they occur via down-regulation of E-selectin and VCAM-1. In conclusion, SFN may provide a useful therapeutic drug to reduce cerebral inflammation in sepsis.
Keywords: Cerebrovascular, Leukocyte, Inflammation, Nrf2, Sulforaphane
Graphical abstract
Introduction
Uncontrolled inflammation is now considered a major component of various widespread diseases including Alzheimer’s disease [1], stroke [2], cancer [3] and sepsis [4]. Whilst it is generally accepted that inflammation is not the primary cause of these diseases, it plays a key role in disease progression, tissue dysfunction and ultimately organ failure. Sepsis is an inflammatory condition involving a complex interaction of multiple pathways. It is the most common causes of death in hospitalized patients, which in part, is due to the susceptibility of the expanding aged population to infection, increased frequency of invasive procedures, and widespread bacterial antibiotic resistance [5, 6] and forecasts are predicting a rise to over one million cases annually by 2020 [1].
Endothelial dysregulation, coagulopathy with microvascular thrombosis, excessive vascular leak and increased leukocyte activation lie at the heart of tissue injury and organ failure during sepsis. When activated, leukocytes undergo a sequential pattern of interaction with vascular endothelial cells, characterized by rolling, adhesion and emigration into inflamed/infected tissue [7]. This inflammatory response which is observed in sepsis [8] is caused within hours by peripheral inflammogens inducing an up-regulation of cell adhesion molecules on the surface of the endothelium (P-selectin, E-selectin, vascular cell adhesion molecule 1 [VCAM-1], and intercellular adhesion molecule 1 [ICAM-1]) and leading to increased adhesion, rolling, and transmigration of circulating leukocytes.
Over half of the cases of sepsis are due to gram-negative bacteria [9], in which endotoxin (lipopolysachharide [LPS]) is a key cell wall component. By interacting with host cell receptors, such as monocyte/macrophage CD14 and toll-like receptor 4 (TLR4), LPS induces proinflammatory mediators e.g. eicosanoids and cytokines, such as tumor necrosis factor-α (TNF-α), interleukin 1 beta (IL-1β), IL-6 [10]. Cytokines are produced by several cell types, including neutrophils, monocytes, macrophages, lymphocytes, and endothelial cells [7]. They are released in response to inflammatory stimuli and are responsible for causing many of the physiological, metabolic and immunological responses associated with inflammation [8]. Despite these effects, therapies directed towards combating the pro-inflammatory cytokines have been unsuccessful in the clinic. Additionally, patients that survive sepsis are sometimes left with long-term cognitive difficulties and neurological defects due to sepsis-associated encephalopathy, which occurs despite the protection provided by the blood-brain barrier [8]. Therefore, there is an urgent need for new and effective therapies to combat sepsis.
Sulforaphane (SFN) is a sulfur-based isothiocyanate found naturally in cruciferous vegetables such as broccoli, brussel sprouts, cabbage and cauliflower [11]. SFN has been shown to induce anti-oxidative mechanisms, and protect against cell inflammatory stress via activation of the NF-erythroid 2-related factor 2 (Nrf2) transcription factor [12]. Under basal conditions, Nrf2 is anchored to the cytoplasm by Kelch-like ECH-associated protein 1 (Keap1). Through interactions with the cysteine residues of Keap1, SFN may induce the release of Nrf2 allowing for its nuclear localization where it binds to the antioxidant response element (ARE) in the promoter region of a variety of different genes involved in antioxidant protection, including heme oxygenases and superoxide dismutase [12, 13]. Although it has been shown that SFN may have anti-inflammatory effects, these anti-inflammatory mechanisms are less well-understood, especially within the context of the cerebral microvasculature. SFN treatment reduces monocyte adhesion to primary endothelial cells via inhibition of nuclear factor kappa B (NF-κB) activation [15] and treatment of cultured Raw 264.7 macrophages with SFN suppresses LPS-induced nitric oxide generation, prostaglandin E2 (PGE2) production, TNF-α secretion, inducible nitric oxide synthase (iNOS) and Cox-2 expression [16]. SFN pre-conditioning has also been shown to protect against blood brain barrier (BBB) disruption and neurological deficits in a rodent model of stroke [17]. As such, SFN treatments or dietary supplements may prove effective in protecting against pathological leukocyte recruitment to the brain.
In this study, we utilized in vivo and in vitro models of leukocyte recruitment to investigate the potential of SFN to reduce cerebral leukocyte recruitment and inflammatory activation. Our results support the premise that SFN plays an important role in protecting the cerebral vasculature from inflammatory insults. We also show for the first time that following systemic inflammatory insults, SFN exerts its protective actions on human cerebral endothelial cells (HBMEC-3) by inhibiting VCAM-1 and E-selectin expression. Furthermore, this protective effect of SFN was lost in Nrf2-KO mice at the lower dose tested, however 50 mg/Kg SFN revealed a partial effect, suggesting SFN works in part independently of Nrf2 activity. In conclusion, SFN may provide a useful therapeutic drug to reduce cerebral inflammation in sepsis.
Materials and methods
Reagents
All reagents were obtained from Sigma-Aldrich, St Louis, MO, USA, unless stated otherwise.
Animal experiments
Drugs and reagents
L-sulforaphane (SFN, 5 mg/kg and 50 mg/kg) was dissolved in corn oil for intraperitoneal (i.p.) injection 24 h prior to LPS/vehicle injection as previously described [18]. LPS (Escherichia coli serotype 0111:B4. 0.5 mg/Kg,) was dissolved in saline for i.p. injection 4 h prior to imaging of the cerebral microcirculation. Rhodamine 6G was dissolved in de-ionized water for i.v. injection.
Animals
Male wild-type (WT) C57BL/6J mice and Nfe2l2tm1Ywk knockout (Nrf2-KO) mice, 25–35g, 12–18 weeks old, were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and maintained on a 12 hour (h) light-dark cycle during which room temperature was maintained at 21– 23°C, and had access to standard chow pellets and water ad libitum. Experimental procedures were reviewed and approved by Louisiana State University Health Sciences Center-Shreveport (LSUHSC-S) Institutional Animal Care and Use Committee, and were performed according to the criteria outlined in the National Institutes of Health guidelines. Animals were randomly assigned to treatment groups (n = 6 animals per group, group size determined with reference to our previous studies in this model [7].
Intravital fluorescence microscopy (IVM)
Animals were anesthetized with an i.p. injection of ketamine (150 mg/kg) and xylazine (7.5 mg/kg) and the jugular vein was cannulated for intravenous (i.v.) administration of rhodamine 6G. Core body temperature was maintained at 37 ± 0.5°C. A craniotomy was performed and the dura matter was not removed as fluorescently labeled blood cells were easily observed and intracranial pressure was well maintained in the absence of this procedure [20]. Artificial cerebrospinal fluid (NaCl 131.9 mM, CaCl2 1.26 mM, CaCl2·2H2O 1.26 mM, KCl 2.95mM, MgCl2·6H2O 0.64 mM, MgCl2 0.5 mM, (NH2)2CO 6.69 mM, C6H12O6 3.69 mM) was placed on the cranial opening. The preparation was allowed to equilibrate for 30 minute (min) before visualization of cerebral microcirculation using a 40 X water-immersion objective attached to a Xanophot IVM microscope (HLX64610; Nikon, Melville, NY, USA). Rhodamine 6G (100 µl, 0.02% v/w in saline, i.p) was used to fluorescently label leukocytes. Sections of un-branched pial post capillary venules 100 µm in length and 30–70 µm in diameter (omitting analysis of dura vessels which are typically 20 µm. [20]) were randomly selected and recordings made for offline analysis using a three charge coupled device color video camera (Hitachi, Woodbury, NY, USA). Rolling velocity (determined from the time required for a leukocyte to roll a given distance along the length of a venule, and is reported as µm/sec), and adhesion (cells that remained adherent for 30 seconds (sec) or longer, and expressed as the number of cells per millimeter of the venular surface, assuming the vessel to be cylindrical as previously described [19]) were assessed offline.
Myeloperoxidase (MPO) activity
Brain homogenates and MPO standards were placed onto a 96-well plate, and 200 µl of o-dianisidine solution and 10 µl of 0.1 % H2O2 were added. Absorbance was read after 5 min at 405 nm and expressed as units per mg (U/mg) of wet tissue.
Cytometric bead array (CBA)
Serum samples were collected from experimental animals and quantitative analysis of multiple cytokines, including: IL-6, IL-10, monocyte chemoattractant protein-1 (MCP-1), interferon gamma (IFN-γ), TNF-α, and IL-12(p70) was performed using a CBA Mouse Inflammation Kit (BD Biosciences, San Jose, CA, USA) as per the manufacturer’s instructions. A dual laser FACSCalibur (BD Biosciences), with CellQuest pro (BD Biosciences) software was used for acquisition and results were analyzed and quantified with reference to the standard curve using FCAP Array software (version 3, Soft Flow Inc, St. Louis Park, MN, USA).
Human neutrophil isolation
Blood collection and neutrophil isolation was performed with ethical approval from the LSUHSC-S Institutional Review Board (STUDY00000261). Blood samples (50ml) were taken from healthy individuals aged 18 – 45 years, with no history of recent acute or chronic illness and non-steroidal anti-inflammatory drug (NSAID) free 2 weeks prior to blood collection at LSUHSC-S. Blood was collected from the anterior cubital fossa with a 21G needle, using 1:10 Acid Citrate Dextrose Solution anticoagulant. All samples were collected and processed within 4 h. Neutrophils were isolated using dextran sedimentation and histopaque 1077 Ficoll gradient-centrifugation as previously described [21].
Flow chamber assay
Human brain endothelial cell line (HBMEC-3) were seeded in IBIDI µ-Slide VI04 flow chambers and cultured for two days until confluent. The cells were treated with SFN (1, 10 and 100 µM) or 0.3% Dimethyl sulfoxide vehicle control 24 h prior to the addition of LPS (500 ng/ml) or saline vehicle control. HBMEC-3 monolayers were stimulated with LPS for 4 h before commencing flow. Neutrophils were fluorescently labeled with 5 µM CellTracker™ Red CMTPX dye (Life Technologies, Grand Island, NY, USA) for 25 min at room temperature. Neutrophils were then centrifuged at 400 x g for 10 min and resuspended to 1×106 cells/ml in Dulbecco’s phosphate-buffered saline (DPBS) with 0.1% BSA before perfusing over HBMEC-3 monolayers at 0.5 dyne/cm2 for 10 min. Leukocyte endothelial interactions were viewed under an Olympus IX71 inverted microscope (x20 objective. Olympus, Saucon, PA, USA), with TRITC filter and 100W high pressure mercury bulb light source. At least 5 random fields per treatment were recorded for offline analysis, using a Sony 3CCD DSP color video camera (Sony, New York, NY, USA), for 10 sec each. Rolling velocity (velocities of 20 consecutive leukocytes in the field of focus were determined by measuring the time required to travel a distance of 100 µm. Reported as µm/sec) and adhesion (those cells that had remained stationary for 10 sec or longer) were quantified during offline analysis as previously described [19].
Immunofluorescent staining
HBMEC-3 seeded in IBIDI µ-Slide VI0.4 flow chambers monolayers were treated with SFN (1, 10 and 100 µM) or vehicle 24 h prior to the addition of LPS (500 ng/ml for 4 h). Cells were washed with PBS and fixed with 4% Paraformaldehyde (PFA) for 10 min before blocking with PBS + 1% BSA, 10% goat serum and 100 mM glycine. Cells were then incubated with primary antibodies for P-selectin (ab54427, 1:100 Abcam, Cambridge, MA, USA,), E-selectin (BBA16, 1:200, R&D systems, Minneapolis, MN, USA), or vascular cell adhesion molecule-1 (VCAM-1, SC1504R, Santa Cruz Biotechnology, Dallas, TX, USA 1:200) in PBS + 1% BSA overnight at 4°C. A Cy5.5 conjugated goat anti-mouse IgG (ab6947 Abcam) or goat anti-rabbit, alexafluor 488 (ab150077, Abcam) both at 1:200 was used as a secondary antibody and cells were mounted in vectasheild + DAPI nuclear stain. Staining was visualized using a using a Nikon Eclipse inverted microscope with Nikon Elements acquisition software (Nikon, Melville, NY, USA), and was quantified using ImageJ software (NIH) and expressed as mean pixel intensity of adhesion molecule expression, normalized to DAPI expression.
NF-κB activation was investigated in HBMEC-3 cells fixed and permeabilized in ice cold acetone before blocking and staining for phosphorylated NF-κB p65 (rabbit anti-NF-kB p65 phospho-S536, ab86299, Abcam, 1:100 in PBS + 1% BSA) for 2 h. Cells were washed with PBS and incubated with goat anti-rabbit alexafluor 488 conjugated secondary (ab150077, 1:200, Abcam). Slides were washed with PBS and mounted with vectasheild + DAPI nuclear stain. Co-localization of DAPI and alexafluor 488 staining was visualized using a using a Nikon Eclipse inverted microscope with Nikon Elements acquisition software. Nuclear mean pixel intensity normalized to area was used to quantify nuclear levels of NF-κB using ImageJ software (NIH).
Nrf2 transcription factor activation
To investigate Nrf2 activation and nuclear localization in HBMEC-3 cells treated with SFN (1, 10 and 100 µM) 24 h prior to stimulation with LPS (10 µg/ml, 4 h), nuclear fractions were isolated using Active Motif Nuclear extract kit (Active Motif, Carlsbad, CA, USA). Nrf2 activity was then assessed in 5 µg nuclear protein using TransAM® Nrf2 transcription factor activation assay (Active Motif) as per the manufacturer’s instructions.
Superoxide assay
To study the effect of SFN treatment on endothelial and neutrophil oxidative state following LPS stimulation (500 ng/ml 4h), CM-H2DCFDA oxidative stress indicator (Life Technologies) was used and fluorescence emission at 525 nm measured over 30 min. Briefly, HBMEC-3 cells grown to confluence in 96-well plates were pre-treated with SFN or vehicle for 24 h before being incubated with 5 µM of the cell permeable CM-H2DCFDA dye for 30 min. 5×105 neutrophils in 100 µl PBS + 1% BSA were added to 96-well plates, blocked with PBS + 1% BSA overnight. Baseline readings were taken using a Biotech Synergy H1 hybrid plate reader (BioTeK, Winooski, VT, USA) at 37°C excitation 490 nm emission 520 nm. Neutrophils were then stimulated with LPS (500 ng/ml) and emission was measured over 30 min. Results expressed as mean maximum relative light units.
Statistical analysis
Results from intravital microscopy experiments were confirmed to follow a normal distribution using Kolmogorov-Smirnov test of normality with Dallal-Wilkinson-Lillie for corrected p value. Data that passed the normality assumption was analyzed using Student’s t-test or ANOVA with Bonferroni post hoc test. Data that failed the normality assumption were analyzed using the non-parametric Mann-Whitney U test. Analysis was conducted using GraphPad Prism5 software. Data are reported as means +/− standard errors of the mean. Differences were considered statistically significant at a value of p < 0.05.
Results
SFN protects against LPS-induced cellular responses in the cerebral microcirculation
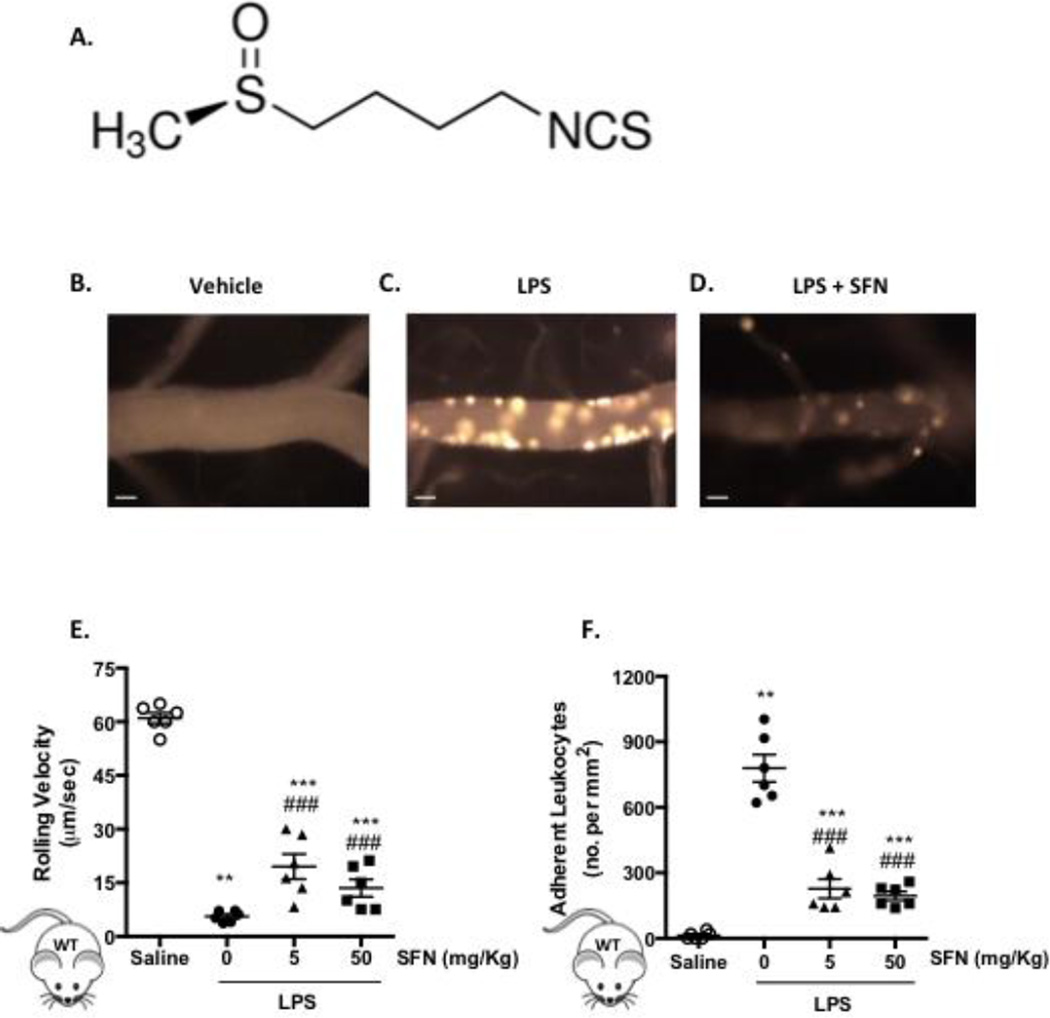
Previously we have shown that i.p. administration of LPS triggers a time-dependent effect on cellular responses in the brain [8]. Based on these findings, LPS was systemically administered for 4 h with and without SFN, in order to assess the therapeutic potential of the isothiocyanate. Using intravital fluorescence microscopy, we were able to visualize and quantify changes in leukocyte behavior within pial vessels, showing a significant (p < 0.001) decrease in leukocyte rolling velocity and an increase in leukocyte adhesion following LPS administration (Figure 1). Pre-treatment with SFN in the absence of an inflammatory stimulus was found to have no effect on any of the leukocyte recruitment parameters measured (Figure 1E+F). However, SFN (5 and 50 mg/Kg) significantly increased the LPS-induced leukocyte rolling velocity and reduced leukocyte adhesion (p < 0.001. Figure 1E+F).
Figure 1. SFN pre-treatment reduces LPS-induced inflammatory cell recruitment into the brain.
A) the chemical structure of SFN. B-D) representative video stills of cerebral microvessels following B) saline treatment, C) LPS treatment (0.5 mg/kg, i.p, 4 h) and D) LPS treatment with 24 h SFN pretreatment (50 mg/Kg). E-G) Leukocyte recruitment in the cerebral microcirculation of C57BL/6J mice following LPS injection (0.5 mg/kg, i.p) and 24 h saline vehicle or SFN pretreatment (5 or 50 mg/Kg) was quantified in terms of: E) rolling velocity (µm/sec), and F) adhesion (cells stationary for 30 sec or longer per mm2). Scale bar = 20 µm. Data are mean ± SEM of 6 mice per group. *p < 0.05 **p < 0.01 & ***p < 0.001 vs. saline. ###p < 0.0001 vs. LPS.
SFN modulates inflammatory markers
Table 1 demonstrates the effect of SFN treatment on circulating levels of cytokines. LPS induced an elevation in pro-inflammatory cytokines: IFN-γ IL-6, MCP-1 and TNF-α, with no effect on the anti-inflammatory cytokine IL-10. SFN (5 mg/Kg) decreased levels of IFN-γ MCP-1 and TNF-α, and increased IL-10 levels. These effects were also mirrored in animals treated with the higher dose of SFN (50 mg/Kg), coupled also with a decrease in IL-6 levels. There was no difference in IL-12 levels in any of the groups. We also found a significant increase in the same four cytokines in whole brain homogenates (Table 2) following LPS, along with a marked increase in MPO (Table 2). SFN treatment was found to reduce the LPS induced increase in brain inflammatory cytokines whilst also elevating IL-10 expression and reducing MPO levels. (LPS: 4.16 ± 0.28 U/mg; SFN (5 mg/Kg)+LPS: 2.68 ± 0.35; SFN (50 mg/Kg)+LPS: 1.73 ± 0.11 U/mg).
Table 1. LPS and SFN treatments modulate serum cytokine levels.
Effect of SFN treatment on serum cytokine levels. Serum levels of A) IFN-γ, IL-6, IL-10, IL-12, MCP-1 and TNF-α following LPS (0.5 mg/Kg) and 24h pre-treatment with SFN (5 and 50 mg/Kg), determined by cytometric bead array.
| IFN-γ (pg/ml) |
IL-6 (ng/ml) |
IL-10 (pg/ml) |
IL-12 (pg/ml) |
MCP-1 (pg/ml) |
TNF-α (pg/ml) |
|
|---|---|---|---|---|---|---|
| Saline | 4.70 ± 0.51 |
2.10 ± 0.31 |
5.21 ± 0.65 |
3.86 ± 0.65 |
9.36 ± 0.82 |
10.3 ± 0.89 |
|
LPS (0.5 mg/Kg) |
40.13 ± 2.12*** |
16.32 ± 1.49*** |
9.92 ± 0.99 |
10.12 ± 1.01* |
27.13 ± 2.34*** |
669.70 ± 90.05*** |
|
SFN+LPS (5 mg/Kg + 0.5 mg/Kg) |
22.75 ± 1.52### |
9.38 ± 0.73 |
19.28 ± 0.88###$$ |
8.67 ± 0.80 |
21.68 ± 198#$ |
487.60 ± 63.48# |
|
SFN+LPS (50 mg/Kg + 0.5 mg/Kg) |
19.48 ± 1.04### |
8.29 ± 0.87### |
28.90 ± 1.15### |
8.68 ± 0.76 |
18.21 ± 1.37# |
465.6 ± 71.72# |
Data are mean ± SEM of 4 mice per group performed in duplicate.
p < 0.05
p < 0.0001 vs. saline.
p < 0.05,
p < 0.001
p < 0.0001 vs. LPS.
p < 0.05
p < 0.001 vs. SFN 50 mg/Kg.
Table 2. LPS and SFN treatments modulate brain homogenate cytokine and MPO levels.
Effect of SFN treatment on brain homogenate MPO and cytokine levels. Brain homogenate levels of IFN-γ, IL-6, IL-10, IL-12, MCP-1, TNF-α and MPO following LPS (0.5 mg/Kg) and 24h pre-treatment with SFN (5 and 50 mg/Kg), determined by cytometric bead array.
 |
IFN-γ (pg/ml) |
IL-6 (ng/ml) |
IL-10 (pg/ml) |
IL-12 (pg/ml) |
MCP-1 (pg/ml) |
TNF-α (pg/ml) |
MPO (U/mg) |
|---|---|---|---|---|---|---|---|
| Saline | 4.63 ± 0.77 |
3.04 ± 0.59 |
5.55 ± 0.96 |
5.19 ± 1.08 |
9.60 ± 1.55 |
0.06 ± 0.60 |
1.78 ± 0.30 |
|
LPS (0.5 mg/Kg) |
45.35 ± 3.50*** |
22.09 ± 2.22** |
9.66 ± 1.00 |
9.74 ± 0.73 |
37.98 ± 4.24** |
866.5 ± 30.74** |
6.12 ± 0.19** |
|
SFN+LPS (5 mg/Kg + 0.5 mg/Kg |
29.13 ± 1.27##$) |
10.69 ± 1.22## |
21.75 ± 1.92## |
10.21 ± 0.93 |
23.05 ± 254#$ |
553.10 ± 61.92# |
3.12 ± 0.23# |
|
SFN+LPS (50 mg/Kg + 0.5 mg/Kg) |
23.61 ± 2.64## |
9.72 ± 0.92## |
29.39 ± 1.79## |
23.72 ± 12.32 |
17.68 ± 1.19## |
434.20 ± 40.20## |
2.13 ± 0.21# |
Data are mean ± SEM of 4 mice per group performed in duplicate.
p < 0.05,
p < 0.001 vs. saline
p < 0.0001.
p < 0.05
p < 0.001 vs. LPS.
p < 0.05
p < 0.001 vs. SFN 50 mg/Kg.
Dose dependent effect of SFN in Nrf2-KO animals
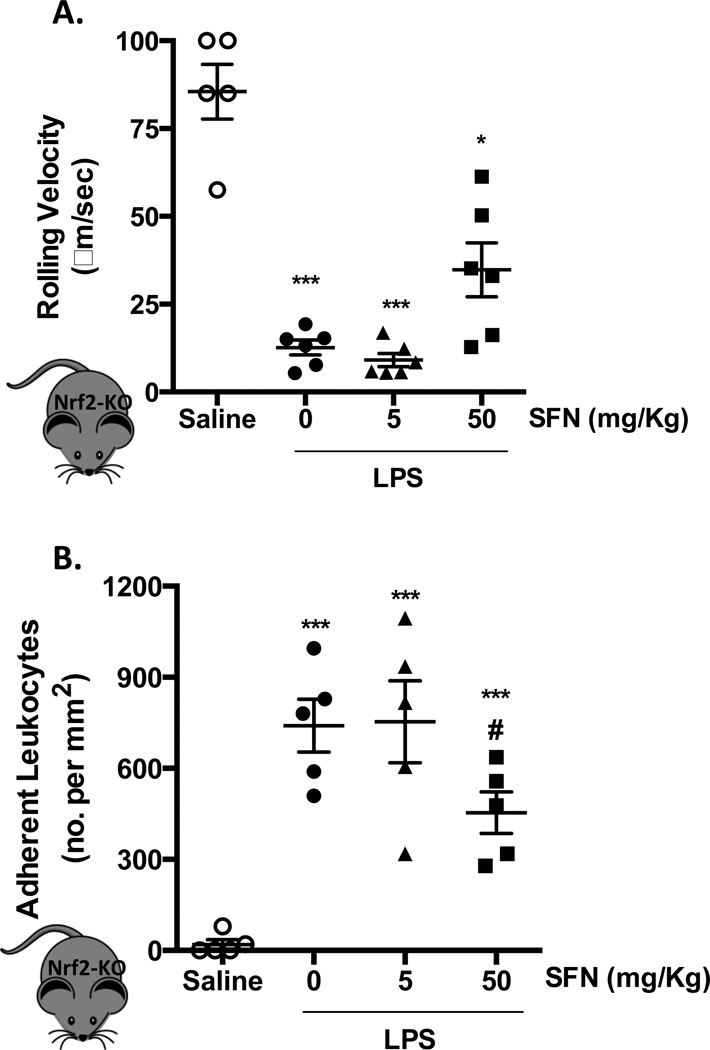
To investigate whether the observed reduction in LPS-induced leukocyte adhesion was due to activation of Nrf2 anti-inflammatory and anti-oxidative pathways, SFN was given to Nrf2-KO mice. Nrf2 gene deletion afforded no difference in the inflammatory response associated with LPS, when compared to the WT counterparts (Figure 1+2). Low dose of SFN (5 mg/Kg) treatment did not influence leukocyte rolling velocity or adhesion in these Nrf2-KO animals (Figure 2A+B). However, the higher SFN dose (50 mg/Kg) increased leukocyte velocity and decreased cellular adhesion (40%) in the brain. LPS produced an increase in the number of rolling cells (cell flux) in WT and Nrf2-KO vs. saline, with SFN having no effect (data not shown).
Figure 2. Dose dependent effect of SFN in Nrf2-KO animals.
Leukocyte recruitment in the cerebral microcirculation of Nrf2-KO mice following LPS injection (0.5 mg/kg, i.p) and 24 h saline vehicle or SFN pretreatment (5 or 50 mg/Kg) was quantified in terms of: A) rolling velocity (µm/sec) and B) adhesion (cells stationary for 30 sec or longer per mm2). Data are mean ± SEM of 5–6 mice per group. *p < 0.05 & ***p < 0.0001 vs. saline. #p < 0.05 vs. LPS.
SFN moderates human neutrophil-endothelial cell interactions
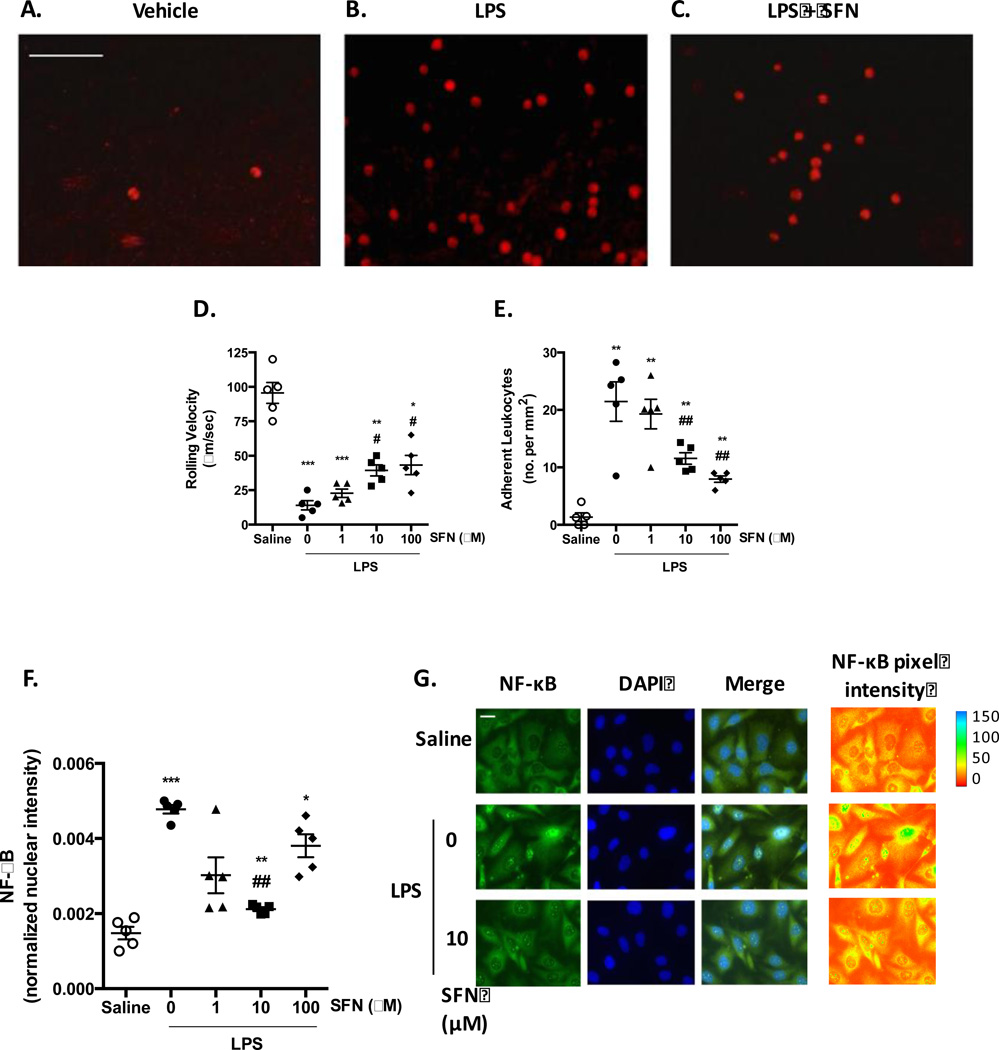
In order to establish whether SFN treatment was effective in reducing recruitment of human neutrophils, in vitro experiments were conducted flowing isolated human neutrophils over LPS activated HBMEC-3 cells, under conditions relevant to venous physiological shear rates. HBMEC-3 cells stimulated with LPS (500 ng/ml) for 4 h (a point that allows sufficient time for de novo synthesis of adhesion molecules [22].) provoked a marked decrease in neutrophil rolling velocity and an increase in the number of adherent cells (Figure 3A–E). Treatments with both 10 and 100 µM SFN had no effect on the number of leukocytes rolling in LPS treated mice, but affected leukocyte adhesion (Figure 3D+E).
Figure 3. Effects of SFN on Human Neutrophil-Endothelium Interaction.
HBMEC-3 cells were treated with saline or 500 ng/ml LPS for 4 h. A-E) human neutrophils were perfused over the stimulated endothelial cells under physiological flow to assess the influence of 24 h saline or SFN (1, 10 or 100 µM) pre-treatment on human neutrophil recruitment. A-C) Representative x20 video stills of fluorescently labeled neutrophils adhering to HBMEC-3 monolayers, scale bar = 100 µm. Neutrophil-endothelial cell interactions were quantified in terms of D) rolling velocity (µm/sec) and E) adhesion (cells stationary for 10 sec or longer per mm2). F+G) LPS-treated HBMEC-3 cells were fixed and permeabilized before staining for p65 NF-κB. F) levels of nuclear localization of fluorescent NF-κB signal were quantified and normalized to nuclear area n = 5 independent experiments. G) representative x60 micrographs demonstrating p65 NF-κB (alexafluor 488) staining. Pixel intensity is illustrated by pseudo color of greyscale NF-kB staining using image J. scale bar = 10 µm. Data are mean ± SEM. *p < 0.05, **p < 0.001, & ***p < 0.0001 vs. saline. #p < 0.05 & ##p < 0.001 vs. LPS.
Numerous studies have shown that the activation of NF-κB is essential for the transcriptional regulation of chemotactic cytokines and vascular adhesion molecules that are critically involved in leukocyte adhesion to endothelium [15]. Thus, we determined the LPS-induced activation of NF-κB signaling by confocal microscopic examination of NF-κB p65 nuclear translocation. Exposure of HBMEC-3 cells to LPS for 4 h increased NF-κB nuclear localization indicating the induction of NF-κB-regulated gene expression (Figure 3F+G). Whilst no significant reduction in NF-κB activation was seen with 1 µM or 100 µM SFN treatment, 10 µM resulted in a significant (p < 0.05) decrease in nuclear fluorescence intensity compared to control (Figure 3H). These results suggest that SFN may inhibit inflammation by suppressing NF-κB signaling in a dose dependent fashion.
SFN treatment reduces LPS-induced expression of endothelial adhesion molecules
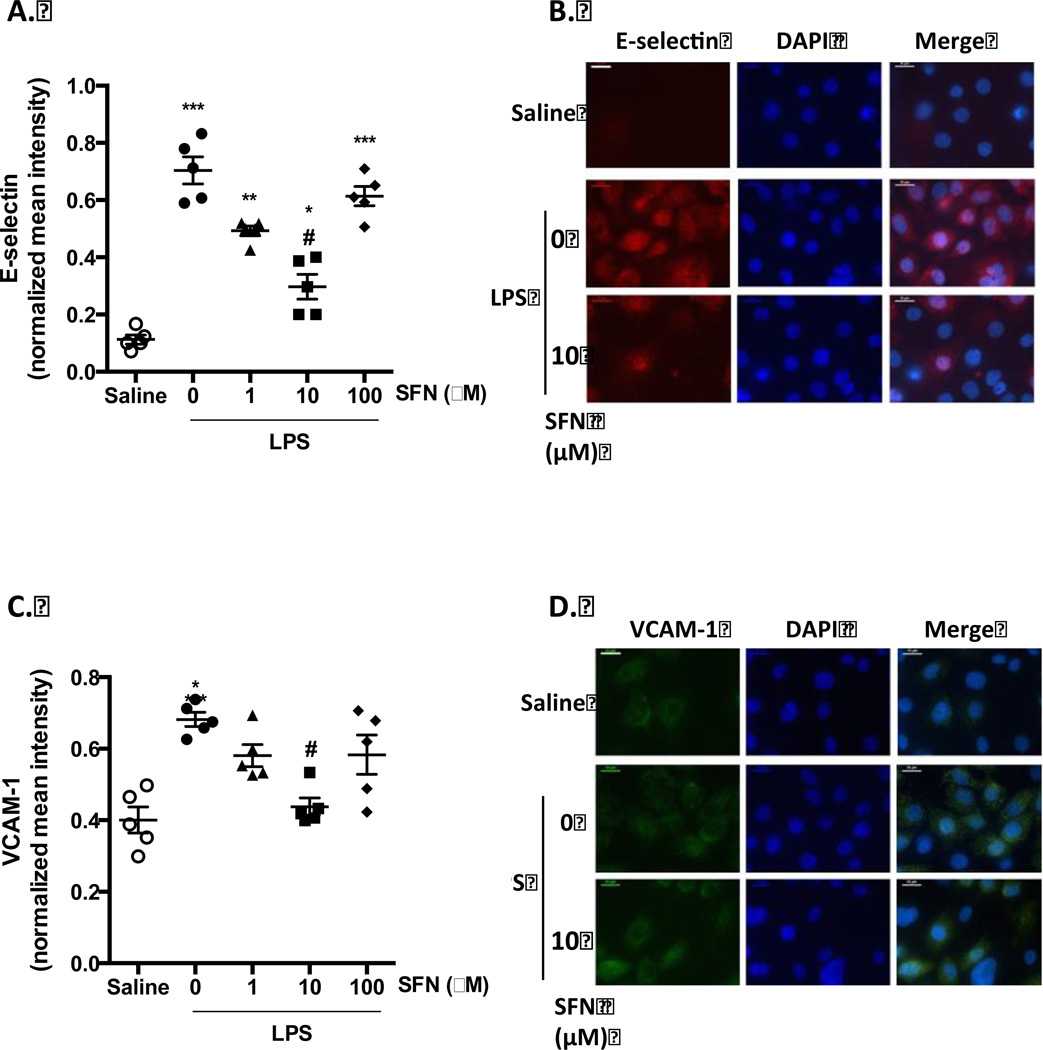
Cell adhesion molecules play an important role during inflammation, and their expression in endothelial cells is a pre-requisite for adhesion of neutrophils. Therefore, the effects of SFN on LPS-induced expression of E-selectin, P-Selectin, ICAM-1 and VCAM-1 were assessed by immunofluorescent staining (Figure 4). Unstimulated cells expressed low levels of the adhesion molecules, however, LPS treatment significantly induced the expression of HBMEC-3 E-selectin and VCAM-1. There was little effect on ICAM-1 expression, and whilst LPS induced a trend towards increased P-selectin expression, it was not statistically significant (data not shown). SFN treatment of 10µM was found to significantly reduce the LPS-induced expression of E-selectin and VCAM-1 (Figure 4A+C), with no statistically significant effects being observed on ICAM-1 and P-selectin expression (data not shown).
Figure 4. SFN reduces endothelial cell expression of adhesion molecules.
Surface expression of A + B) E-selectin and C + D) VCAM-1 were investigated using immune-fluorescent staining of non-permeabilized HBMEC-3 cells following SFN pre-treatment (1, 10 or 100 µM), and 4 h of saline or LPS (500 ng/ml) challenge. Staining was quantified as mean fluorescent intensity and normalized to account for cell number using the mean fluorescent intensity of DAPI staining. Scale bars = 10 µm. Data are mean ± SEM. n = 3 independent experiments for each treatment group. *p < 0.05, **p < 0.001 & ***p < 0.0001 vs. saline. #p < 0.05 vs. LPS.
SFN reduces endothelial cell oxidative state
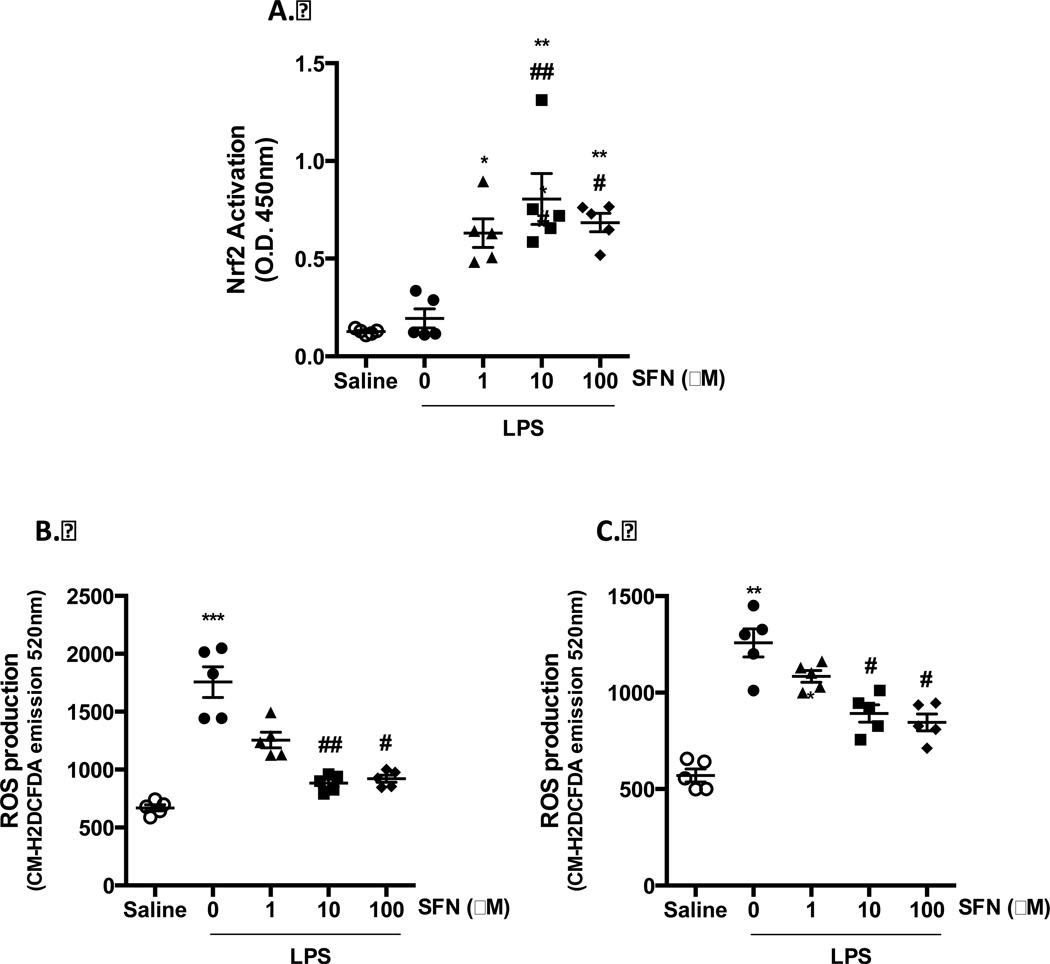
Oxidative stress is a key pathological factor local and systemic inflammatory pathologies. Considering that SFN has previously been shown to induce anti-oxidative enzyme expression via Nrf2 activation both in vivo and in vitro [23], we investigated the influence of SFN treatment on Nrf2 activation in the context of LPS challenge. In nuclear fractions from cortical homogenates of LPS challenged WT mice, no changes in Nrf2 activation was detected across all treatments (data not shown). However, in HBMEC-3, whilst LPS treatment alone had no influence on Nrf2 activation, all concentrations of SFN tested were found to induce significant activation and nuclear translocation of Nrf2, as indicated by enhanced transcription factor activity in nuclear extracts (Figure 5A).
Figure 5. SFN induces Nrf2 activation and protects endothelial cell and neutrophils from oxidative stress.
A) Nrf2 transcription factor activity in HBMEC-3 cells following LPS (500 ng/ml, 4 h) challenge and 24 h SFN pre-treatment as detected by Active moteif TransAM assay. ROS production as determined by CM-H2DCFDA fluorecence was asesed in SFN or saline or LPS treated B) HBMEC-3 cells or C) neutrophils. Data are mean ± SEM. n = 5. *p < 0.05, **p < 0.001 & ***p < 0.0001 vs. saline. #p < 0.05 and ##p < 0.001 vs. LPS.
To correlate the neuroprotective effect of SFN with its ability to induce Nrf2, we measured ROS production from HBMEC-3 using the fluorescent oxidative stress indicator CM-H2DCFDA. LPS treatment (500 ng/ml, 4h) was found to induce significant superoxide production from both neutrophils and HBMEC-3. A similar response was seen in both cell types, with 24 h pretreatment with SFN (10 and 100 µM) significantly inhibiting LPS-induced ROS production (Figure 5B+C).
Discussion
Sepsis is an inflammatory condition involving a complex interaction of multiple pathways. It is the most common cause of death in hospitalized patients, and forecasts are predicting a rise to over one million cases annually by 2020 [6]. Systemic inflammatory responses in sepsis can trigger a diffuse or multifocal cerebral dysfunction even in the absence of any direct brain injury or infection, this is particularly evident in e.g. sepsis-associated encephalopathy (SAE). Thus, the array of sepsis-related complications may lead to multiple organ failure and ultimately death [6]. Efficient anti-inflammatory strategies are therefore required in order to provide therapeutic benefit to patients.
SFN, which is found at high levels in broccoli, is currently one of the most promising natural compounds for clinical implementation [24]. The anti-carcinogenic effects of high doses of SFN are well characterized. Recently it has been reported that SFN can penetrate BBB and exert neuroprotective effects in in vitro and in vivo models of neurological disorders [17, 24], however the protective role of SFN on leukocyte trafficking within the context of the brain remains elusive. Here, using an animal model of endotoxaemia, we reveal a crucial inhibitory role of SFN in modulating leukocyte trafficking in the cerebral microcirculation. Our data show that Nrf2 gene deletion afforded no difference in the inflammatory response associated with LPS, when compared to the WT counterparts, and that SFN works through additional mechanisms other than Nrf2, since SFN’s influence over leukocyte recruitment was only in part abolished at the higher dose tested in Nrf2-KO animals. Furthermore, experiments using HBEMC-3 cells and human neutrophils, demonstrated protective actions of SFN following LPS challenge via down regulation of E-selectin, and VCAM-1, and a decrease in pro-inflammatory cytokines and ROS production.
Leukocyte recruitment to an inflamed site is an important facet of the inflammatory response. Leukocytes undergo a complex process whereby they tether, roll, adhere, spread and finally transmigrate through the endothelium [24]. This process is predominantly mediated by several intracellular signaling events that ultimately up-regulate the expression of pro-inflammatory cyokines, such as MCP-1, and adhesion molecules, including VCAM-1, ICAM-1, and P- and E-selectin [25]. These chemokines and adhesion molecules play key roles in the firm adhesion of leukocytes to activated endothelial cells [15]. However, excessive or inappropriate leukocyte accumulation in tissues contributes to a number of pathologic conditions such as stroke, myocardial infarction and sepsis. SFN has previously been shown to protect against liver injury following intestinal ischemia reperfusion (I/R) injury via Nrf2 signaling, markedly reducing liver tissue MPO activity, which is indicative of reduced neutrophil recruitment [26]. In the brain, Schachtele et al., [27] have demonstrated SFN to reduce macrophage and neutrophil brain infiltration and microglial activation in active viral brain infection. However, the present study is the first to directly quantify the influence of SFN treatment on cerebral leukocyte recruitment following systemic inflammatory challenge. SFN pre-treatment had a potent protective influence on the cerebral microvascular events induced by LPS, being effective in increasing leukocyte rolling (no affect on cell flux) and abrogating leukocyte adhesion and reducing MPO levels. The dose of LPS used here in our study was relatively low. This dose was chosen deliberately to activate the vasculature without causing either mortality or a drastic decrease in microvascular perfusion, as is seen at higher doses and which would complicate the inflammatory response [8].
Sepsis is associated with an excessive production of cytokines (including TNF-α, IL-6, and IL-1β), as well as activation of complement and coagulation cascades following activation of immune cells by endotoxin and other pathogen components [8]. TNF-α increases BBB permeability and the proinflammatory actions of IL-1β and IL-6 in the brain are well documented [28]. Here, LPS induced an increase in circulating pro-inflammatory cytokines (TNF-α, IL-6, IFNγ and MCP-1), which were inhibited when SFN was administered (along with upregulation of IL-10).
Recent studies have demonstrated that the Nrf2/ARE pathway is involved in immune and inflammatory processes, and SFN exerts protective effects via Nrf2 activation, which, under basal conditions, is anchored to the cytoplasm by Keap1. Through interactions with the cysteine residues of Keap1, SFN may induce the release of Nrf2 allowing for its nuclear localization where it binds to the antioxidant response element in a variety of different genes [11–13]. As such, in order to establish whether the observed influence over cerebral leukocyte recruitment was dependent on Nrf2 activation, we conducted experiments using Nrf2-KO mice. In absence of Nrf2, the lower dose of SFN treatment was found to be ineffective in reducing LPS-induced cerebral leukocyte recruitment (i.e. no cerebral leukocyte adhesion in WT mice). These findings concur with other studies that attribute the antioxidant and anti-inflammatory effects of SFN via the Nrf2/ARE pathway [29, 30]. The dependence of SFN’s anti-inflammatory effects on Nrf2 observed here are also supported in investigations by Zakkar et al., [18] who demonstrate SFN to suppress p38 activation and VCAM-1 expression at athero-susceptible sites in the aorta of WT mice, yet not in Nrf2-KO mice. However, we also found that at the higher dose of SFN, the SFN-mediated inhibition of cerebral inflammation was in part independent of the Nrf2/ARE pathway. These findings are supported by other groups describing Nrf2 independent effects of SFN in e.g. in cycle arrest and apoptosis, and inhibition of angiogenesis, histone deacetylases and cytochrome P450 [31]. Recently Greaney et al., 2015 focused studies on inflammasomes, as these have an important role in cytosolic innate immune sensing and pathogen response. They found that SFN-mediated inhibition of inflammasomes is also independent of Nrf2 [31]. Other evidence points to SFN reacting directly with other cellular targets such as toll like receptor 4 (via the suppression of both ligand-induced and ligand-independent oligomerization of TLR4) [32] and tubulin [33]. Jackson and Singletary also showed high SFN doses (≥ 100 µmol/l) have mammary cancer suppressive actions involving mitotic cell cycle arrest and linked to the disruption of normal tubulin polymerization and possible effects on microtubule dynamics at lower SFN doses [34]. Furthermore, it has also been shown that SFN ameliorates skin blistering via Nrf2-dependent and -independent pathways [35]. These findings demonstrate the complex nature of SFN and its ability to mediate its anti-inflammatory and anti-oxidant via various mechanisms.
It is known that local and temporal variations in adhesion molecule expression, such as E- and P-selectin, occur in response to LPS [36, 37]. Furthermore, constitutive and LPS-induced expression of ICAM-1 and VCAM-1 are relatively low in the brain, and the kinetics and relative up-regulation of these adhesion molecules varies between tissues [7]. Therefore, having demonstrated the protective effects of SFN treatment in suppressing cerebral leukocyte recruitment in vivo, we next used the flow chamber assay to assess whether SFN treatment may also prove beneficial in reducing leukocyte-endothelial interactions in human cells, and if so whether the anti-inflammatory effect may be mediated by the inhibition of adhesion molecules. Thus, investigations were made using isolated human neutrophils and HBMEC-3s in an LPS-activated leukocyte recruitment assay under physiological flow. Previously, SFN has been shown to reduce low density lipoprotein-induced endothelial dysfunction, via inducing Nrf2, while suppressing NFκB activation and ICAM-1, VCAM-1, and E-selectin expression [38]. Furthermore SFN reduced TNF-α-induced VCAM-1 and MCP-1 expression in endothelial cells [39] and TNF-α induced adhesion of monocytes [15]. These studies used human umbilical cord vein endothelial cells, however in the present study we investigated anti-inflammatory and anti-oxidative influence of SFN in an endothelial cell line relevant to the unique cells of the cerebral microcirculation (i.e. HBMEC-3). In HBMEC-3, SFN reduced LPS-induced NF-κB activation, as demonstrated by nuclear localization of the phosphorylated p65. Further to the suppression observed in the present study, SFN also interacts with thiol groups to impair redox-sensitive DNA binding and transactivation of NF-κB, via binding to essential Cys residues, or NF-κB related redox regulators [16]. Correlating with decreased NF-κB activation, our studies showed reduced cell surface expression of E-selectin and VCAM-1, along with suppressed LPS-induced neutrophil adhesion under flow conditions. As was found in vivo, SFN treatment had no inhibitory effect on leukocyte rolling, furthermore P-selectin and ICAM-1 expression was found to be unaffected by SFN treatment, suggesting SFN intervention in cerebral leukocyte recruitment is via a suppression of VCAM-1 and E-selectin only.
Activated neutrophils may release large amounts of ROS in a respiratory burst, inducing a high level of oxidative stress, a key pathological factor in local and systemic inflammatory pathologies. This oxidative stress has been implicated in stress, and furthermore, the cerebrovasculature has been demonstrated to be a critical target of oxidative stress in pathological disorders of the brain, but may also be important source of ROS itself [40]. Vascular-derived oxidative stress is a major factor involved in initiating and promoting pathophysiology in a number of disorders of the brain, a process that is evident under inflammatory conditions [40]. Such events can be particularly detrimental in the brain, which lacks robust antioxidant defenses [41]. The neuronal membrane is largely made up of polyunsaturated fatty acids, which are highly susceptible lipid peroxidation [40], additionally ROS accumulation causes altered assembly of the tight junctions, breakdown of the extracellular matrix and loss of BBB integrity [40]. Nrf2 plays a key role in defense against oxidative stress, and as such we investigated the influence of SFN treatment over Nrf2 activation and HBMEC-3 oxidative state, as quantified by CM-H2DCFDA fluorescence. Whilst in brain homogenates from experimental animals, no differences in Nrf2 transcription factor activation was detected, potentialy due to the numerous cell types present in the crude homogenate, SFN was found to activate Nrf2 at all concentrations tested in HBMEC-3, and reduce ROS production at both 10 and 100 µM following LPS challenge (500 ng/ml). SFN (10 and 100 µM) also reduced ROS production from neutrophils post-LPS challenge. Thus, SFN may be beneficial in promoting anti-oxidative mechanisms in the cerebral microcirculation to combat inflammatory increase in damaging ROS.
Blood born cells, the endothelium, resident perivascular cells and parenchymal cells can all influence the inflammatory microenvironment and regulate leukocyte recruitment. In the present study we demonstrate that SFN can have a direct effect on the endothelium by reducing leukocyte recruitment, an effect which was confirmed by our results showing the anti-inflammatory and anti-oxidative effects of SFN in vitro on HBMEC-3s. Nguyen et al., have demonstrated SFN to have beneficial anti-inflammatory effects on leukocytes, reducing NF-κB phosphorylation 50% [42] whilst SFN has also previously been demonstrated to be able to cross the BBB and to accumulate in cerebral tissues [43]. Indeed, SFN treatment has been demonstrated to beneficially effect brain resident cells both in vivo and in vitro [27] and provides a promising therapeutic candidate for the treatment of neuro-inflammatory diseases, in that it may have protective effects not only via anti-inflammatory and anti-oxidative actions on vascular elements and leukocytes (as demonstrated in the present study) but may also influence brain resident cells.
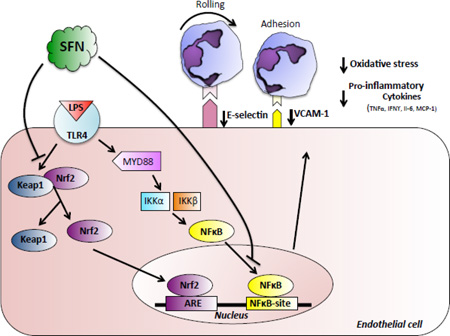
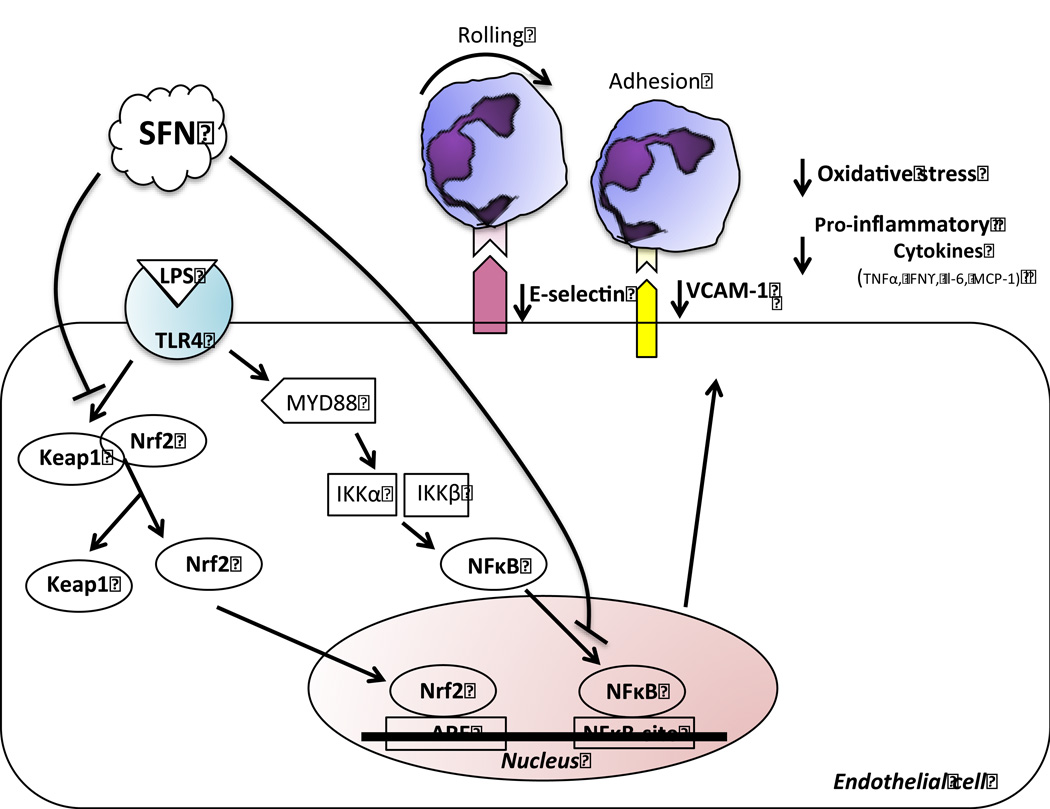
In summary, the present study shows for the first time a fundamental role of SFN in limiting leukocyte endothelial cell interactions in the cerebral microvasculature following a systemic inflammatory challenge. The protective effects of SFN on cerebral vascular inflammation may be, at least in part, independent of Nrf2/ARE pathway and associated with the NF-κB pathway, which in turn leads to a via down-regulation of E-selectin and VCAM-1 (Figure 6). These findings provide evidence suggesting that SFN may be a novel agent to protect the cerebral vasculature against LPS-induced inflammation and dysfunction seen in sepsis.
Figure 6. Proposed mechanism of cerebrovascular neuroprotection elicited by SFN.
SFN mediates its anti-inflammatory and anti-oxidant effects through Keap1/Nrf2 transcriptional activation of the anti-oxidant system, and via the NFκB pathway. In particular, following systemic LPS administration, SFN treatment is able to 1) suppress VCAM-1 and E-selectin adhesion molecules, which in turn leads to a decrease in neutrophil endothelial cell interactions; 2) decrease pro-inflammatory cytokines and 3) decrease ROS production.
Acknowledgments
This work was supported by grants from the NIH/NHLBI (HL125572-01A1) and the German Research Foundation (DFG, BE 5619/1-1). We would like to thank Dr. John A. Vanchiere, Lisa S. Latiolais and Molly J. Linville, LSUHSC-S, for assisting with the collection of human blood samples. We would also like to thank A. Wayne Orr, LSUHSC-S, for providing antibodies and for use of the Nikon Eclipse inverted microscope, and Shaui Yuan, LSUHSC-S for help with endothelial cell experiments.
Abbreviations
- (ARE)
antioxidant response element
- (HBMEC-3)
human brain micro-vascular endothelial cell-3
- (Keap1)
Kelch-like ECH-associated protein 1
- (MCP-1)
monocyte chemoattractant protein-1
- (Nrf2)
NF-erythroid 2-related factor 2
- (SAE)
Sepsis-associated encephalopathy, (SFN), Sulforaphane
- (TNF-α)
tumour necrosis factor-alpha
- (VCAM-1)
vascular cell adhesion molecule-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
P. M. H., S. G., S. A. V., V. N. and F. N. E. G. designed and performed the experiments, analyzed the results and prepared the manuscript. F. B. assisted with analyzing and interpreting the results and preparing the manuscript. J. A. S. and P. C. E helped prepare and edit the manuscript.
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Hickman SE, Allison EK, Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J. Neurosci. 2008;28:8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holloway PM, Smith HK, Renshaw D, Flower RJ, Getting SJ, Gavins FN. Targeting the melanocortin receptor system for anti-stroke therapy. Trends Pharmacol Sci. 2011;32:90–98. doi: 10.1016/j.tips.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Molfino A, Gioia G, Rossi Fanelli F, Laviano A. Contribution of Neuroinflammation to the Pathogenesis of Cancer Cachexia. Mediators Inflamm. 2015:801685. doi: 10.1155/2015/801685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrari GL, Quinto BM, Queiroz KC, Iizuka IJ, Monte JC, Dalboni MA, et al. Effects of simvastatin on cytokines secretion from mononuclear cells from critically ill patients with acute kidney injury. Cytokine. 2011;54:144–148. doi: 10.1016/j.cyto.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Maramattom BV. Sepsis associated encephalopathy. Neurol Res. 2011;29:643–646. doi: 10.1179/016164107X240233. [DOI] [PubMed] [Google Scholar]
- 6.Shukla P, Rao GM, Pandey G, Sharma S, Mittapelly N, Shegokar R, et al. Therapeutic interventions in sepsis: current and anticipated pharmacological agents. Br J Pharmacol. 2014;171:5011–5031. doi: 10.1111/bph.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes EL, Cover PO, Buckingham JC, Gavins FNE. Role and interactions of annexin A1 and oestrogens in the manifestation of sexual dimorphisms in cerebral and systemic inflammation. Br J Pharmacol. 2013;169:539–553. doi: 10.1111/j.1476-5381.2012.02146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gavins FNE, Hughes EL, Buss NA, Holloway PM, Getting SJ, Buckingham JC. Leukocyte recruitment in the brain in sepsis: involvement of the annexin 1-FPR2/ALX anti-inflammatory system. FASEB J. 2012;26:4977–4989. doi: 10.1096/fj.12-205971. [DOI] [PubMed] [Google Scholar]
- 9.Martin GS. Expert Rev Anti Infect Ther. Expert Rev Anti Infect Ther. 2012;10:701–706. doi: 10.1586/eri.12.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anas AA, Hovius JW, van ‘t Veer C, van der Poll T T, de Vos AF. Role of CD14 in a mouse model of acute lung inflammation induced by different lipopolysaccharide chemotypes. PLoS One. 2010;5:e10183. doi: 10.1371/journal.pone.0010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai Y, Wang X, Zhao S, Ma C, Cui J, Zheng Y. Sulforaphane Protects against Cardiovascular Disease via Nrf2 Activation. Oxid Med Cell Longev. 2015;2:9–14. doi: 10.1155/2015/407580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans PC. The influence of sulforaphane on vascular health and its relevance to nutritional approaches to prevent cardiovascular disease. The EPMA Journal. 2011;2:9–14. doi: 10.1007/s13167-011-0064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim J, Wilhelmus MM, de Vries H, Drukarch B, Hoozemans JM, van Horssen J. Antioxidative defense mechanisms controlled by Nrf2: state-of-the-art and clinical perspectives in neurodegenerative diseases. Arch Toxicol. 2014;88:1773–1786. doi: 10.1007/s00204-014-1338-z. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen T, Nioi P, Pickett CB. The Nrf2-Antioxidant Response Element Signaling Pathway and Its Activation by Oxidative Stress. J Biol Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nallasamy P, Si H, Babu PVA, Pan D, Fu Y, Brooke EAS, et al. Sulforaphane reduces vascular inflammation in mice and prevents TNF-α-induced monocyte adhesion to primary endothelial cells through interfering with the NF-κB pathway. Journal of Nutritional Biochemistry. 2014;25:824–833. doi: 10.1016/j.jnutbio.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhäuser C. Nuclear Factor κB Is a Molecular Target for Sulforaphane-mediated Anti-inflammatory Mechanisms. J Biol Chem. 2001;276:32008–32015. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- 17.Alfieri A, Srivastava S, Siow RC, Cash D, Modo M, Duchen MR, et al. Sulforaphane preconditioning of the Nrf2/HO-1 defense pathway protects the cerebral vasculature against blood-brain barrier disruption and neurological deficits in stroke. Free Radic Biol Med. 2013;65:1012–1022. doi: 10.1016/j.freeradbiomed.2013.08.190. [DOI] [PubMed] [Google Scholar]
- 18.Zakkar M, Van der Heiden K, Luong le A, Chaudhury H, Cuhlmann S, Hamdulay SS, et al. Activation of Nrf2 in endothelial cells protects arteries from exhibiting a proinflammatory state. Arterioscler Thromb Vasc Biol. 2009;29:1851–1857. doi: 10.1161/ATVBAHA.109.193375. [DOI] [PubMed] [Google Scholar]
- 19.Holloway PM, Durrenberger PF, Trutschl M, Cvek U, Cooper D, Orr AW AW, et al. Both MC1 and MC3 Receptors Provide Protection From Cerebral Ischemia-Reperfusion- Induced Neutrophil Recruitment. Arterioscler Thromb Vasc Biol. 2015;35:1936–1944. doi: 10.1161/ATVBAHA.115.305348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- 21.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 22.Li J, King MR. Adhesion receptors as therapeutic targets for circulating tumor cells. Front Oncol. 2012;2:79. doi: 10.3389/fonc.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bai Y, Cui W, Xin Y, Miao X, Barati MT, Zhang C, et al. Prevention by sulforaphane of diabetic cardiomyopathy is associated with up-regulation of Nrf2 expression and transcription activation. J Mol Cell Cardiol. 2013;57:82–95. doi: 10.1016/j.yjmcc.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Geisel J, Bruck J, Glocova I, Dengler K, Sinnberg T, Rothfuss O, Walter M, et al. Sulforaphane protects from T cell-mediated autoimmune disease by inhibition of IL-23 and IL-12 in dendritic cells. J Immunol. 2014;192:3530–3539. doi: 10.4049/jimmunol.1300556. [DOI] [PubMed] [Google Scholar]
- 25.Kelly M, Hwang JM, Kubes P. Modulating leukocyte recruitment in inflammation. J Allergy Clin Immunol. 2007;120:3–10. doi: 10.1016/j.jaci.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 26.Zhao HD, Zhang F, Shen G, Li YB, Li YH, Jing HR, et al. Sulforaphane protects liver injury induced by intestinal ischemia reperfusion through Nrf2-ARE pathway. World J Gastroenterol. 2010;16:3002–3010. doi: 10.3748/wjg.v16.i24.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schachtele SJ, Hu S, Lokensgard JR. Modulation of Experimental Herpes Encephalitis-Associated Neurotoxicity through Sulforaphane Treatment. PLoS One. 2012;7:e36216. doi: 10.1371/journal.pone.0036216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rochfort KD, Cummins PM. The blood-brain barrier endothelium: a target for pro-inflammatory cytokines. Biochem Soc Trans. 2015;43:702–706. doi: 10.1042/BST20140319. [DOI] [PubMed] [Google Scholar]
- 29.Dinkova-Kostova AT, Kostov RV. Glucosinolates and isothiocyanates in health and disease. Trends Mol Med. 2012;18:337–347. doi: 10.1016/j.molmed.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Houghton CA, Fassett RG, Coombes JS. Sulforaphane: translational research from laboratory bench to clinic. Nutr Rev. 2013;71:709–26. doi: 10.1111/nure.12060. [DOI] [PubMed] [Google Scholar]
- 31.Greaney AJ, Maier NK, Leppla SH, Moayeri M. Sulforaphane inhibits multiple inflammasomes through an Nrf2-independent mechanism. J Leukoc Biol. 2016;99:189–199. doi: 10.1189/jlb.3A0415-155RR. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Youn HS, Kim YS, Park ZY, Choi NY, Joung SM, et al. Sulforaphane suppresses oligomerization of TLR4 in a thiol-dependent manner. J Immunol. 2010;184:411–419. doi: 10.4049/jimmunol.0803988. [DOI] [PubMed] [Google Scholar]
- 33.Mi L, Xiao Z, Hood BL, Dakshanamurthy S, Wang X, Govind S, et al. Covalent binding to tubulin by isothiocyanates. A mechanism of cell growth arrest and apoptosis. J Biol Chem. 2008;283:22136–22146. doi: 10.1074/jbc.M802330200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson SJ, Singletary KW. Sulforaphane inhibits human MCF-7 mammary cancer cell mitotic progression and tubulin polymerization. J Nutr. 2004;134:2229–2236. doi: 10.1093/jn/134.9.2229. [DOI] [PubMed] [Google Scholar]
- 35.Kerns M, DePianto D, Yamamoto M, Coulombe PA. Differential Modulation of Keratin Expression by Sulforaphane Occurs via Nrf2-dependent and -independent Pathways in Skin Epithelia. Mol Biol Cell. 2010;21:4068–4075. doi: 10.1091/mbc.E10-02-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eppihimer MJ, Wolitzky B, Anderson DC, Labow MA, Granger DN. Heterogeneity of expression of E- and P-selectins in vivo. Circ Res. 1996;79:560–569. doi: 10.1161/01.res.79.3.560. [DOI] [PubMed] [Google Scholar]
- 37.Eppihimer MJ, Schaub RG. P-selectin-dependent inhibition of thrombosis during venous stasis. Arterioscler Thromb Vasc Biol. 2000;20:2483–2488. doi: 10.1161/01.atv.20.11.2483. [DOI] [PubMed] [Google Scholar]
- 38.Huang CS, Lin AH, Liu CT, Tsai CW, Chang IS, Chen HW, et al. Isothiocyanates protect against oxidized LDL-induced endothelial dysfunction by upregulating Nrf2-dependent antioxidation and suppressing NFκB activation. Mol Nutr Food Res. 2013;57:1918–1930. doi: 10.1002/mnfr.201300063. [DOI] [PubMed] [Google Scholar]
- 39.Chen XL, Dodd G, Kunsch C. Sulforaphane inhibits TNF-alpha-induced activation of p38 MAP kinase and VCAM-1 and MCP-1 expression in endothelial cells. Inflamm Res. 2009;58:513–521. doi: 10.1007/s00011-009-0017-7. 2009. [DOI] [PubMed] [Google Scholar]
- 40.Freeman LR, Keller JN. Oxidative stress and cerebral endothelial cells: Regulation of the blood-brain-barrier and antioxidant based interventions. Biochimica et Biophysica Acta (BBA) -Molecular Basis of Disease. 2012;1822:822–829. doi: 10.1016/j.bbadis.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen 2014 have demonstrated SFN to have beneficial anti-inflammatory effects on leukocytes, reducing NF-κB phosphorlyation 50%
- 43.Nguyen DP, Li J, Yadav SS, Tewari AK. Recent insights into NF-κB signalling pathways and the link between inflammation and prostate cancer. BJU Int. 2014;114:168–176. doi: 10.1111/bju.12488. [DOI] [PubMed] [Google Scholar]