Abstract
Direct treatments of cancer such as chemotherapy, radiotherapy and targeted therapy have been shown to depend on recruitment of the immune system for their effectiveness. Recent studies have shown that development of resistance to direct therapies such as BRAF inhibitors in melanoma is associated with suppression of immune responses. We point to emerging data that implicates activation of the polycomb repressive complex 2 (PRC2) and its catalytic component - enhancer of zeste homolog 2 (EZH2) - in progression of melanoma and suppression of immune responses. EZH2 appears to have an important role in differentiation of CD4 T cells and particularly in the function of T regulatory cells, which suppress immune responses to melanoma. We review mechanisms of EZH2 activation at genomic level and from activation of the MAP Kinase, E2F or NF-kB2 pathways. These studies are consistent with activation of EZH2 as a common mechanism for induction of immune suppression in patients failing direct therapies and suggest EZH2 inhibitors may have a role in combination with immunotherapy and targeted therapies to prevent development of immunosuppression.
Keywords: Melanoma, EZH2, epigenetic, immune suppression, treatment resistance
Introduction
Several studies over the past few years have indicated that response to treatment of cancer with chemo and radiotherapy depends not only on direct effects on the tumor cells but on recruitment of the immune system - without which the treatments fail. One of the first of these studies in a murine model suggested that chemotherapy caused the release of high mobility group box 1 (HMGB1) protein from dying tumor cells, which then activated toll like receptor 4 (TLR4) on dendritic cells. The latter then cross-presented tumor antigens to activate CD8 T cells against the tumors. The significance of these findings for cancer patients was supported by shorter relapse free survivals after chemotherapy and radiotherapy in patients with loss of TLR4 function compared to those with normal TLR4 function (Apetoh et al., 2007). Subsequent studies have drawn attention to a number of other mechanisms by which chemotherapy can recruit immune responses against cancer, including type 1 interferon (IFN) signaling and chemokine release (Bracci et al., 2014, Sistigu et al., 2014).
A similar recognition of the importance of the immune system in responses to radiotherapy was first suggested as far back as 1979, when it was found that therapeutic effects of radiotherapy were reduced in mice with low immunity (Stone et al., 1979). It is now known that the efficacy of radiotherapy depends on the recruitment of dendritic cells and CD8 T cells to the tumor via release of complement (Gupta et al., 2012, Surace et al., 2015). As reviewed elsewhere, combinations of radiotherapy and immunotherapy are now the subject of a number of studies in preclinical models and clinical trials (Formenti and Demaria, 2013, Golden et al., 2015).
Given these findings in patients treated with chemotherapy and or radiotherapy, it would not be unexpected that treatments with targeted therapies would also be dependent at least in part on the induction of immune responses. Tumor regression following oncogene withdrawal in c-Myc tumors was reported to be dependent on immune responses, particularly by CD4 T cells (Casey et al., 2014). As reviewed elsewhere (Vanneman and Dranoff, 2012), treatment of breast cancer with Trastuzumab or colon cancer with Cetuximab monoclonal antibodies was associated with increased T cell and NK cell responses against the tumors. We and others have shown that treatment of melanoma with BRAF inhibitors was associated with an influx of T cells into the tumor (Wilmott et al., 2011, Wilmott et al., 2012, Liu et al., 2013). Moreover combining BRAF inhibitors with monoclonal antibodies against melanoma in model systems potentiated the therapeutic effects of BRAF inhibitors (Cooper et al., 2014).
Inhibition of immune responses accompanies resistance of cancers to direct treatments
The increased appreciation of the importance of the immune system in cancer treatment has focused attention on factors that can inhibit immune responses – particularly those associated with treatments directly targeting cancer cells. Studies on mechanisms of BRAF resistance have shown multiple acquired genetic changes (Johnson et al., 2015) but a recent study on non-genomic mechanisms of BRAF resistance identified decreased CD8 T cell numbers and lymphoid enhancer factor 1 (LEF1) nuclear proteins as well as reduced antigen presentation in approximately half the patients that had acquired resistance to BRAFi (Hugo et al., 2015). LEF1 is a transcription factor which together with T cell factor 1 (Tcf1) enhances Th2 differentiation of CD4 T cells, represses Th1 and Th17 differentiation and is involved in silencing CD4 expression in immature CD4/CD8 T cells in the thymus (Steinke and Xue, 2014). LEF1 and Tcf1 may also stabilize regulatory T (Treg) cells but this role and their association with beta-catenin signaling remains controversial. Previous studies have shown involvement of Tcfs and LEF1 in chromatin modification (Hurlstone and Clevers, 2002). The Hugo et al study identified time dependent increased methylation of CpG sites in DNA as correlating with reduction in proteins such as LEF1. This was associated in The Cancer Genome Atlas (TCGA) dataset with decreased β-catenin signaling. These studies have helped focus on the importance of epigenetic factors in acquired resistance but did not identify what these factors may be. Here we review evidence that may implicate the polycomb repressive complex 2 (PRC2) and its catalytic component - enhancer of zeste homolog 2 (EZH2) as an inducer of treatment related immunosuppression.
The PRC2/EZH2 repressive complex as a mediator of immunosuppression
The EZH2 protein and PRC2 complex are conserved across organisms from plants to humans (O'Meara and Simon, 2012, Hobert et al., 1996, Goodrich et al., 1997) and appear to be mainly involved in repression of gene transcription by tri-methylation of lysine 27 on H3 histones (H3K27me3) (Zhang et al., 2012, Muller et al., 2002). The PRC2/EZH2 complex is essential in early development and appears responsible for transcriptional silencing of differentiation genes such as the Hox transcription factors (Rea et al., 2000), in addition to its role in the early steps of mammalian X-chromosome inactivation (Jones and Gelbart, 1990). Several studies have drawn attention to the role of EZH2 in repressing differentiation genes in embryonic stem cells and thereby maintaining their pluripotency, while allowing expression of genes involved in cell division (Simon and Lange, 2008, Lee et al., 2006). Parallels have been drawn to a potentially similar role for EZH2 in promoting self-renewal and impeding differentiation in cancer. In this sense the differentiation genes in embryonic stem cells may be regarded as similar to tumor suppressor genes in cancer cells (Tiffen et al., 2015a, Varambally et al., 2002, Kleer et al., 2003). Importantly EZH2, in the context of PRC2, interacts with DNA methyl transferases (DNMTs) and results in methylation of EZH2 target gene promoters (Vire et al., 2006) providing an additional more stable repressive mechanism.
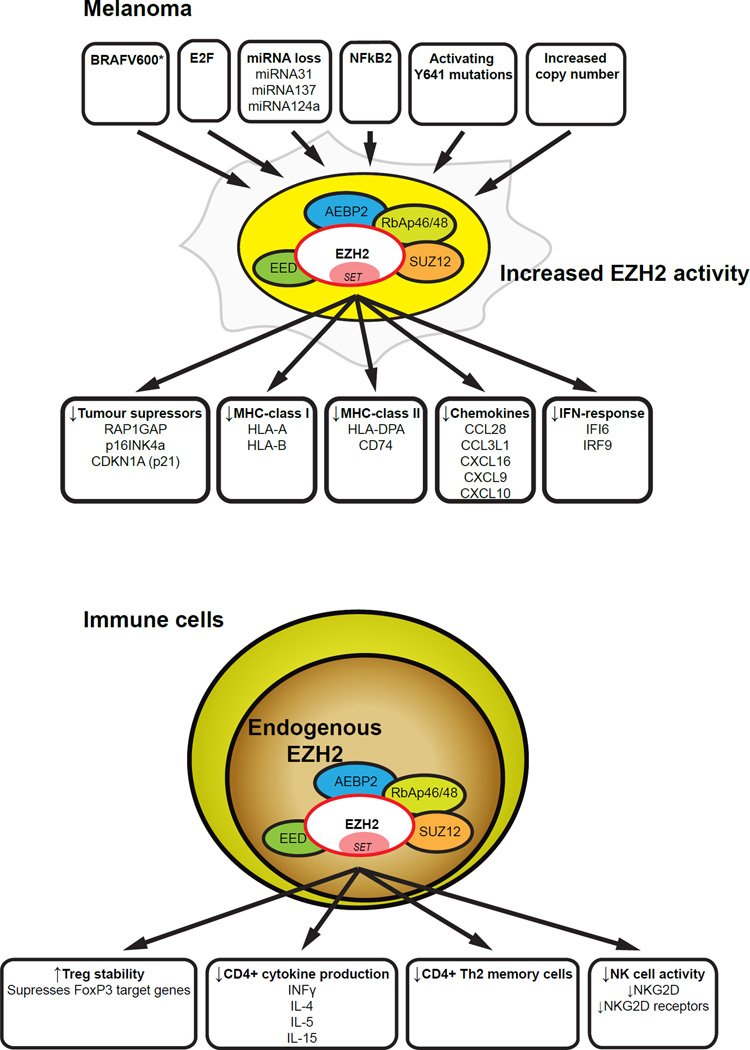
A number of studies have suggested that EZH2 may have an important role in progression of melanoma (Figure 1). High EZH2 expression was shown to be associated with more malignant forms of melanoma (Bachmann et al., 2006, McHugh et al., 2007) and to the repression of several important tumor suppressors in melanoma, including RAP1GAP (Asangani et al., 2012, Zheng et al., 2009, Banerjee et al., 2011), p16INK4a (Delmore et al., 2011) and p21CDKN1A (Fan et al., 2011). Somatic mutations in EZH2 were reported in 3% of samples in the Hodis et al studies (Hodis et al., 2012) that identified drivers of melanoma progression. The recent success of small molecule inhibitors of mutant EZH2 in treatment of lymphoma (McCabe et al., 2012) have made it even more important to understand the role of EZH2 in melanoma.
Figure 1.
EZH2 activity is increased in melanoma by changes occurring during tumorigenesis. The elevated EZH2 expression results in gene suppression that allows the melanoma to suppress and evade immune attack. EZH2 activity in immune cells is also crucial for determining the fate and activity of many types of immune cells.
Inhibition of EZH2 has potent negative effects on the proliferation of human melanoma in vitro particularly in cell lines harboring the Y641 activating mutation within the catalytic component of EZH2 (Tiffen et al., 2015b). EZH2 inhibition was associated with G2/M cell cycle arrest, reduced proliferative capacity in both 2D and 3D culture systems, induction of apoptosis and importantly downregulation of a number of immune response genes. Apoptosis was caspase independent and mediated by the release of apoptosis inducing factor (AIF) from mitochondria. EZH2 inhibition with EZH2 inhibitors or knockdown of EZH2 by siRNA also reactivated several well characterized tumor suppressor genes (Tiffen et al., 2015b).
A comprehensive analysis of genomic and non-genomic changes in 471 melanoma patients in the TCGA dataset revealed hyperactivation of EZH2 and negative control of genes involved in immune responses (Tiffen et al., 2016, Guan et al., 2015). Somatic mutations, somatic copy number alterations, and/or transcriptional upregulation of EZH2 affected approximately 20% of patients in the TCGA melanoma subset. The genomic and epigenomic assessment of EZH2 in the physiological setting of a tumor allowed for insights into target genes. EZH2 performs distinct roles in tumor initiation by silencing tumor suppressor genes (Tiffen et al., 2015b, Zingg et al., 2015, Barsotti et al., 2015, Kampilafkos et al., 2015). It was reasoned that identification of genes that were downregulated in such patients compared to patients with normal or low EZH2 expression would help identify target genes associated with EZH2. This analysis was combined with information about genes that were upregulated in melanoma cell lines treated with inhibitors of EZH2 and which again could be assumed to be genes downregulated by EZH2. 98 genes were common to both analyses, including a number associated with immune responses. Genes associated with antigen presentation such as MHC class 1 antigens HLA-A and HLA- B, the MHC class II antigen HLA-DPA1 and CD74, the MHC class II invariant chain involved in formation and transport of MHC antigens, were downregulated as were a number of chemokines that may be involved in chemo-attraction of lymphocytes (CCL28, CCL3L1) or dendritic cells (CXCL16)(Tiffen et al., 2015b, Tiffen et al., 2016). Levels of the interferon alpha (IFNA) inducible IFI6 gene and the IFNA inducible IRF9 transcription factor (Tahara et al., 2005) were also decreased. IFI6 has been implicated in resistance to apoptosis (Cheriyath et al., 2007) and IRF9 is known to form a complex with Stat1 and Stat2 to form the ISGF3 transcription factor in response to signaling by IFNs (Fink and Grandvaux, 2013).
The role of EZH2 in immune suppression
The idea that EZH2 may have a coordinating role in immune suppression is supported by a number of studies that implicate EZH2 in the differentiation and function of the immune system (Figure 1). Upon encountering antigen in secondary lymphoid organs such as lymph nodes and spleen, naive CD4+ T cells differentiate into discrete subsets of T helper (Th) cells, a process controlled by the cytokines present during activation. Th cell subsets including Th1, Th2, and Th17 then induce different types of immune responses. Expression of lineage-specifying transcription factors is required for Th cell differentiation, e.g. T-bet (encoded by Tbx21) can induce Th1 cell differentiation and Gata3 is required for differentiation of Th2 cells. In addition, a transcription factor related to T-bet called Eomesodermin (Eomes) enhances IFN-γ production in Th1 cells (Tumes et al., 2013). Appropriate immune regulation requires the activity of epigenetic chromatin regulators to allow access of these and other transcription factors to DNA (and the resultant processes such as RNA polymerase II activity). It has been reasoned elsewhere that immune responses may be dependent on different chromatin regulators than those required by cancer cells. Targeting of the chromatin regulators in cancer cells may therefore provide selective treatment opportunities (Prinjha and Tarakhovsky, 2013).
Particular attention has been given to the role of EZH2 in the differentiation and function of Treg cells. Tregs are relatively small subset of T cells but nevertheless have potent effects in preventing autoimmune diseases (Kim et al., 2007). They have been reported to inhibit immune responses against tumors in many animal models (Gallimore and Godkin, 2008). Treg cells are believed to act mainly in secondary lymphoid tissue such as lymph nodes to regulate the priming and differentiation of CD4 T cells (Bolton et al., 2015). This regulation is likely to be essential for rejection of tumors, as CD4 T cells are necessary for the crucial CD8 T cell response. Treg cells may also act peripherally at the site of tumors to directly inhibit the function of CD8 T cells (Mempel et al., 2006). Treg can exist in a resting or activated state (Teh et al., 2015). Activated Treg cells have enhanced ability to migrate and mediate suppression. In comparison to the resting Treg cells, activated Treg cells show distinct surface markers, chemokine receptors, gene expression and epigenetic modifications. Foxp3 expression is essential in the maintenance of both resting and activated Treg cells (Wu et al., 2015).
EZH2 appears crucial for the stability of Tregs after they are activated by a combination of T cell receptor and costimulatory signals. DuPage et al reported that mice with EZH2 deficient Treg cells developed systemic autoimmune and inflammatory disorders associated with lymphocytic infiltration into the organs (DuPage et al., 2015). The deficiency of EZH2 destabilized only the activated Treg cells, suggesting a dynamic epigenetic regulation of Treg cells during activation. Previous studies had also concluded that EZH2 was involved in the function of activated Treg cells in a complex with Foxp3 (Arvey et al., 2014).
EZH2 has also been implicated in differentiation of Th1 and Th2 CD4 cells. EZH2-deficiency in CD4 T cells increased in vitro production of multiple cytokines including IFN gamma, IL-4, IL-5 and IL-13 but not IL-17. However mice in which EZH2 was conditionally knocked out in T cells had increased numbers of CD4 memory phenotype Th2 cell but no increase in cells making IFN gamma (Tumes et al., 2013). EZH2 also has a role in the function of NK cells as EZH2 depletion or inhibition with small molecular weight inhibitors (UNC1999 or EPZ 005687) resulted in increased NK cell activity in both murine and human systems. This was associated with increased NK cell expression of NKG2D and range of receptors for cytokines and chemokines (Yin et al., 2015).
Relatively few studies have focused on the possible immune effects of inhibiting EZH2 in cancers. Studies on human ovarian cancers showed that high levels of EZH2 in the cancer cells was associated with high levels of H3K27me3 and DNMT1 DNA methylation that repressed production of Th1 type chemokines CXCL9 and CXCL10. These chemokines are induced by IFN gamma and are ligands for CXCR3 on T cells (Karin and Wildbaum, 2015). CXCR3 was also shown to be important in homing of T cells to melanoma (Mikucki et al., 2015). Consistent with these data, high EZH2 and DNMT1 were negatively associated with tumor infiltrating CD8 T cells in human ovarian cancers. Treatment of C57/BL6 mice bearing murine ID8 ovarian cancers with a combination of inhibitors of trimethylation (DZNep) and DNA methyltransferases (5-aza-2’-deoxycytidine) resulted in reduction of tumor growth and increased T cell infiltration into the tumors. Moreover this combination augmented the efficacy of immunotherapy with anti-PD1 (Peng et al., 2015).
How is EZH2 activated in melanoma during development of treatment resistance?
A number of pathways central to melanoma biology are involved in EZH2 activity, including MAP Kinase, AKT and E2F1. The most common melanoma mutation, BRAFV600E, which activates the MAP kinase pathway has also been associated with increased expression of EZH2 (Hou et al., 2012). Knockdown of BRAFV600E was shown to profoundly reduce the expression of EZH2 (Hou et al., 2012), suggesting that deregulated BRAF activation may contribute to the overexpression of EZH2 seen in melanoma. Analysis of somatic copy number alterations and mutations in TCGA showed melanoma patients with EZH2 copy number gains were more likely to contain BRAFV600E mutations (Tiffen et al., 2016, Guan et al., 2015). Three out of four EZH2 Y641 mutants in our studies harbored BRAFV600E mutations (Tiffen et al., 2015b). Selection of such patients may occur during treatment and contribute to treatment resistance.
In addition EZH2 is known to be regulated by E2F family transcription factors which are downstream of p16INK4a and p14ARF tumor suppressors commonly inactivated in melanoma (Wu et al., 2010). E2F1 induces increased levels of EZH2 that in turn repress the pro-apoptotic factor Bim. With development of resistance to treatment in melanoma, E2F1 could be expected to increase and thereby recruit EZH2 in the resistance pathway. MicroRNAs appear to be involved in regulation of EZH2 levels – for example loss of microRNA-31 was associated with up-regulation of EZH2 in melanoma, which could be reversed by ectopic overexpression of microRNA-31. Similar experiments have revealed other microRNAs that downregulate EZH2 in melanoma, including miR-137 (Luo et al., 2013) and miR-124a in uveal melanoma (Chen et al., 2013). The extent to which treatment may impact on these microRNAs to upregulate EZH2 is not known.
Other pathways may be involved. Two reports have suggested that EZH2 may be activated in melanoma by the non-canonical NF-kB pathway. The first of these from Iannetti et al found that p52/RelB, a member of the alternative NF-kB pathway, regulated the expression of proteasome subunit alpha 5 (PSMA5) and the anaphase promoting complex (ANAPC1) E3 ligase to decrease the stability of p21WAF1 (CDKNA1) and the tumor suppressor p53 (Iannetti et al., 2014). These combined to upregulate the activity of the retinoblastoma protein, Rb, leading to induction of EZH2 expression which was normally repressed by p53. Chromatin Immunoprecipitation (ChIP) analysis demonstrated that EZH2 was a direct NF-kB target gene. EZH2 antagonized a subset of p53 target genes previously associated with the senescent cell phenotype and thereby suppressed cell senescence in melanoma.
Similar findings were reported by De Donatis et al (De Donatis et al., 2015) who found that the non-canonical pathway was responsible for most of the NF-kB activity in melanoma cells - a finding reported previously by Thu et al who identified the non-canonical-NF-kB pathway as a key regulator of EZH2 expression in melanoma (Thu et al., 2012). There was a strong correlation between NF-kB2 and EZH2 expression in human melanoma metastases. Inhibition of the non-canonical NF-kB pathway by targeting NF-kB2/p52 or the upstream NF-kB inducing kinase NIK restored the senescence program in melanoma cells by decreasing EZH2. Inhibition of the non-canonical NF-kB pathway in murine models also restored senescence and induced a dramatic reduction in tumor growth. We have reported previously that activation of NF-kB occurs during development of resistance to BRAF inhibitors (Mijatov, 2012).
The linkage of NF-kB2 upstream of EZH2 may provide insights into the role EZH2 in melanoma resistance even before exposure to therapy. Melanoma is one of many cancers where NF-kB may be constitutively activated (Franco et al., 2001, Ueda and Richmond, 2006). The clinical spectrum of melanoma includes subsets of melanoma with an inflammatory appearance and systemic symptoms of inflammation as well as an aggressive and infiltrative biology that would be consistent with activation of NF-kB (Gallagher et al., 2014). This is supported by past studies showing an association of activated NF-kB with vascular forms of melanoma (Kashani-Sabet et al., 2004, Kashani-Sabet et al., 2002). Whether this subset may also manifest activation of the β-catenin pathway reported to be upregulated by NIK is as yet unknown (Thu et al., 2012) but requires examination, especially as β-catenin has been implicated in inhibiting immune responses in melanoma (Spranger et al., 2015) and to be linked to LIF1 reported as downregulated in BRAF resistant melanoma in the studies of Hugo et al. (Hugo et al., 2015).
In conclusion the realization that resistance to direct therapies also involves resistance to immune responses acting in concert poses challenges in understanding how the immune system becomes constrained as part of the generation of resistance. This has been shown particularly in patients developing resistance to BRAFi but may apply to other therapies such as chemo and radiotherapy. Activation of EZH2 would appear a prime suspect as the role of PRC2 is largely that of repressing a wide variety of genes with the exception of those involved in cell division. Repression is mediated not only by histone H3K27 trimethylation but also by activation of DNMT3A to methylate CpG dinucleotides in DNA. Evolving studies show that EZH2 has important roles in differentiation and function of the immune system particularly in relation to stabilizing Treg function. Given this association, more attention to the importance of Tregs in treatment resistance induced immunosuppression appears warranted.
Just how EZH2 becomes activated during the development of treatment resistance remains to be established. TCGA data show increased EZH2 activity in at least 20% of melanoma patients and it is possible that treatment may help to select melanoma cells with copy number gains, amplifications and activating mutations of EZH2. Other prime candidates are increased E2F activation in cells acquiring resistance to direct treatments and activation of non-canonical NF-kB which has been linked to BRAFi resistance. Melanoma patients with high levels of EZH2 have a range of immune defects that share similarities to those occurring in patients developing resistance to BRAFi, which raises the question of whether activation of EZH2 is a common factor leading to immune suppression. Inhibitors of EZH2 are now the subject of at least 3 phase I clinical trials (clinicaltrials.gov) so that the use of EZH2 inhibitors to overcome EZH2 induced immune suppression in combination with immunotherapy would be feasible in future studies.
Acknowledgments
Work of the authors described in this article was supported by NHMRC program grant 1093017, NHMRC project 105184 and NHMRC research fellowship to BF and grant CA154887 from the National Institutes of Health, National Cancer Institute to FVF.
References
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- Arvey A, van der Veeken J, Samstein RM, Feng Y, Stamatoyannopoulos JA, Rudensky AY. Inflammation-induced repression of chromatin bound by the transcription factor Foxp3 in regulatory T cells. Nat Immunol. 2014;15:580–587. doi: 10.1038/ni.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asangani IA, Harms PW, Dodson L, Pandhi M, Kunju LP, Maher CA, Fullen DR, Johnson TM, Giordano TJ, Palanisamy N, et al. Genetic and epigenetic loss of microRNA-31 leads to feed-forward expression of EZH2 in melanoma. Oncotarget. 2012;3:1011–1025. doi: 10.18632/oncotarget.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann IM, Halvorsen OJ, Collett K, Stefansson IM, Straume O, Haukaas SA, Salvesen HB, Otte AP, Akslen LA. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J Clin Oncol. 2006;24:268–273. doi: 10.1200/JCO.2005.01.5180. [DOI] [PubMed] [Google Scholar]
- Banerjee R, Mani RS, Russo N, Scanlon CS, Tsodikov A, Jing X, Cao Q, Palanisamy N, Metwally T, Inglehart RC, et al. The tumor suppressor gene rap1GAP is silenced by miR-101-mediated EZH2 overexpression in invasive squamous cell carcinoma. Oncogene. 2011;30:4339–4349. doi: 10.1038/onc.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsotti AM, Ryskin M, Zhong W, Zhang WG, Giannakou A, Loreth C, Diesl V, Follettie M, Golas J, Lee M, et al. Epigenetic reprogramming by tumor-derived EZH2 gain-of-function mutations promotes aggressive 3D cell morphologies and enhances melanoma tumor growth. Oncotarget. 2015;6:2928–2938. doi: 10.18632/oncotarget.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton HA, Zhu E, Terry AM, Guy TV, Koh WP, Tan SY, Power CA, Bertolino P, Lahl K, Sparwasser T, et al. Selective Treg reconstitution during lymphopenia normalizes DC costimulation and prevents graft-versus-host disease. J Clin Invest. 2015;125:3627–3641. doi: 10.1172/JCI76031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21:15–25. doi: 10.1038/cdd.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey SC, Li Y, Fan AC, Felsher DW. Oncogene withdrawal engages the immune system to induce sustained cancer regression. J Immunother Cancer. 2014;2:24. doi: 10.1186/2051-1426-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, He D, Dong XD, Dong F, Wang J, Wang L, Tang J, Hu DN, Yan D, Tu L. MicroRNA-124a is epigenetically regulated and acts as a tumor suppressor by controlling multiple targets in uveal melanoma. Invest Ophthalmol Vis Sci. 2013;54:2248–2256. doi: 10.1167/iovs.12-10977. [DOI] [PubMed] [Google Scholar]
- Cheriyath V, Glaser KB, Waring JF, Baz R, Hussein MA, Borden EC. G1P3, an IFN-induced survival factor, antagonizes TRAIL-induced apoptosis in human myeloma cells. J Clin Invest. 2007;117:3107–3117. doi: 10.1172/JCI31122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZA, Juneja VR, Sage PT, Frederick DT, Piris A, Mitra D, Lo JA, Hodi FS, Freeman GJ, Bosenberg MW, et al. Response to BRAF inhibition in melanoma is enhanced when combined with immune checkpoint blockade. Cancer Immunol Res. 2014;2:643–654. doi: 10.1158/2326-6066.CIR-13-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Donatis GM, Pape EL, Pierron A, Cheli Y, Hofman V, Hofman P, Allegra M, Zahaf K, Bahadoran P, Rocchi S, et al. NF-kB2 induces senescence bypass in melanoma via a direct transcriptional activation of EZH2. Oncogene. 2015 [Google Scholar]
- Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, Kastritis E, Gilpatrick T, Paranal RM, Qi J, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146:904–917. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPage M, Chopra G, Quiros J, Rosenthal WL, Morar MM, Holohan D, Zhang R, Turka L, Marson A, Bluestone JA. The chromatin-modifying enzyme Ezh2 is critical for the maintenance of regulatory T cell identity after activation. Immunity. 2015;42:227–238. doi: 10.1016/j.immuni.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan T, Jiang S, Chung N, Alikhan A, Ni C, Lee CC, Hornyak TJ. EZH2-dependent suppression of a cellular senescence phenotype in melanoma cells by inhibition of p21/CDKN1A expression. Mol Cancer Res. 2011;9:418–429. doi: 10.1158/1541-7786.MCR-10-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink K, Grandvaux N. STAT2 and IRF9: Beyond ISGF3. Jakstat. 2013;2:e27521. doi: 10.4161/jkst.27521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105:256–265. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco AV, Zhang XD, Van Berkel E, Sanders JE, Zhang XY, Thomas WD, Nguyen T, Hersey P. The role of NF-kappa B in TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis of melanoma cells. J Immunol. 2001;166:5337–5345. doi: 10.4049/jimmunol.166.9.5337. [DOI] [PubMed] [Google Scholar]
- Gallagher SJ, Mijatov B, Gunatilake D, Gowrishankar K, Tiffen J, James W, Jin L, Pupo G, Cullinane C, McArthur GA, et al. Control of NF-kB activity in human melanoma by bromodomain and extra-terminal protein inhibitor I-BET151. Pigment Cell Melanoma Res. 2014;27:1126–1137. doi: 10.1111/pcmr.12282. [DOI] [PubMed] [Google Scholar]
- Gallimore A, Godkin A. Regulatory T cells and tumour immunity – observations in mice and men. Immunology. 2008;123:157–163. doi: 10.1111/j.1365-2567.2007.02748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Fenton-Kerimian M, Friedman K, Ponzo F, Babb JS, Goldberg J, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. 2015;16:795–803. doi: 10.1016/S1470-2045(15)00054-6. [DOI] [PubMed] [Google Scholar]
- Goodrich J, Puangsomlee P, Martin M, Long D, Meyerowitz EM, Coupland G. A Polycomb-group gene regulates homeotic gene expression in Arabidopsis. Nature. 1997;386:44–51. doi: 10.1038/386044a0. [DOI] [PubMed] [Google Scholar]
- Guan J, Gupta R, Filipp FV. Cancer systems biology of TCGA SKCM: efficient detection of genomic drivers in melanoma. Sci Rep. 2015;5:7857. doi: 10.1038/srep07857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, Schwendener R, Pruschy M, Knuth A, van den Broek M. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol. 2012;189:558–566. doi: 10.4049/jimmunol.1200563. [DOI] [PubMed] [Google Scholar]
- Hobert O, Jallal B, Ullrich A. Interaction of Vav with ENX-1, a putative transcriptional regulator of homeobox gene expression. Mol Cell Biol. 1996;16:3066–3073. doi: 10.1128/mcb.16.6.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, Nickerson E, Auclair D, Li L, Place C, et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou P, Liu D, Dong J, Xing M. The BRAFV600E causes widespread alterations in gene methylation in the genome of melanoma cells. Cell Cycle. 2012;11:286–295. doi: 10.4161/cc.11.2.18707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo W, Shi H, Sun L, Piva M, Song C, Kong X, Moriceau G, Hong A, Dahlman KB, Johnson DB, et al. Non-genomic and Immune Evolution of Melanoma Acquiring MAPKi Resistance. Cell. 2015;162:1271–1285. doi: 10.1016/j.cell.2015.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlstone A, Clevers H. T-cell factors: turn-ons and turn-offs. Embo j. 2002;21:2303–2311. doi: 10.1093/emboj/21.10.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannetti A, Ledoux AC, Tudhope SJ, Sellier H, Zhao B, Mowla S, Moore A, Hummerich H, Gewurz BE, Cockell SJ, et al. Regulation of p53 and Rb links the alternative NF-kappaB pathway to EZH2 expression and cell senescence. PLoS Genet. 2014;10:e1004642. doi: 10.1371/journal.pgen.1004642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DB, Menzies AM, Zimmer L, Eroglu Z, Ye F, Zhao S, Rizos H, Sucker A, Scolyer RA, Gutzmer R, et al. Acquired BRAF inhibitor resistance: A multicenter meta-analysis of the spectrum and frequencies, clinical behaviour, and phenotypic associations of resistance mechanisms. Eur J Cancer. 2015;51:2792–2799. doi: 10.1016/j.ejca.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RS, Gelbart WM. Genetic analysis of the enhancer of zeste locus and its role in gene regulation in Drosophila melanogaster. Genetics. 1990;126:185–199. doi: 10.1093/genetics/126.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampilafkos P, Melachrinou M, Kefalopoulou Z, Lakoumentas J, Sotiropoulou-Bonikou G. Epigenetic modifications in cutaneous malignant melanoma: EZH2, H3K4me2, and H3K27me3 immunohistochemical expression is enhanced at the invasion front of the tumor. Am J Dermatopathol. 2015;37:138–144. doi: 10.1097/DAD.0b013e31828a2d54. [DOI] [PubMed] [Google Scholar]
- Karin N, Wildbaum G. The Role of Chemokines in Shaping the Balance Between CD4(+) T Cell Subsets and Its Therapeutic Implications in Autoimmune and Cancer Diseases. Front Immunol. 2015;6:609. doi: 10.3389/fimmu.2015.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashani-Sabet M, Liu Y, Fong S, Desprez PY, Liu S, Tu G, Nosrati M, Handumrongkul C, Liggitt D, Thor AD, et al. Identification of gene function and functional pathways by systemic plasmid-based ribozyme targeting in adult mice. Proc Natl Acad Sci U S A. 2002;99:3878–3883. doi: 10.1073/pnas.002025599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashani-Sabet M, Shaikh L, Miller JR, 3rd, Nosrati M, Ferreira CM, Debs RJ, Sagebiel RW. NF-kappa B in the vascular progression of melanoma. J Clin Oncol. 2004;22:617–623. doi: 10.1200/JCO.2004.06.047. [DOI] [PubMed] [Google Scholar]
- Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, Ghosh D, Sewalt RG, Otte AP, Hayes DF, et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci U S A. 2003;100:11606–11611. doi: 10.1073/pnas.1933744100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, Chevalier B, Johnstone SE, Cole MF, Isono K, et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell. 2006;125:301–313. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Peng W, Xu C, Lou Y, Zhang M, Wargo JA, Chen JQ, Li HS, Watowich SS, Yang Y, et al. BRAF inhibition increases tumor infiltration by T cells and enhances the antitumor activity of adoptive immunotherapy in mice. Clin Cancer Res. 2013;19:393–403. doi: 10.1158/1078-0432.CCR-12-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Tetteh PW, Merz PR, Dickes E, Abukiwan A, Hotz-Wagenblatt A, Holland-Cunz S, Sinnberg T, Schittek B, Schadendorf D, et al. miR-137 inhibits the invasion of melanoma cells through downregulation of multiple oncogenic target genes. J Invest Dermatol. 2013;133:768–775. doi: 10.1038/jid.2012.357. [DOI] [PubMed] [Google Scholar]
- McCabe MT, Ott HM, Ganji G, Korenchuk S, Thompson C, Van Aller GS, Liu Y, Graves AP, Della Pietra A, 3rd, Diaz E, et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature. 2012;492:108–112. doi: 10.1038/nature11606. [DOI] [PubMed] [Google Scholar]
- McHugh JB, Fullen DR, Ma L, Kleer CG, Su LD. Expression of polycomb group protein EZH2 in nevi and melanoma. J Cutan Pathol. 2007;34:597–600. doi: 10.1111/j.1600-0560.2006.00678.x. [DOI] [PubMed] [Google Scholar]
- Mempel TR, Pittet MJ, Khazaie K, Weninger W, Weissleder R, von Boehmer H, von Andrian UH. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity. 2006;25:129–141. doi: 10.1016/j.immuni.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Mijatov BSK, Jin L, gallagher S, Hersey P. Cytokines and the NF-kB pathway in resistance of melanoma to BRAF inhibitors. Pigment cell and Melanoma research. 2012;25:875–875. [Google Scholar]
- Mikucki ME, Fisher DT, Matsuzaki J, Skitzki JJ, Gaulin NB, Muhitch JB, Ku AW, Frelinger JG, Odunsi K, Gajewski TF, et al. Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat Commun. 2015;6 doi: 10.1038/ncomms8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J, Hart CM, Francis NJ, Vargas ML, Sengupta A, Wild B, Miller EL, O'Connor MB, Kingston RE, Simon JA. Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell. 2002;111:197–208. doi: 10.1016/s0092-8674(02)00976-5. [DOI] [PubMed] [Google Scholar]
- O'Meara MM, Simon JA. Inner workings and regulatory inputs that control Polycomb repressive complex 2. Chromosoma. 2012;121:221–234. doi: 10.1007/s00412-012-0361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng D, Kryczek I, Nagarsheth N, Zhao L, Wei S, Wang W, Sun Y, Zhao E, Vatan L, Szeliga W, et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature. 2015;527:249–253. doi: 10.1038/nature15520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinjha R, Tarakhovsky A. Chromatin targeting drugs in cancer and immunity. Genes Dev. 2013;27:1731–1738. doi: 10.1101/gad.221895.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea S, Eisenhaber F, O'Carroll D, Strahl BD, Sun ZW, Schmid M, Opravil S, Mechtler K, Ponting CP, Allis CD, et al. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature. 2000;406:593–599. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- Simon JA, Lange CA. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat Res. 2008;647:21–29. doi: 10.1016/j.mrfmmm.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remedios C, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20:1301–1309. doi: 10.1038/nm.3708. [DOI] [PubMed] [Google Scholar]
- Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- Steinke FC, Xue HH. From inception to output, Tcf1 and Lef1 safeguard development of T cells and innate immune cells. Immunol Res. 2014;59:45–55. doi: 10.1007/s12026-014-8545-9. [DOI] [PubMed] [Google Scholar]
- Stone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J Natl Cancer Inst. 1979;63:1229–1235. [PubMed] [Google Scholar]
- Surace L, Lysenko V, Fontana AO, Cecconi V, Janssen H, Bicvic A, Okoniewski M, Pruschy M, Dummer R, Neefjes J, et al. Complement is a central mediator of radiotherapy-induced tumor-specific immunity and clinical response. Immunity. 2015;42:767–777. doi: 10.1016/j.immuni.2015.03.009. [DOI] [PubMed] [Google Scholar]
- Tahara E, Jr, Tahara H, Kanno M, Naka K, Takeda Y, Matsuzaki T, Yamazaki R, Ishihara H, Yasui W, Barrett JC, et al. G1P3, an interferon inducible gene 6–16, is expressed in gastric cancers and inhibits mitochondrial-mediated apoptosis in gastric cancer cell line TMK-1 cell. Cancer Immunol Immunother. 2005;54:729–740. doi: 10.1007/s00262-004-0645-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh PP, Vasanthakumar A, Kallies A. Development and Function of Effector Regulatory T Cells. Prog Mol Biol Transl Sci. 2015;136:155–174. doi: 10.1016/bs.pmbts.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Thu YM, Su Y, Yang J, Splittgerber R, Na S, Boyd A, Mosse C, Simons C, Richmond A. NF-kappaB inducing kinase (NIK) modulates melanoma tumorigenesis by regulating expression of pro-survival factors through the beta-catenin pathway. Oncogene. 2012;31:2580–2592. doi: 10.1038/onc.2011.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffen J, Gallagher SJ, Hersey P. EZH2: an emerging role in melanoma biology and strategies for targeted therapy. Pigment Cell Melanoma Res. 2015a;28:21–30. doi: 10.1111/pcmr.12280. [DOI] [PubMed] [Google Scholar]
- Tiffen JC, Gunatilake D, Gallagher SJ, Gowrishankar K, Heinemann A, Cullinane C, Dutton-Regester K, Pupo GM, Strbenac D, Yang JY, et al. Targeting activating mutations of EZH2 leads to potent cell growth inhibition in human melanoma by derepression of tumor suppressor genes. Oncotarget. 2015b doi: 10.18632/oncotarget.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffen JC, Wilson S, Gallagher SJ, Hersey P, Filipp FV. Somatic copy number amplification and activating somatic mutations of EZH2 drive epigenetic silencing of genes involved in tumor suppression and immune responses in melanoma. Neoplasia. 2016 doi: 10.1016/j.neo.2016.01.003. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumes DJ, Onodera A, Suzuki A, Shinoda K, Endo Y, Iwamura C, Hosokawa H, Koseki H, Tokoyoda K, Suzuki Y, et al. The polycomb protein Ezh2 regulates differentiation and plasticity of CD4(+) T helper type 1 and type 2 cells. Immunity. 2013;39:819–832. doi: 10.1016/j.immuni.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Richmond A. NF-kappaB activation in melanoma. Pigment Cell Res. 2006;19:112–124. doi: 10.1111/j.1600-0749.2006.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneman M, Dranoff G. Combining Immunotherapy and Targeted Therapies in Cancer Treatment. Nature reviews. Cancer. 2012;12:237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–629. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- Vire E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, Morey L, Van Eynde A, Bernard D, Vanderwinden J-M. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871–874. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma R, Thompson JF, Kefford RF, Hersey P, Scolyer RA. Selective BRAF inhibitors induce marked T cell infiltration into human metastatic melanoma. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-11-2479. [DOI] [PubMed] [Google Scholar]
- Wilmott JS, Scolyer RA, Long GV, Hersey P. Combined targeted therapy and immunotherapy in the treatment of advanced melanoma. Oncoimmunology. 2012;1:997–999. doi: 10.4161/onci.19865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Chen Z, Kuchroo VK. Ezh2 lines up the chromatin in T regulatory cells. Immunity. 2015;42:201–203. doi: 10.1016/j.immuni.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZL, Zheng SS, Li ZM, Qiao YY, Aau MY, Yu Q. Polycomb protein EZH2 regulates E2F1-dependent apoptosis through epigenetically modulating Bim expression. Cell Death Differ. 2010;17:801–810. doi: 10.1038/cdd.2009.162. [DOI] [PubMed] [Google Scholar]
- Yin J, Leavenworth JW, Li Y, Luo Q, Xie H, Liu X, Huang S, Yan H, Fu Z, Zhang LY, et al. Ezh2 regulates differentiation and function of natural killer cells through histone methyltransferase activity. Proc Natl Acad Sci U S A. 2015;112:15988–15993. doi: 10.1073/pnas.1521740112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Bardot E, Ezhkova E. Epigenetic regulation of skin: focus on the Polycomb complex. Cell Mol Life Sci. 2012;69:2161–2172. doi: 10.1007/s00018-012-0920-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Gao L, Feng Y, Yuan L, Zhao H, Cornelius LA. Down-regulation of Rap1GAP via promoter hypermethylation promotes melanoma cell proliferation, survival, and migration. Cancer Res. 2009;69:449–457. doi: 10.1158/0008-5472.CAN-08-2399. [DOI] [PubMed] [Google Scholar]
- Zingg D, Debbache J, Schaefer SM, Tuncer E, Frommel SC, Cheng P, Arenas-Ramirez N, Haeusel J, Zhang Y, Bonalli M, et al. The epigenetic modifier EZH2 controls melanoma growth and metastasis through silencing of distinct tumour suppressors. Nat Commun. 2015;6:6051. doi: 10.1038/ncomms7051. [DOI] [PubMed] [Google Scholar]