Abstract
The relationship between P-gp and CD147 in the regulation of MDR in leukemic cells has not been reported. This study aimed to investigate the correlation between CD147 and P-gp in the regulation of drug resistance in the K562/ADR leukemic cell line. The results showed that drug-resistant K562/ADR cells expressed significantly higher P-gp and CD147 levels than drug-free K562/ADR cells. To determine the regulatory effect of CD147 on P-gp expression, anti-CD147 antibody MEM-M6/6 significantly decreased P-gp and CD147 mRNA and protein levels. This is the first report to show that CD147 mediates MDR in leukemia through the regulation of P-gp expression.
Keywords: Multidrug resistance, Leukemia, P-glycoprotein, CD147
Highlights
-
•
High expression levels of P-gp and CD147 in drug-resistant cells.
-
•
MEM-M6/6 antibody decreases both CD147 and P-gp expression.
-
•
CD147 mediates MDR phenotype in leukemia through the regulation of P-gp expression.
1. Introduction
Leukemia, a type of blood cancer, originates from abnormal hematopoietic stem cells and results in a high number of abnormal white blood cells. Chemotherapy, using cytotoxic drugs to destroy cancer cells, has been the most effective treatment. However, multidrug resistance (MDR), the ability of cancer cells to survive exposure to many chemotherapeutic drugs, is the major problem in cancer treatment. Recent studies have revealed that overexpression of drug transporter proteins, such as P-glycoprotein (P-gp), is associated with resistance to multiple chemotherapeutic drugs [1], [2]. P-gp is a 170 kDa transmembrane protein encoded by the MDR1 gene. It functions as a pump to remove anticancer drugs from cells, thereby leading to drug resistance. As reported, P-gp is overexpressed in several drug-resistant cancer cell lines [3], [4], [5]. Thus, P-gp might potentially be a key molecule in MDR cancer. However, the regulatory mechanism of this protein was not elucidated. CD147 is a protein in the immunoglobulin superfamily group [6], [7], [8], [9]; it promotes many properties of cancer cells, including multidrug resistance [10], [11], [12]. A number of studies have shown the high expression level and involvement of CD147 in many MDR cell lines [13], [14]. These studies suggested that CD147 plays an important role in regulating resistance to anticancer drugs. However, the regulatory mechanism of CD147 on the P-gp in leukemic cells remains unclear.
In this study, drug-free K562/ADR cells were established and then used as a drug-resistant phenotype cell control. The CD147 and P-gp demonstrated high expression in drug-resistant K562/ADR cells more than drug-free K562/ADR cells, in both mRNA and protein levels. In addition, the mechanism through which CD147 regulates P-gp in multidrug-resistant leukemic cells was investigated using monoclonal antibodies (mAb) against CD147, MEM-M6/6. MEM-M6/6 down-regulated the expression of MDR1 mRNA and P-gp in drug-resistant K562/ADR cells. The CD147 was purposed to involve in multidrug resistance through regulation of P-gp expression in leukemia cells.
2. Materials and methods
2.1. Antibodies
This study used the following antibodies: mouse anti-CD147 monoclonal antibody clone MEM-M6/6 (Exbio, Vestec, Czech Republic); mouse anti-P-gp monoclonal antibody (MT PGP1) (Biomedical Technology Research Center, Chiang Mai, Thailand) [15], mouse anti-γ4 globin monoclonal antibody (PB1) (Biomedical Technology Research Center) [16], rabbit anti-CD147 polyclonal antibody (Abcam, Cambridge, UK), rabbit anti-GAPDH polyclonal antibody (Merck Millipore, Billeric, MA, USA), goat anti-rabbit IgG-HRP (Abcam), and rabbit anti-mouse Igs-FITC (Dako, Hamburg, Germany).
2.2. Cell lines and culture
The human leukemia cell line, K562/ADR (Adriamycin resistant K562) was purchased from Riken (Riken, Yokohama, Japan) and maintained in RPMI1640 medium (GIBCO™, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS), penicillin, and streptomycin at 37 °C in 5% CO2. Before the experiment, the drug-resistant K562/ADR was cultured in 0.5, 0.75, and 1 μg/mL of Adriamycin (Sigma, St. Louis, MO, USA) to maintain MDR phenotype. Drug-free K562/ADR was generated by growing K562/ADR in culture medium without Adriamycin for at least 5 months to make them sensitive to Adriamycin.
2.3. Antibody treatment
To determine the involvement of the CD147 molecule on the expression and function of P-gp, drug-resistant K562/ADR cells were plated at 2×105 cells/well, then co-cultured with anti-CD147 clone MEM-M6/6 (Exbio) at various concentrations (0–5 µg/mL) for 24–48 h. PB1 antibody against γ4-globin (hemoglobin Bart's) was used as the isotype matched control (IgG1).
2.4. Western blot analysis
Cells were harvested and extracted with RIPA lysis buffer. The total protein concentration was determined using the Folin-Lowry method and 30 μg of total protein were electrophoresed on 10% SDS-PAGE gels. After electrophoresis, the proteins were transferred to polyvinylidene difluoride membranes. The blots were blocked with 5% skim milk-PBS before incubating with 10 μg/mL of mouse monoclonal anti-P-gp antibody (MT-PGP1) (Biomedical Technology Research Center), 1 μg/mL of rabbit polyclonal anti-CD147 antibody (Abcam), or 1 μg/mL of rabbit polyclonal anti-GAPDH antibody (Merck Millipore) for 1 h. Then goat polyclonal anti-rabbit IgG conjugated with HRP (1:20,000 dilution; Abcam) or polyclonal rabbit anti-mouse IgG-HRP (1:5000 dilution; Dako) was added for 2 h. After washing, the signal was detected using Luminata™ forte Western HRP Substrate (Merck Millipore).
2.5. Semi-quantitative reverse transcription-PCR
Total RNA was extracted from cells with TRIzol reagent (Life Technologies) according to the manufacturer's instructions. Single-stranded cDNA was synthesized by reverse transcription from 1 μg of total RNA using the RevertAid strand cDNA Synthesis Kit (Thermo Scientific, USA). Then, 1 μg cDNA was amplified by PCR using a DreamTag Green PCR Master Mix (Thermo ScientificTM). The primer sequences are shown in Table 1. The PCR products were electrophoresed on 1.2% agarose gels, followed by ethidium bromide staining.
Table 1.
| Gene | Primer sequence |
|---|---|
| MDR1 | Sense 5′-AAAGCGACTGAATGTTCAGTGG-3′ |
| Antizense 5′-AATAGATGCCTTTCTGTGCCAG-3′ | |
| BSG | Sense 5′-GCAGCGGTTGGAGGTTGT-3′ |
| Antizense 5′-AGCCACGATGCCCAGGAAAGG-3′ | |
| BIRC5 | Sense 5′-TGGCTGCCATGGATTGAG-3′ |
| Antizense 5′-TCTGAGGAGGCACAGGTGT-3′ | |
| GAPDH | Sense 5′-CAACGTGTCAGTGGTGGACCTG-3′ |
| Antizense 5′-TTACTCCTTGGAGGCCATGTGG-3′ |
2.6. Intracellular rhodamine 123 (Rho123) accumulation assay
After treatment, 2×105 cells were incubated with Rho123 dye at a final concentration of 10 μM for 90 min. After incubation, the cells were harvested and washed twice with ice-cold PBS, and then resuspended in PBS. The fluorescence intensity of the cells was determined using a FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA, USA).
2.7. Statistical analysis
Data were expressed as mean±SEM. Statistical analysis was performed with SPSS 20.0 software. Statistical significance between two groups was determined by Student's t-test. Results from more than two experimental groups were evaluated by one-way Mann-Whitney U test. A difference was considered statistically significant at p<0.05.
3. Results
3.1. Expression of P-gp and CD147 in drug-resistant and drug-free K562/ADR leukemic cell lines
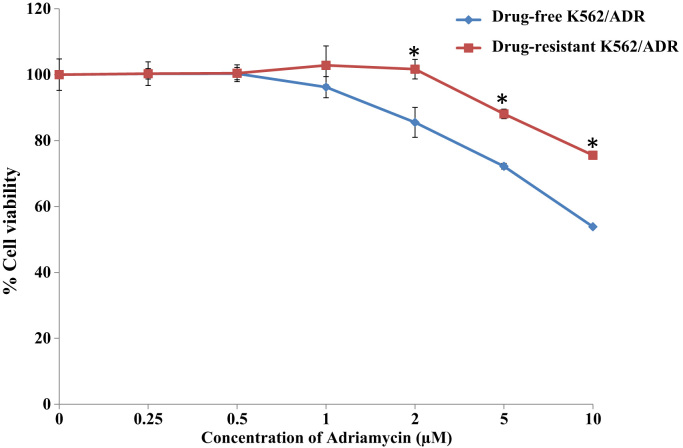
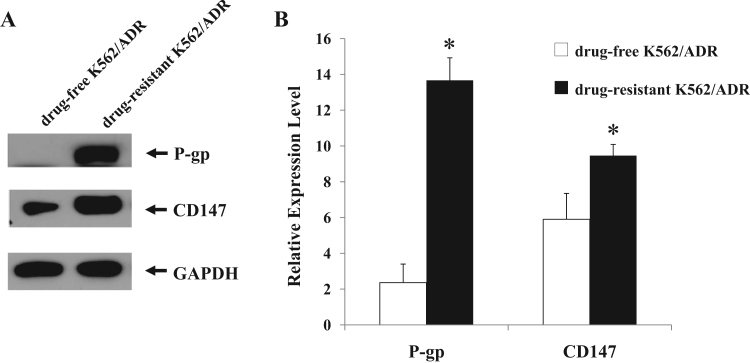
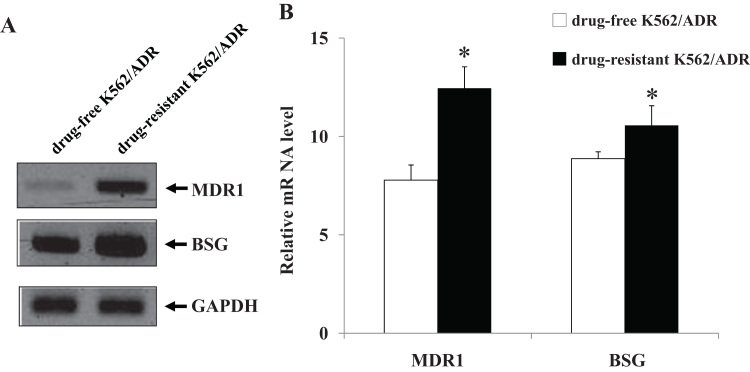
To investigate the multidrug resistance phenotype of drug-resistant K562/ADR and drug-free K562/ADR cell lines, MTT assay was performed to compare the sensitivity of both leukemia cell lines to Adriamycin drug. Drug-resistant K562/ADR and drug-free K562/ADR cell lines were treated with various concentrations of Adriamycin for 48 h. It was found that Adriamycin was more cytotoxic effect to drug-free K562/ADR than drug-resistant K562/ADR cells (Fig. 1). Western blot analysis and semi-quantitative RT-PCR were employed to determine the expression levels of P-gp and CD147 in both drug-resistant K562/ADR and drug-free K562/ADR cells. Representative samples of the immunoblotting results are shown in Fig. 2A. The results showed a higher expression level of P-gp and CD147 proteins in drug-resistant K562/ADR than in drug-free K562/ADR (p<0.05) (Fig. 2B). Furthermore, the results showed significantly higher expression levels of MDR1 and BSG mRNA in drug-resistant K562/ADR compared to drug-free K562/ADR (p<0.05), as shown in Fig. 3. The MDR mRNA, P-gp, and CD147 levels were much higher in drug-resistant K562/ADR than drug-free K562/ADR cells. According to the previous reports, these results confirmed that P-gp and CD147 were involved in the leukemic cell with MDR phenotype.
Fig. 1.
Cytotoxicity of Adriamycin on drug-resistant K562/ADR and drug-free K562/ADR leukemia cell lines by MTT assay. Cells were treated with Adriamycin drug (0–10 μM) for 48 h. and the cytotoxicity was measured by the MTT assay. Data represent as mean±SEM of three independent experiments; ⁎p<0.05 when compared to drug-free K562/ADR cell.
Fig. 2.
The protein expressions of P-gp and CD147 in drug-resistant K562/ADR and drug-free K562/ADR leukemic cell lines. Proteins were extracted drug-resistant K562/ADR and drug-free K562/ADR cell lines and separated by 12% SDS-PAGE and transferred onto PVDF membrane. The membranes were probed with anti-CD147, anti-P-gp and detected by enhanced chemiluminescence. (A) Results are representative of three independent experiments by Western blotting and (B) Quantification of P-gp and CD147 expressions in drug-resistant K562/ADR and drug-free K562/ADR cells; the protein levels were normalized to the loading control GAPDH. The ratio of P-gp and CD147 were calculated for each group. Data are presented as mean±SEM of three independent experiments; ⁎p<0.05 when compared to drug-free K562/ADR cell.
Fig. 3.
The level of MDR1 and BSG mRNA in drug-resistant K562/ADR and drug-free K562/ADR leukemic cell lines. Total RNA was extracted from cells and the reverse transcription reaction were performed using a RevertAid First Strand cDNA Synthesis kit (Thermo Scientific™). The newly synthesized cDNA was amplified by PCR using specific primers. The PCR product were electrophoresed on 1.2% agarose gel and visualized by ethidium bromide staining. (A) Results are representative of three independent experiments by semiquantitative RT-PCR analysis and (B) quantification of MDR1 (multidrug resistance 1) and BSG (Basigin) mRNA expression in drug-resistant K562/ADR and drug-free K562/ADR cells; the mRNA levels were normalized to the loading control GAPDH. The ratio of MDR1 and BSG mRNAs were calculated for each group. Data are presented as mean ±SEM of three independent experiments; ⁎p<0.05 when compared to drug-free K562/ADR cell.
3.2. MEM-M6/6 mAb inhibits the expression of P-gp and CD147 protein in drug-resistant K562/ADR cell line
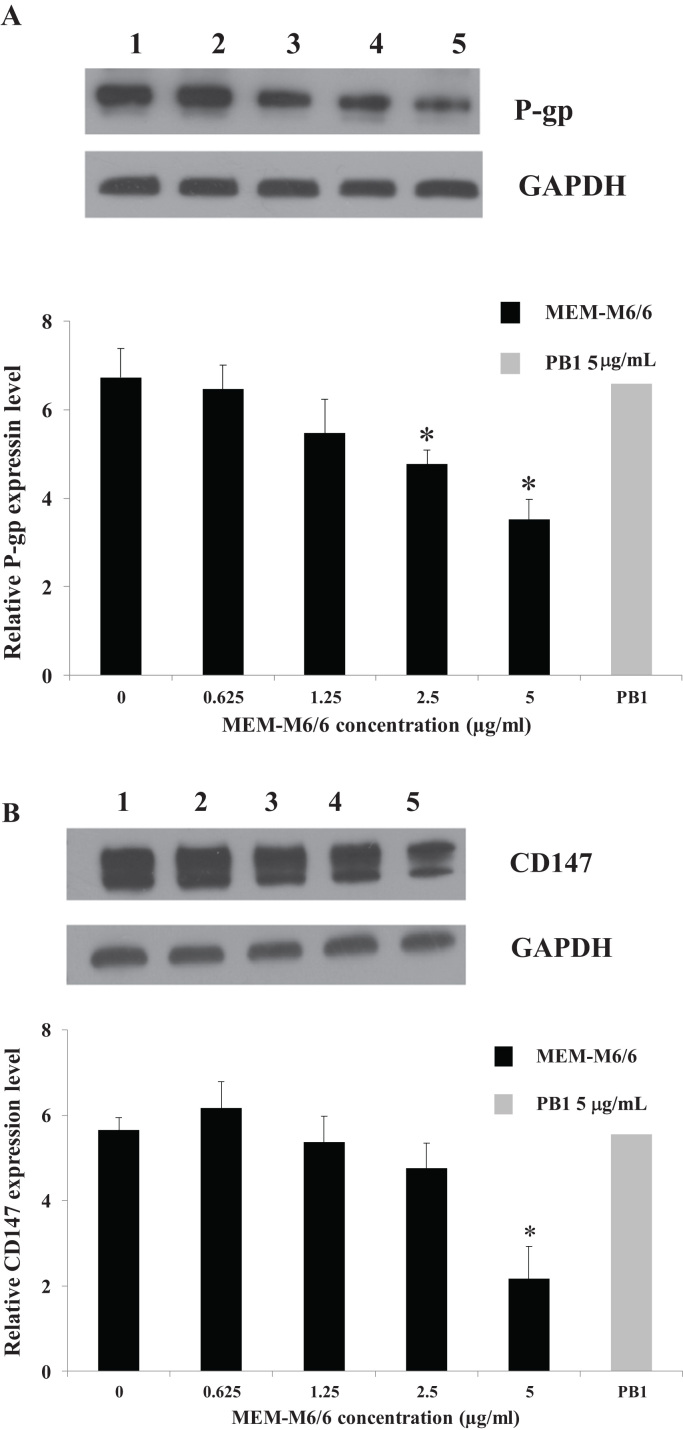
Because CD147 is involved in cancer multidrug resistance, the CD147 was further determined in the regulation of P-gp. Drug-resistant K562/ADR cells were treated with MEM-M6/6 anti-CD147 or PB1 anti-γ4 globin (isotype matched control) for 48 h, then the expression of P-gp and CD147 proteins was examined by Western blotting. As shown in Fig. 4, the results demonstrated that MEM-M6/6 antibody treatment significantly decreased the expression of P-gp and CD147 proteins in a dose-dependent manner. The MEM-M6/6 mAb at 5 μg/mL had no effect on cell viability (data not shown). In contrast, at 5 μg/mL, PB1 anti-γ4 globin mAb showed no difference of all tested proteins expression.
Fig. 4.
Effect of MEM-M6/6 anti-CD147 on P-gp (A) and CD147 (B) protein expression in drug-resistant K562/ADR cell line. The protein levels after treating drug-resistant K562/ADR cell with (1) 0 μg/mL MEM-M6/6, (2) 0.625 μg/mL, (3) 1.25 μg/mL, (4) 2.5 μg/mL, and (5) 5 μg/mL for 48 h were determined by Western blotting. The bands were quantified using a scan densitometer. The protein levels were normalized to the loading control GAPDH. Data are presented as mean±SEM of three independent experiments; ⁎p<0.05 when compared to untreated cell.
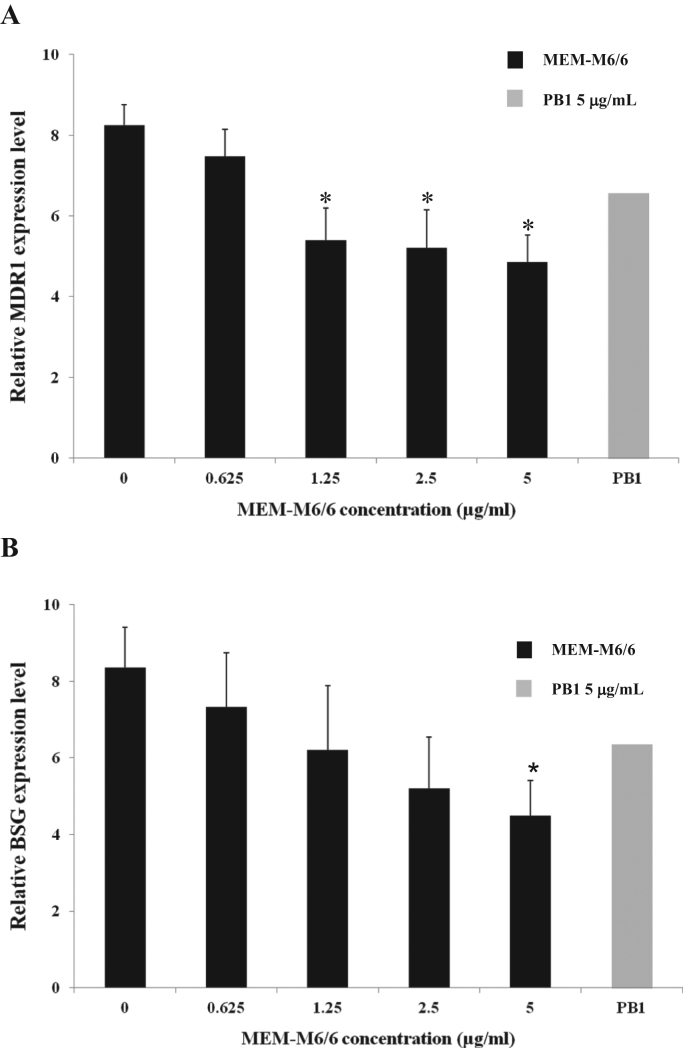
3.3. MEM-M6/6 mAb inhibits the expression of MDR1 and BSG mRNA in drug-resistant K562/ADR cells
The effects of MEM-M6/6 mAb on MDR1 and BSG mRNA expression were also investigated by semi-quantitative RT-PCR. Drug-resistant K562/ADR cells were cultured with MEM-M6/6 mAb for 24 h. The results showed that MEM-M6/6 decreased the expression of MDR1 and BSG mRNA levels in drug-resistant K562/ADR cells in a dose-dependent manner compared to untreated cells (Fig. 5). In addition, 5 μg/mL MEM-M6/6 antibody significantly decreased MDR1 and BSG mRNA levels (p<0.05). For the isotype matched control, the results showed no difference in the mRNA levels in treated and untreated drug-resistant K562/ADR cells. These results suggested that triggering CD147 by anti-CD147 mAb MEM-M6/6 decreased the expression of CD147, leading to a decrease of MDR1 expression.
Fig. 5.
Effect of MEM-M6/6 anti-CD147 on MDR1 (A) and BSG (B) mRNA levels in drug-resistant K562/ADR cell line. The quantification of mRNA expression in the presence of various concentrations of MEM-M6/6 (0–5 µg/mL) were normalized to the loading control GAPDH mRNA. The ratio of mRNA was calculated for each group. Data are presented as mean±SEM of three independent experiments; ⁎p<0.05 when compared to untreated K562/ADR cell.
3.4. Effects of MEM-M6/6 mAb on P-gp function
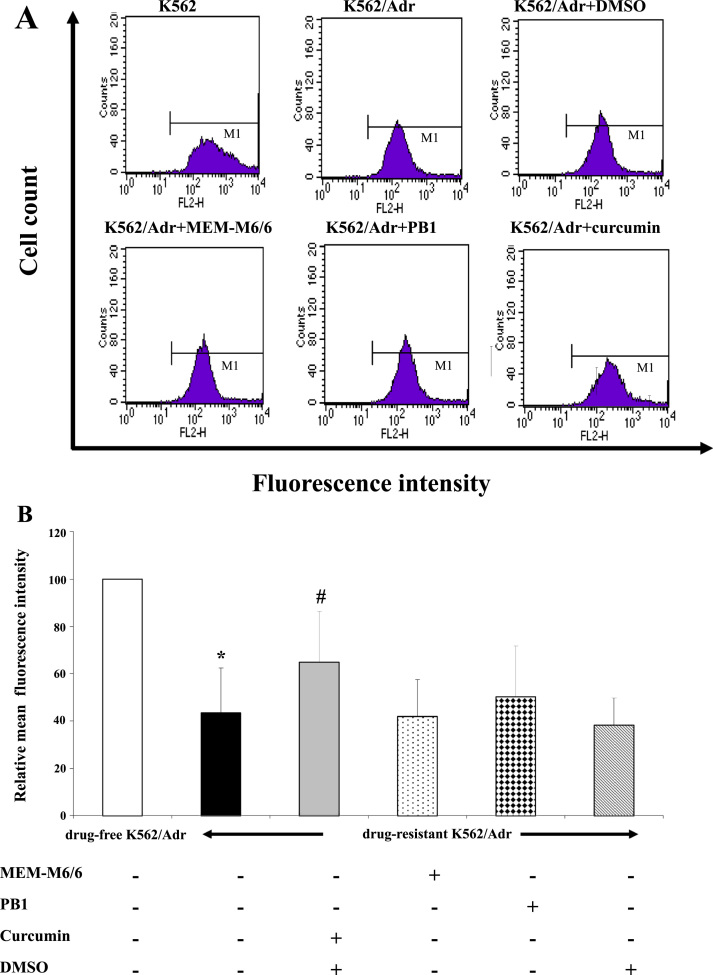
To investigate whether MEM-M6/6 mAb affects P-gp function, the intracellular accumulation of Rho123 in the presence or absence of MEM-M6/6 antibody was determined in drug-resistant K562/ADR and drug-free K562/ADR cells. As shown in Fig. 6, the relative mean fluorescence intensity (MFI) in drug-resistant K562/ADR cells was significantly lower than drug-free K562/ADR cells (p<0.05). For drug-resistant K562/ADR cells, there was no significant difference in Rho123 intracellular accumulation in MEM-M6/6 antibody treatment compared to untreated cells. The accumulation of Rho123 did not alter in PB1 anti-γ4 globin mAb treated cells. However, the MFI of Rho123 was significantly higher in curcumin (MDR modulator) treated K562/ADR cells than untreated cells (p<0.05).
Fig. 6.
Effect of MEM-M6/6 on P-gp function by Rhodamine 123 accumulation assay. Drug-resistant K562/ADR cells were treated with MEM-M6/6 or curcumin for 48 h, then Rho123 was added and incubated for further 90 min. Fluorescence intensity of Rho123 was analyzed by flow cytometry. (A) Results are representative of intracellular Rho123 accumulation and (B) Relative mean fluorescence intensity of various conditions. Results are presented as mean ±SEM of three independent experiments. ⁎p<0.05 when compared to drug-free K562/ADR cell, #p<0.05 when compared to untreated drug-resistant K562/ADR cell.
4. Discussion
There are several mechanisms of MDR, including decreased drug uptake, reduced intracellular drug concentration by efflux pumps, or altered cell-cycle checkpoints [18], [19], [20]. Among these, overexpression of P-gp, ATP-binding cassette (ABC) drug transporters, is an important mechanism [1], [2]. P-gp is a transmembrane glycoprotein that is widely expressed in many human cancers [21]. Levels of P-gp have been correlated with drug resistance in several different cancers [1], [2], [20], [22]. Therefore, understanding MDR is important to help patients for reducing or preventing chemotherapy resistance.
CD147 is a type I transmembrane glycoprotein of the immunoglobulin superfamily. CD147 has been reported to regulate the expression of P-gp and affect apoptosis in cancer cells with MDR phenotype [10], [13], [14]. In the present study, drug-free K562/ADR was first established by culturing K562/ADR in medium without Adriamycin for at least five months. Then Adriamycin drug sensitivities of both drug-resistant and drug-free K562/ADR cells were determined and compared by MTT assay. The results showed that Adriamycin was more cytotoxic in drug-free K562/ADR cells than drug-resistant K562/ADR cells. Moreover, the drug-resistant K562/ADR cell line presented high expression levels of P-gp and CD147, suggesting that both proteins are associated with the MDR phenotype in leukemic cells. This finding is in line with previous studies that have demonstrated high levels of mRNA and protein expression of MDR1 and CD147 in MDR cancer cell lines [13]. Recent studies have revealed CD147 is responsible for the altered multidrug resistance to P-gp substrate drugs [14]. Up-regulation of MDR1 at both transcription and expression levels was found in CD147-transfected MCF7 breast cancer cells, which promotes multidrug resistance to P-gp substrate drugs. In addition, silencing of CD147 led to increase chemosensitivity in MDR cancer cell lines [23], [24]. As previously mentioned, the linkage exists among CD147 and P-gp in leukemia with MDR were further investigated. Drug-resistant K562/ADR cells were co-cultured with MEM-M6/6 anti-CD147 mAb, and then P-gp and CD147 expression were determined by Western blotting and semi-quantitative RT-PCR. The MEM-M6/6 antibody decreased the expression of P-gp and CD147 proteins and mRNA levels, suggesting the regulation of P-gp expression by CD147 molecule in the association of multidrug resistant phenotype in K562/ADR cells. As previously reported, the CD147 molecule contains different bioactive domains responsible for cell functions [25]. Therefore, activation of bioactive domains, by specific mAbs or its natural ligands, induces different cellular responses [25]. MEM-M6/6 was reported as a CD147 mAb, which recognized the membrane-proximal domain of the CD147 molecule [26]. These results suggest that the membrane-proximal domain of the CD147 molecule may be a particular domain that is responsible for the regulation of multidrug resistance. Furthermore, triggering CD147 with MEM-M6/6 decreased the expression of CD147, which in turn down-regulated P-gp expression.
The further investigation was performed by using MEM-M6/6 antibody to observe the linkage exits between CD147 and P-gp activity by intracellular Rho123 accumulation. MEM-M6/6 treatment did not alter Rho123 accumulation in drug-resistant K562/ADR cells. In contrast, the fluorescence intensity of Rho123 was significantly high in K562/ADR cells treated with curcumin, which is a P-gp modulator used as a positive control [27]. These results indicated that the decreased expression of CD147 by anti-CD147 MEM-M6/6 did not affect the P-gp function. It has been suggested that CD147 is involved in leukemia drug resistance by down-regulating Pgp, but not related to its drug efflux function [28], [29].
Triggering of CD147 by MEM-M6/6 anti-CD147 mAb decreased expression of CD147 and caused downregulation of P-gp, but had no effect on P-gp function. Most strategies to reverse MDR phenotype in cancer cells have used drug efflux modulators. Moreover, to avoid MDR could be downregulated the expression of the target gene that involved in MDR such as MDR1 gene. Therefore, we propose regulating CD147 expression as a new strategy to overcome MDR. In summary, the CD147 involved the MDR phenotype of leukemic cells by regulating P-gp expression.
Acknowledgments
This work was supported by the Thailand Research Fund (TRF) (RSA5580030), Chiang Mai University Research Fund (H-M5704), the TRF Senior Research Scholar (RTA5980007), the National Research University Project under Thailand's Office of the Higher Education Commission and supported in part by the grant from Center of Excellence on Medical Biotechnology (CEMB)/PERDO. Biomedical Technology Research Center is a Center of Excellence of Chiang Mai University.
References
- 1.Gottesman M.M., Fojo T., Bates S.E. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 2.Shabbits J.A., Mayer L.D. P-glycoprotein modulates ceramide mediated sensitivity of human breast cancer cells to tubulin binding anticancer drugs. Mol. Cancer Ther. 2002;1:205–213. [PubMed] [Google Scholar]
- 3.Liu F., Liu S., He S., Xie Z., Zu X., Jiang Y. Survivin transcription is associated with P-glycoprotein/MDR1 overexpression in the multidrug resistance of MCF-7 breast cancer cells. Oncol. Rep. 2010;23:1469–1475. doi: 10.3892/or_00000786. [DOI] [PubMed] [Google Scholar]
- 4.Gottesman M.M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev. Biochem. 1993;62:385-–3427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 5.Lo Y.L. Relationships between the hydrophilic-lipophilic balance values of pharmaceutical excipients and their multidrug resistance modulating effect in Caco-2 cells and rat intestines. J. Control Release. 2003;90:37–48. doi: 10.1016/s0168-3659(03)00163-9. [DOI] [PubMed] [Google Scholar]
- 6.Miyauchi T., Kanekura T., Yamaoka A., Ozawa M., Miyazawa S., Muramatsu T. Basigin, a new, broadly distributed member of the immunoglobulin superfamily, has strong homology with both the immunoglobulin V domain and the beta-chain of major histocompatibility complex class II antigen. J. Biochem. 1990;107:316–332. doi: 10.1093/oxfordjournals.jbchem.a123045. [DOI] [PubMed] [Google Scholar]
- 7.Biswas C., Zhang Y., DeCastro R., Guo H., Nakamura T., Kataoka H. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995;55:434–439. [PubMed] [Google Scholar]
- 8.Kasinrerk W., Fiebiger E., Stefanová I., Baumruker T., Knapp W., Stockinger H. Human leukocyte activation antigen M6, a member of the Ig superfamily, is the species homologue of rat OX-47, mouse basigin, and chicken HT7 molecule. J. Immunol. 1992;149:847–854. [PubMed] [Google Scholar]
- 9.Stockinger H., Ebel T., Hansmann C., Koch C., Majdic O., Prager E. CD147 (neurothelin/basigin) workshop panel report. In: Kishimoto T., Kikutani H., Von Dem Borne A.E.G.K., Goyert S.M., Mason D.Y., Miyasaka M., editors. Leucocyte Typing VI. Garland Publishing, Inc; New York: 1997. pp. 760–765. [Google Scholar]
- 10.Misra S., Ghatak S., Zoltan-Jones A., Toole B.P. Regulation of multidrug resistance in cancer cells by hyaluronan. J. Biol. Chem. 2003;278:25285–25288. doi: 10.1074/jbc.C300173200. [DOI] [PubMed] [Google Scholar]
- 11.Iacono K.T., Brown A.L., Greene M.I., Saouaf S.J. CD147 immunoglobulin superfamily receptor function and role in pathology. Exp. Mol. Pathol. 2007;83:283–295. doi: 10.1016/j.yexmp.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanekura T., Chen X., Kanzaki T. Basigin (CD147) is expressed on melanoma cells and induces tumor cell invasion by stimulating production of matrix metalloproteinases by fibroblasts. Int. J. Cancer. 2002;99 doi: 10.1002/ijc.10390. 520-8. [DOI] [PubMed] [Google Scholar]
- 13.Yang J.M., Xu Z., Wu H., Zhu H., Wu X., Hait W.N. Overexpression of extracellular matrix metalloproteinase inducer in multidrug resistant cancer cells. Mol. Cancer Res. 2003;1:420–427. [PubMed] [Google Scholar]
- 14.Li Q.Q., Wang W.J., Xu J.D., Cao X.X., Chen Q., Yang J.M. Involvement of CD147 in regulation of multidrug resistance to P-gp substrate drugs and in vitro invasion in breast cancer cells. Cancer Sci. 2007;98:1064–10649. doi: 10.1111/j.1349-7006.2007.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiampanichayakul S., Anuchapreeda S., Chruewkamlow N., Mahasongkram K., Thanaratanakorn P., Kasinrerk W. Production of monoclonal antibodies to P-glycoprotein: its application in detection of soluble and surface P-glycoprotein of leukemia patients. Int. J. Hematol. 2010;92:326–333. doi: 10.1007/s12185-010-0668-8. [DOI] [PubMed] [Google Scholar]
- 16.Tayapiwatana C., Kuntaruk S., Tatu T., Chiampanichayakul S., Munkongdee T., Winichagoon P. Simple method for screening of alpha-thalassaemia 1 carriers. Int. J. Hematol. 2009;89:559–567. doi: 10.1007/s12185-009-0331-4. [DOI] [PubMed] [Google Scholar]
- 17.Kuang Y.H., Chen X., Su J., Wu L.S., Liao L.Q., Li D. RNA interference targeting the CD147 induces apoptosis of multidrug-resistant cancer cells related to XIAP depletion. Cancer Lett. 2009;276:189–195. doi: 10.1016/j.canlet.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Chai S., To K.K., Lin G. Circumvention of multi-drug resistance of cancer cells by Chinese herbal medicines. Chin. Med. 2010;5:26. doi: 10.1186/1749-8546-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longo-Sorbello G.S., Bertino J.R. Current understanding of methotrexate pharmacology and efficacy in acute leukemias. Use of newer antifolates in clinical trials. Haematologica. 2001;86:121–127. [PubMed] [Google Scholar]
- 20.Gottesman M.M. Mechanisms of cancer drug resistance. Annu Rev. Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 21.Goldstien L.J., Galski H., Fojo A., Willingham M., Lai S.L., Gazdar A. Expression of a multidrug resistance gene in human cancers. J. Natl. Cancer Inst. 1989;81:116–124. doi: 10.1093/jnci/81.2.116. [DOI] [PubMed] [Google Scholar]
- 22.La Porta C.A. Drug resistance in melanoma: new perspectives. Curr. Med Chem. 2007;14:387–391. doi: 10.2174/092986707779941078. [DOI] [PubMed] [Google Scholar]
- 23.Zou W., Yang H., Hou X., Zhang W., Chen B., Xin X. Inhibition of CD147 gene expression via RNA interference reduces tumor cell invasion, tumorigenicity and increases chemosensitivity to paclitaxel in HO-8910pm cells. Cancer Lett. 2007;248:211–218. doi: 10.1016/j.canlet.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Jia L., Xu H., Zhao Y., Jiang L., Yu J., Zhang J. Expression Of Cd147 mediates tumor cells invasion and multidrug resistance in hepatocellular carcinoma. Cancer Investig. 2008;26:977–983. doi: 10.1080/07357900802072723. [DOI] [PubMed] [Google Scholar]
- 25.Chiampanichayakul S., Peng-in P., Khunkaewla P., Stockinger H., Kasinrerk W. CD147 contains different bioactive epitopes involving the regulation of cell adhesion and lymphocyte activation. Immunobiology. 2006;211:167–178. doi: 10.1016/j.imbio.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Koch C., Staffler G., Hüttinger R., Hilgert I., Prager E., Cerný J. T cell activation-associated epitopes of CD147 in regulation of the T cell response, and their definition by antibody affinity and antigen density. Int. Immunol. 1999;11:777–786. doi: 10.1093/intimm/11.5.777. [DOI] [PubMed] [Google Scholar]
- 27.Anuchapreeda S., Leechanachai P., Smith M.M., Ambudkar S.V., Limtrakul P.N. Modulation of P-glycoprotein expression and function by curcumin in multidrug-resistant human KB cells. Biochem. Pharm. 2002;64:573–582. doi: 10.1016/s0006-2952(02)01224-8. [DOI] [PubMed] [Google Scholar]
- 28.Smyth M.J., Krasovskis E., Sutton V.R., Johnstone R.W. The drug efflux protein, P-glycoprotein, additionally protects drug-resistant tumor cells from multiple forms of caspase-dependent apoptosis. Proc. Natl. Acad. Sci. USA. 1998;95:7024–7029. doi: 10.1073/pnas.95.12.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnstone R.W., Cretney E., Smyth M.J. P-glycoprotein protects leukemia cells against caspase-dependent, but not caspase-independent, cell death. Blood. 1999;93:1075–1085. [PubMed] [Google Scholar]