Abstract
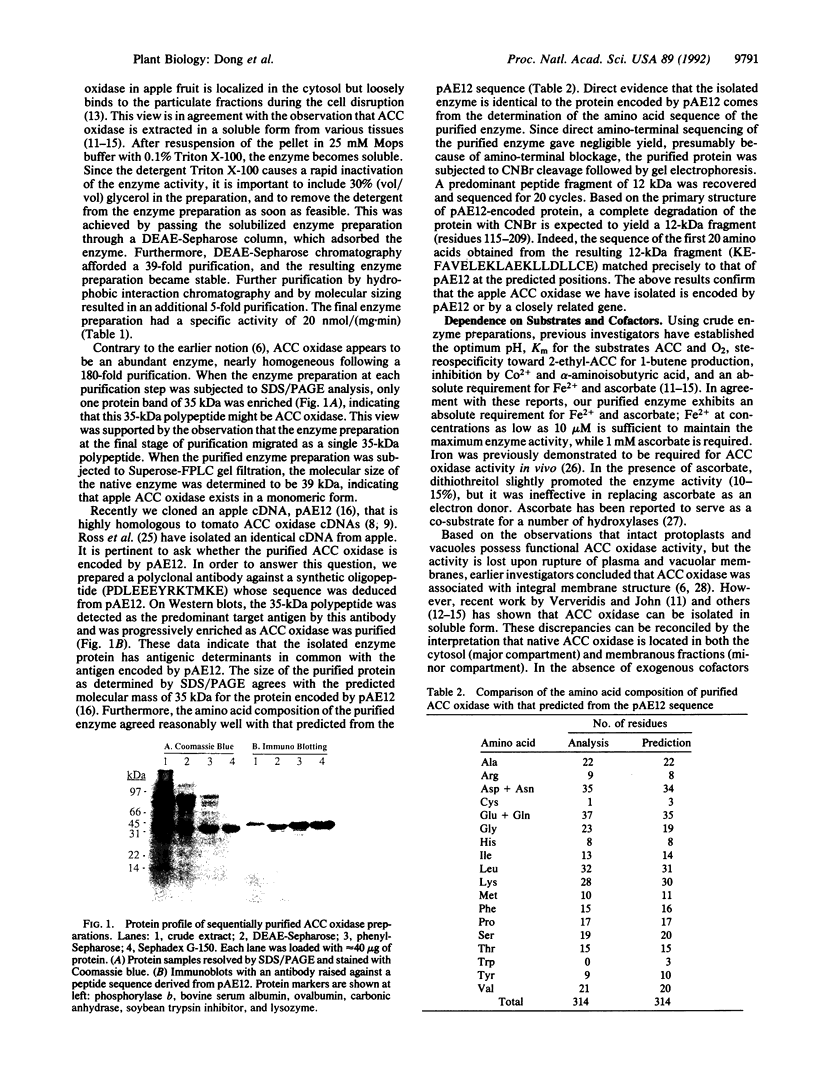
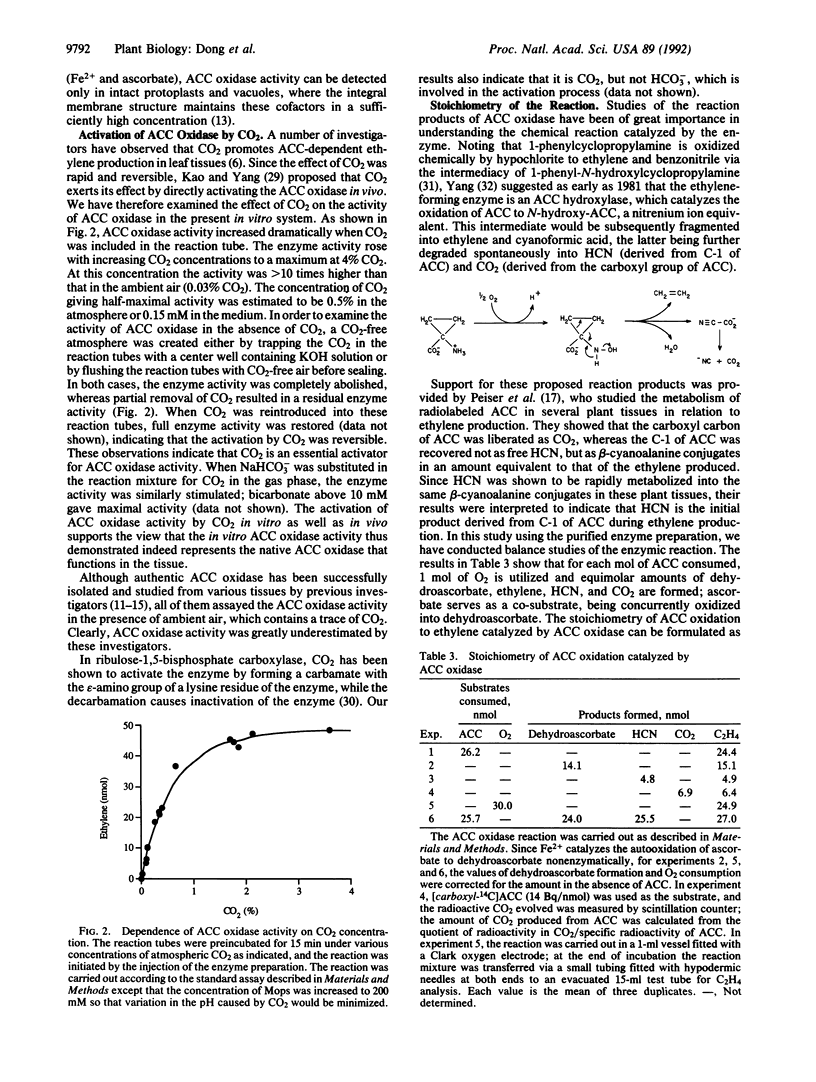
1-Aminocyclopropane-1-carboxylate (ACC) oxidase catalyzes the oxidation of ACC to ethylene. Following conventional column fractionation, the enzyme was purified 180-fold to near homogeneity with a specific activity of 20 nmol/(mg.min). This purified enzyme preparation migrated as a single protein band with an apparent molecular mass of 35 kDa on SDS/PAGE and 39 kDa on gel filtration. As in vivo, the purified enzyme required CO2 for activity. Removal of CO2 from the reaction mixture completely abolished the enzyme activity, while 0.5% atmospheric CO2 (0.15 mM in the medium) gave half-maximal activity. The purified enzyme displayed an absolute requirement for Fe2+ and ascorbate. The stoichiometry of the enzymatic reaction was determined: ACC + ascorbate + O2-->C2H4 + HCN + CO2 + dehydroascorbate + 2 H2O. A polyclonal antibody was raised against a synthetic tridecapeptide (PDLEEEYRKTMKE) whose sequence was deduced from the apple pAE12 cDNA [Dong, J. G., Olson, D., Silverstone, A. & Yang, S. F. (1992) Plant Physiol. 98, 1530-1531], which is homologous to tomato cDNAs encoding ACC oxidase. On a Western blot, this antibody specifically recognized the purified ACC oxidase protein. The amino acid composition of the purified enzyme agreed well with that deduced from the pAE12 sequence. When the protein was cleaved with CNBr and one of the peptide fragments was isolated and sequenced for 20 cycles, its sequence (KEFAVELEKLAEKLLDLLCE) precisely matched that predicted from pAE12 (residues 115-134). When preclimacteric apple fruit was treated with ethylene, a parallel increase in in vivo and in vitro ACC oxidase activities was observed, and this increase was accompanied by a concomitant increase in the level of pAE12 transcript. These observations support the conclusion that the isolated ACC oxidase protein is encoded by pAE12.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dong J. G., Olson D., Silverstone A., Yang S. F. Sequence of a cDNA coding for a 1-aminocyclopropane-1-carboxylate oxidase homolog from apple fruit. Plant Physiol. 1992 Apr;98(4):1530–1531. doi: 10.1104/pp.98.4.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Maculet J. C., Yang S. F. Extraction and partial characterization of the ethylene-forming enzyme from apple fruit. Plant Physiol. 1992 Jun;99(2):751–754. doi: 10.1104/pp.99.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton A. J., Bouzayen M., Grierson D. Identification of a tomato gene for the ethylene-forming enzyme by expression in yeast. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7434–7437. doi: 10.1073/pnas.88.16.7434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu Y., Hoffman N. E., Yang S. F. Promotion by Ethylene of the Capability to Convert 1-Aminocyclopropane-1-carboxylic Acid to Ethylene in Preclimacteric Tomato and Cantaloupe Fruits. Plant Physiol. 1985 Feb;77(2):407–411. doi: 10.1104/pp.77.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizada M. C., Yang S. F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979 Nov 15;100(1):140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- Lorimer G. H., Miziorko H. M. Carbamate formation on the epsilon-amino group of a lysyl residue as the basis for the activation of ribulosebisphosphate carboxylase by CO2 and Mg2+. Biochemistry. 1980 Nov 11;19(23):5321–5328. doi: 10.1021/bi00564a027. [DOI] [PubMed] [Google Scholar]
- Matsuda J., Okabe S., Hashimoto T., Yamada Y. Molecular cloning of hyoscyamine 6 beta-hydroxylase, a 2-oxoglutarate-dependent dioxygenase, from cultured roots of Hyoscyamus niger. J Biol Chem. 1991 May 25;266(15):9460–9464. [PubMed] [Google Scholar]
- McGarvey D. J., Christoffersen R. E. Characterization and kinetic parameters of ethylene-forming enzyme from avocado fruit. J Biol Chem. 1992 Mar 25;267(9):5964–5967. [PubMed] [Google Scholar]
- Numata M., Saito T., Yamazaki T., Fukumori Y., Yamanaka T. Cytochrome P-460 of Nitrosomonas europaea: further purification and further characterization. J Biochem. 1990 Dec;108(6):1016–1021. doi: 10.1093/oxfordjournals.jbchem.a123300. [DOI] [PubMed] [Google Scholar]
- Peiser G. D., Wang T. T., Hoffman N. E., Yang S. F., Liu H. W., Walsh C. T. Formation of cyanide from carbon 1 of 1-aminocyclopropane-1-carboxylic acid during its conversion to ethylene. Proc Natl Acad Sci U S A. 1984 May;81(10):3059–3063. doi: 10.1073/pnas.81.10.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. S., Knighton M. L., Lay-Yee M. An ethylene-related cDNA from ripening apples. Plant Mol Biol. 1992 May;19(2):231–238. doi: 10.1007/BF00027344. [DOI] [PubMed] [Google Scholar]
- Sato T., Oeller P. W., Theologis A. The 1-aminocyclopropane-1-carboxylate synthase of Cucurbita. Purification, properties, expression in Escherichia coli, and primary structure determination by DNA sequence analysis. J Biol Chem. 1991 Feb 25;266(6):3752–3759. [PubMed] [Google Scholar]
- Smith V. A., Gaskin P., Macmillan J. Partial Purification and Characterization of the Gibberellin A(20) 3beta-Hydroxylase from Seeds of Phaseolus vulgaris. Plant Physiol. 1990 Nov;94(3):1390–1401. doi: 10.1104/pp.94.3.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanu P., Reinhardt D., Boller T. Analysis and cloning of the ethylene-forming enzyme from tomato by functional expression of its mRNA in Xenopus laevis oocytes. EMBO J. 1991 Aug;10(8):2007–2013. doi: 10.1002/j.1460-2075.1991.tb07730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada M., Watanabe Y., Kunitomo M., Hayashi E. Differential rapid analysis of ascorbic acid and ascorbic acid 2-sulfate by dinitrophenylhydrazine method. Anal Biochem. 1978 Feb;84(2):604–608. doi: 10.1016/0003-2697(78)90083-0. [DOI] [PubMed] [Google Scholar]
- Van der Straeten D., Van Wiemeersch L., Goodman H. M., Van Montagu M. Cloning and sequence of two different cDNAs encoding 1-aminocyclopropane-1-carboxylate synthase in tomato. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4859–4863. doi: 10.1073/pnas.87.12.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip W. K., Dong J. G., Kenny J. W., Thompson G. A., Yang S. F. Characterization and sequencing of the active site of 1-aminocyclopropane-1-carboxylate synthase. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7930–7934. doi: 10.1073/pnas.87.20.7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip W. K., Dong J. G., Yang S. F. Purification and characterization of 1-aminocyclopropane-1-carboxylate synthase from apple fruits. Plant Physiol. 1991 Jan;95(1):251–257. doi: 10.1104/pp.95.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip W. K., Yang S. F. Cyanide metabolism in relation to ethylene production in plant tissues. Plant Physiol. 1988 Oct;88(2):473–476. doi: 10.1104/pp.88.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]




