Abstract
Background
Maternal obesity is associated with several adverse pregnancy outcomes. This study was conducted aiming to evaluate maternal levels of adipokines and insulin in pregnancies complicated by overweight and obesity and its correlations with maternal and fetal outcomes.
Methods
This cross-sectional study included 72 mother–newborn pairs. Mothers were classified as having normal weight (n = 23), overweight (n = 18), and obesity (n = 31). Maternal adiponectin, leptin, resistin and insulin levels at the end of pregnancy were compared among groups and correlated with maternal and perinatal outcomes. Data were analyzed by ANOVA and correlation tests, with a p value <0.05 being considered as significant.
Results
Obese pregnant women showed higher leptin levels (p = 0.0021). Leptin levels were positively correlated with prepregnancy body mass index—BMI (r = 0.57), gestational (37 or 38 weeks of gestation) BMI (r = 0.39), hypertension (r = 0.27), and hyperglycemia (r = 0.30), and negatively associated with newborns’ abdominal circumference (r = −0.25). Adiponectin concentrations were negatively correlated with gestational BMI (r = −0.29) and newborns’ cephalic circumference (r = −0.27) and positively correlated with birth weight (r = 0.23). Insulin concentrations correlated positively with prepregnancy BMI (r = 0.38), gestational BMI (r = 0.24) and maternal hyperglycemia (r = 0.26).
Conclusions
Our findings support the relationship between markers of obesity and maternal–fetal outcomes. Maternal insulin and adipokines levels showed an independent relationship with mother and newborns outcomes, respectively. In this studied population, the results indirectly reinforce the importance of maternal weight control before and during pregnancy to avoid adverse outcomes to mother and their newborns.
Keywords: Adipokines, Pregnancy, Obesity, Insulin resistance, Hyperglycemia
Background
In recent years the endocrine nature of adipose tissue has been widely recognized. Its cells synthesize and release a number of substances such as many types of adipokines. Adipokines are primarily secreted from adipose tissue and function as important regulators of appetite, glucose homeostasis, immune function, arterial blood pressure and blood coagulation [1–3].
Growing evidence has pointed to a putative and causative relationship between obesity and inflammation. An increase in adipose tissue and consequently in adipokines production triggers a series of physiopathologic processes associated to the genesis of obesity-related pathologies. The production and release of adipokines or inflammatory cytokines characterize obesity as a chronic inflammatory state [1, 4–7]. In the last decade, different adipokines types have been identified, such as leptin, adiponectin, adipsin, resistin, tumoral necrosis factor alpha (TNF-α), plasminogen activator inhibitor-1 (PAI-1), interleukin (IL) 1β, IL-6 and IL-8, insulin-like growth factor 1 (IGF-1), monocyte chemo attractant protein-1 (MCP-1) and visfatin. Except for adiponectin, the production and secretion of these substances is proportional to the degree of obesity. A number of these adipokines, such as adiponectin, leptin, TNF-α, resistin, PAI-1, IL-6 and MCP-1, were found to be directly related to insulin resistance and associated morbidities [8–11].
In pregnant women, several metabolic changes such as progressive increase and redistribution of maternal adipose tissue occur to ensure continuous nutrient supply to fetal development. This affects a series of metabolic functions that characterize a state of dysmetabolism with consequent increase of the risk for adverse pregnancy outcomes [5, 8, 12, 13]. Hormones produced by the placenta, particularly in the second and third trimesters of pregnancy, generally antagonize insulin action, thereby establishing insulin resistance in parallel with an increase in the production of this hormone [14, 15]. It is thought that adipokines participate in the process of insulin resistance during pregnancy, that may be related to the occurrence of gestational diabetes mellitus (GDM), preeclampsia (PE) and intrauterine growth restriction (IUGR). Insulin resistance in pregnancy has been particularly associated with adiponectin and leptin, which are secreted only by the adipose tissue, and IL-6 and TNF-α, which are also secreted by the adipose tissue and other cells [12, 14, 16].
Among adipokines, leptin is known as the “satiety hormone” given that it inhibits food intake and stimulates energy expenditure through activation of receptors in the hypothalamus. High leptin levels are found in obese people, possibly because they exhibit resistance to the action of this hormone [17, 18]. Adiponectin and resistin are adipocytokines with antagonistic actions. Adiponectin increases insulin sensitivity, whereas resistin increases insulin resistance [18–20].
Obesity and pregnancy play individual roles in the development of chronic inflammation and insulin resistance, and their combination can be particularly harmful to the mother and the fetus [21, 22]. Many gaps exist in the understanding the role of obesity during pregnancy and the causal relationship with associated disorders. It is not known which problems arise from obesity itself and which are a result of unhealthy lifestyle such as sedentarism and use of an inadequate diet, which, in turn, can promote obesity [11, 23, 24]. Likewise, the lack of individualization in the studies, considering only overweight or obese mothers without hypertension, hyperglycemia or insulin resistance conditions, may change these results.
To clarify this scenario, adipokines deserve deeper investigation given their potential role as biomarkers of obesity and insulin resistance in pregnancy, and therefore perinatal outcomes.
The present study evaluated the serum levels of adipokines (adiponectin, leptin and resistin) and insulin in overweight and obese pregnant women and correlated them with maternal and perinatal outcomes.
Methods
Study design and subjects
We conducted a cross-sectional study with 72 mother-newborn pairs referred and treated in Diabetes and Pregnancy Care Service of Botucatu Medical School—UNESP (SEDG-FMB/UNESP). The research was approved by the Human Research Ethics Committee of the Medical School of Botucatu (CEP-FMB/UNESP). All included subjects signed the written informed consent.
The women were classified according to prepregnancy BMI into normal weight (18.5–24.9 kg/m2; n = 23), overweight (25.0–29.9 kg/m2; n = 18) and obese (≥30.0 kg/m2; n = 31) [24]. Underweight women and those with multiple pregnancies or malformed fetus were excluded from the study.
Studied variables
Pregnant women enrolled in the study were examined at their first prenatal (before the 20th gestational week) and pre-birth visit (37–38th gestational weeks). They provided information on their age, number of pregnancies, prepregnancy BMI, smoking habits and preexistence of chronic arterial hypertension and diabetes mellitus. In the pre-birth visit (37–38th gestational weeks) maternal body weight, height and blood pressure were evaluated according to the standardization of the Brazilian Ministry of Health [25]. Gestational BMI, weight gain, hypertension complicating pregnancy (yes/no), GDM and mild gestational hyperglycemia (MGH) (yes/no) [26–28] were associated with maternal adiponectin, leptin, resistin and insulin levels in the pre-birth period (37–38th gestational weeks).
The newborns were examined in order to evaluate gestational age at birth by the New Ballard score, weight to gestational age ratio, ponderal index [(weight/length3)*100], length as well as head, thoracic and abdominal circumferences. These evaluations have been conducted by a specialized pediatrician and followed the normalization of our Service, including the New Ballard score. All these variables also were associated with maternal adiponectin, leptin, resistin and insulin levels in the pre-birth period (37–38th gestational weeks).
Collection of maternal blood and analysis of adipokines
Maternal blood samples were collected at in Vacutainer tubes (Becton–Dickinson, USA) and centrifuged at 4 °C for 15 min at 1000×g. The serum samples were stored at −80 °C until adiponectin, leptin, resistin and insulin analysis were performed using multiplex ELISA assays (Millipore Corporation, Massachusetts, USA).
Subjects’ follow-up
Pregnant women with prepregnancy type 1 and type 2 diabetes or those who showed risk of GDM or MGH development [27, 28] were referred to the SEDG-FMB/UNESP. According to the standard protocol used by our service, those pregnant women that did not have the diagnosis of diabetes, performed a 75 g oral glucose tolerance test and had their glycemic profile (GP) determined, between 24 and 26 gestation weeks, to confirm or not the diagnosis GDM or MGH [27, 28]. Pregnant women with MGH presented positive screening for GDM, negative diagnosis for GDM but with hyperglycemia detected in the GP [27]. According to our center’s routine protocol, diabetic pregnant women (type 1-DM or type 2-DM) were immediately managed with glycemic control, individualized nutritional intervention, and light to moderate-intensity exercise program (most frequently walking for 30 min five times a week). Insulin therapy was always maintained (DM-1) or introduced in substitution to oral antidiabetics (DM-2) [27]. Pregnant women who were normoglycemic, but were overweight or obese, received counseling about the importance of lifestyle changes to prevent GDM and MGH, and were promptly assigned to individualized nutritional guidance, walking for 30 min five times a week for weight control during pregnancy. Regardless of these preventive measures, all normoglycemic pregnant women underwent glucose tolerance (75 g-OGTT) and glycemic profile (GP) testing between 24 and 28 weeks of pregnancy for confirmation or ruling out of GDM and MGH. Pregnant women with confirmed GDM or MGH were treated according to the same protocol, relative to diet and exercise, aiming to achieve glycemic control, and insulin therapy was introduced when necessary. Glycemic control and management of diabetes were evaluated by 24-h GP (fasting, pre- and post- prandial glycemic levels) performed at 2-week intervals until week 32, and weekly until delivery. The glycemic mean (GM), calculated by the average of the glycemic levels evaluated in the GP, was used to classify the quality of maternal glycemic control in adequate (GM < 120 mg/dL) or inadequate (GM ≥ 120 mg/dL) [27–30].
Statistical analysis
Data obtained in the medical visits were regularly exported to Microsoft Excel spreadsheets. Statistical analyses were performed using the SAS package for Windows, version 9.2. Adipokines and insulin data were evaluated by ANOVA, followed by the Tukey–Kramer test for multiple comparisons. Data correlation was tested using Pearson or Spearman correlation tests according to distribution normality. Finally, a multiple regression analysis was performed to identify the independent factors to mother and newborn outcomes. The statistical significance was set at 0.05 for all the tests.
Results
According to prepregnancy BMI, out of the 72 pregnant women evaluated, 23 (31.9 %) had normal weight, 18 (25.0 %) were overweight, and 31 (43.1 %) were obese. Independently of nutritional status, most of the pregnant women had between 20 and 35 years old, and at least two previous pregnancies. All types of arterial hypertension complicating pregnancy were observed in 33 (45.8 %) of studied population. A proportion of 16.7 % was smoker and 48 (66.7 %) exhibited some form of hyperglycemia, which was diagnosed as preexisting diabetes (DM2 = 16.7 %), GDM (50.0 %) or MGH (33.3 %). Hypertension and diabetes were more frequently associated with overweight and obesity (Table 1).
Table 1.
Profile of the pregnant women, according to prepregnancy BMI classes
| Normal weight (N = 23) |
Overweight (N = 18) |
Obese (N = 31) |
|
|---|---|---|---|
| Age (year) | |||
| <19 | 9 (39.1) | 1 (5.5) | 0 (0.0) |
| 20–35 | 12 (52.2) | 12 (66.7) | 24 (77.4) |
| >35 | 2 (8.7) | 5 (27.8) | 7 (22.6) |
| Number of pregnancies | |||
| 1 | 9 (39.1) | 3 (16.7) | 3 (9.7) |
| ≥2 | 14 (60.9) | 15 (83.3) | 28 (90.3) |
| Smoking | 5 (21.7) | 0 (0.0) | 7 (22.6) |
| Hypertension (n = 33) | 3 (13.0) | 9 (50.0) | 21 (67.7) |
| Chronic hypertension | 0 (0.0) | 1 (3.0) | 10 (30.3) |
| Preeclampsia | 2 (6.0) | 6 (18.2) | 8 (24.2) |
| Chronic hypertension + PE | 0 (0.0) | 0 (0.0) | 6 (18.2) |
| Gestational hypertension | 1 (3.03) | 2 (6.1) | 3 (9.7) |
| Diabetes mellitus (n = 48) | 6 (26.1) | 11 (61.1) | 31 (100.0) |
| DM-2 | 0 (0.0) | 3 (27.3) | 5 (16.1) |
| GDM | 2 (33.3) | 4 (36.4) | 18 (58.1) |
| MGH | 4 (66.7) | 4 (36.4) | 8 (25.8) |
BMI body mass index, PE preeclampsia, DM-2 type 2 diabetes mellitus, GDM gestational diabetes mellitus, MGD mild gestational hyperglycemia
Maternal and newborn outcomes are shown in Table 2. As indicated by gestational BMI, out of the 72 pregnant women evaluated at the end of pregnancy, 17 (23.6 %) of the women had normal weight, 17 (23.6 %) were overweight, and 38 (52.8 %) obese. Considering gestational BMI, 8/23 (34.8 %) prepregnancy normal weight were classified as overweight or obese, 16/18 (88.9 %) prepregnancy overweight as overweight or obese, and 31/31 (100.0 %) prepregnancy obese were overweight or obese. Of the 72 newborns, 97.2 % were born to term, and 80.6 % had normal weight. Only one was underweight (<2500 g) and 8.3 % were classified as large for gestational age. Figure 1 shows adiponectin, leptin, resistin, and insulin levels according to weight status. Compared to normal weight pregnant women, leptin levels were significantly higher in obese women (p = 0.0021).
Table 2.
Maternal and newborn outcomes, according to prepregnancy BMI classes
| Normal weight (N = 23) |
Overweight (N = 18) |
Obese (N = 31) |
|
|---|---|---|---|
| Maternal | |||
| Gestational BMI (kg/m2) | |||
| Adequate | 15 (65.2) | 2 (11.1) | 0 |
| Overweight | 6 (26.1) | 9 (50.0) | 2 (6.5) |
| Obesity | 2 (8.7) | 7 (38.9) | 29 (93.5) |
| Newborn | |||
| GA at birth (new Ballard score) | |||
| <37 weeks | 0 | 1 (5.6) | 1 (3.2) |
| ≥37 weeks | 23 (100.0) | 17 (94.4) | 30 (96.8) |
| Weight (g) | |||
| <2500 | 0 | 0 | 1 (3.2) |
| 2500–4000 | 21 (91,3) | 17 (94.4) | 27 (87.1) |
| ≥4000 | 2 (8.7) | 1 (5.6) | 3 (9.7) |
| Weight to GA ratio | |||
| Small (SGA) | 2 (8.7) | 4 (22.2) | 2 (6.4) |
| Adequate (AGA) | 19 (82.6) | 13 (72.2) | 26 (83.9) |
| Large (LGA) | 2 (8.7) | 1 (5.6) | 3 (9.7) |
| Normal weight | Overweight | Obese | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Maternal | |||
| Weight gain (kg) | 14.6 ± 4.5 | 13.8 ± 6.1 | 10.4 ± 8,1 |
| Newborn | |||
| Weight (g) | 3340 ± 462.8 | 3220.6 ± 430.7 | 3333.9 ± 492.2 |
| Ponderal index (g/cm3) | 2.8 ± 0.3 | 2.8 ± 0.3 | 2.9 ± 0.3 |
| Length (cm) | 48.9 ± 2.5 | 48.4 ± 1.7 | 48.5 ± 2,1 |
| Head circumference (cm) | 34.5 ± 1.7 | 34.5 ± 1.5 | 34.9 ± 1,5 |
| Thoracic circumference (cm) | 33.6 ± 1.9 | 33.0 ± 1.6 | 33.3 ± 2.2 |
| Abdominal circumference (cm) | 31.9 ± 2.0 | 31.4 ± 1.8 | 34.9 ± 1.5 |
BMI body mass index, GA gestational age
Fig. 1.
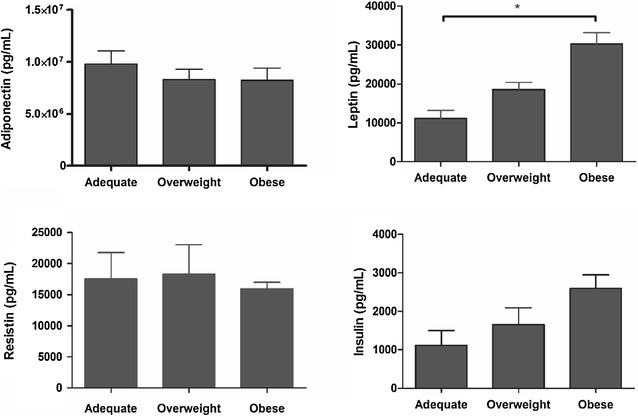
Adiponectin, leptin, resistin and insulin levels according to weight classes of the pregnant women. (Values as mean ± standard error of the mean; *p < 0.05—ANOVA, followed by the Tukey–Kramer test)
The correlation between maternal outcomes and markers of insulin resistance is shown in Table 3. Adiponectin levels were negatively correlated with gestational BMI (r = −0.29, p = 0.013), and leptin levels positively correlated with prepregnancy BMI (r = 0.57, p < 0.0001), gestational BMI (r = 0.39, p = 0.0007), hyperglycemia (r = 0.31, p = 0.0086) and hypertension (r = 0.27, p = 0.0196). Insulin levels were positively correlated with prepregnancy BMI (r = 0.38, p = 0.0009), gestational BMI (r = 0.24, p = 0.0466) and hyperglycemia (r = 0.26, p = 0.0252). Resistin levels were not correlated with the evaluated maternal outcomes. Multiple regression analysis confirmed only one independent relationship that is maternal insulin levels with prepregnancy BMI (p = 0.0343) and weight gain (p = 0.0417), and a tendency with gestational BMI (p = 0.0570) (Table 4).
Table 3.
Correlation analysis, coefficient (r) and p value, for the relationship between adipokine/insulin levels and maternal–newborn variables
| Adiponectin | Leptin | Resistin | Insulin | ||
|---|---|---|---|---|---|
| Maternal | |||||
| Prepregnancy BMIa | (r) | 0.0522 | 0.5689 | −0.1378 | 0.3843 |
| p | 0.6630 | <0.0001 | 0.2483 | 0.0009 | |
| Gestational BMIb | (r) | −0.2893 | 0.3886 | −0.0720 | 0.2354 |
| p | 0.0137 | 0.0007 | 0.5477 | 0.0466 | |
| Weight gaina | (r) | −0.1742 | 0.0948 | −0.0620 | 0.0608 |
| p | 0.1434 | 0.4284 | 0.6047 | 0.6118 | |
| Hypertensionb | (r) | −0.0669 | 0.2746 | 0.0732 | −0.0527 |
| p | 0.5767 | 0.0196 | 0.5413 | 0.6601 | |
| DM and MGHb | (r) | −0.1389 | 0.3076 | −0.0206 | 0.2637 |
| p | 0.2445 | 0.0086 | 0.8639 | 0.0252 | |
| Newborn | |||||
| Weighta | (r) | 0.2340 | −0.1216 | −0.1536 | 0.1097 |
| p | 0.0478 | 0.3089 | 0.1975 | 0.3590 | |
| Lengtha | (r) | −0.1604 | −0.2037 | −0.1003 | 0.0750 |
| p | 0.1785 | 0.0862 | 0.4019 | 0.5309 | |
| Head circumferencea | (r) | −0.2704 | −0.1373 | −0.2194 | 0.0440 |
| p | 0.0216 | 0.2501 | 0.0641 | 0.7133 | |
| Thoracic circumferencea | (r) | −0.1777 | −0.1845 | −0.0572 | −0.0254 |
| p | 0.1354 | 0.1208 | 0.6332 | 0.8325 | |
| Abdominal circumferencea | (r) | −0.1637 | −0.2488 | −0.0809 | 0.0049 |
| p | 0.1694 | 0.0350 | 0.4991 | 0.9677 | |
| Ponderal indexa | (r) | −0.1605 | 0.0856 | −0.1098 | 0.0655 |
| p | 0.1779 | 0.4744 | 0.3584 | 0.5845 | |
Statistically significant results (p < 0.05) highlighted in italic
DM prepregnancy and gestational diabetes, MGH mild gestational hyperglycemia
aPearson’s correlation test
bSpearman’s correlation test
Table 4.
Multiple regression analysis, p value, for the relationship between adipokine/insulin levels and maternal-newborn variables
| Adiponectin p value |
Leptin p value |
Resistin p value |
Insulin p value |
|
|---|---|---|---|---|
| Maternal—intercept | 0.0348 | 0.0109 | 0.0130 | 0.0452 |
| Prepregnancy BMI | 0.1598 | 0.9971 | 0.3654 | 0.0343 |
| Gestational BMI | 0.1904 | 0.7297 | 0.4166 | 0.0570 |
| Weight gain | 0.1181 | 0.9258 | 0.3662 | 0.0417 |
| GM at third trimester | 0.4076 | 0.4172 | 0.8201 | 0.0894 |
| Newborn—intercept | 0.0781 | 0.0262 | 0.1369 | 0.3185 |
| Weight at birth | 0.4478 | 0.2378 | 0.5439 | 0.1291 |
| Head circumference | 0.1904 | 0.4507 | 0.1910 | 0.9480 |
| Abdominal circumference | 0.6045 | 0.0270 | 0.4548 | 0.1522 |
| Ponderal index | 0.6533 | 0.1536 | 0.7648 | 0.8645 |
Statistically significant results (p < 0.05) highlighted in italics
BMI body mass index, GM glycemic mean
Relative to newborn outcomes, maternal adiponectin levels were positively correlated with newborns weight (r = 0.23, p = 0.0487) and inversely correlated with newborns head circumference (r = −0.27, p = 0.0216). Leptin levels were inversely correlated with abdominal circumference (r = −0.25, p = 0.0350). Maternal resistin and insulin levels were not correlated with newborns measures (Table 3). In the multiple regression analysis, only leptin levels remained as independent variable associated with the abdominal circumference of the newborn (p = 0.0270) (Table 4).
Discussion
In this study, we have found significant associations between adipokines and insulin levels with maternal and newborn outcomes. Leptin levels increased in obese pregnant women, showing a positive correlation with maternal BMI, hyperglycemia and hypertension and an inverse correlation with newborn abdominal circumference. Adiponectin levels, in turn, were negatively correlated with gestational BMI and newborns head circumference, and positively correlated with weight at birth. Resistin levels were not associated with any maternal or fetal outcomes. Also, insulin levels were positively related to weight status and maternal hyperglycemia, but not associated with newborn outcomes. Finally, the multiple regression analysis showed that, in this pregnant women population, only maternal insulin levels were independently associated to prepregnancy BMI and maternal weight gain, with a tendency in gestational BMI (p = 0.0570). Likewise, only leptin levels remained as an independent variable with the abdominal circumference of the newborns.
The positive association between insulin and prepregnancy BMI (r = +0.38), gestational BMI (r = +0.24), and hyperglycemic disorders (r = +0.26) met the metabolic syndrome diagnosis criteria, whose physiopathologic basis is the association between obesity and insulin resistance [31]. The association between metabolic syndrome and gestational hyperglycemia was identified by Bo et al. [32] and recently reproduced by our research group [33, 34]. In our study the results were not different; pregnant women with overweight or obesity showed more hypertension and hyperglycemic disorders, and insulin levels were positively correlated with prepregnancy BMI, gestational BMI, and hyperglycemia. Moreover, insulin levels remained as an independent variable associated with maternal BMI and weight gain during pregnancy.
Leptin is known as the “satiety hormone” given that it inhibits food intake and stimulates energy expenditure through activation of receptors in the hypothalamus. High leptin levels are found in obese people, possibly because they exhibit resistance to the action of this hormone [17, 18]. In the present study, leptin levels were significantly higher in obese mothers, exhibiting a direct association with their BMI (r = +0.56 prepregnancy; r = +0.38 gestational period), hyperglycemic disorders (r = +0.31), and maternal hypertension (r = +0.27). These results confirm the role of leptin in energy metabolism, inflammatory state, and insulin resistance associated with obesity, that has already been found in other studies [6, 35, 36].
Adiponectin and resistin are adipocytokines with antagonistic actions. Adiponectin increases insulin sensitivity, while resistin increases insulin resistance [19, 20]. For some authors, resistin is a marker of maternal hyperglycemia, increases in late pregnancy and during the immediate post-birth period, and is associated with the HOMA-IR index [10, 15, 37, 38]. Conversely, other authors have found no differences in serum resistin levels in pregnant women with GDM, although they show higher BMI and higher levels of insulin resistance markers [39].
In the present study, the negative association between adiponectin levels and gestational BMI (r = −0.28) suggests that the higher the degree of obesity, the higher the insulin resistance, with lower adiponectin and higher resistin levels expected. Despite this, our results showed that adiponectin and resistin levels exhibited no differences among maternal BMI classes. This apparent controversy has already been described in the literature. Adiponectin concentrations were lower in insulin-resistant states such as obesity and type 2 DM [40] and pregnancy complicated by GDM [8, 23]. In contrast, adipocytokines levels (including adiponectin, leptin, and resistin), although associated with HOMA-IR, had no difference among normal or intermediate tolerance glucose and GDM pregnant women [19]. Except for leptin levels, a recent study also showed no difference in maternal levels of adiponectin and resistin between obese and non-obese pregnant women [41]. In our study, overweight or obese mothers showed 85.7 % (42/49) of hyperglycemic disorders; thus maternal obesity associated with hyperglycemia and insulin resistance could explain these results. The controversy in the literature therefore persists and may be more explored. Perhaps, the individual analysis of overweight or obese pregnant women, with and without diabetes, may solve this question.
Regarding our perinatal results, adiponectin was positively associated with fetal weight (r = +0.23) and negatively with head circumference (r = −0.27), and leptin was negatively correlated with abdominal circumference (r = −0.25). Adiponectin levels were statistically similar in normal, overweight, and obese mothers and only maternal leptin levels were statistically different and defined as an independent variable to fetal abdominal circumference. Pregnant women who are obese or have GDM typically have low circulating levels of adiponectin associated with increased fetal growth [7, 8, 42]. Therefore, the positive association between maternal adiponectin and fetal weight, as well as the negative association between maternal adiponectin and head circumference, observed in our study, was not expected.
According to a recent review, the underlying mechanisms remain largely unknown [43]. Some of these mechanisms include the action of adiponectin possibly produced by the placenta [44], the role of fetal adiponectin [45], and an antagonistic action of this hormone in the placenta inducing insulin resistance [43]. All these factors could influence and modulate fetal growth and thus alter the expected results of its biomarkers.
Corroborating our controversial findings, no association was found between maternal levels of resistin or adiponectin and weight at birth [46]. Some investigators suggested that these adipokines are markers of maternal diabetes but not of fetal weight [47], and according to others, maternal adiponectin levels are inversely related to weight at birth in both healthy pregnant women and GDM [7, 8, 13]. The maternal milieu compromised by overweight or obesity may program fat neonatal accretion, and intrauterine insulin resistance and weight gain. In this context, the evaluation of adipokines in maternal and cord blood could clarify these questions. This is one of our study limitations.
Other limitations in the present study must be considered. The sample size, probably small, should be mentioned. Maternal characteristics, with 66.7 % (48/72) presenting hyperglycemic disorders, 45.8 % (33/72) with hypertension, and 68.0 % (49/72) with overweight or obesity may have influenced the results. Targeted interventions for maternal hyperglycemia and overweight or obesity control may have also affected our results. The rates of SGA and LGA newborns in overweight (22.2 %) and obese (9.7 %) mothers, respectively; the small weight gain of overweight or obese mothers, and fetal growth markers, similar among groups, indirectly reinforce the positive effects of these interventions in our studied population.
Finally, our results have shown an independent association between maternal insulin levels and BMI status. Likewise, an independent relationship between maternal leptin and the fetal abdominal circumference was defined. To the best our knowledge, these relationships have not been presented in the literature yet, pointing new perspectives for future research. These results highlight the need of individualized evaluation considering overweight or obese mothers with and without hyperglycemic or insulin resistance conditions, not observed in most studies. Besides this, they indirectly reinforce the positive effects of specific interventions to obese and hyperglycemic mothers.
Conclusions
Our findings support the relationship between markers of obesity and maternal–fetal outcomes. Maternal insulin and adipokines levels showed an independent relationship with respectively mother and newborns outcomes. In this studied population, the results indirectly reinforce the importance of maternal weight control before and during pregnancy to avoid adverse outcomes to mother and their newborns.
Authors’ contributions
IMPC conceived, devised and coordinated the study. JMV and JBM researched data, wrote, discussed and reviewed/edited the manuscript. RAAC, CAN, MVCR, and IMPC contributed to the discussion and reviewed/edited the manuscript. All authors read and approved the final manuscript.
Acknowledgements
To the São Paulo Research Foundation for providing a research grant (FAPESP Grant number 2012/51257-9; PP-SUS edict/2012).
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The present study was approved by the Research Ethics Committee of the Botucatu Medical School (FMB-UNESP), according to Official Letter no. 3900/2011. All participants signed informed consent forms prior to beginning any study procedure.
Funding
This work was supported by the São Paulo Research Foundation (FAPESP Grant number 2012/51257-9; PP-SUS edict/2012).
Contributor Information
Joice Monaliza Vernini, Email: joice.vernini@hotmail.com.
Jusciéle Brogin Moreli, Email: juscielemoreli@gmail.com.
Roberto Antônio Araújo Costa, Email: rcosta@fmb.unesp.br.
Carlos Antonio Negrato, Phone: +55 (14) 38801383, Email: carlosnegrato@uol.com.br.
Marilza Vieira Cunha Rudge, Email: marilzarudge@gmail.com.
Iracema Mattos Paranhos Calderon, Phone: +55 (14) 38801383, Email: calderon@fmb.unesp.br, Email: iracema.calderon@gmail.com.
References
- 1.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/BJN20041213. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson B, Lof M, Olausson H, Forsum E. Body fat, insulin resistance, energy expenditure and serum concentrations of leptin, adiponectin and resistin before, during and after pregnancy in healthy Swedish women. Br J Nutr. 2010;103:50–57. doi: 10.1017/S0007114509991371. [DOI] [PubMed] [Google Scholar]
- 3.Svensson H, Wetterling L, Bosaeus M, Odén B, Odén A, Jennische E, Edén S, Holmäng A, Lönn M. Body fat mass and the proportion of very large adipocytes in pregnant women are associated with gestational insulin resistance. Int J Obes. 2016;40:646–653. doi: 10.1038/ijo.2015.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajala MW, Scherer PE. Minireview: the adipocyte—at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–3773. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- 5.Zavalza-Gomez AB, Anaya-Prado R, Rinconsanchez AR, Mora-Martinez JM. Adipokines and insulin resistance during pregnancy. Diabetes Res Clin Pract. 2008;80:8–15. doi: 10.1016/j.diabres.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Madan JC, Damman O, Allan W, Quinn R, Davis J. Maternal obesity and markers of inflammation and pregnancy. Cytokine. 2009;47:61–64. doi: 10.1016/j.cyto.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Lowe LP, Metzger BE, Lowe WL, Jr, Dyer AR, McDade TW, McIntyre HD. Inflammatory mediators and glucose in pregnancy: results from a subset of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. J Clin Endocrinol Metab. 2010;95:5427–5434. doi: 10.1210/jc.2010-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atègbo JM, Grissa O, Yessoufou A, et al. Modulation of adipokines and cytokines in gestational diabetes and macrosomia. J Clin Endocrinol Metab. 2006;91:4137–4143. doi: 10.1210/jc.2006-0980. [DOI] [PubMed] [Google Scholar]
- 9.Briana DD, Malamitsi-Puchner A. Reviews: adipocytokines in normal and complicated pregnancies. Reprod Sci. 2009;16:921–937. doi: 10.1177/1933719109336614. [DOI] [PubMed] [Google Scholar]
- 10.D’Ippolito S, Tersigni C, Scambia G, Di Simone N. Adipokines, an adipose tissue and placental product with biological functions during pregnancy. BioFactors. 2012;38:14–23. doi: 10.1002/biof.201. [DOI] [PubMed] [Google Scholar]
- 11.Fain JN. Release of inflammatory mediators by human adipose tissue is enhanced in obesity and primarily by the nonfat cells: a review. Mediat Inflamm. 2010 doi: 10.1155/2010/513948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haugen F, Ranheim T, Harsem NK, et al. Increased plasma levels of adipokines in preeclampsia: relationship to placenta and adipose tissue gene expression. Am J Physiol Endocrinol Metab. 2006;290:326–333. doi: 10.1152/ajpendo.00020.2005. [DOI] [PubMed] [Google Scholar]
- 13.Catalano PM, Hoegh M, Minium J, Huston-Presley L, Bernard S, Kalhan S, et al. Adiponectin in human pregnancy: implications for regulation of glucose and lipid metabolism. Diabetologia. 2006;49:1677–1685. doi: 10.1007/s00125-006-0264-x. [DOI] [PubMed] [Google Scholar]
- 14.Ryan EA, Enns L. Role of gestational hormones in the induction of insulin resistance. J Clin Endocrinol Metab. 1988;67:341–347. doi: 10.1210/jcem-67-2-341. [DOI] [PubMed] [Google Scholar]
- 15.Lacroix M, Kina E, Hivert MF. Maternal/fetal determinants of insulin resistance in women during pregnancy and in offspring over life. Curr Diab Rep. 2013;13:238–244. doi: 10.1007/s11892-012-0360-x. [DOI] [PubMed] [Google Scholar]
- 16.Briana DD, Boutsikou M, Gourgiotis D, et al. Role of visfatin, insulin-like growth factor-I and insulin in fetal growth. J Perinat Med. 2007;35:326–329. doi: 10.1515/JPM.2007.071. [DOI] [PubMed] [Google Scholar]
- 17.Ahima RS. Adipose tissue as an endocrine organ. Obesity. 2006;14:242S–249S. doi: 10.1038/oby.2006.317. [DOI] [PubMed] [Google Scholar]
- 18.Lecke SB, Morsch DM, Spritzer PM. Leptin and adiponectin in the female life course. Braz J Med Biol Res. 2011;44:381–387. doi: 10.1590/S0100-879X2011000500001. [DOI] [PubMed] [Google Scholar]
- 19.Skvarca A, Tomazic M, Krhin B, Blagus R, Janez A. Adipocytokines and insulin resistance across various degrees of glucose tolerance in pregnancy. J Int Med Res. 2012;40:583–589. doi: 10.1177/147323001204000220. [DOI] [PubMed] [Google Scholar]
- 20.Skvarca A, Tomazic M, Blagus R, Krhin B, Janez A. Adiponectin/leptin ratio and insulin resistance in pregnancy. J Int Med Res. 2013;41:123–128. doi: 10.1177/0300060513476409. [DOI] [PubMed] [Google Scholar]
- 21.Torloni MR, Betran AP, Horta BL, Nakamura MU, Atallah AN, Moron AF, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009;10:194–203. doi: 10.1111/j.1467-789X.2008.00541.x. [DOI] [PubMed] [Google Scholar]
- 22.Waxman A. Prevention of chronic diseases: WHO global strategy on diet, physical activity and health. Food Nutr Bull. 2003;24:281–284. doi: 10.1177/156482650302400306. [DOI] [PubMed] [Google Scholar]
- 23.Retnakaran R, Qi Y, Connelly PW, et al. Low adiponectin concentration during pregnancy predicts postpartum insulin resistance, beta cell dysfunction and fasting glycaemia. Diabetologia. 2010;53:268–276. doi: 10.1007/s00125-009-1600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO . Obesity: preventing and managing the global epidemic. Report of a WHO consultation. WHO Technical Report Series 894. Geneva: World Health Organization; 2000. [PubMed] [Google Scholar]
- 25.Brasil. Ministério da Saúde. Secretaria de Atenção à Saúde. Departamento de Atenção Básica. Atenção ao pré-natal de baixo risco/Ministério da Saúde. Secretaria de Atenção à Saúde. Departamento de Atenção Básica. – Brasília: Editora do Ministério da Saúde, 2012. p. 318.
- 26.Atalah SE, Castilho LC, Castro SR, Aldea PA. Propuesta de um nuevo estándar de evalución nutricional en embarazadas. Rev Med Chil. 1997;125:1429–1436. [PubMed] [Google Scholar]
- 27.Rudge MVC, Calderon IMP, Ramos MD, Brasil MAM, Rugolo LMSS, Bossolan G, et al. Hiperglicemia materna diária diagnosticada pelo perfil glicêmico: um problema de saúde pública materno e perinatal. Rev Bras Ginecol Obstet. 2005;27:691–697. [Google Scholar]
- 28.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl 1):S62–S69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudge MV, Calderon IM, Ramos MD, Abbade JF, Rugolo LM. Perinatal outcome of pregnancies complicated by diabetes and by maternal daily hyperglycemia not related to diabetes. A retrospective 10-year analysis. Gynecol Obstet Investig. 2000;50:108–112. doi: 10.1159/000010293. [DOI] [PubMed] [Google Scholar]
- 30.American Diabetes Association Standards of medical care in diabetes—2015. Diabetes Care. 2015;38(Suppl 1):S1–S90. [PubMed] [Google Scholar]
- 31.International Diabetes Association (IDF). The IDF consensus worldwide definition of the metabolic syndrome. 2006. http://www.idf.org/webdata/docs/MetS_def_update2006.pdf. Accessed Mar 2014.
- 32.Bo S, Menato G, Gallo ML, et al. Mild gestational hyperglycemia, the metabolic syndrome and adverse neonatal outcomes. Acta Obstet Gynecol Scand. 2004;83:335–340. doi: 10.1111/j.0001-6349.2004.00314.x. [DOI] [PubMed] [Google Scholar]
- 33.Negrato CA, Javanovic L, Tambascia MA, et al. Mild gestational hyperglycemia as a risk factor for metabolic syndrome in pregnancy and adverse perinatal outcomes. Diabetes Metab Res Rev. 2008;24:324–330. doi: 10.1002/dmrr.815. [DOI] [PubMed] [Google Scholar]
- 34.Negrato CA, Javanovic L, Tambascia MA, et al. Association between insulin resistance, glucose intolerance, and hypertension in pregnancy. Metab Syndr Relat Disord. 2009;7:53–59. doi: 10.1089/met.2008.0043. [DOI] [PubMed] [Google Scholar]
- 35.Tessier DR, Ferraro ZM, Gruslin A. Role of leptin in pregnancy: consequences of maternal obesity. Placenta. 2013;34:205–211. doi: 10.1016/j.placenta.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 36.Chehab FF. 20 years of leptin—leptin and reproduction: past milestones, present undertakings, and future endeavors. J Endocrinol. 2014;223:T37–T48. doi: 10.1530/JOE-14-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Simone N, Di Nicuolom F, Marzioni D, et al. Resistin modulates glucose uptake and glucose transporter-1 (GLUT-1) expression in trophoblast cells. J Cell Mol Med. 2009;13:388–397. doi: 10.1111/j.1582-4934.2008.00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vitoratos N, Deliveliotou A, Dimitrakaki A, et al. Maternal serum resistin concentrations in gestational diabetes mellitus and normal pregnancies. J Obstet Gynaecol Res. 2011;37:112–118. doi: 10.1111/j.1447-0756.2010.01327.x. [DOI] [PubMed] [Google Scholar]
- 39.Akdeniz N, Kuyumcuoglu U, Kale A, Arikan S, Kele E, Erdemoglu M. Resistin may not associate with gestational diabetes mellitus although insulin resistance. Clin Exp Obstet Gynecol. 2011;38:236–238. [PubMed] [Google Scholar]
- 40.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 41.Solis-Paredes M, Espino Y Sosa S, Estrada-Gutierrez G, et al. Maternal and fetal lipid and adipokine profiles and their association with obesity. Int J Endocrinol. 2016;2016:7015626. doi: 10.1155/2016/7015626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jansson N, Nilsfelt A, Gellerstedt M, Wennergren M, Rossander-Hulthen L, Powell TL, et al. Maternal hormones linking maternal body mass index and dietary intake to birth weight. Am J Clin Nutr. 2008;87:1743–1749. doi: 10.1093/ajcn/87.6.1743. [DOI] [PubMed] [Google Scholar]
- 43.Aye ILMH, Powell TL, Jansson T. Review: adiponectin—the missing link between maternal adiposity, placental transport and fetal growth? Placenta. 2013;34:S40–S45. doi: 10.1016/j.placenta.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lappas M, Yee K, Permezel M, Rice GE. Release and regulation of leptin, resistin and adiponectin from human placenta, fetal membranes, and maternal adipose tissue and skeletal muscle from normal and gestational diabetes mellitus complicated pregnancies. J Endocrinol. 2005;186:457–465. doi: 10.1677/joe.1.06227. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Wang SH, Shang LX, et al. Relationship of adiponectin and resistin levels in umbilical and maternal serum with fetal macrosomia. J Obstet Gynaecol Res. 2010;36:533–537. doi: 10.1111/j.1447-0756.2010.01228.x. [DOI] [PubMed] [Google Scholar]
- 46.Cortelazzi D, Corbetta S, Ronzoni S, et al. Maternal and fetal resistin and adiponectin concentrations in normal and complicated pregnancies. Clin Endocrinol. 2007;66:447–453. doi: 10.1111/j.1365-2265.2007.02761.x. [DOI] [PubMed] [Google Scholar]
- 47.Ornoy A. Biomarkers of maternal diabetes and its complication in pregnancy. Reprod Toxicol. 2012;34:174–179. doi: 10.1016/j.reprotox.2012.05.005. [DOI] [PubMed] [Google Scholar]


