Abstract
Objective
To assess CD4+ T cell responsiveness to IL-7 and IFN-α in HIV infected patients who experience poor recovery of CD4 T cell counts during therapy (immune failure subjects).
Design
Responses to IL-7 and IFN-α were compared between HIV infected immune failure (CD4 counts < 379) subjects and immune success (CD4 counts >500) as well as healthy control subjects.
Methods
Flow cytometry was used to assess peripheral blood mononuclear cells for IL-7 induced proliferation, CD25 expression and signaling (P-STAT5 and P-Akt) in CD4+ T cells. Freshly isolated cells were characterized by expression of IL-7Rα (CD127) among CD4+ T cell maturation subsets by flow cytometry and sorted CD3+ T cells were assessed for expression of IFN-α and interferon stimulated genes (OAS1 and MxA) by qRT-PCR. Responses to IFN-α were assessed by induction of P-STAT1 and inhibition of IL-7-induced CD4+ T cell proliferation.
Results
IL-7-induced proliferation and CD25 expression were decreased in CD4+ T cells from immune failure subjects. CD127 expressing CD4+ T cells were decreased while expression of OAS1, MxA and IFN-α mRNA were increased in total CD3+ T cells from immune failure subjects. CD127 expression correlated with CD25 induction but not proliferation, whereas T cell IFN-α mRNA was associated with reduced proliferation in CD4+ T cells from immune failure subjects. IFN-α mediated induction of P-STAT1 and inhibition of proliferation were not diminished in CD4+ T cells from immune failure subjects.
Conclusion
IL-7 responsiveness is impaired in immune failure subjects and may be related to expression of CD127 and IFN-α.
Keywords: HIV infection, CD4 lymphocyte count, interleukin-7, CD127, interferons
Introduction
Up to 30% of HIV-1 infected individuals receiving ART fail to recover CD4 T cells counts to normal levels despite sustained HIV suppression [1]. Such patients are referred to as immunologic non-responders or immune failures (IF). Persistently low CD4 T cell counts are associated with increased risk of non-AIDS related morbidities [2–4]. One mechanism that may contribute to poor CD4 recovery during ART is chronic immune activation as suggested by studies linking IF to increased CD4 T cell activation and cycling as well increased plasma markers of inflammation (sCD14 and IL-6) [5]. Furthermore, increased expression of interferon stimulated genes (ISGs) has been observed in T cells from IF patients [6]. Although chronic type 1 interferon (IFN-I) expression has been implicated in models of HIV/SIV pathogenesis [7–9], it is not known whether IFN-I contributes to impaired CD4 recovery in treated patients.
Recovery of T cells in lymphopenic conditions is mediated by thymic output and homeostatic proliferation in response to IL-7 [10, 11]. Ligation of the IL-7 receptor, which is comprised of CD127 and CD132, transmits signals that promote CD4+ T cell survival and proliferation [11–13]. IL-7-induced STAT5 phosphorylation (P-STAT5) results in the rapid induction of an anti-apoptotic protein, bcl-2, and up-regulation of IL-2Rα (CD25) [14]. IL-7 induced Akt phosphorylation (P-Akt) is linked to cell cycle progression and T cell proliferation [15, 16]. Plasma IL-7 levels in IF patients are increased when compared to IL-7 levels in patients who recovered CD4 T cells [17]. In contrast, the capacity of CD4 T cells to respond to IL-7 stimulation as measured by rapid induction of P-STAT5 is diminished in IF patients and associated with reduced expression of CD127 [18]. Thus, diminished IL-7 responsiveness may occur in CD4 T cells of IF patients, impairing recovery.
We consider the possibility that persistent IFN-I expression could contribute to poor IL-7 responsiveness in IF subjects. We have demonstrated that P-Akt induction is delayed following exposure of peripheral CD4 T cells to IL-7 and that IFN-I impairs this signaling activity [19]. Furthermore, IFN-α inhibits CD4 T cell proliferation responses to IL-7 [19, 20]. Thus, it is possible that IFN-α produced in HIV disease diminishes homeostatic proliferation in response to IL-7 and plays a role in poor CD4 T cell recovery.
We compared IL-7 responsiveness in CD4+ T cells from immune failure (IF), immune success (IS; persons who experience CD4 recovery over 500 cells/µL during ART) and healthy control subjects (HC). We assessed CD127 protein expression as well as IFN-α mRNA and ISGs (OAS1 and MxA) mRNA expression in order to determine if IFN-I expression and exposure are related to IL-7 responsiveness. In addition, we evaluated the capacity of T cells from IF subjects to respond to IFN-α stimulation since IFN-I tolerance has been reported in HIV disease [21]. Our results suggest that IFN-α responsiveness is largely maintained while IL-7 responsiveness is impaired in T cells from IF patients. Furthermore, our data suggest that IFN-α may play a role in mediating poor IL-7 responsiveness in T cells from IF patients.
Methods
Subjects
This work was approved by the institutional review board at University Hospitals. After obtaining written informed consent, blood was drawn into heparin tubes. IF subjects were selected based on past clinical history of CD4 cell counts ≤350 cells/µL with treatment. Since participation was based on past medical history, some patients had achieved slightly higher CD4 cell counts at the time of blood draw, with no IF subjects exceeding 378 cells/µl as indicated in Table 1. All IS subjects had achieved CD4 cell counts >500 cells/µL leading up to the study. All HIV+ donors had plasma HIV RNA below detection limits (typically 50 copies/mL) for at least 2 years leading up to the study. Both CD4 nadir and median age of the subjects, which are recognized risk factors for IF, were not different between IF and IS or HC (Table 1).
Table 1.
Clinical characteristics and immune phenotypesa
| Clinical Characteristics | IF (n=13) |
IS (n=12) |
HC (n=9) |
|---|---|---|---|
| % male | 85 | 50 | 89 |
| Age (years) | |||
| At study enrollment | 54 (34–64) | 49 (32–60) | 46 (30–61) |
| At ART initiation | 44 (19–49) | 35 (25–53) | - |
| CD4 T cells/mL | 294 (141–378) | 745 (566–1919) | - |
| Nadir CD4 (cells/mL) | 56 (0–180) | 60 (4–354) | - |
| Years with undetectable plasma HIV RNA |
7 (2–17) | 6 (2–15) | - |
| Co-infection (seropositive; n(%)) |
|||
| CMV | 12 (92) | 9 (82) | - |
| Hepatitis B | 1 (8) | 0 (0) | - |
| Hepatitis C | 2 (17) | 0 (0) | - |
| Immune phenotypesb | |||
| % CD4+/CD3+c,d | 34 (9–42) | 46 (26–66) | 74 (42–79) |
| % naïvec,e | 24 (4–48) | 35 (2–56) | 56 (30–71) |
| % CM | 46 (29–82) | 46 (30–79) | 37 (22–59) |
| % EMc,e | 9 (6–62) | 11 (7–21) | 7 (3–15) |
| % TM | 2 (0.1–10) | 1.4 (0.1–8) | 0.4 (0.2–0.9) |
| % Ki-67+ in total CD4c,d | 4 (2–6)f | 2 (1–4) | |
| % Ki-67 in naïve CD4c | 0.4 (0.2–2) | 0.2 (0–1) | |
| % Ki-67 in CM CD4c,d | 5 (2–8) | 2 (1–7) | |
| % Ki-67 in EM CD4c | 7 (3–16) | 4 (3–7) | |
| % CD38+ in memoryd | 29 (19–66) | 16 (5–38) | 21 (11–32) |
Median (range)
central memory, CM, CD45RA-CD27+; effector memory, EM, CD45RA-CD27−; terminal memory, TM, CD45RA+CD27−; naïve (CD45RA+CD27+) Statistically significant comparisons (p<0.05):
IF vs HC;
IF vs IS;
IS vs HC
n=12
Peripheral blood mononuclear cell and T cell isolation
PBMCs were isolated from whole blood by centrifugation over a Ficoll-Hypaque cushion. For T cell isolation, the pan T cell isolation kit (Miltenyi) was used resulting in a mean purity of 94%, as assessed by flow cytometric analysis.
mRNA analyses
RNA was prepared from cell lysates using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA). Total RNA was reverse transcribed into cDNA using the QuantiTect Reverse Transcription kit (Qiagen). Equal quantities of cDNA for each experimental condition were amplified by real-time PCR using iQ SYBR Green Supermix (Bio-Rad). Primer pairs used for genes encoding for GAPDH, OAS1, MxA were previously published [21], including IFN-α [7]. Samples were amplified using a hot start at 95°C for 3 min, followed by 50 cycles of 10 s at 95°C, 10 s at 59°C, and 30 s at 72°C, and a post-amplification melting curve ramping from 65°C to 95°C in increments of 0.5°C per 5 s. Abundances of transcripts were calculated relative to Gapdh expression using the formula 2−[Ct(target gene)−Ct(Gapdh)].
Stimulation conditions
To assess proliferation, PBMCs were labeled with carboxyfluorescein succinimidyl ester (CFSE; 0.25 mM at 37°C for 10 minutes; Molecular Probes Invitrogen, Grand Island, NY). Staining was quenched with fetal bovine serum (FBS) for 5 minutes on ice and then cells were washed with RPMI (10% FBS). PBMCs were resuspended in X-VIVO 15 serum-free medium supplemented with 1% penicillin streptomycin and incubated at a concentration of 1 million cells/ml ± rIL-7 (Cytheris; 5 ng/ml) ± IFN-α (PBL; 500 U/ml).
Flow cytometry
Freshly isolated PBMCs were incubated with fluorochome-labeled monoclonal antibodies for 30 minutes: anti-CD3 peridinin chorophyll protein (Percp), anti-CD45RA phycoerythrin-cyanine 7 (PE-Cy7), anti-CD8 allophycocyanin-cyanine (APC-Cy7), anti-CD27 alexafluor-700 (AF-700), anti-CD127 fluorescein isothiocyanate (FITC), anti-CD38 phycoerythrin-cyanine 5 (PE-Cy5) (all from BD Bioscience, San Jose, CA) and anti-CD4 pacific blue (Biolegend, San Diego, CA). Cells were washed, fixed and permeabilized with 2× perm/wash buffer (BD Bioscience, San Jose, CA) and incubated with anti-Ki-67 APC for 45 minutes. For proliferation assays, CFSE stained PBMCs were incubated with antibodies specific for CD3, CD4 and CD45RA following incubation with Live/Dead Fixable Yellow Dead Cell dye (Invitrogen, Grand Island, NY, USA). For apoptosis studies, cells were washed with cold PBS, resuspended in 1× binding buffer (BD Bioscience, San Jose, CA) and then incubated with annexin-V PE (BD Bioscience, San Jose, CA) at room temperature for 15 minutes. Intracellular detection of P-Akt and P-STAT5 was performed via the BD phosflow protocol and incubated with the following fluorochome-labeled monoclonal antibodies for 30 minutes: anti-CD3, anti-CD4, anti-CD45RA, anti-STAT5 [7](pY649) APC and anti-Akt (pS473) PE (BD Bioscience, San Jose, CA). Flow cytometric analyses were performed with FlowJo software (FlowJo LLC, Ashland, OR). Doublets, debris and dead cells were excluded from analyses.
Statistical analyses
Prism 5 software (Graphpad, La Jolla, CA) was used to generate figures and perform statistical analyses. Nonparametric tests (Kruskal-Wallis and Dunn’s multiple comparison post test) were used to assess differences between IF and IS or HC. Wilcoxon signed rank test was used to assess differences in paired data. All the tests were 2-sided with a significance p value cutoff of 0.05.
Results
IL-7 mediated proliferation and induction of CD25 are diminished in CD4+ T cells from immune failure subjects
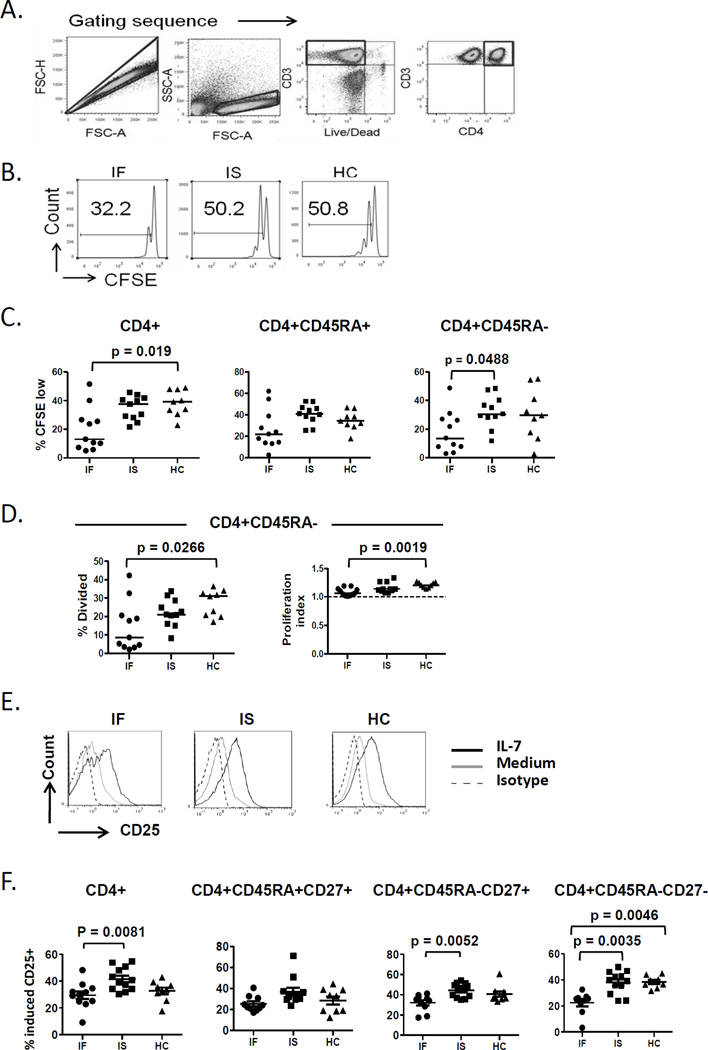
To test IL-7 responsiveness in CD4+ T cells, PBMCs were stimulated with rIL-7 and proliferation (CFSE dye dilution at day 7), cell signaling (P-STAT5 and P-Akt at day 3) and cell surface induction of CD25 expression (at day 1) were measured in CD4+ T cells by flow cytometry. Total CD4+ T cells from IF but not IS patients exhibited diminished proliferation (%CFSE low) in response to rIL-7 compared to cells from HC (Fig. 1A–C). CD4+ T cells incubated in medium alone had median %CFSE low values of <1% and these median values were not significantly different between the groups (data not shown). Furthermore, CD3+CD4− T cells from IF subjects also displayed diminished proliferation (% CFSE low) in response to IL-7 stimulation when compared to cells from IS and HC subjects and diminished proliferation indices and % divided cells when compared to cells from HC (Supplemental Fig. 1).
Fig. 1. CD4+ T cell responses to IL-7 are diminished in IF subjects.
(A–D) PBMCs were labeled with CFSE and incubated in the presence or absence of rIL-7. After 7 days, cells were assessed by flow cytometry. (A) The gating sequence is provided. Doublets were excluded from all analysis by the FSC-A and FSC-H gate and lymphocytes were identified by forward and side scatter. (B) Representative histograms showing proliferation response by %CSFE low cell population in CD4+ cells. (C) Summary data of proliferation response in CD4+ cells (IF n=11, IS n=10 and HC n= 9) as well as CD45RA+ and CD45RA− CD4 subsets. (D) Summary data showing % divided and proliferation index in the CD45RA− subset in cells from subjects that were analyzed in 1C. (E and F) PBMCs were incubated in the presence or absence of IL-7. After 24 hours, cells were assessed for surface CD25 expression by flow cytometry. (E) Representative histograms showing CD25 expression in IL-7 treated CD4+ T cells in comparison with untreated and isotype control. (F) Summary data of % IL-7 induced CD25+ cells in CD4+ T cells (IF n= 11, IS n= 12, HC n= 9) and maturation subsets (naive defined by CD45RA+CD27+, central memory defined by CD45RA−CD27+ and effector memory defined by CD45RA−CD27−). % IL-7 induced CD25 is the difference in CD25 expression between IL-7 treated and untreated. Abbreviations: immune failure, IF; immune success, IS; healthy control, HC.
We also assessed proliferation responses among CD4+ T cell maturation subsets. Proliferation responses were diminished among the memory (CD45RA−) CD4+ T cells and a similar trend that was not statistically significant was observed in naïve (CD45RA+) CD4+ T cells (p=0.059) from IF compared to cells from controls (Fig. 1C). To further understand the proliferation defect in the CD4+ cells, we calculated the average number of divisions per divided cell (proliferation index) and the proportion of precursor cells that divided at least once (% divided). Both indices were diminished in the memory (CD45RA−) subset of IF subjects compared to HC (Fig. 1D). The proliferation index and % divided in the total CD4+ and CD45RA+ subset and were not statistically different between subject groups (data not shown). Consistent with the above findings, absolute numbers of CD4+ T cells at the end of the cell culture also suggested that T cells from control subjects underwent greater expansion in response to IL-7 than cells from IF subjects (data not shown).
CD4+ T cell proliferation in response to IL-7 stimulation is dependent on cell signaling mediated by P-STAT5 and P-Akt [22]. Therefore, we assessed IL-7 induced P-STAT5 and P-Akt following 3 days of stimulation. P-STAT5 and P-Akt levels in CD4+ and CD4− T cells following IL-7 stimulation were not different between IF and IS or HC subjects (Supplemental Fig. 2).
IL-7-induced P-STAT5 results in upregulation of CD25 expression [14]. We compared IL-7 induced cell surface CD25 expression between IF, IS and HC subjects. PBMCs isolated from subjects were incubated in the presence or absence of rIL-7 overnight. Surface expression of CD25 was measured by flow cytometry after gating on CD3+CD4+ T cells (Fig. 1E). CD25 induction in response to IL-7 was diminished in total CD4+ T cells and naive subset from IF subjects. Among memory CD4+ T cells, induction of CD25 expression by IL-7 was reduced in IF subjects, particularly among the effector memory subset (Fig. 1F).
CD4 T cells from immune failure subjects exhibit decreased CD127 expression and increased immune activation
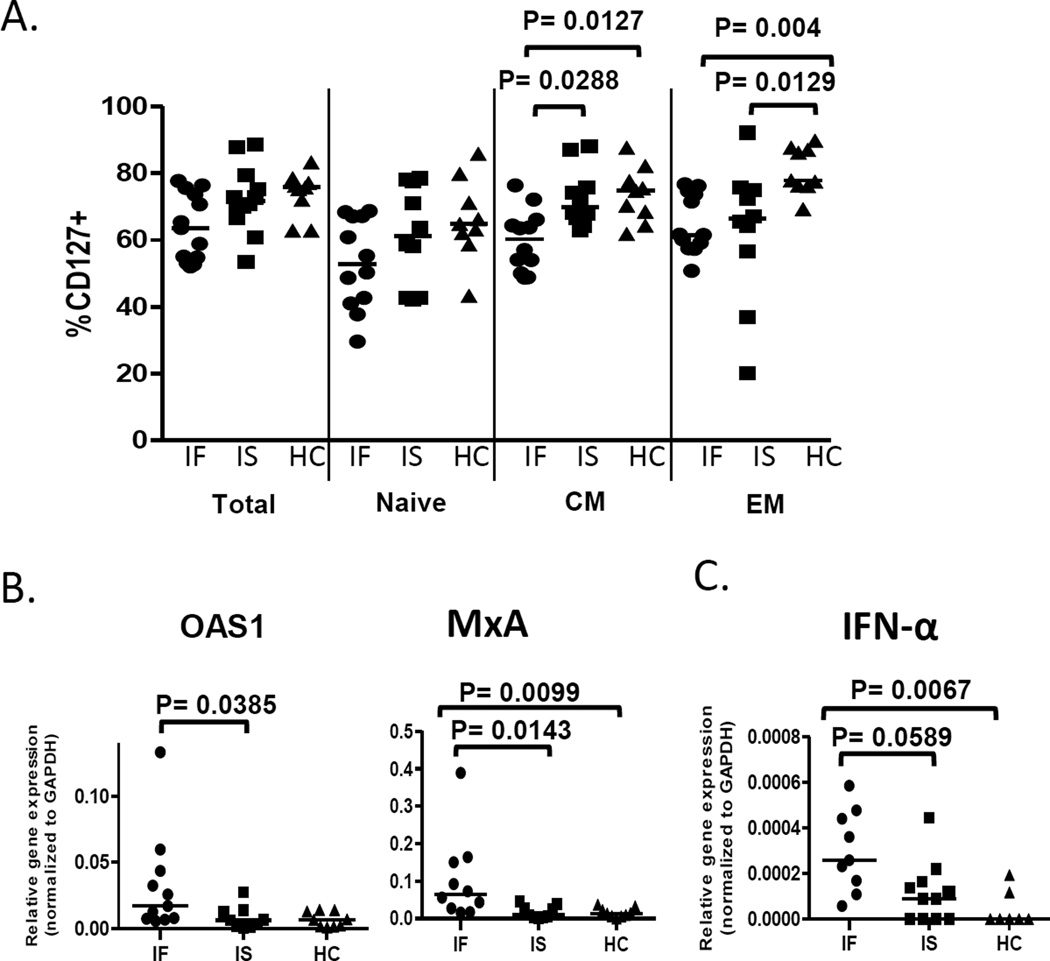
To characterize freshly isolated CD4+ T cells from IF subjects and to investigate underlying mechanisms that may contribute to poor IL-7 responses in IF subjects, we measured immune activation (CD38 expression), cell cycling (Ki-67 expression), and IL-7 receptor-α (CD127 expression) as well as proportions of maturation subsets among the CD4+ T cells (Table 1 and Figure 2A). Among the CD4+ T cells, IF subjects had decreased proportions of naive cells but increased proportions of effector memory cells when compared to HC subjects. CD4+ T cells from IF subjects displayed diminished expression of CD127 within memory T cell subsets as measured by either proportion of CD127+ cells (Fig. 2A) or MFI of CD127 expression (not shown). Compared to IS subjects, IF subjects had increased proportions of activated memory T cells (Table 1). Furthermore, IF subjects had increased proportions of cycling cells comparing IF to HC (naïve, CM, EM) and comparing IF to IS (CM). These data are consistent with previous observations and suggest that CD4+ T cells from IF subjects are activated and cycling and may be less responsive to IL-7 stimulation due to lower CD127 expression [5].
Fig. 2. T cells from IF subjects exhibit increased expression of ISGs and IFN-α.
(A) Freshly isolated PBMC were examined by flow cytometry for percentages of CD127+ cells in total, naïve, CM and EM CD3+CD4+ T cells. (B–C) T cells were purified from freshly isolated PBMCs and gene expression of ISGs and IFN-α was analyzed by qRT-PCR. (B) Messenger RNA expression of OAS1 and MxA (IF n=10, IS n= 9, HC n= 9) ISGs (C) IFN-α mRNA analyses (IF n=9, IS n= 11, HC n= 7). Abbreviations: 2'-5'-Oligoadenylate Synthetase-1, OAS-1; Myxovirus resistance A protein, MxA; Interferon-alpha, IFN-α; immune failure, IF; immune success, IS; healthy control, HC.
ISG and IFN-α mRNA expression are increased in CD3+ T cells from immune failure subjects
We have previously reported the inhibitory effect of IFN-α on IL-7 induced proliferation [19]. To address the possibility that T cells from IF subjects were exposed to IFN-I in vivo, we measured ISG expression in T cells from our subjects and found that mRNA expression of OAS1 and MxA were increased in CD3+ T cells from IF subjects (Fig. 2B). To determine if T cells could be a source of IFN-I, expression of IFN-α mRNA was also assessed. We found evidence of increased mRNA expression of IFN-α in T cells from IF subjects (Fig. 2C).
Diminished IL-7 responsiveness in immune failure subjects is related to expression of CD127 and IFN-α
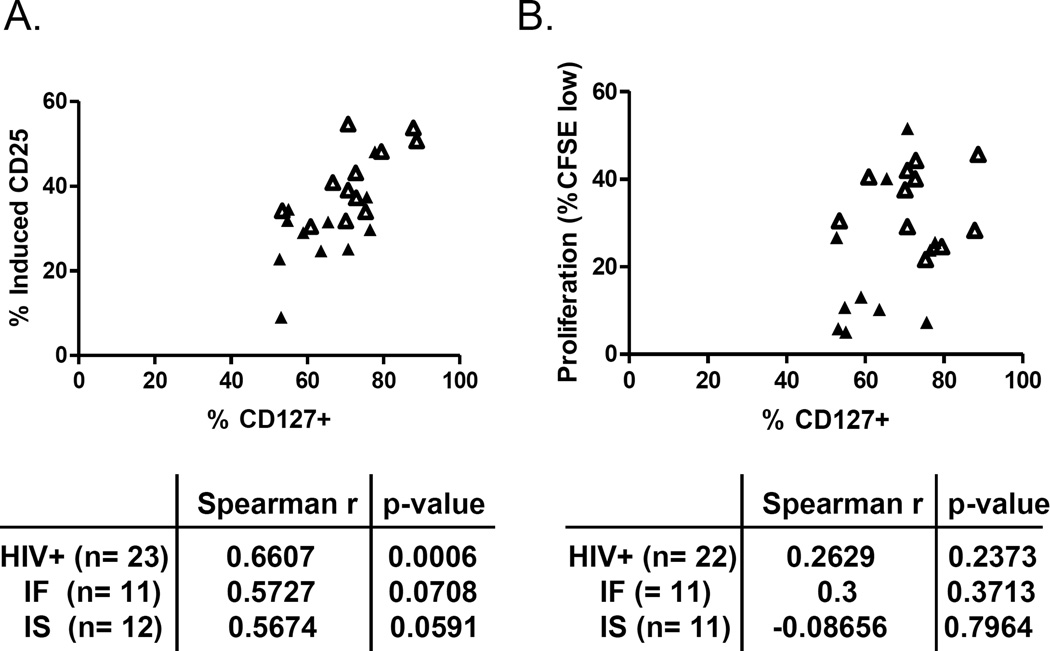
CD127 expression is a strong correlate of IL-7 responsiveness (P-STAT5 induction) in cells from IF patients [18]. Therefore, we performed correlation analyses between CD127 expression and IL-7 responsiveness (CD25 induction and proliferation responses). We assessed these relationships using all HIV infected subjects to provide greater analytic power and then examined the relationships in IF and IS subjects separately. CD25 induction in response to IL-7 directly correlated with CD127 expression in the HIV infected subjects. When assessed in the IF or IS subjects independently, the relationships remained positive and trending but not significant (Fig. 3A). In contrast, proliferation responses to IL-7 stimulation were poorly correlated with CD127 expression (Fig. 3B) suggesting that diminished CD127 expression may not be a key determinant for diminished proliferation responses observed in CD4+ T cells from IF subjects. We also considered the possibility that IFN-α or ISG mRNA expression might be related to proliferation responses to IL-7 stimulation. ISG expression did not correlate with either IL-7 induced CD25 expression or proliferation response (data not shown) while IFN-α mRNA expression in total T cells was inversely correlated with proliferation in the HIV infected subjects (Spearman r= -0.6379, p= 0.0033, n= 18). The relationship was not significant although trending in the same direction among IF subjects (Spearman r= −0.4667, p= 0.2125, n= 9).
Fig. 3. IL-7 induced CD25 expression is related to expression of CD127.
(A) Relationship between CD127 expression and induction of CD25 in response to overnight IL-7 treatment. (B) Relationship between CD127 expression and proliferation (%CFSE) in response to 7 days of IL-7 stimulation. Open symbols represent IS subjects and closed symbols represent IF subjects. Abbreviations: immune failure, IF; immune success, IS; healthy control, HC.
IFN-α responsiveness is not diminished CD4+ T cells from immune failure subjects
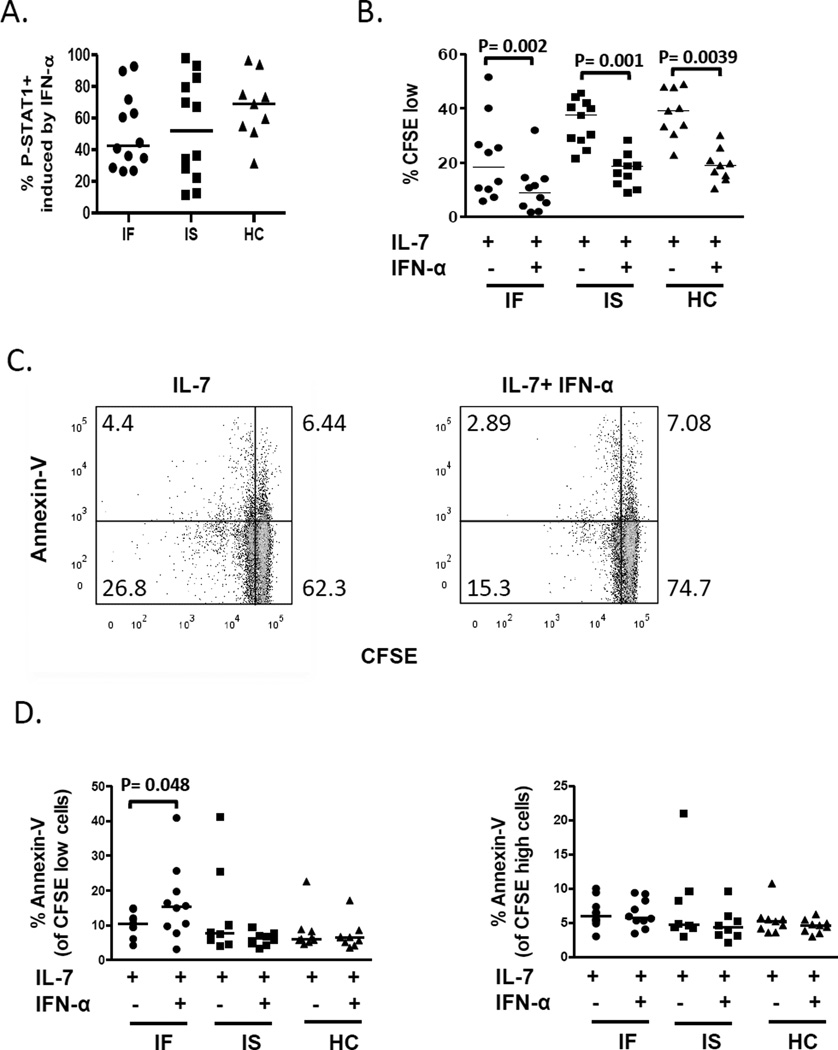
Previous studies suggest that exposure to IFN-I in vivo during HIV or SIV infection may lead to tolerance and reduced IFN-I responsiveness [8, 21]. To assess IFN-α responsiveness in CD4+ T cells, we measured both the rapid induction of P-STAT1 in freshly isolated PBMCs and the capacity of IFN-α to inhibit T cell proliferation in cells from all subjects. We found that IFN-α induced P-STAT1 levels were not different between the subjects (Fig. 4A). IFN-α significantly inhibited IL-7 induced proliferation in CD4+ T cells from all subject groups (Fig. 4B). The magnitude of IFN-α mediated inhibition of IL-7 induced proliferation was also similar between groups (median % inhibition equal 55, 53 and 51 for IF, IS and HC, respectively). Since IFN-Is have also been implicated in the induction of apoptosis in HIV disease [23], we also assessed cell death by annexin-V binding following IFN-α, IL-7 or IL-7 + IFN-α simulation. Cells incubated in IFN-α alone did not proliferate above background and did not reveal significant differences in cell death between the subject groups (median percentages of CD4+ T cells that were annexin V-bound was 7.3, 6.1 and 8.1 for IF, IS and HC subject groups, respectively; p = 0.79). Interestingly, when comparing cells incubated with IL-7 to cells incubated with IL-7 + IFN-α, we found an increase in cell death in the presence of IFN-α among divided (CFSE low) cells but not undivided (CFSE high) cells from IF but not IS or HC subjects (Fig. 4C–D). Subset analyses of CD45RA+ and CD45RA− cells indicated that adding IFN-α to IL-7-treated cells resulted in significant increases in cell death of divided cells within the naïve-enriched (CD45RA+) T cell subset of IF subjects (Supplemental Figure 3). To ascertain if this observation might be explained by increased frequencies of Terminal memory (CD45RA+CD27−) cells within the CD45RA+ T cell subset of IF subjects, we performed correlation analyses between cell death and frequencies of Terminal memory T cells within the CD4+CD45RA+ subset that had been measured in the freshly isolated PBMC samples. We did not find a significant relationship between these indices in cells from IF subjects (Spearman r= 0.32, p= 0.37, n= 10), suggesting that Terminal memory T cell frequencies are not likely to explain the increased susceptibility to IFN-α-mediated cell death in CD45RA+ cells from IF subjects. Overall, these observations indicate that despite evidence of increased IFN-I exposure in vivo, CD4+ T cells from IF subjects maintained sensitivity to IFN-α mediated induction of signaling as well as anti-proliferative and pro-apoptotic activity.
Fig. 4. CD4+ T cell responses to IFN-α were not diminished in IF subjects. PBMCs from subjects were incubated in the presence or absence of IFN-α.
All data shown are gated from CD3+CD4+ cells. (A) P-STAT1 induction following 15 minutes of IFN-α treatment (IF n= 12, IS n= 12, HC n= 9). (B-D) PBMCs were CFSE stained and assessed for proliferation and cell death in cells treated with IL-7 in the absence or presence of IFN-α for 7 days. (B) Compares %CFSE low (IF n= 10, IS n= 11, HC n= 9) and (C) are representative dot plots comparing cell death as measured by annexin-V binding CD4+ T cells that were not excluded by viability dye. (D) Summary data of cell death in divided cells (%CFSE low) and undivided cells (CFSE high) (IF n= 10, IS n= 8, HC n= 9). P-values were obtained by Wilcoxon signed rank test. Abbreviations: immune failure, IF; immune success, IS; healthy control, HC.
Discussion
IL-7 responsiveness is important for the recovery of CD4 T cells in ART treated patients as suggested by evidence linking the rate of CD4 recovery to CD127 polymorphisms [24]. The importance of IL-7 responsiveness is also raised by studies showing that CD4+ T cells from IF subjects respond poorly to IL-7 stimulation as measured by rapid induction of P-STAT5 signaling [18]. In addition to diminished IL-7 responsiveness, it is also possible that fibrosis of lymphoid tissues further contributes to poor CD4 recovery during ART by limiting access to IL-7 within reticular networks [25]. Thus, both limited access to IL-7 and limited capacity to respond to IL-7 stimulation may contribute to poor CD4 recovery in treated HIV.
Here, we describe additional impairments in IL-7 responsiveness in CD4+ T cells from IF patients that are characterized by diminished induction of proliferation over a 7 day stimulation and diminished induction of CD25 after overnight stimulation. Similar to the previous reports of diminished P-STAT5 signaling in T cells from IF subjects [18], we found a direct relationship between induction of CD25 and levels of CD127 expression among CD4+ T cells from HIV-infected subjects. These data and the observations that there are increased proportion of CD57+ "senescent" CD4+ T cells in IF subjects [18] point to a mechanism whereby the accumulation of cells poorly equipped to proliferate in response to IL-7 stimulation may contribute to poor CD4 recovery in treated HIV disease. Unlike the previously reported observation of a defect in IL-7 induced P-STAT5 in CD4 T cells from IF patients [18], we did not find evidence of diminished P-STAT5 signaling in CD4 T cells from IF subjects. This may be related to differences in approach as we assessed P-STAT5 following 3 days of IL-7 stimulation compared to 15 minutes in the previous work. We examined the later time point in order to allow a comparison between P-STAT5 signaling and Akt signaling since Akt signaling is consistently measurable after 3 days but not after 15 minutes of IL-7 stimulation [19]. It is possible that early P-STAT5 signaling defects in IF are transient, or alternatively, cells that fail to induce P-STAT5 signals early are lost over a few days of culture.
Consistent with previous observations [18], we found that CD127 expression was diminished in T cells from IF subjects and other studies indicate that IL-7 is increased in plasma of IF subjects [26]. It is possible, therefore, that reduced CD127 expression is a consequence of increased chronic exposure of T cells to high concentrations of IL-7 in vivo leading to IL-7 hyporesponsiveness. While CD127 expression correlated with CD25 induction mediated by IL-7, it was a poor predictor of T cell proliferation responses to IL-7 stimulation. This suggests that the mechanism of impaired IL-7 induced proliferation in CD4+ T cells from IF patients is likely to be complex and not simply a reflection of reduced CD127 expression.
We also confirm a previous report that T cells from IF subjects display increased ISG mRNA expression [6] and add that these cells also express increased IFN-α mRNA. Notably, the analyses of ISGs and IFN-α mRNA were limited to total CD3+ T cells. Thus, determining the contribution of CD4 and CD8 T cells as well as the contribution of memory and naive cell subsets to IFN-α mRNA will be important in future studies. While T cells are not necessarily recognized as a predominant producer of IFN-I, previous studies in HIV+ patients indicate that various cell types, including T cells, can contribute to IFN-α production [27]. It is possible that a small contaminating population of cells may have accounted for the IFN-α mRNA signal in our assays, however, we did not observe a relationship between the purity of the sample and the expression levels of IFN-α mRNA (not shown). Although limited by a small sample size, the increased IFN-α mRNA signal observed in T cells from IF subjects and the trending inverse relationship with T cell proliferation responses to IL-7 leads us to speculate that autocrine IFN-α production could contribute to poor IL-7 responsiveness in T cells from IF patients. Interestingly, this relationship may reflect an indirect mechanism since there was no discernible relationship between ISG expression and IL-7-induced proliferation. Further studies will be important to confirm and expand on these observations.
Increased levels of ISG and IFN-α mRNA expression in T cells from IF subjects suggest that IFN-I exposure may be sustained in IF. In certain cell types, chronic exposure to IFN-I in vivo can lead to IFN-I tolerance. For example, monocytes from untreated HIV infected but not healthy control subjects are refractory to IFN-α2a mediated induction of OAS and MxA presumably due to chronic IFN-I exposure during HIV infection [21]. Diminished expression of ISGs also has been observed following IFN-α2a prolonged administration in rhesus macaques [8]. Our data suggest that IFN-α responsiveness as measured by P-STAT1 or inhibition of IL-7-induced proliferation was not impaired in CD4+ T cells from IF subjects. Furthermore, CD4+ T cells from IF subjects that have divided in response to IL-7 appear more susceptible to IFN-α enhanced cell death, revealing a potential mechanism that could limit homeostatic recovery of CD4+ T cells in these subjects.
Supplementary Material
(A) %CFSE low (IF n= 11, IS n= 11, HC n= 9). (B) %Divided (IF n= 11; IS n= 10; HC n= 9). (C) Proliferation index (IF n= 11; IS n= 10; HC n= 9). Data shown are CFSE dye dilution response to IL-7 when gating in CD4− cells under the experimental conditions described in figure 1. Abbreviations: immune failure, IF; immune success, IS; healthy control, HC.
PBMCs were incubated in the presence or absence of IL-7. After 3 days, cells were assessed for P-STAT5 and P-Akt by flow cytometry. (A) Graphs show responses in CD4+ (%P-STAT5+ IF n= 12, IS n= 12, HC n= 9; %P-Akt+ IF n= 13, IS n= 12, HC n= 9) and (B) shows CD4− T cells (%P-STAT5+ IF n= 11, IS n= 12, HC n= 9; %P-Akt+ IF n= 12, IS n= 12, HC n= 9). Abbreviations: immune failure, IF; immune success, IS; healthy control, HC.
Maturation subsets were based on (A) CD45RA+ (naive) and (B) CD45RA− (memory). Summary data of cell death in divided cells (CFSE low) and undivided cells (CFSE high) (IF n= 10, IS n= 8, HC n= 9). P-values were obtained by Wilcoxon signed rank test. Abbreviations: immune failure, IF; immune success, IS; healthy control, HC.
Acknowledgments
We thank the patients who participated in these studies.
Funding: This work was supported by NIH grants AI-104480 and AI-36219 (the CWRU Center for Aids Research).
Footnotes
Author contributions: T.P.N. and S.F.S. designed the studies. T.P.N. and S.S. performed laboratory experiments; R.A. collected patient samples and collected clinical data; T.P.N. and S.S. analyzed data; T.P.N., S.F.S., S.S., M.L.F., M.M.L. and C.V.H. evaluated and interpreted data; T.P.N. wrote the manuscript; S.F.S., M.M.L., M.L.F. and C.V.H. contributed to manuscript revisions.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Gazzola L, Tincati C, Bellistri GM, Monforte A, Marchetti G. The absence of CD4+ T cell count recovery despite receipt of virologically suppressive highly active antiretroviral therapy: clinical risk, immunological gaps, and therapeutic options. Clin Infect Dis. 2009;48:328–337. doi: 10.1086/595851. [DOI] [PubMed] [Google Scholar]
- 2.Lichtenstein KA, Ward DJ, Moorman AC, Delaney KM, Young B, Palella FJ, Jr, et al. Clinical assessment of HIV-associated lipodystrophy in an ambulatory population. AIDS. 2001;15:1389–1398. doi: 10.1097/00002030-200107270-00008. [DOI] [PubMed] [Google Scholar]
- 3.Yong MK, Elliott JH, Woolley IJ, Hoy JF. Low CD4 count is associated with an increased risk of fragility fracture in HIV-infected patients. J Acquir Immune Defic Syndr. 2011;57:205–210. doi: 10.1097/QAI.0b013e31821ecf4c. [DOI] [PubMed] [Google Scholar]
- 4.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 5.Lederman MM, Calabrese L, Funderburg NT, Clagett B, Medvik K, Bonilla H, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis. 2011;204:1217–1226. doi: 10.1093/infdis/jir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez S, Tanaskovic S, Helbig K, Rajasuriar R, Kramski M, Murray JM, et al. CD4+ T-cell deficiency in HIV patients responding to antiretroviral therapy is associated with increased expression of interferon-stimulated genes in CD4+ T cells. J Infect Dis. 2011;204:1927–1935. doi: 10.1093/infdis/jir659. [DOI] [PubMed] [Google Scholar]
- 7.Hardy GA, Sieg S, Rodriguez B, Anthony D, Asaad R, Jiang W, et al. Interferon-alpha is the primary plasma type-I IFN in HIV-1 infection and correlates with immune activation and disease markers. PLoS One. 2013;8:e56527. doi: 10.1371/journal.pone.0056527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandler NG, Bosinger SE, Estes JD, Zhu RT, Tharp GK, Boritz E, et al. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature. 2014;511:601–605. doi: 10.1038/nature13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herbeuval JP, Grivel JC, Boasso A, Hardy AW, Chougnet C, Dolan MJ, et al. CD4+ T-cell death induced by infectious and noninfectious HIV-1: role of type 1 interferon-dependent, TRAIL/DR5-mediated apoptosis. Blood. 2005;106:3524–3531. doi: 10.1182/blood-2005-03-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mackall CL, Fry TJ, Bare C, Morgan P, Galbraith A, Gress RE. IL-7 increases both thymic-dependent and thymic-independent T-cell regeneration after bone marrow transplantation. Blood. 2001;97:1491–1497. doi: 10.1182/blood.v97.5.1491. [DOI] [PubMed] [Google Scholar]
- 11.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 12.Lai SY, Molden J, Goldsmith MA. Shared gamma(c) subunit within the human interleukin-7 receptor complex. A molecular basis for the pathogenesis of X-linked severe combined immunodeficiency. J Clin Invest. 1997;99:169–177. doi: 10.1172/JCI119144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li T, Wu N, Dai Y, Qiu Z, Han Y, Xie J, et al. Reduced thymic output is a major mechanism of immune reconstitution failure in HIV-infected patients after long-term antiretroviral therapy. Clin Infect Dis. 2011;53:944–951. doi: 10.1093/cid/cir552. [DOI] [PubMed] [Google Scholar]
- 14.John S, Robbins CM, Leonard WJ. An IL-2 response element in the human IL-2 receptor alpha chain promoter is a composite element that binds Stat5, Elf-1, HMG-I(Y) and a GATA family protein. EMBO J. 1996;15:5627–5635. [PMC free article] [PubMed] [Google Scholar]
- 15.Barata JT, Cardoso AA, Nadler LM, Boussiotis VA. Interleukin-7 promotes survival and cell cycle progression of T-cell acute lymphoblastic leukemia cells by down-regulating the cyclin-dependent kinase inhibitor p27(kip1) Blood. 2001;98:1524–1531. doi: 10.1182/blood.v98.5.1524. [DOI] [PubMed] [Google Scholar]
- 16.Dadi H, Ke S, Roifman CM. Activation of phosphatidylinositol-3 kinase by ligation of the interleukin-7 receptor is dependent on protein tyrosine kinase activity. Blood. 1994;84:1579–1586. [PubMed] [Google Scholar]
- 17.Shive CL, Clagett B, McCausland MR, Mudd JC, Funderburg NT, Freeman ML, et al. Inflammation perturbs the IL-7 axis, promoting senescence and exhaustion that broadly characterize immune failure in treated HIV infection. J Acquir Immune Defic Syndr. 2015 doi: 10.1097/QAI.0000000000000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaskovic S, Fernandez S, Price P, French MA. Interleukin-7 signalling defects in naive CD4+ T cells of HIV patients with CD4+ T-cell deficiency on antiretroviral therapy are associated with T-cell activation and senescence. AIDS. 2014;28:821–830. doi: 10.1097/QAD.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen TP, Bazdar DA, Mudd JC, Lederman MM, Harding CV, Hardy GA, et al. Interferon-alpha inhibits CD4 T cell responses to interleukin-7 and interleukin-2 and selectively interferes with Akt signaling. J Leukoc Biol. 2015;97:1139–1146. doi: 10.1189/jlb.4A0714-345RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cha L, de Jong E, French MA, Fernandez S. IFN-alpha exerts opposing effects on activation-induced and IL-7-induced proliferation of T cells that may impair homeostatic maintenance of CD4+ T cell numbers in treated HIV infection. J Immunol. 2014;193:2178–2186. doi: 10.4049/jimmunol.1302536. [DOI] [PubMed] [Google Scholar]
- 21.Hardy GA, Sieg SF, Rodriguez B, Jiang W, Asaad R, Lederman MM, et al. Desensitization to type I interferon in HIV-1 infection correlates with markers of immune activation and disease progression. Blood. 2009;113:5497–5505. doi: 10.1182/blood-2008-11-190231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol. 2011;11:330–342. doi: 10.1038/nri2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fraietta JA, Mueller YM, Yang G, Boesteanu AC, Gracias DT, Do DH, et al. Type I interferon upregulates Bak and contributes to T cell loss during human immunodeficiency virus (HIV) infection. PLoS Pathog. 2013;9:e1003658. doi: 10.1371/journal.ppat.1003658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajasuriar R, Booth DR, Gouillou M, Spelman T, James I, Solomon A, et al. The role of SNPs in the alpha-chain of the IL-7R gene in CD4+ T-cell recovery in HIV-infected African patients receiving suppressive cART. Genes Immun. 2012;13:83–93. doi: 10.1038/gene.2011.65. [DOI] [PubMed] [Google Scholar]
- 25.Zeng M, Smith AJ, Wietgrefe SW, Southern PJ, Schacker TW, Reilly CS, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shive CL, Clagett B, McCausland MR, Mudd JC, Funderburg NT, Freeman ML, et al. Inflammation Perturbs the IL-7 Axis, Promoting Senescence and Exhaustion that Broadly Characterize Immune Failure in Treated HIV Infection. J Acquir Immune Defic Syndr. 2016;71:483–492. doi: 10.1097/QAI.0000000000000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nascimbeni M, Perie L, Chorro L, Diocou S, Kreitmann L, Louis S, et al. Plasmacytoid dendritic cells accumulate in spleens from chronically HIV-infected patients but barely participate in interferon-alpha expression. Blood. 2009;113:6112–6119. doi: 10.1182/blood-2008-07-170803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) %CFSE low (IF n= 11, IS n= 11, HC n= 9). (B) %Divided (IF n= 11; IS n= 10; HC n= 9). (C) Proliferation index (IF n= 11; IS n= 10; HC n= 9). Data shown are CFSE dye dilution response to IL-7 when gating in CD4− cells under the experimental conditions described in figure 1. Abbreviations: immune failure, IF; immune success, IS; healthy control, HC.
PBMCs were incubated in the presence or absence of IL-7. After 3 days, cells were assessed for P-STAT5 and P-Akt by flow cytometry. (A) Graphs show responses in CD4+ (%P-STAT5+ IF n= 12, IS n= 12, HC n= 9; %P-Akt+ IF n= 13, IS n= 12, HC n= 9) and (B) shows CD4− T cells (%P-STAT5+ IF n= 11, IS n= 12, HC n= 9; %P-Akt+ IF n= 12, IS n= 12, HC n= 9). Abbreviations: immune failure, IF; immune success, IS; healthy control, HC.
Maturation subsets were based on (A) CD45RA+ (naive) and (B) CD45RA− (memory). Summary data of cell death in divided cells (CFSE low) and undivided cells (CFSE high) (IF n= 10, IS n= 8, HC n= 9). P-values were obtained by Wilcoxon signed rank test. Abbreviations: immune failure, IF; immune success, IS; healthy control, HC.