ABSTRACT
Genetic approaches in C. elegans are complementing the biochemical and antibody based strategies traditionally used to study the molecular underpinnings of fertilization in other organisms. A pair of worm studies, one based on forward genetics and one based on reverse genetics, converge on the sperm immunoglobulin superfamily molecule SPE-45. Loss of spe-45 function leads to the production of sperm that cannot fertilize wild-type eggs. This is a strikingly similar phenotype as those seen in mice lacking the immunoglobulin superfamily protein Izumo1. This work sets the stage for leveraging the power of the C. elegans model system to learn more about Izumo-like molecular function but also for the discovery of additional deeply conserved components of fertility pathways.
KEYWORDS: egg, fertilization, immunoglobulin, Izumo, SPE-45, sperm
Most of us have seen generic images of sperm and eggs interacting. What surprises most people is that our understanding of the genetic and molecular underpinnings of fertilization is quite limited. We do not have microscopes that can show us individual molecules such as cell receptors or hormones in action. The many ethical and experimental limitations to studying human fertility directly add additional challenges.
A little more than a century ago, research on fertilization was conducted mostly on marine invertebrates whose gametes are relatively easy to obtain.1 This tradition has lived on with the biochemical purification of many molecules hypothesized to function on the sperm or egg surface during fertilization.2 In mammals, sperm and eggs are difficult to obtain and manipulate.3 However, biochemical and antibody based approaches have still identified candidate molecules with a role in gamete interactions during mammalian fertilization.4 Unfortunately, genetic evidence for a direct role in gamete interactions at fertilization exists for only a few genes.3,5 At present, the only mammalian sperm protein both genetically and biochemically verified to be necessary and sufficient for binding to the egg is Izumo1 (named after a Japanese shrine dedicated to marriage).6,7 The paucity of fertility gene discovery in marine invertebrates and mammals could be due to the challenges of applying mutant screening strategies and the difficulty of maintaining strains of animals with fertility defects.
Recently, the nematode worm Caenorhabditis elegans has been emerging as a model system for understanding the molecular underpinnings of fertilization.4,8 Both forward and reverse genetic strategies have been used to identify molecules required for worm fertilization.9-11 Most of the molecules discovered in C. elegans sit on the surface of sperm or eggs and have features that support the idea that they are involved in sperm-egg signaling, binding, and/or fusion.8 However, the sequences of these genes have not matched up very well with the few other sperm and egg molecules discovered in traditional fertilization model systems. The lack of known deep molecular conservation of fertility genes has now changed with the publication of 2 complementary reports in Current Biology describing the discovery of the spe-45 gene in C. elegans.
Through differing approaches, one forward genetics and one reverse genetics, our laboratory and the laboratory of Dr. Steven L'Hernault converged on the finding that C. elegans have an Izumo-like protein that is required for fertilization.12,13 Harnessing the genetic power of C. elegans, our lab carried out a forward genetic screen to isolate C. elegans mutants defective in fertilization and generate a more complete inventory of the molecules required for gamete interactions. The first mutant that we characterized from our screen was a temperature-sensitive allele that we mapped to the predicted gene F28D1.8. At the same time, Nishimura et al. took a reverse genetic approach and asked whether C. elegans have an Izumo-like protein required for fertilization, specifically addressing the hypothesis that some fertility molecules are conserved between mammalian and non-mammalian model systems. Their search of the C. elegans genome and follow up phenotypic analysis led them to F28D1.8. We later showed that the allele isolated from our screen fails to complement the deletion allele of F28D1.8 characterized by Nishimura et al., confirming that the same gene was responsible for the sterile phenotype observed in both labs. Originally named oig-7 based on the predicted protein structure, F28D1.8 has been renamed spe-45 due to its sperm-specific function.
SPE-45 is functionally similar to Izumo1
Loss of spe-45 function in C. elegans produces a sterile phenotype restricted to male germline function.12,13 Hermaphrodites lacking spe-45 are self-sterile, which could be due to a defect in sperm, eggs, or both, but 2 pieces of evidence suggest only sperm are affected. First, males lacking spe-45 are unable to produce cross progeny when mated to hermaphrodites, as one would expect if sperm function is impaired. Second, spe-45 hermaphrodites can produce progeny when mated to males that make wild-type sperm, indicating that they have fertilization- and developmentally-competent eggs. Loss of spe-45 function does not result in any noticeable somatic defects. Thus, SPE-45 is required for fertility and acts exclusively in the sperm.
So what is wrong with spe-45 sperm? We can observe sperm within the spermathecae of spe-45 hermaphrodites and dissected spe-45 males release large numbers of spermatids that are morphologically indistinguishable from wild-type; so production of sperm is not the problem. Likewise, spe-45 sperm do not display any defects in sperm activation. When C. elegans sperm are activated, they form a pseudopod, acquire motility, and become fertilization-competent.14 Sperm dissected out of spe-45 hermaphrodites appear identical to the activated spermatozoa dissected from wild-type hermaphrodites. Male spe-45 sperm can also be activated in vitro with activation rates and efficiencies that are comparable to controls. Finally, mating experiments additionally confirm that spe-45 sperm are phenotypically normal up until the point of fertilization. By mating spe-45 males to feminized worms that don't produce any self-sperm, transfer and proper migration of sperm to the spermathecae (the site of fertilization) can be observed. The sperm from spe-45 males can even out-compete the self-sperm of fertile hermaphrodites, as seen by a reduction in the number of self-progeny even though no cross-progeny are produced.
Despite having no observable defects up to the point of contact, spe-45 sperm fail to fertilize eggs. We were unable to observe any paternal DNA in the eggs dissected from the uteri of spe-45 hermaphrodites. Another indicator that these eggs are not fertilized is their single large DNA mass. Unfertilized eggs undergo endomitotic DNA replication, but since sperm are responsible for delivering the centrosomes, the DNA remains as a single mass in the absence of sperm entry. Nishimura et al. also showed that the self-sperm of spe-45 hermaphrodites are retained in the spermathecae past the point when wild-type animals have normally depleted their store of sperm. Since sperm production is limited to a specific stage of larval development, hermaphrodites have a finite supply of self-sperm for fertilizing their eggs. As progeny are produced this supply of sperm is used up. Consequently, wild-type unmated hermaphrodites have almost no sperm remaining in the spermathecae by the third day of adulthood. In contrast, unmated spe-45 hermaphrodites still had numerous sperm in their spermathecae as 3-day adults. This is consistent with spe-45 sperm being unable to fertilize eggs and thus not being depleted in the manner observed in fertile animals.
Overall, we and Nishimura et al. showed that SPE-45 is required specifically for C. elegans sperm to successfully fertilize eggs. The phenotype that we observed for spe-45 animals is incredibly similar to the phenotype of mice deficient for the immunoglobulin super-family protein Izumo1. In the absence of Izumo1, mice are completely healthy, females are fertile, but males are sterile.7 In addition, sperm from Izumo1-deficient males have normal morphology, can migrate to the egg, undergo the acrosome reaction, and penetrate the zona pellucida (egg coat).7 So just like sperm from spe-45 worms, sperm from Izumo1-deficient mice appear completely normal up to the point of contacting the plasma membrane of the egg, but fertilization fails to occur. Based on these data, we propose that SPE-45 and Izumo1 are carrying out similar functions mediating sperm-egg binding and/or fusion. But it takes more than phenotypic similarities to drive the hypothesis that these proteins are functional homologs demonstrating deep molecular conservation in the process of fertilization.
SPE-45 has a similar molecular architecture to Izumo1
After the identification of Izumo1 in mice, Nishimura et al. predicted that C. elegans might also have an Izumo1-like protein that functions during fertilization. However, no Izumo1 orthologs are detectable in the C. elegans genome by BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi). This led them to carry out a more in depth search based on the known domain structure and expression pattern of Izumo1. Mouse Izumo1 contains a single transmembrane domain, a single immunoglobulin (Ig)-like domain, and shows testis-specific expression (Fig. 1).7 Using the SMART (Simple Modular Architecture Research Tool) program15, they identified 8 genes in the C. elegans genome that meet the criteria of having one transmembrane domain and one Ig-like domain. Expression data shows that only one of these 8 genes, F28D1.8, is enriched in the male germline.12,16 The fact that a single gene, F28D1.8 (now known as spe-45), has the same molecular attributes and expression pattern as Izumo1, led us and our colleagues to conclude that C. elegans does possess an Izumo1-like protein (Fig. 1). The similarity in knockout phenotype, as discussed above, points to a deep evolutionary conservation of this particular molecule as a mediator of fertilization. Together the evidence strongly supports the hypothesis that SPE-45 is orthologous to mouse Izumo1.
Figure 1.
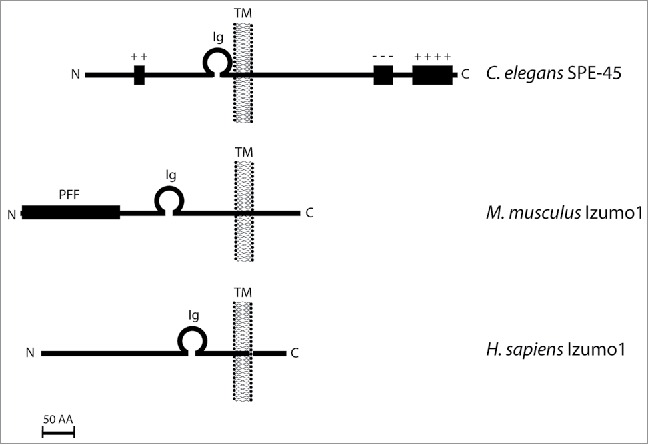
Schematic diagram showing the topology of Izumo-like proteins from Caenorhabditis elegans, Mus musculus, and Homo sapiens. C. elegans SPE-45 has positively and negatively charged regions indicated by + and - respectively. PFF refers to the “putative functional fragment” shown to be sufficient for binding to the egg.6 Ig, immunoglobulin-like domain; TM, transmembrane domain; AA, amino acid.
Since reproductive proteins are known to evolve quite rapidly,17 the low sequence identity between the 2 proteins and the inability to find spe-45 through a straightforward BLAST search, is not surprising. But despite the limited sequence identity, Nishimura et al. showed that a chimeric SPE-45 protein containing the mouse Izumo1 Ig-like domain in place of the SPE-45 Ig-like domain can function in place of the wild-type protein. This is in contrast to a SPE-45 protein where the Ig-like domain had been replaced with the Ig-like domain of a somatic C. elegans protein, which could not rescue the spe-45 phenotype. Thus, the Ig-like domain of Izumo1 and SPE-45 share a functional specificity that cannot be explained by the general presence of an Ig-like domain, lending further support to the orthologous nature of these 2 proteins.
Moving forward worms will continue to impact our understanding of fertilization
Questions still remain regarding the mechanism of SPE-45 function. Based on the spe-45 phenotype and the role of Izumo1, we predict that SPE-45 is likewise important for sperm adhesion with the egg. As such, we expect that SPE-45 should localize to the plasma membrane of the sperm where it would be available to interact with surface molecules on the egg. Unfortunately, localization of the protein has not been determined up to this point, despite numerous attempts. It is our hope that alternative strategies will eventually answer this persisting question.
Dissection of the functional domains of SPE-45, besides the Ig-like domain, can also complement our knowledge of Izumo1. It is much easier to test in vivo function of chimeric or truncated forms of the protein in C. elegans compared to mammalian systems. For example, we do not currently know if the intracellular region is important for function. Nishimura et al. also hypothesized that additional extracellular regions may act in the formation of SPE-45 multimers. Izumo1 is proposed to dimerize after initial binding with its receptor on the egg, so this may be another common attribute of both proteins.6,18,19 As the functionally important regions of SPE-45 are identified, they may help direct future studies of Izumo1 in mammals.
Finally, one of the critical aims for the future is to identify the SPE-45 binding partner(s) on eggs. It took nearly a decade after the identification of Izumo1 to find its binding partner — Juno — on mammalian eggs.20 By applying the same techniques that led to the discovery of Juno, it should take significantly less time to find the C. elegans egg receptor for SPE-45. And while the presence and function of Izumo1/SPE-45 on sperm is conserved, we are likely to discover that the egg binding partner differs between taxa.
Ig-like domains probably evolved to mediate interactions at the cell surface.21 Additionally, Ig-like cell adhesion molecules are one of the most ancient and diverse families of cell adhesion proteins.22 So it is easy to see how a member of this protein class was co-opted early in the evolution of sexual reproduction to mediate sperm binding to an egg. In addition to our finding of SPE-45 in C. elegans, a recent study identified potential Izumo1 orthologs in birds, fish, and reptiles lending more support to the deep evolutionary conservation of this molecule.23 That same study however, suggests that Juno is only present in mammals. Immunoglobulin superfamily (IgSF) proteins are known to interact with cell surface proteins of multiple different classes.22 As such, it presently appears that different species have evolved to utilize different proteins on the egg to interact with the sperm's Izumo1-like protein. Growing evidence also suggests that fertilization is not mediated by a single ligand-receptor pair, but by a complex network of multiple proteins. Even if Juno became an egg receptor specifically during mammalian evolution, we may find that the binding partner of SPE-45 still has a homolog with a supporting role in mammalian fertilization.
C. elegans has the largest repertoire of genes that have been genetically verified to be essential for fertilization.8 The discovery of spe-45 and the deep molecular conservation that it implies between C. elegans fertilization and mammalian fertilization indicates the broader significance of gene discovery in our worm model system. C. elegans lends itself particularly well to forward genetic screens making it a powerful tool for the continued discovery of fertilization molecules with genetic validation. Since the same cellular events - gamete recognition, adhesion, and fusion - underlie successful fertilization across taxa is it unlikely that SPE-45/IZUMO1 is the only example of conserved molecules mediating these events. As more genes are discovered they can complement ongoing studies in other model systems and provide new candidates for study in other organisms.
Abbreviation
- Ig
immunoglobulin
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Hitoshi Nishimura and Steve L'Hernault for sharing spe-45 results prior to publication and members of the Singson lab for helpful discussion.
Funding
This work was supported by a grant from the NIH (R01 HD054681) to AS and ARK is supported by a NIGMS K12 IRACDA postdoctoral fellowship (1K12GM093854).
References
- [1].Lillie FR. Studies of fertilization. VI. The mechanism of fertilization in arbacia. J Exp Zool 1914; 16:523-90; http://dx.doi.org/ 10.1002/jez.1400160404 [DOI] [Google Scholar]
- [2].Yanagimachi R. Mammalian fertilization. New York, NY: Raven Press, 1994. [Google Scholar]
- [3].Wright GJ, Bianchi E. The challenges involved in elucidating the molecular basis of sperm-egg recognition in mammals and approaches to overcome them. Cell Tissue Res 2016; 363:227-35; PMID:26224538; http://dx.doi.org/ 10.1007/s00441-015-2243-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Singson A. Every sperm is sacred: fertilization in Caenorhabditis elegans. Dev Biol 2001; 230:101-9; PMID:11161565; http://dx.doi.org/ 10.1006/dbio.2000.0118 [DOI] [PubMed] [Google Scholar]
- [5].Schultz R, Williams C. Developmental biology: sperm-egg fusion unscrambled. Nature 2005; 434:152-3; PMID:15758983; http://dx.doi.org/ 10.1038/434152a [DOI] [PubMed] [Google Scholar]
- [6].Inoue N, Hamada D, Kamikubo H, Hirata K, Kataoka M, Yamamoto M, Ikawa M, Okabe M, Hagihara Y. Molecular dissection of IZUMO1, a sperm protein essential for sperm-egg fusion. Development 2013; 140:3221-9; PMID:23824580; http://dx.doi.org/ 10.1242/dev.094854 [DOI] [PubMed] [Google Scholar]
- [7].Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 2005; 434:234-8; PMID:15759005; http://dx.doi.org/ 10.1038/nature03362 [DOI] [PubMed] [Google Scholar]
- [8].Krauchunas AR, Marcello MR, Singson A. The molecular complexity of fertilization: Introducing the concept of a fertilization synapse. Mol Reprod Dev 2016; PMID:26970099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Marcello MR, Singaravelu G, Singson A. Fertilization. Adv Exp Med Biol 2013; 757:321-50; PMID:22872482; http://dx.doi.org/ 10.1007/978-1-4614-4015-4_11 [DOI] [PubMed] [Google Scholar]
- [10].Nishimura H, L'Hernault SW. Spermatogenesis-defective (spe) mutants of the nematode Caenorhabditis elegans provide clues to solve the puzzle of male germline functions during reproduction. Dev Dyn 2010; 239:1502-14; PMID:20419782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Geldziler B, Kadandale P, Singson A. Molecular genetic approaches to studying fertilization in model systems. Reproduction 2004; 127:409-16; PMID:15047931; http://dx.doi.org/ 10.1530/rep.1.00009 [DOI] [PubMed] [Google Scholar]
- [12].Nishimura H, Tajima T, Comstra HS, Gleason EJ, L'Hernault SW. The Immunoglobulin-like Gene spe-45 Acts during Fertilization in Caenorhabditis elegans like the Mouse Izumo1 Gene. Curr Biol 2015; 25:3225-31; PMID:26671669; http://dx.doi.org/ 10.1016/j.cub.2015.10.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Singaravelu G, Rahimi S, Krauchunas A, Rizvi A, Dharia S, Shakes D, Smith H, Golden A, Singson A. Forward Genetics Identifies a Requirement for the Izumo-like Immunoglobulin Superfamily spe-45 Gene in Caenorhabditis elegans Fertilization. Curr Biol 2015; 25:3220-4; PMID:26671668; http://dx.doi.org/ 10.1016/j.cub.2015.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ellis RE, Stanfield GM. The regulation of spermatogenesis and sperm function in nematodes. Semin Cell Dev Biol 2014; 29:17-30; PMID:24718317; http://dx.doi.org/ 10.1016/j.semcdb.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Letunic I, Doerks T, Bork P. SMART: recent updates, new developments and status in 2015. Nucleic Acids Res 2015; 43:D257-60; PMID:25300481; http://dx.doi.org/ 10.1093/nar/gku949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Reinke V, Gil IS, Ward S, Kazmer K. Genome-wide germline-enriched and sex-biased expression profiles in Caenorhabditis elegans. Development 2004; 131:311-23; PMID:14668411; http://dx.doi.org/ 10.1242/dev.00914 [DOI] [PubMed] [Google Scholar]
- [17].Swanson WJ, Vacquier VD. The rapid evolution of reproductive proteins. Nat Rev Genet 2002; 3:137-44; PMID:11836507; http://dx.doi.org/ 10.1038/nrg733 [DOI] [PubMed] [Google Scholar]
- [18].Ellerman DA, Pei J, Gupta S, Snell WJ, Myles D, Primakoff P. Izumo is part of a multiprotein family whose members form large complexes on mammalian sperm. Mol Reprod Dev 2009; 76:1188-99; PMID:19658160; http://dx.doi.org/ 10.1002/mrd.21092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Inoue N, Hagihara Y, Wright D, Suzuki T, Wada I. Oocyte-triggered dimerization of sperm IZUMO1 promotes sperm-egg fusion in mice. Nat Commun 2015; 6:8858; PMID:26568141; http://dx.doi.org/ 10.1038/ncomms9858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bianchi E, Doe B, Goulding D, Wright GJ. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature 2014; 508:483-7; PMID:24739963; http://dx.doi.org/ 10.1038/nature13203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Barclay AN. Ig-like domains: evolution from simple interaction molecules to sophisticated antigen recognition. Proc Natl Acad Sci U S A 1999; 96:14672-4; PMID:10611269; http://dx.doi.org/ 10.1073/pnas.96.26.14672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shapiro L, Love J, Colman DR. Adhesion molecules in the nervous system: structural insights into function and diversity. Annu Rev Neurosci 2007; 30:451-74; PMID:17600523; http://dx.doi.org/ 10.1146/annurev.neuro.29.051605.113034 [DOI] [PubMed] [Google Scholar]
- [23].Grayson P. Izumo1 and Juno: the evolutionary origins and coevolution of essential sperm-egg binding partners. R Soc Open Sci 2015; 2:150296; PMID:27019721; http://dx.doi.org/ 10.1098/rsos.150296 [DOI] [PMC free article] [PubMed] [Google Scholar]


