Abstract
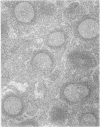
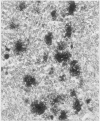
Sixteen lymph nodes from 14 patients with rheumatoid arthritis were examined immunohistochemically and morphometrically and compared with 10 control nodes showing follicular hyperplasia from patients without rheumatoid disease. Frozen material was available from nine patients and all controls. The nodes from patients with rheumatoid arthritis seemed to share characteristic features. The most striking of these was follicular hyperplasia in which the germinal centres, in spite of being quite large, showed relatively sparse proliferative activity. The nodes often showed infiltration of germinal centres by CD8 positive T lymphocytes and contained fewer IL2R positive cells in the paracortex than controls. These and other features may have some correlation with disease activity. Lymphadenopathy in rheumatoid arthritis may not just be a manifestation of joint inflammation but an active component of this multisystem disease and may reflect a widespread immunological abnormality.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchan G., Barrett K., Fujita T., Taniguchi T., Maini R., Feldmann M. Detection of activated T cell products in the rheumatoid joint using cDNA probes to Interleukin-2 (IL-2) IL-2 receptor and IFN-gamma. Clin Exp Immunol. 1988 Feb;71(2):295–301. [PMC free article] [PubMed] [Google Scholar]
- CRUICKSHANK B. Lesions of lymph nodes in rheumatoid disease and in disseminated lupus erythematosus. Scott Med J. 1958 Mar;3(3):110–119. doi: 10.1177/003693305800300302. [DOI] [PubMed] [Google Scholar]
- Dvoretsky P., Wood G. S., Levy R., Warnke R. A. T-lymphocyte subsets in follicular lymphomas compared with those in non-neoplastic lymph nodes and tonsils. Hum Pathol. 1982 Jul;13(7):618–625. doi: 10.1016/s0046-8177(82)80003-8. [DOI] [PubMed] [Google Scholar]
- Janossy G., Tidman N., Selby W. S., Thomas J. A., Granger S., Kung P. C., Goldstein G. Human T lymphocytes of inducer and suppressor type occupy different microenvironments. Nature. 1980 Nov 6;288(5786):81–84. doi: 10.1038/288081a0. [DOI] [PubMed] [Google Scholar]
- Kelly C. A., Malcolm A. J., Griffiths I. Lymphadenopathy in rheumatic patients. Ann Rheum Dis. 1987 Mar;46(3):224–227. doi: 10.1136/ard.46.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy T. D., Plater-Zyberk C., Partridge T. A., Woodrow D. F., Maini R. N. Morphometric comparison of synovium from patients with osteoarthritis and rheumatoid arthritis. J Clin Pathol. 1988 Aug;41(8):847–852. doi: 10.1136/jcp.41.8.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitas G. D., Salmon M., Farr M., Gaston J. S., Bacon P. A. Deficient interleukin 2 production in rheumatoid arthritis: association with active disease and systemic complications. Clin Exp Immunol. 1988 Aug;73(2):242–249. [PMC free article] [PubMed] [Google Scholar]
- LEA A. J. AN ASSOCIATION BETWEEN THE RHEUMATIC DISEASES AND THE RETICULOSES. Ann Rheum Dis. 1964 Nov;23:480–484. doi: 10.1136/ard.23.6.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOTULSKY A. G., WEINBERG S., SAPHIR O., ROSENBERG E. Lymph nodes in rheumatoid arthritis. AMA Arch Intern Med. 1952 Nov;90(5):660–676. doi: 10.1001/archinte.1952.00240110086009. [DOI] [PubMed] [Google Scholar]
- Mageed R. A., Walker M. R., Jefferis R. Restricted light chain subgroup expression on human rheumatoid factor paraproteins determined by monoclonal antibodies. Immunology. 1986 Nov;59(3):473–478. [PMC free article] [PubMed] [Google Scholar]
- McCusker C. T., Singal D. P. Molecular relationships between the class II HLA antigens and susceptibility to rheumatoid arthritis. J Rheumatol. 1988 Jul;15(7):1050–1053. [PubMed] [Google Scholar]
- Modlin R. L., Meyer P. R., Hofman F. M., Mehlmauer M., Levy N. B., Lukes R. J., Parker J. W., Ammann A. J., Conant M. A., Rea T. H. T-lymphocyte subsets in lymph nodes from homosexual men. JAMA. 1983 Sep 9;250(10):1302–1305. [PubMed] [Google Scholar]
- Muirden K. D. Lymph node iron in rheumatoid arthritis. Histology, ultrastructure, and chemical concentration. Ann Rheum Dis. 1970 Jan;29(1):81–88. doi: 10.1136/ard.29.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadimitriou G. M., Bacon P. A., Hall N. D. Circulating activated lymphocytes in rheumatoid arthritis: a marker of synovial inflammation. J Rheumatol. 1982 Mar-Apr;9(2):217–223. [PubMed] [Google Scholar]
- Said J. W., Shintaku I. P., Teitelbaum A., Chien K., Sassoon A. F. Distribution of T-cell phenotypic subsets and surface immunoglobulin-bearing lymphocytes in lymph nodes from male homosexuals with persistent generalized adenopathy: an immunohistochemical and ultrastructural study. Hum Pathol. 1984 Aug;15(8):785–790. doi: 10.1016/s0046-8177(84)80171-9. [DOI] [PubMed] [Google Scholar]
- Salmon M., Bacon P. A. A cellular deficiency in the rheumatoid one-way mixed lymphocyte reaction. Clin Exp Immunol. 1988 Jan;71(1):79–84. [PMC free article] [PubMed] [Google Scholar]
- Symmons D. P., Ahern M., Bacon P. A., Hawkins C. F., Amlot P. L., Jones E. L., Prior P., Scott D. L. Lymphoproliferative malignancy in rheumatoid arthritis: a study of 20 cases. Ann Rheum Dis. 1984 Apr;43(2):132–135. doi: 10.1136/ard.43.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood G. S., Burns B. F., Dorfman R. F., Warnke R. A. In situ quantitation of lymph node helper, suppressor, and cytotoxic T cell subsets in AIDS. Blood. 1986 Mar;67(3):596–603. [PubMed] [Google Scholar]