Abstract
Insulin-dependent diabetes mellitus (IDDM) is associated with serum antibodies that precipitate a 64-kDa pancreatic islet cell protein reported to be glutamic acid decarboxylase (GAD; glutamate decarboxylase, EC 4.1.1.15). Previously, antibodies to GAD were found in the rare neurological disorder stiff man syndrome. To demonstrate directly antibodies to GAD, enzymatically active GAD was first purified from fresh human cerebellum. Brain GAD activity was precipitated by noninhibitory antibodies in the sera of 16/26 (62%) subjects defined as having preclinical IDDM (islet cell antibody-positive first-degree relatives of a person with IDDM), 3/13 (23%) with recent-onset IDDM, and 3/3 with the stiff man syndrome. In addition, sera of 5/26 (19%) preclinical and 2/13 (15%) recent-onset IDDM subjects contained antibodies that precipitated GAD but inhibited its activity. Thus, overall, 21/26 (81%) preclinical and 5/13 (38%) recent-onset IDDM subjects had antibodies that precipitated GAD activity. Antibodies to GAD were not detected in sera from subjects with other autoimmune diseases (n = 29) or healthy controls (n = 14). GAD affinity-purified to homogeneity (specific activity, 58 units/mg) was specifically immunoprecipitated as a single 60-kDa species by the IDDM sera. In an ELISA incorporating whole mouse brain GAD captured by the GAD-6 monoclonal antibody the frequencies of GAD antibodies for all subject groups were indistinguishable from those found by precipitation of human brain enzymatic activity. We conclude that (i) GAD is an (auto)antigen in a majority of subjects operationally defined as having preclinical IDDM, (ii) pancreatic islet and brain GAD are likely to be cross-reactive, and (iii) the majority of GAD antibodies are directed away from the catalytic site of the brain enzyme. The lower frequency of GAD antibodies in recent-onset IDDM subjects indicates either that immunoreactivity is lost with near-total beta-cell destruction or that GAD antibodies denote a low risk of progression to clinical disease.
Full text
PDF

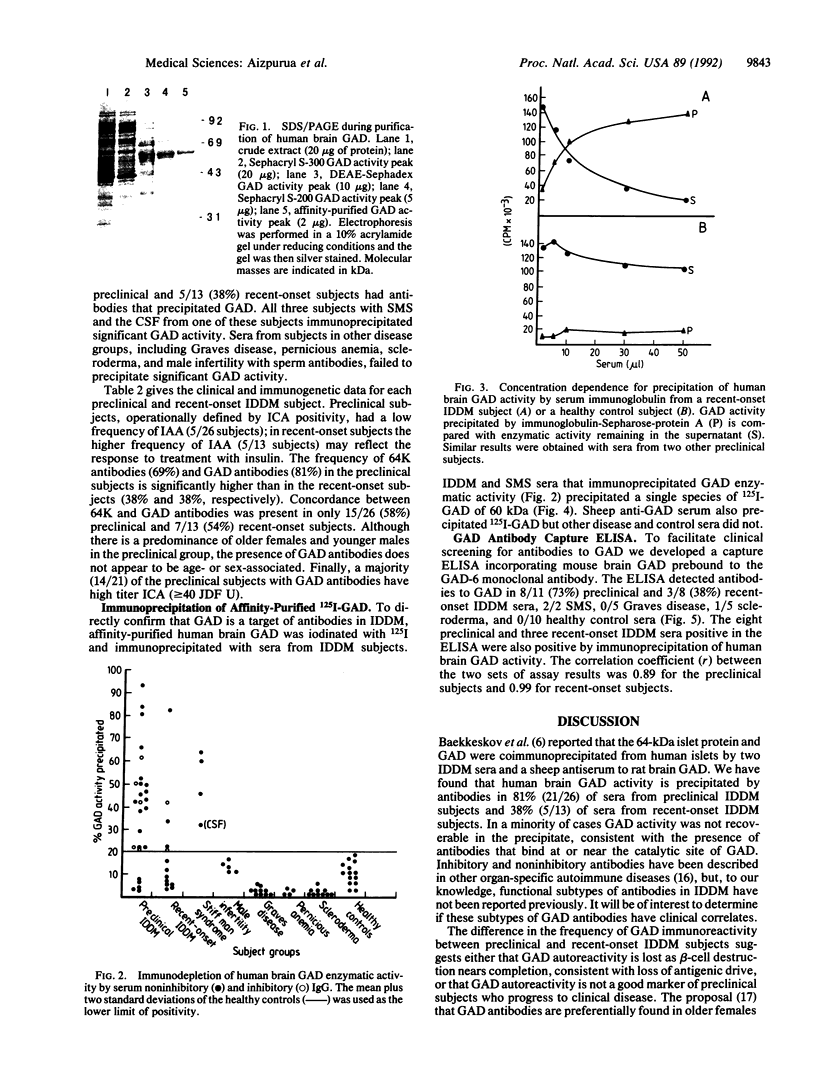
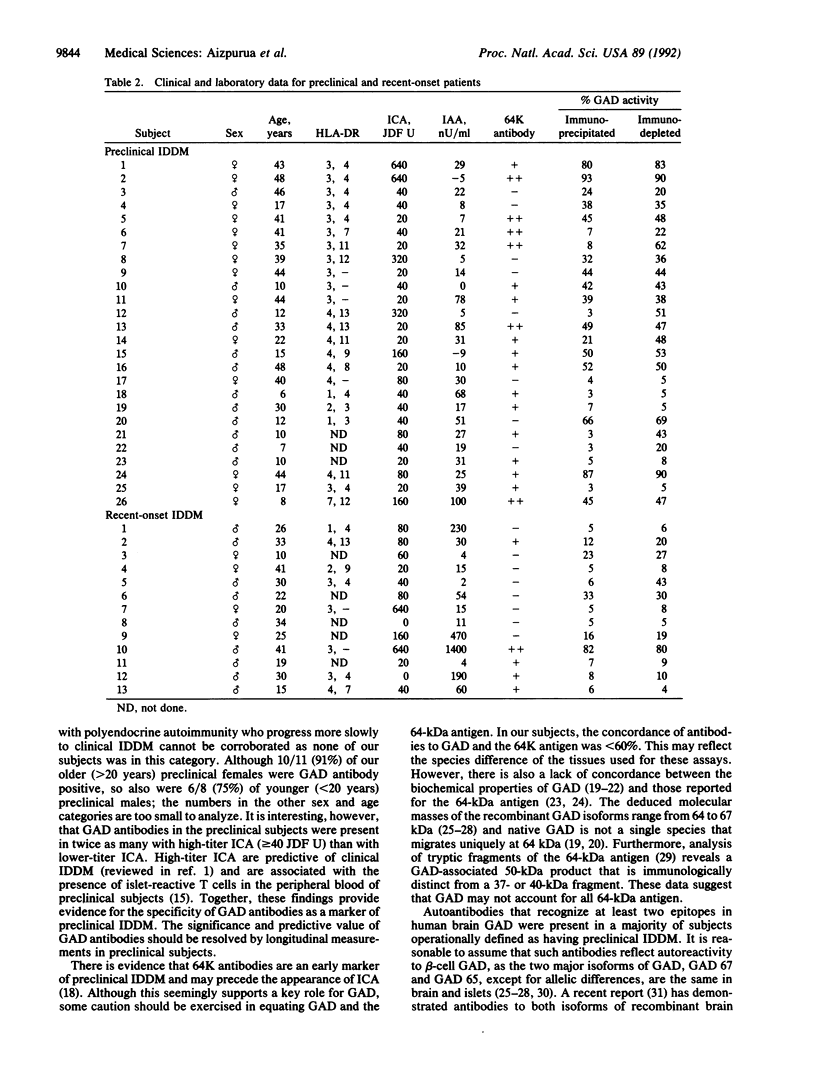
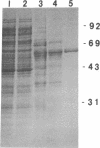
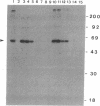
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERS R. W., BRADY R. O. The distribution of glutamic decarboxylase in the nervous system of the rhesus monkey. J Biol Chem. 1959 Apr;234(4):926–928. [PubMed] [Google Scholar]
- Atkinson M. A., Maclaren N. K., Scharp D. W., Lacy P. E., Riley W. J. 64,000 Mr autoantibodies as predictors of insulin-dependent diabetes. Lancet. 1990 Jun 9;335(8702):1357–1360. doi: 10.1016/0140-6736(90)91241-2. [DOI] [PubMed] [Google Scholar]
- Baekkeskov S., Aanstoot H. J., Christgau S., Reetz A., Solimena M., Cascalho M., Folli F., Richter-Olesen H., De Camilli P., Camilli P. D. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990 Sep 13;347(6289):151–156. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- Baekkeskov S., Landin M., Kristensen J. K., Srikanta S., Bruining G. J., Mandrup-Poulsen T., de Beaufort C., Soeldner J. S., Eisenbarth G., Lindgren F. Antibodies to a 64,000 Mr human islet cell antigen precede the clinical onset of insulin-dependent diabetes. J Clin Invest. 1987 Mar;79(3):926–934. doi: 10.1172/JCI112903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekkeskov S., Nielsen J. H., Marner B., Bilde T., Ludvigsson J., Lernmark A. Autoantibodies in newly diagnosed diabetic children immunoprecipitate human pancreatic islet cell proteins. Nature. 1982 Jul 8;298(5870):167–169. doi: 10.1038/298167a0. [DOI] [PubMed] [Google Scholar]
- Baekkeskov S., Warnock G., Christie M., Rajotte R. V., Larsen P. M., Fey S. Revelation of specificity of 64K autoantibodies in IDDM serums by high-resolution 2-D gel electrophoresis. Unambiguous identification of 64K target antigen. Diabetes. 1989 Sep;38(9):1133–1141. doi: 10.2337/diab.38.9.1133. [DOI] [PubMed] [Google Scholar]
- Blindermann J. M., Maitre M., Ossola L., Mandel P. Purification and some properties of L-glutamate decarboxylase from human brain. Eur J Biochem. 1978 May;86(1):143–152. doi: 10.1111/j.1432-1033.1978.tb12293.x. [DOI] [PubMed] [Google Scholar]
- Bu D. F., Erlander M. G., Hitz B. C., Tillakaratne N. J., Kaufman D. L., Wagner-McPherson C. B., Evans G. A., Tobin A. J. Two human glutamate decarboxylases, 65-kDa GAD and 67-kDa GAD, are each encoded by a single gene. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2115–2119. doi: 10.1073/pnas.89.6.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. C., Gottlieb D. I. Characterization of the proteins purified with monoclonal antibodies to glutamic acid decarboxylase. J Neurosci. 1988 Jun;8(6):2123–2130. doi: 10.1523/JNEUROSCI.08-06-02123.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. C., Gottlieb D. I. Characterization of the proteins purified with monoclonal antibodies to glutamic acid decarboxylase. J Neurosci. 1988 Jun;8(6):2123–2130. doi: 10.1523/JNEUROSCI.08-06-02123.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman P. G., Nayak R. C., Campbell I. L., Eisenbarth G. S. Binding of cytoplasmic islet cell antibodies is blocked by human pancreatic glycolipid extracts. Diabetes. 1988 May;37(5):645–652. doi: 10.2337/diab.37.5.645. [DOI] [PubMed] [Google Scholar]
- Cram D. S., Barnett L. D., Joseph J. L., Harrison L. C. Cloning and partial nucleotide sequence of human glutamic acid decarboxylase cDNA from brain and pancreatic islets. Biochem Biophys Res Commun. 1991 May 15;176(3):1239–1244. doi: 10.1016/0006-291x(91)90418-7. [DOI] [PubMed] [Google Scholar]
- Erlander M. G., Tillakaratne N. J., Feldblum S., Patel N., Tobin A. J. Two genes encode distinct glutamate decarboxylases. Neuron. 1991 Jul;7(1):91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- Harrison L. C., Chu S. X., DeAizpurua H. J., Graham M., Honeyman M. C., Colman P. G. Islet-reactive T cells are a marker of preclinical insulin-dependent diabetes. J Clin Invest. 1992 Apr;89(4):1161–1165. doi: 10.1172/JCI115698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsen A. E., Hagopian W. A., Grubin C. E., Dube S., Disteche C. M., Adler D. A., Bärmeier H., Mathewes S., Grant F. J., Foster D. Cloning and primary structure of a human islet isoform of glutamic acid decarboxylase from chromosome 10. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8337–8341. doi: 10.1073/pnas.88.19.8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman D. L., Erlander M. G., Clare-Salzler M., Atkinson M. A., Maclaren N. K., Tobin A. J. Autoimmunity to two forms of glutamate decarboxylase in insulin-dependent diabetes mellitus. J Clin Invest. 1992 Jan;89(1):283–292. doi: 10.1172/JCI115573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitre M., Blindermann J. M., Ossola L., Mandel P. Comparison of the structures of L-glutamate decarboxylases from human and rat brains. Biochem Biophys Res Commun. 1978 Dec 14;85(3):885–890. doi: 10.1016/0006-291x(78)90626-5. [DOI] [PubMed] [Google Scholar]
- Martino G. V., Tappaz M. L., Braghi S., Dozio N., Canal N., Pozza G., Bottazzo G. F., Grimaldi L. M., Bosi E. Autoantibodies to glutamic acid decarboxylase (GAD) detected by an immuno-trapping enzyme activity assay: relation to insulin-dependent diabetes mellitus and islet cell antibodies. J Autoimmun. 1991 Dec;4(6):915–923. doi: 10.1016/0896-8411(91)90054-g. [DOI] [PubMed] [Google Scholar]
- Michelsen B. K., Petersen J. S., Boel E., Møldrup A., Dyrberg T., Madsen O. D. Cloning, characterization, and autoimmune recognition of rat islet glutamic acid decarboxylase in insulin-dependent diabetes mellitus. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8754–8758. doi: 10.1073/pnas.88.19.8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner M. L., Harrison L. C., Campbell I. L., Heyma P., Cook J. J. Detection of insulin antibodies in newly-diagnosed type 1 diabetic children after "acid-stripping" of sera. Diabetes Res. 1987 Sep;6(1):1–4. [PubMed] [Google Scholar]
- Reetz A., Solimena M., Matteoli M., Folli F., Takei K., De Camilli P. GABA and pancreatic beta-cells: colocalization of glutamic acid decarboxylase (GAD) and GABA with synaptic-like microvesicles suggests their role in GABA storage and secretion. EMBO J. 1991 May;10(5):1275–1284. doi: 10.1002/j.1460-2075.1991.tb08069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. Methods for assessing immunologic and biologic properties of iodinated peptide hormones. Methods Enzymol. 1975;37:223–233. doi: 10.1016/s0076-6879(75)37018-3. [DOI] [PubMed] [Google Scholar]
- Solimena M., Folli F., Denis-Donini S., Comi G. C., Pozza G., De Camilli P., Vicari A. M. Autoantibodies to glutamic acid decarboxylase in a patient with stiff-man syndrome, epilepsy, and type I diabetes mellitus. N Engl J Med. 1988 Apr 21;318(16):1012–1020. doi: 10.1056/NEJM198804213181602. [DOI] [PubMed] [Google Scholar]
- Spink D. C., Porter T. G., Wu S. J., Martin D. L. Characterization of three kinetically distinct forms of glutamate decarboxylase from pig brain. Biochem J. 1985 Nov 1;231(3):695–703. doi: 10.1042/bj2310695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spink D. C., Wu S. J., Martin D. L. Multiple forms of glutamate decarboxylase in porcine brain. J Neurochem. 1983 Apr;40(4):1113–1119. doi: 10.1111/j.1471-4159.1983.tb08101.x. [DOI] [PubMed] [Google Scholar]
- Vardi P., Dib S. A., Tuttleman M., Connelly J. E., Grinbergs M., Radizabeh A., Riley W. J., Maclaren N. K., Eisenbarth G. S., Soeldner J. S. Competitive insulin autoantibody assay. Prospective evaluation of subjects at high risk for development of type I diabetes mellitus. Diabetes. 1987 Nov;36(11):1286–1291. doi: 10.2337/diab.36.11.1286. [DOI] [PubMed] [Google Scholar]
- Wu J. Y., Matsuda T., Roberts E. Purification and characterization of glutamate decarboxylase from mouse brain. J Biol Chem. 1973 May 10;248(9):3029–3034. [PubMed] [Google Scholar]