Abstract
Pancreatic islet transplantation is a promising therapy for diabetes, but acute rejection of the islets by host effector T cells has hindered clinical application. Here we addressed the mechanisms of CD8+ effector T cell migration to islet grafts since interrupting this step is key to preventing rejection. We found that effector T cell migration to re-vascularized islet transplants in mice is dependent on non-self Ag recognition rather than signaling via Gαi-coupled chemokine receptors. Presentation of non-self Ag by donor cells was necessary for migration, whereas Ag presentation by recipient cells was dispensable. We also observed that deficiency of SKAP1, an immune cell adaptor downstream of the TCR and important for integrin activation, prolongs allograft survival but does not reduce effector T cell migration to the graft. Therefore, effector T cell migration to transplanted islets is Ag-, not chemokine-, driven but SKAP1 does not play a critical role in this process.
Introduction
Isolated pancreatic islets transplanted into either mice or humans reverse diabetes mellitus, but rejection of the islets by host effector T cells remains a major impediment to full-scale clinical application (1). In the mouse, host effector T cells specific to donor non-self Ag migrate to the islet graft and reject it after they are generated in secondary lymphoid tissues (2, 3). Both CD4+ and CD8+ effector T cells contribute to the rejection process, with the latter playing a key role (4, 5). Elucidating the mechanisms by which CD8+ effector T cells migrate to the graft is therefore an important step towards preventing islet rejection.
Effector T cell migration to islet grafts has been traditionally attributed to inflammatory chemokines produced at the site of transplantation (6). Chemokines bind to the surface of endothelial cells and trigger the activation of integrin molecules on effector T cells via Gαi-coupled receptors. Activated integrins then cause firm arrest and transendothelial migration of the T cells into the tissue (7). Recent experiments however have shown that the migration of auto-reactive CD4+ T cells to native islets in the mouse pancreas is not interrupted by pre-treating T cells with pertussis toxin (PTx), which irreversibly blocks Gαi-dependent signaling, but is in fact dependent on engagement of the TCR by cognate antigen (8). Similarly, the migration of donor Ag-specific CD8+ effector T cells to heart and kidney allografts in the mouse occurs when T cells bind cognate Ag presented by either the graft endothelial or dendritic cells, independently of Gαi signaling (9). In both cases, binding of integrins on the T cell surface to their ligands on endothelial or dendritic cells was still required for migration. Therefore, migration of effector T cells that cause end organ damage in either autoimmunity or transplantation appears to be driven by Ag and not chemokines. This is a highly plausible scenario because TCR engagement triggers inside-out signaling that leads to integrin activation (10), although the signaling molecules involved are distinct from those employed by Gαi-coupled chemokine receptors (11). The adaptor SKAP1 is an immune cell-specific signaling adaptor that mediates TCR inside-out signaling needed for the activation of integrins such as LFA-1. It contributes to the activation of naive T cells by stabilizing contacts between T cells and Ag-presenting DC (12, 13). These contacts are mediated by binding of the integrin LFA-1 on T cells to its ligand ICAM-1 on DCs. Whether SKAP1 is also important for migration and arrest of Ag-specific effector T cells in target tissues is not known.
Islet transplantation is distinct from whole organ transplantation in several aspects that could influence effector T cell migration. One important distinction is that islet grafts are re-vascularized de novo (neo-vascularized) after transplantation, while whole organ grafts are transplanted with intact donor vasculature displaying a multitude of donor antigens. Neo-vessels in islet grafts are most likely of both donor and recipient origin (14), but the extent to which they express or present donor antigens is not known. Moreover, it is unclear whether T cells exit readily from neo-vessels and infiltrate islet grafts independent of either chemokines or Ag. Therefore, inferring from studies on native islets or whole organ grafts the roles of chemokines and Ag in the migration of effector T cells to islet grafts is not straightforward. We addressed this problem here by imaging the migration of CD8+ effector T cells to islet grafts transplanted under the mouse kidney capsule using two-photon intra-vital microscopy. We also investigated the role of SKAP1 in the same model.
Methods
Mice
B6 (C57BL/6J; Thy1.2, CD45.2, H-2Kb), B6 Act-OVA (C57BL/6J-Tg[CAG-OVA]916Jen/J; CD45.2, H-2Kb), OT-I (C57BL/6-Tg[TcraTcrb]1100Mjb/J; CD45.2, H-2Kb), and B6 CD11c-YFP mice were purchased from The Jackson Laboratory, B6 H-2Kb−/− mice from Taconic, B6-Ly5.2/Cr mice (Thy1.2, CD45.1, H-2Kb) from NCI, and BALB/c mice (BALB/cAnNCrl; CD45.2, H-2Kd) from Charles River. B6 Act-OVA.H-2Kb−/− and B6 CD11c-YFP.H-2Kb−/− mice were generated in our animal facility by breeding B6 Act-OVA and B6 CD11c-YFP mice with B6 H-2Kb−/− mice. OT-I mice were bred onto RAG−/− Thy1.1 and RAG−/− Thy1.2 backgrounds. B6 SKAP1−/− mice were provided by C. E. Rudd (13). B6 OT-I.SKAP1−/− mice were generated by breeding B6 SKAP1−/− mice with B6 OT-I.RAG−/− mice to generate B6 OT-I.SKAP1−/−RAG+/− and B6 OT-1.SKAP1−/−.RAG−/− mice. Mice were maintained under SPF conditions. Animal studies were approved by University of Pittsburgh IACUC.
Islet transplantation
Mouse pancreatic islets were isolated and transplanted as established by Bertera et al. (15). Briefly, pancreata were perfused with collagenase V (Sigma), harvested, and digested. Islets were separated over a Ficoll gradient, handpicked with a Pasteur pipet, and cultured overnight in complete RPMI medium at 37 °C and 5% CO2. For graft survival experiments, islets were injected immediately into the left renal subcapsular space of diabetic mice. For imaging experiments, cultured islets were labeled with 10 μM Cell Tracker Orange (CTO; Invitrogen) for 1 hr before transplantation. Diabetes was induced by injecting 230 mg/Kg streptozotocin i.p. 4–5 days prior to transplantation and confirmed by elevated blood glucose levels (>300 mg/dl). Blood glucose <150 mg/dl after transplantation was considered engraftment, and >300 mg/dl was considered islet graft non-engraftment or rejection. In mice that received immunosuppression, 200 μg of CTLA-4-Ig (BioXCell) was administrated i.p. on days 0 and day 2 after transplantation.
Generation and adoptive transfer of OT-I effector and memory T cells
Spleens from OT-I.RAG−/− (CD45.2), OT-I.SKAP1−/−, or OT-I.SKAP1−/−.RAG−/− (CD45.2) mice were processed to single leukocyte suspensions and 1 or 5 × 106 splenocytes were transferred i.v. to B6 (CD45.1) mice. 6 hr later, recipient mice were immunized with 25 μg of OVA-conjugated anti-Dec205 antibody (Dec205-OVA) i.v. plus 50 μg of agonistic anti-CD40 (FGK45) i.p. to generate OT-1 effector cells (16). Spleens were harvested 6 days later (for effector experiments) or 6 weeks later (for memory experiments) and CD45.2+CD4−CD45.1− (CD11cCD11bTer119CD16/32B220F4/80DX5)− cells were sorted on a high-speed cell sorter (BD Aria). Flow cytometry confirmed that >95% of sorted cells were CD8+CD44hi and >97% of these were OT-I cells (Vα2 and Vβ5.1/5.2 double positive). Sorted cells were incubated with PTx (Sigma) (200 ng/ml for 30 min at 37 °C and 5% CO2), or not, after which they were labeled with 2.5 μM Cell Tracker Violet (CTV; Invitrogen) for 20 min or 1.5 μM Carboxyfluorescein Succinimidyl Ester (CFSE; Invitrogen) for 10 min. 5 × 106 PTx-treated and/or 5 × 106 untreated OT-I effector cells were transferred i.v. 9 – 10 days after islet transplantation. Imaging was performed 16–24 hours after transfer. The same cell numbers (5 × 106 of each) and labeling procedures were used in experiments in which WT and SKAP1−/− OT-I effectors were co-transferred.
Two-photon intravital microscopy
Two-photon intravital microscopy was performed on islet grafts transplanted under the left kidney capsule. An Olympus FluoView FV1000 microscope equipped with a Mai Tai DeepSee femtosecond-pulsed laser (Spectra-Physics) tuned and mode-locked to 830nm was used for all experiments. Mice were anesthetized with isoflurane and oxygen, and core body temperature was maintained at 37°C with a homeothermic controller (CWE Inc.). Animals were kept hydrated by injecting 1 ml of 5% dextrose lactated ringer’s solution s.c. every 60 min. Blood vessels were visualized by injecting Evans Blue (Sigma). The kidney housing the transplanted islets was extraverted from its original location with intact vascular connection and immobilized in a custom cup mount (17). Z-stacks were visualized with a 25× water immersion objective (NA: 1.05) 20–70 μm below the kidney capsule. A total of 12 slices per stack were acquired at a step size of 4.5 μm. Brightness and laser power were adjusted based on imaging depth and kept below phototoxic level. Dwell time was set to 8 μs/pixel and resolution of 512 × 512 pixels. Approximately 36-second-long stacks were repeatedly scanned up to 60 times for a maximum imaging time of 36 min per location. Multiple different locations per islet graft were imaged. All acquired movies were analyzed using Imaris software (Bitplane). Drift was corrected using islets as a reference point. All CTV+ or CFSE+ cells detected during the imaging time were enumerated. Cells were determined to be extravascular if the majority or all of the cell body had moved outside the vessel lumen.
Statistics
Statistical analysis of allograft survival was calculated using the log-rank test. All other experiments were analyzed using 2-tailed unpaired t test for samples with normal distribution and 2-tailed Mann-Whitney test for samples with non-Gaussian distribution. All statistical calculations were made using GraphPad Prism version 6.0c. A p-value <0.05 was considered significant.
Results
CD8+ effector T cell migration to islet grafts is dependent on cognate Ag, not Gαi-coupled chemokine receptor signaling
To test the roles of cognate Ag and Gαi-coupled chemokine receptor signaling in the migration of CD8+ effector T cells, we transplanted B6 Act-OVA (referred to thereafter as B6.OVA) transgenic islets, which express the non-self antigen ovalbumin (OVA), or syngeneic B6 islets, which do not express OVA, under the kidney capsule of B6 recipients rendered diabetic by streptozotocin injection. PTx-treated and untreated TCR-transgenic OT-I CD8+ effector T cells, which recognize the SIINFEKL OVA peptide in the context of the B6 H-2Kb class I molecule, were co-transferred 9 – 10 days after transplantation, and the grafts were imaged 16 – 24 hours later. Delaying the transfer of effector T cells was necessary to allow sufficient time for the grafted islets to re-vascularize. The early imaging time point after cell transfer was selected to ensure that the number of OT-I cells present in the graft reflects their migration to instead of proliferation within the graft (9). As shown in Fig. 1A (supplemental movie 1), both PTx-treated and untreated OT-I cells migrated to B6.OVA but not B6 islet grafts and were readily detected in the peri-islet as well as the intra-islet areas of B6.OVA grafts. The number of PTx-treated OT-I cells in the graft was comparable to that of untreated cells (Fig. 1B), and the majority of OT-I cells, whether PTx-treated or not, had already transmigrated out of the vessel lumen, but significantly less had done so in the PTx-treated group (p = 0.04) (Fig. 1C). On average, 83% of OT-I cells were in the extravascular space in the PTx-treated group compared to 98% in the untreated group. Markedly diminished migration of PTx-treated OT-I cells to the lymph nodes of the imaged mice confirmed that Gαi was adequately blocked in these cells (Fig. 1D). As observed with OT-I effectors, migration of memory OT-I cells to B6.OVA islet grafts was not hindered by PTx treatment (Fig. 1B). Migration of naïve OT-I cells to the graft on the other hand was insignificant (Fig. 1B), consistent with the inability of naïve lymphocytes to enter non-lymphoid tissues. Motility analysis revealed small but statistically significant differences between PTx-treated and untreated OT-I effector cells (greater median velocity and displacement and lower arrest coefficient in the treated group) (Fig. 1E). The results shown in Fig. 1 therefore imply that cognate non-self Ag is necessary for the migration of CD8+ effector T cells to islet grafts while Gαi-coupled chemokine receptor signaling is dispensable. Gαi-signaling however contributes to the trans-endothelial migration (Fig. 1C) and firm arrest (Fig. 1E) of effector T cells in the graft, albeit it to a minor degree.
FIGURE 1. Role of cognate Ag and Gαi-coupled chemokine receptor signaling in CD8+ effector T cell migration.
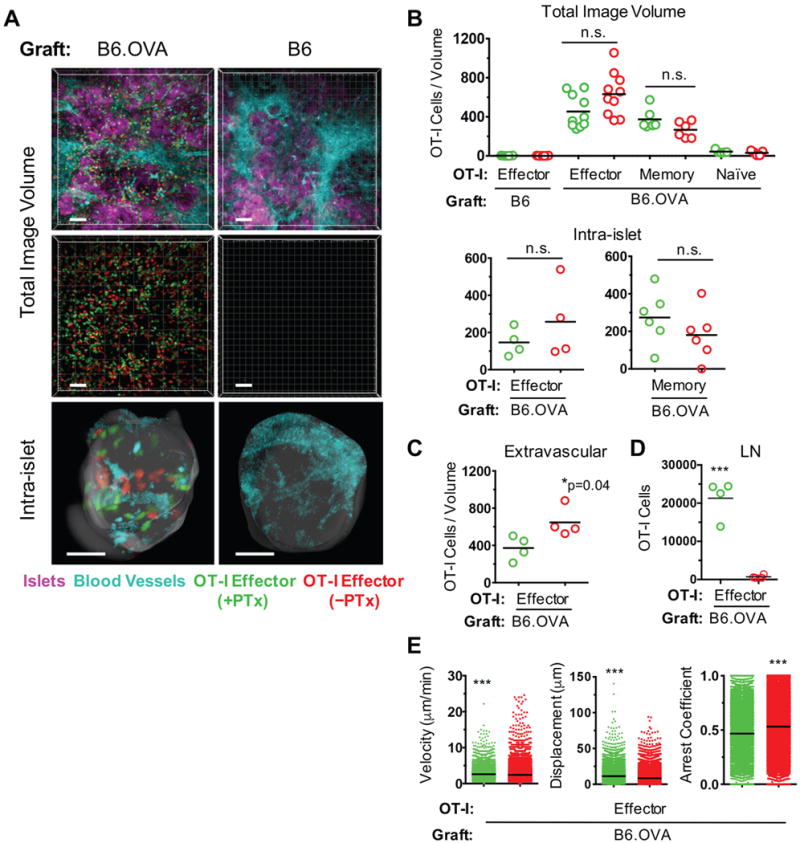
B6 Act-OVA transgenic (B6.OVA) or non-transgenic B6 islets were transplanted under the kidney capsule of diabetic B6 recipients. PTx-treated (green) and untreated (red) OT-I effector, naïve, or memory T cells were co-transferred 9 – 10 days after transplantation and imaged by two-photon intravital microscopy 16 – 24 hr later. All OT-I cells were from B6 OT1.RAG−/− donors. (A) Representative, volume-rendered images depicting PTx-treated and untreated OT-I effectors present in either the total or intra-islet image volumes. Blood vessel and islet channels were subtracted from middle panels to better visualize infiltrating T cells. Scale bar = 50 μm. (B) Enumeration of OT-I cells detected in total and intra-islet image volumes. Each data point represents number of cells in one movie (image volume). For OT-I effector migration, 5 mice in the B6.OVA group and 3 mice in the B6 group were imaged. For analysis of naïve and memory OT-I migration, 2 mice were imaged in each group. (C) Enumeration of extravascular OT-I effectors in the total image volume. (D) Enumeration of OT-I effectors in lymph nodes of recipient mice. (E) Motility parameters of OT-I effectors in total image volume. *p<0.05; ***p<0.0001; n.s. = not significant.
Cognate Ag presentation by donor graft cells is necessary for CD8+ effector T cell migration
Since neo-vessels that form in the islet graft are of both donor and recipient origin (14), and because the graft is populated by recipient DC soon after transplantation, we investigated which graft cells, the donor’s or the recipient’s, are responsible for presenting cognate non-self Ag to effector T cells. We analyzed the migration of PTx-treated OT-I effector cells to B6.OVA islet grafts in which donor cells, recipient cells, or both lacked the MHC class I molecule H-2Kb required for presenting the SIINFEKL OVA peptide to OT-I cells. Cell transfer and imaging was performed at the same time points as the prior experiments. As expected, OT-I cells were not detected in B6.OVA.H-2Kb−/− islets transplanted to B6.H-2Kb−/− mice as cognate Ag presentation was incapacitated in both donor and recipient cells (Fig. 2). When donor cells alone were H2-Kb sufficient (H-2Kb+/+ B6.OVA islets transplanted to B6.H-2Kb−/− mice), OT-I cell migration was restored to that observed in H-2Kb+/+ B6.OVA islets transplanted to H-2Kb+/+ B6 mice (Fig. 2). In contrast, transplanting B6.OVA.H-2Kb−/− islets to H-2Kb+/+ B6 mice, such that only recipient cells expressed H-2Kb, did not restore OT-I cell migration (Fig. 2). Therefore, cognate Ag presentation by donor graft cells is necessary for the migration of effector T cells to islet grafts.
FIGURE 2. Cognate Ag presentation by donor graft cells is necessary for CD8+ effector T cell migration.
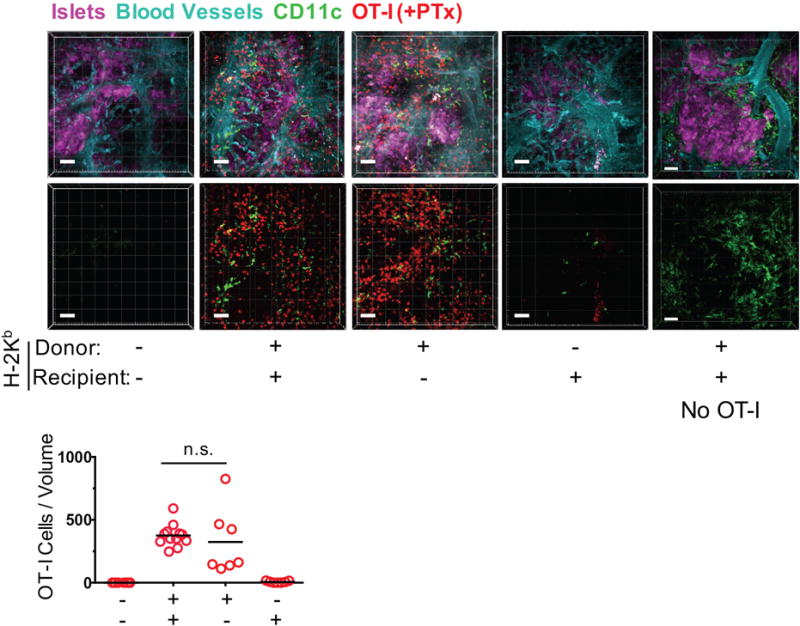
Migration of PTx-treated OT-I effector T cells to B6.OVA islet grafts (in which recipient cells (N = 5 mice, 7 movies), donor cells (N = 3 mice, 7 movies), or both (N = 2 mice, 8 movies) lacked the H-2Kb MHC class I molecule required for presenting OVA peptide to OT-I cells) was imaged and enumerated by two-photon intravital microscopy as described in Fig. 1, except that recipients also carried the YFP transgene under control of the CD11c promoter to visualize recipient-derived DC in the graft. Grafts in which both donor and recipient cells expressed H-2Kb served as positive controls (N = 4 mice, 12 movies). Additional grafts in which both donor and recipient cells expressed H-2Kb but no OT-I cells were transferred were also imaged (far right panels; N = 2 mice, 10 movies). Representative volume-rendered images are shown. Blood vessel and islet channels were subtracted from bottom panels to better visualize infiltrating T cells. n.s. = not significant. Scale bar = 50 μm.
Since recipient mice in these experiments carried the YFP gene knocked into the CD11c locus (cD11c-YFP), we were also able to visualize recipient YFP+ cells, comprised of CD11c+ DC and macrophages, that populated the islet grafts. As shown in the micrographs in Fig. 2, YFP+ cells, depicted in green color, were present namely in grafts heavily infiltrated with OT-I cells, suggesting that ongoing rejection drives CD11c+ cell accumulation. YFP+ cells were also present in H-2Kb+/+ B6.OVA grafts transplanted to H-2Kb+/+ B6 recipients that did not receive OT-I cells (Fig. 2, far right panels), indicating that the endogenous immune response to the OVA Ag was sufficient for driving the accumulation of CD11c+ cells. The paucity of CD11c+ cells in H-2Kb+/+ B6 recipients of H-2Kb−/− B6.OVA suggests that Ag presentation by donor cells is also required for the endogenous immune response.
SKAP1 deficiency prolongs allograft survival in CTLA-4-Ig-treated recipients but does not interfere with CD8+ effector T cell migration
SKAP1 is an adaptor protein expressed predominantly in T cells where it plays a key role in the TCR-mediated inside-out signaling pathway that leads to integrin activation (10). Since the integrins VLA-4 and LFA-1 play a central role in the migration of effector T cells to transplanted organs (9, 18), we tested the hypothesis that SKAP1 is important for Ag-driven effector T cell entry into islet grafts. We first investigated whether genetic deficiency of SKAP1 in recipient mice delays allograft rejection. We found that B6 SKAP1−/− mice rejected BALB/c islet allografts at the same rate as B6 WT recipients, but that the administration of a suboptimal immunosuppressive regimen (CTLA-4-Ig × 2 doses) significantly prolonged allograft survival in SKAP1−/− but not WT recipients (Fig. 3A). Similarly, we found that B6 SKAP1−/− recipients that received SKAP1−/− OT-I cells rejected B6.OVA islet grafts significantly slower than B6 WT recipients that received SKAP1+/+ OT-I cells (Fig. 3B). Second, we co-transferred PTx-treated SKAP1+/+ and SKAP1−/− effector OT-I cells to B6 recipients of B6.OVA islet grafts to investigate whether SKAP1 deficiency affects Ag-dependent effector T cell migration. As shown in Fig. 3C, we observed comparable numbers of SKAP1+/+ and SKAP1−/− OT-I cells in the grafts. Comparable numbers were also found in the recipients’ spleens, indicating equal transfer of SKAP1+/+ and SKAP1−/− OT-I cells (data not shown). Analogous migration results were obtained using shRNA to knock down SKAP1 expression in OT-I effector cells (data not shown). Motility analysis of OT-I effectors that migrated to the graft showed a slight but significant reduction in median velocity and displacement of SKAP1−/− cells leading to a higher arrest coefficient (Fig. 3D). Therefore, SKAP1 does not play an essential role in effector T cell migration to islet grafts but contributes to allograft rejection, likely via a different mechanism.
FIGURE 3. SKAP1 deficiency delays allograft rejection but does not influence CD8+ effector T cell migration.
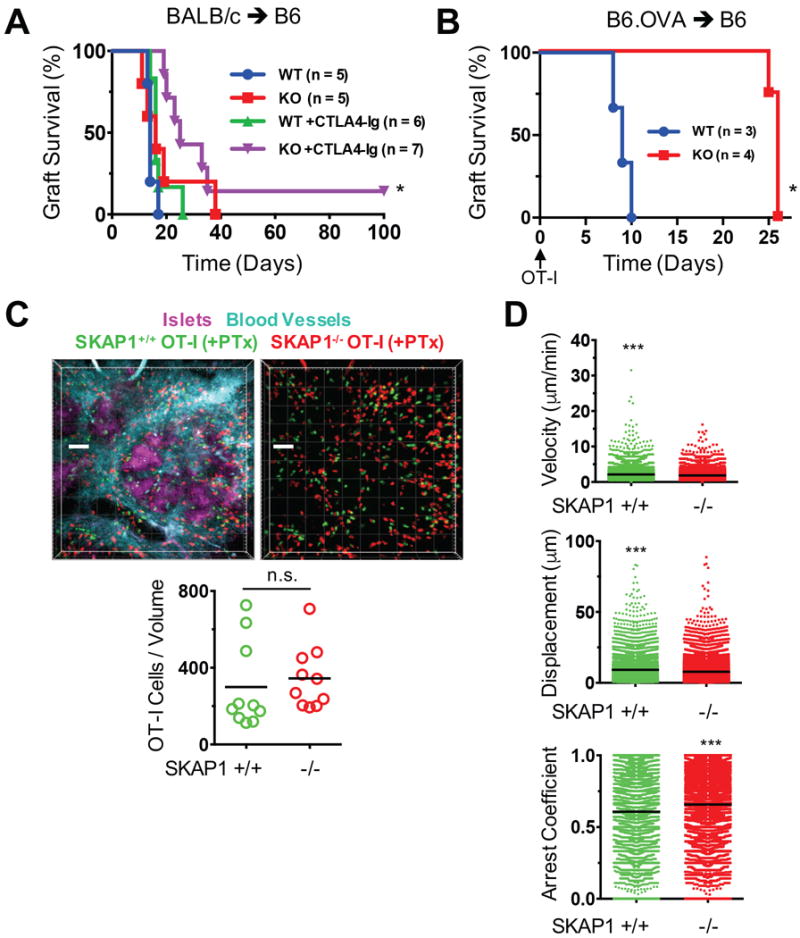
(A) BALB/c islet allograft survival in wildtype (WT) or SKAP1−/− (KO) B6 recipients that were either untreated or received limited immunosuppression immediately after transplantation (CTLA-4-Ig 200 μg on days 0 and 2). (B) B6.OVA islet graft survival in WT or KO B6 recipients that were not immunosuppressed but received 5 × 106 naïve SKAP1+/+ or SKAP1−/− OT-I cells, respectively, immediately after transplantation. (C) B6.OVA islet grafts were transplanted to B6 mice and the migration of co-transferred, PTx-treated SKAP1+/+ and SKAP1−/− OT-I effector T cells to the grafts was imaged and enumerated by two-photon intravital microscopy as described in Fig. 1 (N = 3 mice, 10 movies). In these experiments, SKAP1−/− OT-I cells transferred were from B6 OT-I.SKAP1−/−.RAG+/− donors; however, the transfer of B6 SKAP1−/−. RAG−/− OT-I effectors yielded the same results (n = 2 mice, 4 movies; data not shown). Representative, volume-rendered images are shown. Blood vessel and islet channels were subtracted from right panel to better visualize infiltrating T cells. Scale bar = 50 μm. (C) Motility parameters of SKAP1+/+ and SKAP1−/− OT-I cells detected in the total image volume. *p<0.05 compared to each of the other groups; ***p<0.0001; n.s. = not significant.
Discussion
We provide in this manuscript direct evidence that the migration of CD8+ effector T cells to islet grafts is dependent on cognate Ag and not chemokines. Signaling via Gαi-coupled receptors, the principal pathway of chemokine attraction and activation of T cells (19), enhanced transvascular migration and increased effector T cell arrest in the graft, but these contributions were relatively minor and unessential. Therefore, the Ag-dependent paradigm of effector T cell migration applies to cellular grafts that are neo-vascularized after transplantation. This finding has important relevance to clinical transplantation as it underscores the need to target Ag-TCR interactions, rather than chemokines and chemokine receptors, to achieve significant interruption of T cell entry into islet grafts.
The observation that Ag presentation by donor cells is sufficient for T cell migration suggests that indirect Ag presentation by recipient-derived cells, whether endothelial cells that line the neo-vessels or host dendritic cells and macrophages that infiltrate the graft, may not be adequate for driving the migration process. Instead, the data suggest that donor-derived H-2Kb-OVA peptide complexes, either on graft cells or potentially on cross-dressed host-derived DCs, are detected directly by OT-I cells, leading to their migration. These possibilities warrant further investigation.
Discovering the TCR signaling pathways that are key to effector T cell migration should shed light on novel potential ways to block graft rejection. Strategies that target integrins on T cells, for example antibodies to LFA-1 or VLA-4, have proven effective but unsafe in the clinic because they interfere with the immune surveillance functions of memory T cells leading to the reactivation of latent infections (21, 22). Targeting molecules downstream of the TCR on the other hand could potentially be more specific, singling out only those effector or memory cells that are engaging Ag at the time. In this manuscript, we tested the hypothesis that SKAP1 is essential for Ag-dependent T cell migration because it preferentially mediates TCR-triggered rather than chemokine receptor-induced integrin activation (23). Its involvement was particularly evident in responses to low receptor occupancy or lower affinity Ag such that high levels of TCR ligation could overcome the need for the adaptor (24). We found that SKAP1 was dispensable in our model. One explanation is that the strong avidity by which OVA-specific T cells in our model, and possibly alloreactive T cells in general, engage Ag leads to alternate signaling that bypasses the need for SKAP1. Therefore, prolongation of graft survival in partially immunosuppressed recipients in our study, although potentially an important therapeutic lead, is likely mediated by other actions of SKAP1 - for example, its role in naive T cell activation (24). The ability of CTLA-4-Ig to cooperate with the absence of SKAP1 in prolonging graph survival might involve other mechanisms. In this regard, SKAP1 possesses a PH domain that mediates binding to membranes in cells, an event influenced by CD28 (25), which is a target of CTLA-4-Ig.
Although based on studies of TCR-transgenic CD8+ T cells, our findings likely apply to the migration of CD4+ as well as polyclonal effector T cells. Unanue and colleagues demonstrated that PTx does not inhibit the entry of self-reactive CD4+ T cell into native pancreatic islets (8), and we have previously shown that the same is true for the migration of polyclonal CD4 and CD8 effector or memory T cells to solid organ allografts (9). Importantly, polyclonal effector T cells cause rapid graft rejection in the absence of Gαi-coupled receptor signaling (9), further underscoring the primacy of non-chemokine pathways in the homing of effector T cells to allografts.
Acknowledgments
We wish to thank Dr. Jiyun Kim and the staff of the two photon intravital microscopy core at the Starzl Transplantation Institute and Dr. Simon Watkins and the staff of the Center for Biologic Imaging at the University of Pittsburgh School of Medicine for their technical support.
Footnotes
This work was supported by NIH grant AI049466 (to FGL) and the Frank & Athena Sarris Chair in Transplantation Biology (to FGL).
References
- 1.Ricordi C, Strom TB. Clinical islet transplantation: advances and immunological challenges. Nat Rev Immunol. 2004;4:259–268. doi: 10.1038/nri1332. [DOI] [PubMed] [Google Scholar]
- 2.Pericin M, Althage A, Freigang S, Hengartner H, Rolland E, Dupraz P, Thorens B, Aebischer P, Zinkernagel RM. Allogeneic beta-islet cells correct diabetes and resist immune rejection. Proc Natl Acad Sci U S A. 2002;99:8203–8206. doi: 10.1073/pnas.122241299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L, Han R, Lee I, Hancock AS, Xiong G, Gunn MD, Hancock WW. Permanent survival of fully MHC-mismatched islet allografts by targeting a single chemokine receptor pathway. J Immunol. 2005;175:6311–6318. doi: 10.4049/jimmunol.175.10.6311. [DOI] [PubMed] [Google Scholar]
- 4.Makhlouf L, Yamada A, Ito T, Abdi R, Ansari MJ, Khuong CQ, Winn HJ, Auchincloss H, Jr, Sayegh MH. Allorecognition and effector pathways of islet allograft rejection in normal versus nonobese diabetic mice. J Am Soc Nephrol. 2003;14:2168–2175. doi: 10.1097/01.asn.0000079041.15707.a9. [DOI] [PubMed] [Google Scholar]
- 5.Sleater M, Diamond AS, Gill RG. Islet allograft rejection by contact-dependent CD8+ T cells: perforin and FasL play alternate but obligatory roles. Am J Transplant. 2007;7:1927–1933. doi: 10.1111/j.1600-6143.2007.01889.x. [DOI] [PubMed] [Google Scholar]
- 6.Abdi R, Means TK, Luster AD. Chemokines in islet allograft rejection. Diabetes Metab Res Rev. 2003;19:186–190. doi: 10.1002/dmrr.362. [DOI] [PubMed] [Google Scholar]
- 7.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 8.Calderon B, Carrero JA, Miller MJ, Unanue ER. Cellular and molecular events in the localization of diabetogenic T cells to islets of Langerhans. Proc Natl Acad Sci U S A. 2011;108:1561–1566. doi: 10.1073/pnas.1018973108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walch JM, Zeng Q, Li Q, Oberbarnscheidt MH, Hoffman RA, Wiliams AL, Rothstein DM, Shlomchik WD, Kim JV, Camirand G, Lakkis FG. Cognate antigen directs CD8+ T cell migration to vascularized transplants. J Clin Invest. 2013;123:2663–2671. doi: 10.1172/JCI66722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dustin ML, Bromley SK, Kan Z, Peterson DA, Unanue ER. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc Natl Acad Sci USA. 1997;94:3909–3913. doi: 10.1073/pnas.94.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raab M, Wang H, Lu Y, Smith X, Wu Z, Strebhardt K, Ladbury JE, Rudd CE. T cell receptor “inside-out” pathway via signaling module SKAP1-RapL regulates T cell motility and interactions in lymph nodes. Immunity. 2010;32:541–556. doi: 10.1016/j.immuni.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jo EK, Wang H, Rudd CE. An essential role for SKAP-55 in LFA-1 clustering on T cells that cannot be substituted by SKAP-55R. J Exp Med. 2005;201:1733–1739. doi: 10.1084/jem.20042577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Liu H, Lu Y, Lovatt M, Wei B, Rudd CE. Functional defects of SKAP-55-deficient T cells identify a regulatory role for the adaptor in LFA-1 adhesion. Mol Cell Biol. 2007;27:6863–6875. doi: 10.1128/MCB.00556-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brissova M, Fowler M, Wiebe P, Shostak A, Shiota M, Radhika A, Lin PC, Gannon M, Powers AC. Intraislet endothelial cells contribute to revascularization of transplanted pancreatic islets. Diabetes. 2004;53:1318–1325. doi: 10.2337/diabetes.53.5.1318. [DOI] [PubMed] [Google Scholar]
- 15.Bertera S, Balamurugan AN, Bottino R, He J, Trucco M. Increased yield and improved transplantation outcome of mouse islets with bovine serum albumin. J Transplant. 2012;2012 doi: 10.1155/2012/856386. 856386 (article ID) (published online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camirand G, Li Q, Demetris AJ, Watkins SC, Shlomchik WD, Rothstein DM, Lakkis FG. Multiphoton intravital microscopy of the transplanted mouse kidney. Am J Transplant. 2011;11:2067–2074. doi: 10.1111/j.1600-6143.2011.03671.x. [DOI] [PubMed] [Google Scholar]
- 18.Kitchens WH, Haridas D, Wagener ME, Song M, Kirk AD, Larsen CP, Ford ML. Integrin antagonists prevent costimulatory blockade-resistant transplant rejection by CD8+ memory T cells. Am J Transplant. 2012;12:69–80. doi: 10.1111/j.1600-6143.2011.03762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luther SA, Cyster JG. Chemokines as regulators of T cell differentiation. Nat Immunol. 2001;2:102–107. doi: 10.1038/84205. [DOI] [PubMed] [Google Scholar]
- 20.Grau V, Herbst B, Steiniger B. Dynamics of monocytes/macrophages and T lymphocytes in acutely rejecting rat renal allografts. Cell Tissue Res. 1998;291:117–126. doi: 10.1007/s004410050985. [DOI] [PubMed] [Google Scholar]
- 21.Berger JR, Koralnik IJ. Progressive multifocal leukoencephalopathy and natalizumab–unforeseen consequences. N Engl J Med. 2005;353:414–416. doi: 10.1056/NEJMe058122. [DOI] [PubMed] [Google Scholar]
- 22.Lowe MC, Badell IR, Turner AP, Thompson PW, Leopardi FV, Strobert EA, Larsen CP, Kirk AD. Belatacept and sirolimus prolong nonhuman primate islet allograft survival: adverse consequences of concomitant alefacept therapy. Am J Transplant. 2013;13:312–319. doi: 10.1111/j.1600-6143.2012.04341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Lu Y, Rudd CE. SKAP1 is dispensable for chemokine-induced migration of primary T-cells. Immunol Lett. 2010;128:148–153. doi: 10.1016/j.imlet.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Moon EY, Azouz A, Wu X, Smith A, Schneider H, Hogg N, Rudd CE. SKAP-55 regulates integrin adhesion and formation of T cell-APC conjugates. Nat Immunol. 2003;4:366–374. doi: 10.1038/ni913. [DOI] [PubMed] [Google Scholar]
- 25.Raab M, Smith X, Matthess Y, Strebhardt K, Rudd CE. SKAP1 protein PH domain determines RapL membrane localization and Rap1 protein complex formation for T cell receptor (TCR) activation of LFA-1. J Biol Chem. 2011;286:29663–29670. doi: 10.1074/jbc.M111.222661. [DOI] [PMC free article] [PubMed] [Google Scholar]


