Abstract
Yeast vacuole fusion requires the activation of cis-SNARE complexes through priming carried out by Sec18p/NSF and Sec17p/α-SNAP. The association of Sec18p with vacuolar cis-SNAREs is regulated in part by phosphatidic acid (PA) phosphatase production of diacylglycerol (DAG). Inhibition of PA phosphatase activity blocks the transfer of membrane associated Sec18p to SNAREs. Thus, we hypothesized that Sec18p associates with PA-rich membrane microdomains before transferring to cis-SNARE complexes upon PA phosphatase activity. Here we examined the direct binding of Sec18p to liposomes containing PA or DAG. We found that Sec18p preferentially bound to liposomes containing PA compared to those containing DAG by approximately five-fold. Additionally, using a specific PA-binding domain blocked Sec18p binding to PA-liposomes and displaced endogenous Sec18p from isolated vacuoles. Moreover, the direct addition of excess PA blocked the priming activity on isolated vacuoles in a manner similar to chemically inhibiting PA phosphatase activity. These data suggest that the conversion of PA to DAG facilitates the recruitment of Sec18p to cis-SNAREs. Purified vacuoles from yeast lacking the PA phosphatase Pah1p showed reduced Sec18p association with cis-SNAREs and complementation with plasmid-encoded PAH1 or recombinant Pah1p restored the interaction. Taken together this demonstrates that regulating PA concentrations by Pah1p activity controls SNARE priming by Sec18p.
Keywords: Phosphatidic acid, Diacylglycerol, Lipin, Pah1p, SNARE, Fusion, Sec17, Priming
Synopsis
In this study we found that the yeast NSF orthologue Sec18p resides in an inactive pool bound to phosphatidic acid on the vacuolar/lysosomal membrane. The association of Sec18p with phosphatidic acid is disrupted by vacuolar phosphatidic acid phosphatase activity leading to the transfer of Sec18p to cis-SNARE complexes for the priming reaction. In the presence of excess phosphatidic acid or the lack of phosphatidic acid phosphatase activity results in the sequestration of Sec18p away from cis-SNARE complexes.
Introduction
Eukaryotic membrane fusion is a process vital to cellular homeostasis. Trafficking of transport vesicles among organelles and the plasma membrane is governed by of a series of events that is highly conserved in all eukaryotes from yeast to humans (1). The proteins that carry out this process are diverse, but all fusion machinery ultimately facilitates a series of membrane contact, fusion, and compartment mixing events (2). We use vacuoles from Saccharomyces cerevisiae as a model to examine the regulation of the eukaryotic fusion machinery.
Yeast homotypic vacuole fusion has been well described and is characterized by a series of experimentally defined stages. The fusion cascade begins with the priming stage in which the AAA+ ATPase Sec18p (NSF) and its co-chaperone Sec17p (α-SNAP) disassemble inactive cis-SNARE complexes. Priming requires ATP hydrolysis by Sec18p and results in the disassociation of Sec17p and the soluble SNARE Vam7p from the membrane (3, 4). During the tethering/docking stage, the Rab GTPase Ypt7p and its effector complex HOPS interact to bring two vacuolar compartments together (5, 6). Vam7p rebinds the vacuolar membrane through interactions with HOPS and the regulatory lipid phosphatidylinositol 3-phosphate (PI3P) leading to the formation of trans-SNARE pairing between apposing membranes (3, 7). At this stage the two membranes are closely associated and are enriched in regulatory proteins and lipids at the periphery of their interface named the “vertex ring” microdomain (8–10). The fusion cascade proceeds through the formation of a hemifusion intermediate before pore formation and luminal content mixing occur (2, 11).
Maintenance of SNARE proteins is crucial for turnover of eukaryotic membrane fusion events. Upon fusion and compartment mixing, SNAREs exist in a stable cis-SNARE complex in which all SNAREs reside on a single membrane rendering them unable to carry out subsequent rounds of docking. During priming, homohexameric Sec18p/NSF associates with cis-SNARE complexes through binding to Sec17p/SNAPs and generates the necessary force to disassociate inactive complexes into active individual SNAREs (4). Recent evidence suggests that NSF disrupts cis-SNARE complexes through a “loaded-spring” mechanism (12, 13). In this model, NSF ATPase activity leads to large conformational changes in the NSF N-terminal and D1 domains, which exert enough force on SNAPs to disassemble the SNAREs. Direct regulation of this Sec18p/NSF priming activity remains mostly unknown, although protein kinase C has been implicated in negative regulation of NSF association with cis-SNARE complexes (14). Of interest, NSF has been found to bind phosphatidic acid (PA), a regulatory lipid required for vacuole fusion; however, the effects of Sec18p/NSF lipid association are still not well understood (15–17).
Regulatory phospholipids on the cytosolic face of the vacuolar membrane serve as key regulators of the fusion machinery. A presence of phosphatidylcholine (PC), phosphatidylethanolamine (PE), PA, diacylglycerol (DAG), and PI3P is essential for vacuolar fusion to occur and serve as the minimal lipid requirements of the fusion reaction (16). Additional lipids including PI4P, PI(4,5)P2 and ergosterol are also required for optimal homotypic vacuolar fusion (8, 18–20). Specific lipid head groups provide a scaffold for the binding and recruitment of proteins that actively carry out the stages of fusion. This function relies on direct protein-lipid interactions between the membrane and protein machinery as exemplified by the binding of PI3P by the Vam7p PX domain (21). Specific lipids also affect the fusion machinery physically through changes to membrane dynamics. This role is demonstrated by the dependence of SNARE function on lipid microdomain formation and potential allosteric effects exerted on the transmembrane domains of SNARE proteins (8, 22). Reversible changes to the concentration of specific lipids within microdomains also mediate protein-lipid interactions at the vertex ring and dynamics of vacuole associated actin.
The interconversion of regulatory lipids is catalyzed by a variety of lipid modifying proteins. These enzymes respond to varied growth conditions through coordination of genetic and biochemical mechanisms. The yeast PA phosphatase Pah1p plays an important role in the regulation of lipid synthesis. Pah1p has been shown to have a role in mediation of the transcription of the lipid synthesis genes INO1, INO2 and OPI3 through its phosphatase activity (23, 24). This is critical for organelle replication for daughter cells during mitosis as the nuclear envelope and endoplasmic reticulum must expand. Unphosphorylated Pah1p generates DAG, which is involved in the synthesis of triacylglycerol, PC, and PE (25, 26). Recently, it was shown that Pah1p localizes to the nuclear vacuole junction at the diauxic shift during acute glucose starvation (27).
A number of lipid modifying enzymes have been implicated in vacuole fusion or fission events and display abnormal morphologies or other observable defects when deleted or mutated. Both PA and DAG are essential lipids for vacuole fusion and have been implicated in organelle transport and fusion activities. For instance DAG plays a role in Golgi to ER transport and the formation of COPI vesicles (28), where as PA is important for sporulation and stimulating the fusion activity of the SNARE Spo20p (29, 30). In addition, the PA phosphatase activity of Pah1p is necessary for endolysosomal maturation and vacuole homotypic fusion (17). Deletion of PAH1 leads to the fragmentation of immature vacuoles and severely abrogates fusion activity. This phenotype is proposed to come from two defects involving the fusion machinery. First, vacuoles harvested from pah1Δ yeast contain strikingly reduced levels of factors involved in regulating vacuole fusion including the PI-3 kinase Vps34p and its product PI3P, Ypt7p and its guanine exchange factor Mon1p-Ccz1p, as well as the HOPS subunit Vps39p (17, 31). Second, deletion of PAH1 or inhibition of PA phosphatase activity led to a measurable decrease in priming and SNARE-associated Sec18p. Importantly, neither PAH1 deletion nor chemical inhibition of PA phosphatase activity alters the total levels of Sec18p associated with vacuoles. Thus, we posit that inactive (i.e. SNARE-free) Sec18p remains associated with vacuoles through interactions with PA and that modification of PA releases Sec18p to cis-SNAREs to enable priming. In this study, we examined the interactions of Sec18p and PA in the regulation of priming. We report that like its mammalian orthologue NSF, Sec18p specifically binds to PA. Additionally, both increased levels of PA and the absence of Pah1p on the vacuole inhibited Sec18p-dependent priming and prevent recruitment of Sec18p to cis-SNARE complexes.
RESULTS
Sec18p binds preferentially to membranes containing phosphatidic acid
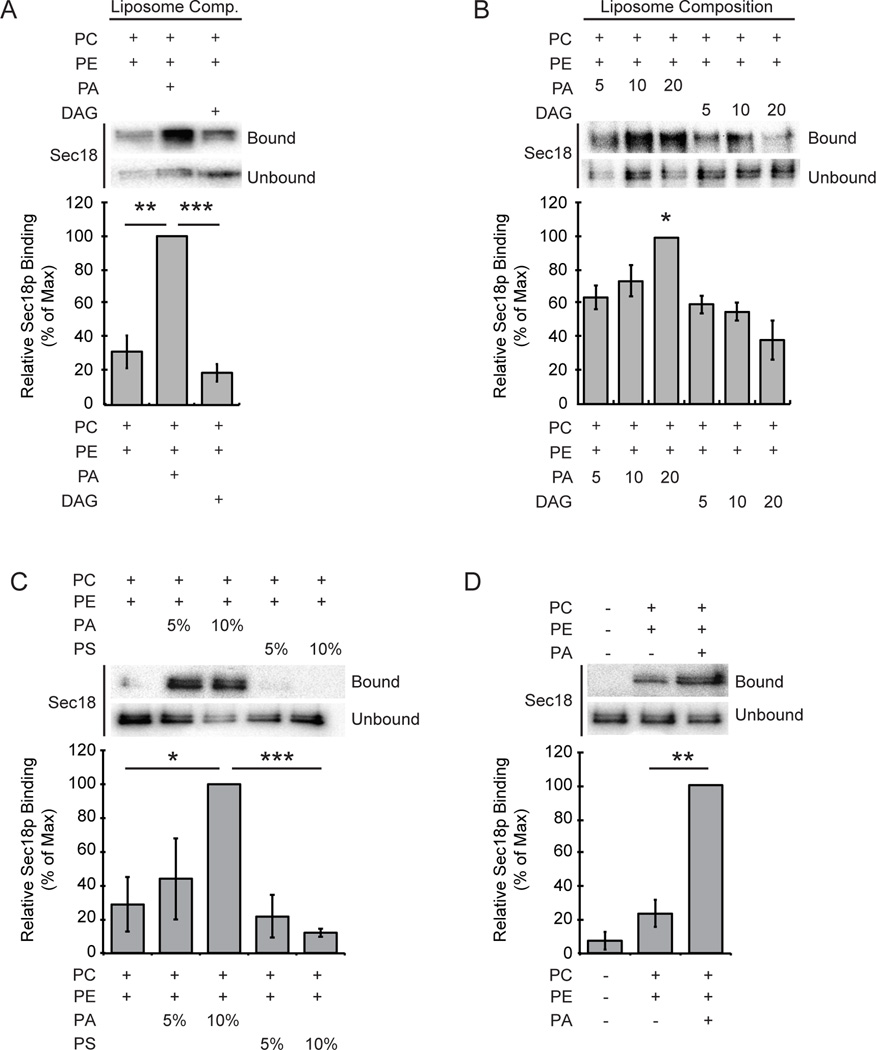
The yeast PA phosphatase Pah1p regulates endolysosomal maturation leading to impaired homotypic vacuolar fusion (17). Inhibition of PA phosphatase activity with the small molecule propranolol suggested that phosphatidic acid potentially had a specific role in mediating the priming activity of the SNARE chaperone. Treatment with propranolol blocked the binding of Sec18p to cis-SNARE complexes, while the total levels of vacuole associated Sec18p remained constant. Importantly, vacuoles from pah1Δ cells harbor wild type levels of Sec18p. Thus, we hypothesized that the conversion of PA to DAG led to a transition of Sec18p from a PA bound state to a cis-SNARE bound state. The Sec18p mammalian orthologue NSF was previously shown to specifically bind resin-conjugated PA, but the purpose of this binding remained unknown (15). Here we asked if Sec18p also binds PA, a relationship that could be controlled by PA phosphatase activity. To test this we used a liposome-binding assay to detect Sec18p binding to membranes of different lipid compositions. Recombinant Sec18p was incubated with liposomes containing mixtures of the lipids PC, PE, PA, and DAG. Liposome-bound Sec18p was separated from unbound protein by floatation. Sec18p showed light binding to PC:PE liposomes alone, however this binding was significantly weaker compared to liposomes containing PA (Figure 1A). Liposomes containing DAG also showed little Sec18p binding relative to liposomes containing PA. This stark difference in binding between PA and DAG containing membranes is consistent with a requirement for Pah1p activity in regulating Sec18p recruitment from the membrane to cis-SNARE complexes. Next, we tested to see if the concentration of PA or DAG specifically had an effect on Sec18p binding to liposomes. Similar liposome floatation assays were carried out but included liposomes of increasing PA or DAG concentrations. Membrane-bound Sec18p was present at higher levels in liposomes that contained elevated levels of PA (Figure 1B). Strikingly, an increase in the concentration of DAG in the liposomes had a significant negative effect on the level of Sec18p membrane association (Figure 1B). These data suggest that Sec18p binds preferentially to membranes containing PA and that the presence of DAG may promote the release of Sec18p from binding to membrane lipids directly. Taken together, this suggests that the conversion of PA to DAG may affect Sec18p membrane localization dynamics and Pah1p may specifically play a role in controlling the state Sec18p membrane lipid association.
Figure 1. Sec18p preferentially binds to liposomes containing phosphatidic acid.
Recombinant His6-Sec18p (2 µg) was incubated with liposomes of the indicated compositions for 10 min at 30°C. Liposomes were isolated by centrifugation and washed with 1X PBS before bound proteins were resolved by SDS-PAGE. Bar graphs show average normalized densitometry values measured for 3 separate experiments. Binding was observed in liposomes with PA or DAG (A), with increasing concentrations of PA or DAG (B), with increasing concentrations of PA or PS (C), and with no liposomes (D). * P<0.05; ** P<0.001; *** P<0.0001
We next tested whether the specificity of Sec18p binding to membranes extends beyond a nonspecific electrostatic interaction. The cytosolic face of the yeast vacuolar membrane contains a wide array of regulatory anionic phospholipids (32). Because of this we asked if Sec18p would show preferential binding to PA or if it would also bind to other vacuolar membrane lipids carrying a negative charge. We performed liposome-binding assays with liposomes that contained PS, an anionic phospholipid that, like PA, localizes to the outer leaflet of the yeast vacuolar membrane. We again observed preferential Sec18p binding to liposomes of increasing PA concentration (Figure 1C). Sec18p binding to liposomes containing PS showed no significant binding suggesting that Sec18p lipid binding has specificity for PA and not anionic lipids in general. It is also important to note that the absence of liposomes in our binding assays gave no signal and detection of liposome-associated Sec18p was non-random (Figure 1D). These results highlight a specific binding interaction between Sec18p and PA that is likely present at the yeast vacuolar membrane.
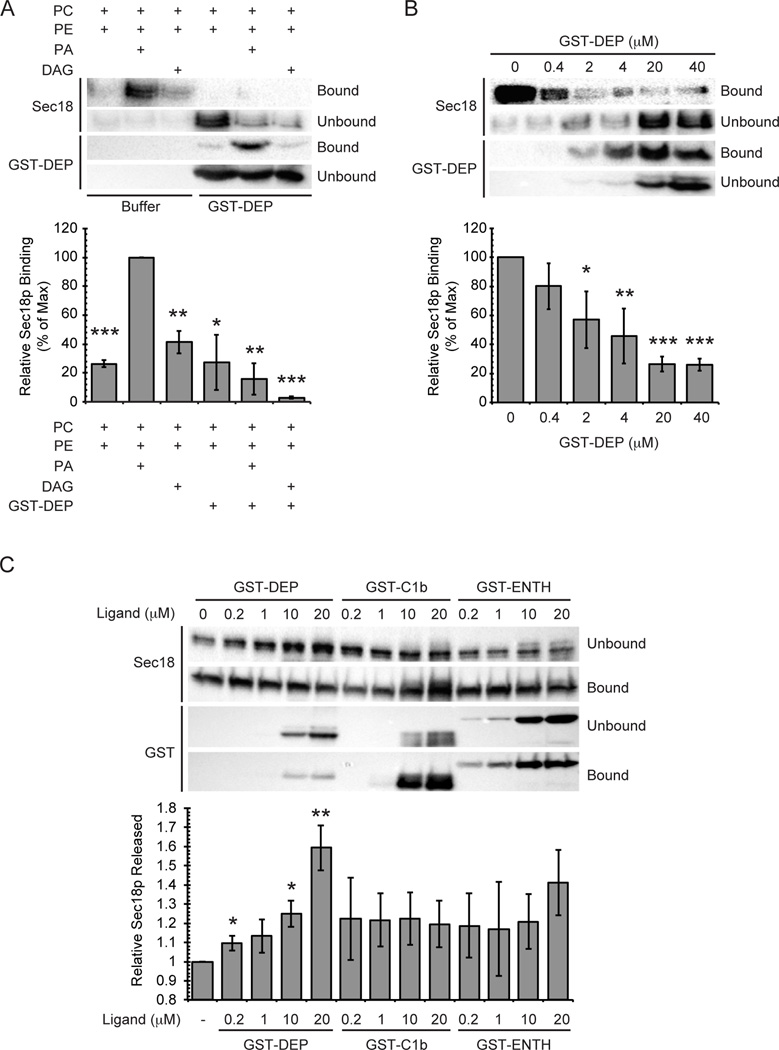
To further probe Sec18p binding to specific membrane lipids, we performed binding competition assays in the presence of the PA-specific binding domain GST-DEP from the mouse protein Dvl2 (33). The addition of GST-DEP was expected to disrupt Sec18p binding to liposomes containing PA by occupying potential binding sites on the membrane. The addition of GST-DEP showed no significant effect on the level of Sec18p bound to PC:PE liposomes, but severely affected the ability of Sec18p to bind liposomes containing PA (Figure 2A, P<0.005). This suggests that the observed membrane binding of Sec18p was specifically dependent on the presence of PA. Surprisingly, the addition of GST-DEP also lowered the level of Sec18p binding to membranes containing DAG (P<0.05). We suspect that GST-DEP has some non-negligible amount of binding to DAG as many characterized PA-binding domains contain essential hydrophobic residues that embed in membranes (34). The absence of a large headgroup on DAG could allow for GST-DEP to sit very close to the acyl chain region within the bilayer promoting hydrophobic residue interactions with DAG or adjacent lipids. Considering this and the significant increase in binding to membranes containing PA when compared to those containing DAG, the data suggest that Sec18p membrane lipid binding is dependent on the presence of PA. To further characterize this interaction, we performed a binding competition assay that utilized PA-containing liposomes and the addition of increasing concentrations of GST-DEP. In the absence of competitor, significant binding of Sec18p to the liposomes was observed, and this binding was perturbed by the addition of GST-DEP in a dose-dependent manner (Figure 2B). These data further indicate that Sec18p has specific binding to PA.
Figure 2. The PA-binding domain DEP reduces Sec18p binding to membranes.
(A) Liposomes of the indicated compositions were incubated without Dvl2-DEP or with 20 µM Dvl2-DEP for 5 min at 30°C. Recombinant His6-Sec18p (2 µg) was added to the sample and incubated for 10 min at 30°C. (B) GST-DEP titration and Sec18p binding to liposomes was performed as described above in the presence of the indicated concentrations of GST-DEP. (C) Vacuoles were harvested from wild type DKY6281 and incubated with GST-DEP, GST-C1b domain, or GST-ENTH domain. Bound and unbound fractions were separated by centrifugation and resolved by SDS PAGE. Bar graphs show the percentage of unbound Sec18p in each sample normalized to wild type control. * P<0.05; ** P<0.001; *** P<0.0001.
To probe for specific Sec18p-lipid interactions on vacuole membranes, we used a binding competition assay that contained purified vacuoles in place of liposomes. Vacuoles were harvested from yeast and incubated on ice with the lipid-specific binding domains GST-DEP, GST-C1b (protein kinase C βII), and GST-ENTH (rat epsin 1) to bind to PA, DAG, and PI(4,5)P2 respectively and displace vacuolar proteins bound to each (35, 36). C1b and ENTH have been previously shown to cause the release of HOPS and Vam7p from vacuoles (8, 37). Samples were separated into bound and unbound fractions by centrifugation and Sec18p levels were assayed by Western blot. The percentage of Sec18p in the unbound fraction significantly increased with the addition of GST-DEP in a dose dependent manner when compared to the control similar to what was seen in the liposome-binding assays described above (Figure 2C). Addition of GST-C1b showed a slight increase in the proportion of unbound Sec18p from the untreated control condition however this release was independent of the concentration added and was statistically insignificant. Addition of GST-ENTH led to an increase of Sec18p release at the highest concentration used; however, the amount of released Sec18p was not statistically significant. Taken together, these data suggest that disruption of Sec18p binding to membrane lipids is most affected when PA is specifically occupied. This further supports the notion that Sec18p binding to vacuolar membranes occurs in a PA dependent manner. From this experiment we cannot rule out potential interactions between Sec18p and vacuolar DAG or PI(4,5)P2. However, considering the lack of dose independent effect using GST-C1b along with our previous observations, the direct binding of Sec18p to DAG seems unlikely. Sec18p interactions with PI(4,5)P2 are an interesting possibility that were not further investigated. However, it is not difficult to envision Sec18p having specific interactions with multiple regulatory phospholipids at different points of regulation or perhaps even during a single binding event. It is important to note that ENTH and C1b disrupt vertex microdomain organization (8), thus the limited release of Sec18p caused by binding PI(4,5)P2 and DAG here could be due to the redistribution of PA on vacuoles.
Phosphatidic acid inhibits vacuolar fusion during the priming stage
Sec18p, like its mammalian orthologue NSF, performs the necessary priming activity within the membrane fusion cascade. During this priming step, Sec18p hydrolyzes ATP to disassemble inactive cis-SNARE complexes and reactivate them for additional rounds of SNARE-pairing and fusion. Sec18p priming activity is necessary for membrane fusion to occur within the cell, and its importance to organelle trafficking and maintenance is highlighted by the fact that its null mutant strains are inviable. Because its function is vital for fusion activity and cell survival, we believe Sec18p is a target for a key regulation point within the vacuolar fusion cascade. This combined with the PA-specific binding previously seen and the regulatory role of Pah1p has in membrane fusion led us to investigate whether the lipids PA and DAG had an effect on Sec18p activity and vacuolar fusion.
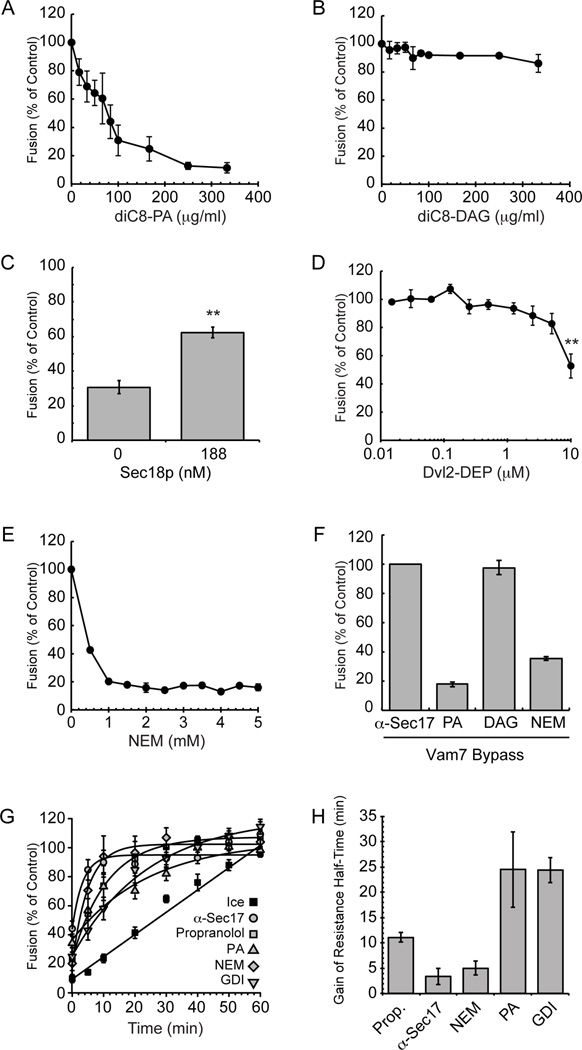
To measure vacuolar fusion we employed the well-characterized proPho8p maturation vacuole content mixing assay as previously described (38). To test the effects of specific lipids on fusion, we used dioctanoyl-PA (diC8-PA) and diC8-DAG (39, 40). We first assayed if the addition of diC8-PA or diC8-DAG showed a significant effect on overall vacuolar fusion activity. Addition of diC8-PA led to potent inhibition of vacuolar content mixing in a dose-dependent manner (Figure 3A), whereas adding the same concentrations of diC8-DAG did not affect fusion (Figure 3B). Importantly, inhibition by diC8-PA could be reversed by the addition of recombinant Sec18p suggesting a direct interaction between the protein and lipid (Figure 3C). We then wanted to test if sequestering PA with the PA-specific binding domain GST-DEP would have a measureable effect on fusion activity. Addition GST-DEP had a minimal effect on fusion except at the highest concentration tested (10 µM), which reduced fusion by approximately 50% (Figure 3D). Higher concentrations were not used due to buffer interference with the fusion assay. The inhibition of fusion by GST-DEP is in keeping with the vacuole competition binding assay in Figure 2C where GST-DEP at ≥10 µM significantly displaced Sec18p from the membrane. We reason that fusion inhibition caused by addition of GST-DEP may occur at higher concentrations because of a decrease of the overall Sec18p level on the membrane to below a threshold required for priming activity to occur. Alternatively, this inhibition may be due to general crowding on the membrane and not from an inaccessibility of PA to the fusion machinery. Taken together the data suggest that increased concentrations of PA within the vacuolar membrane have an inhibitory effect on membrane fusion activities but increased concentrations of DAG do not.
Figure 3. Addition of exogenous phosphatidic acid or N-ethylmaleimide causes a severe membrane fusion defect.
Vacuoles were harvested from wild type BJ3505 and DKY6281 and tested for fusion by luminal mixing and proPho8p maturation. Fusion reactions containing 3 µg of each vacuole type were incubated in the presence of diC8-PA (A), or diC8-DAG (B) at the indicated concentrations. (C) Fusion reactions containing 100 µg/ml diC8-PA were incubated in the presence of 188 nM recombinant Sec18p. Fusion reactions containing 3 µg of each vacuole type were incubated in the presence of GST-DEP (D) or NEM (E) at the indicated concentrations. Fusion results were normalized to untreated wild type vacuoles at standard conditions. (F) Exogenous recombinant Vam7p (200 nM) was used to bypass fusion inhibitors. (G) Gain of resistance kinetics assays were performed in the presence of 140 µg/ml α-Sec17p IgG, 2 mM propranolol, 300 µM diC8-PA, 1 mM NEM, or PS buffer. Data was fit using first-order exponential decay with weights and errors. (H) Calculated half-times from first-order exponential decay fit. ** P<0.001.
Previous work suggested that Pah1p activity regulates Sec18p priming activity through its control of PA concentrations in the vacuolar membrane (17). Because we observed Sec18p-PA binding and the inhibition of fusion by excess PA, we expected that this lipid could be potentially blocking the priming stage of the fusion cascade. To test this, we wanted to use an inhibitor that would block Sec18p activity for comparison. NEM is a known inhibitor of NSF priming activity however its effects on Sec18p-mediated priming activity have not been fully investigated. Because NEM is known to alkylate NSF we reasoned that a similar modification could take place with Sec18p and perturb priming activity. We found that NEM potently inhibited fusion at ≥1 mM suggesting that NEM might modify and inhibit Sec18p (Figure 3E). To test whether diC8-PA, dic8-DAG, or NEM had priming specific inhibition we employed a variant of the content mixing assay that utilizes a Vam7p bypass of priming. Previous work has shown that priming-specific inhibition with an inhibitor such as α-Sec17p IgG can be rescued by the addition of recombinant Vam7p, a soluble SNARE protein (41, 42). We expected that a priming-specific defect caused by addition of diC8-PA or NEM should also be rescued in this manner. Surprisingly, a Vam7p bypass did not rescue fusion activity in the presence of diC8-PA or NEM (Figure 3F). These data suggest that diC8-PA and NEM could additionally be affecting stages downstream of priming in the fusion cascade. It should be noted that Vam7p function is related to the presence of the ABC transporter Ycf1p that contains multiple free Cys required for its activity (43, 44). Thus, alkylating Ycf1p could prevent Vam7p bypass of the effect of NEM on Sec18p. To probe whether diC8-PA or NEM could be inhibiting a later fusion stage we performed a temporal acquisition of resistance assay in which inhibitors were added at different time points throughout the fusion reaction (4, 45). Here fusion reactions gain resistance to an inhibitor once enough time had passed for its target to carry out its relevant activity. Therefore, fusion would recover at earlier time points for inhibitors that specifically target machinery early in the fusion reaction (e.g. α-Sec17p IgG) while inhibitors targeting later stages would gain resistance later in the reaction (e.g. GDI). We compared diC8-PA inhibition kinetics to that of α-Sec17p IgG, propranolol, NEM, and GDI through calculated gain of resistance half-times using first-order exponential decay fitting (Figure 3G) (4, 17). Interestingly, diC8-PA showed similar recovery of fusion to that of the tethering stage inhibitor GDI (Figure 3H). This suggests that PA could be inhibiting fusion during a stage downstream of priming, independent of Sec18p, however we cannot rule out that diC8-PA may be blocking additional rounds of fusion. It is important to note that these results can only point to the last stage of fusion that is targeted by an inhibitor and that PA may be regulating multiple fusion stages including priming. NEM showed early recovery of fusion similar to that of α-Sec17p IgG and propranolol suggesting that it likely has an inhibitory effect on priming activity.
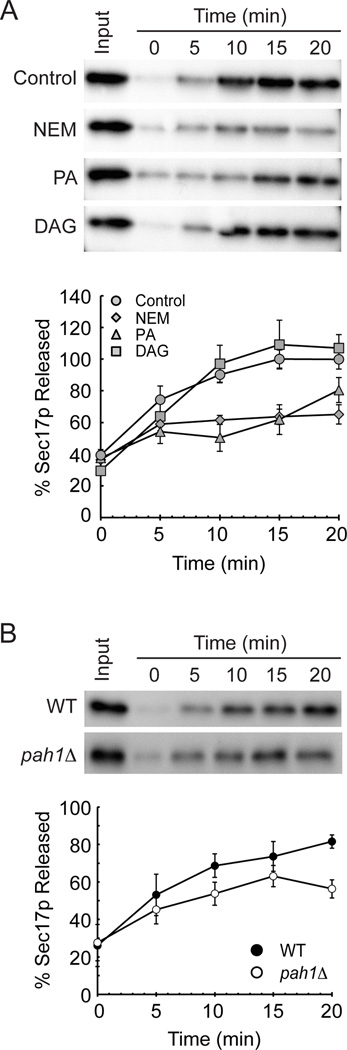
Next we determined if increased levels of PA had a noticeable effect on Sec18p activity during the priming stage. To do this we examined the release of Sec17p upon Sec18p-mediated priming (4). Sec17p associates with the membrane through binding to cis-SNARE complexes, which recruits Sec18p to stimulate its priming activity. Upon recruitment Sec18p hydrolyzes ATP to disassemble the cis-SNAREs leading to Sec17p dissociation from the membrane. Here we monitored Sec17p release by fractionating soluble and membrane-bound proteins followed by Western blotting. Vacuolar fusion assays were carried out in the presence of diC8-PA, diC8-DAG, or NEM after which we monitored the release of Sec17p from vacuolar membranes as a measure of priming. We found that NEM potently reduced Sec17p release relative to the untreated control condition, suggesting that Sec18p activity was blocked by NEM in a similar fashion to what is seen with NSF (Figure 4A). When diC8-PA was tested, we found that Sec17p release was severely inhibited. This is in keeping with the notion that diC8-PA inhibits priming by sequestering Sec18p. In contrast, diC8-DAG had no effect as predicted based on the content mixing assays. A previous study showed that NEM did not have an effect on Sec18p ATPase activity, however priming activity was not specifically examined (46). It is possible that Sec18p alkylation by NEM may affect its ability to disassemble cis-SNARE complexes in a manner independent of its ATPase activity. It is clear that in the presence of the full fusion machinery Sec18p priming activity is inhibited by NEM. We then tested Sec18p activity on pah1Δ deletion vacuoles using the same priming assay to further examine the effect of increased PA on priming. As expected, vacuoles lacking pah1Δ showed significantly decreased levels of Sec17p release when compared to the wild type control (Figure 4B). Taken together, these data illustrate that priming activity is blocked by an increase in PA at the vacuole and that this inhibition is specific.
Figure 4. Addition of exogenous phosphatidic acid but not diacylglycerol inhibits the priming activity of Sec18p.
(A) Vacuoles from BJ3505 yeast were assayed for priming activity as detected by the release of Sec17p from the membrane fraction. Fusion reactions of 3 µg of vacuoles were incubated in the presence of buffer, 1 mM NEM, 300 µM diC8-PA, or 300 µM diC8-DAG. Vacuoles were pelleted by centrifugation at the indicated times and proteins in the supernatant fraction were resolved by SDS-PAGE and imaged by Western blot. Densitometry values were normalized against input sample for each condition. Graphs show the normalized averages (n=3). (B) Vacuoles from BJ3505 and pah1Δ yeast were assayed for priming activity as described in (A). Graph shows the normalized averages (n=3).
Phosphatidic acid regulates Sec18p recruitment to cis-SNARE complexes
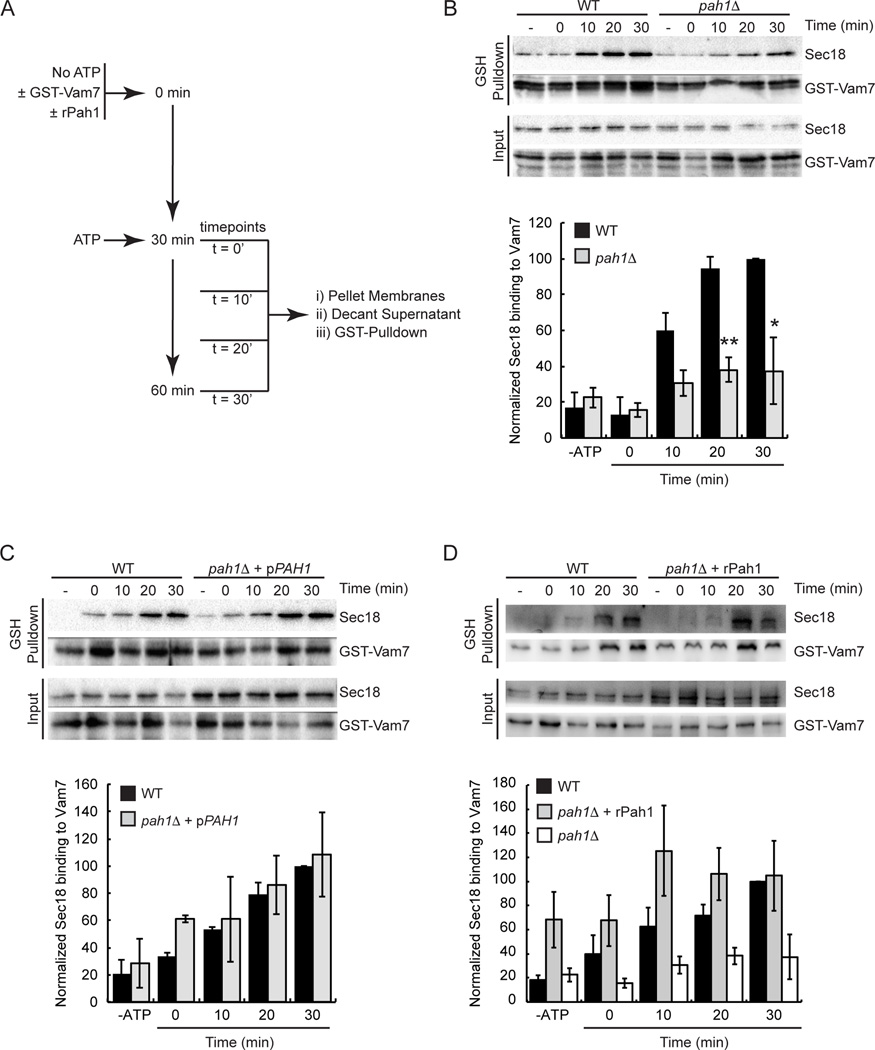
Thus far we have found that cis-SNARE priming by Sec18p is blocked by the presence of increased PA while unaffected by the presence of increased DAG. This relationship supports the idea that the conversion of PA to DAG through PA phosphatase activity may have a regulatory role in the priming stage of fusion. Previous work showed that the PA phosphatase Pah1p had a regulatory role in endolysosomal maturation and was also proposed to regulate the localization of Sec18p on the membrane (17). Sec18p has been shown to stay associated with the membrane throughout priming while its co-chaperone Sec17p is released (4). This suggests that Sec18p stays attached to the membrane independently of Sec17p and SNAREs. Given our previous observations we identified PA as an alternate Sec18p membrane-binding site and asked if shifting dynamic membrane PA and DAG levels would also affect the association of Sec18p to SNAREs. To address this question, we utilized cis-SNARE pulldown assays in which recombinant GST-tagged Vam7p was introduced to fusion reactions in the absence of ATP to allow the formation of cis-SNARE complexes with free cognate SNAREs (47). It was previously shown that all vacuole-associated Vam7p is bound in cis-SNARE complexes suggesting SNARE complexes formed were appropriate Sec18p priming targets (48). The fusion reactions were then started with the addition of ATP and pulldowns of the GST-Vam7p containing cis-SNARE complexes were performed across various time points. The level of Sec18p associated with the newly formed cis-SNARE complexes was analyzed by Western blot. To further test the effect of altering the PA:DAG ratio on priming we harvested vacuoles from pah1Δ strains to remove PA phosphatase activity relevant to fusion. The recruitment of Sec18p to cis-SNARE complexes was severely inhibited on pah1Δ vacuoles compared to wild type vacuoles across at each time point tested (Figure 5A-B). To confirm that the effect on Sec18p was only due to the lack of Pah1p, we used vacuoles from pah1Δ yeast complemented with plasmid-encoded PAH1 (17). Complementation fully reversed Sec18p recruitment to cis-SNARE complexes (Figure 5C). Addition of recombinant Pah1p also fully reversed the recruitment defect seen on pah1Δ vacuoles (Figure 5D). Taken together these data suggest that PA levels in the membrane play a significant role in the regulation of Sec18p recruitment to cis-SNARE complexes for priming.
Figure 5. Vacuoles from a pah1Δ deletion strain have a decrease in the level of Sec18p bound to cis-SNARE complexes.
Vacuoles were harvested from BJ3505, RFY17 (BJ3505 pah1Δ), and RFY19 (BJ3505 pah1Δ, pPAH1) strains and assayed for Sec18p binding to cis-SNARE complexes. (A) Recombinant GST-Vam7p was added to fusion reactions and incubated at 27°C for 30 min in the absence of ATP to allow formation of cis-SNARE complexes containing GST-Vam7p. Next, ATP regenerating system was added and the samples were incubated at 27°C for the indicated times before vacuoles were isolated by centrifugation and solubilized. Glutathione Sepharose was used to pull down GST-Vam7p and attached proteins were resolved by SDS-PAGE and imaged by Western blot. (B) WT versus pah1Δ vacuoles. (C) WT versus pah1Δ + pPAH1 vacuoles. (D) WT versus pah1Δ vacuoles + 200 µg/ml rPah1p. Densitometry values for Sec18p pull down were normalized against the corresponding pull down Vam7p value. Graph shows the normalized average ratios (n=3). The white bars represent the pah1Δ pulldown data from panel B. This is to facilitate visualization of the effect of adding recombinant Pah1. * P<0.05; ** P<0.001.
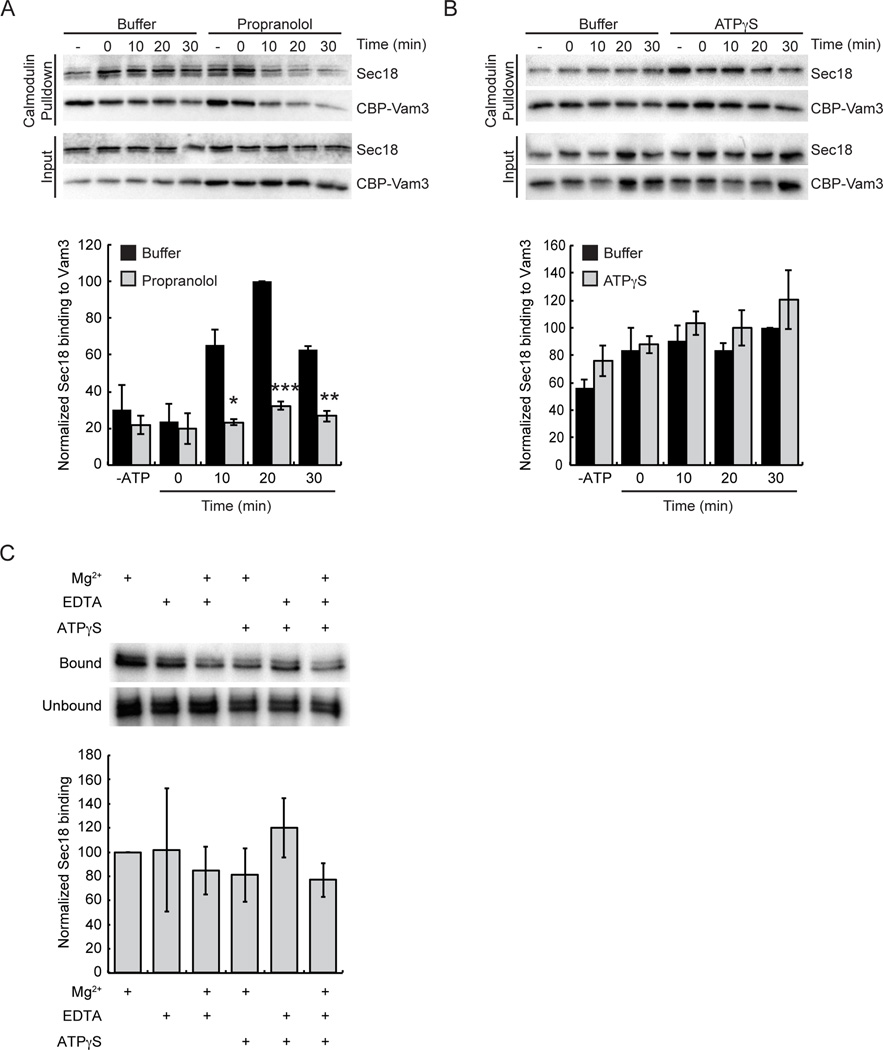
To further examine the potential role PA could have in Sec18p recruitment to cis-SNAREs we employed a similar pulldown approach but included the addition of the small molecule inhibitor propranolol. Here we reasoned that the addition of propranolol, a known inhibitor of PA phosphatase activity, should lead to similar Sec18p-cis-SNARE association levels as our pah1Δ vacuoles. Because propranolol inhibition of vacuolar fusion can be rescued by the addition of exogenous Vam7p we instead carried out pulldowns using vacuoles that harbor the syntaxin orthologue Vam3p containing an internal calmodulin-binding domain (CBP-Vam3p) (49). As expected, inhibition of Pah1p activity by the addition of propranolol significantly decreased the levels of Sec18p associated with cis-SNARE complexes relative to control levels (Figure 6A). This is consistent with the effect seen in pah1Δ vacuoles and further supports a role for PA in the regulation of SNARE priming.
Figure 6. Vacuoles treated with the Pah1p inhibitor propranolol have a decreased level of Sec18p bound to cis-SNARE complexes.
Vacuoles were harvested from BJ3505 yeast expressing CBP-Vam3p and assayed for Sec18p binding to cis-SNARE complexes. Fusion reactions were incubated at 27°C for the indicated times in the presence of propranolol or ATPγS or fusion buffer. At the indicated times, vacuoles were isolated by centrifugation and solubilized. Calmodulin agarose was used to pull down CBP-Vam3p and attached proteins were resolved by SDS-PAGE and imaged by immunoblotting. Fusion reactions were treated with PS buffer, 2 mM propranolol (A) or 1 mM ATPγS (B). (C) Liposome binding and effect of the nucleotide-binding state of Sec18p was tested using liposomes containing 10% PA. His6-Sec18p (2 µg) was incubated with PA liposomes in binding buffer alone or in the presence of 1 mM Mg2+, 1 mM EDTA, or 1 mM ATPγS. Densitometry values for Sec18p pull down were normalized against the corresponding pull down Vam7p value. Graph shows the normalized average ratios (n=3). * P<0.05; ** P<0.001; *** P<0.0001.
A previous study showed that mammalian NSF bound PA in a nucleotide-dependent fashion wherein the ADP bound state of the protein had greater binding to PA than the ATP bound state (15). We asked if the nucleotide-bound state might also play a role in Sec18p binding to PA and ultimately its recruitment to cis-SNARE complexes. We reasoned that if ATP is replaced with the slowly hydrolyzable analog ATPγS, then PA binding by Sec18p could be lessened and a larger pool would be available to bind to cis-SNARE complexes. Sec18p is known to bind to the cis-SNAREs in an ATP-bound state so we expected levels associated with cis-SNARE complexes to be at wild type levels or greater. Using either our GST-Vam7p (not shown) or CBP-Vam3p pulldown assays, we tested the effect of ATPγS on Sec18p recruitment to cis-SNARE complexes. Sec18p association to SNAREs was similar to control conditions with ATP (Figure 6B). We next tested Sec18p interactions with PA directly using a liposome floatation assay in the presence of Mg2+, EDTA, or ATPγS to assay the dependence of the nucleotide-bound state (Figure 6C). We observed no significant difference between the nucleotide states tested suggesting that unlike NSF, binding of Sec18p to PA was independent of such effects.
Phosphatidic acid blocks the recruitment of Sec18p to cis-SNARE complexes
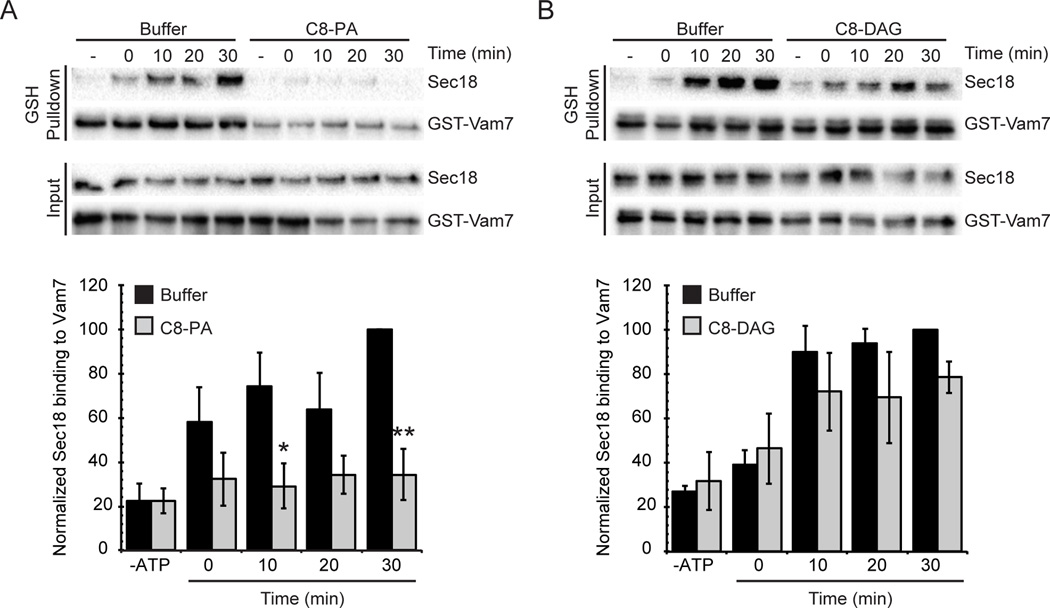
We next wanted to determine if Pah1p could regulate Sec18p association with cis-SNAREs through its maintenance of membrane PA and DAG levels. The absence of Pah1p from the membrane likely increased the level of PA present, allowing the lipid to bind to Sec18p and sequester it from cis-SNAREs ultimately stalling its priming activity. To test this, we utilized the GST-Vam7p pulldowns of cis-SNAREs as mentioned above, in the presence of inhibitory concentrations of diC8-PA or the equivalent concentration of diC8-DAG. Addition of diC8-PA abolished Sec18p association with cis-SNARE complexes when compared to the untreated vacuoles (Figure 7A). The total level of membrane-bound Sec18p was not affected. Addition of the same concentration of diC8-DAG did not significantly affect the level of Sec18p associated to cis-SNARE complexes compared to the control (Figure 7B). These findings support the idea that Pah1p could regulate Sec18p recruitment to cis-SNARE complexes through its maintenance of PA and DAG levels on the membrane. By controlling the dynamic levels of PA and DAG present in the vacuolar membrane, Pah1p could regulate the priming stage by affecting the localization of Sec18p.
Figure 7. Vacuoles treated with diC8-PA have a decrease in the level of Sec18p bound to cis-SNARE complexes.
Vacuoles were harvested from BJ3505 and assayed for Sec18p binding to cis-SNARE complexes. Recombinant GST-Vam7p was added to fusion reactions and incubated at 27°C for 30 min in the absence of ATP to allow formation of cis-SNARE complexes containing GST-Vam7p. Next, ATP regenerating system was added and the samples were incubated at 27°C for the indicated times before vacuoles were isolated by centrifugation and solubilized. Glutathione Sepharose was used to pull down GST-Vam7p and protein complexes were resolved by SDS-PAGE and examined by Western blot. Fusion reactions were treated with PS buffer, 300 µM diC8-PA (A) or 300 µM diC8-DAG (B). Densitometry values for Sec18p pull down were normalized against the corresponding pull down Vam7p value. Graph shows the normalized average ratios (n=3). * P<0.05; ** P<0.001.
Discussion
The protein machinery involved in the regulation of membrane fusion has been well characterized, however the regulatory effects of phospholipids on these proteins are still not well understood. The dynamic changes that these lipids undergo throughout the fusion cascade are only beginning to be explored. Yeast homotypic vacuole fusion utilizes a set of regulatory lipids that helps mediate each stage in a series of defined events. Lipids likely fulfill multiple roles during membrane fusion events including controlling the physical properties of the membrane, and providing a scaffold for the protein fusion machinery to bind, and organization of proteins into microdomains where fusion occurs. These lipids are currently known to include ergosterol, phosphoinositides, DAG, and PA, which are maintained and modified by a diverse group of kinases, phosphatases, and lipases.
PA has a regulatory role in multiple fusion pathways including Glut4 trafficking, mitochondrial fusion, and prospore membrane fusion (30, 50, 51). Additionally, PA has been identified as one of the minimum lipid requirements required for SNARE-dependent fusion of reconstituted proteoliposomes (RPLs) and promotes the association of Sec18p and HOPS with vacuolar SNARE RPLs (16). However, it remains uncertain whether PA directly interacts with these proteins or if it participates in establishing a conducive environment for optimal protein function. PA is converted to DAG during the fusion cascade and inhibition of PA phosphatase activity blocks fusion at the priming stage. The importance of DAG in vacuole fusion is demonstrated in part by the effect of the DAG-binding domain C1b, which disrupts the organization of the vertex microdomain resulting in the potent inhibition of fusion (8, 52). DAG is a fusogenic lipid due to its negative curvature and ability to disrupt lipid bilayers (53, 54). These features also allow DAG to trigger the fusion of protein-free liposomes (55, 56). While the potential importance of PA and DAG to vacuole fusion has been highlighted, no specific regulatory mechanisms have been described. Previously, it was found that the yeast PA phosphatase Pah1p has a significant regulatory role in the vacuolar fusion cascade suggesting that the balance of PA and DAG at the site of fusion is critical in mediating fusion related events (17, 31).
Our earlier studies suggested that Pah1p regulated fusion in part by triggering the priming stage of the fusion cascade (17). We theorized that prior to engaging cis-SNARE complexes Sec18p resided as part of a resting pool of protein bound to the membrane in a PA-dependent manner. Pah1p activity would convert PA to DAG leading to the release of Sec18p from the membrane facilitating its recruitment to cis-SNARE bundles bound to Sec17p. In keeping with this model this study showed that deletion of PAH1 or chemical inactivation of its phosphatase activity led to the retention of Sec18p bound to the PA enriched membrane and blocking its translocation to Sec17p-bound cis-SNAREs. This is further bolstered by our findings that Sec18p binds preferentially to liposome membranes that contain PA. This interaction is specific as replacement of PA with other acidic or small head group lipids abolishes Sec18p binding. PA is a unique phospholipid in that it is both anionic and promotes negative membrane curvature, a property favorable for protein insertion at the head group region of the membrane bilayer (57). It is likely that a Sec18p lipid-binding site would interact with the vacuolar membrane in a way dependent on both electrostatic forces and curvature effects, however we did not detect significant binding of the protein to membranes that contained DAG, another lipid that promotes negative curvature. Considering this and the finding that Sec18p does not bind other anionic phospholipids we expect that the lipid association we observed is specific for PA. These results parallel previous work that showed the Sec18p mammalian orthologue NSF is also a PA binding protein (15). While no sequence orthologue has been described for PA binding regions, most that have been described contain a combination of adjacent basic and hydrophobic amino acids (57). The basic residues in PA binding domains are able to preferentially bind the lipid phosphomonoester head group through hydrogen bonding as described by the electrostatic-hydrogen bond switch model (34). The presence of hydrophobic residues often allows for PA binding proteins to insert into the membrane and interact with adjacent lipid acyl chains (58). Based on our data, we propose that Sec18p association with PA requires electrostatic interactions between a lipid-binding region on the protein and the phosphomonoester head group of PA. This binding is likely enhanced by surrounding hydrophobic interactions but these interactions alone are not strong enough to support the entire binding event. We propose that upon Pah1p-dependent conversion of PA to DAG, crucial electrostatic interactions between Sec18p and PA are interrupted and Sec18p is released from the membrane.
Specific lipids have been shown to be of crucial importance in regulating the different stages of membrane fusion (3, 8, 17, 18, 22, 31, 37, 52). In this study, we show that PA has an inhibitory effect during the priming stage of fusion while DAG does not. Previous studies have shown that addition of ergosterol ligands or antibody against PI(4,5)P2 also potently inhibit Sec18p priming activity (19, 20). Interestingly, mammalian NSF does not bind PI(4,5)P2 alone (15). It is possible that Sec18p and NSF have distinct lipid interactions, however it is also important to consider the roles of both ergosterol and PI(4,5)P2 at the site of fusion. Each of these lipids is a requirement for functional microdomain formation at the vertex ring (8). Disruption of these microdomains with exogenous ligands may compromise the organization of necessary protein and lipid machinery at the site of fusion ultimately resulting in a priming defect. We have demonstrated direct physical interaction between Sec18p and PA, and we believe this association accounts for PA-specific priming inhibition.
There are two primary ways that we considered PA could inhibit the priming activity of Sec18p directly. First, PA could decrease the ability of Sec18p to disassociate cis-SNARE complexes through allosteric effects. Alternatively, PA could act to sequester Sec18p away from cis-SNARE complexes effectively stalling priming. In this study, we provide evidence in accord with the latter possibility. We observed a decrease in recruitment of Sec18p to cis-SNAREs on vacuoles from a pah1Δ deletion strain, as well as by inhibition of PA phosphatase activity with propranolol, and upon addition of exogenous PA. Taken together, we propose that dynamic PA and DAG levels mediate Sec18p localization on the vacuolar membrane and recruitment to cis-SNARE complexes.
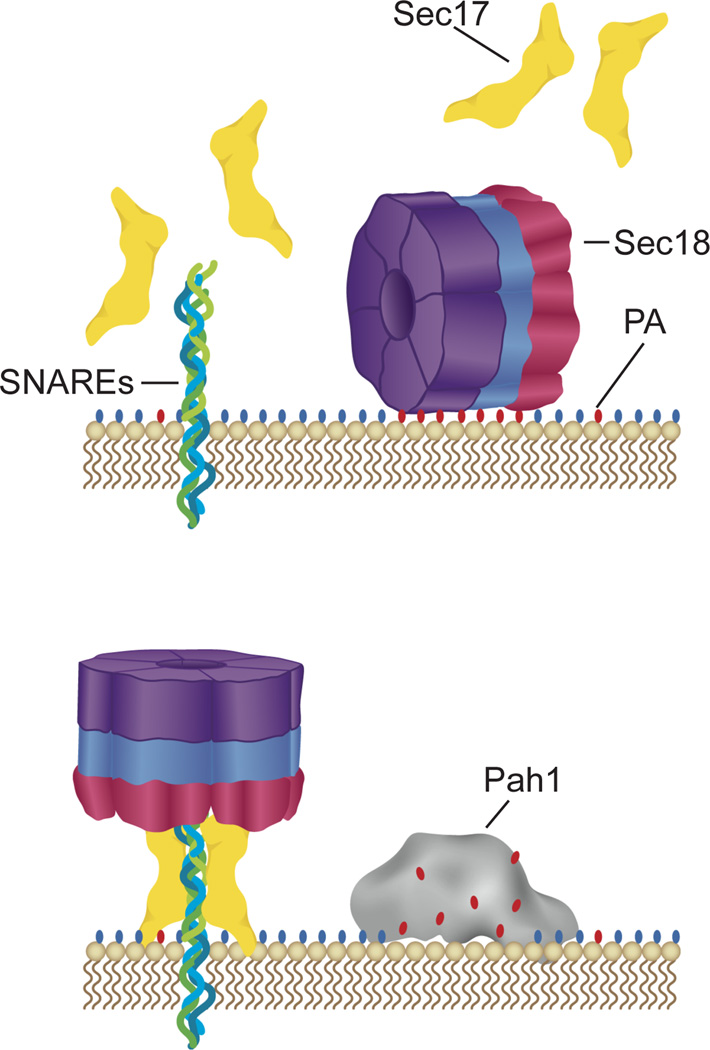
The stark contrast in effects that PA and DAG have on priming activity frame Pah1p as a potential initiator of the fusion cascade. Reactivation of SNAREs and turnover of fusion may be controlled by Pah1p through its modulation of local PA concentrations at the vertex ring. We propose a model in which Sec18p is initially recruited to the vacuolar membrane through binding to organized PA pools (Figure 8). Once recruited, Sec18p remains bound to PA pools where it is unable to access SNAREs during non-priming stages of fusion. Upon completion of compartment fusion and generation of cis-SNAREs, Pah1p converts pools of PA to DAG effectively lowering local PA concentrations. Sec18p is freed from the membrane and is able to be recruited to cis-SNARE complexes for priming. It is possible that conversion of PA to other regulatory phospholipids through a CDP-DAG intermediate may also serve as an initiator of priming. Under this model, PA could serve as a global regulator of Sec18p localization in the cell. However, previous work has shown that Sec18p levels at the vacuole are not increased in pah1Δ cells (17). It is possible that there is an upper limit to PA dependent membrane association of Sec18p, or there may be compensatory enzymatic activity of other lipid modifiers such as Phospholipase D. Further work is needed to investigate these possibilities and probe organelle specificity of Sec18p regulation by PA.
Figure 8. Working model of Sec18p regulation by PA.
Sec18p associates with vacuole membranes through direct interactions with PA. Upon PA hydrolysis by Pah1p, Sec18p is no longer sequestered on the membrane away from cis-SNAREs and is recruited to them to carry out priming activity. Red ovals depict PA’s phosphate headgroup. Blue ovals depict generic lipid head groups.
While the PA binding region of Sec18p/NSF is not clear, the potential effects of this lipid interaction are interesting to consider. The role of each of the three domains in Sec18p/NSF has been previously described, and binding of any of these to PA could have inhibitory effects on its overall function. The nucleotide binding domains of Sec18p/NSF (D1 and D2) are responsible for ATPase activity and formation of a homohexamer respectively (59, 60). It is possible that PA binds to the D1 domain and disrupts the ability of Sec18p to hydrolyze ATP making it unable to generate the necessary force for cis-SNARE complex disruption. It is also possible that PA binds to the D2 domain and blocks the formation of the active Sec18p homohexamer. This could force Sec18p into a monomeric state preventing it from effectively associating with cis-SNARE complexes. The N-terminal domain of Sec18p is responsible for association with Sec17p and cis-SNARE complexes (61). Binding of the N-terminal domain to PA could block the basic groove needed for Sec17p binding preventing recruitment of Sec18p to cis-SNARE complexes. This potential association of the N-terminal domain to PA is consistent with our observations and model, however additional work is necessary to identify the specific PA binding region of Sec18p to understand the mechanism of this regulation.
It is clear that phosphatidic acid can modulate membrane fusion through its control of Sec18p priming activity at the vacuole. Additional work is needed to identify the PA-binding region on Sec18p. Also of interest will be determining the upstream regulation of PA content on the vacuole. PA is produced from DAG by Dgk1p, from PC by Spo14p, and from PE by PE-PLD making all of these enzymes potential regulators of SNARE priming (62–64). Sec18p regulation by phosphatidic acid provides another example of the highly organized interplay between protein and lipid machinery that helps to carry out membrane fusion and trafficking events.
Materials and Methods
Reagents
All reagents were diluted in PS buffer (20 mM PIPES-KOH pH 6.8, 200 mM sorbitol) to a working concentration before use in experiments. Antibodies against Sec17p (65) and Sec18p have been described previously. N-ethylmaleimide (NEM), propranolol hydrochloride, and ATPγS were purchased from Sigma Aldrich and dissolved in PS buffer. The lipids used in this study: POPA (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphate), POPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylcholine), POPE (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylethanolamine), POG/DAG (1-palmitoyl-2-oleoyl-sn-glycerol), diC8-PA (1,2-dioctanoyl-sn-glycero-3-phosphate), and diC8-DAG (1,2-dioctanoyl-sn-glycerol) were purchased from Avanti Polar Lipids as chloroform stock solutions and stored at −20°C.
Recombinant proteins
Recombinant His6-Sec18p was purified from Escherichia coli as previously described (46). Briefly, PQE9-His6-Sec18p plasmid was transformed into Rosetta-2 (DE3) pLysS (EMD Biosciences) competent cells and plated on LB agar plates containing 100 µg/ml ampicillin and 35 µg/ml chloramphenicol. Transformed cells were grown for 14 h in 100 ml LB at 37°C. Culture flasks containing 1 liter Terrific Broth were inoculated with 50 ml of the pre-culture and grown at 37°C to A600 = 0.6. Cells were induced with 1 mM 1-thio-β-D-galactopyranoside at 16°C for 14 h before being collected by centrifugation and washed with lysis buffer (10 mM HEPES-KOH pH 7.0, 500 mM KCl, 5 mM ATP, 5 mM MgCl2, 2 mM β-mercaptoethanol, 1 mM PMSF and protease inhibitor cocktail). Cells were lysed with four passes through a French pressure cell. Lysates were cleared by centrifugation (200,000 x g, 30 min, 4°C), and the cleared lysate was loaded onto a Ni-NTA agarose column (Thermo Scientific) equilibrated with lysis buffer. The resin was washed with 20 column volumes of lysis buffer containing 10% glycerol and 50 mM Imidazole. His6-Sec18p was eluted from with lysis buffer containing 10% glycerol and 350 mM Imidazole. The eluted protein was further purified by gel filtration in GF buffer (20 mM PIPES-KOH pH 6.8, 200 mM Sorbitol, 125 mM KCl, 5 mM MgCl2, 2 mM ATP, 2 mM DTT and 10% glycerol) and 1 ml fractions were collected. Sec18p elutes from the column as three distinct peaks: the hexameric pool with a molecular mass of 640 kDa elutes early (approximately 50 ml post injection), and dimer and monomeric pools each elute later (approximately 90 and 105 ml post-injection, respectively). The hexameric pool of Sec18p was used in all experiments. The recombinant His6-SUMO-Pah1p construct used was created for this study. The PAH1 gene was cloned from BJ3505 genomic DNA via PCR amplification using the primers: Forward 5’ – TACTTCCAATCCAATGCAATGCAGTACGTAGGAA – 3’ and Reverse 5’ – TTATCCACTTCCAATGTTATTATTAATCTTCGAATTCATCTTCG – 3’. The amplified gene was inserted into pET His6 Sumo TEV LIC cloning vector (2S-T) (Addgene plasmid #29711) using the restriction enzyme SspI and the LIC method previously described to create the plasmid pSP1 (66). Three liters of Rosetta2(DE3)pLysS (EMD Millipore) cells transformed with pSP1 were grown in auto-inducing media supplemented with 2 mM MgSO4 at 37°C to an OD600 of 4.0, and cells were harvested by centrifugation (67). Cells were lysed by freeze-thaw and sonication in buffer containing 50 mM Tris-HCl, pH 7.5, 300 mM NaCl, 2 mM MgCl2, 10 mM imidazole, and 1mM phenylmethanesulfonyl fluoride (PMSF). Lysates were centrifuged for 1 hour at 140,000 x g, and the supernatant was applied to Ni-NTA agarose resin (Invitrogen). The resin was washed with lysis buffer supplemented with 25 mM imidazole and 0.5 mM ATP, and bound proteins were eluted with lysis buffer supplemented with 300 mM imidazole. Eluted protein was dialyzed into PS buffer containing 125 mM KCl. Recombinant GST-DEP, GST-C1b, GST-ENTH and GST-Vam7p were purified as described and dialyzed into PS buffer containing 125 mM KCl (33, 35, 36, 42). The plasmid for GST-DEP expression was a gift from Dr. D. Capelluto (Virginia Tech University).
Strains
BJ3505 (MATα pep4::HIS3 prb1-Δ1.6R his3-200 lys2-801 trp1Δ101(gal3) ura3-52 gal2 can1) and DKY6281 (MATα leu2-3 leu2-112 ura3-52 his3-Δ200 trpΔ1-901 lys 2-801) were used for standard vacuole fusion assays (38). BJ3505-CBP-Vam3p vacuoles were used for the isolation of Sec18p-SNARE complexes (49). PAH1 deleted strains RFY17 and RFY18 and WT Pah1p plasmid complemented strain RFY19, RFY20 have previously been described (17).
Vacuole Isolation and in vitro vacuole fusion assay
Vacuoles were isolated from yeast strains by density gradient floatation as previously described (38). Fusion reactions (30 µl) contained 3 µg each of vacuoles from BJ3505 (pep4Δ PHO8) and DK6281 (PEP4 pho8Δ), fusion assay buffer (125 mM KCl, 5 mM MgCl2, 20 mM PIPES-KOH pH 6.8, 200 mM sorbitol), ATP regenerating system (1 mM ATP, 29 mM creatine phosphate, 0.1 mg/ml creatine kinase), 10 µM CoA, and 283 nM Pbi2p. Reactions were incubated at 27°C for 90 min and the Pho8p activity was measured in 250 mM Tris-Cl PH 8.5, 0.4% Triton X-100, 10 mM MgCl2, 1 mM p-nitrophenyl phosphate. Fusion-dependent alkaline phosphatase maturation was measured by the amount of p-nitrophenylate produced. p-Nitrophenylate absorbance was measured at 400 nm.
Liposome Preparation and Co-Floatation Assay
Small unilamellar liposomes containing various lipid compositions were prepared using the sonication method (16). Briefly, stock lipids in chloroform were mixed to produce a lipid mixture with the desired lipid mole percentages of 2.6 µmoles of total phospholipids. The lipid mixture was dried under a gentle stream of nitrogen and dried in a speed-vacuum for an additional 60 min. The tubes were placed under vacuum in a desiccator for an additional 14 h. To the dried lipids, 2.6 ml of 1X PBS solution was added. Tubes were covered with parafilm and incubated at room temperature for 1 h. The lipids were resuspended with vortexing and disrupted in a water bath sonicator for 30 min. To measure protein binding to the liposomes we used a floatation assay as described (68). Briefly, 40 µl of lipid binding domain/PBS mixture was incubated with 150 µl of the 1 mM liposome suspension for 5 minutes at 30°C before 20 µg of recombinant His6-Sec18p was added. Samples were incubated for an additional 10 min at 30°C and 630 µl of 1.65 M sucrose (PBS) was added. Samples were loaded into the bottom of a centrifuge tube and layered with 840 µl of 0.75 M sucrose (PBS), and 1X PBS to the top of the tube. Samples were centrifuged (200,000 x g, 90 min, 4°C) and 200 µl of floated liposomes were recovered from the top of the 0.75 M sucrose layer. The bottom 100 µl fraction was recovered and SDS sample buffer was added to sample unbound protein levels. Liposomes were resuspended in 1 ml of 1X PBS and isolated by centrifugation (16,000 x g, 10 min, 4°C). SDS sample buffer was added to the final liposome pellet and bound proteins were resolved by SDS-PAGE. The proteins were transferred to nitrocellulose and probed by Western blotting. Images were acquired using a ChemiDoc MP Imaging System (Bio-Rad).
Vacuole Competition Binding Assay
Vacuoles were harvested from DKY6281. Each sample contained 12 µg of vacuoles suspended in binding assay buffer (125 mM KCl, 5 mM MgCl2, 20 mM PIPES-KOH pH 6.8, 200 mM sorbitol) to a final volume of 60 µl. Recombinant GST-DEP, GST-C1b, or GST-ENTH in binding assay buffer was added to the indicated concentrations and samples were incubated at 4°C for 10 min. Whole vacuole samples with no added lipid binding domain were saved for input controls. Supernatant and pellet fractions were isolated by centrifugation (16,000 x g, 5 min, 4°C) and suspended in 1X SDS sample buffer. Samples were heated at 95°C for 5 min, resolved by SDS-PAGE, and transferred to nitrocellulose. Presence of Sec18p in the input controls and supernatant fractions was probed by Western blot.
Priming Assay
Priming activity of Sec18p was assayed as previously described (4). Briefly, vacuoles were harvested from BJ3505. The equivalent of two standard fusion reactions was incubated at 27°C with buffer, NEM, diC8-PA, or diC8-DAG. At the indicated times, vacuoles were removed by centrifugation (16,000 x g, 5 min, 4°C) and SDS sample buffer was added to the supernatants. Samples were heated at 95°C for 5 min, resolved by SDS-PAGE, transferred to nitrocellulose, and probed by Western blot.
Sec18p-SNARE Complex Detection using CBP-Vam3p Pull-down
Analysis of the formation of Sec18p-cis-SNARE complexes was assayed as previously described (17). Briefly, vacuoles used were isolated from BJ3505-CBP-Vam3p. Isolation of Sec18p-cis-SNARE complexes was performed in large-scale fusion reactions (480 µl) using 96 µg of vacuoles. Reactions were incubated at 27°C for the indicated time and treated with buffer or propranolol. Reactions treated with ATPγS were treated with buffer and ATP regenerating system containing ATPγS in place of ATP. Separate reactions were kept on ice as 0 min samples and without ATP regenerating added as No ATP samples. After incubation, samples were placed on ice for 5 min and 30 µl samples were withdrawn to assay fusion activity. Remaining samples were centrifuged (13,000 x g, 15 min, 4°C) and supernatant fractions were removed. The membrane fractions were gently resuspended in ice-cold solubilization buffer (20 mM Tris-Cl, pH 7.5, 150 mM NaCl, 1 mM MgCl2, 0.5% Nonidet P-40 alternative, 10% glycerol) with protease inhibitors (0.46 µg/ml leupeptin, 3.5 µg/ml pepstatin, 2.4 µg/ml Pefabloc-SC, 1 mM PMSF). Solubilization buffer was added to a final volume of 600 µl and samples were mixed by nutation (20 min, 4°C). Insoluble debris was removed by centrifugation (16,000 x g, 15 min, 4°C) and supernatants were transferred to fresh tubes. A 60 µl sample was withdrawn for input samples. The remaining extracts were brought to 2 mM CaCl2 and incubated with 50 µl of equilibrated calmodulin-Sepharose 4B (GE Healthcare) (4°C, 14 h, nutating). Beads were collected by centrifugation (4,000 g, 2 min, 4 °C) and washed 4 times in solubilization buffer. Bound proteins were eluted with SDS sample buffer containing 5 mM EGTA and heated at 95°C for 5 min. The samples were resolved using SDS-PAGE, transferred to nitrocellulose, and probed by Western blot.
Sec18p-SNARE Complex Detection using GST-Vam7p Pull-down
Analysis of the formation of Sec18p-cis-SNARE complexes in mutant strains was assayed essentially the same as in CBP-Vam3p containing strains with some modifications. Vacuoles were isolated from pah1Δ strains (RFY17 and RFY18) or pPAH1-complemented strains (RFY19 and RFY20). Large-scale reactions were used (480 µl) using 96 µg of vacuoles. Recombinant GST-Vam7p was added to reactions (200 nM) prior to addition of ATP regenerating system and incubated at 27°C for 30 min. ATP regenerating system was then added to samples and incubated for the indicated times at 27°C. Fusion assay buffer was added in place of ATP regenerating system in the “no-ATP” sample. The 0 min sample was kept on ice throughout the duration of the incubation step. Solubilization buffer differed from the CBP-Vam3p solubilization buffer (20 mM HEPES-KOH, 100 mM NaCl, 2mM EDTA, 0.5% Nonidet P-40 alternative, 1 mM DTT, 20% glycerol, 1 mM PMSF, 0.46 µg/ml leupeptin, 3.5 µg/ml pepstatin, and 2.4 µg/ml Pefabloc-SC) and Glutathione Sepharose 4B resin (GE Healthcare) was used for the pull-down of complexes. The same method was used for Sec18p-SNARE complex detection in diC8-PA and diC8-DAG treated samples using vacuoles isolated from BJ3505.
Statistical analysis
All statistical analysis was calculated using one-way ANOVA. P values of ≤ 0.05 were considered significant. Half-times for the gain of resistance fusion assay were calculated using first-order exponential decay fitting with weights and errors (OriginPro 9.1 software).
Acknowledgments
We thank Dr. William Wickner and for generous gifts of antisera and Dr. Daniel Capelluto for plasmid to express Dvl2-DEP. This research was supported by a grant from the National Institutes of Health (GM101132) to RAF. MLS was partially supported by an NIGMS-NIH Chemistry-Biology Interface Training Grant (5T32-GM070421).
Abbreviations
- CBP
calmodulin-binding peptide
- DAG
diacylglycerol
- diC8
dioctanoyl
- GDI
guanosine nucleotide dissociation inhibitor
- HOPS
homotypic fusion and vacuole protein sorting complex
- NEM
N-ethylmaleimide
- NSF
NEM sensitive factor
- PA
phosphatidic acid
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PI
phosphatidylinositol
- PS
phosphatidylserine
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptors
- YPD
yeast extract/peptone/dextrose
References
- 1.Jahn R, Sudhof TC. Membrane fusion and exocytosis. Annu. Rev. Biochem. 1999:68863–68911. doi: 10.1146/annurev.biochem.68.1.863. [DOI] [PubMed] [Google Scholar]
- 2.Jahn R, Lang T, Südhof TC. Membrane fusion. Cell. 2003;112(4):519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 3.Boeddinghaus C, Merz AJ, Laage R, Ungermann C. A cycle of Vam7p release from and PtdIns 3-P-dependent rebinding to the yeast vacuole is required for homotypic vacuole fusion. J. Cell Biol. 2002;157(1):79–89. doi: 10.1083/jcb.200112098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayer A, Wickner W, Haas A. Sec18p (NSF)-driven release of Sec17p (alpha-SNAP) can precede docking and fusion of yeast vacuoles. Cell. 1996;85(1):83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 5.Mayer A, Wickner W. Docking of yeast vacuoles is catalyzed by the Ras-like GTPase Ypt7p after symmetric priming by Sec18p (NSF) J. Cell Biol. 1997;136(2):307–317. doi: 10.1083/jcb.136.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seals DF, Eitzen G, Margolis N, Wickner WT, Price A. A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc. Natl. Acad. Sci. U. S. A. 2000;97(17):9402–9407. doi: 10.1073/pnas.97.17.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ungermann C, Sato K, Wickner W. Defining the functions of trans-SNARE pairs. Nature. 1998;396(6711):543–548. doi: 10.1038/25069. [DOI] [PubMed] [Google Scholar]
- 8.Fratti RA, Jun Y, Merz AJ, Margolis N, Wickner W. Interdependent assembly of specific regulatory lipids and membrane fusion proteins into the vertex ring domain of docked vacuoles. J. Cell Biol. 2004;167(6):1087–1098. doi: 10.1083/jcb.200409068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Seeley ES, Wickner W, Merz AJ. Vacuole Fusion at a Ring of Vertex Docking Sites Leaves Membrane Fragments within the Organelle. Cell. 2002;108(3):357–369. doi: 10.1016/s0092-8674(02)00632-3. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Merz AJ, Collins KM, Wickner W. Hierarchy of protein assembly at the vertex ring domain for yeast vacuole docking and fusion. J. Cell Biol. 2003;160(3):365–374. doi: 10.1083/jcb.200209095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reese C, Mayer A. Transition from hemifusion to pore opening is rate limiting for vacuole membrane fusion. J. Cell Biol. 2005;171(6):981–990. doi: 10.1083/jcb.200510018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryu JK, Min D, Rah SH, Kim SJ, Park Y, Kim H, Hyeon C, Kim HM, Jahn R, Yoon TY. Spring-loaded unraveling of a single SNARE complex by NSF in one round of ATP turnover. Science. 2015;347(6229):1485–1489. doi: 10.1126/science.aaa5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao M, Wu S, Zhou Q, Vivona S, Cipriano DJ, Cheng Y, Brunger AT. Mechanistic insights into the recycling machine of the SNARE complex. Nature. 2015;518(7537):61–67. doi: 10.1038/nature14148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matveeva EA, Whiteheart SW, Vanaman TC, Slevin JT. Phosphorylation of the N-ethylmaleimide-sensitive factor is associated with depolarization-dependent neurotransmitter release from synaptosomes. J. Biol. Chem. 2001;276(15):12174–12181. doi: 10.1074/jbc.M007394200. [DOI] [PubMed] [Google Scholar]
- 15.Manifava M, Thuring JW, Lim ZY, Packman L, Holmes AB, Ktistakis NT. Differential binding of traffic-related proteins to phosphatidic acid- or phosphatidylinositol (4,5)- bisphosphate-coupled affinity reagents. J. Biol. Chem. 2001;276(12):8987–8994. doi: 10.1074/jbc.M010308200. [DOI] [PubMed] [Google Scholar]
- 16.Mima J, Wickner W. Complex lipid requirements for SNARE-and SNARE chaperone dependent membrane fusion. J. Biol. Chem. 2009:28427114–28427122. doi: 10.1074/jbc.M109.010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasser T, Qiu QS, Karunakaran S, Padolina M, Reyes A, Flood B, Smith S, Gonzales C, Fratti RA. Yeast lipin 1 orthologue pah1p regulates vacuole homeostasis and membrane fusion. J. Biol. Chem. 2012;287(3):2221–2236. doi: 10.1074/jbc.M111.317420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karunakaran S, Fratti R. The Lipid Composition and Physical Properties of the Yeast Vacuole Affect the Hemifusion-Fusion Transition. Traffic. 2013;14(6):650–662. doi: 10.1111/tra.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato M, Wickner W. Ergosterol is required for the Sec18/ATP-dependent priming step of homotypic vacuole fusion. EMBO J. 2001;20(15):4035–4040. doi: 10.1093/emboj/20.15.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayer A, Scheglmann D, Dove S, Glatz A, Wickner W, Haas A. Phosphatidylinositol 4,5-bisphosphate regulates two steps of homotypic vacuole fusion. Mol Biol Cell. 2000;11(3):807–817. doi: 10.1091/mbc.11.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheever ML, Sato TK, de Beer T, Kutateladze TG, Emr SD, Overduin M. Phox domain interaction with PtdIns(3)P targets the Vam7 t-SNARE to vacuole membranes. Nat Cell Biol. 2001;3(7):613–618. doi: 10.1038/35083000. [DOI] [PubMed] [Google Scholar]
- 22.Karunakaran S, Sasser T, Rajalekshmi S, Fratti RA. SNAREs, HOPS, and regulatory lipids control the dynamics of vacuolar actin during homotypic fusion. J. Cell Sci. 2012:14650–14662. doi: 10.1242/jcs.091900. [DOI] [PubMed] [Google Scholar]
- 23.Han GS, Siniossoglou S, Carman GM. The cellular functions of the yeast lipin homolog PAH1p are dependent on its phosphatidate phosphatase activity. J. Biol. Chem. 2007;282(51):37026–37035. doi: 10.1074/jbc.M705777200. [DOI] [PubMed] [Google Scholar]
- 24.O’Hara L, Han GS, Peak-Chew S, Grimsey N, Carman GM, Siniossoglou S. Control of phospholipid synthesis by phosphorylation of the yeast lipin Pah1p/Smp2p Mg2+-dependent phosphatidate phosphatase. J. Biol. Chem. 2006;281(45):34537–34548. doi: 10.1074/jbc.M606654200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karanasios E, Han GS, Xu Z, Carman GM, Siniossoglou S. A phosphorylation-regulated amphipathic helix controls the membrane translocation and function of the yeast phosphatidate phosphatase. Proc. Natl. Acad. Sci. U. S. A. 2010;107(41):17539–17544. doi: 10.1073/pnas.1007974107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi HS, Su WM, Morgan JM, Han GS, Xu Z, Karanasios E, Siniossoglou S, Carman GM. Phosphorylation of phosphatidate phosphatase regulates its membrane association and physiological functions in Saccharomyces cerevisiae: identification of SER(602), THR(723), AND SER(744) as the sites phosphorylated by CDC28 (CDK1)-encoded cyclin-dependent kinase. J. Biol. Chem. 2011;286(2):1486–1498. doi: 10.1074/jbc.M110.155598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbosa AD, Sembongi H, Su WM, Abreu S, Reggiori F, Carman GM, Siniossoglou S. Lipid partitioning at the nuclear envelope controls membrane biogenesis. Mol Biol Cell. 2015;26(20):3641–3657. doi: 10.1091/mbc.E15-03-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez-Ulibarri I, Vilella M, Lazaro-Dieguez F, Sarri E, Martinez SE, Jimenez N, Claro E, Merida I, Burger KN, Egea G. Diacylglycerol Is Required for the Formation of COPI Vesicles in the Golgi-to-ER Transport Pathway. Mol Biol Cell. 2007;18(9):3250–3263. doi: 10.1091/mbc.E07-04-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu S, Wilson KA, Rice-Stitt T, Neiman AM, McNew JA. In vitro fusion catalyzed by the sporulation-specific t-SNARE light-chain Spo20p is stimulated by phosphatidic acid. Traffic. 2007;8(11):1630–1643. doi: 10.1111/j.1600-0854.2007.00628.x. [DOI] [PubMed] [Google Scholar]
- 30.Nakanishi H, Morishita M, Schwartz CL, Coluccio A, Engebrecht J, Neiman AM. Phospholipase D and the SNARE Sso1p are necessary for vesicle fusion during sporulation in yeast. J. Cell Sci. 2006;119(Pt 7):1406–1415. doi: 10.1242/jcs.02841. [DOI] [PubMed] [Google Scholar]
- 31.Lawrence G, Brown CC, Flood BA, Karunakaran S, Cabrera M, Nordmann M, Ungermann C, Fratti RA. Dynamic association of the PI3P-interacting Mon1-Ccz1 GEF with vacuoles is controlled through its phosphorylation by the type-1 casein kinase Yck3. Mol Biol Cell. 2014;25(10):1608–1619. doi: 10.1091/mbc.E13-08-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li SC, Kane PM. The yeast lysosome-like vacuole: endpoint and crossroads. Biochim. Biophys. Acta. 2009;1793(4):650–663. doi: 10.1016/j.bbamcr.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Capelluto DG, Zhao X, Lucas A, Lemkul JA, Xiao S, Fu X, Sun F, Bevan DR, Finkielstein CV. Biophysical and molecular-dynamics studies of phosphatidic acid binding by the Dvl-2 DEP domain. Biophys. J. 2014;106(5):1101–1111. doi: 10.1016/j.bpj.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kooijman EE, Tieleman DP, Testerink C, Munnik T, Rijkers DT, Burger KN, de Kruijff B. An electrostatic/hydrogen bond switch as the basis for the specific interaction of phosphatidic acid with proteins. J. Biol. Chem. 2007;282(15):11356–11364. doi: 10.1074/jbc.M609737200. [DOI] [PubMed] [Google Scholar]
- 35.Johnson JE, Giorgione J, Newton AC. The C1 and C2 domains of protein kinase C are independent membrane targeting modules, with specificity for phosphatidylserine conferred by the C1 domain. Biochemistry. 2000;39(37):11360–11369. doi: 10.1021/bi000902c. [DOI] [PubMed] [Google Scholar]
- 36.Rosenthal JA, Chen H, Slepnev VI, Pellegrini L, Salcini AE, Di Fiore PP, De Camilli P. The epsins define a family of proteins that interact with components of the clathrin coat and contain a new protein module. J. Biol. Chem. 1999;274(48):33959–33965. doi: 10.1074/jbc.274.48.33959. [DOI] [PubMed] [Google Scholar]
- 37.Stroupe C, Collins KM, Fratti RA, Wickner W. Purification of active HOPS complex reveals its affinities for phosphoinositides and the SNARE Vam7p. EMBO J. 2006;25(8):1579–1589. doi: 10.1038/sj.emboj.7601051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haas A, Conradt B, Wickner W. G-protein ligands inhibit in vitro reactions of vacuole inheritance. J. Cell Biol. 1994;126(1):87–97. doi: 10.1083/jcb.126.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong XP, Shen D, Wang X, Dawson T, Li X, Zhang Q, Cheng X, Zhang Y, Weisman LS, Delling M, Xu H. PI(3,5)P2 controls membrane trafficking by direct activation of mucolipin Ca(2+) release channels in the endolysosome. Nat Commun. 2010:138. doi: 10.1038/ncomms1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siddiqui RA, English D, Harvey K, Cui Y, Martin MI, Wentland J, Akard L, Jansen J, Thompson J, Garcia JG. Phorbol ester-induced priming of superoxide generation by phosphatidic acid-stimulated neutrophils and granule-free neutrophil cytoplasts. J Leukoc Biol. 1995;58(2):189–195. doi: 10.1002/jlb.58.2.189. [DOI] [PubMed] [Google Scholar]
- 41.Fratti RA, Wickner W. Distinct Targeting and Fusion Functions of the PX and SNARE Domains of Yeast Vacuolar Vam7p. J. Biol. Chem. 2007;282(17):13133–13138. doi: 10.1074/jbc.M700584200. [DOI] [PubMed] [Google Scholar]
- 42.Fratti RA, Collins KM, Hickey CM, Wickner W. Stringent 3Q: 1R composition of the SNARE 0-layer can be bypassed for fusion by compensatory SNARE mutation or by lipid bilayer modification. J. Biol. Chem. 2007;282(20):14861–14867. doi: 10.1074/jbc.M700971200. [DOI] [PubMed] [Google Scholar]
- 43.Sasser TL, Lawrence G, Karunakaran S, Brown C, Fratti RA. The Yeast ABC Transporter Ycf1p Enhances the Recruitment of the Soluble SNARE Vam7p to Vacuoles for Efficient Membrane Fusion. J. Biol. Chem. 2013:28818300–28818310. doi: 10.1074/jbc.M112.441089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei W, Smith N, Wu X, Kim H, Seravalli J, Khalimonchuk O, Lee J. YCF1-mediated cadmium resistance in yeast is dependent on copper metabolism and antioxidant enzymes. Antioxid Redox Signal. 2014;21(10):1475–1489. doi: 10.1089/ars.2013.5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haas A, Scheglmann D, Lazar T, Gallwitz D, Wickner W. The GTPase Ypt7p of Saccharomyces cerevisiae is required on both partner vacuoles for the homotypic fusion step of vacuole inheritance. EMBO J. 1995;14(21):5258–5270. doi: 10.1002/j.1460-2075.1995.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steel GJ, Laude AJ, Boojawan A, Harvey DJ, Morgan A. Biochemical analysis of the Saccharomyces cerevisiae SEC18 gene product: implications for the molecular mechanism of membrane fusion. Biochemistry. 1999;38(24):7764–7772. doi: 10.1021/bi990315v. [DOI] [PubMed] [Google Scholar]
- 47.Thorngren N, Collins KM, Fratti RA, Wickner W, Merz AJ. A soluble SNARE drives rapid docking, bypassing ATP and Sec17/18p for vacuole fusion. EMBO J. 2004:232765–232776. doi: 10.1038/sj.emboj.7600286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collins KM, Thorngren NL, Fratti RA, Wickner WT. Sec17p and HOPS, in distinct SNARE complexes, mediate SNARE complex disruption or assembly for fusion. EMBO J. 2005;24(10):1775–1786. doi: 10.1038/sj.emboj.7600658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Collins KM, Wickner WT. Trans-SNARE complex assembly and yeast vacuole membrane fusion. Proc. Natl. Acad. Sci. U. S. A. 2007;104(21):8755–8760. doi: 10.1073/pnas.0702290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vicogne J, Vollenweider D, Smith JR, Huang P, Frohman MA, Pessin JE. Asymmetric phospholipid distribution drives in vitro reconstituted SNARE-dependent membrane fusion. Proc. Natl. Acad. Sci. U. S. A. 2006;103(40):14761–14766. doi: 10.1073/pnas.0606881103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choi SY, Huang P, Jenkins GM, Chan DC, Schiller J, Frohman MA. A common lipid links Mfn-mediated mitochondrial fusion and SNARE-regulated exocytosis. Nat Cell Biol. 2006;8(11):1255–1262. doi: 10.1038/ncb1487. [DOI] [PubMed] [Google Scholar]
- 52.Jun Y, Fratti RA, Wickner W. Diacylglycerol and its formation by Phospholipase C regulate Rab- and SNARE- dependent yeast vacuole fusion. J. Biol. Chem. 2004:27953186–27953195. doi: 10.1074/jbc.M411363200. [DOI] [PubMed] [Google Scholar]
- 53.Das S, Rand RP. Diacylglycerol causes major structural transitions in phospholipid bilayer membranes. Biochem. Biophys. Res. Commun. 1984;124(2):491–496. doi: 10.1016/0006-291x(84)91580-8. [DOI] [PubMed] [Google Scholar]
- 54.Seddon JM. An inverse face-centered cubic phase formed by diacylglycerol-phosphatidylcholine mixtures. Biochemistry. 1990;29(34):7997–8002. doi: 10.1021/bi00486a031. [DOI] [PubMed] [Google Scholar]
- 55.Sánchez-Migallón MP, Aranda FJ, Gómez-Fernández JC. The dissimilar effect of diacylglycerols on Ca(2+)-induced phosphatidylserine vesicle fusion. Biophys. J. 1995;68(2):558–566. doi: 10.1016/S0006-3495(95)80217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Villar AV, Alonso A, Goñi FM. Leaky vesicle fusion induced by phosphatidylinositol-specific phospholipase C: observation of mixing of vesicular inner monolayers. Biochemistry. 2000;39(46):14012–14018. doi: 10.1021/bi992515c. [DOI] [PubMed] [Google Scholar]
- 57.Kooijman EE, Burger KN. Biophysics and function of phosphatidic acid: a molecular perspective. Biochim. Biophys. Acta. 2009;1791(9):881–888. doi: 10.1016/j.bbalip.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 58.van den Brink-van der , Laan E, Chupin V, Killian JA, de Kruijff B. Small alcohols destabilize the KcsA tetramer via their effect on the membrane lateral pressure. Biochemistry. 2004;43(20):5937–5942. doi: 10.1021/bi0496079. [DOI] [PubMed] [Google Scholar]
- 59.Whiteheart SW, Rossnagel K, Buhrow SA, Brunner M, Jaenicke R, Rothman JE. N-ethylmaleimide-sensitive fusion protein: a trimeric ATPase whose hydrolysis of ATP is required for membrane fusion. J. Cell Biol. 1994;126(4):945–954. doi: 10.1083/jcb.126.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hanson PI, Roth R, Morisaki H, Jahn R, Heuser JE. Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell. 1997;90(3):523–535. doi: 10.1016/s0092-8674(00)80512-7. [DOI] [PubMed] [Google Scholar]
- 61.Babor SM, Fass D. Crystal structure of the Sec18p N-terminal domain. Proc. Natl. Acad. Sci. U. S. A. 1999;96(26):14759–14764. doi: 10.1073/pnas.96.26.14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Han GS, O’Hara L, Carman GM, Siniossoglou S. An unconventional diacylglycerol kinase that regulates phospholipid synthesis and nuclear membrane growth. J. Biol. Chem. 2008:28320433–28320442. doi: 10.1074/jbc.M802903200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sreenivas A, Patton-Vogt JL, Bruno V, Griac P, Henry SA. A role for phospholipase D (Pld1p) in growth, secretion, and regulation of membrane lipid synthesis in yeast. J. Biol. Chem. 1998;273(27):16635–16638. doi: 10.1074/jbc.273.27.16635. [DOI] [PubMed] [Google Scholar]
- 64.Waksman M, Tang X, Eli Y, Gerst JE, Liscovitch M. Identification of a novel Ca2+-dependent, phosphatidylethanolamine-hydrolyzing phospholipase D in yeast bearing a disruption in PLD1. J. Biol. Chem. 1997;272(1):36–39. doi: 10.1074/jbc.272.1.36. [DOI] [PubMed] [Google Scholar]
- 65.Haas A, Wickner W. Homotypic vacuole fusion requires Sec17p (yeast alpha-SNAP) and Sec18p (yeast NSF) EMBO J. 1996;15(13):3296–3305. [PMC free article] [PubMed] [Google Scholar]
- 66.Aslanidis C, de Jong PJ. Ligation-independent cloning of PCR products (LIC-PCR) Nucleic Acids Res. 1990;18(20):6069–6074. doi: 10.1093/nar/18.20.6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Studier FW. Protein production by auto-induction in high density shaking cultures. Protein Expr Purif. 2005;41(1):207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 68.Matsuoka K, Morimitsu Y, Uchida K, Schekman R. Coat assembly directs v-SNARE concentration into synthetic COPII vesicles. Mol Cell. 1998;2(5):703–708. doi: 10.1016/s1097-2765(00)80168-9. [DOI] [PubMed] [Google Scholar]