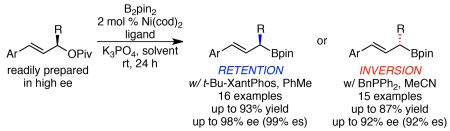
Abstract
We have developed a stereospecific, nickel-catalyzed Miyaura borylation of allylic pivalates, which delivers highly enantioenriched α-stereogenic γ-aryl allylboronates with good yields and regioselectivities. Our complementary sets of conditions enable access to either enantiomer of allylboronate product from a single enantiomer of readily prepared allylic pivalate substrate. Excellent functional group tolerance, yields, regioselectivities, and stereochemical fidelities are observed. The stereochemical switch from stereoretention to stereoinversion largely depends upon solvent, and can be explained by competitive pathways for the oxidative addition step. Our mechanistic investigations support a stereoretentive pathway stemming from a directed oxidative addition and a stereoinvertive pathway that is dominant when MeCN blocks coordination of the directing group by binding the nickel catalyst.
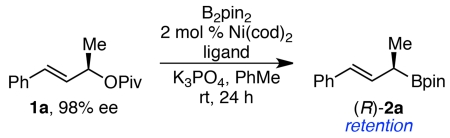
Graphical abstract
INTRODUCTION
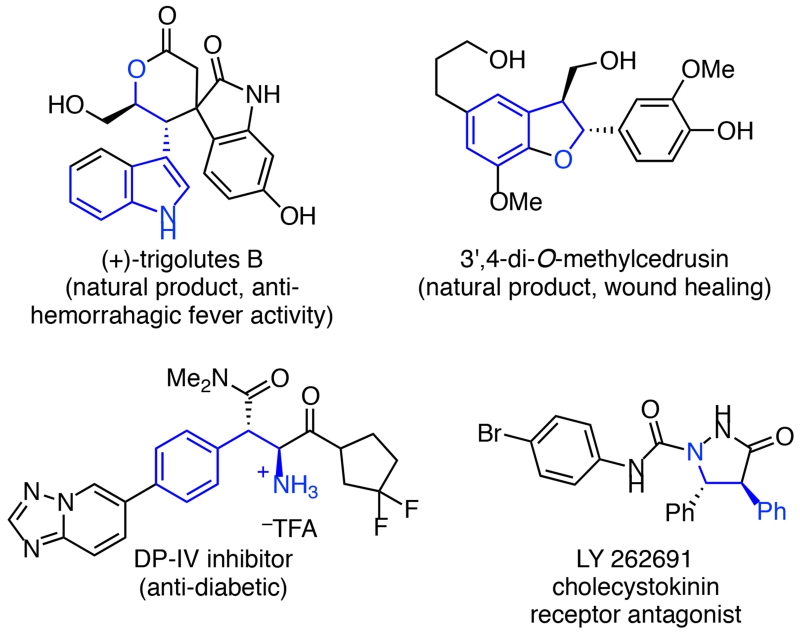
α-Stereogenic allylic boronates are powerful synthetic intermediates.1 Allylic transposition reactions of these versatile molecules enable construction of C–C bonds with exquisite control over product stereochemistry.2 In particular, allylboration of aldehydes and imines gives rise to homoallylic alcohols and amines that have found wide use in the preparation of natural products, pharmaceuticals, and other bioactive compounds. As illustrated by the examples in Figure 1, numerous bioactive molecules contain a homobenzylic alcohol or amine fragment,3 which are accessible via aldehyde or imine hydroboration using α-stereogenic γ-aryl allylic boronates.2d-i, 4, 5 α-Stereogenic γ-substituted allylic boronates can also be elaborated to a range of arylated products via stereospecific palladium-catalyzed cross couplings that occur with allylic transposition.2d, 6 These powerful allylic transposition reactions make such allylic boronates highly valuable intermediates.
Figure 1.
Bioactive homobenzylic alcohol and amine derivatives.
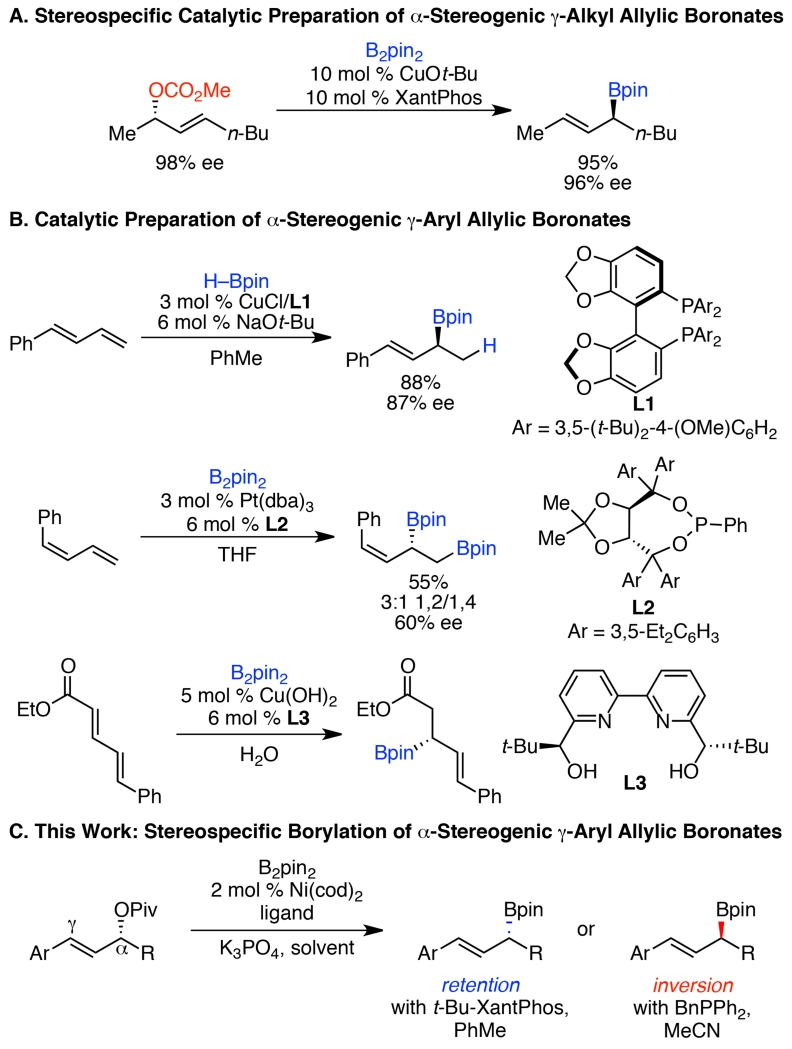
Given their utility, it is not surprising that robust methods have been developed to prepare these highly useful compounds. Matteson homologations and related reactions enable access to a wide range of enantioenriched allylic boronates.2f, 2h, 7 However, these routes require use of Grignard or lithium reagents, and typically stoichiometric chiral agents. Although alternative methods, including catalytic variants, have been developed for the preparation of α-stereogenic allylic boronates with terminal alkenes,8, 5 relatively few catalytic methods for the preparation of enantioenriched γ-substituted α-stereogenic allylic boronates have been reported. The majority of these methods deliver only γ-alkyl allylic boronates.9 Most closely related to this report, Ito and Sawamura have elegantly demonstrated a stereospecific, copper-catalyzed borylation of allylic carbonates to deliver highly enantioenriched γ-alkyl allylic boronates (Scheme 1A),9a but such methodology is absent for the preparation of γ-aryl allylic boronates. Only three catalytic methods deliver enantioenriched γ-aryl allylic boronates, all of which rely on 1,3-dienes as substrates and have limited scope in the preparation of γ-aryl products (Scheme 1B).4a, 9b, 10
Scheme 1. Catalytic Preparation of Enantioenriched α-Stereogenic γ-Substituted Allylic Boronates.
Given these limitations, as well as the high synthetic value of these γ-aryl allylic boronates, we endeavored to develop an efficient, catalytic route to their preparation. We recently developed a stereospecific, nickel-catalyzed arylation of γ-aryl allylic pivalates,11 and envisioned that such a strategy may enable a stereospecific Miyaura borylation to deliver γ-aryl α-stereogenic allylic boronates.12 Herein, we report the successful development of a stereospecific borylation of allylic pivalates to deliver highly enantioenriched α-stereogenic γ-aryl allylic boronates (Scheme 1C). This method harnesses readily prepared, enantioenriched secondary allylic alcohol derivatives to deliver a wide range of γ-aryl allylic boronates with broad functional group tolerance. Excitingly, largely by optimization of reaction solvent, we discovered complementary conditions that result in either stereoinversion or stereoretention, thereby enabling efficient access to either enantiomer of allylic boronate product from a single enantiomer of starting material.13 Our mechanistic investigations suggest that the primary role of solvent over the stereochemical outcome stems from a dual role of MeCN as both solvent and ligand.14
RESULTS AND DISCUSSION
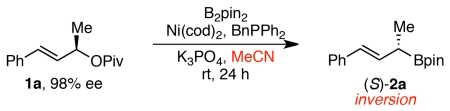
We began by examining the borylation of pivalate 1. Such pivalates are derived from allylic alcohol precursors, which are readily prepared in high enantiomeric excess via either CBS reduction or kinetic resolution.15 Using conditions similar to the optimal conditions for the stereospecific allylic arylation (Ni(cod)2, BnPPh2, K3PO4, MeCN), high yield and stereochemical fidelity was observed in the borylation (Table 1, entry 1). Consistent with the arylation, the major product resulted from inversion of absolute configuration. Optimization of these conditions revealed that reduction of the catalyst loading to 2 mol % Ni(cod)2 and 4.4 mol % BnPPh2 led to similar yields with slightly higher regioselectivity and enantiospecificity (entry 2).
Table 1. Optimization of stereoinvertive borylationa.
Conditions: 1a (98% ee, 0.2 mmol, 1.0 equiv), B2pin2 (2.0 equiv), Ni(cod)2, BnPPh2 (ratio of Ni(cod)2:BnPPh2 = 1:2.2), K3PO4 (2.0 equiv), MeCN (0.4 M), rt, 24 h.
Determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as internal standard.
ee’s of the subsequent alcohol (3a), formed via oxidation with H2O2 and NaOH. Determined by HPLC analysis using a chiral stationary phase.
es = eeproduct/eestarting material.
However, when PhMe replaced MeCN as solvent, a remarkable switch in stereospecificity was observed; the major product was formed in 49% ee via retention of absolute configuration (Table 2, entry 1). To obtain high levels of stereochemical fidelity with retention of configuration, optimization of the ligand proved critical. Use of P(o-Tol)3 resulted in high yield and a dramatic increase in stereochemical fidelity (entry 2). However, the small amount of remaining pivalate 1a proved difficult to separate from allylic boronate 2a. To enable effective purification of boronate 2, we further optimized the ligand to achieve quantitative conversion. t-BuXPhos provided some increase in yield (entry 3), but t-BuXantPhos proved superior, delivering nearly quantitative yield of 2a (entry 5). Notably, other bidentate ligands proved inferior (e.g., entry 4).
Table 2. Optimization of stereoretentive borylationa.
| entry | ligand (mol%) | yield (%)b |
α:γb | ee (%)c |
es (%)d |
|---|---|---|---|---|---|
| 1e | BnPPh2 (11) | 89 | >20:1 | 49 | 50 |
| 2 | P(o-Tol)3 (4.4) | 90 | >20:1 | 94 | 96 |
| 3 | t-BuXPhos (4.4) | 95 | >20:1 | 95 | 97 |
| 4 | dppb (2.2) | 55 | >20:1 | 75 | 77 |
| 5 | t-BuXantPhos (2.2) | 99 | >20:1 | 95 | 97 |
1a (98% ee, 0.2 mmol, 1.0 equiv), B2pin2 (2.0 equiv), Ni(cod)2, ligand, K3PO4 (2.0 equiv), PhMe (0.4 M), rt, 24 h.
Determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as internal standard.
ee’s of the subsequent alcohol (3a), formed via oxidation with H2O2 and NaOH. Determined by HPLC analysis using a chiral stationary phase.
es = eeproduct/eestarting material.
5 mol % Ni(cod)2.
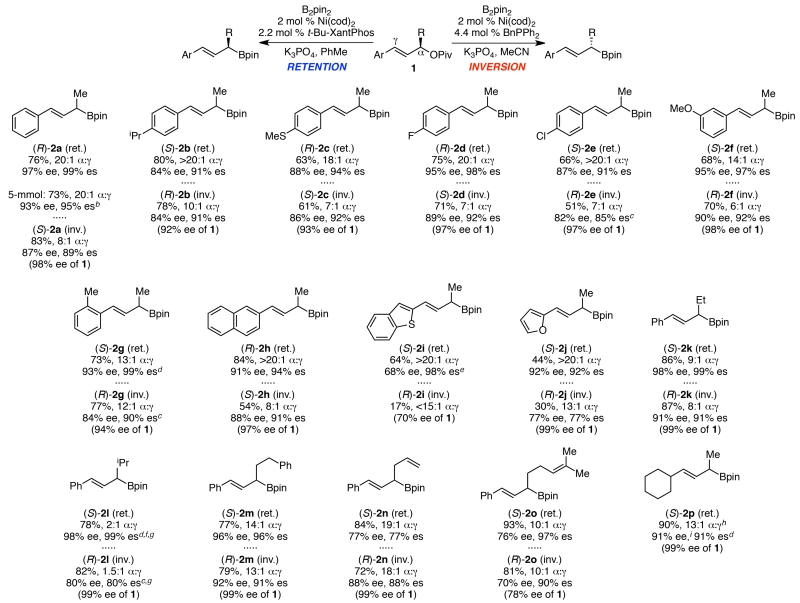
A wide range of γ-aryl allylic pivalates underwent borylation under both sets of conditions (Scheme 2). In general, the regioselectivity and stereochemical fidelity were slightly higher under the stereoretentive conditions (e.g., 2a). However, synthetically useful yields, regioselectivities and stereospecificities were generally observed under both sets of conditions with each substrate. Borylation of 1a proceeded to give high isolated yields of boronate 2a. Further, this reaction was performed on a 5-mmol scale nearly identical yield, regioselectivity and stereochemical fidelity. A variety of functional groups were well tolerated on the γ-aryl substituent, including thioether (2c), fluoro (2d), chloro (2e), and ether (2f). Products with both electron-rich (2b, 2c, 2g, 2i, 2j) and electron-poor (2d–2f) aryl substituents were formed in high yields, regioselectivities and ee’s. In addition, pivalates with sterically bulky o-tolyl (2g), as well as 2-naphthyl (2h), substituents reacted well. Heteroaryl groups can also be incorporated. Under stereoretentive conditions, both benzothiofuran 2i and furan 2j were formed in excellent regioselectivity and high ee’s, albeit in somewhat lower yields. Under stereoinvertive conditions, however, the yields of these heteroaryl products, as well as the stereochemical fidelity of furan 2j, were diminished. With respect to the alkyl substituent (R in Scheme 2), high yields and stereochemical fidelity were observed with increased steric hindrance (Et and i-Pr, 2k and 2l). However, lower regioselectivity was observed, particularly in the formation of 2l. Boronates with both phenethyl (2m) and allylic (2n) groups were prepared in high yields. Notably, borylation of allyl-substituted 1n resulted in lower levels of stereochemical fidelity (77 and 88% ee), whereas prenol 1o reacted to give 2o in much higher levels of stereochemical fidelity. This contrast suggests that the vinyl group of 1n may bind to the nickel catalyst, changing the mechanism of the stereospecific reaction and/or accelerating an epimerization pathway. In addition to γ-aryl allylic boronates, this method can be used to prepare γ-alkyl allylic boronates in high yields, regioselectivities, and enantiopurity, as long as there is a significant steric difference between the α and γ alkyl substituents. For example, cyclohexyl-substituted allylic boronate 2p was formed in 90% yield, 13:1 regioselectivity, and 91% es under the stereoretentive conditions.
Scheme 2. Scope of allylic borylation.a.
a Retention conditions: 1 (0.4 mmol, 1.0 equiv), B2pin2 (2.0 equiv), Ni(cod)2 (2 mol %), t-Bu-XantPhos (2.2 mol %), K3PO4 (2.0 equiv), PhMe (0.4 M), rt, 24 h. Inversion Conditions: 1 (0.4 mmol, 1.0 equiv), B2pin2 (2.0 equiv), Ni(cod)2 (2 mol %), BnPPh2 (4.4 mol %), K3PO4 (2.0 equiv), MeCN (0.4 M), rt, 32 h. Average isolated yields, α:γ ratios (determined by 1H NMR of isolated allylic boronates 2), and ee’s (of the subsequent alcohol 3 formed via oxidation with H2O2 and NaOH, determined by HPLC analysis using a chiral stationary phase) of two duplicate runs. b 5 mmol scale, single experiment. c 5 mol % Ni(cod)2, 11 mol % BnPPh2. d 5 mol % Ni(cod)2, 5.5 mol % t-Bu-XantPhos. e Single experiment. f 0.3 mmol scale. g 40 °C. h Ratio of subsequent alcohol (3p). i ee of p-nitrobenzoate of alcohol 3p.
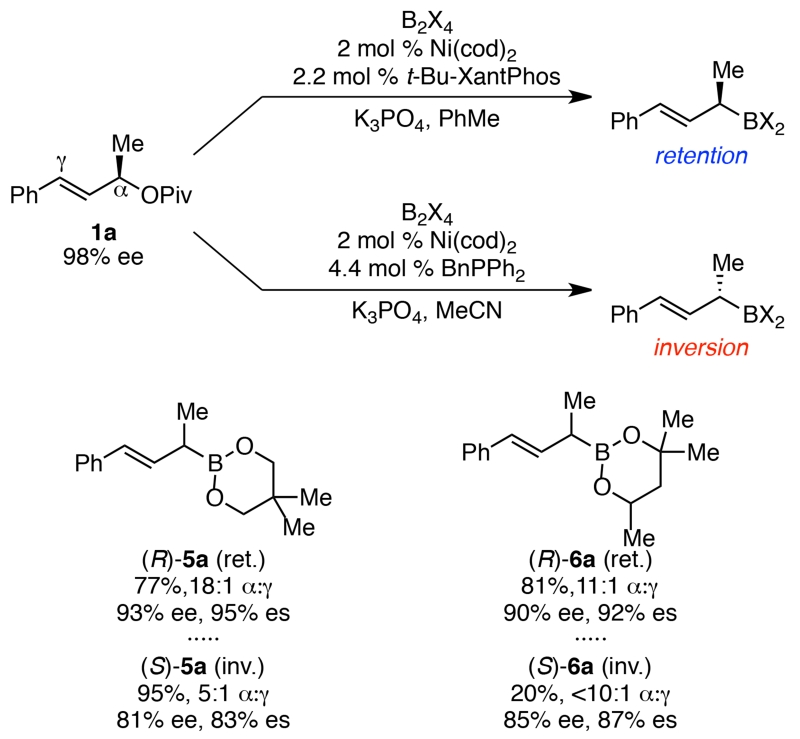
In addition to wide scope in the pivalate partner, various diboranes can be employed (Scheme 3). The use of bis(neopenthyl glycolato)diboron led to high yields under both stereoretentive and stereoinvertive conditions. Bis(hexylene glycolato)diboron proved more sensitive to reaction conditions, only resulting in high yields under the stereoretention conditions. However, these boronates proved more difficult to isolate due to decomposition upon purification by silica gel chromatography, limiting the utility of borylations with these diboranes to cases where the allylic boronate can be used directly without purification.
Scheme 3. Alternative diboranesa.
a Retention conditions: 1a (0.2 mmol, 1.0 equiv), B2X2 (2.0 equiv), Ni(cod)2 (2 mol %), t-Bu-XantPhos (2.2 mol %), K3PO4 (2.0 equiv), PhMe (0.4 M), rt, 24 h. Inversion Conditions: 1 (0.2 mmol, 1.0 equiv), B2pin2 (2.0 equiv), Ni(cod)2 (2 mol %), BnPPh2 (4.4 mol %), K3PO4 (2.0 equiv), MeCN (0.4 M), rt, 32 h. Yields and α:γ ratios determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as an internal standard. Ee’s of the subsequent alcohol 3 formed via oxidation with H2O2 and NaOH, determined by HPLC analysis using a chiral stationary phase.
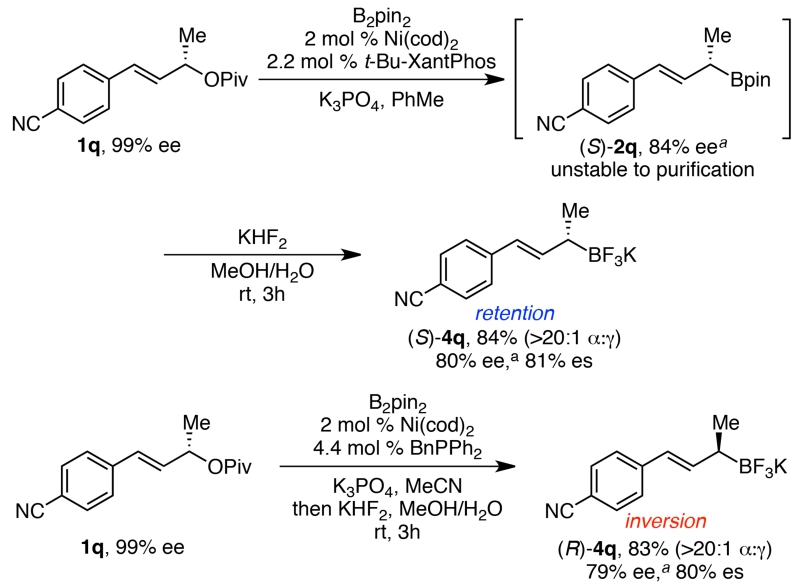
In some cases, purification of the allylic boronate esters was hindered due to their decomposition on silica gel. This problem was overcome by conversion of the boronate ester to a potassium trifluoroborate salt.16 For example, borylation of nitrile 1q under the stereoretentive conditions produced (S)-2q in 84% ee (Scheme 4). Although 2q could not be purified via silica gel chromatography, the subsequent trifluoroborate (4q) was cleanly isolated in 83% yield as a single regioisomer with minimal loss in enantiopurity. Similarly, trifluoroborate (R)-4q was formed in 77% yield and 77% ee via borylation using the stereoinvertive conditions, followed by conversion to the trifluoroborate.
Scheme 4. Formation of allylic potassium trifluoroborate salts.
a ee of the subsequent alcohol (3q).
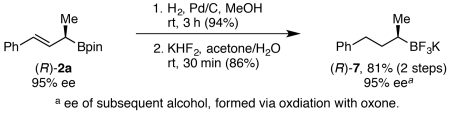
In addition to the allylic transposition reactions, these allylic boronates can be reduced to highly enantioenriched secondary alkyl boronates. Reduction of boronate 2a and subsequent conversion to trifluoroborate 7 proceeded in 81% yield with no loss of enantiopurity (eq 1). Via stereospecific, palladium-catalyzed cross couplings with aryl chlorides, trifluoroborate 7 can be converted to a number of highly enantioenriched products with tertiary benzylic stereocenters.17
 |
(1) |
With respect to the mechanism of this reaction, we were intrigued by the strong stereochemical dependence on solvent. Examples of switchable stereospecificity have been reported with both allylic and benzylic electrophiles. In their copper-catalyzed alkylation of allylic phosphates, Ohmiya and Sawamura observed a stereospecificity switch dependent on base and solvent.13b Kurosawa’s group reported a solvent-controlled stereochemical switch in the oxidative addition of Pd2(dba)3 into allylic chlorides.13c In her nickel-catalyzed arylation of benzylic carbamates, Jarvo observed that ligand identity could flip the stereochemical outcome.13a, 13e These groups all attribute the stereoretention to a mechanism in which the metal catalyst is directed to the allylic or benzylic fragment by the leaving group (phosphate, chloride, or carbamate). The inversion product is hypothesized to arise via a non-directed SN2’ addition. Support for these mechanistic proposals lies largely in the comparison of reaction parameters for the two pathways; no systematic study to support the dual role of the leaving group as also a directing group has been published. We thought that this allylic borylation would provide a suitable platform for such studies, particularly given the fact that solvent was providing our stereospecificity switch.
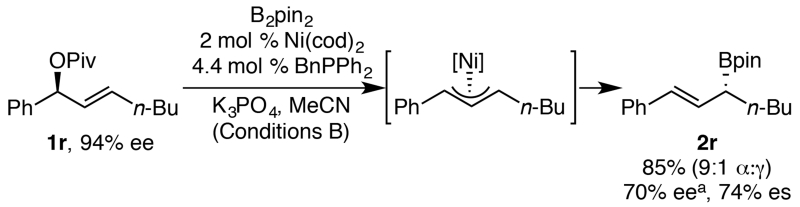
To begin our mechanistic study, we first hypothesized that the reaction proceeds via a π-allyl nickel intermediate. The observation of minor amounts of the γ-regioisomer is consistent with this hypothesis (see Scheme 2 above). In addition, we subjected pivalate 1r to the borylation conditions. Boronate 2r was formed as a 9:1 ratio of regioisomers, with the major isomer mirroring that formed when pivalates 1a–1w are used (Scheme 5). The styrenyl isomer is likely favored due to conjugation of the olefin with the aryl group. Similar regioselectivity and support for a π-allyl intermediate was also obtained in the analogous arylation of γ-aryl allylic pivalates.11
Scheme 5. Regioselective borylation consistent with π-allyl nickel intermediate.
a ee of subsequent alcohol, formed via oxidation by H2O2 and NaOH.
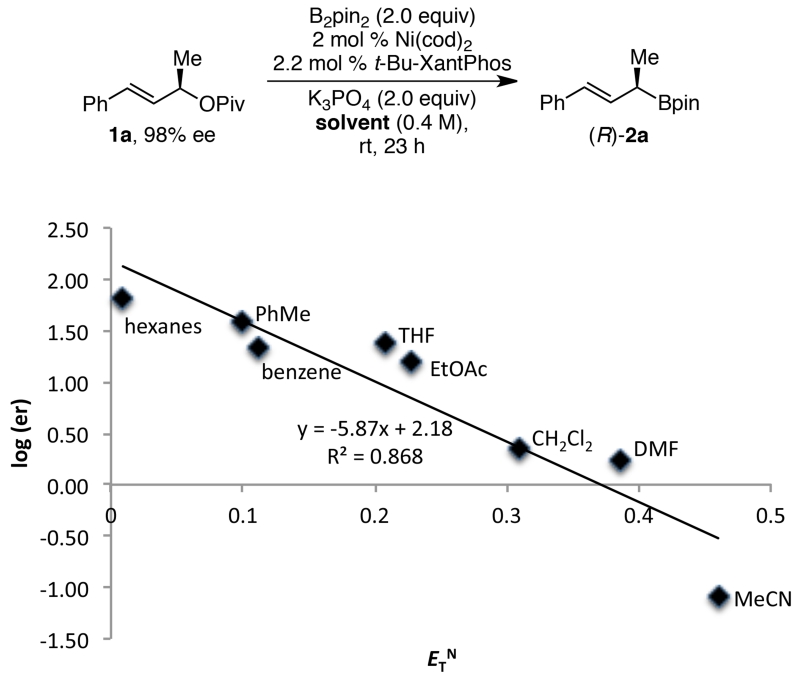
We next investigated the dramatic solvent effect. By performing the borylation of 1a under the conditions optimized for retention of configuration in a variety of solvents, we observed a general trend that the stereochemical fidelity of the reaction decreases as the polarity of the solvent increases, as shown in Figure 2, where ETN is an empirical parameter of solvent polarity.18 Similar trends are observed using other solvent parameters, including εr and the Kirkwood function. Notably, however, the result in MeCN falls distinctly off this trend, suggesting that MeCN’s role goes beyond a medium effect.
Figure 2.
Plot of log er vs. ETN.
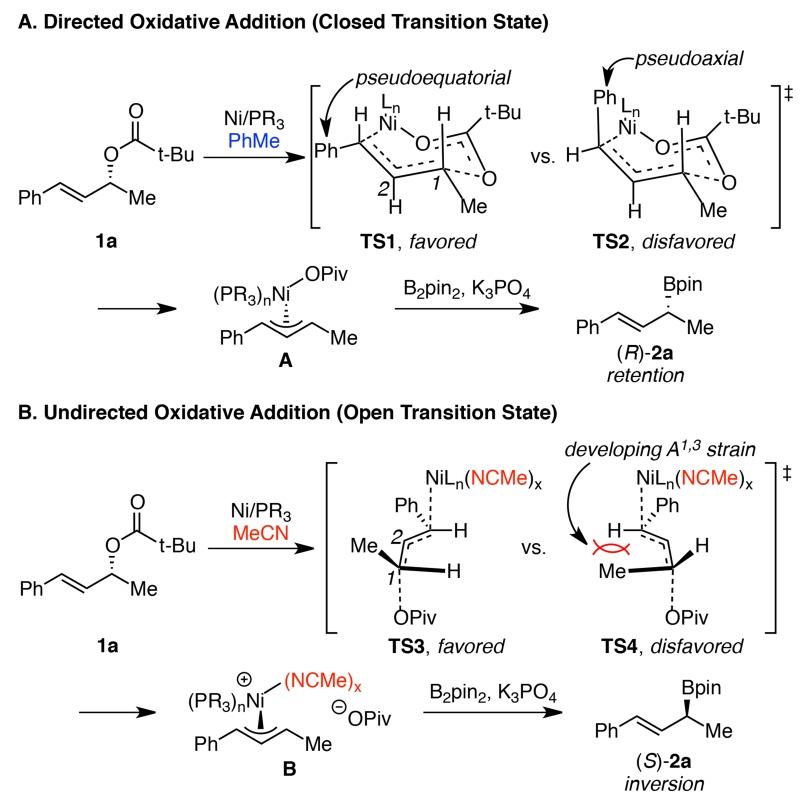
Consistent with previous hypotheses for stereoretentive oxidative addition into benzylic and allylic electrophiles,13a, 13c we propose that the pivalate leaving group directs the nickel catalyst in the oxidative addition when the reaction is run in nonpolar solvents, such as PhMe (Scheme 6A). Two 7-membered-ring, chair-like structures must be considered for this closed transition state. In TS1, the nickel catalyst adds to the re face of the alkene. Via rotation around the C1–C2 bond, the nickel catalyst adds to the si face in TS2. We propose that TS1 will be favored, because both the phenyl and methyl substituents reside in pseudoequatorial positions. This oxidative addition leads to overall retention of stereochemistry. In more polar solvents, an undirected, SN2’-type oxidative addition, which proceeds via the charge-separated intermediate B, begins to compete with Path A (Scheme 6B). However, it is only when MeCN is used as solvent that the stereochemical outcome flips from stereoretention to stereoinversion. Given MeCN’s propensity to act as a ligand for transition metals,14 we hypothesize that MeCN coordinates nickel, prohibiting the directed oxidative addition. In this case, two open transition states are possible. We propose that TS3 will be favored, because of destabilizing A1,3 strain between methyl and hydrogen in TS4. Oxidative addition via TS3 leads to the observed inversion of configuration.
Scheme 6. Proposed mechanism of allylic borylation with retention and inversion.
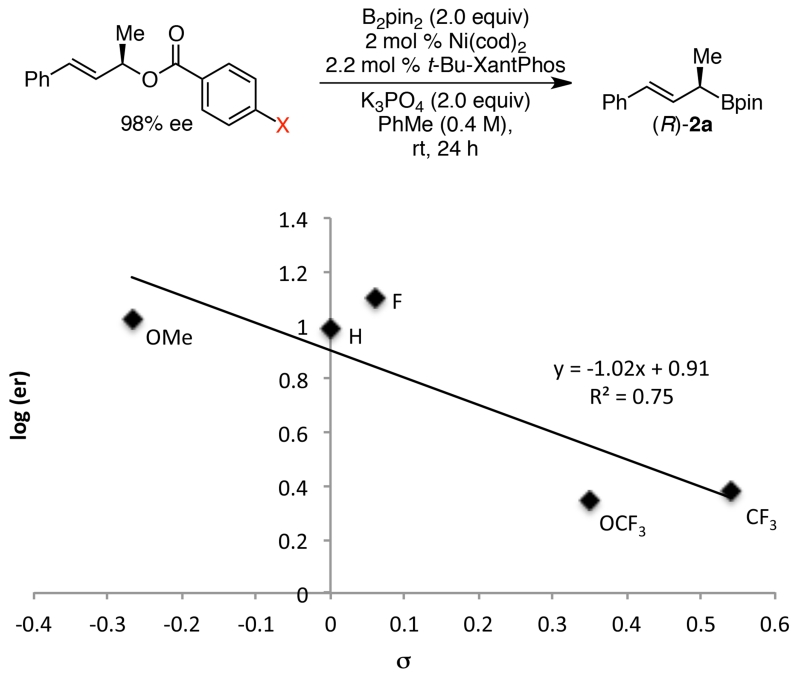
To support this mechanistic hypothesis, we undertook a series of experiments to probe the role of both carboxylate leaving group and nitrile additive. For these experiments, we have assumed that each enantiomer of product forms via a single pathway (Path A or B, Scheme 6). We can then use the enantiomeric ratio to reflect the relative rates between Path A and B. To probe the directing ability of the carboxylate, we replaced pivalate with a series of substituted benzoates in the borylation under conditions that lead to stereoretention (Figure 3). Under these conditions, higher stereochemical fidelity was observed for benzoates with more electron-donating groups. This trend is consistent with coordination of the carboxylate to the nickel catalyst in the stereoretentive pathway; more strongly coordinating carboxylates lead to higher stereochemical fidelity. We hypothesize that the moderate R2 value may be due to the fact that the carboxylate is both a directing group and a leaving group. Changing the leaving group identity affects both Path A and Path B.
Figure 3.
Hammett correlation between benzoate substituents and enantiomeric ratio.
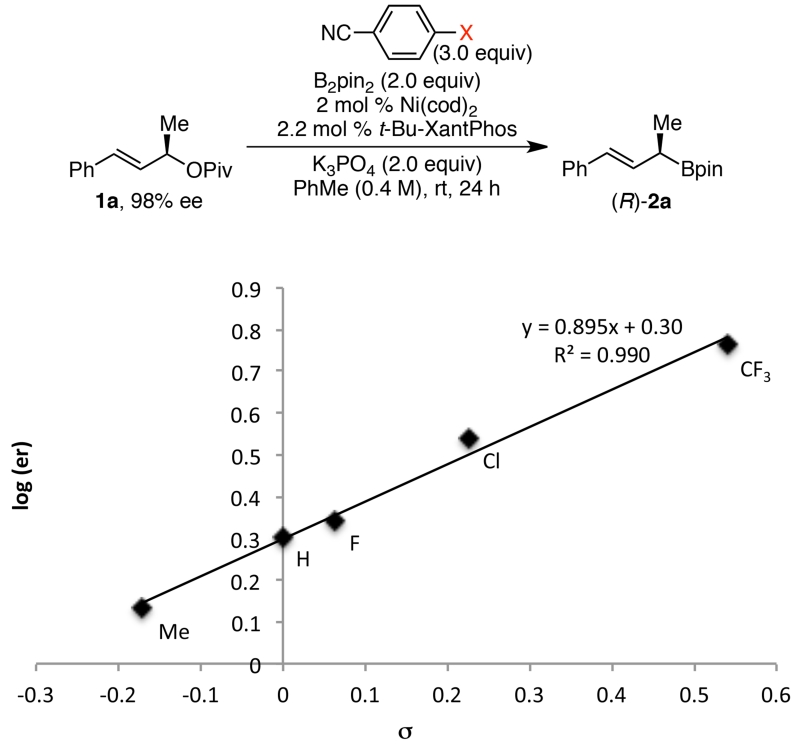
We also investigated the importance (and geometry) of nitrile coordination. Under the conditions optimized for stereoretention (Ni(cod)2, t-Bu-XantPhos, K3PO4, PhMe), we added para-substituted benzonitriles (3.0 equiv) to probe their effect on the stereochemical fidelity (Figure 4). Excellent correlation (R2 = 0.990) was observed between the electron-donating ability of the substituent and the stereospecificity. With more electron-rich benzonitriles, lower stereochemical fidelity is observed, consistent with acceleration of Path B via η1 coordination of the nitrile to the nickel catalyst. This acceleration makes Path B competitive with the stereoretentive Path A, leading to low ee’s. In sum, these correlations support dueling mechanisms involving directed vs. undirected oxidative addition steps, with both polar solvents and saturation of the nickel coordination sphere favoring the undirected stereoinvertive pathway.
Figure 4.
Hammett correlation between benzonitrile substituents and enantiomeric ratio.
CONCLUSION
In conclusion, we have developed complementary reaction conditions to enable the borylation of secondary allylic pivalates to deliver either enantiomer of highly enantioenriched α-stereogenic γ-aryl allylic boronates. This reaction harnesses allylic pivalates that are readily prepared in high enantiopurity, as well as commercially available nickel sources and ligands. Excellent scope and functional group tolerance were observed. The dramatic solvent effect over the stereospecificity appears to arise from competitive oxidative addition mechanisms. Support for the directing ability of the carboxylate, as well as the importance of nitrile coordination, was gained via a series of Hammett correlations. This is the first example of the synthesis of enantioenriched α-stereogenic γ-aryl allylic boronates via borylation of an allylic electrophile, and offers an efficient route to these versatile synthetic intermediates.
Supplementary Material
ACKNOWLEDGMENT
NIH (R01 GM111820) is gratefully acknowledged. NMR and other data were acquired at UD on instruments obtained with assistance of NSF and NIH funding (NSF CHE0421224, CHE1229234, CHE0840401, and CHE1048367; NIH P20GM104316, P20 GM103541, and S10OD016267).
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Experimental details and data (PDF)
The authors declare no competing financial interest.
REFERENCES
- 1.Lachance H, Hall DG. Organic Reactions. Vol. 73. John Wiley & Sons, Inc.; 2009. p. 1. [Google Scholar]
- 2.(a) Carreira EM, Kvaerno L. Classics in Stereoselective Synthesis. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim: 2009. p. 153. [Google Scholar]; (b) Roush WR. In: Methods in Organic Chemistry. Ahlbrecht H, Helmchean G, Arend M, Herrman R, editors. E 21. Georg Thieme Verlag; Stuttgart: 1995. p. 1410. [Google Scholar]; (c) Yamamoto Y, Asao N. Chem. Rev. 1993;93:2207. [Google Scholar]; (d) Chausset-Boissarie L, Ghozati K, LaBine E, Chen JLY, Aggarwal VK, Crudden CM. Chem. Eur. J. 2013;19:17698. doi: 10.1002/chem.201303683. [DOI] [PubMed] [Google Scholar]; (e) Chen JLY, Scott HK, Hesse MJ, Willis CL, Aggarwal VK. J. Am. Chem. Soc. 2013;135:5316. doi: 10.1021/ja401564z. [DOI] [PubMed] [Google Scholar]; (f) Althaus M, Mahmood A, Suarez JR, Thomas SP, Aggarwal VK. J. Am. Chem. Soc. 2010;132:4025. doi: 10.1021/ja910593w. [DOI] [PubMed] [Google Scholar]; (g) Ditrich K, Bube T, Stürmer R, Hoffmann RW. Angew. Chem., Int. Ed. 1986;25:1028. [Google Scholar]; (h) Fang GY, Aggarwal VK. Angew. Chem., Int. Ed. 2007;46:359. doi: 10.1002/anie.200603659. [DOI] [PubMed] [Google Scholar]; (i) Hoffmann RW, Dresely S. Tetrahedron Lett. 1987;28:5303. [Google Scholar]; (j) Peng F, Hall DG. Tetrahedron Lett. 2007;48:3305. [Google Scholar]
- 3.(a) Ma S-S, Mei W-L, Guo Z-K, Liu S-B, Zhao Y-X, Yang D-L, Zeng Y-B, Jiang B, Dai H-F. Org. Lett. 2013;15:1492. doi: 10.1021/ol4002619. [DOI] [PubMed] [Google Scholar]; (b) Huang J-Z, Zhang C-L, Zhu Y-F, Li L-L, Chen D-F, Han Z-Y, Gong L-Z. Chem. Eur. J. 2015;21:8389. doi: 10.1002/chem.201500349. [DOI] [PubMed] [Google Scholar]; (c) Pieters L, de Bruyne T, Claeys M, Vlietinck A, Calomme M, vanden Berghe D. J. Nat. Prod. 1993;56:899. doi: 10.1021/np50096a013. [DOI] [PubMed] [Google Scholar]; (d) Edmondson SD, Mastracchio A, Mathvink RJ, He J, Harper B, Park Y-J, Beconi M, Di Salvo J, Eiermann GJ, He H, Leiting B, Leone JF, Levorse DA, Lyons K, Patel RA, Patel SB, Petrov A, Scapin G, Shang J, Roy RS, Smith A, Wu JK, Xu S, Zhu B, Thornberry NA, Weber AE. J. Med. Chem. 2006;49:3614. doi: 10.1021/jm060015t. [DOI] [PubMed] [Google Scholar]; (e) Howbert JJ, Lobb KL, Britton TC, Mason NR, Bruns RF. Bioorg. Med. Chem. Lett. 1993;3:875. [Google Scholar]
- 4.(a) For the preparation of enantioenriched γ-aryl allylic boronates via a Johnson-Claisen rearrangement and their elaboration, see: Pietruszka J, Schöne N. Eur. J. Org. Chem. 2004;2004:5011. Tao Z-L, Li X-H, Han Z-Y, Gong L-Z. J. Am. Chem. Soc. 2015;137:4054. doi: 10.1021/jacs.5b00507. Das A, Alam R, Eriksson L, Szabó KJ. Org. Lett. 2014;16:3808. doi: 10.1021/ol501699x.
- 5.For the preparation and of γ-aryl allylic boronates from allylic boronates with terminal alkenes via olefin cross metathesis, see: Goldberg SD, Grubbs RH. Angew. Chem., Int. Ed. 2002;41:807. doi: 10.1002/1521-3773(20020301)41:5<807::aid-anie807>3.0.co;2-v. Hemelaere R, Carreaux F, Carboni B. Chem. Eur. J. 2014;20:14518. doi: 10.1002/chem.201403954. Hemelaere R, Carreaux F, Carboni B. Eur. J. Org. Chem. 2015;2015:2470. doi: 10.1021/jo400872x.
- 6.Ding J, Rybak T, Hall DG. Nature Communications. 2014;5:5474. doi: 10.1038/ncomms6474. [DOI] [PubMed] [Google Scholar]
- 7.(a) Beckmann E, Desai V, Hoppe D. Synlett. 2004:2275. [Google Scholar]; (b) Chen M, Roush WR. Org. Lett. 2010;12:2706. doi: 10.1021/ol1007444. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Matteson DS. Tetrahedron. 1998;54:10555. [Google Scholar]; (d) Matteson DS, Ray R. J. Am. Chem. Soc. 1980;102:7590. [Google Scholar]; (e) Peng F, Hall DG. J. Am. Chem. Soc. 2007;129:3070. doi: 10.1021/ja068985t. [DOI] [PubMed] [Google Scholar]; (f) Pulis AP, Aggarwal VK. J. Am. Chem. Soc. 2012;134:7570. doi: 10.1021/ja303022d. [DOI] [PubMed] [Google Scholar]
- 8.(a) Burks HE, Liu S, Morken JP. J. Am. Chem. Soc. 2007;129:8766. doi: 10.1021/ja070572k. [DOI] [PubMed] [Google Scholar]; (b) Carosi L, Hall DG. Angew. Chem., Int. Ed. 2007;46:5913. doi: 10.1002/anie.200700975. [DOI] [PubMed] [Google Scholar]; (c) Flamme EM, Roush WR. J. Am. Chem. Soc. 2002;124:13644. doi: 10.1021/ja028055j. [DOI] [PubMed] [Google Scholar]; (d) Guzman-Martinez A, Hoveyda AH. J. Am. Chem. Soc. 2010;132:10634. doi: 10.1021/ja104254d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Hicks JD, Flamme EM, Roush WR. Org. Lett. 2005;7:5509. doi: 10.1021/ol052322j. [DOI] [PubMed] [Google Scholar]; (f) Ito H, Ito S, Sasaki Y, Matsuura K, Sawamura M. J. Am. Chem. Soc. 2007;129:14856. doi: 10.1021/ja076634o. [DOI] [PubMed] [Google Scholar]; (g) Park JK, Lackey HH, Ondrusek BA, McQuade DT. J. Am. Chem. Soc. 2011;133:2410. doi: 10.1021/ja1112518. [DOI] [PubMed] [Google Scholar]; (h) Pelz NF, Woodward AR, Burks HE, Sieber JD, Morken JP. J. Am. Chem. Soc. 2004;126:16328. doi: 10.1021/ja044167u. [DOI] [PubMed] [Google Scholar]
- 9.(a) Ito H, Kawakami C, Sawamura M. J. Am. Chem. Soc. 2005;127:16034. doi: 10.1021/ja056099x. [DOI] [PubMed] [Google Scholar]; (b) Kliman LT, Mlynarski SN, Ferris GE, Morken JP. Angew. Chem., Int. Ed. 2012;51:521. doi: 10.1002/anie.201105716. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Potter B, Szymaniak AA, Edelstein EK, Morken JP. J. Am. Chem. Soc. 2014;136:17918. doi: 10.1021/ja510266x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Noh D, Yoon SK, Won J, Lee JY, Yun J. Chem. Asian J. 2011;6:1967. doi: 10.1002/asia.201100146. [DOI] [PubMed] [Google Scholar]; (b) Kitanosono T, Xu P, Kobayashi S. Chem. Commun. 2013;49:8184. doi: 10.1039/c3cc44324h. [DOI] [PubMed] [Google Scholar]
- 11.Srinivas HD, Zhou Q, Watson MP. Org. Lett. 2014;16:3596. doi: 10.1021/ol5016724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.For a related benzylic borylation, see: Basch CH, Cobb KM, Watson MP. Org. Lett. 2016;18:136. doi: 10.1021/acs.orglett.5b03455.
- 13.(a) For a ligand-controlled stereochemical switch, see: Harris MR, Hanna LE, Greene MA, Moore C, Jarvo ER. J. Am. Chem. Soc. 2013;135:3303. doi: 10.1021/ja311783k. (b) For a solvent- and base-dependent stereochemical switch, see: Nagao K, Yokobori U, Makida Y, Ohmiya H, Sawamura M. J. Am. Chem. Soc. 2012;134:8982. doi: 10.1021/ja302520h. (c) For a solvent-controlled stereochemical switch, see: Kurosawa H, Ogoshi S, Kawasaki Y, Murai S, Miyoshi M, Ikeda I. J. Am. Chem. Soc. 1990;112:2813. (d) For an acidic additive-controlled stereochemical switch, see: Awano T, Ohmura T, Suginome M. J. Am. Chem. Soc. 2011;133:20738. doi: 10.1021/ja210025q. (e) See also: Zhou Q, Srinivas HD, Dasgupta S, Watson MP. J. Am. Chem. Soc. 2013;135:3307. doi: 10.1021/ja312087x.
- 14.Rach SF, Kühn FE. Chem. Rev. 2009;109:2061. doi: 10.1021/cr800270h. [DOI] [PubMed] [Google Scholar]
- 15.(a) Corey EJ, Bakshi RK. Tetrahedron Lett. 1990;31:611. [Google Scholar]; (b) Corey EJ, Helal CJ. Tetrahedron Lett. 1995;36:9153. [Google Scholar]; (c) Sasaki M, Ikemoto H, Kawahata M, Yamaguchi K, Takeda K. Chem. Eur. J. 2009;15:4663. doi: 10.1002/chem.200802322. [DOI] [PubMed] [Google Scholar]
- 16.(a) Darses S, Genet J-P. Eur. J. Org. Chem. 2003;2003:4313. [Google Scholar]; (b) Molander GA, Figueroa R. Aldrichimica Acta. 2005;38:49. [Google Scholar]
- 17.(a) Li L, Zhao S, Joshi-Pangu A, Diane M, Biscoe MR. J. Am. Chem. Soc. 2014;136:14027. doi: 10.1021/ja508815w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang C-Y, Derosa J, Biscoe MR. Chem. Sci. 2015;6:5105. doi: 10.1039/c5sc01710f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reichardt C. Solvents and Solvent Effects in Organic Chemistry. 3rd ed. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim: 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.