Abstract
A major drawback with cancer chemotherapy is its severe toxic effects on non-target tissues. Assessment of natural products for their protective effect against anticancer drugs-induced toxicity is gaining importance in cancer biology. The present study was aimed at assessing the protective effect of hydroethanolic extract of Indian propolis (HEIP) against mitomycin C (MMC)-induced genotoxicity and cytotoxicity. Swiss albino mice were injected with various doses of HEIP (100, 200, 300, 400, 600 and 800 mg/kg b. wt., i.p) 1 h prior to MMC (8 mg/kg, i.p.) injection. The geno- and cyto-toxicities were evaluated in mice by performing bone marrow micronucleus and TUNEL assays. In vitro antioxidant and lipid peroxidation inhibitory assays were carried out to understand the mechanism of the protective effects. The significant increase in the frequency of micronculeated cells (12.51 ± 0.48), apoptotic cells (23.43 ± 1.86) and reduction in P/N ratio (0.69 ± 0.04) compared with control indicated the potential geno- and cytotoxic effects of MMC in bone marrow. Pretreatment with HEIP resulted in the significant recovery of the toxic effects induced by MMC. HEIP at 400 mg/kg b. wt. was found to be the optimum dose imparting the maximum protective effects. The in vitro antioxidant and lipid peroxidation inhibitory assays suggest that the extract possesses substantial free radical scavenging activities. In conclusion, HEIP possesses substantial geno- and cyto-protective properties against MMC, which could be mediated through efficient free radical scavenging and inhibitory effect on lipid peroxidation.
Keywords: Genotoxicity, Micronucleus, Apoptosis, Antioxidant activity
Introduction
Chemotherapy is still an inevitable treatment modality for cancer patients, although some advancements have been achieved in the treatment strategies. Unfortunately, patients have to experience severe side effects since many of the anticancer drugs induce genotoxicity and cytotoxicity in healthy cells. Mitomycin C (MMC), an aziridine-containing agent derived from Streptomyces caespitosus or S. lavendulae, is used as a chemotherapeutic agent for esophageal carcinoma, breast cancer, bladder tumors, gastric, cervical, and pancreatic adenocarcinoma (Crooke and Bradner 1976). MMC also has its therapeutic application in glaucoma and strabismus surgeries (Iwao et al. 2014). The drug acts by two mechanisms, namely bio-reductive alkylation and generation of free radicals such as superoxide and hydroxyl radicals through its metabolic activation (Dorr et al. 1985; Dusre et al. 1989). The major side effects of MMC are bone marrow depression, lung fibrosis and renal damage (Crooke and Bradner 1976). Considering the therapeutic usefulness of MMC it is of practical relevance to find out the possible strategy to prevent/minimize its toxic effects to non-target tissues/cells. In this context, explorations of new beneficial properties and scientific validation of natural products used in the traditional medicinal systems are gaining research attention in the recent years.
Propolis, a resinous mixture composed of various plant products collected by honey bees to construct the hive, is known for certain medicinal values (Kimoto et al. 1999). Earlier studies have ascertained that propolis extracts possess antimicrobial (Astani et al. 2013), anticancer (Watanabe et al. 2011), immunomodulatory, anti-inflammatory (Bolfa et al. 2013) and radioprotective activities (Benkovic et al. 2009). It is enriched with potent antioxidant molecules, including flavonoids and polyphenols (Kurek-Górecka et al. 2013; Pellati et al. 2013; Piccinelli et al. 2013). Therefore, it may have the beneficial role in preventing the normal tissue from MMC induced genotoxicity. Propolis extracts have been evaluated for their genoprotective effect against certain xenobiotics (Fu et al. 2004; Benguedouar et al. 2008; Abdulrhman et al. 2012; Rizk et al. 2014). However, the possible protective effect of propolis extracts against the potent genotoxic agent MMC has not been explored. Further, propolis collected from different geographical regions varies in their chemical/antioxidant composition (Piccinelli et al. 2013). In this context, the present study was undertaken to assess the protective role of hydroethanolic extract of Indian propolis (HEIP) against MMC-induced geno- and cytotoxic effects in bone marrow using Swiss albino mice as the experimental model.
Methods
Chemicals
Mitomycin C (C15H18N4O5; CAS RN 50-07-7), manufactured and marketed by Kyowa, Biochem Pharmaceutical Industries Limited (Mumbai, India) was used as the genotoxic agent. Linoleic acid and trolox (6-hydroxy-2,5,7,8-tetramethylchroman 2- carboxylic acid) were purchased from HiMedia Laboratories Pvt. Ltd. (Mumbai, India). 2,2′-azobis-(2-amidinopropane) dihydrochloride (AAPH) was procured from Sigma-Aldrich Inc. (St. Louis, MO, USA). All other chemicals and reagents were obtained from Merck India (Delhi, India) and SRL India (Maharashtra, India).
Mitomycin C (MMC)
The MMC powder was reconstituted with sterile phosphate-buffered saline (PBS, pH- 7.4) to obtain a concentration of 1 mg/mL.
Hydroethanolic extract of propolis (HEIP)
After complete separation of honey from the locally collected bee hive, the resinous material was chopped into small pieces and enclosed in Whatman filter paper. Cold extraction of propolis was performed at room temperature by immersing the bee hive in freshly prepared 50 % ethanol for 48 h. The extract thus collected was filtered using cotton bed, concentrated in rotary evaporator and freeze dried by lyophilization. The final extract was protected from light and stored at 4 °C till its use. Each time, fresh HEIP solution was prepared by dissolving the lyophilized extract in PBS (pH-7.4) just before the treatment.
Animal model
Male Swiss albino mice were obtained from the Central Animal Research Facility (Kasturba Medical College, Manipal University, Manipal, India). Mice were housed under standard animal husbandry conditions (temperature: 23 ± 2 °C, humidity: 55 ± 5 %) and allowed access to standard commercial diet and acidified tap water ad libitum. Prior approval (IAEC/KMC/80/2013) was obtained from the Institutional Animal Ethics Committee for the present study.
Dose and treatment schedule
Male Swiss albino mice (8 weeks old, body weight 30 ± 2 g) were divided into 4 groups of 6 animals each and were treated as follows.
Control: Phosphate buffered saline (i.p)
MMC alone: 8 mg/kg b. wt. (i.p)
HEIP alone: HEIP at 100, 200, 300, 400, 600 and 800 mg/kg b. wt (i.p)
HEIP + MMC: Injected with various doses (100, 200, 300, 400, 600 and 800 mg/kg) of HEIP (i.p), followed by i.p. injection of MMC at 8 mg/kg b. wt. 1 h later
Micronucleus (MN) assay
The mice were sacrificed at 24 h after various treatments and magnitude of the chromosomal damage was assessed by determining the frequency of micronucleated cells in bone marrow as described by Jagetia and Reddy (2002) with minor modifications. Briefly, bone marrow from tibia and femur was flushed with PBS (pH-6.9) to obtain a fine cell suspension. The cell suspension was centrifuged at 1000 rpm for 10 min. A part of the suspension was subjected to hypotonic treatment with 0.56 % of KCl for 2 min followed by centrifugation at 1000 rpm for 4 min. The pellet obtained after decanting the supernatant liquid, was fixed in Carnoy’s fixative (Methanol:acetic acid::3:1) and incubated at 4 °C for 30 min. The cell suspension was centrifuged again and mixed with freshly prepared Carnoy’s fixative. After processing, a thin layer of bone marrow suspension was made on the chilled microscopic slides. The slides were air dried at room temperature and coded to avoid any observer bias. Smears were stained with Acridine orange solution (0.002 %) in Sorenson’s buffer (pH 6.8) for 2 min followed by washing in Sorenson’s buffer to remove any non-specific staining. A total of 2000 cells per animal were scored under fluorescence microscope (Zeiss, Oberkochen, Germany) to assess the frequency of micronucleated cells (MacGregor et al. 1987) and the data were expressed in percentage.
Polychromatic erythrocyte (PCE) and normochromatic erythrocytes (NCE) ratio (P/N ratio)
The remaining part of the bone marrow was gently dispersed in a test tube containing 2 mL of fetal calf serum (FCS) to achieve a fine single cell suspension. The cells were centrifuged at 1000 rpm for 10 min and the resultant pellet was resuspended in a drop of FCS. Smears were prepared on the clean slides, and air dried. After 24 h, slides were stained with May-Grunwald/Giemsa as described by Schmid (1975). In order to determine the cytotoxicity, PCE/NCE (P/N) ratio was determined by scoring 2000 polychromatic erythrocytes (PCE) and corresponding normochromatic erythrocytes (NCE).
TUNEL assay
The bone marrow cells were flushed in PBS with 0.1 % BSA and centrifuged at 1000 rpm for 10 min. The cells were then fixed on a clean glass slide using 4 % paraformaldehyde (PFA) at 4 °C for 20 min. Cells were washed in PBS and incubated with permeabilization buffer (0.5 % Triton X-100 and 0.1 % sodium citrate in PBS with 0.1 % BSA) at room temperature for 1 h. After washing with PBS containing 0.1 % BSA (3 × 5 min), the cells were incubated with TUNEL mixture (TUNEL reaction mixture: labeling solution = 1:20) for 1 h at 37 °C. The cells were then washed in PBS (3 × 5 min), counterstained with DAPI (4 μg/ml) and observed under fluorescence microscope (Imager-A1, Zeiss). A total of 1000 cells was scored for the frequency of TUNEL positive cells and values were expressed as percentage of apoptotic cells.
Screening of antioxidant/free radical scavenging activity
DPPH free radical scavenging activity
Radical scavenging (hydrogen donating) ability of HEIP was determined using the stable α,α-diphenyl-β-picrylhydrazyl (DPPH) radical as described by Brand-Williams et al. (1995) with minor modifications (Yoo et al. 2008). A series of test tubes containing blank, standards (S1–S2) and tests (T1–T2) were added with ascorbic acid and HEIP, respectively, in a concentration ranging from 5, 10, 25, 50 and 75 µg/mL. Volume in all test tubes was made up to 2.0 mL with distilled water. One mL of DPPH (0.4 mg/mL) was then added to all test tubes and the tubes were mixed well with the help of cyclomixer. These solution mixtures were incubated at 37 °C for 30 min in dark. Optical density (OD) was measured at 520 nm using Double beam UV–Vis spectrophotometer.
The ability of the extract and positive control to scavenge DPPH free radical was calculated using the formula: Radical scavenging rate (%) = [1 − (A1 − A2)/A0] × 100, where A0 is the absorbance of the control (without sample) and A1 is the absorbance in the presence of the sample, A2 is the absorbance of sample without DPPH radical.
Evaluation of total antioxidant activity (TAA)
The TAA of HEIP extract was evaluated by method described by Prieto et al. (1999), which is based on the principle of reduction of molybdenum (Mo) (VI) to Mo (V) giving rise to phosphomolybdenum complex. Briefly, 1.0 ml of freshly prepared reagent (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate) was added to a series of test tubes containing blank, standards (S1–S5) and tests (T1–T5). The standards and tests were added with ascorbic acid and HEIP, respectively, at concentrations ranging from 5, 10, 25, 50, 75 and 100 µg/mL. The tubes were capped and incubated in a boiling water bath at 95 °C for 90 min. After the samples were cooled to room temperature, the absorbance was measured at 695 nm against the blank using UV–visible spectrophotometer. The total antioxidant activity was expressed as percentage activity by using the formula, percentage of antioxidant activity = A(s) − A(b)/A(b) × 100, where A(s) is Absorbance of the sample and A(b) is the Absorbance of the blank. Calibration curve was constructed taking % of inhibition on Y axis against concentration gradient and EC50 was determined and expressed as µg/mL.
Ferric reducing antioxidant power (FRAP) assay
Ability of the HEIP to reduce ferric ions was determined following the method described by Benzie and Strain (1996) with minor modifications. Briefly, 2.7 mL of FRAP reagent [300 mM acetate buffer (pH 3.6), 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ) in 40 mM hydrochloric acid and 20 mM ferric chloride were mixed in ratio of 10:1:1] in a series of test tubes (Blank, S1–S2 and T1–T2). Calibration curve was constructed for the standard (ascorbic acid) and test sample (HEIP) in a concentration range of 20, 40, 60 80, 100 and 120 µg/mL. Test tubes were incubated in a water bath for 30 min at 37 °C and the absorbance of the samples was determined against blank at 593 nm. The effective concentrations (EC50) for the standard and HEIP were determined from the calibration graph providing 0.5 of absorbance at 593 nm. The assay was carried out in triplicate and the values obtained were expressed as mg/mL ± standard deviation.
Lipid peroxidation inhibitory activity
Lipid peroxidation inhibitory activity of the HEIP was determined by employing the method of Liegeois et al. (2000). Briefly, the lipid peroxidation of linoleic acid was induced by water soluble azo compound 2,2′-azobis(2-amidinopropane) dihydrochloride which acts as alkylperoxy free radicals (AAPH). Freshly prepared aqueous solution (30 μL) of 16 mM linoleic acid was added to the test tubes. The peroxidation reaction was initiated at 37 °C under air by adding 150 μL of 40 mM AAPH solution (in the absence of free radical, the rate of spontaneous oxidation in the air at 37 °C is considered negligible). Effect of HEIP on the oxidation of linoleic acid by AAPH was carried out by adding the same in the concentration range of 10, 100, 200, 500 and 1000 μg/mL. The final volume in each test was made up to 2.8 mL by adding 0.05 mM phosphate buffer (pH 7.4). The experiment was also performed for the trolox solution (10, 100, 200, 500 and 1000 μg/mL) taken as the reference standard. Rate of the production of conjugated diene hydroperoxide by oxidation of linoleic acid incubated at 37 °C for 10 min was monitored by measuring the OD at 234 nm in UV–visible spectrophotometer. The percentage inhibition of lipid peroxidation was calculated by the following formula: Percentage of Inhibition (%) = (C–T) ÷ C × 100, where C is the absorbance of the control reaction and T is the absorbance in the presence of the samples (HEIP/trolox).
HPTLC fingerprinting of HEIP
HPTLC conditions
Stationary phase consisted of 10 × 10 cm, 0.2 mm thick, pre-coated silica gel 60F254 HPTLC plates (E. Merck) with aluminum as a supporting material. CAMAG’s Linomat-5 automatic sample applicator (Camag, Muttenz, Switzerland) was used to apply sample solution on the plate, with the help of 100 μL (Hamilton, Bonaduz, Switzerland) syringe. Sample application was performed with nitrogen gas. Mobile phase 10 mL was prepared in CAMAG glass twin trough chamber (15 × 15 cm). Chamber was saturated with mobile phase for 20 min. Densitometric scan was performed using CAMAG TLC Scanner 3 operated by WINCAT software. Photo documentation was carried out using CAMAG Reprostar-3 at 366 nm and visible light.
Fingerprint of HEIP by HPTLC
Mobile phase used was toluene: acetone: methanol: formic acid (5:3:1:1). Sample was prepared (2 mg/mL) in methanol and applied in the form of band (6 × 0.45 mm). Plate was developed up to the distance of 85 mm from the bottom. Plate was air dried and scanned at 365 nm. Plate was derivatised with anisaldehyde sulphuric acid reagent. After derivatisation plate was heated at 115 °C for 10 min, scanned at 590 nm and Rf values were reported (Thirugnanasampandan et al. 2012).
Quantification of quercetin in HEIP
Quercetin was quantified in the extract using HPTLC by following standardized method. Standard quercetin (98 %) solution was prepared at the concentration of 100 μg/mL in methanol. HEIP was dissolved in methanol to get concentration of 6 mg/mL. 10 μL of sample was applied in the form of band (6 × 0.45 mm). Plate was developed in toluene: ethyl acetate: formic acid (5:4:1) and scanned at 375 nm and peak area was noted. Rf value for quercetin was found to be 0.60. Percentage content of quercetin was reported as described by Tandon and Sharma (2010).
Statistical analysis
The statistical analysis of the results was performed with the help of GraphPadInStat 3 package (GraphPad Software Inc, San Diego, CA, USA). One-way ANOVA test was applied to compare the differences among the groups. Differences were considered to be statistically significant if P < 0.05. All the antioxidant assays were performed in triplicates and standard deviation was applied for the mean values.
Results
Effect of HEIP on MMC-induced cytotoxicity
The ratio of PCE and NCE in bone marrow of control mice was 0.94 ± 0.01 (Table 1). Administration of HEIP did not induce any cytotoxic effect as the P/N ratio was similar to that of the control (0.97–1.07). MMC exhibited a severe cytotoxic effect as indicated by the significant decrease in the P/N ratio to 0.69 ± 0.04 (P < 0.001). Administration of HEIP 1 h before MMC injection was able to reduce the cytotoxic effect of MMC in a dose-dependent manner. HEIP at 300 and 400 mg/kg b.wt., imparted the maximum protective effect against MMC-induced cytotoxicity (P < 0.05). However, the cytoprotective effect of HEIP was in declining trend from 400 mg/kg onwards.
Table 1.
Effect of various doses of hydroalcoholic extract of Indian propolis extract (HEIP) on MMC-induced micronucleus in bone marrow cells of Swiss albino mice at 24 h after injection (N = 12)
| Groups | MN incidence (%) | P/N ratio |
|---|---|---|
| Control | 0.31 ± 0.07 | 0.94 ± 0.01 |
| HEIP 100 | 0.33 ± 0.33 | 0.97 ± 0.06 |
| HEIP 200 | 0.05 ± 0.05 | 1.05 ± 0.02 |
| HEIP 300 | 0.03 ± 0.03 | 1.02 ± 0.02 |
| HEIP 400 | 0.18 ± 0.06 | 1.07 ± 0.04 |
| HEIP 600 | 0.21 ± 0.07 | 1.04 ± 0.04 |
| HEIP 800 | 0.02 ± 0.02 | 1.06 ± 0.02 |
| MMC (8 mg/kg) | 12.51 ± 0.48a | 0.69 ± 0.04d |
| HEIP 100 + MMC | 8.13 ± 0.45c | 0.71 ± 0.02 |
| HEIP 200 + MMC | 7.74 ± 0.44c | 0.69 ± 0.01 |
| HEIP 300 + MMC | 7.49 ± 0.81c | 0.81 ± 0.03e |
| HEIP 400 + MMC | 6.93 ± 0.66c | 0.82 ± 0.02e |
| HEIP 600 + MMC | 9.91 ± 0.93b | 0.70 ± 0.04 |
| HEIP 800 + MMC | 11.57 ± 0.54 | 0.64 ± 0.01 |
a P < 0.001 compared to control
b P < 0.05, c P < 0.001 compared to MMC alone for MN
d P < 0.001 compared to control
e P < 0.05 compared to MMC alone for P/N ratio
Effect of HEIP on MMC-induced micronucleus formation
The bone marrow of control mice had 0.31 ± 0.07 % micronucleated cells (Table 1). Administration of HEIP alone did not show any significant genotoxic effect even at the highest dose (800 mg/kg). However, in the MMC treated mice, a significantly higher percentage of micronucleated cells (12.51 ± 0.48) were observed when compared with the control group (P < 0.001). Administration of HEIP 1 h before MMC injection resulted in a dose-dependent inhibition of MMC-induced MN formation up to 400 mg/kg body weight above which, the protective effect started declining. Thus, the optimum protective effect of HEIP was observed at 400 mg/kg b. wt., in which bone marrow had almost two times lower percentage of micronucleated cells (6.93 ± 0.66) compared to MMC alone treated group (P < 0.001). However, above this dose of HEIP, the protective effect started decreasing with further increase in the dose.
Effect of HEIP on MMC-induced apoptosis
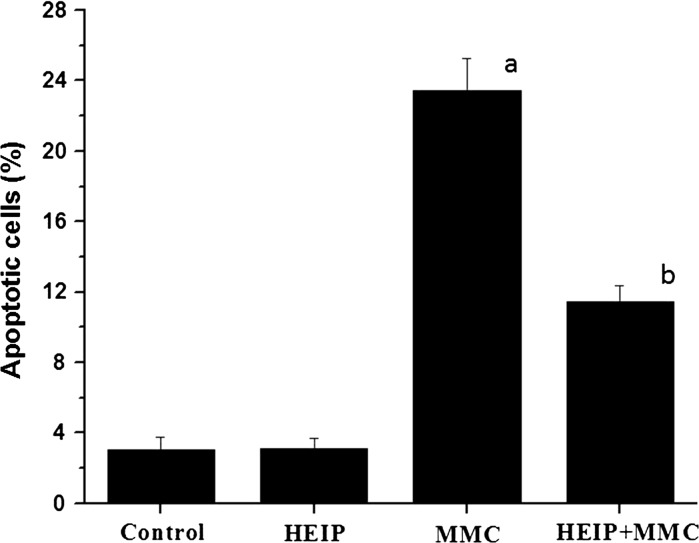
The frequency of apoptotic cells in bone marrow of control mice was 3.08 ± 0.68 % which did not differ in HEIP group (3.13 ± 0.6) (Fig. 1). However, the MMC administration resulted in a significant increase in the percentage of apoptotic cells (23.43 ± 1.86, P < 0.001) compared with the control. The frequency of apoptotic cells was significantly reduced (P < 0.001) as indicated by the comparison between MMC alone treated and MMC with HEIP treated group.
Fig. 1.
Effect of hydroalcoholic extract of Indian propolis (HEIP) against MMC (8 mg/kg b. wt.) induced apoptosis in bone marrow of Swiss albino mice: TUNEL assay (N = 6). a P < 0.001 versus Control; b P < 0.001 versus MMC
DPPH radical scavenging activity
HEIP possesses a good DPPH radical scavenging activity as indicated by increase in the percentage of inhibition with increase in the concentration of the extract (Table 2). The percentage of inhibition from the lowest (5 µg/mL) to the highest concentration (75 µg/mL) of the HEIP ranges between 12.9 ± 0.89 and 95.6 ± 2.54. The EC50 of HEIP was found to be 16.56 ± 1.29 µg/mL, which is almost similar to the value obtained for ascorbic acid (12.39 ± 1.34 µg/mL), taken as the reference standard.
Table 2.
Scavenging effect of hydroethanolic extract of Indian propolis (HEIP) and ascorbic acid on 2,2-diphenyl-1-picrylhydrazyl free radical (DPPH)
| Conc. (µg) | HEIP | Positive control (ascorbic acid) | ||
|---|---|---|---|---|
| % of inhibition (mean ± SD) | EC50 (µg/mL) (mean ± SD) | % of inhibition (mean ± SD) | EC50 (µg/mL) (mean ± SD) | |
| 5 | 12.9 ± 0.89 | 16.56 ± 1.29 | 27.56 ± 1.34 | 12.39 ± 1.34 |
| 10 | 29.8 ± 1.08 | 41.43 ± 0.92 | ||
| 25 | 69.4 ± 1.76 | 82.24 ± 1.67 | ||
| 50 | 82.7 ± 1.56 | 93.49 ± 2.08 | ||
| 75 | 95.6 ± 2.54 | 97.85 ± 1.87 | ||
Total antioxidant activity
The total antioxidant activity expressed as EC50 values was calculated based on percent inhibition (Table 3). As the concentration of HEIP increased from 5 to 100 µg/mL, the activity was increased as shown by percentage inhibition from 18.69 to 96.8. The EC50 value determined from the calibration curve was found to be 27.51 ± 1.56. L-ascorbic acid, the positive control exhibited a good antioxidant activity showing the EC50 value of 13.72 ± 1.89. In comparison, HEIP is not as effective as ascorbic acid in possessing the total antioxidant activity.
Table 3.
Total antioxidant activity of hydroethanolic extract of Indian propolis (HEIP) and ascorbic acid
| Conc. (µg) | HEIP | Ascorbic acid | ||
|---|---|---|---|---|
| % of inhibition (mean ± SD) | EC50 (µg/mL) (mean ± SD) | % of inhibition (mean ± SD) | EC50 (µg/mL) (mean ± SD) | |
| 5 | 18.69 ± 1.79 | 27.51 ± 1.56 | 24.63 ± 2.49 | 13.72 ± 1.89 |
| 10 | 31.16 ± 2.37 | 37.30 ± 3.11 | ||
| 25 | 47.33 ± 2.82 | 78.43 ± 2.88 | ||
| 50 | 78.77 ± 2.74 | 94.67 ± 2.58 | ||
| 75 | 93.21 ± 4.24 | 97.05 ± 1.45 | ||
| 100 | 96.80 ± 1.43 | 98.19 ± 1.56 | ||
FRAP assay
HEIP exhibited free radical scavenging activity in terms of ferric reducing antioxidant power as indicated by increase in the absorbance at 593 nm with increase in the concentration (Table 4). The lowest concentration of HEIP (20 µg/mL) showed the absorbance 0.081 ± 0.001, while that of the highest concentration (120 µg/mL) was found to be 0.573 ± 0.003. EC50 value derived from the calibration curve was found to be 105.73 ± 2.47 µg/mL. The reference standard, ascorbic acid gave the EC50 value, 56.29 ± 3.21 µg/mL, indicating that HEIP has FRAP almost half to that of ascorbic acid.
Table 4.
Ferric reducing antioxidant power (FRAP) assay for hydroethanolic extract of Indian propolis (HEIP) and ascorbic acid
| Conc. (µg) | HEIP | Ascorbic acid | ||
|---|---|---|---|---|
| Absorbance at 593 nm (mean ± SD) | EC50 (µg/mL) (mean ± SD) | Absorbance at 593 nm (mean ± SD) | EC50 (µg/mL) (mean ± SD) | |
| 20 | 0.081 ± 0.001 | 105.73 ± 2.47 | 0.177 ± 0.003 | 56.29 ± 3.21 |
| 40 | 0.193 ± 0.001 | 0.371 ± 0.004 | ||
| 60 | 0.281 ± 0.002 | 0.521 ± 0.002 | ||
| 80 | 0.376 ± 0.004 | 0.723 ± 0.006 | ||
| 100 | 0.486 ± 0.002 | 0.874 ± 0.003 | ||
| 120 | 0.573 ± 0.003 | 1.131 ± 0.007 | ||
Lipid peroxidation inhibitory activity
HEIP exhibited a dose-dependent inhibitory activity on lipid peroxidation (Table 5) which ranged from 2.83 ± 0.84 to 61.48 ± 2.24 %. The calibration curve showed the EC50 value of HEIP for its lipid peroxidation inhibitory activity being 710.00 ± 4.53 µg/mL. Though, HEIP was found to possess a substantial lipid peroxidation inhibitory activity, it is not as potent as trolox, used as the reference standard, which yielded the EC50 value of 170.00 ± 2.84 (µg/mL).
Table 5.
Inhibition of lipid peroxidation activity by hydroethanolic extract of Indian propolis (HEIP) and trolox
| Conc. (µg) | HEIP | Trolox | ||
|---|---|---|---|---|
| % Of Inhibition (Mean ± Sd) | EC50 (µg/mL) (mean ± SD) | % of inhibition (mean ± SD) | EC50 (µg/mL) (mean ± SD) | |
| 10 | 2.83 ± 0.84 | 710.00 ± 4.53 | 7.34 ± 0.93 | 170.00 ± 2.84 |
| 100 | 8.76 ± 1.78 | 24.13 ± 1.49 | ||
| 200 | 19.32 ± 1.96 | 53.36 ± 3.23 | ||
| 500 | 43.73 ± 2.43 | 79.43 ± 2.54 | ||
| 1000 | 61.48 ± 2.24 | 91.31 ± 4.03 | ||
HPTLC fingerprinting of HEIP
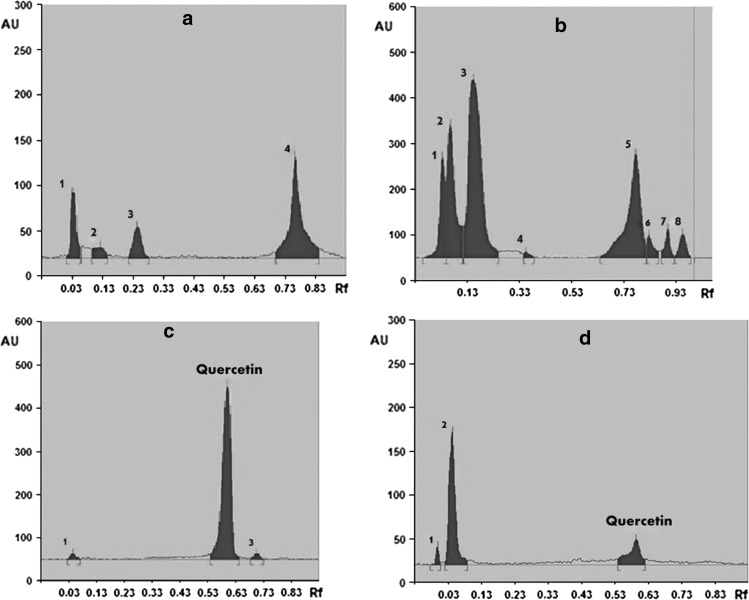
Chromatography allows the separation and identification of phyto-constituents in the extracts/mixture of constituents. It is also useful to develop finger printing profile for a particular extract. In this study, we have developed fingerprint for HEIP. Various mobile phase compositions were attempted to get a better separation of the compounds. A better separation was obtained with mobile phase containing toluene, acetone, methanol, and formic acid. We were able to separate few polar compounds using this mobile phase. Wavelength of 365 nm was found to be more appropriate for visualization of different compounds in this mobile phase (Fig. 2a). Rf values of different peaks of the extracts are given in Table 6. This fingerprint pattern can be used as quality control tool for propolis extracts. Further, the HEIP used in the present study contained 0.12 % of quercetin (Fig. 2b), as quantified using HPTLC.
Fig. 2.
a Finger print profile of hydroalcoholic extract of Indian propolis (HEIP) at the wavelength of 365 nm. b Finger print profile of hydroalcoholic extract of Indian propolis (HEIP) at the wavelength of 590 nm. c HPTLC chromatogram of quercetin standard. d HPTLC chromatogram of hydroalcoholic extract of Indian propolis (HEIP) for quercetin
Table 6.
Rf values of hydroethanolic extract of Indian propolis (HEIP) at 365 nm and 590 nm using HPTLC
| Wavelength | Peak number | Rf value | Area (%) |
|---|---|---|---|
| 365 nm | 1 | 0.03 | 18.88 |
| 2 | 0.12 | 6.60 | |
| 3 | 0.24 | 13.68 | |
| 4 | 0.76 | 60.84 | |
| 590 nm | 1 | 0.03 | 9.48 |
| 2 | 0.06 | 18.36 | |
| 3 | 0.15 | 40.36 | |
| 4 | 0.35 | 0.59 | |
| 5 | 0.78 | 23.46 | |
| 6 | 0.83 | 2.40 | |
| 7 | 0.90 | 2.53 | |
| 8 | 0.96 | 2.82 |
Discussion
Hydroethanolic extract of Indian propolis containing both polar and non-polar constituents, was evaluated for the protective effect against MMC-induced genotoxicity and cytotoxicity. The yield of the extract was 21.34 % (weight/weight). As depicted in Table 1, a high frequency of micronucleated cells (in both PCE and NCE) observed in MMC treated mice compared with the control (P < 0.001) confirms the genotoxic potency of the drug which agrees with previous reports (Fu et al. 2004; Ortega-Gutiérrez et al. 2009). Earlier studies have demonstrated that the genotoxic effect of MMC is also mediated through generation of free radicals (Dorr et al. 1985; Dusre et al. 1989), specifically, hydroxyl and superoxide radicals. In addition to its clastogenic action, MMC also induces the formation of MN through spindle damage (Miller et al. 1991).
The genoprotective effect of HEIP against MMC was clearly evident in terms of significant reduction in the frequency of micronucleated cells and also reduction in the percentage of apoptotic cells (Fig. 1). This observation is comparable to earlier reports where propolis extracts were shown to reduce the genotoxic effect against various positive agents both in vitro (Türkez et al. 2010, 2012, 2013) and in vivo (Fu et al. 2004). Though HEIP exhibited the genoprotective effect at all doses (except at the highest dose), 400 mg/kg of HEIP was found to be the optimum dose imparting the maximum effect against MMC (8 mg/kg) which is equivalent to 32.43 mg/kg body weight when extrapolated to human (Reigner and Blesch 2002). Probably, at higher doses some of the constituents of propolis extract act as prooxidants rather than the antioxidants. Similar observation was made by an earlier study where, an increase in the dose of protective/antioxidant agents beyond the optimum dose was not effective against an alkylating agent-induced genotoxicity (Prasad et al. 2002). Bouayed and Bohn (2010) discussed in their review that higher doses of exogenous antioxidants may act as prooxidants and disrupt redox balance. Since the extract alone did not induce the formation of MN cells even at 800 mg/kg b. wt., the extract may not possess the prooxidant activity on its own at higher concentration. It is, however, possible that few of the constituents present in the extract may interfere with the free-radical scavenging reaction of antioxidants at higher concentration, which will be interesting to study further.
One of the mechanisms of propolis-induced genoprotective effect against MMC is through its antioxidant/free radical scavenging activity, which has been demonstrated by earlier studies (Kurek-Górecka et al. 2013; Pellati et al. 2013; Piccinelli et al. 2013). Since oxidation is a very complex process with different mechanisms of origin, we assessed the antioxidant efficacy of HEIP using various in vitro methods, and compared with L-ascorbic acid for its efficacy.
In all the three in vitro antioxidant assays, HEIP exhibited the potential free-radical scavenging activity as efficient as that of L-ascorbic acid (Tables 2, 3, 4). In addition, it exhibited substantial anti-lipid peroxidation properties (Table 5). Indian propolis is composed of several antioxidant molecules, including flavonoids such as pinocembrin and galangin and, polyphenolic compounds (Kimoto et al. 1999). Kumazawa et al. (2004) screened propolis derived from different geographic regions for their antioxidant property and found that propolis containing kaempferol (a flavonoid) and phenethylcaffeate (a phenolic) showed relatively strong activity. These two active principles may also be present in Indian propolis, which needs to be screened to give further insight about their roles in HEIP-induced geno-protective effects.
Decrease in polychromatic erythrocytes (PCE) in comparison to mature (normochromatic) erythrocytes (NCE) generally indicates mito-depressive effect and bone marrow toxicity (Suzuki et al. 1989). A significant reduction in P/N ratio compared with the control (P < 0.001) was observed in MMC treated mice. It confirms the mitotic-depressive/cytotoxic effect of MMC, and this observation is in parallel with the previous reports (Liu et al. 2002; Borges et al. 2013). Since the most common complication in MMC treated patients is bone marrow depression (Molyneux et al. 2005), one of the objectives of the current study was to evaluate the possible protective effect of HEIP against MMC-induced cytotoxicity. A significant rise in P/N ratio (recovery) in combination treatment comparing with the drug alone administered group is a clear indication that HEIP protects the bone marrow cells from the cytotoxic effect of MMC (Table 1). More importantly, HEIP did not induce any drastic change in P/N ratio from the dose 100–800 mg/kg b. wt. when compared with the control indicating that it is not cytotoxic even up to 800 mg/kg b. wt. Comparison of P/N ratio between MMC treated group and MMC with HEIP pretreated group at different doses indicates that there was no significant increase in P/N ratio by HEIP at lower concentrations (100 and 200 mg/kg b. wt.). Increase in P/N ratio at significant level (P < 0.05) was observed at 300 and 400 mg/kg b. wt., and the effect was almost similar for both doses of HEIP. Since increasing the concentration further did not lead to any further enhance in the protective effect, HEIP at a dose of 300 mg/kg b. wt. can be considered as the threshold dose required for the recovery against MMC (8 mg/kg)- induced mitotic depression. And, the same dose can also be considered as the optimum considering that no more enhancement in recovery beyond this dose was observed.
This is the first study of its kind reporting the ameliorating effect of propolis extract on mitotic depression in bone marrow by determination of P/N ratio. However, there are many reports on the protective effect of plant extracts enriched with antioxidants, against MMC-induced cytotoxicity in terms of recovery of P/N ratio (Prasad et al. 2002; Borges et al. 2013). The cytoprotective effect observed in the present study, to some extent, is in line with a previous report where ethanolic extract of propolis was shown to prevent necrosis of the gastric mucosa in rats (Liu et al. 2002). Since MMC produces free-radicals through its metabolism, the antioxidant rich HEIP can counteract the free-radicals preventing them from cytotoxicity. There are several reports on antioxidant/free radical scavenging-mediated protection against a wide variety of cytotoxic agents (Yoo et al. 2008; Rajan et al. 2014; Sharma et al. 2014) and their mechanism of action (Mathers et al. 2004). Our data on antioxidant/free radical scavenging potency of HEIP in vitro, justifies its cytoprotective effect against MMC (8 mg/kg), for which the threshold dose of the extract required was found to be 300 mg/kg b. wt. With a current set of data, it is difficult to explain why at higher dose (>600 mg/kg) HEIP did not enhance the cytoprotective effect and rather reversed the effect, which is interesting to study further. Possibly, some of the polar and/or non-polar constituents present in HEIP may have antagonistic effect on the cytoprotectivity by antioxidants.
Considering together the geno- and cyto-protective effects of HEIP against MMC, lipid peroxidation is an important basis for the justification. It is known that lipid peroxidation is a crucial event in oxidative stress-induced cellular damages—mutation and cytotoxicity (Park et al. 2002). Indian propolis extracts have been reported to possess the significant lipid peroxidation inhibitory property (Benguedouar et al. 2008; Thirugnanasampandan et al. 2012). In the present study, the observed geno- and cyto-protective effects of HEIP is probably due to the inhibition of lipid peroxidation-mediated cytogenetic damages induced by MMC, since the latter is a potential inducer of lipid peroxidation via free radical generation (Siddique et al. 2010).
Conclusions
The hydroethanolic extract of Indian propolis has a significant protective effect against genotoxic and cytotoxic stress induced by mitomycin C. Strong free radical scavenging activity of the extract indicates that it is enriched with antioxidants, which might be responsible for ameliorating the MMC-induced toxic effects on proliferating cells in bone marrow. Thus, HEIP may be regarded as a protective agent to minimize the MMC-induced toxicities (side effects) in non-target cells/tissues. Further investigations are necessary to understand whether HEIP has any significant role to play in DNA repair mechanisms to minimize genotoxicity.
Compliance with ethical standards
Conflict of interest
Authors declare that there are no conflicts of interest.
References
- Abdulrhman M, El Barbary NS, Ahmed AD, Saeid ER. Honey and a mixture of honey, beeswax, and olive oil-propolis extract in treatment of chemotherapy-induced oral mucositis: a randomized controlled pilot study. Pediatr Hematol Oncol. 2012;29:285–292. doi: 10.3109/08880018.2012.669026. [DOI] [PubMed] [Google Scholar]
- Astani A, Zimmermann S, Hassan E, Reichling J, Sensch KH, Schnitzler P. Antimicrobial activity of propolis special extract GH 2002 against multidrug-resistant clinical isolates. Pharmazie. 2013;68:695–701. [PubMed] [Google Scholar]
- Benguedouar L, Boussenane HN, Wided K, Alyane M, Rouibah H, Lahouel M. Efficiency of propolis extract against mitochondrial stress induced by antineoplasic agents (doxorubicin and vinblastin) in rats. Ind J Exp Biol. 2008;46:112–119. [PubMed] [Google Scholar]
- Benkovic V, Knezevic AH, Orsolic N, Basic I, Ramic S, Viculin T, Knezevic F, Kopjar N. Evaluation of radioprotective effects of propolis and its flavonoid constituents: in vitro study on human white blood cells. Phytother Res. 2009;23:1159–1168. doi: 10.1002/ptr.2774. [DOI] [PubMed] [Google Scholar]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bolfa P, Vidrighinescu R, Petruta A, Dezmirean D, Stan L, Vlase L, Damian G, Catoi C, Filip A, Clichici S. Photoprotective effects of Romanian propolis on skin of mice exposed to UVB irradiation. Food Chem Toxicol. 2013;62:329–342. doi: 10.1016/j.fct.2013.08.078. [DOI] [PubMed] [Google Scholar]
- Borges FF, Machado TC, Cunha KS, Pereira KC, Costa EA, De Paula JR, Chen-Chen L. Assessment of the cytotoxic, genotoxic, and antigenotoxic activities of Celtis iguanaea (Jacq.) in mice. An Acad Bras Cienc. 2013;85:955–964. doi: 10.1590/S0001-37652013005000054. [DOI] [PubMed] [Google Scholar]
- Bouayed J, Bohn T. Exogenous antioxidants- Double edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev. 2010;3:228–237. doi: 10.4161/oxim.3.4.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Food Sci Technol. 1995;28:25–30. [Google Scholar]
- Crooke ST, Bradner WT. Mitomycin C: a review. Cancer Treat Rev. 1976;3:121–139. doi: 10.1016/S0305-7372(76)80019-9. [DOI] [PubMed] [Google Scholar]
- Dorr RT, Bowden GT, Alberts DS, Liddil JD. Interactions of mitomycin C with mammalian DNA detected by alkaline elution. Cancer Res. 1985;45:3510–3516. [PubMed] [Google Scholar]
- Dusre L, Covey JM, Collins C, Sinha BK. DNA damage, cytotoxicity and free radical formation by mitomycin C in humancells. Chem Biol Interact. 1989;71:63–78. doi: 10.1016/0009-2797(89)90090-2. [DOI] [PubMed] [Google Scholar]
- Fu JY, Xia Y, Zheng YY. Antimutagenicity of propolis against some mutagens in vivo and in vitro. Biomed Environ Sci. 2004;17:469–475. [PubMed] [Google Scholar]
- Iwao K, Inatani M, Seto T, Takihara Y, Ogata-Iwao M, Okinami S, Tanihara H. Long-term outcomes and prognostic factors for trabeculectomy with mitomycin C in eyes with uveitic glaucoma: a retrospective cohort study. J Glaucoma. 2014;23:88–94. doi: 10.1097/IJG.0b013e3182685167. [DOI] [PubMed] [Google Scholar]
- Jagetia GC, Reddy TK. The grapefruit flavanone naringin protects against the radiation-induced genomic instability in the mice bone marrow: a micronucleus study. Mutat Res. 2002;519:37–48. doi: 10.1016/S1383-5718(02)00111-0. [DOI] [PubMed] [Google Scholar]
- Kimoto N, Hirose M, Kawabe M, Satoh T, Miyataka H, Shirai T. Post-initiation effects of a super critical extract of propolis in a rat two stage carcinogenesis model in female F344 rats. Cancer Lett. 1999;147:221–227. doi: 10.1016/S0304-3835(99)00305-5. [DOI] [PubMed] [Google Scholar]
- Kumazawa S, Tomoko H, Tsutomu N. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004;84:329–339. doi: 10.1016/S0308-8146(03)00216-4. [DOI] [Google Scholar]
- Kurek-Górecka A, Rzepecka-Stojko A, Górecki M, Stojko J, Sosada M, Swierczek-Zieba G. Structure and antioxidant activity of polyphenols derived from propolis. Molecules. 2013;19:78–101. doi: 10.3390/molecules19010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liegeois C, Lermusieau G, Collin S. Measuring antioxidant efficiency of wort, malt, and hops against the 2,2'-azobis(2-amidinopropane) dihydrochloride-induced oxidation of an aqueous dispersion of linoleic acid. J Agric Food Chem. 2000;48:1129–1134. doi: 10.1021/jf9911242. [DOI] [PubMed] [Google Scholar]
- Liu CF, Lin CC, Lin MH, Lin YS, Lin SC. Cytoprotection by propolis ethanol extract of acute absolute ethanol-induced gastric mucosal lesions. Am J Chin Med. 2002;30:245–254. doi: 10.1142/S0192415X02000387. [DOI] [PubMed] [Google Scholar]
- MacGregor JT, Heddle JA, Hite M, Margolin BH, Ramel C, Salamone MF, Tice RR, Wild D. Guidelines for the conduct of micronucleus assays in mammalian bone marrow erythrocytes. Mutat Res. 1987;189:103–112. doi: 10.1016/0165-1218(87)90016-4. [DOI] [PubMed] [Google Scholar]
- Mathers J, Fraser JA, McMahon M, Saunders RD, Hayes JD, McLellan LI. Antioxidant and cytoprotective responses to redox stress. Biochem Soc Symp. 2004;71:157–176. doi: 10.1042/bss0710157. [DOI] [PubMed] [Google Scholar]
- Miller BM, Zitzelsberger HF, Weier HU, Adler ID. Classification of micronuclei in murine erythrocytes: immunofluorescent staining using CREST antibodies compared to in situ hybridization with biotinylated gamma satellite DNA. Mutagenesis. 1991;6:297–302. doi: 10.1093/mutage/6.4.297. [DOI] [PubMed] [Google Scholar]
- Molyneux G, Gibson FM, Gordon-Smith EC, Pilling AM, Liu KC, Rizzo S, Sulsh S, Turton JA. The haemotoxicity of mitomycin in a repeat dose study in the female CD-1 mouse. Int J Exp Pathol. 2005;86:415–430. doi: 10.1111/j.0959-9673.2005.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Gutiérrez S, López-Vicente M, Lostalé F, Fuentes-Broto L, Martínez-Ballarín E, García JJ. Protective effect of melatonin against mitomycin C induced genotoxic damage in peripheral blood of rats. J Biomed Biotechnol. 2009;2009:1–6. doi: 10.1155/2009/791432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JE, Yang JH, Yoon SJ, Lee JH, Yang ES, Park JW. Lipid peroxidation-mediated cytotoxicity and DNA damage in U937 cells. Biochimie. 2002;84:1199–1205. doi: 10.1016/S0300-9084(02)00039-1. [DOI] [PubMed] [Google Scholar]
- Pellati F, Prencipe FP, Bertelli D, Benvenuti S. An efficient chemical analysis of phenolic acids and flavonoids in raw propolis by microwave-assisted extraction combined with high-performance liquid chromatography using the fused-core technology. J Pharm Biomed Anal. 2013;81–82:126–132. doi: 10.1016/j.jpba.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Piccinelli AL, Mencherini T, Celano R, Mouhoubi Z, Tamendjari A, Aquino RP. RastrelliL. Chemical composition and antioxidant activity of Algerian propolis. J Agric Food Chem. 2013;61:5080–5088. doi: 10.1021/jf400779w. [DOI] [PubMed] [Google Scholar]
- Prasad S, Naik P, Vijayalaxmi KK. Efficiency of Coleus aromaticus extract in modifying cyclophosphamide and mitomycin-C induced clastogenicity in mouse bone marrow cells. Ind J Exp Biol. 2002;40:1020–1025. [PubMed] [Google Scholar]
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Rajan I, Rabindran R, Jayasree PR, Kumar PR. Antioxidant potential and oxidative DNA damage preventive activity of unexplored endemic species of Curcuma. Ind J Exp Biol. 2014;52:133–138. [PubMed] [Google Scholar]
- Reigner B, Blesch K. Estimating the starting dose for entry into humans: principles and practice. Eur J Clin Pharmacol. 2002;57:835–845. doi: 10.1007/s00228-001-0405-6. [DOI] [PubMed] [Google Scholar]
- Rizk SM, Zaki HF, Mina MA. Propolis attenuates Doxorubicin-induced testicular toxicity in rats. Food Chem Toxicol. 2014;67:176–186. doi: 10.1016/j.fct.2014.02.031. [DOI] [PubMed] [Google Scholar]
- Schmid W. The micronucleus test. Mutat Res. 1975;31:9–15. doi: 10.1016/0165-1161(75)90058-8. [DOI] [PubMed] [Google Scholar]
- Sharma H, Kanwal R, Bhaskaran N, Gupta S. Plant flavone apigenin binds to nucleic Acid bases and reduces oxidative DNA damage in prostate epithelial cells. PLoS One. 2014;9:e91588. doi: 10.1371/journal.pone.0091588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique YH, Ara G, Beg T, Gupta J, Afzal M. Assessment of cell viability, lipid peroxidation and quantification of DNA fragmentation after the treatment of anticancerous drug mitomycin C and curcumin in cultured human blood lymphocytes. Exp Toxicol Pathol. 2010;62:503–508. doi: 10.1016/j.etp.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Nagae Y, Li J, Sakaba H, Mozawa K. Takahashi A, Shimizu H. The micronucleus test and electrophoresis. Effects of erythropoietin and mutagen on the ratio of polychromatic to normochromatic erythrocytes (P/N ratio) Mutagenesis. 1989;4:420–424. doi: 10.1093/mutage/4.6.420. [DOI] [PubMed] [Google Scholar]
- Tandon N, Sharma M. Quality standards of Indian medicinal plants. Indian Council of Medical Research (ICMR): New Delhi; 2010. pp. 32–36. [Google Scholar]
- Thirugnanasampandan R, Raveendran SB, Jayakumar R. Analysis of chemical composition and bioactive property evaluation of Indian propolis. Asian Pac J Trop Biomed. 2012;2:651–654. doi: 10.1016/S2221-1691(12)60114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Türkez H, Yousef MI, Geyikoglu F. Propolis prevents aluminium-induced genetic and hepatic damages in rat liver. Food Chem Toxicol. 2010;48:2741–2746. doi: 10.1016/j.fct.2010.06.049. [DOI] [PubMed] [Google Scholar]
- Türkez H, Yousef MI, Geyikoglu F. Propolis protects against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity in rat hepatocytes. Food Chem Toxicol. 2012;50:2142–2148. doi: 10.1016/j.fct.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Türkez H, Geyikoğlu F, Yousef MI, Toğar B, Vançelik S. Propolis alleviates 2,3,7,8-Tetrachlorodibenzo-p-dioxin-induced histological changes, oxidative stress and DNA damage in rat liver. Toxicol Ind Health. 2013;29:677–685. doi: 10.1177/0748233712440139. [DOI] [PubMed] [Google Scholar]
- Watanabe MA, Amarante MK, Conti BJ, Sforcin JM. Cytotoxic constituents of propolis inducing anticancer effects. J Pharm Pharmacol. 2011;63:1378–1386. doi: 10.1111/j.2042-7158.2011.01331.x. [DOI] [PubMed] [Google Scholar]
- Yoo KM, Lee CH, Lee H, Moon BK, Lee CH. Relative antioxidant and cytoprotective activities of common herbs. Food Chem. 2008;106:929–936. doi: 10.1016/j.foodchem.2007.07.006. [DOI] [Google Scholar]