Abstract
The need to have a better and safer culture condition for expansion of human mesenchymal stem cells (MSCs) is crucial particularly to prevent infection and immune rejection. This is normally associated with the use of animal-based serum in the culture media for cell expansion. The aim of this study is to investigate alternative culture conditions which may provide better and safer environment for cell growth. In the present study, human adipose-derived stem cells (ASCs) at passage 3 were subjected to treatment in 4 conditions: (1) 21 % O2 with fetal bovine serum (FBS), (2) 21 % O2 without FBS, (3) 2 % O2 with FBS and (4) 2 % O2 without FBS followed by subsequent analysis of their phenotype, viability and functionality. We observed that ASCs cultured in all conditions present no significant phenotypic changes. It was found that ASCs cultured in 2 % O2 without serum showed an increase in viability and growth to a certain extent when compared to those cultured in 21 % O2 without serum. However, ASCs cultured in 2 % O2 without serum displayed a relatively low adipogenic and osteogenic potential. On the other hand, interestingly, there was a positive enhancement in chondrogenic differentiation of ASCs cultured in 21 % O2 without serum. Our findings suggest that different culture conditions may be suitable for different indications. In summary, ASCs cultured in serum-free condition can still survive, proliferate and undergo subsequent adipogenic, osteogenic and chondrogenic differentiation. Therefore, FBS is feasible to be excluded for culture of ASCs, which avoids clinical complications.
Keywords: Human adipose-derived stem cells, 2 % O2, 21 % O2, Fetal bovine serum, Serum-free condition
Introduction
MSCs are capable of self-renewal and differentiation into various types of cells, particularly osteogenic, chondrogenic and adipogenic cells (Doulatov and Daley 2013). Due to these characteristics, MSCs are widely used in medical research and tissue engineering by avoiding the ethical concerns related to embryonic stem cells (Young 2000). MSCs can be readily obtained from various sources in human body e.g., bone marrow, adipose tissues and periosteum (Ferretti et al. 2012; Yong et al. 2015b). Recently, adipose tissue has been identified as an attractive source of MSCs (Gimble et al. 2007) due to its abundance and ease of harvest. Although MSCs have emerged as a promising tool for clinical applications, but they need to be expanded in order to acquire sufficient cells for clinical applications (Pittenger 1999) and this requires the development of effective cell expansion protocols. To this end, MSCs would require a suitable culture environment for them to proliferate and grow at a rate sufficient for timely therapeutic use.
There are several culture environments (e.g., low oxygen tension and serum supplementation) that promote cell growth and proliferation. In general, oxygen concentration is varied (1–10 %) depending on the tissue type in human body. For instance, oxygen concentration in human adipose tissue is <4 % (Matsumoto et al. 2005; Pasarica et al. 2008), whereas oxygen concentration in bone tissue is 4–7 % (Xu et al. 2007). As articular cartilage is avascular, therefore oxygen tension in cartilage can reach as low as 1 % (Fermor et al. 2007; Wang et al. 2005). However, oxygen tension in the culture seems to be often overlooked when conducting in vitro study. Cells were normally cultured at atmospheric oxygen tension (20–21 %) instead of physiological oxygen tension (1–10 %) experienced by them in the human body (Ivanovic 2009). In fact, oxygen concentration can be adjusted in vitro to culture cells for different indications. For instance, 2 % O2 which was reported to enhance proliferation rate of human ASCs (Choi et al. 2014a; Lee et al. 2009), can be used to expand undifferentiated ASCs extensively to get sufficient cells for cell-based therapies (e.g., graft-vs-host-disease). On the other hand, MSCs can be cultured in a condition mimicking physiological oxygen tension in native adipose, bone and cartilage tissues to enhance their adipogenic, osteogenic and chondrogenic differentiation in vitro respectively (Valorani et al. 2012; Xu et al. 2007), for transplantation and tissue engineering approach.
Besides low oxygen tension, the use of serum, e.g., FBS also has been reported to promote cell growth. However, FBS may contain pathogens which pose a risk of infection, and animal-based proteins which induce severe immune reactions, in recipients following cell transplantation (Lindroos et al. 2009). To avoid such adverse reactions, FBS is recommended to be excluded from cell culture medium. To this end, Parker et al. (2007) have performed a study to evaluate the proliferation and differentiation potential of MSCs cultured in serum-free condition. It was found that serum-free condition may not support proliferation and differentiation (adipogenic and osteogenic) of MSCs. This study was carried out in atmospheric oxygen tension only. Therefore, studies on the effects of serum deprivation and hypoxia on MSCs were performed, but it was only reported that such condition may reduce viability of MSCs (Huang et al. 2009; Zhu et al. 2006). To date, the exact concomitant effect of hypoxia and serum deprivation on MSCs in terms of stemness properties (proliferation and differentiation potential) is still relatively unknown.
Herein, we evaluated the effects of hypoxia on stemness properties of ASCs cultured in serum-free condition thoroughly by providing an insight into the molecular changes that may occur following such treatment. The findings from this study would impact the establishment of an effective culture method which avoids the use of animal-based serum.
Methods
Isolation and culture of human ASCs
Adipose tissue samples were collected from 6 different female donors aged 25–35 years during elective caesarean section surgery in University of Malaya Medical Centre (UMMC) posterior to informed consent from the donors. This study was approved by the Medical Ethics Committee of UMMC (Reference No: 996.46). After collection, the tissue was cleaned and washed with phosphate-buffered saline (PBS) (Sigma-Aldrich, St Louis, MO, USA) and minced. It was then digested using 0.3 % collagenase type I (Worthington, Freehold, NJ, USA) at 37 °C with agitation for 20 min. The digested tissue was then centrifuged and the resulting pellet was washed followed by resuspension in culture medium containing Dulbecco’s Modified Eagle’s Medium (DMEM)/Ham F-12 (Gibco, Grand Island, NY, USA) supplemented with 10 % FBS (Gibco), 1 % antibiotic–antimycotic (Gibco), 1 % glutamax (Gibco) and 1 % vitamin C (Gibco). Cells were expanded in 5 % CO2, 21 % O2 and 37 °C until passage 3 (P3). ASCs at P3 were divided into 4 groups: (1) 21 % O2 with FBS, (2) 21 % O2 without FBS, (3) 2 % O2 with FBS, and (4) 2 % O2 without FBS. ASCs at P3 have been shown to be relatively homogenous (contaminated with only an insignificant proportion of hematopoietic cells) (Choi et al. 2015; Hsiao et al. 2013) compared to those at previous passage. All groups were cultured and observed for morphological changes until they reached the cell density needed for the following assays and analysis. Each experiment was conducted in triplicates per donor.
Cell viability and proliferation assay
ASCs from all groups were subjected to a trypan blue exclusion assay for cell viability analysis. Total live cells and dead cells were counted and percentage of cell viability was calculated. On the other hand, a Resazurin reduction assay was performed to evaluate the proliferation potential of the cells. In brief, ASCs were seeded onto a 24-well plate at 5 × 104 cells per well and incubated overnight for cell attachment. Resazurin reduction assay was performed after 24 h (day 1), and on days 3 and 7. The absorbance signals were quantified at wavelength of 570 nm and 595 nm using FLUOstar Optima microplate reader (BMG Labtech, Offenburg, Germany). Finally, the cell numbers on each day of culture were determined.
Immunophenotyping
Immunophenotyping was used to determine the expression of surface markers in ASCs. To perform immunophenotyping, the cells were stained with specific antibodies tagged with fluorochrome as follows: FITC-conjugated CD105, CD90, CD45, CD34, HLA DR DQ DP and PE-conjugated CD73, CD14, and CD19 (Becton–Dickinson, San Jose, CA, USA). Then, the data were acquired using a flow cytometry system (BD FACSCanto II, Becton–Dickinson). Finally, analysis was performed using a FlowJo software (Treestar, Ashland, OR, USA) with 10,000 cells per sample.
Cell differentiation assay
Adipogenic induction
ASCs were cultured for 21 days in adipogenic induction medium containing DMEM/F12 supplemented with 10 % FBS, 0.5 μM isobutyl-1-methy xanthine (Sigma-Aldrich), 1 μM dexamethasone (Sigma-Aldrich) and 10 μM insulin (Sigma-Aldrich). Differentiation of ASCs to adipogenic cells was indicated by the formation of lipid droplets stained by Oil red O (Sigma-Aldrich).
Osteogenic induction
ASCs were cultured for 21 days in osteogenic induction medium containing DMEM/F12 supplemented with 10 % FBS, 100 nM dexamethasone, 10 mM β-glycerophosphate (Sigma-Aldrich) and 0.05 mM ascorbic acid-2-phosphate (Sigma-Aldrich). Differentiation of ASCs to osteogenic cells was indicated by the formation of calcium deposits stained by Alizarin red (Sigma-Aldrich).
Chondrogenic induction
ASCs were cultured for 21 days in chondrogenic induction medium containing DMEM/F12 supplemented with 1 % FBS, 1 % antibiotic–antimycotic, 1 % vitamin, 1 % glutamax, ITS premix (Becton–Dickinson), 50 μg/ml ascorbate-2-phosphate, 100 nM dexamethasone, 40 μg/ml l-proline (Sigma-Aldrich), 10 ng/ml TGF-β1 (Peprotech, Rocky Hill, NJ, USA) and 50 ng/ml IGF-1 (Peprotech). Differentiation of ASCs to chondrogenic cells was indicated by the formation of proteoglycan stained by Alcian blue (Sigma-Aldrich).
Images of each staining were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA). ASCs before differentiation induction for each culture condition were used as control for normalization. Data were expressed as percentage (%) of stained area for Oil red O, Alizarin red and Alcian blue, respectively. Further, the expression of specific genes for each differentiation was determined using quantitative Real-Time polymerase chain reaction (qPCR) to compare the differentiation potential of ASCs in each culture condition.
RNA extraction, cDNA synthesis and qPCR
ASCs from all groups were subjected to RNA extraction using TRI reagent (Ambion, Austin, TX, USA). The extraction was carried out according to the manufacturer’s protocol. RNA were converted to cDNA using a high capacity RNA-to-cDNA synthesis kit (Applied Biosystems, Foster City, CA, USA). A qPCR assay was carried out using TaqMan gene expression assays (Applied Biosystems) and a Real-Time PCR system (StepOnePlus, Applied Biosystems). The thermal cycling profile for Real-Time PCR was as follows: 95 °C for 20 s (polymerase activation) followed by 40 cycles of 95 °C for 1 s (denaturation) and 60 °C for 20 s (primer annealing/extending). Genes analysed were adipogenic genes: peroxisome proliferator-activated receptor gamma (PPAR-γ) (Hs01115513_m1), lipoprotein lipase (LPL) (Hs00173425_m1) and fatty-binding protein 4 (FBP4) (Hs01086177); Osteogenic genes: alkaline phosphatase (ALPL) (Hs01029144_m1), osteocalcin (OSC) (Hs015878914_m1) and runt-related transcription factor 2 (RUNX2) (Hs00231692_m1); and Chondrogenic genes: aggrecan (ACAN) (Hs00153936_m1), collagen type II (COL-2) (Hs00264051_m1) and Sry-related HMG box-9 (SOX-9) (Hs00165814_m1). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Hs99999905_m1) was used as a reference gene for normalization. Data were expressed as fold change relative to control group (ASCs before differentiation induction).
Statistical analysis
Statistical analysis was performed with SPSS 18.0 software using One-Way ANOVA with post hoc tukey test to compare the data from all groups. A paired t test was used to compare data before and after the differentiation induction in the gene expression study. All data were expressed as mean ± SE of mean (SEM) and a p value of <0.05 was accepted as statistically significant.
Results
Effects of hypoxia and serum deprivation on morphology of ASCs
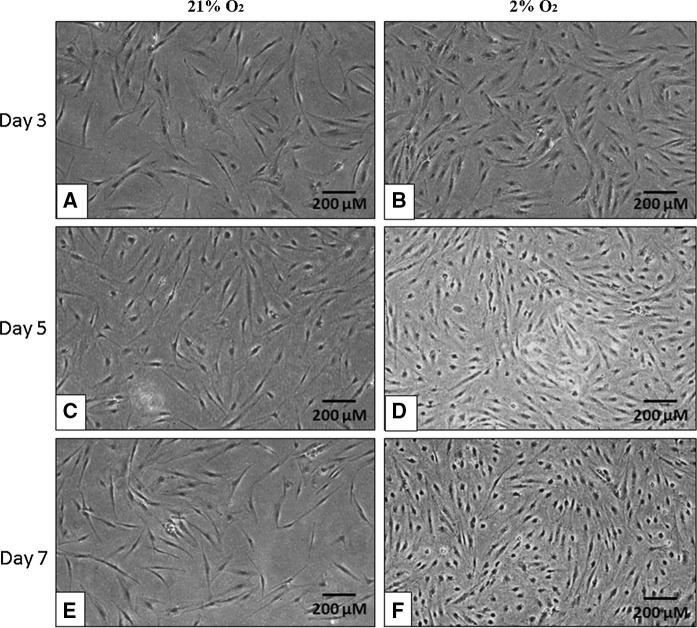
Microscopic examination revealed that ASCs cultured with serum (Fig. 1) in 21 % O2 and 2 % O2 showed spindle-like and fibroblastic morphology. The cell density continued to grow as the culture period increased from day 3 to day 7. On the other hand, ASCs cultured without serum supplementation at P3 (Fig. 2) in both 21 % O2 and 2 % O2 still maintained their spindle-like and fibroblastic morphology. However, through microscopic observation, the cell density was decreased particularly in 21 % O2 on day 7. Interestingly, the cell density in 2 % O2 without serum supplementation was increased on day 7. The density of cells in 2 % O2 without serum supplementation was observed to be lower than those cultured in 2 % O2 with serum.
Fig. 1.
Representative images of ASCs cultured in 21 % O2 and 2 % O2 with serum on days 3, 5 and 7. ASCs displayed their fibroblastic appearance and increased cell density. Magnification ×100. Scale bars 200 µm
Fig. 2.
Representative images of ASC cultured in 21 % O2 and 2 % O2 without serum on days 3, 5, and 7. ASCs still maintained their fibroblastic appearance but the cell density was getting lower. Magnification ×100. Scale bars 200 µm
Effects of hypoxia and serum deprivation on viability of ASCs
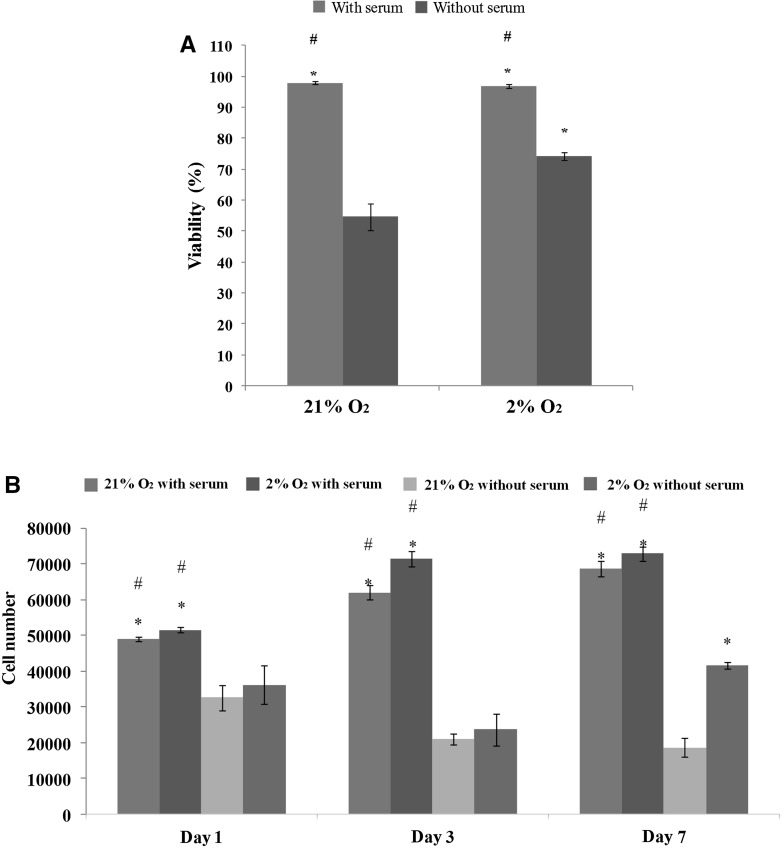
To assess cell viability, we performed Trypan blue exclusion assay. It was found that the viability of ASCs cultured in 21 % O2 with serum (98 ± 0.49 %) was significantly (p < 0.05) higher than that of ASCs cultured in 21 % O2 without serum (54 ± 4.2 %). Similarly, viability of ASCs cultured in 2 % O2 with serum (97 ± 0.6 %) was significantly (p < 0.05) higher than for those cultured without serum (74 ± 1.3 %). Surprisingly, viability of ASCs cultured without serum in 2 % O2 was significantly (p < 0.05) increased as compared to those cultured without serum in 21 % O2 (Fig. 3a). This indicates that 2 % O2 may support cell viability when cultured in serum-free condition.
Fig. 3.
Viability and proliferation rate of ASCs cultured with serum and without serum in both 21 % O2 and 2 % O2. a ASCs cultured with serum had higher viability as compared to those cultured without serum in both 21 % O2 and 2 % O2. ASCs cultured without serum in 2 % O2 had higher viability as compared to those cultured without serum in 21 % O2. b ASCs cultured with serum in 2 % O2 had the highest number of cells as compared to ASCs in other conditions. At day 7, ASCs cultured without serum in 2 % O2 showed a significant (p < 0.05) increase in cell number as compared to ASCs cultured without serum in 21 % O2. *p < 0.05 relative to 21 % O2 without serum; #p < 0.05 relative to 2 % O2 without serum
Effects of hypoxia and serum deprivation on proliferation of ASCs
To evaluate proliferation rate of ASCs, we conducted Resazurin reduction assay. It was found that ASCs cultured with serum in either 2 or 21 % O2 displayed significantly (p < 0.05) higher number of cells than those culture without serum from day 1 to day 7. Interestingly, we found that ASCs cultured without serum in 2 % O2 showed a significantly (p < 0.05) greater cell number than those cultured without serum in 21 % O2 on day 7 (Fig. 3b). This result indicates that 2 % O2 may assist in maintaining cell growth of ASCs in the absence of serum, but this condition is still less superior to 2 % O2 with serum. Therefore, a longer period of culture in 2 % O2 without serum is needed to achieve similar numbers of ASCs as those cultured in 2 % O2 supplemented with serum.
Effects of hypoxia and serum deprivation on surface markers expression of ASCs
ASCs in all groups were evaluated for surface marker expression (Table 1), except for those cultured in 21 % O2 without serum due to insufficient cell numbers for immunophenotyping. ASCs in all tested groups were positive (>90 % cells) for mesenchymal-associated markers such as CD90, CD73, CD105, and CD44 while negative (<2 % cells) for hematopoietic cell makers such as CD19, CD14, CD34 and CD45, which met the minimal criteria proposed by the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy for defining human MSCs (Dominici et al. 2006). It is worth to note that there was a significant (p < 0.05) increase of CD14 and CD34 in ASCs cultured in 2 % O2 without serum.
Table 1.
Expression of surface markers of ASCs cultured in various conditions
| CD antigen | Percentage of expression (%) | ||
|---|---|---|---|
| 21 % O2 with serum | 2 % O2 with serum | 21 % O2 without serum | |
| CD90 | 98.90 ± 1.04 | 99.90 ± 0.0 | 99.90 ± 0.0 |
| CD73 | 95.47 ± 7.25 | 91.57 ± 2.73 | 99.70 ± 0.10 |
| CD105 | 89.50 ± 1.65 | 89.37 ± 1.36 | 90.43 ± 1.38 |
| CD44 | 92.90 ± 5.38 | 92.53 ± 0.78 | 91.37 ± 1.04 |
| CD19 | 0.10 ± 0.0 | 0.33 ± 0.06 | 0.23 ± 0.06 |
| CD14 | 0.43 ± 0.12 | 0.27 ± 0.06 | 0.70 ± 0.20* |
| CD34 | 0.17 ± 0.06 | 0.33 ± 0.06 | 0.53 ± 0.15* |
| CD45 | 0.40 ± 0.10 | 0.60 ± 0.10 | 0.67 ± 0.15 |
* p < 0.05 relative to ASCs cultured in 21 % O2 with serum
Effects of hypoxia and serum deprivation on differentiation potential of ASCs
Adipogenic potential
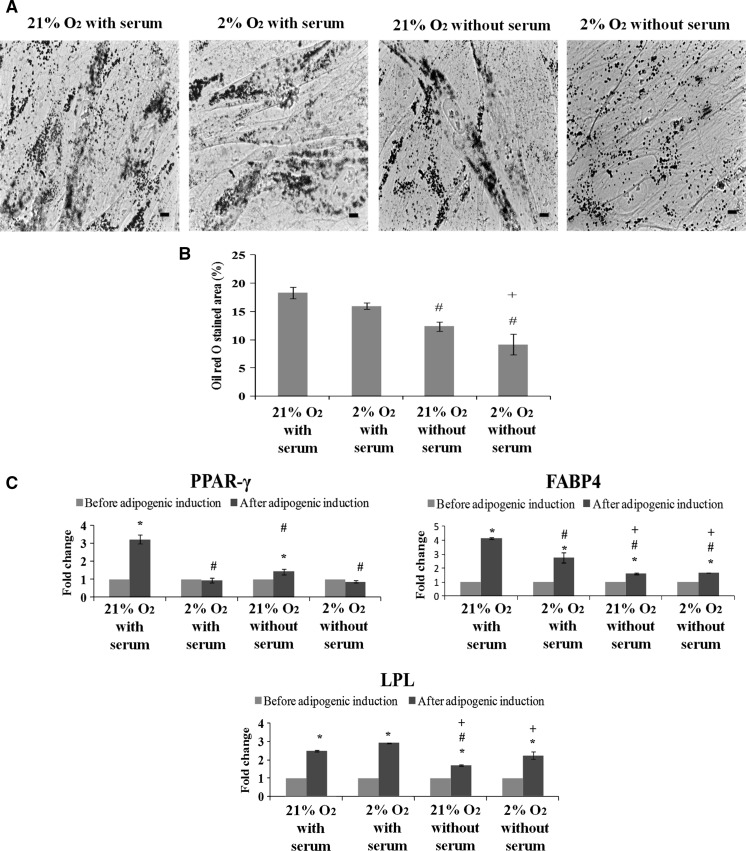
To evaluate adipogenic potential of ASCs, we performed Oil red O staining and gene expression analysis. We observed that ASCs cultured in all conditions displayed formation of lipid droplets which were stained positively by Oil red O (Fig. 4a). More lipid droplets were seen in ASCs cultured with serum, as indicated by a significantly (p < 0.05) higher percentage of Oil red O stained area (Fig. 4b). Further, it was found that ASCs cultured in 2 or 21 % O2 with serum displayed significantly (p < 0.05) higher gene expression levels of FABP4 and LPL than those cultured without serum. Gene expression of PPAR-γ was found to be significantly (p < 0.05) highest in ASCs cultured in 21 % O2 with serum (Fig. 4c). These results indicate that adipogenic potential was relatively higher when ASCs were cultured with serum supplementation.
Fig. 4.
Serum enhances adipogenesis of ASCs cultured in 2 % O2 and 21 % O2. a Formation of lipid droplets stained by Oil red O. Magnification ×400. Scale bars 100 µm. b High percentage of Oil red O stained area was observed in ASCs cultured with serum. c ASCs cultured in 21 % O2 with serum expressed high levels of adipogenic genes PPAR-γ, FABP4 and LPL. *p < 0.05 relative to before adipogenic induction; #p < 0.05 relative to 21 % O2 with serum; +p < 0.05 relative to 2 % O2 with serum
Osteogenic potential
To assess osteogenic capacity of ASCs, we conducted Alizarin red staining and gene expression analysis. We observed that only ASCs cultured in 21 % O2 showed deposition of calcium which was positively stained by Alizarin red (Fig. 5a). There was no significant (p > 0.05) difference in terms of percentage of Alizarin red stained area between ASCs cultured in 21 % O2 with serum and without serum (Fig. 5b). This finding is in accordance with the gene expression analysis (Fig. 5c). In addition, it was found that ASCs cultured in 21 % O2 with or without serum showed significantly (p < 0.05) higher gene expression levels of osteogenic markers (ALPL, OSC and RUNX2) than those cultured in 2 % O2 (Fig. 5c). Taken together, these results indicate that hypoxia could reduce osteogenic potential of ASCs.
Fig. 5.
High oxygen tension promotes osteogenesis of ASCs cultured with and without serum. a Calcium deposits stained by Alizarin red were observed only in ASCs cultured in 21 % O2. Magnification ×100. Scale bars 100 µm. b High percentage of Alizarin red stained area was seen in ASCs cultured in 21 % O2 with or without serum. c ASCs cultured in 21 % O2 with or without serum expressed high levels of osteogenic genes ALPL, OSC and RUNX2. *p < 0.05 relative to before osteogenic induction; #p < 0.05 relative to 21 % O2 with serum; +p < 0.05 relative to 21 % O2 without serum
Chondrogenic potential
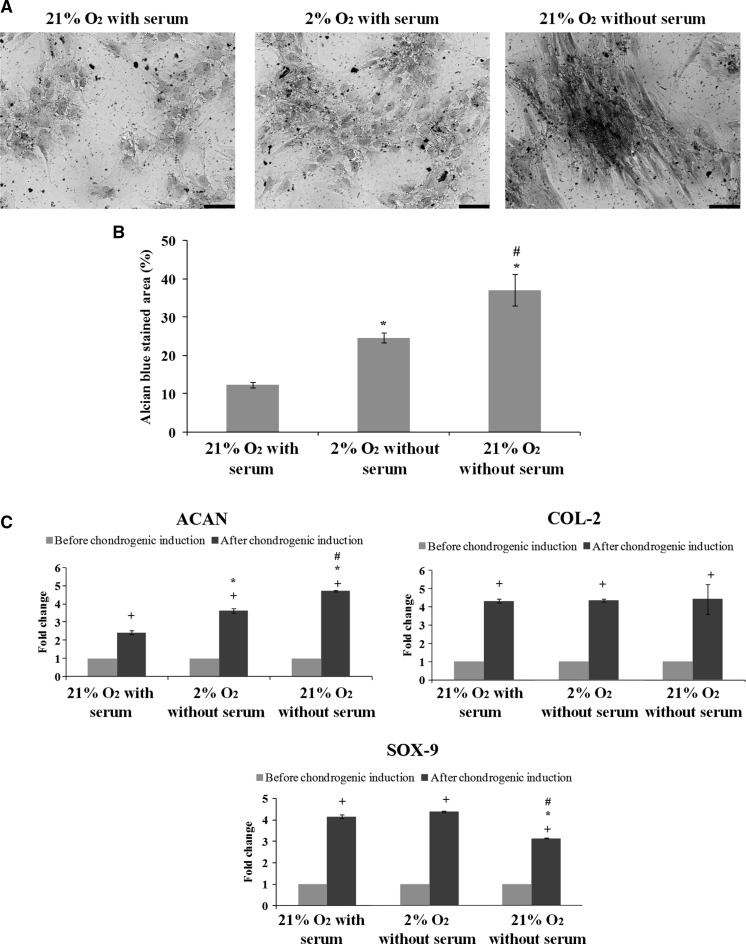
To assess chondrogenic potential of ASCs, we performed Alcian blue staining and gene expression analysis. Upon chondrogenic induction, it was observed that ASCs cultured in all conditions except for those cultured in 2 % O2 without serum, demonstrated formation of proteoglycans which were positively stained by Alcian blue (Fig. 6a). Chondrogenic potential of ASCs cultured in 2 % O2 without serum could not be evaluated due their inability to survive in the chondrogenic induction medium in the low oxygen tension environment.
Fig. 6.
Serum-free condition enhances chondrogenesis of ASCs in cultured 21 % O2. a Formation of proteoglycan stained by Alcian blue. Magnification ×100. Scale bars 100 µm. b High percentage of Alcian blue stained area was observed in ASCs cultured in 21 % O2 without serum. c ASCs cultured in 21 % O2 without serum expressed high levels of chondrogenic genes ACAN and COL-2, which help in chondrogenic maturation. +p < 0.05 relative to before chondrogenic induction; *p < 0.05 relative to 21 % O2 with serum; #p < 0.05 relative to 2 % O2 with serum
Serum-free condition seems to enhance chondrogenic differentiation the most, as indicated by a significantly (p < 0.05) highest percentage of Alcian blue stained area (Fig. 6b). qPCR revealed that ASCs in all tested groups displayed significantly (p < 0.05) increased gene expression levels of chondrogenic markers (ACAN, COL-2 and SOX-9) as compared to those before chondrogenic induction. In addition, ASCs cultured in 21 % O2 without serum expressed a significantly (p < 0.05) higher level of ACAN and lower level of SOX-9 than those cultured with serum. There was no significant (p > 0.05) difference in terms of gene expression level of COL-2 among ASCs in those tested groups (Fig. 6c). These results suggest that serum-free culture condition promotes chondrogenic maturation of ASCs, as indicated by high levels of late chondrogenic markers (ACAN and COL-2) and a low level of early chondrogenic marker (SOX-9).
Discussion
In the present study, ASCs were expanded in 21 % O2 until P3 to get sufficient cell density for treatment with 2 % O2 and serum deprivation, as well as subsequent analysis. Most studies have demonstrated that ASCs expanded in 21 % O2 within P3 are functionally intact and safe for clinical use due to their low risk of chromosomal aberration and tumourigenesis (Choi et al. 2015; Froelich et al. 2013; Yong et al. 2015a, c). After reaching P3, ASCs were divided into 4 culture conditions: (1) 2 % O2 supplemented with 10 % FBS, (2) 2 % O2 without FBS, (3) 21 % O2 supplemented with 10 % FBS (control group) and (4) 21 % O2 without FBS. This investigation was to search for a better culture environment without having to rely on animal-based products such as FBS, which has been used widely in cell culture media. FBS has been reported to exert immune reaction in the form of anaphylaxis (Mackensen et al. 2000). Further, the use of FBS has been associated with pathogenic contamination of clinically used cells and increase of immunogenicity of the cells (Lalu et al. 2012; Shahdadfar et al. 2005; Spees et al. 2004). This indicates that cells should be cultured under serum free condition or cultured in autologous serum before cells can be applied therapeutically in human. In addition, if serum is still needed to expand the cells, human platelet lysate has been suggested as a replacement for FBS for clinical-scale expansion of human MSCs (Schallmoser et al. 2007).
Under the current prescribed conditions, ASCs showed a similar morphological appearance with fibroblastic and spindle-like features. Further, cell density was also observed to increase in 2 % O2 including those cultured without serum, to a certain extent. This indicates that 2 % O2 does facilitate cell growth and viability (Choi et al. 2015; Grayson et al. 2007) even without the use of serum in cell culture. However, the limited expansion of cells in 2 % O2 without serum was observed, which could be due to increased cell death, as prolonged concomitant hypoxia and serum deprivation in culture has been reported to induce apoptosis in MSCs (Potier et al. 2007; Zhu et al. 2006).
Through surface marker evaluation, our findings show that 2 % O2 or serum deprivation does not affect expression of mesenchymal-associated markers (including CD90, CD73, CD44 and CD105) in ASCs. However, ASCs cultured in 2 % O2 and serum-free condition displayed a relatively high expression of CD14 and CD34. CD14 is a marker that is expressed in non-tumorigenic cell line (Lobba et al. 2012) and also known as a surface protein that is expressed in monocytes or macrophages (Simmons et al. 1989). This indicates that there may be an increase of immunogenicity of ASCs when cultured in 2 % O2 and in serum free condition. In addition, increase of CD14 expression may indicate that there is a low risk of tumorigenic potential in ASCs cultured in 2 % O2 and in serum-free condition. Increase of CD34, an endothelial marker (Muller et al. 2002), may indicate that ASCs cultured in 2 % O2 and in serum-free condition has become more committed to a specific cell lineage, e.g., endothelial cells. However, CD34 may also play a role in the increase of immunogenicity as CD34 has been reported to be involved in promoting lymphocyte antigens (Nielsen and McNagny 2008). Taken together, ASCs in all culture conditions displayed no significant phenotypic changes.
Upon adipogenic induction, ASCs cultured in 21 % O2 with serum displayed more lipid droplets and higher levels of expression in terms of adipogenic genes PPAR-γ, FABP4 and LPL than those cultured without serum, suggesting serum-free condition may reduce adipogenic potential of ASCs. This suggests that serum may contain components which help to maintain expression of adipogenic markers in ASCs, particularly PPAR-γ, which helps to regulate adipogenic differentiation and maintain adipocytic phenotype (Kawai and Rosen 2010). These findings are in accordance with Parker et al. (2007), who reported that serum enhanced adipogenesis of ASCs. Taken together, serum supplementation seems to play an essential role in adipogenic differentiation of ASCs. In spite of that, ASCs cultured in either 2 or 21 % O2 with serum-free condition were still capable of undergoing adipogenic differentiation, but with lower capacity than those cultured in serum.
Interestingly, ASCs that were cultured in 21 % O2 without serum showed an increase in osteogenic differentiation ability, as indicated by the increase in deposition of calcium and expression of the osteogenic genes, including OSC, RUNX2 and ALPL. However, ASCs expanded in 2 % O2 without serum did not show any positive enhancement in the subsequent osteogenic differentiation, as indicated by no formation of calcium deposit and a decrease in the gene expression of ALPL and RUNX2. It has been reported that low oxygen tension could upregulate hypoxia-inducible factor 1-alpha (HIF-1α) in ASCs, which in turn downregulates osteogenic genes (e.g., RUNX2) to inhibit osteogenic differentiation, as supported by Xu et al. (2013) and Choi et al. (2014b). Moreover, osteogenic differentiation of ASCs is believed to take place in an environment with high oxygen tension, as bones are situated closely to the blood vessels which provide great oxygen supply (Fehrer et al. 2007). In short, serum-free condition is still capable of supporting osteogenic differentiation of ASCs, but requires high oxygen tension.
On the other hand, ASCs cultured in 21 % O2 without serum demonstrated increased expression of chondrogenic genes ACAN, COL-2 and SOX-9 upon chondrogenic induction. A higher expression of ACAN in ASCs cultured without serum than those cultured with serum was observed, suggesting that serum-free condition may enhance chondrogenic maturation of ASCs. Many studies have shown that oxygen deprivation helps in chondrogenic differentiation and maturation (Choi et al. 2014b; Merceron et al. 2010; Weijers et al. 2011), but the beneficial effect of serum deprivation on chondrogenic differentiation is reported for the first time. It has been reported that high expression of CD90, CD73 and CD 105 are associated with positive enhancement in chondrogenic differentiation and maturation of MSCs (Arufe et al. 2010; Campbell and Pei 2012; Jiang et al. 2010). Our findings show that there is no significant (p < 0.05) difference in terms of expression of those markers in ASCs cultured in all conditions including those cultured in 21 % O2 without serum, suggesting that chondrogenic differentiation enhancement in serum deprivation condition may be affected by other factors. We suggest that serum may contain components which delay maturation of chondrogenic cells derived from ASCs and this requires further investigation.
Conclusion
In summary, 2 % O2 can help to increase the viability and growth of ASCs even without the use of serum although it may take a longer period of time to achieve sufficient number of cells. However, ASCs cultured in 2 % O2 without serum displayed low adipogenic potential and inhibition of osteogenic differentiation. On the other hand, ASCs cultured in 21 % O2 without serum seem to show a better enhancement in chondrogenic potential, and capable of undergoing adipogenic and osteogenic differentiation. Overall, our findings show that different culture conditions may be suitable for different indications. For instance, 2 % O2 may help to expand undifferentiated ASCs cultured in serum-free condition for clinical applications. On the other hand, ASCs cultured in 21 % O2 without serum can be induced to differentiate into osteogenic or chondrogenic cells in vitro prior to transplantation into human body for bone or cartilage regeneration. Taken together, animal-based serum is feasible to be excluded from cell culture medium to avoid xenogeneic immune rejection. Further investigation is needed to determine the active components in FBS so that these components can be replaced by non animal-based product to further enhance the stemness properties of ASCs in serum-free condition for better therapeutic efficacy.
Acknowledgments
This study was supported by University of Malaya (University of Malaya Research Grant (UMRG: RP040B-15HTM) and the Ministry of Higher Education (MOHE) of Malaysia (MOHE High Impact Research Grant: UM.C/HIR/MOHE/ENG/44). The sponsors have no involvement in the study design, research conduct, the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Compliance with ethical standards
Conflict of interest
All authors declare that there is no conflict of interest.
References
- Arufe MC, De la Fuente A, Fuentes I, de Toro FJ, Blanco FJ. Chondrogenic potential of subpopulations of cells expressing mesenchymal stem cell markers derived from human synovial membranes. J Cell Biochem. 2010;111:834–845. doi: 10.1002/jcb.22768. [DOI] [PubMed] [Google Scholar]
- Campbell DD, Pei M. Surface markers for chondrogenic determination: a highlight of synovium-derived stem cells. Cells. 2012;1:1107–1120. doi: 10.3390/cells1041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JR, Pingguan-Murphy B, Abas WABW, Azmi MAN, Omar SZ, Chua KH, Safwani WKZW. Hypoxia promotes growth and viability of human adipose-derived stem cells with increased growth factors secretion. J Asian Sci Res. 2014;4:328–338. [Google Scholar]
- Choi JR, Pingguan-Murphy B, Wan Abas WA, Noor Azmi MA, Omar SZ, Chua KH, Wan Safwani WK. Impact of low oxygen tension on stemness, proliferation and differentiation potential of human adipose-derived stem cells. Biochem Biophys Res Commun. 2014;448:218–224. doi: 10.1016/j.bbrc.2014.04.096. [DOI] [PubMed] [Google Scholar]
- Choi JR, Pingguan-Murphy B, Wan Abas WA, Yong KW, Poon CT, Noor Azmi MA, Omar SZ, Chua KH, Xu F, Wan Safwani WK. In situ normoxia enhances survival and proliferation rate of human adipose tissue-derived stromal cells without increasing the risk of tumourigenesis. PLoS One. 2015;10:e0115034. doi: 10.1371/journal.pone.0115034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Doulatov S, Daley GQ. Development. A stem cell perspective on cellular engineering. Science. 2013;342:700–702. doi: 10.1126/science.1238363. [DOI] [PubMed] [Google Scholar]
- Fehrer C, Brunauer R, Laschober G, Unterluggauer H, Reitinger S, Kloss F, Gully C, Gassner R, Lepperdinger G. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007;6:745–757. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- Fermor B, Christensen SE, Youn I, Cernanec JM, Davies CM, Weinberg JB. Oxygen, nitric oxide and articular cartilage. Eur Cell Mater. 2007;13:56–65. doi: 10.22203/ecm.v013a06. [DOI] [PubMed] [Google Scholar]
- Ferretti C, Borsari V, Falconi M, Gigante A, Lazzarini R, Fini M, Di Primio R, Mattioli- Belmonte M. Human periosteum-derived stem cells for tissue engineering applications: the role of VEGF. Stem Cell Rev Rep. 2012;8:882–890. doi: 10.1007/s12015-012-9374-7. [DOI] [PubMed] [Google Scholar]
- Froelich K, Mickler J, Steusloff G, Technau A, Ramos Tirado M, Scherzed A, Hackenberg S, Radeloff A, Hagen R, Kleinsasser N. Chromosomal aberrations and deoxyribonucleic acid single-strand breaks in adipose-derived stem cells during long-term expansion in vitro. Cytotherapy. 2013;15:767–781. doi: 10.1016/j.jcyt.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358:948–953. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- Hsiao ST, Lokmic Z, Peshavariya H, Abberton KM, Dusting GJ, Lim SY, Dilley RJ. Hypoxic conditioning enhances the angiogenic paracrine activity of human adipose derived stem cells. Stem Cells Dev. 2013;22:1614–1623. doi: 10.1089/scd.2012.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YC, Yang ZM, Chen XH, Tan MY, Wang J, Li XQ, Xie HQ, Deng L. Isolation of mesenchymal stem cells from human placental decidua basalis and resistance to hypoxia and serum deprivation. Stem Cell Rev. 2009;5:247–255. doi: 10.1007/s12015-009-9069-x. [DOI] [PubMed] [Google Scholar]
- Ivanovic Z. Hypoxia or in situ normoxia: the stem cell paradigm. J Cell Physiol. 2009;219:271–275. doi: 10.1002/jcp.21690. [DOI] [PubMed] [Google Scholar]
- Jiang T, Liu W, Lv X, Sun H, Zhang L, Liu Y, Zhang WJ, Cao Y, Zhou G. Potent in vitro chondrogenesis of CD105 enriched human adipose-derived stem cells. Biomaterials. 2010;31:3564–3571. doi: 10.1016/j.biomaterials.2010.01.050. [DOI] [PubMed] [Google Scholar]
- Kawai M, Rosen CJ. PPARgamma: a circadian transcription factor in adipogenesis and osteogenesis. Nat Rev Endocrinol. 2010;6:629–636. doi: 10.1038/nrendo.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:25. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EY, Xia Y, Kim WS, Kim MH, Kim TH, Kim KJ, Park BS, Sung JH. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 2009;17:540–547. doi: 10.1111/j.1524-475X.2009.00499.x. [DOI] [PubMed] [Google Scholar]
- Lindroos B, Boucher S, Chase L, Kuokkanen H, Huhtala H, Haataja R, Vemuri M, Suuronen R, Miettinen S. Serum-free, xeno-free culture media maintain the proliferation rate and multipotentiality of adipose stem cells in vitro. Cytotherapy. 2009;11:958–972. doi: 10.3109/14653240903233081. [DOI] [PubMed] [Google Scholar]
- Lobba AR, Forni MF, Carreira AC, Sogayar MC. Differential expression of CD90 and CD14 stem cell markers in malignant breast cancer cell line.s. Cytometry A. 2012;81:1084–1091. doi: 10.1002/cyto.a.22220. [DOI] [PubMed] [Google Scholar]
- Mackensen A, Drager R, Schlesier M, Mertelsmann R, Lindemann A. Presence of IgE antibodies to bovine serum albumin in a patient developing anaphylaxis after vaccination with human peptide-pulsed dendritic cells. Cancer Immunol Immunother. 2000;49:152–156. doi: 10.1007/s002620050614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A, Matsumoto S, Sowers AL, Koscielniak JW, Trigg NJ, Kuppusamy P, Mitchell JB, Subramanian S, Krishna MC, Matsumoto K-i. Absolute oxygen tension (pO2) in murine fatty and muscle tissue as determined by EPR. Magn Reson Med. 2005;54:1530–1535. doi: 10.1002/mrm.20714. [DOI] [PubMed] [Google Scholar]
- Merceron C, Vinatier C, Portron S, Masson M, Amiaud J, Guigand L, Cherel Y, Weiss P, Guicheux J. Differential effects of hypoxia on osteochondrogenic potential of human adipose-derived stem cells. Am J Physiol Cell Physiol. 2010;298:25. doi: 10.1152/ajpcell.00398.2009. [DOI] [PubMed] [Google Scholar]
- Muller AM, Hermanns MI, Skrzynski C, Nesslinger M, Muller KM, Kirkpatrick CJ. Expression of the endothelial markers PECAM-1, vWf, and CD34 in vivo and in vitro. Exp Mol Pathol. 2002;72:221–229. doi: 10.1006/exmp.2002.2424. [DOI] [PubMed] [Google Scholar]
- Nielsen JS, McNagny KM. Novel functions of the CD34 family. J Cell Sci. 2008;121:3683–3692. doi: 10.1242/jcs.037507. [DOI] [PubMed] [Google Scholar]
- Parker A, Shang H, Khurgel M, Katz A. Low serum and serum-free culture of multipotential human adipose stem cells. Cytotherapy. 2007;9:637–646. doi: 10.1080/14653240701508452. [DOI] [PubMed] [Google Scholar]
- Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2008;58:718–725. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Potier E, Ferreira E, Meunier A, Sedel L, Logeart-Avramoglou D, Petite H. Prolonged hypoxia concomitant with serum deprivation induces massive human mesenchymal stem cell death. Tissue Eng. 2007;13:1325–1331. doi: 10.1089/ten.2006.0325. [DOI] [PubMed] [Google Scholar]
- Schallmoser K, Bartmann C, Rohde E, Reinisch A, Kashofer K, Stadelmeyer E, Drexler C, Lanzer G, Linkesch W, Strunk D. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion. 2007;47:1436–1446. doi: 10.1111/j.1537-2995.2007.01220.x. [DOI] [PubMed] [Google Scholar]
- Shahdadfar A, Fronsdal K, Haug T, Reinholt FP, Brinchmann JE. In vitro expansion of human mesenchymal stem cells: choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells. 2005;23:1357–1366. doi: 10.1634/stemcells.2005-0094. [DOI] [PubMed] [Google Scholar]
- Simmons DL, Tan S, Tenen DG, Nicholson-Weller A, Seed B. Monocyte antigen CD14 is a phospholipid anchored membrane protein. Blood. 1989;73:284–289. [PubMed] [Google Scholar]
- Spees JL, Gregory CA, Singh H, Tucker HA, Peister A, Lynch PJ, Hsu SC, Smith J, Prockop DJ. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol Ther. 2004;9:747–756. doi: 10.1016/j.ymthe.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Valorani MG, Montelatici E, Germani A, Biddle A, D’Alessandro D, Strollo R, Patrizi MP, Lazzari L, Nye E, Otto WR, Pozzilli P, Alison MR. Pre-culturing human adipose tissue mesenchymal stem cells under hypoxia increases their adipogenic and osteogenic differentiation potentials. Cell Prolif. 2012;45:225–238. doi: 10.1111/j.1365-2184.2012.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DW, Fermor B, Gimble JM, Awad HA, Guilak F. Influence of oxygen on the proliferation and metabolism of adipose derived adult stem cells. J Cell Physiol. 2005;204:184–191. doi: 10.1002/jcp.20324. [DOI] [PubMed] [Google Scholar]
- Weijers EM, Van Den Broek LJ, Waaijman T, Van Hinsbergh VW, Gibbs S, Koolwijk P. The influence of hypoxia and fibrinogen variants on the expansion and differentiation of adipose tissue-derived mesenchymal stem cells. Tissue Eng Part A. 2011;17:2675–2685. doi: 10.1089/ten.tea.2010.0661. [DOI] [PubMed] [Google Scholar]
- Xu N, Liu H, Qu F, Fan J, Mao K, Yin Y, Liu J, Geng Z, Wang Y. Hypoxia inhibits the differentiation of mesenchymal stem cells into osteoblasts by activation of Notch signaling. Exp Mol Pathol. 2013;94:33–39. doi: 10.1016/j.yexmp.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Xu Y, Malladi P, Chiou M, Bekerman E, Giaccia AJ, Longaker MT. In vitro expansion of adipose-derived adult stromal cells in hypoxia enhances early chondrogenesis. Tissue Eng. 2007;13:2981–2993. doi: 10.1089/ten.2007.0050. [DOI] [PubMed] [Google Scholar]
- Yong KW, Pingguan-Murphy B, Xu F, Abas WA, Choi JR, Omar SZ, Azmi MA, Chua KH, Wan Safwani WK. Phenotypic and functional characterization of long-term cryopreserved human adipose-derived stem cells. Sci Rep. 2015;5:9596. doi: 10.1038/srep09596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong KW, Wan Safwani WK, Xu F, Wan Abas WA, Choi JR, Pingguan-Murphy B. Cryopreservation of human mesenchymal stem cells for clinical applications. Curr Methods Chall Biopreserv Biobank. 2015;13:231–239. doi: 10.1089/bio.2014.0104. [DOI] [PubMed] [Google Scholar]
- Yong KW, Wan Safwani WK, Xu F, Zhang X, Choi JR, Wan Abas WA, Omar SZ, Azmi MA, Chua KH, Pingguan-Murphy B (2015c) Assessment of tumourigenic potential in long-term cryopreserved human adipose-derived stem cells. J Tissue Eng Regen Med (in press) [DOI] [PubMed]
- Young FE. A time for restraint. Science. 2000;287:1424. doi: 10.1126/science.287.5457.1424. [DOI] [PubMed] [Google Scholar]
- Zhu W, Chen J, Cong X, Hu S, Chen X. Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Stem Cells. 2006;24:416–425. doi: 10.1634/stemcells.2005-0121. [DOI] [PubMed] [Google Scholar]