Abstract
Medium-chain fatty acids (MCFAs) have been suggested as an alternative to the use of antibiotics in animal nutrition with promising results. First, we studied the sensitivity of Salmonella Enteritidis and an enteropathogenic Escherichia coli strain against caprylic (C8), capric (C10) and lauric (C12) acids. A porcine in vitro model using the porcine cell line IPEC-J2 was used to test the effects of MCFAs on structural and immunological traits without and with a concomitant challenge with E. coli or S. Enteritidis. The three MCFAs exerted an inhibitory effect on bacterial growth, stronger for C12 than C8 or C10, S. Enteritidis being more sensitive than the E. coli strain. Flow cytometry showed a numeric concentration dependent increase in the adhesion of E. coli or S. Enteritidis to IPEC-J2 cells. Measurement of transepithelial electrical resistance after bacterial challenge showed negative effects of all MCFAs on IPEC-J2 cells at the highest concentrations. Immune parameters were affected by C8, since a concentration dependent effect starting at 5 mM was observed for mRNA expression of IL-6 and TLR-4 (up-regulated) and IL-8 (down-regulated). TLR-4 was up-regulated with C10 at 2 and 5 mM. The three MCFAs affected also the epithelial morphology through down-regulation of Occludin and up-regulation of Claudin-4 expression. In conclusion, the three MCFAs under study influenced bacterial growth rates and modified the gene expression to a different degree in the cell line IPEC-J2 but the effect on the morphological structure and response of the cells after bacterial challenge could not be assessed. Although these tests show a prior estimation of MCFAs effects in intestinal epithelium, in vivo confirmation is still needed.
Keywords: Medium-chain fatty acids, IPEC-J2, Escherichia coli, Salmonella Enteritidis
Introduction
Gastrointestinal disorders are one of the major causes of losses in pig production and antibiotics have been successfully used for decades to fight against them. However, in the last two decades, there is an increasing concern regarding public health due to the rise of antibiotic-resistant bacteria (Davies and Davies 2010; Heuer et al. 2011) besides to the impact on the environment (Kemper 2008). High efforts are carried out in the search of alternatives to substitute or decrease the use of antibiotics in animal production (Allen et al. 2013; Seal et al. 2013). In pig production, a diverse range of feed additives has been tested like probiotics, zinc, organic acids, plant extracts, microbial by-products or dairy products and others (Roselli et al. 2005; González-Ortiz et al. 2014; Pluske 2013; Spitzer et al. 2014).
Medium-chain fatty acids (MCFAs) are saturated fatty acids with 6–12 carbon atoms. They are found in milk fat of some species (Breckenridge and Kuksis 1967) and in some vegetable sources like coconut and palm kernel oil (Marten et al. 2006) or some seed fats of the Lythraceaea (Cuphea species) and Lauraceae families (Graham and Knapp 1989; Chow 1992). Different in vitro studies have shown antimicrobial effects against some algae, fungi, protozoa, viruses and Gram-positive bacteria (Desbois and Smith 2010) but results on their effect against Gram-negative bacteria are contradictory (Zentek et al. 2011). MCFAs have been tested as an alternative for antibiotics in pig production with promising results (Dierick et al. 2002a, b; Decuypere and Dierick 2003). Caprylic (C8) and capric (C10) acids could work as modulators of the gastric microbiota in weaned piglets (Zentek et al. 2012). MCFAs also seem to positively affect the intestinal morphology in pigs. In this sense, Dierick et al. (2003) found that the use of diets with Cuphea seeds (which are rich in C10) in 3-weeks old piglets newly weaned increased the villus/crypt ratio and decreased the presence of intraepithelial lymphocytes in the small intestine. In addition, their possible influence on the immune response has been studied in rats (Kono et al. 2009) and also in different human cell lines like human fetal intestinal cells (Andoh et al. 2000), adipocytes (Nagasaki et al. 2012) but especially the colon-derived line Caco-2 (Tanaka et al. 2001; Hoshimoto et al. 2002). However, their effect on the gut-associated immune system in pigs has not been assessed.
Nowadays, alternatives are being developed to reduce the use of animals in scientific studies. Intestinal epithelial cell lines give an approach to the mechanisms of signaling pathways related with the interaction between the epithelial cell and bacteria or viruses. The IPEC-J2 cell line is one of the most often used porcine intestinal in vitro model. IPEC-J2 is a non-transformed intestinal cell line isolated from the jejunum of a neonatal piglet (Berschneider 1989). This cell line has been used for morphological studies regarding the integrity of the epithelial monolayer in microbiological studies (Schierack et al. 2006; Brosnahan and Brown 2012) or the response to bacterial challenge after pretreatment with different substances (Spitzer et al. 2016). They have also been used to study the epithelial innate immune responses by measuring the expression of epithelial and immune-related genes (Brosnahan and Brown 2012; Støy et al. 2013).
In this study we studied a porcine in vitro model to test the effects of the MCFAs on immunological as well as morphological functionality and in response to a challenge with two of the most spread bacteria responsible of gastrointestinal disorders such as Escherichia coli and Salmonella Enteritidis.
Materials and methods
Bacterial strains
Bacteria used in the trials were an E. coli 0147:K89:K88 strain, positive for the virulence factors fan, fae, est-Ib, elt-Ia (Institute of Animal Nutrition, Freie Universität Berlin) and a Salmonellaenterica subsp. enterica DSMZ serovar Enteritidis (S. Enteritidis; Leibniz Institute DSMZ-German collection of microorganisms and cell cultures, Braunschweig, Germany). Bacteria were taken from cryo-culture 2 days prior challenge and grown in brain–heart-infusion broth (BHI medium; Carl Roth GmbH, Karlsruhe, Germany) under constant shaking at 37 °C. One day before the experiments, the bacterial cultures were transferred twice into fresh and sterile BHI medium.
Cell culture conditions
The IPEC-J2 cells (kindly provided by Prof. Dr. Michael Fromm (Charité CBF, Berlin, Germany)) were grown in plastic flasks (Greiner Bio-One International GmbH, Kremsmünster, Austria) at 37 °C in an atmosphere of 5 % CO2, and maintained in Dulbecco's modified Eagle medium (DMEM)/Ham's-F12 supplemented with 5 % pig serum, 100 units penicillin/mL and 100 mg streptomycin/mL (all from Biochrom GmbH, Berlin, Germany). IPEC-J2 cells from passage 20 to 24 were used for all experiments.
Medium-chain fatty acids (MCFAs)
The three MCFAs tested in this trial were caprylic acid (C8), capric acid (C10) and lauric acid (C12), all of them from Sigma-Aldrich (Steinheim, Germany). The stock solutions were prepared in Ethanol 70 % in a dilution of 1:10 (w/v) for C8. C10 and C12 were diluted 1:20 (w/v) due to the low solubility. To prepare working concentrations, the stock solutions were mixed with antibiotic-free DMEM for measurements of bacterial growth, fluorescence-activated cells sorting (FACS) as well as transepithelial electrical resistance (TEER), and with penicillin/streptomycin DMEM for gene expression of IPEC-J2 cells.
Bacterial growth
Bacteria were taken from exponentially growing cultures and counted in a Thoma chamber. They were then diluted with different concentrations of MCFAs having a final density of 1 × 105 cells/mL. The solutions were then transferred in 96-well microtiter plates in triplicates. Turbidity readings were monitored in a Tecan microtiter plate reader (Tecan Austria GmbH, Salzburg, Austria) at 690 nm for 24 h with constant shaking at 37 °C. Turbidity data were transformed into growth curves and specific growth and lag time were calculated as growth parameters using a sigmoidal 3 parameter equation.
Bacterial adhesion assays
IPEC-J2 cells were transferred from the culture flasks to 24-well plates (Greiner BioOne). Cells were washed twice with phosphate-buffered saline (PBS Dulbecco, Biochrom GmbH) and treated with Trypsin/EDTA (Biochrom GmbH). Once detached, cells were re-suspended in DMEM and counted in a Neubauer chamber. Final seeding was set to a density of 1 × 105 cells/well in 1 mL total volume per well.
A confluent monolayer of IPEC-J2 cells was visible after 24 h of growth and cells were then washed with PBS. Different treatments of MCFAs were administered: 1.5, 3 and 7 mM for C8; 0.5, 1 and 2 mM for C10 and C12 and additionally 0.75 mM for C12. Higher concentrations of the three MCFAs induced negative effects on monolayer integrity, as tested prior to the assay. Cells in MCFAs medium were further incubated for 24 h before challenge with bacterial cultures. All experiments with IPEC-J2 cells were repeated five times.
Bacteria were stained with the fluorescent dye 5,6-carboxymethyl fluorescein diacetate succinimidyl ester (CFDA-SE; Sigma Aldrich). In short: CFDA-SE was diluted 1:20 into dimethyl sulfoxide (DMSO, Sigma Aldrich) and 50 µL were added to 5 mL of bacterial suspension and incubated at 37 °C for 2 h. After centrifugation and resuspension in PBS, bacteria were diluted to a MOI of 100 (1 × 102 Bacteria/IPEC-J2 cell). Challenged cell cultures were incubated at 37 °C for 90 min and then washed three times with PBS. This procedure ensures that only adherent bacterial cells are measured by subsequent flow cytometry. Cell culture cells were then detached by trypsin treatment, re-suspended in antibiotic-free DMEM, centrifuged at 390×g for 5 min at 4 °C and re-suspended in 300 µL of antibiotic-free DMEM. Cells were then subjected to flow cytometry using a FACSCalibur™ instrument (Becton–Dickinson, San José, CA, USA) equipped with a 15 mW argon ion laser emitting light at a fixed wavelength of 488 nm. Data were analysed using the CellQuest™ software (Becton–Dickinson). The forward scattered (FSC) and side scattered (SCC-90° side angle) light was displayed according to size and granularity using scatter plots. A gate region was drawn to specify characteristics of desired cells. Cells were analysed at a rate of 500–750 events per second until 10,000 events in the region of interest were collected. Noise, cell debris and non-adherent bacteria outside of the gate were discriminated.
Transepithelial electrical resistance (TEER)
IPEC-J2 cells at passage 20–24 were transferred as described above to ThinCert™ cell culture inserts (polyethylene terephthalate capillary pore membranes; 0.4 µm pore size; Greiner BioOne) compatible with 6-well plates (Greiner BioOne). Cells were seeded at a density of 5 × 105 cells/well. They were maintained in the plates during 7 days (37 °C; 5 % CO2) changing to fresh medium every second day, until they reached confluency, using the value of the TEER as indicator (Schierack et al. 2006). Once confluency was achieved (with values over 2 kΩ × cm2), they were incubated overnight with the different MCFAs solutions, which were added only in the inner well (insert). The concentrations used were the following: 0.5, 1, 2, 3 and 5 mM for C8, 0.5 and 1 mM for C10 and 0.5 mM for C12. Higher concentrations could not be used due to a loss of monolayer integrity, as tested prior to the assay. It was determined that the used concentrations did not compromise cell culture growth as compared to controls without MCFAs.
Bacteria were counted and incubated for 30 min in the respective MCFAs solutions. Bacterial suspensions were then administered at a MOI of 100. Two hours after challenge, TEER was measured every hour until 9 h post-challenge. Measurement was carried out using an epithelial Voltohmmeter EVOM2 with a chopstick electrode STX2 (World Precision Instruments Inc, Sarasota, FL, USA). Five repetitions were used for challenge with E. coli and four repetitions were used for challenge with S. Enteritidis.
Relative gene expression
IPEC-J2 cells were transferred from the culture flasks into 6-well plates. Passages between 21 and 24 were used. Cells were grown for 1 week to reach confluency and then incubated for 4 h in medium containing different MCFAs concentrations (0.5, 1, 2, 5, 8 and 10 mM for C8; 0.5, 1, 2 and 5 mM for C10; 0.5, 1 and 2 mM for C12) and collected by washing twice with PBS, trypsin detachment, resuspension in fresh DMEM and centrifugation at 390×g for 5 min at 4 °C. The centrifugate was then resuspended in a RNA stabilization reagent (RNAlater; Qiagen GmbH, Hilden, Germany). Previously performed tests showed that a 4 h incubation was suitable to detect changes in IPEC-J2 cells and also let to establish the concentrations that could be used without inducing visible cell damage or death (data not shown).
Total RNA from IPEC-J2 cells was extracted using NucleoSpin RNA II kit (Macherey–Nagel GmbH & Company KG, Düren, Germany) according to the instructions of the manufacturer. RNA quality and quantity was determined with the Agilent RNA 6000 Nano Kit in an Agilent 2100 Bioanalyzer (Agilent Technologies, Waldbronn, Germany). Transcription into cDNA was performed using the SuperScript III Reverse Transcriptase First-Strand complementary DNA Synthesis System (Invitrogen) in a Sure Cycler 8800 (Agilent Technologies). Quantitative real-time PCR (qPCR) was performed using Brilliant II SYBR Green QPCR Master Mix with Low ROX (Agilent Technologies) on a Stratagene MX3000p (Agilent Technologies). 60S ribosomal protein L13 (RPL13), succinate dehydrogenase subunit A (SDHA) and ß2-microglobulin (ß2-glob) were selected as housekeeping genes and used for data normalisation. Primer sequences and annealing temperatures are shown in Table 1.
Table 1.
Primers used in this study
| Gene name | Primer sequences (5′ 3′) | AT |
|---|---|---|
| 60S ribosomal protein L13 (RPL-13) | F: CCGTCTCAAGGTGTTCGATG | 60 °C |
| R: GGATCTTGGCCTTCTCCTTC | ||
| Succinate dehydrogenase subunit A (SDHA) | F: CAAACTCGCTCCTGGACCTC | 60 °C |
| R: CCGGAGGATCTTCTCACAGC | ||
| β2-Microglobulin (β2-glob) | F: CCCCGAAGGTTCAGGTTTAC | 60 °C |
| R: CGGCAGCTATACTGATCCAC | ||
| Interleukin 6 (IL-6) | F: CCACCGGTCTTGTGGAGTTT | 59 °C |
| R: TCTGCACAGCCTCGACATTT | ||
| Interleukin 8 (IL-8) | F: GGTCTGCCTGGACCCCAAGGAA | 60 °C |
| R: TGGGAGCCACGGAGAATGGGTT | ||
| Toll-like receptor 4 (TLR-4) | F: AGGCCGTCATTAGTGCGTCAGT | 60 °C |
| R: AGCCCACAAAAAGCAACAAGTGGA | ||
| Occludin (OCLN) | F: CAGGTGCACCCTCCAGATTG | 60 °C |
| R: CAGCGGGTCACCTGATCTTC | ||
| Zonula Occludens 1 (ZO-1) | F: ACAGTGCCCAGAGACCAAGA | 60 °C |
| R: CATTTCCTCGGGGTAGGGGT | ||
| Claudin 4 (CLDN-4) | F: CTCTCTTCGGACGCTGACTG | 60 °C |
| R: GGGTCTAGGAGCTGGAAGGA |
A T annealing temperature
Statistical analysis
Data from bacterial growth were analysed using Sigma Plot 11.0 (Systat Software, Erkrath, Germany). Data from FACS and TEER were analysed using the program SPSS Statistics (Version 21; IBM, Somers, NY, USA). Normality was tested using the Shapiro–Wilk test. Normally distributed data were subjected to one-way analysis of variance (ANOVA) followed by Tukey post hoc test. Differences with p value <0.05 were considered significant.
Data from relative gene expression were analysed with the program REST 2009 (Relative Expression Software Tool V2.0.13, Qiagen GmbH).
Results
Effect of MCFAs on bacterial growth
The bactericidal effect of the three MCFAs under study was tested against E.coli and S. Enteritidis.
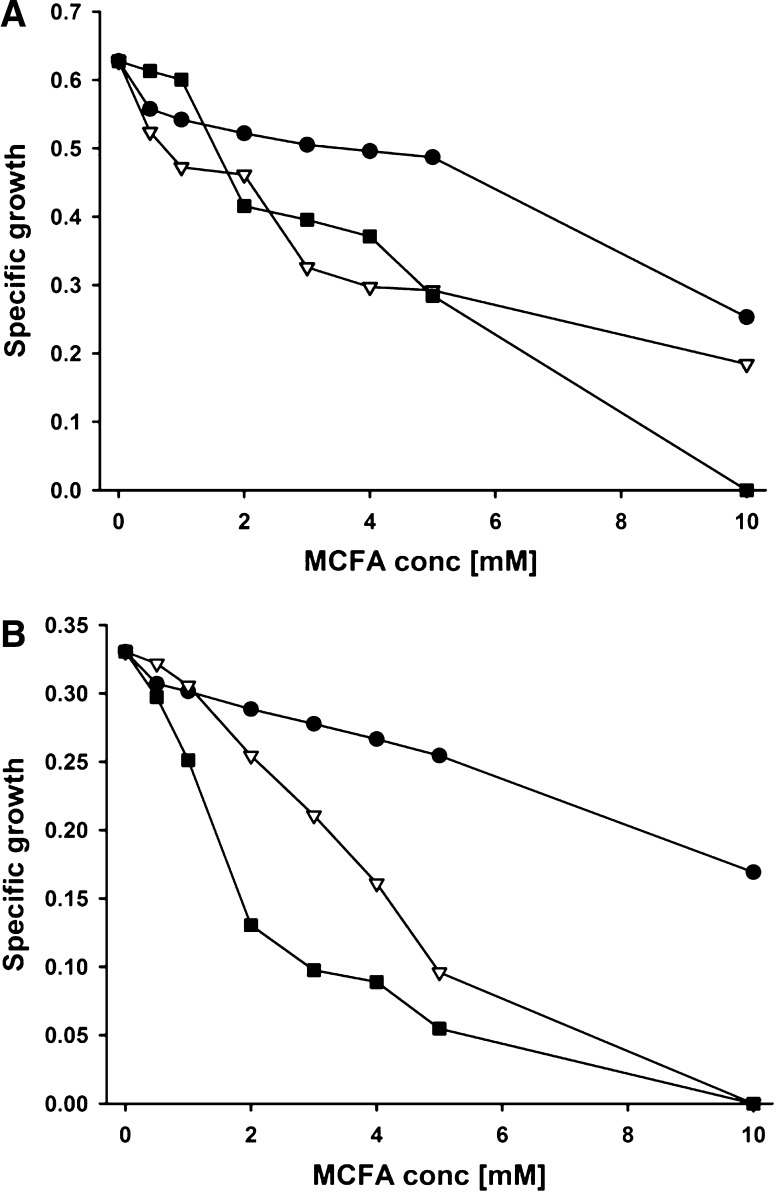
Specific growth of E. coli and S. Enteritidis in the presence of MCFAs is shown in Fig. 1a, b. All MCFAs inhibited bacterial growth in a concentration dependent manner. C8 presented the least inhibitory effect, followed by C10 and C12, which showed the most drastic inhibition even at lower concentrations. Both strains were unable to grow in C12 at a concentration of 10 mM; the S. Enteritidis strain was also unable to grow at 5 mM C10. In general, the Salmonella strain seemed to be more sensitive to MCFAs than the E. coli strain, because lower specific growth was observed at increasing MCFA concentrations.
Fig. 1.
Effect of MCFA on specific growth (1/h) of E. coli and S. Enteritidis in vitro. Bacteria were diluted with different concentrations of MCFAs to a final density of 1 × 105 cells/mL. Turbidity readings were monitored at 690 nm for 24 h with constant shaking at 37 °C. A = E. coli; B = S. Enteritidis. ● Caprylic acid; Capric acid; ■ Lauric acid
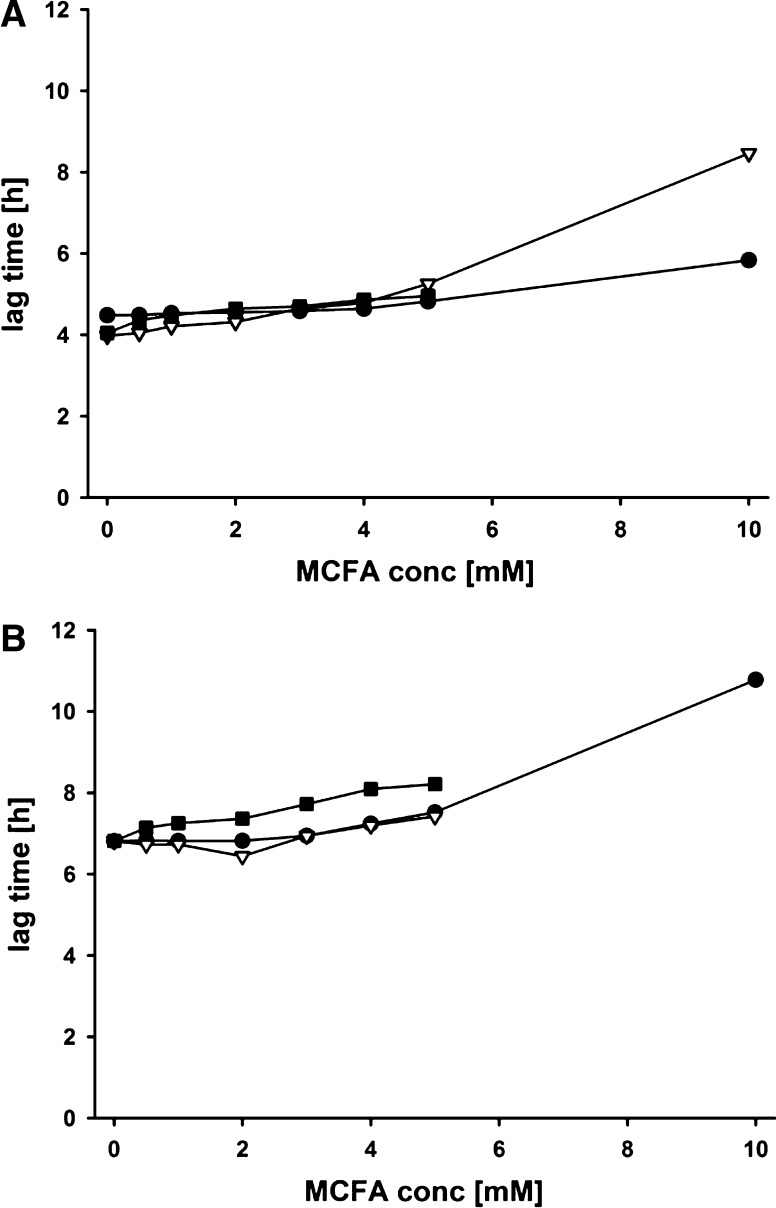
Figure 2 shows the lag time for both strains. Lag time is the initial period in the life of a bacterial population when cells are adjusting to a new environment before starting exponential growth (Rolfe et al. 2012). Interestingly, only very minor differences in lag time were observed for C8 and C10 up to 5 mM MCFAs for both strains. This was also true for the E. coli strain and C12, while the Salmonella strain showed increased lag with this MCFAs. In general, initial growth was only visibly reduced at concentrations of 10 mM MCFAs.
Fig. 2.
Effect of MCFA on lag time of E. coli and S. Enteritidis in vitro. Bacteria were diluted with different concentrations of MCFAs to a final density of 1 × 105 cells/mL. Turbidity readings were monitored at 690 nm for 24 h with constant shaking at 37 °C. A = E. coli; B = S. Enteritidis. ● Caprylic acid; Capric acid; ■ Lauric acid
Bacterial adhesion assays
The rate of adhesion of E.coli and S. Enteritidis to IPEC-J2 cells was measured by flow cytometry to determine the possible protective effect of MCFAs against bacterial challenge.
Table 2 shows the relative adhesion observed after correction with controls. Significant differences to the control were observed for both strains at concentrations of 3 mM and higher for C8, of 1 mM and higher for C10 and of 0.75 mM and higher for C12. A numeric concentration dependent increase in adhesion was observed for all MCFAs.
Table 2.
Effect of MCFAs on adhesion of pathogenic bacteria to IPEC-J2 cells
| E. coli | S. Enteritidis | |||
|---|---|---|---|---|
| Relative adhesion (%) | SEM | Relative adhesion (%) | SEM | |
| Caprylic acid (C8) | ||||
| Control | 100a | 0.39 | 100a | 0.87 |
| 1.5 mM | 105a | 1.97 | 109a | 2.41 |
| 3 mM | 141b | 4.53 | 160b | 5.08 |
| 7 mM | 149b | 4.24 | 172b | 3.93 |
| Capric acid (C10) | ||||
| Control | 100a | 0.71 | 100a | 1.12 |
| 0.5 mM | 93.5a | 2.26 | 96.5a | 5.36 |
| 1 mM | 141b | 3.66 | 159b | 3.19 |
| 2 mM | 148b | 3.71 | 168b | 3.29 |
| Lauric acid (C12) | ||||
| Control | 100a | 1.08 | 100a | 0.96 |
| 0.5 mM | 96.7a | 2.57 | 107a | 2.18 |
| 0.75 mM | 128b | 4.34 | 131b | 1.12 |
| 1 mM | 175c | 2.13 | 182c | 5.32 |
| 2 mM | 166c | 3.34 | 198c | 7.15 |
a, b, cWithin a column, means not sharing any common superscript are significantly different (P < 0.05)
Trans-epithelial electrical resistance (TEER) of IPEC-J2 cells after challenge with pathogenic bacteria
The TEER of the cells after incubation with different concentrations of the three MCFAs under study was measured to determine the integrity of the monolayer and the possible protective effect of the MCFAs against a bacterial challenge.
Table 3 shows the development of the TEER in the IPEC-J2 cells challenged with E. coli (3a) or S. Enteritidis (3b). The cell culture controls in MCFAs showed lower TEER for 5 mM C8 and 1 mM C10. In both adhesion experiments, the highest concentrations of the three MCFAs seemed to be more harmful to the IPEC-J2 cells. However, this effect was also observed in the non-challenged cells with the C8 5 mM and C10 1 mM (data not shown).
Table 3.
Evolution of the TEER (kΩ × cm2) of IPEC-J2 cells after overnight incubation in different MCFAs and challenge with E. coli (a) or S. Enteritidis (b)
| Challenge (h) | Control | C8 | C10 | C12 | |||||
|---|---|---|---|---|---|---|---|---|---|
| 0 mM | 0.5 mM | 1 mM | 2 mM | 3 mM | 5 mM | 0.5 mM | 1 mM | 0.5 mM | |
| (a) | |||||||||
| 0 | 2546a | 2425ab | 2487a | 2421a | 2026a | 1489bc | 2367a | 1808bc | 1980a |
| 2 | 2547a | 2627a | 2680a | 2590a | 2280a | 1784ab | 2470a† | 2282a | 2162a |
| 3 | 2326ab† | 2636a | 2741a | 2559a | 2291a | 1926a | 2422a | 2213ab† | 2210a |
| 4 | 2146ab | 2455a† | 2555a† | 2507a | 2129a | 1847ab† | 2229ab | 1869b | 2031a† |
| 5 | 1914bc | 2204ab | 2286ab | 1982ab† | 1804ab† | 1591ab | 1855bc | 1472c | 1618ab |
| 6 | 1604cd | 2014abc | 1728bc | 1626b | 1362bc | 1194c | 1519cd | 1003d | 1119bc |
| 7 | 1380de | 1825abc | 1345cd | 1205bc | 839cd | 815d | 1142de | 452e | 694cd |
| 8 | 1163de | 1520bc | 951d | 858c | 526d | 451e | 724e | 180e | 378d |
| 9 | 991e | 1306bc | 714d | 674c | 362d | 290e | 473e | 102e | 239d |
| (b) | |||||||||
| 0 | 2302a | 2093ab | 1924cd | 1870bcd | 1895 | 1068bcd | 1787 | 1155ab | 1502 |
| 2 | 2365a | 2322a | 2353abc | 2349ab | 2170 | 1245abcd | 1885† | 1414a | 1462† |
| 3 | 2348a | 2484a | 2545a | 2474a | 2346 | 1668a | 2053 | 1646a | 1700 |
| 4 | 2337a | 2412a | 2505a | 2466a | 2323 | 1686a | 2083 | 1677a | 1685 |
| 5 | 2127ab | 2389a† | 2492a | 2427a | 2321 | 1656a | 2178 | 1690a† | 1706 |
| 6 | 2056ab | 2356a | 2443ab† | 2349ab† | 2336 | 1570abc† | 2122 | 1625a | 1670 |
| 7 | 1936ab | 2119ab | 2248abc | 2138abc | 2123† | 1351abc | 1932 | 1382ab | 1567 |
| 8 | 1876ab | 1941ab | 1997bc | 1812cd | 1857 | 1038cd | 1674 | 1082ab | 1362 |
| 9 | 1659b† | 1669b | 1654d | 1384d | 1566 | 683d | 1292 | 729b | 1134 |
a, b, c, d, eWithin a column, means not sharing any common superscript are significantly different (P < 0.05)
† Significantly different to control (p ≤ 0.05) from this time point on
The data showed that S. Enteritidis adhered to the cells much later than the E. coli strain in controls. The differences in TEER between challenged and non- challenged cells in control were significant for E. coli after 3 h, but only after 9 h for S. Enteritidis. In the treated wells, the differences in TEER between challenged and non- challenged cells became significant at 4–5 h with C8 and C12 and 3 h in C10 after challenge with E. coli. However, our results show that the use of MCFAs increased the negative effect of the Salmonella strain, since the effect of the adhesion began to be statistically detectable at 6–7 h for C8 and even much earlier with C10 and C12, appearing differences at 2 h post-challenge for the concentration 0.5 mM.
Gene expression
The effects of the three MCFAs on the relative gene expression of different genes in IPEC-J2 cells involved in both immune response and morphology of the epithelium are shown in Table 4.
Table 4.
Relative gene expression of selected genes in IPEC-J2 cells after incubation for 4 h in different MCFAs
| Immunological response | Morphological response | ||||||
|---|---|---|---|---|---|---|---|
| IL-6 | IL-8 | IL-12 | TLR-4 | OCLN | ZO-1 | CLDN-4 | |
| C8 (mM) | |||||||
| 0.5 | 1.090 | 0.818 | 1.508 | 0.877 | 0.811* | 0.981 | 1.122 |
| 1.0 | 0.921 | 0.733 | 1.494 | 0.972 | 0.728* | 0.762* | 1.072 |
| 2.0 | 0.906 | 0.770 | 1.454 | 1.124 | 0.762* | 0.801* | 1.303* |
| 5.0 | 1.383* | 0.625* | 1.036 | 1.332* | 0.878 | 0.891 | 2.291* |
| 8.0 | 1.908* | 0.449* | 0.998 | 1.449* | 0.820 | 0.893 | 3.177* |
| 10.0 | 2.132* | 0.328* | 0.934 | 1.589* | 0.746* | 0.838 | 2.573* |
| C10 (mM) | |||||||
| 0.5 | 0.833* | 0.666* | 1.115 | 0.989 | 0.766* | 0.746* | 0.984 |
| 1.0 | 0.831* | 0.690* | 1.155 | 1.111 | 0.779* | 0.821a | 1.211a |
| 2.0 | 0.866a | 0.676* | 1.166 | 1.287* | 0.619* | 0.787a | 1.442* |
| 5.0 | 1.390* | 0.652* | 0.751a | 1.185a | 0.512* | 0.966 | 1.392* |
| C12 (mM) | |||||||
| 0.5 | 0.723* | 0.700* | 1.261 | 0.864 | 0.921 | 0.933 | 1.265* |
| 1.0 | 0.822* | 0.843 | 1.551 | 0.952 | 0.808* | 0.933 | 1.182 |
| 2.0 | 0.973 | 0.638* | 1.246 | 0.902 | 0.738* | 0.863 | 1.467* |
* Significantly different to control (p ≤ 0.05)
aTrend for significant difference to control (p ≤ 0.1)
Regarding the effect on the immune response, there seems to be a concentration dependent effect of C8 starting at 5 mM for IL-6, IL-8 and TLR-4, but not for IL-12. While IL-6 and TLR-4 were upregulated, IL-8 was downregulated with C8. On the other hand, C10 and C12 showed a different trend: the relative gene expression of IL-6 and IL-8 was already down-regulated at 0.5 mM and up to 5 mM for C10, while IL-12 was only down-regulated at 5 mM for C10, but showed no differences for C12. Similarly, TLR-4 was only significantly up-regulated for C10 at 2 and 5 mM, but not for C12.
Regarding the genes related with the morphology of the epithelium, there was a clear effect of the three MCFAs in down-regulation of occludin and up-regulation of claudin-4, while the zonula occludens-1 gene was only down-regulated at lower concentrations of C8 and C10.
Discussion
This study investigated the effects of MCFAs on physiological response of the porcine IPEC-J2 cell line after challenge with pathogenic bacteria and its immunological as well as morphological functionality in the presence of MCFAs alone. Prior to in vitro challenge, however, we studied the impact of MCFAs on the IPEC-J2 cell line and pathogenic bacteria separately.
As reviewed by Zentek et al. (2011), the results obtained in different in vitro studies using C8 and C10 against the bacterial species also used in this study are contradictory regarding both the presence or absence of effect and the concentration needed to inhibit growth of bacteria. Marounek et al. (2002) found a significant in vitro antimicrobial activity of C8 and C10 against some Gram-positive and Gram-negative bacteria present in gastrointestinal tract of ruminants and rabbits. This activity was also found against E. coli by the same group (Marounek et al. 2003) being stronger in this case in C8 than in C10 (around 2 and 7 mM, respectively, were needed), after a 1-day incubation. On the contrary, Bergsson et al. (2002) did not find killing activity of none of the three MCFAs against E. coli or Salmonella spp. after 30 min of incubation with a concentration of 10 mM. However, in the same study, the authors found an inactivation of Helicobacter pylori with quite high concentrations of C8 and C10 (10 and 5–10 mM, respectively). Also C12 showed a stronger effect against H. pylori, with concentrations between 1.25 and 10 mM.
There is a general problem to compare studies since different methodologies were used. As stated by Zentek et al. (2011), the undissociated forms of the MCFAs have generally the stronger effects, but at neutral pH they would be in the dissociated form. For instance, Marounek et al. (2003) found no effect on the viable E. coli count after short term incubation with C8 or C10 at pH 6.5 but a strong decrease was observed at a pH of 5.2. Furthermore, Sun et al. (2003) found that even at a concentration of 1 mM C8, bactericidal effects were visible between pH 3.5 and pH 5.5. However, Petschow et al. (1996) testing the bactericidal activity of C12 against H. pylori did not find differences using pH ranging from 3 to 7. A pH dependent effect could well be the reason for the observed results on growth in our study. We found a decrease in specific growth at quite low concentrations, but the start of exponential growth (lag time) was only delayed at the highest test concentrations (10 mM). Under our experimental conditions, the buffering capacity of the media was most probably sufficient to keep the MCFA in their undissociated form at the start, thus no differences in lag time were observed. During growth, bacterial metabolites will acidify the media and convert the MCFA into their undissociated forms, leading to reduced specific growth in the exponential phase. Nevertheless, consistent with previous studies, C12 seemed to exert a much higher inhibitory power on bacterial growth than C8 and C10.
Secondly, to observe how the MCFAs could affect the functionality of IPEC-J2 cells, we studied the expression of selected genes involved in immune response (IL-6, IL8, IL-12, TLR-4) and morphological integrity (OCLN, ZO-1, CLDN-4). In this assay, since the exposition to the MCFAs was much shorter in time than in the others, we were able to test higher concentrations without inducing visible cell damage or death. In fact, compared to controls, we did not find growth inhibition of the IPEC-J2 cells in the presence of MCFAs at the employed concentrations, but MCFAs apparently modified the functionality of the cell culture.
One result confirmed earlier studies, as IL-10, an anti-inflammatory cytokine, is not expressed in IPEC-J2 cells (Schierack et al. 2006; Mariani et al. 2009). Since C8 was easily solubilized in the DMEM medium, higher concentrations of this fatty acid could be used in the trial while C10 and C12 showed problems with solubility at concentrations higher than 2 and 1 mM, respectively. Furthermore, higher concentrations than 7 mM of C8 led to cell detachment after the incubation and cells were completely removed during the washing process.
Previous studies have characterized the IPEC-J2 cell line studying the gene expression of some cellular and immunological parameters (Brosnahan and Brown 2012). However, information regarding the effect of the MCFAs on gene expression is only found for in vitro cell lines other than IPEC-J2. These former results point to the idea expressed by Georgiadi and Kersten (2012) that these fatty acids play a role as signaling molecules influencing biological processes. In fact, Talukdar et al. (2011) concluded that fatty acids play important roles as endocrine regulators. Glass and Olefsky (2012) defined saturated fatty acids as pro-inflammatory lipid compounds. However, our results on the IPEC-J2 cell line are contradictory regarding this effect. On one side, there was a clear inhibition of IL-8, a pro-inflammatory cytokine responsible for neutrophil chemotaxis by all MCFAs under study. The same observation was recorded by Hoshimoto et al. (2002) incubating Caco-2 cells for 24 h with a concentration of 1.3 mM of C8, although other authors found opposite results for that cell line (Andoh et al. 2000; Tanaka et al. 2001). Another pro-inflammatory cytokine affected by the MCFAs was IL-6, but the effect of the MCFAs in this case was more complex, since only the lowest concentrations of C12 induced a down-regulation. On the other side, there was an up-regulation of IL-6 and TLR-4 with the highest concentrations of C8 (from 5 mM to 10 mM). We cannot support or even compare these results with other studies, since there is no available information on IPEC-J2 and MCFAs. In fact, regarding human diseases, Laroui et al. (2012) indicated that the role of MCFAs in inflammation is not clear, because the size of the carbon chain is intermediate between small and long chains.
The effects of MCFAs (mainly C10 and C12) on the epithelial structure have been more deeply studied although the mechanism of action is not yet clear. Again, the IPEC-J2 cell line has not been studied as thoroughly as other cell lines. Coyne et al. (2003) attributed the enhancement of the permeability to the alteration of the intestinal tight junctions (TJ) barrier function in primary airway cells from human subjects. It has been demonstrated that C10 regulates paracellular permeability in Caco-2 cells (Anderberg et al. 1993). Lindmark et al. (1998) stated that C12 also exhibits this effect, although it did not induce detectable changes after immunofluorescent staining as C10, which induced redistribution of ZO-1 and occludin. Our results indicate that not only C10 and C12, but also C8 had a clear effect on gene expression of occludin, although the response to C8 was quite similar at all concentrations, C10 and C12 presented a concentration dependent effect. However, no clear effect on ZO-1 was found. Coyne et al. (2003) observed also a redistribution of some structural components of the TJ incubating primary airway cells with C10 and C12, but, as in our study, ZO-1 distribution remained quite unchanged when observed under confocal microscopy. They also found a redistribution of claudin-4 which, in our case, is the structural component most strongly affected by the three MCFAs.
Finally, the effect of the three MCFAs on the response of IPEC-J2 cells to the adhesion of pathogenic bacteria was studied. There is not much available information in the literature about the effect of the MCFAs on IPEC-J2 cells and their response to an in vitro bacterial challenge. The results from this study seem to point to a negative effect of these MCFAs on the capacity of the cells to cope with bacterial challenge, since the percentage of cells with adhered bacteria observed after incubation with the three of them was equal or higher than the control. In fact, the bactericidal effect that the MCFAs showed in bacterial growth experiments was not reflected in these results.
Since quite a long time, MCFAs and their salts have been tested as permeability enhancers to improve drug absorption and delivery (Shima et al. 1997; Aungst 2012). However, most studies have been performed using the human derived cell line Caco-2, and information on IPEC-J2 cells cannot be found to our best knowledge. Lindmark et al. (1995) observed that the sodium salts of C8, C10 and C12 increased permeability of [14C]-mannitol, a radioactive marker used to observe changes in the epithelial integrity.
A mix of dyes was used by Brayden et al. (2014) revealing that C8, C10 and C12 increased the intracellular calcium (from the concentrations 30, 5 and 1 mM, respectively) and the plasma membrane permeability (40, 8.5 and 2 mM, respectively) after 60 min of exposure. The authors also found that MCFAs displayed cytotoxicity, as previously reported by other studies (Kamm et al. 2000; Endo et al. 2002), with concentrations higher than 9.5 mM of C10 and 5 mM for C12 after 1 h of exposure and from 7.5 mM for C10 and 2.5 mM for C12 after 8 h. Transepithelial resistance also seemed to be impaired when MCFAs are used in Caco-2 cells (Lindmark et al. 1998).
In our study, TEER measurements sharply decreased after medium change. The 16-h incubation seems not to be sufficient to allow the total recovery of the initial TEER measurements, because TEER values continued to increase slightly until 3–4 h after the bacterial challenge. Thus, our TEER measurements fall in line with studies on other cell lines that found a cytotoxic effect at higher concentrations. In fact, preliminary studies on the subject showed that overnight incubations with concentrations even higher than 5 mM in C8, 1 mM in C10 and 0,5 mM in C12 led to a complete breakdown of monolayer integrity. Even C8 at 5 mM and C10 at 1 mM seemed to impair the monolayer integrity since the TEER did not recover completely during the whole experimental period.
However, these observations do not completely explain the increase in the rate of adhesion registered on FACS, since some of the apparently non-cytotoxic concentrations in TEER also led to high adhesion rates. Apparently, there are other factors involved in the increased adhesion rate with MCFAs. Prior to visible reduction in cell integrity, the morphological structure of the monolayer may already be compromised. This would improve the chance for bacterial adhesion.
To summarize all the results, we can state that the three MCFAs under study influenced bacterial growth rates and also modified the gene expression to a different degree in the cell line IPEC-J2. Furthermore, as the cell culture was not compromised in growth, but functionality at the used MCFAs concentrations, we can conclude that the impact of MCFAs on pathogenic bacteria is multifactorial in the sense that a change in cell functionality may increase the resistance against bacterial adhesion.
Finally, we consider that IPEC-J2 seems to be an adequate model to measure the immune- and morphological responses to MCFAs treatment at transcriptional level. However, the effect of these fatty acids on morphological structure and response of IPEC-J2 cells after a bacterial challenge could not be assessed and other alternative methodologies should be used. In addition, although these in vitro tests show a prior estimation of the possible effects of MCFAs in intestinal epithelium, in vivo confirmation is still needed, since there are many other factors influencing intestinal epithelium function which cannot be considered in cell models.
Acknowledgments
We would like to thank Petra Huck for her invaluable assistance with IPEC-J2 cells.
References
- Allen HK, Levine UY, Looft T, Bandrick M, Casey TA. Treatment, promotion, commotion: antibiotic alternatives in food-producing animals. Trends Microbiol. 2013;21:114–119. doi: 10.1016/j.tim.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Anderberg EK, Lindmark T, Artursson P. Sodium caprate elicits dilatations in human intestinal tight junctions and enhances drug absorption by the paracellular route. Pharm Res. 1993;10:857–864. doi: 10.1023/A:1018909210879. [DOI] [PubMed] [Google Scholar]
- Andoh A, Takaya H, Araki Y, Tsujikawa T, Fujiyama Y, Bamba T. Medium- and long-chain fatty acids differentially modulate interleukin-8 secretion in human fetal intestinal epithelial cells. J Nutr. 2000;130:2636–2640. doi: 10.1093/jn/130.11.2636. [DOI] [PubMed] [Google Scholar]
- Aungst BJ. Absorption enhancers: applications and advances. AAPS J. 2012;14:10–18. doi: 10.1208/s12248-011-9307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsson G, Steingrímsson O, Thormar H. Bactericidal effects of fatty acids and monoglycerides on Helicobacter pylori. Int J Antimicrob Ag. 2002;20:258–262. doi: 10.1016/S0924-8579(02)00205-4. [DOI] [PubMed] [Google Scholar]
- Berschneider H. Development of normal cultured small intestinal epithelial cell lines which transport Na and Cl. Gastroenterology. 1989;96:A41. [Google Scholar]
- Brayden DJ, Gleeson J, Walsh EG. A head-to-head multi-parametric high content analysis of a series of medium chain fatty acid intestinal permeation enhancers in Caco-2 cells. Eur J Pharm Biopharm. 2014;88:830–839. doi: 10.1016/j.ejpb.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Breckenridge WC, Kuksis A. Molecular weight distributions of milk fat triglycerides from seven species. J Lipid Res. 1967;8:473–478. [PubMed] [Google Scholar]
- Brosnahan AJ, Brown DR. Porcine IPEC-J2 intestinal epithelial cells in microbiological investigations. Vet Microbiol. 2012;156:229–237. doi: 10.1016/j.vetmic.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow CK. Fatty acids in foods and their health implications. New York: Marcel Dekker Inc; 1992. [Google Scholar]
- Coyne CB, Ribeiro CMP, Boucher RC, Johnson LG. Acute mechanism of medium chain fatty acid-induced enhancement of airway epithelial permeability. J Pharmacol Exp Ther. 2003;305:440–450. doi: 10.1124/jpet.102.047654. [DOI] [PubMed] [Google Scholar]
- Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol R. 2010;74:417–433. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decuypere JA, Dierick NA. The combined use of triacylglycerols containing medium-chain fatty acids and exogenous lipolytic enzymes as an alternative to in-feed antibiotics in piglets: concept, possibilities and limitations. An overview. Nutr Res Rev. 2003;16:193–209. doi: 10.1079/NRR200369. [DOI] [PubMed] [Google Scholar]
- Desbois AP, Smith VJ. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl Microbiol Biotechnol. 2010;85:1629–1642. doi: 10.1007/s00253-009-2355-3. [DOI] [PubMed] [Google Scholar]
- Dierick NA, Decuypere JA, Molly K, Van Beek E, Vanderbeke E. The combined use of triacylglycerols containing medium-chain fatty acids (MCFAs) and exogenous lipolytic enzymes as an alternative for nutritional antibiotics in piglet nutrition: I. In vitro screening of the release of MCFAs from selected fat sources by selected exogenous lipolytic enzymes under simulated pig gastric conditions and their effects on the gut flora of piglets. Lives Prod Sci. 2002;75:129–142. doi: 10.1016/S0301-6226(01)00303-7. [DOI] [Google Scholar]
- Dierick NA, Decuypere JA, Molly K, Van Beek E, Vanderbeke E. The combined use of triacylglycerols (TAGs) containing medium chain fatty acids (MCFAs) and exogenous lipolytic enzymes as an alternative to nutritional antibiotics in piglet nutrition: II. In vivo release of MCFAs in gastric cannulated and slaughtered piglets by endogenous and exogenous lipases; effects on the luminal gut flora and growth performance. Lives Prod Sci. 2002;76:1–16. doi: 10.1016/S0301-6226(01)00331-1. [DOI] [Google Scholar]
- Dierick NA, Decuypere JA, Degeyter I. The combined use of whole Cuphea seeds containing medium chain fatty acids and an exogenous lipase in piglet nutrition. Arch Anim Nutr. 2003;57:49–63. doi: 10.1080/0003942031000086626. [DOI] [PubMed] [Google Scholar]
- Endo Y, Hanada K, Miyake M, Ogawara KI, Higaki K, Kimura T. Mechanisms of cytoprotective effect of amino acids on local toxicity caused by sodium laurate, a drug absorption enhancer, in intestinal epithelium. J Pharm Sci. 2002;91:730–743. doi: 10.1002/jps.10049. [DOI] [PubMed] [Google Scholar]
- Georgiadi A, Kersten S. Mechanisms of gene regulation by fatty acids. Adv Nutr. 2012;3:127–134. doi: 10.3945/an.111.001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15:635–645. doi: 10.1016/j.cmet.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Ortiz G, Pérez JF, Hermes RF, Molist F, Jiménez-Díaz R, Martín-Orúe SM. Screening the ability of natural feed ingredients to interfere with the adherence of enterotoxigenic Escherichia coli (ETEC) K88 to the porcine intestinal mucus. Brit J Nutr. 2014;111:633–642. doi: 10.1017/S0007114513003024. [DOI] [PubMed] [Google Scholar]
- Graham SA, Knapp SJ. Cuphea: a new plant source of medium-chain fatty acids. Crit Rev Food Sci. 1989;28:139–173. doi: 10.1080/10408398909527495. [DOI] [PubMed] [Google Scholar]
- Heuer H, Schmitt H, Smalla K. Antibiotic resistance gene spread due to manure application on agricultural fields. Curr Opin Microbiol. 2011;14:236–243. doi: 10.1016/j.mib.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Hoshimoto A, Suzuki Y, Katsuno T, Nakajima H, Saito Y. Caprylic acid and medium-chain triglycerides inhibit IL-8 gene transcription in Caco-2 cells: comparison with the potent histone deacetylase inhibitor trichostatin. Brit J Pharmacol. 2002;136:280–286. doi: 10.1038/sj.bjp.0704719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm W, Jonczyk A, Jung T, Luckenbach G, Raddatz P, Kissel T. Evaluation of absorption enhancement for a potent cyclopeptidic ανβ3-antagonist in a human intestinal cell line (Caco-2) Eur J Pharm Sci. 2000;10:205–214. doi: 10.1016/S0928-0987(99)00092-5. [DOI] [PubMed] [Google Scholar]
- Kemper N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol Indic. 2008;8:1–13. doi: 10.1016/j.ecolind.2007.06.002. [DOI] [Google Scholar]
- Kono H, Fujii H, Ishii K, Hosomura N, Ogiku M. Dietary medium-chain triglycerides prevent chemically induced experimental colitis in rats. Transl Res. 2009;155:131–141. doi: 10.1016/j.trsl.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Laroui H, Ingersoll SA, Liu HC, Baker MT, Ayyadurai S, Charania MA, Laroui F, Yan Y, Sitaraman SV, Merlin D. Dextran Sodium Sulfate (DSS) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PLoS One. 2012;7:e32084. doi: 10.1371/journal.pone.0032084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindmark T, Nikkilä T, Artursson P. Mechanisms of absorption enhancement by medium chain fatty acids in intestinal epithelial Caco-2 cell monolayer. J Pharmacol Exp Ther. 1995;275:958–964. [PubMed] [Google Scholar]
- Lindmark T, Kimura Y, Artursson P. Absorption enhancement through intracellular regulation of tight junction permeability by medium chain fatty acids in Caco-2 cells. J Pharmacol Exp Ther. 1998;284:362–369. [PubMed] [Google Scholar]
- Mariani V, Palermo S, Fiorentini S, Lanubile A. Gene expression study of two widely used pig intestinal epithelial cell lines: IPEC-J2 and IPI-21. Vet Immunol Immunopathol. 2009;131:278–284. doi: 10.1016/j.vetimm.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Marounek M, Skrinaková V, Savka O. Effect of caprylic, capric and oleic acid on growth of rumen and rabbit caecal bacteria. J Anim Feed Sci. 2002;11:507–516. [Google Scholar]
- Marounek M, Skrinaková V, Rada V. Susceptibility of Escherichia coli to C2-C18 fatty acids. Folia Microbiol. 2003;48:731–735. doi: 10.1007/BF02931506. [DOI] [PubMed] [Google Scholar]
- Marten B, Pfeuffer M, Schrezenmeir J. Medium-chain triglycerides. Int Dairy J. 2006;16:1374–1382. doi: 10.1016/j.idairyj.2006.06.015. [DOI] [Google Scholar]
- Nagasaki H, Kondo T, Fuchigami M, Hashimoto H, Sugimura Y, Ozaki N, Arima H, Ota A, Oiso Y, Hamada Y. Inflammatory changes in adipose tissue enhance expression of GPR84, a medium-chain fatty acid receptor TNFa enhances GPR84 expression in adipocytes. FEBS Lett. 2012;586:368–372. doi: 10.1016/j.febslet.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Petschow BW, Batema RP, Ford LL. Susceptibility of Helicobacter pylori to bactericidal properties of medium-chain monoglycerides and free fatty acids. Antimicrob Agents Ch. 1996;40:302–306. doi: 10.1128/aac.40.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluske J. Feed- and feed additives-related aspects of gut health and development in weanling pigs. J Anim Sci Biotechnol. 2013;4:1. doi: 10.1186/2049-1891-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe MD, Rice CJ, Lucchini S, Pin C, Thompson A, Cameron ADS, Mark Alston M, Stringer MF, Betts RP, Baranyi J, Peck MW, Hinton JCD. Lag phase is a distinct growth phase that prepares bacteria for exponential growth and involves transient metal accumulation. J Bacteriol. 2012;194:686–701. doi: 10.1128/JB.06112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli M, Finamore A, Britti MS, Bosi P, Oswald I, Mengheri E. Alternatives to in-feed antibiotics in pigs: evaluation of probiotics, zinc or organic acids as protective agents for the intestinal mucosa. A comparison of in vitro and in vivo results. Anim Res. 2005;54:203–218. doi: 10.1051/animres:2005012. [DOI] [Google Scholar]
- Schierack P, Nordhoff M, Pollmann M, Weyrauch KD, Amasheh S, Lodemann U, Jores J, Tachu B, Kleta S, Blikslager A, Tedin K, Wieler LH. Characterization of a porcine intestinal epithelial cell line for in vitro studies of microbial pathogenesis in swine. Histochem Cell Biol. 2006;125:293–305. doi: 10.1007/s00418-005-0067-z. [DOI] [PubMed] [Google Scholar]
- Seal BS, Lillehoj HS, Donovan DM, Gay CG. Alternatives to antibiotics: a symposium on the challenges and solutions for animal production. Anim Health Res Rev. 2013;14:78–87. doi: 10.1017/S1466252313000030. [DOI] [PubMed] [Google Scholar]
- Shima M, Yohdoh K, Yamaguchi M, Kimura Y, Adachi S, Matsuno R. Effects of medium-chain fatty acids and their acylglycerols on the transport of penicillin V across Caco-2 cell monolayers. Biosci Biotech Biochem. 1997;61:1150–1155. doi: 10.1271/bbb.61.1150. [DOI] [PubMed] [Google Scholar]
- Spitzer F, Vahjen W, Pieper R, Martínez-Vallespín B, Zentek J. A standardised challenge model with an enterotoxigenic F4 + Escherichia coli strain in piglets assessing clinical traits and faecal shedding of fae and est-II toxin genes. Arch Anim Nutr. 2014;68:448–459. doi: 10.1080/1745039X.2014.968701. [DOI] [PubMed] [Google Scholar]
- Spitzer F, Speiser S, Vahjen W, Zentek J. Effect of different feed ingredients and additives on IPEC-J2 cells challenged with an enterotoxigenic Escherichia coli strain. Cytotechnology. 2016;68:1463–1471. doi: 10.1007/s10616-015-9905-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Støy ACF, Heegaard PMH, Sangild PT, Østergaard MV, Skovgaard K (2013) Gene expression analysis of the IPEC-J2 cell line: a simple model for the inflammation-sensitive preterm intestine. ISRN Genom Article ID 980651. doi:10.1155/2013/980651
- Sun CQ, O´Connor CJ, Roberton AM. Antibacterial actions of fatty acids and monoglycerides against Helicobacter pylori. FEMS Immunol Med Mic. 2003;36:9–17. doi: 10.1016/S0928-8244(03)00008-7. [DOI] [PubMed] [Google Scholar]
- Talukdar S, Olefsky JM, Osborn O. Targeting GPR120 and other fatty acid-sensing GPCRs ameliorates insulin resistance and inflammatory diseases. Trends Pharmacol Sci. 2011;32:543–550. doi: 10.1016/j.tips.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Saitoh O, Tabata K, Matsuse R, Kojima K, Sugi K, Nakagawa K, Kayazawa M, Teranishi T, Uchida K, Hirata I, Katsu KI. Medium-chain fatty acids stimulate interleukin-8 production in Caco-2 cells with different mechanisms from long-chain fatty acids. J Gastroen Hepatol. 2001;16:748–754. doi: 10.1046/j.1440-1746.2001.02537.x. [DOI] [PubMed] [Google Scholar]
- Zentek J, Buchheit-Renko S, Ferrara F, Vahjen W, Van Kessel AG, Pieper R. Nutritional and physiological role of medium-chain triglycerides and medium-chain fatty acids in piglets. Anim Health Res Rev. 2011;12:83–93. doi: 10.1017/S1466252311000089. [DOI] [PubMed] [Google Scholar]
- Zentek J, Buchheit-Renko S, Männer K, Pieper R, Vahjen W. Intestinal concentrations of free and encapsulated dietary medium-chain fatty acids and effects on gastric microbial ecology and bacterial metabolic products in the digestive tract of piglets. Arch Anim Nutr. 2012;66:14–26. doi: 10.1080/1745039X.2011.644916. [DOI] [PubMed] [Google Scholar]