Abstract
The P2X7 and Wnt/β-catenin signaling pathways regulate osteoblast differentiation and are critical for the anabolic responses of bone to mechanical loading. However, whether these pathways interact to control osteoblast activity is unknown. The purpose of this study was to investigate the effects of P2X7 activation on Wnt/β-catenin signaling in osteoblasts. Using MC3T3-E1 cells, we found that combined treatment with Wnt3a and the P2X7 agonist 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate (BzATP) elicited more sustained β-catenin nuclear localization than that induced by Wnt3a alone. Wnt3a-induced increases in β-catenin transcriptional activity were also potentiated by treatment with BzATP. Consistent with involvement of P2X7, a high ATP concentration (1 mM) potentiated Wnt3a-induced β-catenin transcriptional activity, whereas a low concentration (10 μM) of ATP, adenosine 5′-diphosphate (ADP), or uridine 5′-triphosphate (UTP) failed to elicit a response. The potentiation of β-catenin transcriptional activity elicited by BzATP was also inhibited by two distinct P2X7 antagonists: A 438079 and A 740003. Furthermore, responses to Wnt3a in calvarial cells isolated from P2rx7 knockout mice were significantly less than in cells from wild-type controls. In MC3T3-E1 cells, BzATP increased inhibitory phosphorylation of glycogen synthase kinase 3β (GSK3β), a process that was blocked by A 438079 and diminished by inhibition of protein kinase C. Thus, P2X7 signaling may potentiate the canonical Wnt pathway through GSK3β inhibition. Taken together, we show that P2X7 activation prolongs and potentiates Wnt/β-catenin signaling. Consequently, cross-talk between P2X7 and Wnt/β-catenin pathways may modulate osteoblast activity in response to mechanical loading.
Keywords: P2X7 receptors, Wnt, β-catenin, Osteoblast, Glycogen synthase kinase 3β
Introduction
Mechanical loading stimulates bone formation by osteoblasts and inhibits bone resorption by osteoclasts, leading to increased bone mass and improved skeletal strength [1]. The canonical Wnt/β-catenin pathway plays a critical role in osteoblast differentiation, bone formation, and in the response of the skeleton to mechanical loading [2, 3]. Specifically, mechanical loading in mice reduces levels of the LRP5/6 antagonist sclerostin (SOST) and activates canonical Wnt signaling to promote bone formation [4, 5]. In osteoblast and osteocyte culture systems, β-catenin can be activated following fluid shear stress or tensile strain [6, 7]. At the same time, the canonical Wnt antagonist dickopff 1 (DKK1) fails to inhibit β-catenin nuclear localization elicited by tensile strain [8], suggesting that mechanisms independent of canonical Wnt signaling contribute to β-catenin activation in cells of the osteoblast lineage.
Extracellular nucleotides, released in response to mechanical stimuli, signal through the P2Y family of G protein-coupled receptors and the P2X family of ligand-gated cation channels [9]. Mice in which P2X7 receptor function is disrupted (knockout) exhibit diminished periosteal bone formation and increased trabecular bone resorption [10] and impaired anabolic responses of the skeleton to mechanical load [11]. In cells of the osteoblast lineage, stimulation of the P2X7 receptor promotes differentiation and matrix mineralization [12]. Additionally, P2X7 is required to initiate signaling following fluid shear stress in cultures of osteoblast-like and osteocyte-like cells [11]. In cerebellar granule neurons, stimulation of the P2X7 receptor leads to inhibitory phosphorylation of glycogen synthase kinase 3β (GSK3β) [13]. However, it is not known whether P2X7 can inhibit GSK3β in osteoblasts and thereby elicit activation of β-catenin.
In the present study, we show that stimulation of P2X7 in MC3T3-E1 osteoblast-like cells promotes transient β-catenin nuclear localization with no detectable change in transcriptional activity. On the other hand, P2X7 activation prolongs β-catenin nuclear localization and potentiates transcriptional activity elicited by canonical Wnt signaling. Mechanistically, this potentiation is associated with inhibitory phosphorylation of GSK3β induced by activated P2X7 receptors, suggesting that P2X7 may promote Wnt/β-catenin signaling through GSK3β. Taken together, these data show that P2X7 activation potentiates Wnt/β-catenin signaling, a mechanism through which osteoblast activity may be modulated in response to mechanically-induced ATP release.
Materials and methods
Materials and solutions
α-Minimum essential medium (α-MEM), heat-inactivated fetal bovine serum (FBS), antibiotic solution (10,000 U/ml penicillin, 10,000 μg/ml streptomycin, and 25 μg/ml amphotericin B), trypsin solution, Dulbecco’s phosphate-buffered saline (DPBS), and Dulbecco’s modified Eagle medium (high glucose) (DMEM) were obtained from GIBCO (Life Technologies Inc., Burlington, ON, Canada). β-catenin (L54E2) mouse monoclonal and phospho-GSK3α/β (pSer21/9) rabbit polyclonal antibodies were from Cell Signaling Technologies Inc. (Danvers, MA, USA). GSK3α/β mouse monoclonal, horseradish peroxidase (HRP)-conjugated goat anti-rabbit, and HRP-conjugated goat anti-mouse antibodies were obtained from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). Alexa Fluor® 488 goat anti-mouse antibody was from Molecular Probes (Life Technologies Inc.). Vectashield mounting medium with 4,6-diamidino-2-phenylindole (DAPI) was obtained from Vector Laboratories Inc. (Burlingame, CA, USA). BCA Protein Assay Kit, SuperSignal West Pico Chemiluminescent Substrate, and Restore PLUS Western Blot Stripping Buffer were obtained from Pierce (Thermo Fisher Scientific, Inc., Rockland, IL, USA). Precision Plus ProteinTM Kaleidoscope Standards and nitrocellulose transfer membranes were from Bio-Rad Laboratories Inc. (Hercules, CA, USA). Phospho-GSK3α/β (pTyr279/216) rabbit polyclonal antibody, NuPAGE® LDS Sample Buffer, 4×, NuPAGE® Sample Reducing Agent, 10×, NuPAGE® Antioxidant, NuPAGE® MOPS SDS Running Buffer, 20×, NuPAGE® Transfer Buffer, 20×, and NuPAGE® Bis-Tris Gels, 1.5 mm, 10 well, were obtained from Novex (Life Technologies Inc.). Gel blot paper was from Whatman (GE Healthcare Life Sciences, Piscataway, NJ, USA). KODAK® BioMax® light autoradiography film was obtained from VWR International (Mississauga, ON, Canada). Mini-Complete Protease Inhibitor tablets, FuGENE 6, and X-tremeGENE 9 were from Roche Diagnostics (Laval, QC, Canada). Passive Lysis Buffer, 5×, and Bright-GloTM Luciferase Assay System were obtained from Promega (Madison, WI, USA). Bovine albumin (BSA), Fraction V was from Fisher Scientific (Thermo Fisher Scientific Inc.). Recombinant mouse Wnt3a was obtained from either R&D Systems, Inc. (Minneapolis, MN, USA) or Abcam, Inc. (Cambridge, MA, USA). Normal goat serum, collagenase type II, Phosphatase Inhibitor Cocktail II, ATP disodium salt, adenosine 5′-diphosphate (ADP) disodium salt, uridine 5′-triphosphate (UTP) trisodium salt hydrate, and 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate (BzATP) triethylammonium salt were obtained from Sigma-Aldrich (St. Louis, MO, USA). 3-[[5-(2,3-Dichlorophenyl)-1H-tetrazol-1-yl]methyl]pyridine hydrochloride (A 438079 HCl) and N-[1-[[(cyanoamino)(5-quinolinylamino) methylene]amino]-2,2-dimethylpropyl]-3,4-dimethoxybenzeneacetamide (A 740003) were from Tocris Bioscience (Ellisville, MO, USA). Phosphatase Inhibitor Cocktail IV, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY 294002), bisindolylmaleimide I (Bis I), and bisindolylmaleimide V (Bis V) were from Calbiochem (EMD Biosciences, San Diego, CA).
RIPA buffer (for protein isolation) consisted of 50 mM Tris pH 7.5, 150 mM NaCl, 1 % Triton X-100, 1 % sodium deoxycholate, 0.1 % sodium dodecyl sulphate (SDS), and 2 mM EDTA, pH 8.0 supplemented with two Mini-Complete Protease Inhibitor tablets (Roche Diagnostics), 200 μl Phosphatase Inhibitor Cocktail II (Sigma-Aldrich), and 400 μl Phosphatase Inhibitor Cocktail IV (Calbiochem) in 20 ml total volume.
Animals and cell culture
The P2X7 loss-of-function (knockout) mouse was previously generated by Solle and colleagues [14] and obtained from Pfizer. Though P2X7 is present in this mouse model, the protein is truncated at its C-terminus resulting in little-to-no receptor function [15]. Wild-type and knockout mouse colonies were maintained on a mixed genetic background (129/Ola × C57BL/6 × DBA/2). All procedures were approved by the Council on Animal Care at the University of Western Ontario and in accordance with the guidelines of the Canadian Council on Animal Care.
Calvarial osteoblasts were isolated from 5- to 7-day-old mice using sequential collagenase digestion, as previously described [12]. Freshly isolated osteoblasts were then plated at 1.0–1.5 × 104 cells/cm2 on Nunc 6-well plates (Thermo Fisher Scientific, Rochester, NY, USA) and maintained in α-MEM supplemented with 10 % FBS and 1 % antibiotic solution (culture medium) at 37 °C and 5 % CO2. When confluence was reached after 3–5 days, cells were trypsinized and plated for experiments. The MC3T3-E1 osteoblast-like cell line (subclone 4) was obtained from the American Type Culture Collection (Rockville, MD, USA). MC3T3-E1 cells were subcultured twice weekly and maintained in culture medium at 37 °C and 5 % CO2. These cells endogenously express P2X7 and Wnt signaling components and are responsive to Wnt3a [16–18].
Immunofluorescence localization of β-catenin
MC3T3-E1 cells were plated at a density of 1.5 × 104 cells/cm2 on 12-mm glass coverslips in Falcon 24-well plates in culture medium. After 2 days, cells were placed in serum-free medium and incubated overnight. On the day of the experiment, cells were incubated with test substances for the indicated times. Cells were then fixed with paraformaldehyde (4 %) in sucrose solution (2 %), permeabilized with 0.1 % Triton X-100 in DPBS for 10 min, and blocked for 1 h with 1 % normal goat serum in DPBS (blocking solution). To detect subcellular localization of β-catenin, cells were first incubated for 1 h with a mouse monoclonal antibody (1:200 in blocking solution) followed by 2-h incubation with an Alexa Fluor® 488 goat anti-mouse antibody (1:200 in blocking solution) at room temperature. Stained samples were then sealed using Vectashield mounting medium with DAPI and visualized by confocal microscopy (model LSM 510; Carl Zeiss Inc., Jena, Germany) using a Zeiss Plan-Apochromat 40× objective (1.2 NA) at a slice thickness of 2 μm with 488-nm Ar+ ion laser excitation and emission wavelengths filtered at 500–550 nm band pass. To quantify subcellular localization of β-catenin, the average fluorescence intensity of an area in the nucleus (FN) and the average fluorescence intensity of an area of equal size in the cytosol (FC) were determined. Values of the ratio FN/FC greater than or equal to 1.25 were taken to indicate nuclear localization of β-catenin protein. Twelve images were obtained per coverslip for each treatment, and the FN/FC ratios for all cells within a field were analyzed (∼20–30 cells per field).
Luciferase reporter assay for β-catenin transcriptional activity
The β-catenin luciferase reporter plasmid (pBARL) was obtained from Dr. Randall Moon (University of Washington, Seattle, WA, USA). This plasmid consists of 12 TCF response elements (or β-catenin binding sites) cloned upstream of Promega’s minP© minimal promoter [19]. MC3T3-E1 cells and calvarial osteoblasts were transfected in suspension with the β-catenin luciferase reporter vector using FuGENE 6 or X-tremeGENE 9 according to manufacturers’ instructions. Cells were subsequently plated at a density of 3.0 × 104 cells/cm2 on Falcon 48-well plates (or Nunc 48-well plates for calvarial cells) in culture medium. At 1 day post-transfection, cells were placed in serum-free medium and incubated overnight. On the day of the experiment, cells were treated with test substances and subsequently incubated for 24 h. Cell lysates were then prepared by incubation with 65 μl (or 100 μl for calvarial cells) of passive lysis buffer, 1× per well at room temperature for a minimum of 30 min with agitation. To assess luminescence, 15 μl of lysate was combined with 15 μl of Bright-Glo Luciferase Reagent in a 96-well white plate (Greiner Bio-One, Monroe, NC, USA). Reactions for each sample were performed in triplicate. Luminescence was measured using 2-s integration per well on a LMAX II384 microplate reader (Molecular Devices, Downingtown, PA, USA).
Western blot analysis of GSK3α/β phosphorylation
MC3T3-E1 cells were plated at a density of 1.5 × 104 cells/cm2 on Falcon 60-mm dishes in culture medium. After 1 day, cells were placed in serum-free medium and incubated overnight. On the day of the experiment, cells were incubated with test substances for the indicated times. Cellular protein was then collected by lysing cells on ice in RIPA lysis buffer supplemented with protease and phosphatase inhibitors. Cell lysates were homogenized by sonication at 20 % for 5 s on ice using a Sonic Dismembrator (Model 500; Fisher Scientific) followed by sedimentation at 13,000×g for 10 min at 4 °C. The resulting supernatant was used for Western blot analysis. Protein concentration was determined with the Pierce BCA protein assay kit. Equivalent amounts of protein (10–15 μg) were prepared with NuPAGE® Sample Reducing Agent (1×) and NuPAGE® LDS Sample Buffer (1×) and resolved on NuPAGE® Bis-Tris polyacrylamide gels in NuPAGE® MOPS SDS Running Buffer (1×). Proteins were then transferred to nitrocellulose membranes by electroblotting at 30 V for 90 min at 4 °C.
After transfer, membranes were washed for 5 min in Tris-buffered saline (TBS) with 0.05 % Tween 20 (TBST; washing solution) before being blocked for 1 h with 5 % BSA in TBST (blocking solution) at RT. To detect phospho-GSK3α/β (pSer21/9 or pTyr279/216) or total GSK3α/β, membranes were incubated overnight at 4 °C with a phospho-GSK3α/β (pSer21/9 or pTyr216/279) rabbit polyclonal antibody (1:1000 in blocking solution) or GSK3α/β mouse monoclonal antibody (1:5000 in blocking solution), respectively. The next day, membranes were subjected to three 5-min washes in TBST, incubated with the appropriate secondary antibody at a dilution of 1:20,000 in blocking solution at RT and washed three more times for 5 min each in TBST. Antibody/protein complexes were visualized by 1, 3, or 5-min incubations of the membrane in SuperSignal West Pico Chemiluminescent Substrate followed by exposure using KODAK® BioMax® light autoradiography film and a KODAK® M35A X-OMAT processor. Band intensities on immunoblots were quantified by densitometry using Quantity One v4.5.2 (Bio-Rad Laboratories Inc.) and normalized to total GSK3α/β signal (loading control). For repeated probing, membranes were stripped with Restore PLUS Western Blot Stripping Buffer for 5–20 min at RT (or 37 °C) followed by one wash with TBST.
Statistical analyses
Data are shown as means ± SEM. Differences between two groups were assessed using t tests. Differences among three or more groups were evaluated by one-way analysis of variance (ANOVA) followed by a Tukey multiple comparisons test or two-way ANOVA followed by a Bonferroni multiple comparisons test. Differences were accepted as statistically significant at p < 0.05.
Results
Effect of the P2X7 agonist BzATP on Wnt-induced β-catenin nuclear localization
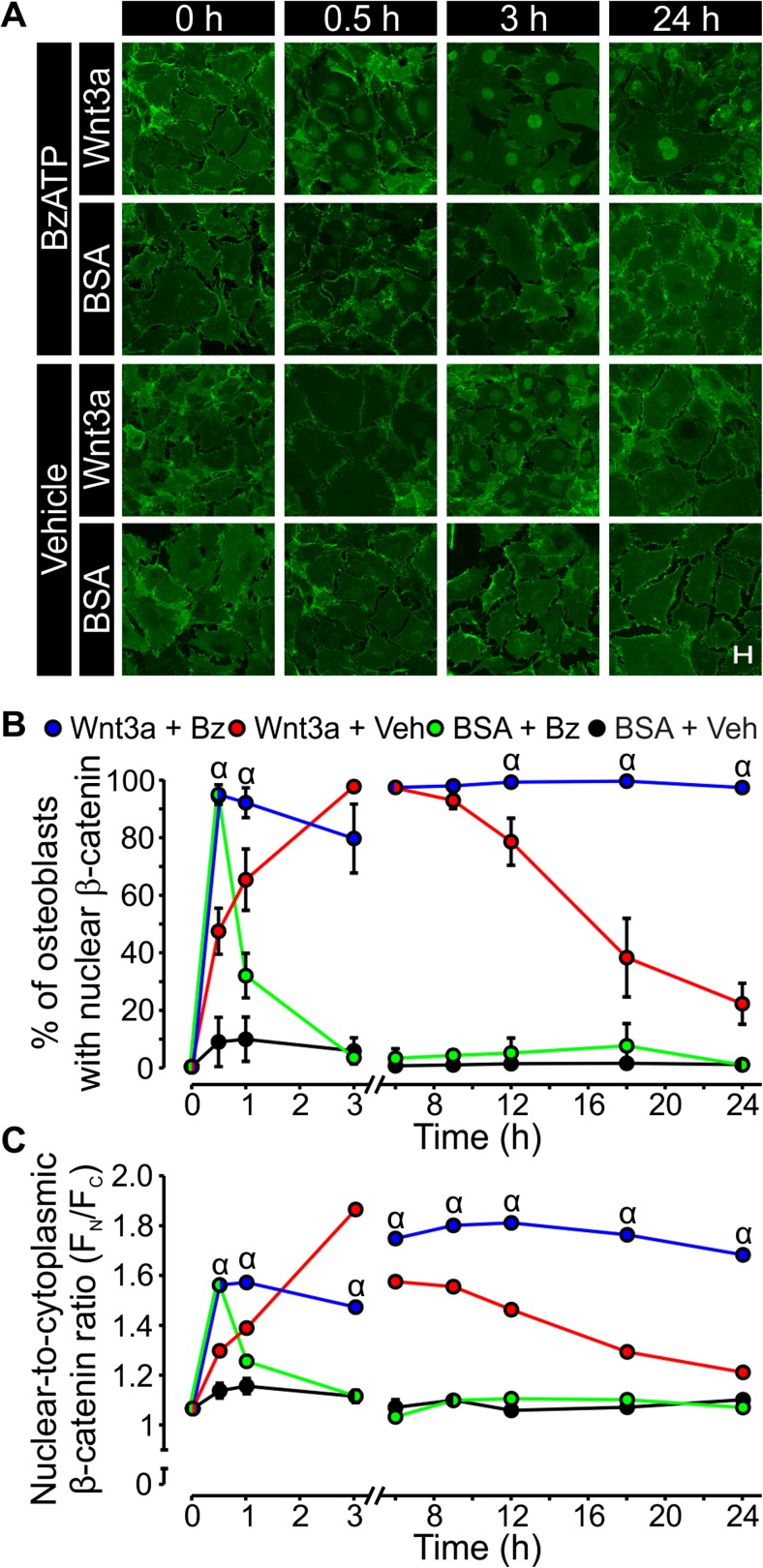
In resting cells, β-catenin forms a complex with GSK3β and other proteins, resulting in its phosphorylation and subsequent degradation [20]. Upon binding of canonical Wnt ligands to the Frizzled receptor (Fzd)-lipoprotein receptor-related protein (LRP) 5/6 co-receptor complex, GSK3β activity is suppressed and β-catenin translocates to the nucleus to activate target gene expression [20]. To assess whether P2X7 receptor signaling could influence Wnt-induced nuclear localization of β-catenin, MC3T3-E1 cells were treated with Wnt3a (20 ng/ml) or its vehicle, and the P2X7 receptor agonist BzATP (300 μM) or its vehicle. Samples were fixed at various time points, processed for β-catenin immunofluorescence, and visualized by confocal microscopy (Fig. 1a). To quantify β-catenin subcellular localization, average pixel intensity for areas of equal size within the nucleus (FN) and the cytosol (FC) were determined. Values of FN/FC exceeding 1.25 were taken to indicate nuclear localization (Fig. 1b). In the absence of agonists, β-catenin localized to cell junctions and the cytoplasm, with little or no fluorescence in the nucleus (Fig. 1a, b). Wnt3a alone elicited a gradual increase in the percentage of osteoblasts with β-catenin nuclear localization, which peaked at 3 h and slowly returned to baseline by 24 h (Fig. 1a, b). In contrast, BzATP alone caused transient nuclear localization in nearly all cells only at 0.5 h after treatment (Fig. 1a, b). Notably, when cells were treated with Wnt3a and BzATP in combination, β-catenin nuclear localization was more rapid and sustained compared to Wnt3a alone (Fig. 1a, b).
Fig. 1.
The P2X7 agonist BzATP potentiates β-catenin nuclear localization elicited by Wnt3a. a MC3T3-E1 osteoblast-like cells were seeded at a density of 1.5 × 104 cells/cm2 on glass coverslips and cultured for 2 days. Cells were then placed in serum-free media and incubated overnight. The next day, cells were treated under serum-free conditions with recombinant mouse Wnt3a (20 ng/ml) or its vehicle (0.2 % BSA; BSA) in the presence of either BzATP (300 μM) or its vehicle (Vehicle or Veh) and fixed at the indicated times. To observe changes in subcellular localization of β-catenin, immunofluorescence was performed using a β-catenin monoclonal antibody (green) and visualized by confocal microscopy. Data are representative images from four independent preparations each performed in duplicate. Scale bar is 20 μm. b β-catenin nuclear localization was assessed by measuring the average pixel intensity of an area in the nucleus (F N) and an area of equal size in the cytosol (F C). The proportion of all cells within 24 fields (12 per coverslip) exhibiting β-catenin nuclear localization was analyzed for each condition. Values of the ratio F N/F C greater than or equal to 1.25 were taken as indicating nuclear localization. The number of cells exhibiting nuclear localization was expressed as a percentage of the total number of cells for each treatment group. c The ratio of F N/F C (an indication of the intensity of β-catenin nuclear localization) for all cells analyzed was plotted for each treatment at times indicated. For both b and c, α indicates a significant effect of BzATP on Wnt3a-induced β-catenin nuclear localization compared to Wnt3a alone at the same time point (p < 0.05). Data are means ± SEM (n = 4 independent preparations each performed in duplicate)
To assess the degree (or intensity) of β-catenin nuclear localization at each time point, the average value for FN/FC in a field was determined and plotted. In keeping with observations made when assessing the percentage of responding cells, the intensity of β-catenin nuclear localization was significantly greater at all time points other than 3 h in cultures treated with Wnt3a and BzATP compared to Wnt3a alone (Fig. 1a, c). Thus, stimulation of P2X7 alone promotes brief, transient β-catenin nuclear localization, whereas activated P2X7 receptors prolong and increase nuclear localization of β-catenin elicited by canonical Wnt signaling.
Effect of BzATP on Wnt-induced β-catenin transcriptional activity
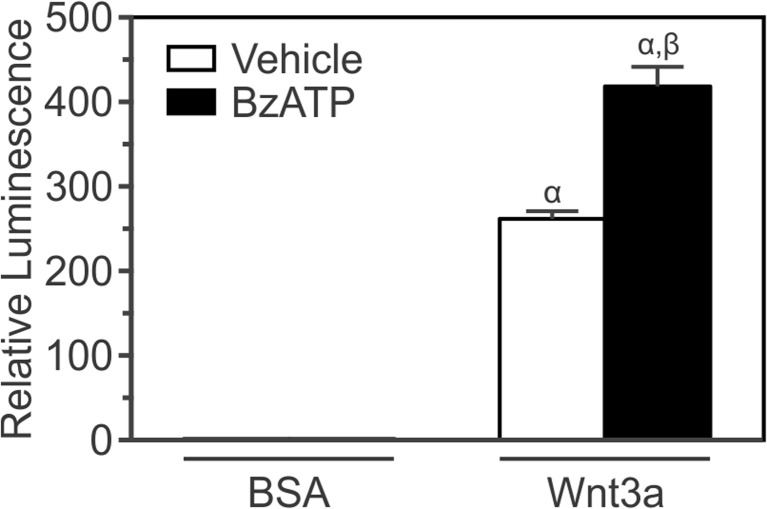
To determine if BzATP affects β-catenin transcriptional activity elicited by canonical Wnt signaling, MC3T3-E1 cells were transfected with a β-catenin luciferase reporter and treated with Wnt3a (20 ng/ml) or its vehicle, and BzATP (300 μM) or its vehicle. After 24 h, cell lysates were collected and luminescence was assessed as a measure of β-catenin transcriptional activity (Fig. 2). As expected, Wnt3a alone induced an increase in β-catenin transcriptional activity (Fig. 2). On the other hand, though BzATP alone did induce nuclear localization of β-catenin at 0.5 h, it did not increase β-catenin transcriptional activity in these cells. In keeping with the analysis of β-catenin subcellular localization, the combination of Wnt3a and BzATP significantly enhanced transcriptional activity compared to that induced by Wnt3a alone (Fig. 2).
Fig. 2.
BzATP potentiates β-catenin transcriptional activity elicited by Wnt3a. MC3T3-E1 osteoblast-like cells were transfected in suspension with a β-catenin luciferase reporter plasmid, seeded at a density of 3.0 × 104 cells/cm2 in a 48-well plate, and cultured for 1 day. Cells were then placed in serum-free media and incubated overnight. The next day, cells were treated under serum-free conditions with recombinant mouse Wnt3a (20 ng/ml) or its vehicle (0.2 % BSA; BSA) in the presence of either BzATP (300 μM) or its vehicle (Vehicle). After 24 h, cell lysates were collected and luminescence was assessed as a measure of β-catenin transcriptional activity. The luminescence for each treatment was expressed relative to BSA + Vehicle. No effect on transcriptional activity was seen for BzATP alone. α indicates a significant effect of treatment on β-catenin transcriptional activity compared to BSA + Vehicle (p < 0.05). β indicates significant effect of BzATP on Wnt3a-induced β-catenin transcriptional activity (p < 0.05). Data are means ± SEM (n = 17-21 samples from seven independent preparations)
P2X7 is essential for mediating the effects of nucleotides on Wnt-induced β-catenin transcriptional activity
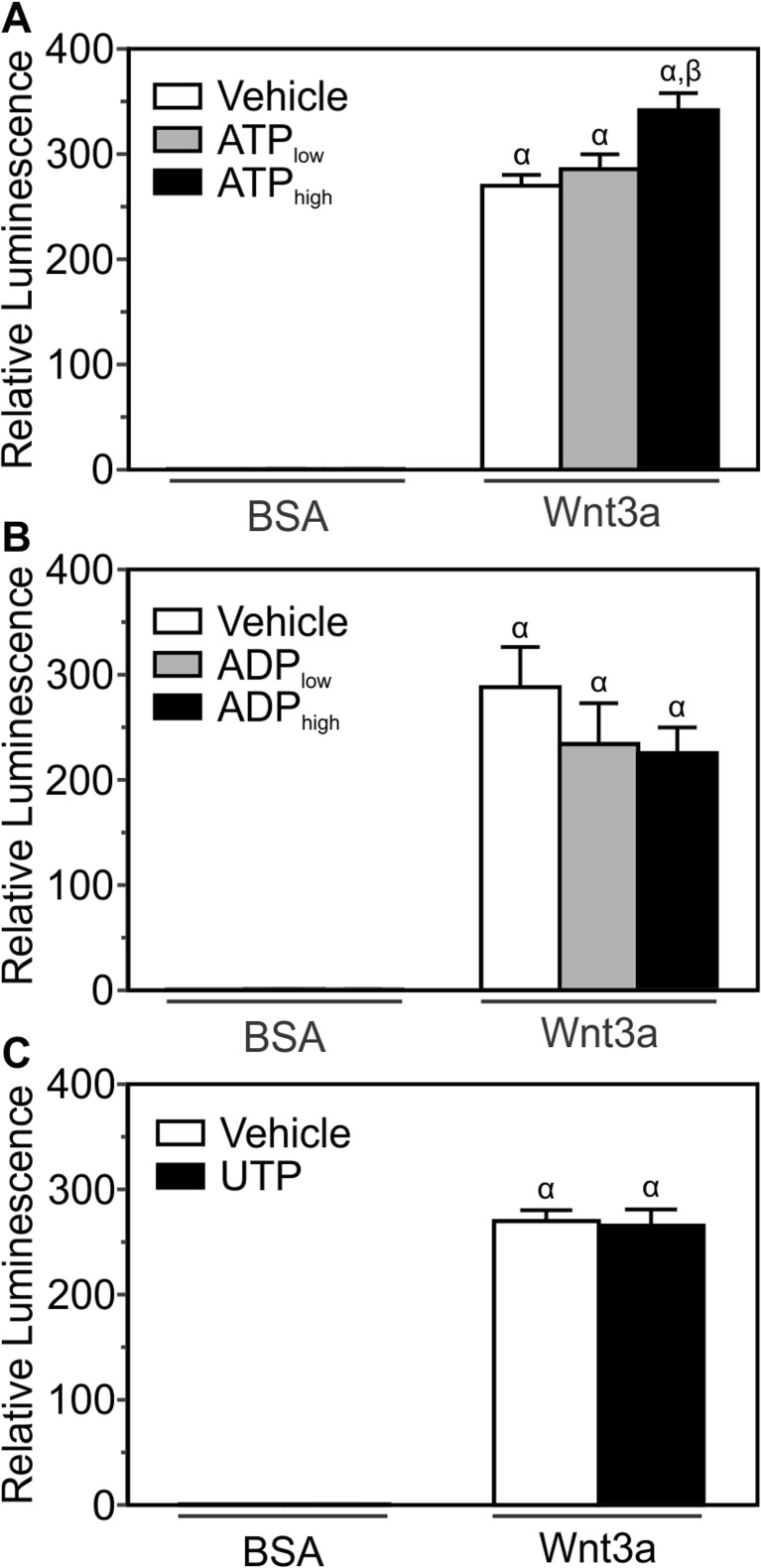
Since BzATP can activate other P2 nucleotide receptors in addition to P2X7 [21], the effects of additional P2 agonists on Wnt-induced β-catenin transcriptional activity were assessed. MC3T3-E1 cells transfected with the β-catenin luciferase reporter were treated with Wnt3a (20 ng/ml) or its vehicle and varying concentrations of ATP, ADP, UTP, or their vehicle (Fig. 3a–c). Whether in the absence or presence of Wnt3a, neither ADP nor UTP significantly altered β-catenin transcriptional activity (Fig. 3b, c). A low concentration of ATP (10 μM) also failed to elicit a response (Fig. 3a). In contrast, a high ATP concentration (1 mM) significantly potentiated β-catenin transcriptional activity compared to Wnt3a alone (Fig. 3a), consistent with involvement of the low-affinity P2X7 receptor.
Fig. 3.
P2X7 receptor agonists potentiate β-catenin transcriptional activity elicited by Wnt3a. MC3T3-E1 osteoblast-like cells transfected with the β-catenin luciferase reporter plasmid were treated under serum-free conditions. a Cells were treated with recombinant mouse Wnt3a (20 ng/ml) or its vehicle (0.2 % BSA; BSA) in the presence of either 10 μM ATP (ATP low), 1 mM ATP (ATP high), or their vehicle (Vehicle). After 24 h, cell lysates were collected and luminescence was assessed. Luminescence for each treatment was expressed relative to BSA + Vehicle. b Cells were treated with recombinant canonical Wnt3a (20 ng/ml) or its vehicle (0.2 % BSA; BSA) in the presence of either 10 μM ADP (ADP low), 1 mM ADP (ADP high) or their vehicle (Vehicle). After 24 h, luminescence was assessed as described in a. c Cells were treated with recombinant canonical Wnt3a (20 ng/ml) or its vehicle (0.2 % BSA; BSA) in the presence of either 10 μM UTP (UTP) or its vehicle (Vehicle). After 24 h, luminescence was assessed as described in a. For a, b, and c, there was no effect of nucleotide treatment alone on transcriptional activity. α indicates a significant effect of treatment on β-catenin transcriptional activity compared to BSA + Vehicle (p < 0.05). β indicates significant effect of nucleotide on Wnt3a-induced β-catenin transcriptional activity (p < 0.05). Data are means ± SEM (n = 9-21 samples from three-to-seven independent experiments)
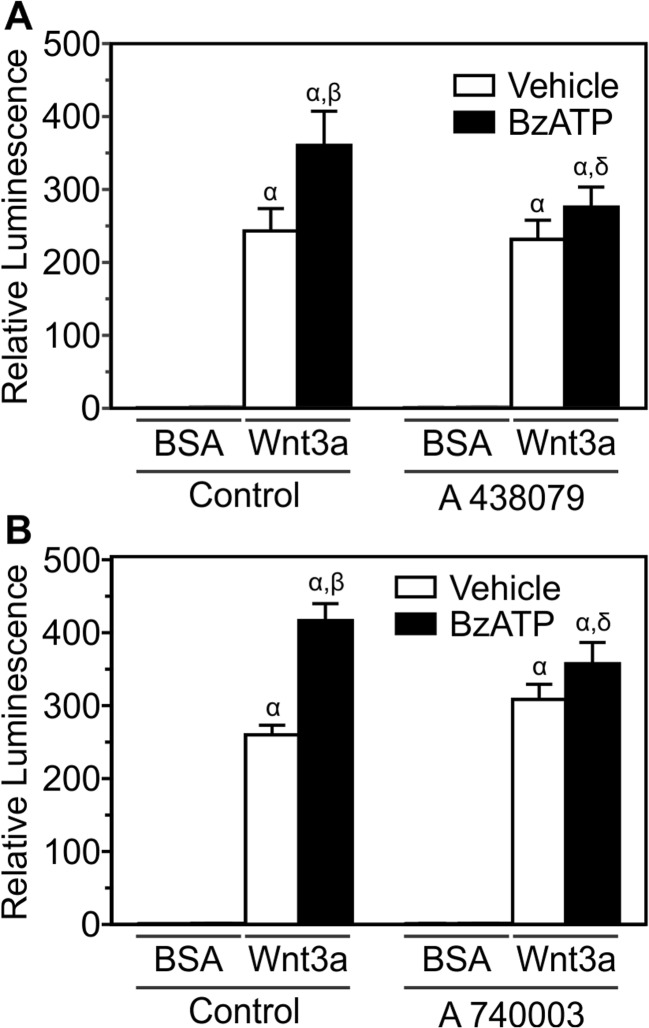
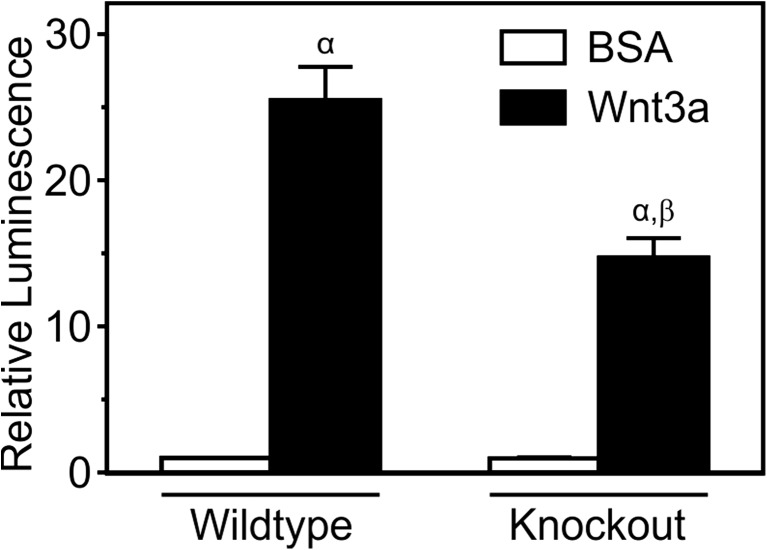
To further confirm the role of P2X7, pharmacological and genetic approaches were utilized. MC3T3-E1 cells transfected with the β-catenin luciferase reporter were first treated with Wnt3a (20 ng/ml) or its vehicle and BzATP (300 μM) or its vehicle, in the presence or absence of the P2X7 antagonists A 438079 (10 μM) or A 740003 (10 μM). A 438079 (Fig. 4a) and A 740003 (Fig. 4b) both inhibited BzATP-induced potentiation of Wnt3a signaling. Next, changes in Wnt signaling were assessed in osteoblasts isolated from wild-type mice and P2rx7 knockout mice. Both wild-type and knockout calvarial osteoblasts transfected with the β-catenin reporter displayed significant increases in β-catenin transcriptional activity in response to Wnt3a (20 ng/ml) (Fig. 5). However, the response to Wnt3a was significantly less in cells from knockout mice than in cells from wild-type controls (Fig. 5). Taken together, these findings demonstrate that activated P2X7 receptors potentiate Wnt/β-catenin signaling in cells of the osteoblast lineage.
Fig. 4.
P2X7 receptor antagonists block the effects of BzATP on Wnt3a-induced β-catenin transcriptional activity. MC3T3-E1 osteoblast-like cells transfected with the β-catenin luciferase reporter plasmid were treated under serum-free conditions. Cells were incubated for 5–10 min in the absence or presence of the specific P2X7 antagonist A 438079 (10 μM, panel a) or a second P2X7 antagonist A 740003 (10 μM, panel b). Next, cells were treated with recombinant mouse Wnt3a (20 ng/ml) or its vehicle (0.2 % BSA; BSA) in the presence of either BzATP (300 μM) or its vehicle (Vehicle) in the continued presence of antagonist. After 24 h, cell lysates were collected and luminescence was assessed. Luminescence for each treatment was expressed relative to Control + BSA + Vehicle. For both a and b, α indicates a significant effect of treatment on β-catenin transcriptional activity compared to Control + BSA + Vehicle (p < 0.05). β indicates significant effect of BzATP on Wnt3a-induced β-catenin transcriptional activity (p < 0.05). δ indicates significant effect of the antagonist (p < 0.05). Data are means ± SEM (n = 11-21 samples from five-to-seven independent experiments)
Fig. 5.
Osteoblasts from P2rx7 knockout mice exhibit smaller response to Wnt3a. Calvarial osteoblasts from wild-type and P2rx7 knockout mice were transfected in suspension with a β-catenin luciferase reporter plasmid, seeded at a density of 3.0 × 104 cells/cm2 in a 48-well plate, and cultured for 1 day. Cells were then placed in serum-free media and incubated overnight. The next day, cells were treated under serum-free conditions with recombinant mouse Wnt3a (20 ng/ml) or its vehicle (0.2 % BSA; BSA). After 24 h, cell lysates were collected and luminescence was assessed as a measure of β-catenin transcriptional activity. The luminescence for each treatment was expressed relative to BSA treatment in wild-type cells. α indicates a significant effect of Wnt3a on β-catenin transcriptional activity compared to BSA treatment (p < 0.05). β indicates significant effect of genotype (p < 0.05). Data are means ± SEM (n = 18-21 samples from seven independent experiments)
Activation of P2X7 increases inhibitory phosphorylation of GSK3α/β
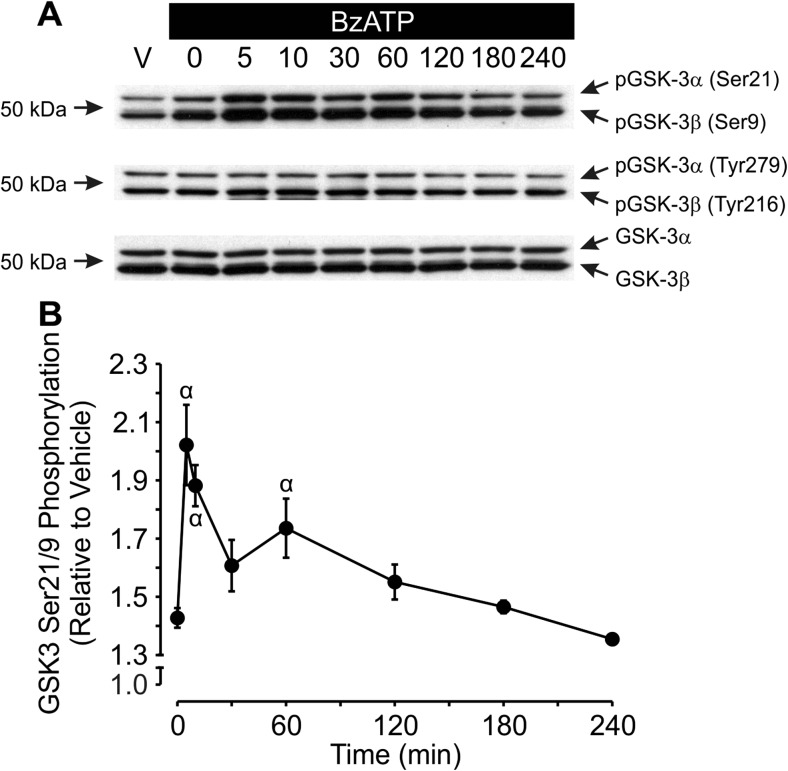
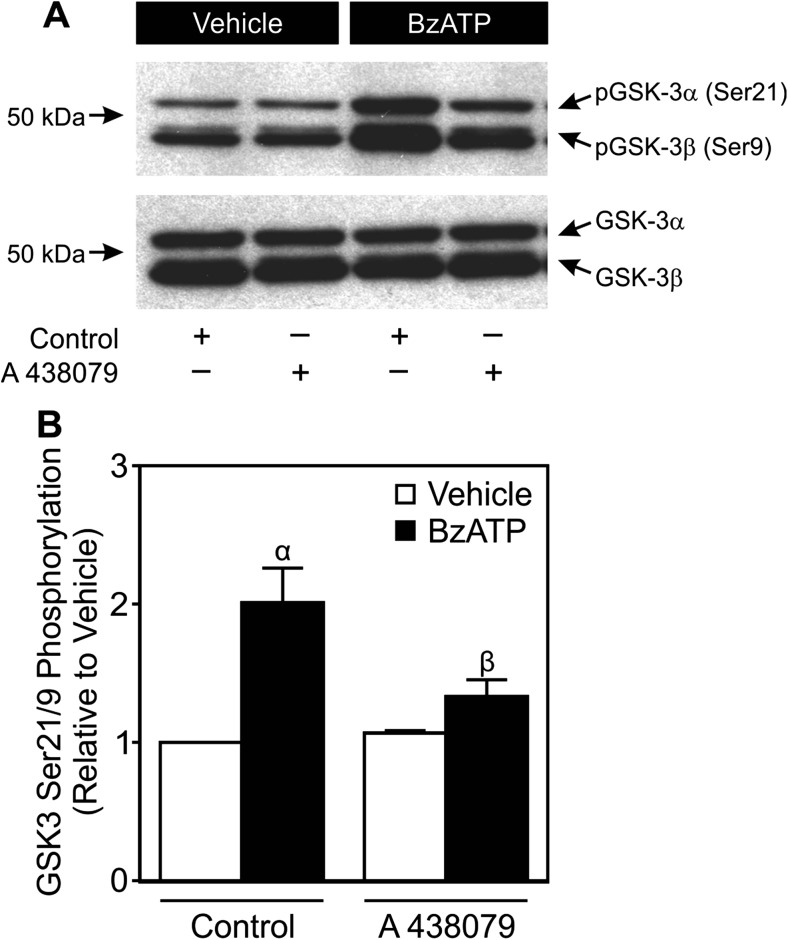
During canonical Wnt signaling, stabilization of β-catenin is achieved in part through inhibition of GSK3β [20]. To determine whether P2X7 couples to inhibition of GSK3β in osteoblastic cells, the effects of BzATP on GSK3α/β phosphorylation were examined (Fig. 6a, b). Treatment of MC3T3-E1 cells with BzATP (300 μM) led to a significant increase in inhibitory phosphorylation of both GSK3α and GSK3β at serines 21 and 9, respectively (Fig. 6a, b). On the other hand, BzATP had no discernable effect on the phosphorylation of tyrosine residues 279 (GSK3α) or 216 (GSK3β), both of which are associated with activation of GSK3 (Fig. 6a). Consistent with involvement of P2X7, A 438079 (10 μM) suppressed the inhibitory phosphorylation of GSK3α/β induced by BzATP (Fig. 7a, b). Thus, P2X7 may potentiate canonical Wnt signaling through inhibition of GSK3β, though further experiments will be required to investigate this possibility.
Fig. 6.
BzATP induces inhibitory phosphorylation of GSK3α/β. a MC3T3-E1 osteoblast-like cells were seeded at a density of 1.5 × 104 cells/cm2 in a 6-well plate and cultured for 1 day. Cells were then placed in serum-free media and incubated overnight. The next day, cells were treated under serum-free conditions with vehicle (V) or BzATP (300 μM; BzATP) for the times indicated, and total protein was harvested. Samples were subjected to immunoblot analyses using specific pSer21/9 and pTyr279/216 GSK3α/β antibodies. As a loading control, blots were also probed for total GSK3α/β. Bands were visualized by the ECL method. Images are representative blots from three to five independent preparations. b pSer21/9, pTyr279/216, and total GSK3α/β bands were evaluated by densitometry. The ratio of pGSK3α/β to total GSK3α/β was determined for serine and tyrosine residues, and results were normalized to vehicle. Treatment with BzATP had no significant effect on the phosphorylation status of tyrosine 279/216 (quantification not shown). α indicates a significant difference from 0 min (p < 0.05). Data are means ± SEM (n = 3–4 independent preparations)
Fig. 7.
A P2X7 antagonist blocks BzATP-induced inhibitory phosphorylation of GSK3α/β. a MC3T3-E1 osteoblast-like cells were incubated for 20 min in the presence of the P2X7 antagonist A 438079 (10 μM) or its vehicle (Control). Next, cells were treated with vehicle or BzATP (300 μM; BzATP) for 5 min in the continued presence of antagonist. Total protein was subsequently harvested, and samples were subjected to immunoblot analyses using a specific pSer21/9 GSK3α/β antibody. As a loading control, blots were also probed for total GSK3α/β. Bands were visualized by the ECL method. Images are representative blots from three independent preparations. b pSer21/9 and total GSK3α/β bands were evaluated by densitometry. The ratio of pGSK3α/β to total GSK3α/β was determined, and results were normalized to Control + Vehicle. α indicates a significant difference from Control + Vehicle (p < 0.05). β indicates a significant effect of the antagonist (p < 0.05). Data are means ± SEM (n = 3 independent preparations)
Effects of inhibition of phosphatidylinositol 3-kinase and protein kinase C on P2X7-induced inhibitory phosphorylation of GSK3α/β
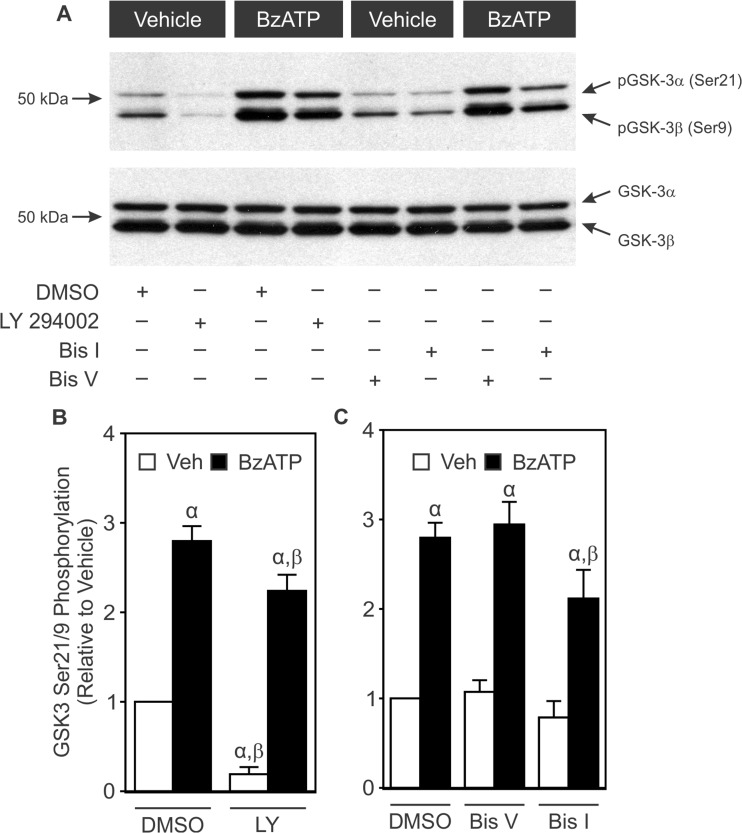
In cerebellar granule neurons, activation of P2X7 has been shown to inhibit GSK3β through mechanisms dependent on protein kinase C (PKC) [13, 22]. Previous work from our lab has demonstrated that stimulation of P2X7 in osteoblasts activates Ca2+ signaling and the phosphatidylinositol 3-kinase (PI3K) pathway [17, 23]. To investigate the pathways responsible for mediating P2X7-induced inhibitory phosphorylation of GSK3α/β, MC3T3-E1 cells were treated with BzATP (300 μM) in the presence or absence of the PI3K inhibitor LY 294002 (30 μM), the PKC inhibitor Bis I (0.5 μM), or its inactive analog Bis V (0.5 μM) (Fig. 8). LY 294002 significantly inhibited basal levels of GSK3α/β phosphorylation, but BzATP still induced inhibitory phosphorylation (Fig. 8a, b). On the other hand, Bis I partially blocked the inhibitory phosphorylation of GSK3α/β induced by BzATP. In contrast, Bis V had no significant effect, arguing against the possibility of a nonspecific action of Bis I (Fig. 8a, c). Taken together, these data implicate PKC, but not PI3K, in coupling P2X7 activation to GSK3α/β inhibition.
Fig. 8.
Effects of PI3K and PKC inhibitors on BzATP-induced inhibitory phosphorylation of GSK3α/β. Changes in inhibitory phosphorylation of GSK3α/β were monitored in MC3T3-E1 cells as described in the legend to Figure 6. Cells were incubated for 20 min in the presence or absence of the PI3K inhibitor LY 294002 (30 μM; LY), its vehicle (DMSO), the PKC inhibitor bisindolylmaleimide I (0.5 μM; Bis I), its inactive analogue bisindolylmaleimide V (0.5 μM; Bis V), or their vehicle (DMSO). Next, cells were treated with vehicle (Veh) or BzATP (300 μM; BzATP) for 5 min, and total protein was subsequently harvested. Samples were subjected to Western blot analyses using a specific pSer21/9 GSK3α/β antibody. As a loading control, blots were also probed for total GSK3α/β. Bands were visualized by the ECL method. a Immunoblot representative of three independent preparations. b, c pSer21/9 and total GSK3α/β bands were evaluated by densitometry. The ratio of pGSK3α/β to total GSK3α/β was determined, and results were normalized to Veh + DMSO. α indicates a significant difference from Veh + DMSO (p < 0.05). β indicates a significant effect of the inhibitor (p < 0.05). Data are means ± SEM (n = 3 independent preparations)
Discussion
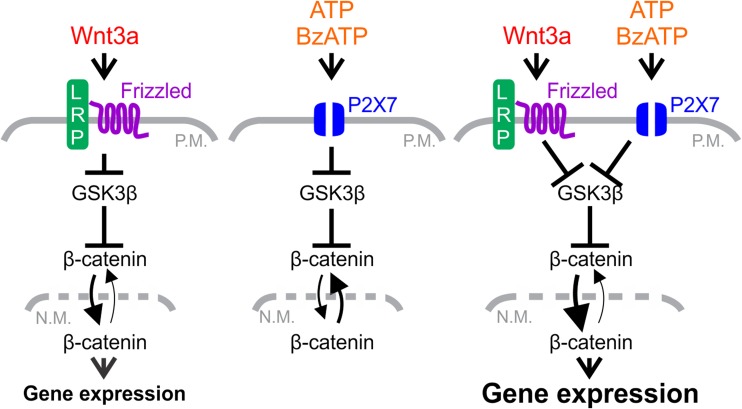
In the present study, we investigated the effects of P2X7 receptor activation on Wnt/β-catenin signaling in osteoblasts. We show that activation of P2X7 by exogenous nucleotides promotes transient β-catenin nuclear localization. Notably, stimulation of P2X7 prolongs β-catenin nuclear localization and potentiates transcriptional activation elicited by canonical Wnt signaling (Fig. 9). This potentiation is associated with inhibitory phosphorylation of GSK3β induced by P2X7 activation and mediated in part by PKC. Thus, P2X7 receptors may potentiate Wnt/β-catenin signaling through GSK3β. Taken together, P2X7-induced potentiation of Wnt/β-catenin signaling is a novel mechanism through which osteoblast activity may be modulated in response to mechanically induced ATP release in bone.
Fig. 9.
Possible mechanism for cross talk between P2X7 nucleotide receptor and canonical Wnt signaling pathways in cells of the osteoblast lineage. The P2X7 and Wnt/β-catenin pathways regulate osteoblast differentiation and are critical for anabolic responses of the skeleton to mechanical load. Activation of canonical Wnt signaling by Wnt3a alone elicits a sustained increase in β-catenin nuclear localization and transcriptional activity (left panel). Activation of P2X7 receptor signaling causes inhibitory phosphorylation of GSK3β and promotes transient nuclear localization of β-catenin but fails to elicit changes in β-catenin transcriptional activity (middle panel). Notably, stimulation of P2X7 receptors in the presence of Wnt3a leads to a dramatic potentiation of β-catenin nuclear localization and transcriptional activity compared to Wnt3a alone (right panel). Mechanistically, this potentiation may be mediated in part through mutual inhibition of GSKβ. Taken together, this study provides the first evidence of cross talk between the P2X7 and Wnt/β-catenin pathways, a novel mechanism through which osteoblast activity may be modulated in response to mechanically-induced ATP release in bone
P2X7 potentiates the Wnt/β-catenin pathway
Various P2 receptors have been shown to modulate signaling mediated by growth factors and hormones in cells of the osteoblast lineage. P2Y2 potentiates c-fos expression elicited by parathyroid hormone (PTH)/cAMP signaling [24]. ATP also enhances platelet-derived growth factor (PDGF) and insulin-like growth factor (IGF)-1-induced proliferation of human MG-63 osteoblast-like cells through an unidentified P2 receptor [25]. In the present study, we provide the first evidence that activation of P2X7 potentiates Wnt/β-catenin signaling. Stimulation of the ADP-sensitive P2Y13 receptor has also been shown by others to promote β-catenin nuclear localization in cerebellar granule neurons [26]. Given that transcripts for P2Y13 are present in MC3T3-E1 cells and rodent calvarial osteoblasts [27], it is possible that P2Y13 may also modulate canonical Wnt signaling in our system. However, we found that low concentrations of ATP, UTP, and ADP did not potentiate β-catenin transcriptional activity in the presence or absence of Wnt3a. In contrast, both BzATP and high concentrations of ATP elicited potentiation, consistent with P2X7 alone modulating canonical Wnt signaling in osteoblasts. This was confirmed using P2X7 receptor antagonists. Interestingly, Wnt-induced β-catenin transcriptional activity was greater in wild-type than in knockout cells, suggesting that endogenous P2X7 activity potentiates Wnt/β-catenin signaling in wild-type cells.
Many bone anabolic pathways modulate Wnt/β-catenin signaling to regulate osteoblast proliferation, differentiation, and function. In the present study, we demonstrated that P2X7 receptors cause rapid increases in β-catenin nuclear localization just minutes after treatment. The speed with which β-catenin is activated suggests that P2X7 regulates the stability of β-catenin as opposed to its gene expression at early time points. In this regard, we showed that P2X7 activation in osteoblast-like cells rapidly inhibits GSK3β in a PKC-dependent manner in agreement with previous findings in cerebellar granule neurons [13, 22]. Interestingly, the PTH-PTH 1 receptor complex has also been shown to facilitate canonical Wnt signaling through inhibition of GSK3β in osteoblast-like SaOS-2 cells [28].
In the present study, we assessed changes in β-catenin subcellular localization by immunolabeling and comparing the fluorescence signal in the nucleus relative to that in the cytoplasm. In addition to a cytoplasmic pool, β-catenin is sequestered at cell-cell junctions by cadherins, an interaction that stabilizes these adhesions while concomitantly promoting β-catenin inactivation [29]. Therefore, it is possible that rapid β-catenin activation could be elicited by disruption of cellular adhesions. In this regard, activation of the P2X7 receptor induces cell retraction and membrane blebbing in cells of the osteoblast lineage [17, 30, 31]. In the present study, some cellular retraction was seen in BzATP-treated cells, which might promote the liberation of β-catenin. However, membrane blebbing was not observed due to the presence of divalent cations in the culture medium. Our previous live-cell imaging studies demonstrated that extensive membrane blebbing of osteoblasts is only observed when cells are incubated in medium containing a low concentration of divalent cations [17, 30]. Importantly, as images were collected in the present study with an optical slice of 2 μm using confocal microscopy, changes in cell shape would not influence measurements of fluorescence intensity.
In addition to its transient effects, stimulation of P2X7 prolonged Wnt3a-induced β-catenin nuclear localization compared to Wnt3a alone for up to 24 h post-treatment. As nucleotides are rapidly degraded once released into the extracellular milieu [32, 33], it is likely that the prolonged potentiation of canonical Wnt signaling is due at least in part to changes in expression of secondary factors or pathway components. One possibility is that activation of P2X7 receptors leads to alterations in the expression of Wnt pathway components. In this regard, PTH signaling has been shown to decrease expression of Wnt antagonists such as SOST and DKK1 [34, 35]. Additionally, BMP-2 has been shown to stimulate β-catenin transcriptional activity leading to increased expression of Wnt ligands and their receptors in differentiating cultures of calvarial osteoblasts [36]. Previously, we demonstrated that prolonged Ca2+/NFATc1 signaling downstream of P2X7 leads to expression of cyclooxygenase-2 (COX-2) [23]. Interestingly, NFATc1 has been shown to function downstream of strontium ranelate in vitro to elicit expression of canonical Wnt3a [37]. A second possibility is that Wnt signaling might suppress expression of cell surface ectonucleotidases to prolong ATP signaling. Taken together, whereas transient activation of β-catenin downstream of P2X7 is likely due to modulation of β-catenin stability or localization, prolonged potentiation may be mediated by longer term changes in the expression of canonical Wnt pathway components or mediators of extracellular nucleotide metabolism.
Prolonged nuclear occupancy of β-catenin is required for transcriptional activity
In the present study, we found that, although BzATP alone induces transient nuclear localization of β-catenin, it fails to increase β-catenin transcriptional activity. In contrast, prolonged β-catenin nuclear localization elicited by Wnt3a alone, or more so by a combination of Wnt3a and BzATP, was associated with enhanced transcriptional activity. These observations are consistent with our previous findings, in which we demonstrated that prolonged but not transient Ca2+/NFATc1 signaling in osteoblasts resulted in increased expression of NFAT target genes [23]. Taken together, these findings highlight the positive association between duration of nuclear occupancy by a transcription factor (such as NFAT or β-catenin) and expression of its target genes.
P2X7 promotes inhibitory phosphorylation of GSK3β
One mechanism through which anabolic factors such as PTH modulate β-catenin activity is by inhibiting GSK3β through cAMP/PKA and PI3K/AKT signaling [38]. In the present study, we found that activation of P2X7 receptors leads to inhibitory phosphorylation of GSK3β, mediated in part through PKC. These findings are consistent with observations in cultures of cerebellar granule neurons, in which activation of P2X7 inhibits GSK3β in a manner dependent upon PKC signaling [13]. Moreover, in osteoclasts, P2X7 couples to activation of various Ca2+-sensitive PKC isoforms including PKCα and PKCβ [39]. We have shown previously that activation of P2X7 in osteoblast-like cells couples to PI3K/AKT signaling [17], a pathway associated with GSK3β inhibition [38]. However, we found that PI3K plays a role in regulating basal but not P2X7-stimulated phosphorylation of GSK3β.
Physiological roles of P2X7-induced potentiation of canonical Wnt signaling in osteoblasts
Homozygous deletion of either P2X7 or canonical Wnt signaling components in mice reduces anabolic responses of the skeleton to mechanical load [11, 40, 41]. The cross talk identified between these two pathways in the present study may help to explain how each increases osteoblast differentiation and bone formation during mechanotransduction. Experimental evidence suggests that ATP acts upstream of LRP5-dependent signaling as an initial transducer of mechanical stimuli in cells of the osteoblast lineage [40]. Thus, ATP signaling through P2X7 may be required to sensitize quiescent cells to mechanical stimuli to permit activation of the Wnt/β-catenin pathway. In this regard, microstrain increases expression of Wnt/β-catenin target genes in MC3T3-E1 osteoblast-like cells, an effect that can be potentiated by pretreatment with Wnt ligand [42]. Given that ATP is released from these cultures in response to mechanical stimuli [11, 40], this potentiation may in fact be mediated by signaling through P2X7.
Acknowledgments
We thank Tom Chrones and Drs. Stephen Sims and Kim Beaucage for valuable assistance and advice. This work was funded by the Canadian Institutes of Health Research (CIHR) [grant number 102542]. M.W.G. was supported by a CIHR Frederick Banting and Charles Best Canada Graduate Scholarship Doctoral Award. P.J.B. was supported by studentships from the Canadian Arthritis Network and the Joint Motion Program, a CIHR Strategic Training Program in Musculoskeletal Health Research and Leadership.
References
- 1.Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng. 2006;8:455–498. doi: 10.1146/annurev.bioeng.8.061505.095721. [DOI] [PubMed] [Google Scholar]
- 2.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19:179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 4.Lin C, Jiang X, Dai Z, et al. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009;24:1651–1661. doi: 10.1359/jbmr.090411. [DOI] [PubMed] [Google Scholar]
- 5.Tu X, Rhee Y, Condon KW, et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone. 2012;50:209–217. doi: 10.1016/j.bone.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sen B, Styner M, Xie Z, et al. Mechanical loading regulates NFATc1 and beta-catenin signaling through a GSK3beta control node. J Biol Chem. 2009;284:34607–34617. doi: 10.1074/jbc.M109.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Case N, Sen B, Thomas JA, et al. Steady and oscillatory fluid flows produce a similar osteogenic phenotype. Calcif Tissue Int. 2011;88:189–197. doi: 10.1007/s00223-010-9448-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Case N, Ma M, Sen B, et al. Beta-catenin levels influence rapid mechanical responses in osteoblasts. J Biol Chem. 2008;283:29196–29205. doi: 10.1074/jbc.M801907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ke HZ, Qi H, Weidema AF, et al. Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol Endocrinol. 2003;17:1356–1367. doi: 10.1210/me.2003-0021. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Liu D, Ke HZ, et al. The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J Biol Chem. 2005;280:42952–42959. doi: 10.1074/jbc.M506415200. [DOI] [PubMed] [Google Scholar]
- 12.Panupinthu N, Rogers JT, Zhao L, et al. P2X7 receptors on osteoblasts couple to production of lysophosphatidic acid: a signaling axis promoting osteogenesis. J Cell Biol. 2008;181:859–871. doi: 10.1083/jcb.200708037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ortega F, Pérez-Sen R, Delicado EG, Miras-Portugal MT. P2X7 nucleotide receptor is coupled to GSK-3 inhibition and neuroprotection in cerebellar granule neurons. Neurotox Res. 2009;15:193–204. doi: 10.1007/s12640-009-9020-6. [DOI] [PubMed] [Google Scholar]
- 14.Solle M, Labasi J, Perregaux DG, et al. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 15.Masin M, Young C, Lim K, et al. Expression, assembly and function of novel C-terminal truncated variants of the mouse P2X7 receptor: re-evaluation of P2X7 knockouts. Br J Pharmacol. 2012;165:978–993. doi: 10.1111/j.1476-5381.2011.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spencer GJ, Utting JC, Etheridge SL, et al. Wnt signalling in osteoblasts regulates expression of the receptor activator of NFkappaB ligand and inhibits osteoclastogenesis in vitro. J Cell Sci. 2006;119:1283–1296. doi: 10.1242/jcs.02883. [DOI] [PubMed] [Google Scholar]
- 17.Grol MW, Zelner I, Dixon SJ. P2X7-mediated calcium influx triggers a sustained, PI3K-dependent increase in metabolic acid production by osteoblast-like cells. Am J Physiol Endocrinol Metab. 2012;302:E561–75. doi: 10.1152/ajpendo.00209.2011. [DOI] [PubMed] [Google Scholar]
- 18.Caverzasio J, Biver E, Thouverey C. Predominant role of PDGF receptor transactivation in Wnt3a-induced osteoblastic cell proliferation. J Bone Miner Res. 2013;28:260–270. doi: 10.1002/jbmr.1748. [DOI] [PubMed] [Google Scholar]
- 19.Biechele TL, Moon RT. Assaying beta-catenin/TCF transcription with beta-catenin/TCF transcription-based reporter constructs. Methods Mol Biol. 2008;468:99–110. doi: 10.1007/978-1-59745-249-6_8. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 22.Ortega F, Pérez-Sen R, Morente V, et al. P2X7, NMDA and BDNF receptors converge on GSK3 phosphorylation and cooperate to promote survival in cerebellar granule neurons. Cell Mol Life Sci. 2010;67:1723–1733. doi: 10.1007/s00018-010-0278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grol MW, Pereverzev A, Sims SM, Dixon SJ. P2 receptor networks regulate signaling duration over a wide dynamic range of ATP concentrations. J Cell Sci. 2013;126:3615–3626. doi: 10.1242/jcs.122705. [DOI] [PubMed] [Google Scholar]
- 24.Bowler WB, Dixon CJ, Halleux C, et al. Signaling in human osteoblasts by extracellular nucleotides. Their weak induction of the c-fos proto-oncogene via Ca2+ mobilization is strongly potentiated by a parathyroid hormone/cAMP-dependent protein kinase pathway independently of mitogen-activated protein kinase. J Biol Chem. 1999;274:14315–14324. doi: 10.1074/jbc.274.20.14315. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura E, Uezono Y, Narusawa K, et al. ATP activates DNA synthesis by acting on P2X receptors in human osteoblast-like MG-63 cells. Am J Physiol Cell Physiol. 2000;279:C510–9. doi: 10.1152/ajpcell.2000.279.2.C510. [DOI] [PubMed] [Google Scholar]
- 26.Ortega F, Pérez-Sen R, Miras-Portugal MT. Gi-coupled P2Y-ADP receptor mediates GSK-3 phosphorylation and beta-catenin nuclear translocation in granule neurons. J Neurochem. 2008;104:62–73. doi: 10.1111/j.1471-4159.2007.05021.x. [DOI] [PubMed] [Google Scholar]
- 27.Orriss IR, Burnstock G, Arnett TR. Purinergic signalling and bone remodelling. Curr Opin Pharmacol. 2010;10:322–330. doi: 10.1016/j.coph.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki A, Ozono K, Kubota T, et al. PTH/cAMP/PKA signaling facilitates canonical Wnt signaling via inactivation of glycogen synthase kinase-3beta in osteoblastic Saos-2 cells. J Cell Biochem. 2008;104:304–317. doi: 10.1002/jcb.21626. [DOI] [PubMed] [Google Scholar]
- 29.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panupinthu N, Zhao L, Possmayer F, et al. P2X7 nucleotide receptors mediate blebbing in osteoblasts through a pathway involving lysophosphatidic acid. J Biol Chem. 2007;282:3403–3412. doi: 10.1074/jbc.M605620200. [DOI] [PubMed] [Google Scholar]
- 31.Noronha-Matos JB, Coimbra J, Sá-e-Sousa A, et al. P2X7-induced zeiosis promotes osteogenic differentiation and mineralization of postmenopausal bone marrow-derived mesenchymal stem cells. FASEB J. 2014;28:5208–5222. doi: 10.1096/fj.14-257923. [DOI] [PubMed] [Google Scholar]
- 32.Zimmermann H, Zebisch M, Sträter N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8:437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noronha-Matos JB, Costa MA, Magalhães-Cardoso MT, et al. Role of ecto-NTPDases on UDP-sensitive P2Y(6) receptor activation during osteogenic differentiation of primary bone marrow stromal cells from postmenopausal women. J Cell Physiol. 2012;227:2694–2709. doi: 10.1002/jcp.23014. [DOI] [PubMed] [Google Scholar]
- 34.Bellido T, Ali AA, Gubrij I, et al. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146:4577–4583. doi: 10.1210/en.2005-0239. [DOI] [PubMed] [Google Scholar]
- 35.Yao G-Q, Wu J-J, Troiano N, Insogna K. Targeted overexpression of Dkk1 in osteoblasts reduces bone mass but does not impair the anabolic response to intermittent PTH treatment in mice. J Bone Miner Metab. 2011;29:141–148. doi: 10.1007/s00774-010-0202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, Whetstone HC, Youn A, et al. Beta-catenin signaling pathway is crucial for bone morphogenetic protein 2 to induce new bone formation. J Biol Chem. 2007;282:526–533. doi: 10.1074/jbc.M602700200. [DOI] [PubMed] [Google Scholar]
- 37.Fromigué O, Haÿ E, Barbara A, Marie PJ. Essential role of nuclear factor of activated T cells (NFAT)-mediated Wnt signaling in osteoblast differentiation induced by strontium ranelate. J Biol Chem. 2010;285:25251–25258. doi: 10.1074/jbc.M110.110502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitase Y, Barragan L, Qing H, et al. Mechanical induction of PGE2 in osteocytes blocks glucocorticoid-induced apoptosis through both the β-catenin and PKA pathways. J Bone Miner Res. 2010;25:2657–2668. doi: 10.1002/jbmr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armstrong S, Pereverzev A, Dixon SJ, Sims SM. Activation of P2X7 receptors causes isoform-specific translocation of protein kinase C in osteoclasts. J Cell Sci. 2009;122:136–144. doi: 10.1242/jcs.031534. [DOI] [PubMed] [Google Scholar]
- 40.Sawakami K, Robling AG, Ai M, et al. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem. 2006;281:23698–23711. doi: 10.1074/jbc.M601000200. [DOI] [PubMed] [Google Scholar]
- 41.Zhao L, Shim JW, Dodge TR, et al. Inactivation of Lrp5 in osteocytes reduces Young’s modulus and responsiveness to the mechanical loading. Bone. 2013;54:35–43. doi: 10.1016/j.bone.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, et al. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281:31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]