Abstract
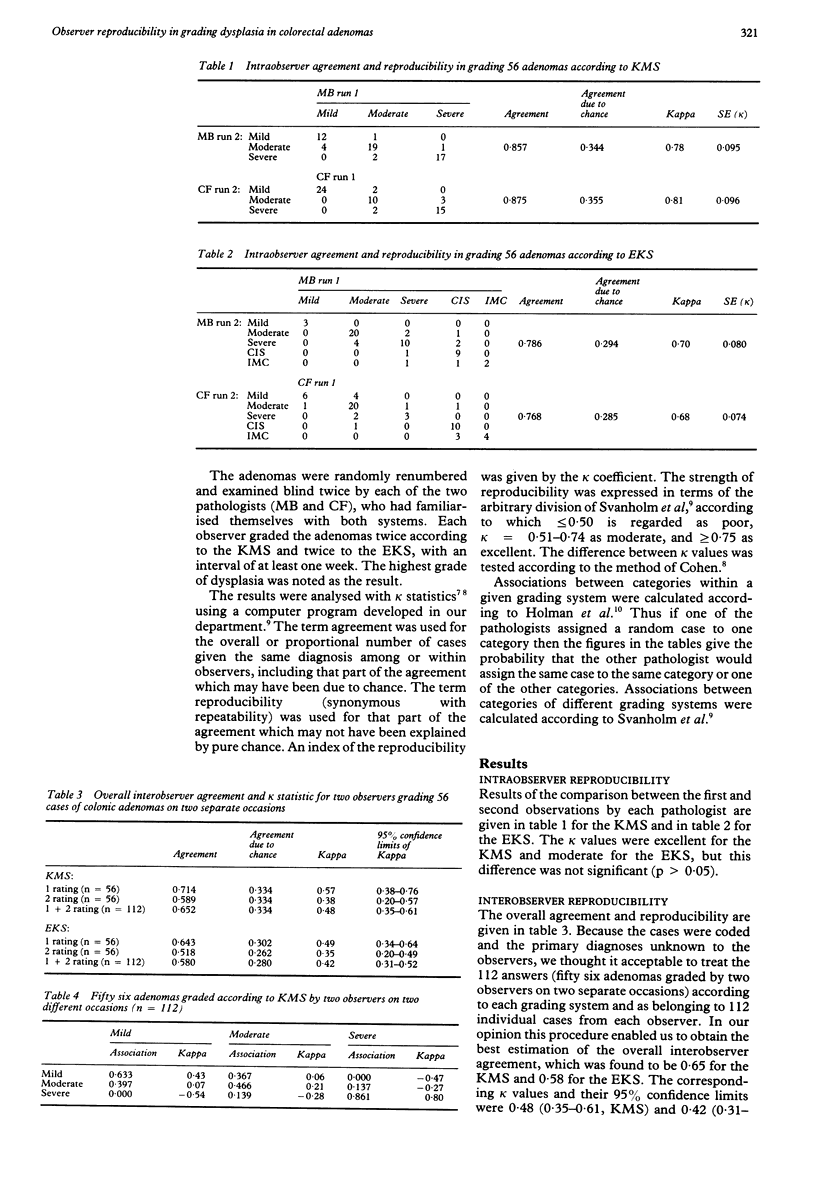
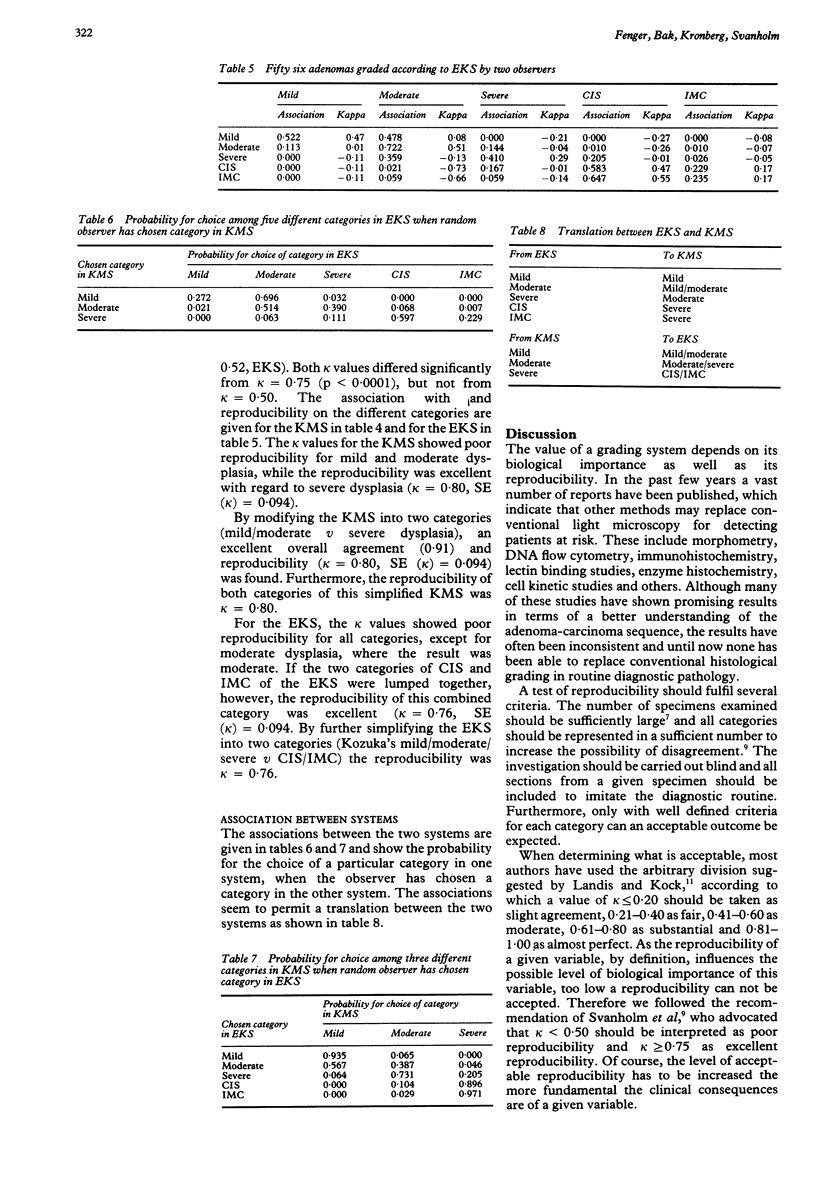
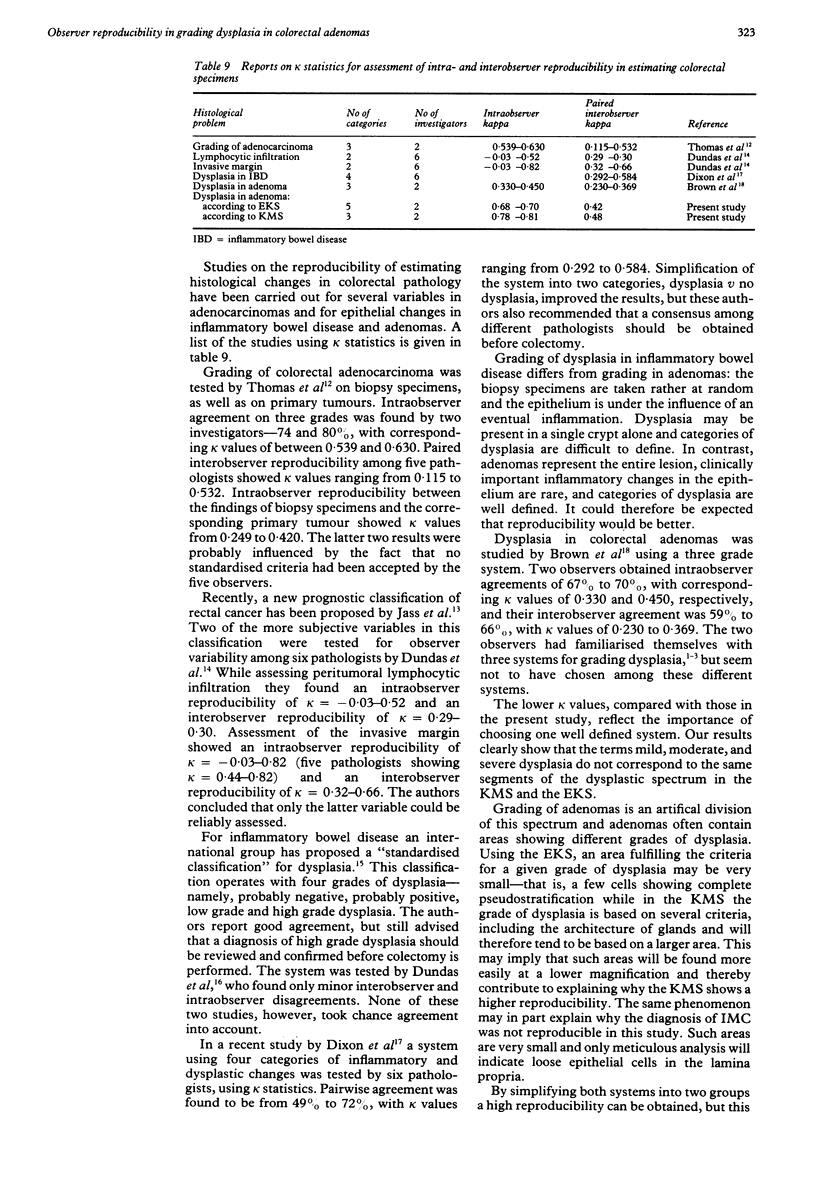
The two most well known and well defined grading systems for dysplasia in colorectal adenomas were compared with regard to reproducibility. The Konishi-Morson system (KMS) operates with several histological and cytological variables and grades of mild, moderate, and severe dysplasia. The Kozuka system is based on the extent of nuclear pseudostratification and also has three grades of dysplasia (III-V). As the group of severe dysplasia is very large in this system, it was extended with two higher grades, similarly based on individual histological criteria, known hereafter as the extended Kozuka system (EKS). Fifty six adenomas were graded by two observers, each observer grading twice according to the KMS criteria and twice according to EKS criteria. Intraobserver reproducibility was excellent for the KMS and moderate for the EKS, but this was not significant. The overall interobserver reproducibility was similar (moderate) for the KMS and for the EKS. Kappa values for interobserver reproducibility on individual categories were excellent for severe dysplasia according to the KMS, but low for all other categories in both systems. By simplifying both systems into two groups a high reproducibility can be obtained, but this implies that all the original grades (III-V) for the EKS must be grouped together. It is therefore recommended that a simplified KMS is used for further studies on the biological importance of dysplasia and for comparison between histological changes and other markers for colorectal neoplasia.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown L. J., Smeeton N. C., Dixon M. F. Assessment of dysplasia in colorectal adenomas: an observer variation and morphometric study. J Clin Pathol. 1985 Feb;38(2):174–179. doi: 10.1136/jcp.38.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon M. F., Brown L. J., Gilmour H. M., Price A. B., Smeeton N. C., Talbot I. C., Williams G. T. Observer variation in the assessment of dysplasia in ulcerative colitis. Histopathology. 1988 Oct;13(4):385–397. doi: 10.1111/j.1365-2559.1988.tb02055.x. [DOI] [PubMed] [Google Scholar]
- Dundas S. A., Kay R., Beck S., Cotton D. W., Coup A. J., Slater D. N., Underwood J. C. Can histopathologists reliably assess dysplasia in chronic inflammatory bowel disease? J Clin Pathol. 1987 Nov;40(11):1282–1286. doi: 10.1136/jcp.40.11.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dundas S. A., Laing R. W., O'Cathain A., Seddon I., Slater D. N., Stephenson T. J., Underwood J. C. Feasibility of new prognostic classification for rectal cancer. J Clin Pathol. 1988 Dec;41(12):1273–1276. doi: 10.1136/jcp.41.12.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekelund G., Lindström C. Histopathological analysis of benign polyps in patients with carcinoma of the colon and rectum. Gut. 1974 Aug;15(8):654–663. doi: 10.1136/gut.15.8.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio C. M., Kaye G. I., Lane N. Distribution of human colonic lymphatics in normal, hyperplastic, and adenomatous tissue. Its relationship to metastasis from small carcinomas in pedunculated adenomas, with two case reports. Gastroenterology. 1973 Jan;64(1):51–66. [PubMed] [Google Scholar]
- Greaves P., Filipe M. I., Abbas S., Ormerod M. G. Sialomucins and carcinoembryonic antigen in the evolution of colorectal cancer. Histopathology. 1984 Sep;8(5):825–834. doi: 10.1111/j.1365-2559.1984.tb02398.x. [DOI] [PubMed] [Google Scholar]
- Holman C. D., James I. R., Heenan P. J., Matz L. R., Blackwell J. B., Kelsall G. R., Singh A., ten Seldam R. E. An improved method of analysis of observer variation between pathologists. Histopathology. 1982 Sep;6(5):581–589. doi: 10.1111/j.1365-2559.1982.tb02752.x. [DOI] [PubMed] [Google Scholar]
- Jass J. R., Love S. B., Northover J. M. A new prognostic classification of rectal cancer. Lancet. 1987 Jun 6;1(8545):1303–1306. doi: 10.1016/s0140-6736(87)90552-6. [DOI] [PubMed] [Google Scholar]
- Konishi F., Morson B. C. Pathology of colorectal adenomas: a colonoscopic survey. J Clin Pathol. 1982 Aug;35(8):830–841. doi: 10.1136/jcp.35.8.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozuka S. Premalignancy of the mucosal polyp in the large intestine: I. Histologic gradation of the polyp on the basis of epithelial pseudostratification and glandular branching. Dis Colon Rectum. 1975 Sep;18(6):483–493. doi: 10.1007/BF02587217. [DOI] [PubMed] [Google Scholar]
- Landis J. R., Koch G. G. The measurement of observer agreement for categorical data. Biometrics. 1977 Mar;33(1):159–174. [PubMed] [Google Scholar]
- Riddell R. H., Goldman H., Ransohoff D. F., Appelman H. D., Fenoglio C. M., Haggitt R. C., Ahren C., Correa P., Hamilton S. R., Morson B. C. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983 Nov;14(11):931–968. doi: 10.1016/s0046-8177(83)80175-0. [DOI] [PubMed] [Google Scholar]
- Silcocks P. B. Measuring repeatability and validity of histological diagnosis--a brief review with some practical examples. J Clin Pathol. 1983 Nov;36(11):1269–1275. doi: 10.1136/jcp.36.11.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. D., Dixon M. F., Smeeton N. C., Williams N. S. Observer variation in the histological grading of rectal carcinoma. J Clin Pathol. 1983 Apr;36(4):385–391. doi: 10.1136/jcp.36.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]


