Significance
Contact-dependent growth inhibition (CDI) systems produce toxins that inhibit competing bacteria and immunity proteins that protect against self-inhibition. The CDI toxin deployed by Escherichia coli 536 is a nuclease that only cleaves transfer RNA (tRNA) molecules when bound to the biosynthetic enzyme O-acetylserine sulfhydrylase (CysK). Here, we present crystal structures of the activated CysK/toxin binary complex and the neutralized CysK/toxin/immunity protein ternary complex. CysK significantly increases toxin thermostability and promotes its interaction with tRNA substrates. Collectively, our results indicate that CysK stabilizes the toxin fold, thereby organizing the nuclease active site for substrate recognition and catalysis. We propose that the E. coli 536 toxin may need to unfold when transferred between bacteria and that its interaction with CysK could ensure reactivation after entry into target cells.
Keywords: bacterial competition, structural biology, toxin chaperone, tRNase activity
Abstract
Contact-dependent growth inhibition (CDI) is a widespread mechanism of bacterial competition. CDI+ bacteria deliver the toxic C-terminal region of contact-dependent inhibition A proteins (CdiA-CT) into neighboring target bacteria and produce CDI immunity proteins (CdiI) to protect against self-inhibition. The CdiA-CTEC536 deployed by uropathogenic Escherichia coli 536 (EC536) is a bacterial toxin 28 (Ntox28) domain that only exhibits ribonuclease activity when bound to the cysteine biosynthetic enzyme O-acetylserine sulfhydrylase A (CysK). Here, we present crystal structures of the CysK/CdiA-CTEC536 binary complex and the neutralized ternary complex of CysK/CdiA-CT/CdiIEC536. CdiA-CTEC536 inserts its C-terminal Gly-Tyr-Gly-Ile peptide tail into the active-site cleft of CysK to anchor the interaction. Remarkably, E. coli serine O-acetyltransferase uses a similar Gly-Asp-Gly-Ile motif to form the “cysteine synthase” complex with CysK. The cysteine synthase complex is found throughout bacteria, protozoa, and plants, indicating that CdiA-CTEC536 exploits a highly conserved protein–protein interaction to promote its toxicity. CysK significantly increases CdiA-CTEC536 thermostability and is required for toxin interaction with tRNA substrates. These observations suggest that CysK stabilizes the toxin fold, thereby organizing the nuclease active site for substrate recognition and catalysis. By contrast, Ntox28 domains from Gram-positive bacteria lack C-terminal Gly-Tyr-Gly-Ile motifs, suggesting that they do not interact with CysK. We show that the Ntox28 domain from Ruminococcus lactaris is significantly more thermostable than CdiA-CTEC536, and its intrinsic tRNA-binding properties support CysK-independent nuclease activity. The striking differences between related Ntox28 domains suggest that CDI toxins may be under evolutionary pressure to maintain low global stability.
Bacteria have evolved diverse mechanisms to communicate and compete with neighboring microorganisms. One such mechanism is contact-dependent growth inhibition (CDI), which mediates the transfer of protein toxins between Gram-negative bacterial cells. CDI systems are distributed throughout α-, β-, and γ-proteobacteria and are particularly common in pathogens (1). CDI is mediated by the CdiB/CdiA family of two-partner secretion proteins. CdiB is an Omp85-family transporter that exports and assembles CdiA effectors onto the cell surface. CdiA proteins share homology with filamentous hemagglutinin adhesins and are thought to form long filaments projecting from the inhibitor cell. CdiA recognizes specific receptors on susceptible bacteria and delivers its toxic C-terminal domain (CdiA-CT) to inhibit target-cell growth (2). CDI+ bacteria also produce CdiI immunity proteins, which bind CdiA-CT domains and neutralize their toxic activity to protect against self-inhibition. The CdiA-CT region is highly polymorphic between bacteria, with sequences diverging abruptly after the Val-Glu-Asn-Asn (VENN) peptide motif within the conserved pretoxin domain (Pfam: PF04829) (3, 4). CdiA-CT diversity reflects the variety of toxins deployed during CDI, with most experimentally characterized toxins exhibiting distinct nuclease activities (3, 5–7). CdiI immunity proteins are also variable and only neutralize their cognate CdiA-CT toxins. Thus, CDI is thought to mediate interstrain competition, with toxin/immunity protein variability providing a mechanism to discriminate between self and non-self (1, 2).
Previous studies on the CDI toxin from uropathogenic Escherichia coli 536 (EC536) revealed that it possesses latent anticodon nuclease activity against all tRNA isoacceptors (8). The CdiA-CTEC536 region is composed of two domains that have distinct functions during CDI (9). The extreme C-terminal domain is an Ntox28 RNase family member (Pfam: PF15605) and is responsible for growth-inhibition activity (3, 8). The N-terminal domain facilitates translocation of the tethered nuclease into the cytosol of target bacteria (9). Although CdiA-CTEC536 rapidly cleaves tRNA in vivo, the purified toxin has no detectable nuclease activity in vitro (8). Using biochemical approaches, we discovered that CdiA-CTEC536 is activated when bound to the biosynthetic enzyme O-acetylserine sulfhydrylase-A (CysK). CysK is one of two isoenzymes (along with CysM) that catalyze the final reaction in cysteine synthesis. In bacteria and plants, CysK is found in the “cysteine synthase” complex together with CysE—the serine O-acetyltransferase responsible for the penultimate step of cysteine synthesis (10). Formation of the cysteine synthase complex requires the C-terminal tail of CysE, which inserts into the CysK active site (11). The C-terminal Ile residue of CysE is particularly critical and interacts with the CysK active site using the same contacts as the enzyme substrate O-acetylserine (12, 13). Although CysK and CysM share 58% sequence identity, CysE does not interact stably with the CysM isoenzyme (14). The CdiA-CTEC536 toxin carries a C-terminal Gly-Tyr-Gly-Ile (GYGI) peptide motif that appears to mimic the Gly-Asp-Gly-Ile (GDGI) tail of E. coli CysE. Moreover, O-acetylserine blocks the binding of both CysE and CdiA-CTEC536 to CysK (8, 10), indicating that CdiA-CTEC536 also inserts its C-terminal tail into the CysK active-site cleft. Remarkably, other proteins mimic CysE to bind CysK (15). In Bacillus species, the CymR transcription factor uses its C-terminal Met-Phe-Tyr-Ile tail to bind CysK, and modulation of this interaction controls the cys regulon (16). Perhaps more intriguing is EGL-9, an O2-sensing prolyl hydroxylase from Caenorhabditis elegans that binds a CysK homolog (CYSL-1) using a C-terminal Ile residue (17). The resulting complex senses O2 tension indirectly through hydrogen sulfide, which accumulates during hypoxia. Sulfide binds the CYSL-1 active site and displaces the C terminus of EGL-9. Once liberated, EGL-9 hydroxylates HIF-1 to initiate transcriptional responses to hypoxia (17). Thus, CysK and its homologs have been coopted to regulate gene expression in bacteria and eukaryotes.
To gain mechanistic insight into toxin activation, we solved crystal structures of the CdiA-CTEC536 toxin in binary complex with CysK, and in ternary complex with CysK and CdiIEC536 immunity protein. The nuclease domain forms a small four-helix bundle with no structural similarity to other known RNase families. Two toxins bind to each CysK homodimer, and the C-terminal GYGI peptide of the nuclease inserts into the CysK active-site cleft as predicted by previous studies (8). Structure-guided mutagenesis revealed a putative catalytic triad of Asp155, His178, and Glu181 in the nuclease domain. The predicted nuclease active site is occluded by immunity protein in the CysK/CdiA-CT/CdiIEC536 structure, suggesting that CdiIEC536 blocks the binding of tRNA substrates to the toxin. Intriguingly, Ntox28 homologs from Gram-positive bacteria lack the C-terminal GYGI motif, suggesting that they do not require CysK-mediated activation. We tested this prediction using Tox28Rlac from Ruminococcus lactaris and confirmed that the domain possesses CysK-independent tRNase activity. Moreover, Tox28Rlac is significantly more stable to thermal denaturation than CdiA-CTEC536 and possesses intrinsic tRNA-binding activity. By contrast, CysK is required for tRNA binding to CdiA-CTEC536. Collectively, these findings suggest that CysK is recruited to stabilize CdiA-CTEC536, thereby organizing the active site to promote substrate binding and catalysis.
Results
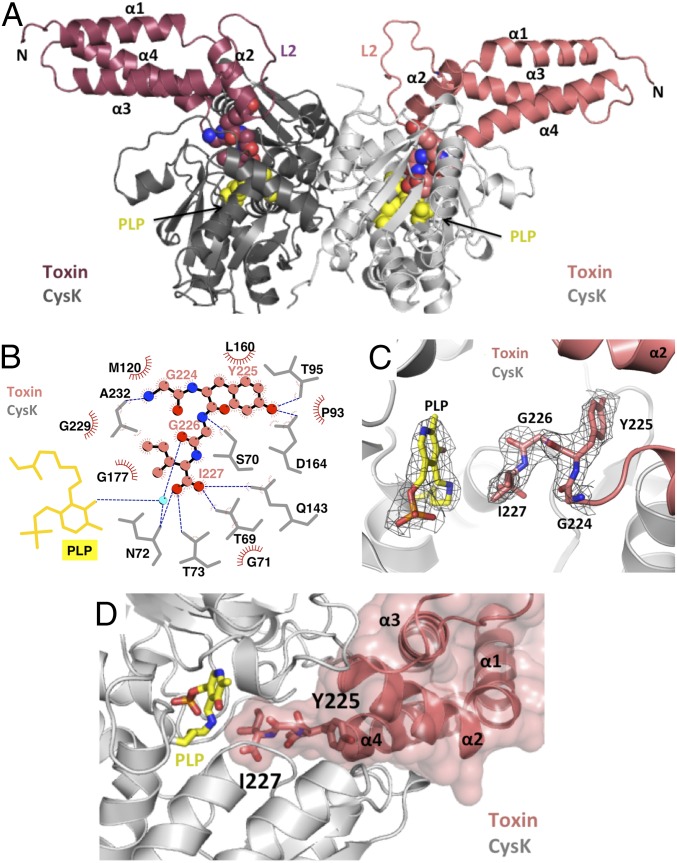
We generated a binary complex of CysK bound to inactive CdiA-CTEC536 toxin that contains the His178Ala mutation (numbered from Val1 of the VENN motif). The CysK/CdiA-CT(H178A)EC536 complex crystallized in space group P41, and the structure was solved to 2.7 Å resolution (Table S1). Like other O-acetylserine sulfhydrylases (18), E. coli CysK is homodimeric and contains a pyridoxal 5′-phosphate (PLP) cofactor in Schiff-base linkage to Lys42. CysK in the binary complex has an “open” active-site conformation, similar to the structure of unliganded CysK from Salmonella Typhimurium (rmsd of 0.5 Å over all 312 α-carbons) (18). Thus, the toxin does not induce the “closed” conformation observed when CysK contains substrate covalently bound to PLP in the active site (19). As we have found in other CdiA-CT toxin structures (5, 6, 20), only the C-terminal nuclease domain (residues Lys127–Ile227) is resolved in the final model. The CdiA-CTEC536 nuclease domain consists of four α-helices. Three long helices (α1, α3, and α4) form a bundle capped by the shorter helix α2 (Fig. 1A). Helices α2 and α3 are connected by the long flexible loop L2, which was modeled predominately as Ala residues. Two CdiA-CTEC536 nuclease domains bind to the CysK dimer, but the toxins make no contact with one another (Fig. 1A), suggesting that they bind independently. The C-terminal tail of the nuclease domain inserts into the CysK active site, with the GYGI peptide backbone forming a network of hydrogen bonds (H-bonds) with CysK (Fig. 1B and Table S2). The toxin's C-terminal Ile227 residue is positioned in close proximity to the active-site PLP and forms H-bonds with Thr69, Asn72, Thr73, and Gln143 of CysK (Fig. 1 B and C). These latter contacts have been observed in structures of CysE C-terminal peptides bound to the CysK active site (13, 21, 22). The side-chain of Tyr225 H-bonds to CysK residues Thr95 and Asp164 (Fig. 1B and Table S2). The GYGI tail interactions are buttressed by additional H-bonds and salt bridges emanating from toxin helices α2, α3, and α4 (Table S2). Residues within α2 (Leu160, Ile156, Ile157, and Met163), α3 (Met179 and Leu186), and α4 (Leu222) also form hydrophobic interactions with CysK, and the toxin helical bundle exploits shape complementarity to fit into the CysK active-site cleft (Fig. 1D). Overall, the complex interface is 1,280 Å2, burying 9.2% and 19.4% of the solvent-accessible surface areas of CysK and CdiA-CTEC536, respectively.
Table S1.
X-ray diffraction data and refinement statistics for CysK/CdiA-CTEC536 complexes
| CysK/CdiA-CT(H178A)EC536 | CysK/CdiA-CT/CdiIEC536* | |
| Space group | P41 | C2221 |
| Unit cell dimensions, Å | 64.01 x 64.01 x 365.37 | 81.25 x 195.54 x 175.06 |
| pH of crystallization condition | 7.9 | 7.1 |
| Protein concentration, mg/mL | 20 | 20 |
| Data collection | ||
| Wavelength, Å | 1.0 | 0.9795 |
| Resolution range | 44.92–2.7 | 50–2.75 |
| Unique reflections (total) | 39,795 (303,136) | 33,877 (483,433) |
| Completeness, %† | 99.4 | 100.0 |
| Redundancy† | 12.8 (13.4) | 14.3 (14.5) |
| Rmerge†‡ | 0.163 (0.734) | 0.279 (1.159) |
| Rmeas†§ | 0.176 (0.791) | 0.289 (1.201) |
| Rp.i.m.†¶ | 0.066 (0.293) | 0.077 (0.314) |
| CC1/2† | 0.996 (0.952) | 0.995 (0.817) |
| I/σ† | 19.96 (15.94) | 10.41 (2.75) |
| NCS copies | 2 | 2 |
| Model refinement | ||
| Resolution range, Å | 44.89–2.70 | 48.83–2.75 |
| No. of reflections | 39,640 | 36,673 |
| No. of protein + ligand atoms | 6,220 | 8,197 |
| No. of water molecules | 110 | 101 |
| Missing residues | CdiA-CT:1–126 CysK:315–323 | CdiA-CT:1–126 CysK:315–323 CdiI:125–128# |
| Rwork/Rfree, %|| | 20.2/22.4 | 19.4/24.2 |
| rms deviations | ||
| Bond lengths, Å | 0.009 | 0.011 |
| Bond angles | 1.225 | 1.072 |
| Ramachandran plot | ||
| Most favorable region, % | 97.08 | 95.83 |
| Additional allowed region, % | 2.92 | 3.90 |
| Disallowed region | 0 | 0.27 |
| PDB ID code | 5J43 | 5J5V |
CdiA-CT/CdiIEC536 complex is an SeMet derivative.
Statistics for the highest resolution shell are given in parentheses.
Rmerge = ΣhklΣi |Ii(hkl) – (I(hkl))|/ΣhklΣi Ii(hkl).
Rmeas = Σhkl {N(hkl)/[N(hkl) – 1]}1/2 Σi |Ii(hkl) – (I(hkl))|/ΣhklΣi Ii(hkl).
Rp.i.m (precision-indicating Rmerge) = Σhkl {1/[N(hkl) – 1]} 1/2 Σi |Ii(hkl) – (I(hkl))|/ΣhklΣi Ii(hkl).
Missing residues for one CdiI protomer. The other CdiI protomer is missing only residue 128.
Rwork = Σ|Fobs − Fcalc|/ΣFobs. Rfree was computed identically except where all reflections belong to a test set of 5% randomly selected data.
Fig. 1.
The CysK/CdiA-CTEC536 binary complex. (A) Crystal structure of the CysK/CdiA-CTEC536 complex. Secondary structure elements of the toxin nuclease domain are indicated together with flexible loop L2. The C-terminal GYGI peptides of CdiA-CTEC536 and CysK-bound pyridoxyl 5′-phosphate (PLP) are rendered as spheres. (B) GYGI peptide interaction network. CdiA-CTEC536 residues Gly224, Tyr225, Gly226, and Ile227 are shown in spheres, and CysK residues and PLP are rendered as gray and yellow sticks, respectively. A water molecule is shown as a cyan sphere. Red arcs represent hydrophobic interactions, and blue dashed lines indicate H-bonds. The interaction network was produced using LigPlot. (C) Interaction between CdiA-CTEC536 C-terminal GYGI peptide and the CysK active site. The CdiA-CTEC536 GYGI peptide and CysK PLP are shown in stick representation. The 2Fo − Fc electron density map of the CdiA-CTEC536 GYGI peptide and CysK PLP is shown in gray mesh and contoured at 1.0 σ. (D) The CdiA-CTEC536 toxin domain exploits shape complementarity to bind the CysK active-site cleft. Residues Gly224–Ile227 and PLP are shown in stick representation.
Table S2.
Hydrogen bonds and salt bridges between CdiA-CTEC536 and CysK in the binary complex
| CdiA-CTEC536 | CysK | Distance, Å |
| LYS 161 [NZ] | THR 95 [O] | 3.09 |
| GLY 165 [O] | ALA 310 [N] | 3.02 |
| LYS 166 [NZ] | SER308 [OG] | 3.40 |
| GLN 183 [NE2] | PRO 224 [O] | 3.11 |
| GLN 183 [NE2] | ASP 207 [OD2] | 3.22 |
| ARG 190 [NH2] | PRO 222 [O] | 2.95 |
| SER 220 [O] | LYS 121 [N] | 3.17 |
| ALA 221 [O] | MET 120 [N] | 2.90 |
| GLY 224 [N] | ALA 232 [O] | 2.77 |
| TYR 225 [OH] | THR 95 [OH] | 3.00 |
| GLY 226 [N] | SER 70 [OG] | 2.64 |
| ILE 227 [O] | THR 69 [OG1] | 2.62 |
| ILE 227 [O] | GLN 143 [NE2] | 3.04 |
| ILE 227 [OXT] | THR 73 [N] | 3.14 |
| ILE 227 [OXT] | ASN 72 [N] | 3.35 |
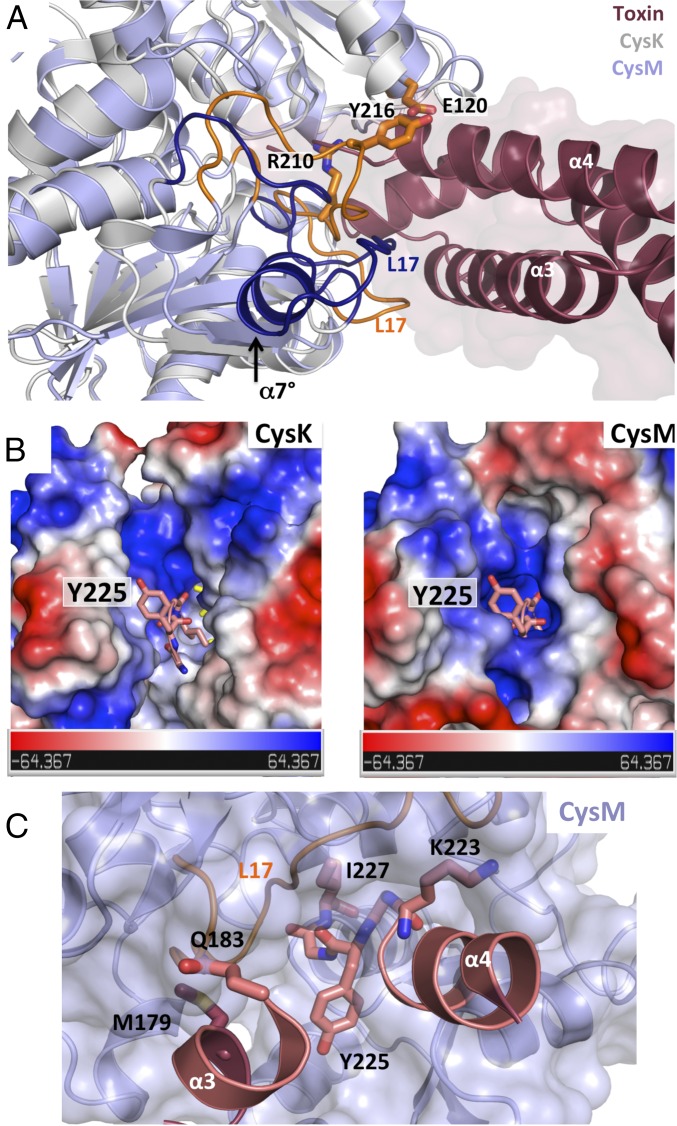
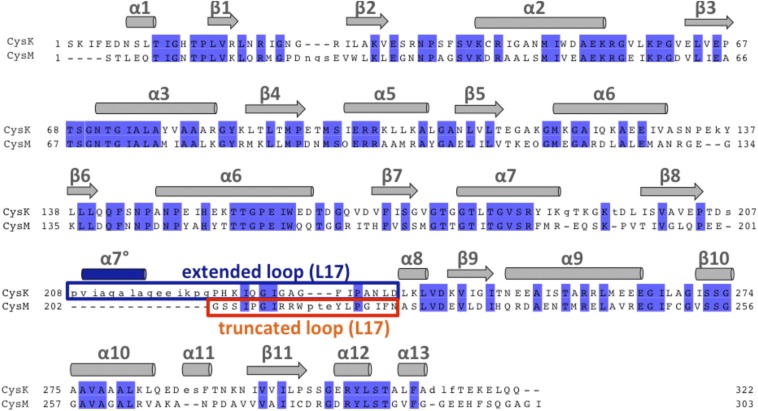
E. coli CysK and CysM share related structures (rmsd of 1.8 Å over 285/292 α-carbons) but differ significantly in the loop L17 region at the entrance of the active-site cleft (Fig. 2A). CysK contains 16 extra residues in loop L17 that introduce an additional α-helix (α7°) (Fig. 2A and Fig. S1). The differences in loop L17 have profound effects on the landscape and electrostatic surface potential of each active-site cleft (Fig. 2B) (12, 23). Superimposition of CdiA-CTEC536 onto the CysM structure produces several clashes with the C-terminal GYGI peptide (Fig. 2B). Moreover, the CysM active site contains no hydrophobic pockets capable of accommodating the side-chains of toxin residues Ile227 and Tyr225. Residues Lys223 and Gly224 at the end of toxin helix α4 and Met179 and Gln183 within helix α3 each clash with the surface of CysM loop L17 (Fig. 2C). Thus, CdiA-CTEC536 and CysE both exploit differences in loop L17 sequence and structure to bind CysK specifically.
Fig. 2.
Comparison of E. coli CysK and CysM structures. (A) Superimposition of E. coli CysM (PDB ID code 2BHT) onto the CysK/CdiA-CTEC536 binary complex. The divergent loop L17 regions are highlighted in orange for CysM and dark blue for CysK. The additional helix α7° in CysK is indicated, as are CysM residues that clash with the surface of the nuclease domain. (B) Electrostatic surface representations of CysK and CysM. Electric isopotentials of +64.4 kT/e and –64.4 kT/e are shown in blue and red, respectively. The C-terminal GYGI peptide of CdiA-CTEC536 is shown in stick representation. (C) CdiA-CTEC536 modeled onto the CysM structure. Toxin residues that clash with loop L17 of CysM are shown in stick representation.
Fig. S1.
Structure and sequence alignment of E. coli CysK and CysM. CysK and CysM sequences were aligned using Clustal omega. Identical residues are highlighted in blue. Secondary structure elements are presented above the alignment. CysK contains an extended loop L17 region (boxed in blue) and an additional helix (α7° in blue) compared with CysM (orange).
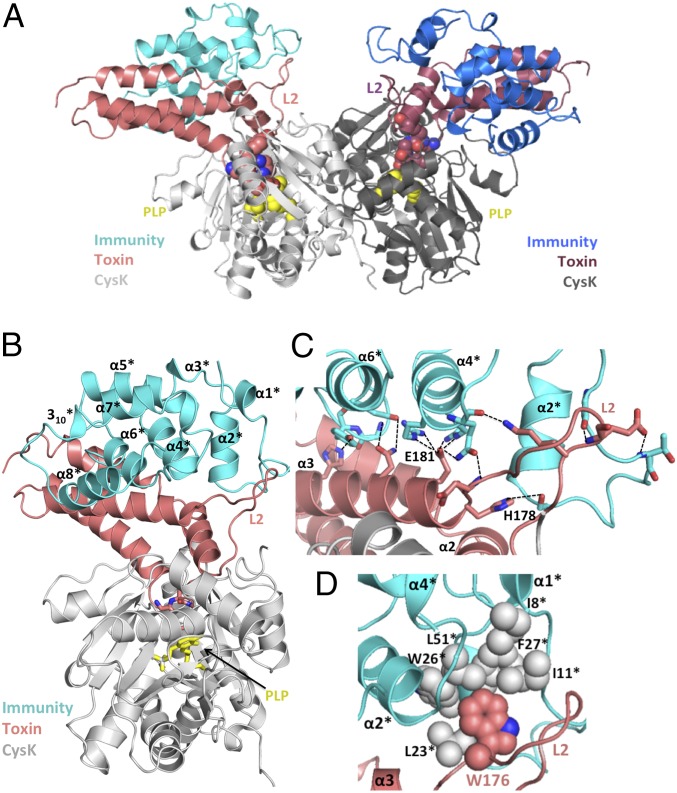
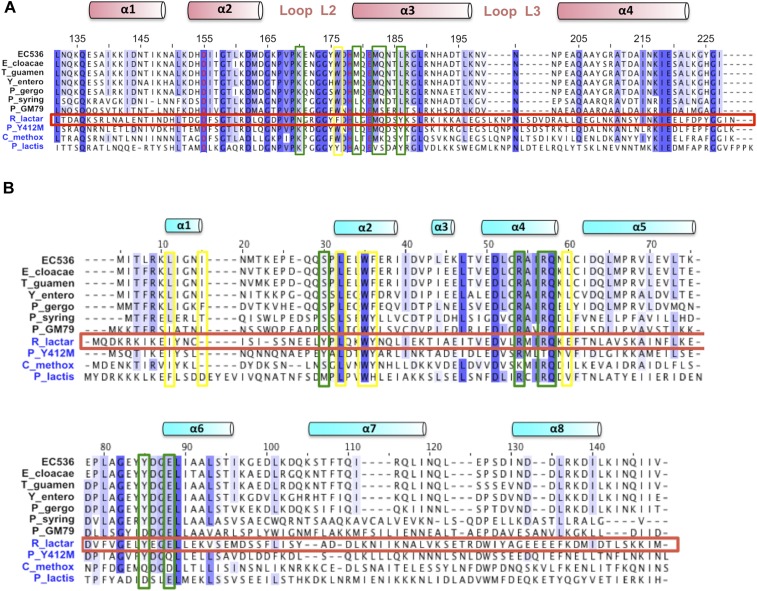
The CdiIEC536 immunity protein binds to CysK/CdiA-CTEC536, forming a neutralized ternary complex (8). The crystal structure of this ternary complex shows that immunity protein binds to each toxin to form a dimer of heterotrimers (Fig. 3A). The CysK and CdiA-CTEC536 structures and interactions are very similar in the binary and ternary complexes (rmsd of 0.25 Å over all α-carbons; Table S3), indicating that the immunity protein does not grossly alter toxin conformation. CdiIEC536 is a single domain composed of one 310- and eight α-helices arranged in four stacked layers to form an antiparallel spiral (Fig. 3B). A DALI server (24) search indicates that CdiIEC536 does not share structural homology with other known antitoxins or immunity proteins (Table S4). The immunity protein interacts exclusively with the nuclease domain of CdiA-CTEC536 and makes no contacts with CysK (Fig. 3A). Flexible loop L2 of the nuclease domain interacts extensively with CdiIEC536, stabilizing the loop and allowing its side-chains to be fully resolved in the ternary complex (Fig. 3C). Fifteen H-bonds and ion pairs connect CdiA-CTEC536 helix α3 to helices α2*, α4*, and α6* of CdiIEC536 (Fig. 2C and Table S5). Additionally, toxin residue Trp176 from loop L2 fits into a hydrophobic pocket formed by Ile8, Ile11, Leu23, Trp26, Phe27, and Leu51 of CdiIEC536 (Fig. 3D). The toxin/immunity protein interface is 1,034 Å2, burying 16.6% and 12.7% of the solvent-accessible surface areas of CdiA-CTEC536 and CdiIEC536, respectively. Comparisons of different Ntox28 domains and their predicted immunity proteins reveal that many of the interacting residues are conserved throughout the toxin/immunity family (Fig. S2).
Fig. 3.
The CysK/CdiA-CT/CdiIEC536 ternary complex. (A) Crystal structure of the CysK/CdiA-CT/CdiIEC536 complex. The C-terminal GYGI peptide of CdiA-CTEC536 and CysK-bound pyridoxyl 5′-phosphate (PLP) are rendered as spheres. The ternary complex is presented in the same orientation as Fig. 1A. (B) Monomeric version of the ternary complex. CdiIEC536 secondary structure elements are outlined and indicated with superscripted asterisks (*). The C-terminal GYGI peptide of the toxin and PLP are shown as sticks. (C) H-bonding network between CdiA-CTEC536 and CdiIEC536. Interacting residues are shown in stick representation, and dashed lines indicate H-bonds. Active-site residues His178 and Glu181 from L2 and α3 of CdiA-CTEC536 interact with α2*, α4*, and α6* of CdiIEC536. (D) Hydrophobic interactions between CdiA-CTEC536 and CdiIEC536. CdiA-CTEC536 Trp176 binds into a hydrophobic pocket formed by CdiIEC536.
Table S3.
Hydrogen bonds and salt bridges between CdiA-CTEC536 and CysK in the ternary complex
| CdiA-CTEC536 | CysK | Distance, Å |
| LYS 161 [NZ] | THR 95 [O] | 2.71 |
| GLY 165 [O] | ALA 310 [N] | 2.76 |
| LYS 166 [NZ] | SER308 [OG] | 3.40 |
| GLN 183 [NE2] | PRO 224 [O] | 3.90 |
| GLN 183 [NE2] | ASP 207 [OD2] | 3.69 |
| ARG 190 [NH2] | PRO 222 [O] | 2.70 |
| SER 220 [O] | LYS 121 [N] | 3.12 |
| ALA 221 [O] | MET 120 [N] | 3.12 |
| GLY 224 [N] | ALA 232 [O] | 2.92 |
| TYR 225 [OH] | THR95 [OG1] | 2.86 |
| GLY 226 [N] | SER 70 [OG] | 2.65 |
| ILE 227 [O] | THR 69 [OG1] | 2.86 |
| ILE 227 [O] | GLN 143 [NE2] | 3.26 |
| ILE 227 [OXT] | THR 73 [N] | 3.57 |
| ILE 227 [OXT] | ASN 72 [N] | 3.57 |
Table S4.
DALI server search results
| Search input | Structural homolog | Sequence identity, % | PDB ID code | Z-score | rmsd, Å |
| CdiA-CTEC536 | Importin subunit alpha-1 | 7 | 4ZDU | 6.5 | 4.1 (108/416)* |
| Unc-45 | 11 | 3NOW | 6.3 | 2.6 (85/768) | |
| Gastric intrinsic factor | 13 | 2PMV | 6.3 | 4.4 (92/267) | |
| Beta-catenin | 7 | 4EV9 | 6.3 | 4.0 (108/492) | |
| Plakoglobin | 11 | 3IFG | 6.3 | 2.5 (79/546) | |
| Histone binding protein N1/N2 | 7 | 1PJN | 6.2 | 3.2 (105/427) | |
| CdiIEC536 | BAG family chaperone regulator 4 | 8 | 4HWH | 8.3 | 3.2 (79/84) |
| Syntaxin 6 | 9 | 1LVF | 8.3 | 3.0 (86/104) | |
| Heat shock 70 kDa protein 1 | 8 | 3A8Y | 7.5 | 2.8 (76/100) | |
| Spastin | 6 | 3EAB | 7.5 | 3.5 (83/88) | |
| Diaphanous homolog 1 | 5 | 3OBV | 7.2 | 3.5 (81/419) | |
| Stata protein | 7 | 1UUR | 7.2 | 3.2 (86/461) |
rmsd, root-mean-square deviation.
Aligned over number of α-carbons out of number of residues.
Table S5.
Hydrogen bonds and salt bridges between CdiA-CTEC536 and CdiIEC536 in the ternary complex
| CdiA-CTEC536 | CdiIEC536 | Distance, Å |
| LYS 170 [NZ] | GLN 49 [O] | 2.81 |
| GLU 171 [N] | ILE 11 [O] | 2.64 |
| GLU 171 [OE2] | THR 14 [N] | 3.70 |
| ASP 177 [N] | GLN 49 [OE1] | 3.00 |
| ASP 177 [O] | GLN 49 [NE2] | 2.89 |
| HIS 178 [NE2] | SER 21 [OG] | 3.40 |
| GLU 181 [OE1] | ARG 45 [NH1] | 3.03 |
| GLU 181 [OE1] | ARG 48 [NE] | 3.57 |
| GLU 181 [OE1] | ARG 48 [NH2] | 3.01 |
| GLU 181 [OE2] | GLN49 [NE2] | 2.90 |
| GLU 181 [OE2] | ARG 48 [NE] | 3.06 |
| GLU 181 [OE2] | ARG 48 [NH2] | 3.79 |
| ASN 184 [ND2] | GLU 77 [OE2] | 2.93 |
| ASN 184 [ND2] | TYR 74 [OH] | 2.77 |
| HIS 192 [NE2] | TYR 73 [O] | 2.80 |
Fig. S2.
Alignments of Ntox28 family members and their cognate immunity proteins. (A) Ntox28 domain sequences were aligned using Clustal omega. Secondary structure elements and residue numbers correspond to the CdiA-CTEC536 nuclease domain. Sequences are from E. coli 536 (WP_000554175), Enterobacter cloacae B2 (WP_046887392), Trabulsiella guamensis ATCC 49490 (WP_038156365), Yersinia enterocolitica (CFB70688), Pluralibacter gergoviae FB2 (WP_043081994), Pseudomonas syringae UB246 (WP_049826804), Pseudomonas sp. GM79 (WP_008076692), Ruminococcus lactaris ATCC 29176 (EDY33923), Paenibacillus sp. Y412MC10 (ACX63382), Clostridium methoxybenzovorans SR3 (WP_024345457), and Paenibacillus lactis 154 (EHB62722). (B) Predicted Imm28 immunity protein sequences were aligned using Clustal omega. Secondary structure elements correspond to CdiIEC536. Sequences are from E. coli 536 (WP_000631802), E. cloacae B2 (WP_046887372), T. guamensis ATCC 49490 (WP_038156368), Y. enterocolitica (CFB70689), P. gergoviae FB2 (WP_043081995), P. syringae UB246 (unannotated), Pseudomonas sp. GM79 (WP_050549280), R. lactaris ATCC 29176 (WP_005609771), Paenibacillus sp. Y412MC10 (ACX63383), C. methoxybenzovorans SR3 (WP_024345456), and P. lactis 154 (EHB62723). Yellow boxes indicate residues that mediate hydrophobic interactions between CdiA-CTEC536 and CdiIEC536. Green boxes indicate residues involved in H-bonds or ion-pair interactions between CdiA-CTEC536 and CdiIEC536. The R. lactaris toxin and immunity sequences are boxed in red.
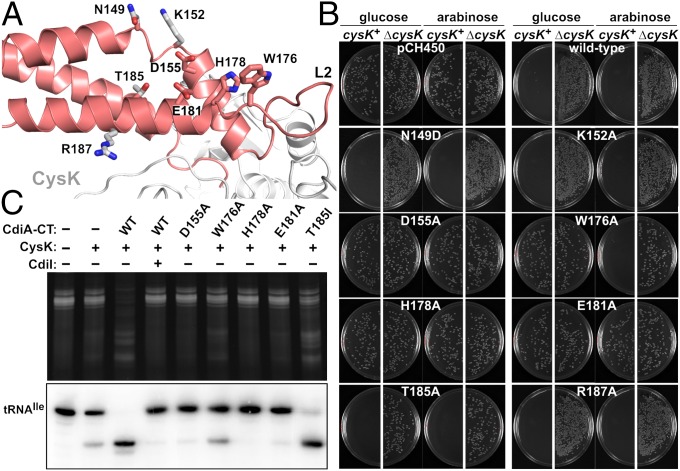
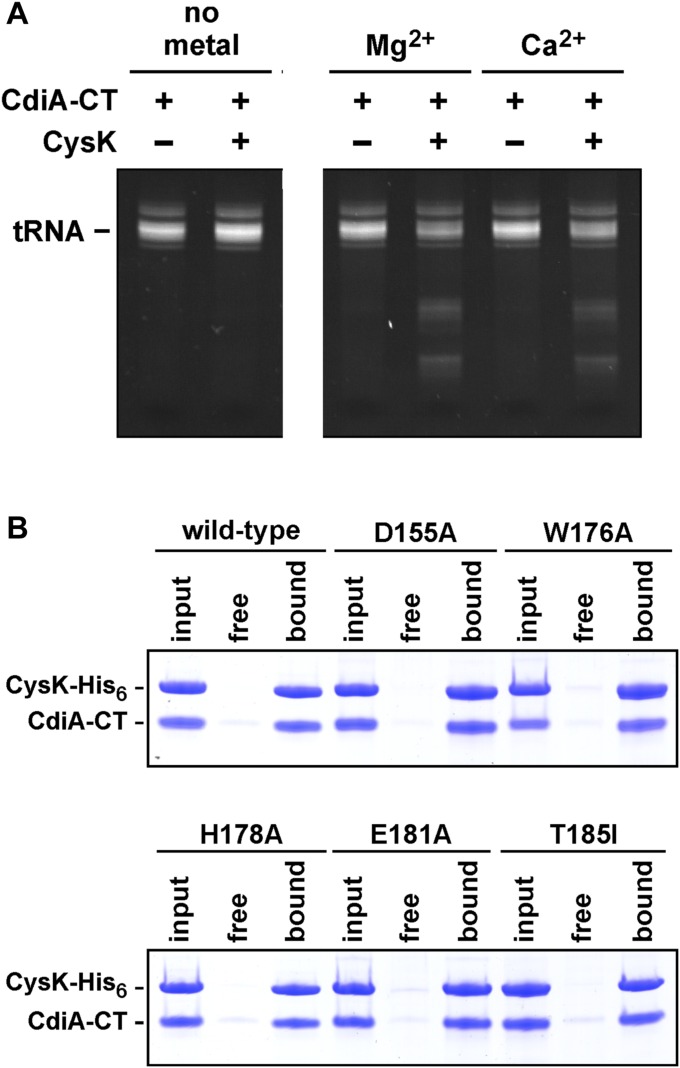
A DALI search indicates that the CdiA-CTEC536 nuclease domain does not share structural homology with other known RNases (Table S4). The domain also lacks an obvious tRNA-binding pocket, but previous work suggests that His178 is an active-site residue (Fig. 4A and Fig. S2A) (8). Therefore, we used site-directed mutagenesis to probe residues in the vicinity of His178 for roles in growth inhibition. CdiA-CTEC536 expression plasmids were introduced into E. coli cysK+ and ∆cysK strains, and transformants were selected on media supplemented with either glucose to suppress, or arabinose to induce, toxin expression. The WT construct was lethal when introduced into cysK+ cells under any condition but had no effect on ∆cysK cell growth even when induced with arabinose (Fig. 4B). This result illustrates cysK-dependent toxicity for comparison with mutated CdiA-CTEC536 variants. CdiA-CTEC536 residues Asp155 and Glu181 cluster near His178 (Fig. 4A) and are completely conserved in known Ntox28 domains (Fig. S2A), suggesting functional importance. Asp155Ala and Glu181Ala mutations abolished growth inhibition activity, showing the same phenotype as the His178Ala mutation (Fig. 4B). Thr185 is also positioned near His178, but mutation of this residue had no discernible effect on toxicity (Fig. 4B). Asn149, Lys152, and Arg187 were tested for potential contributions to tRNA binding, but mutations at these positions also had no effect on growth inhibition (Fig. 4B). Lastly, we examined loop L2 residue Trp176, which is located at the junction with helix α3 near the putative catalytic triad of Asp155, His178, and Glu181 (Fig. 4A). Aromatic residues are conserved at this position in Ntox28 domains (Fig. S2A), suggesting that Trp176 may contribute to tRNA substrate recognition. The Trp176Ala expression plasmid was maintained stably in cysK+ cells under repressive conditions, but cell growth was inhibited upon induction with arabinose (Fig. 4B). In vitro nuclease assays with purified toxins showed that the Asp155Ala, His178Ala, and Glu181Ala mutations each blocked activity (Fig. 4C). Additionally, we found that CdiA-CTEC536 anticodon nuclease activity requires divalent cations (Fig. S3A), suggesting that Asp155 and Glu181 may contribute to catalysis by coordinating Mg2+. The Thr185Ile mutation had a minor effect, but the activity of the Trp176Ala variant was significantly attenuated (Fig. 4C). To exclude the possibility that the mutations interfere with CysK binding, we confirmed that each CdiA-CTEC536 variant interacts with CysK-His6 using Ni2+-affinity copurification (Fig. S3B). Together, these results suggest that Asp155, His178, and Glu181 form a catalytic triad required for tRNase activity.
Fig. 4.
Identification of nuclease active-site residues. (A) Mutated residues of CdiA-CTEC536. (B) Growth inhibition activity of CdiA-CTEC536 variants. Arabinose-inducible expression plasmids were introduced into E. coli cysK+ and ∆cysK cells, and transformants were selected on media supplemented with glucose or arabinose. Plasmid pCH450 is the empty vector. (C) In vitro nuclease activity of CdiA-CTEC536 variants. Purified CdiA-CTEC536 proteins were incubated with total cellular RNA in the presence of CysK and CdiIEC536 where indicated. Reactions were run on denaturing polyacrylamide gels and visualized by ethidium bromide staining (Top) and Northern blot hybridization (Bottom).
Fig. S3.
CdiA-CTEC536 anticodon nuclease activity is metal-dependent, and the CdiA-CTEC536 variants interact stably with CysK. (A) Total E. coli RNA was incubated with purified CdiA-CTEC536 and CysK. Reactions were supplemented with 1 mM MgCl2 or CaCl2 where indicated. Reactions were analyzed by denaturing PAGE and ethidium bromide staining. (B) Purified CdiA-CTEC536 toxins were mixed with CysK-His6 (input lanes) and subjected to Ni2+-affinity chromatography. Proteins that did not bind the Ni2+-NTA agarose matrix are shown in free lanes, and the bound lanes contain proteins eluted with imidazole.
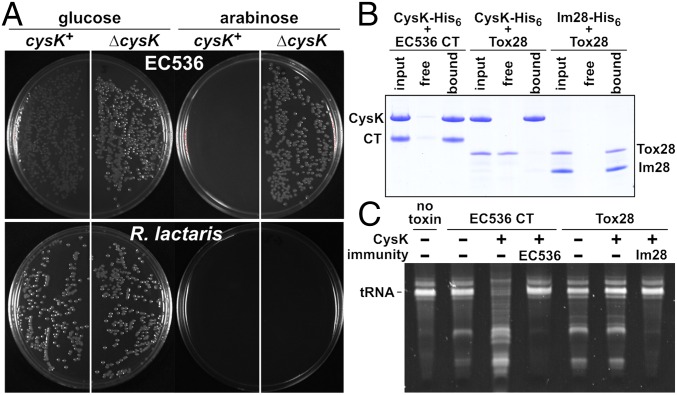
Several CdiA proteins carry Ntox28 domains, and these toxins are also found at the C terminus of Tyr-Asp-repeat and WXG100 proteins from Gram-positive bacteria (4). Gram-positive Ntox28 domains have insertions in loop L3 and lack C-terminal G(Y/H)GI sequences (Fig. S2A), suggesting that they do not interact with CysK. We tested this prediction using the Ntox28/immunity protein pair encoded by the RUMLAC_00243/00244 loci of R. lactaris ATCC 29176. We first confirmed that Tox28Rlac inhibits cell growth using controlled proteolysis to degrade ssrA(DAS)-tagged ImmRlac immunity protein, thereby liberating the toxin domain inside E. coli cells. Tox28Rlac inhibited the growth of both cysK+ and ∆cysK cells whereas expression of CdiA-CTEC536 using the same approach had no inhibitory effect on ∆cysK cells (Fig. 5A). We also found that CysK does not bind to Tox28Rlac with high affinity although the toxin forms a stable complex with the ImmRlac immunity protein (Fig. 5B). In vitro nuclease assays revealed that purified Tox28Rlac cleaves tRNA, but, in contrast to CdiA-CTEC536, the addition of CysK failed to stimulate nuclease activity (Fig. 5C). Together, these results demonstrate that Tox28Rlac shares tRNase activity with CdiA-CTEC536 but does not require activation by CysK or other proteins.
Fig. 5.
R. lactaris Tox28 is a CysK-independent tRNase. (A) CdiA-CT/CdiIEC536-DAS and Tox/ImmRlac-DAS expression constructs were introduced into E. coli cysK+ and ∆cysK cells, and transformants were selected on media supplemented with glucose or arabinose. (B) Protein binding assays. The indicated proteins were mixed and subjected to Ni2+-affinity chromatography. The free lanes represent proteins that failed to bind the affinity matrix, and the bound lanes show proteins eluted with imidazole. (C) In vitro nuclease assays. Purified CdiA-CTEC536 or Tox28Rlac was incubated with total cellular RNA. Reactions were supplemented with purified CysK, CdiIEC536, or ImmRlac where indicated.
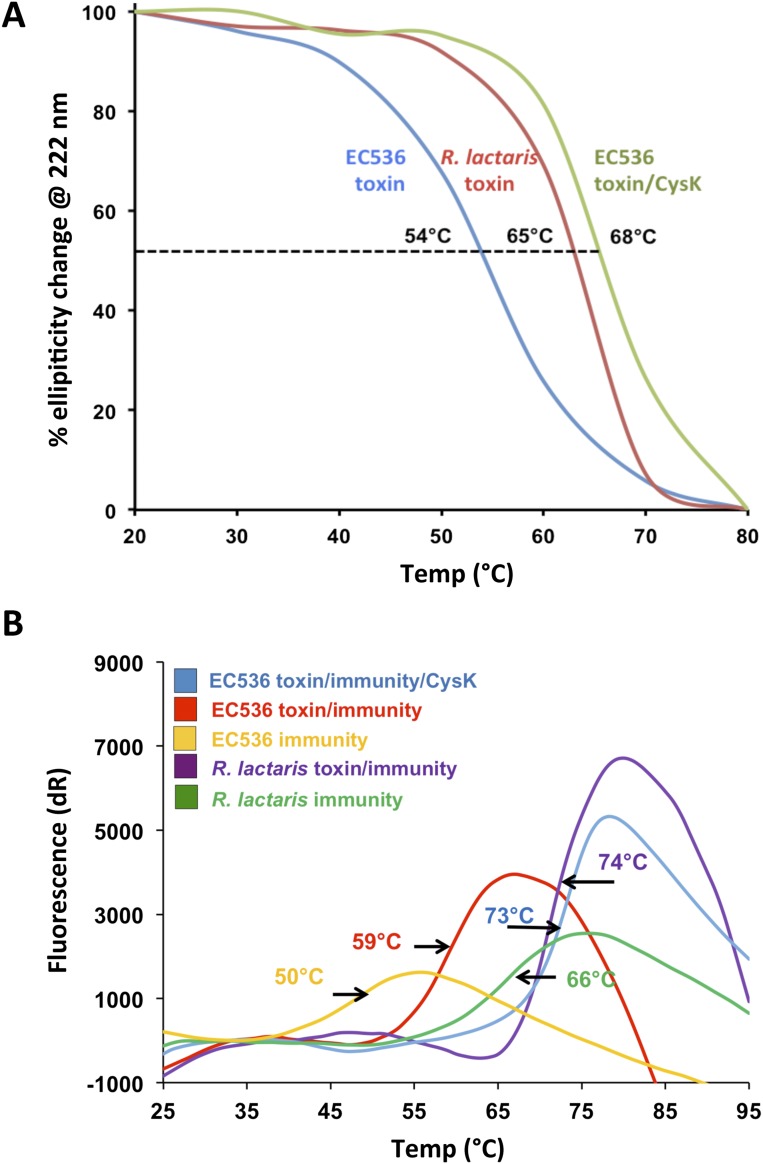
The autonomy of the Tox28Rlac domain raises the question of why CdiA-CTEC536 requires activation. Because the CdiA-CTEC536 nuclease domain is small and lacks an extensive hydrophobic core, we explored the possibility that CysK stabilizes the toxin fold. We first monitored the stability of each toxin to thermal denaturation using circular dichroism (CD) spectroscopy. These analyses revealed that the melting temperature (Tm) for Tox28Rlac is ∼10 °C higher than that of the CdiA-CTEC536 toxin (Table S6 and Fig. S4A). We then examined the thermostability of the CysK/CdiA-CTEC536 complex using both CD spectroscopy and differential scanning fluorimetry (DSF). Both experimental approaches showed that the CysK/CdiA-CTEC536 complex has a Tm of ∼66 °C, which is very similar to the value obtained for Tox28Rlac (Table S6 and Fig. S4A). The Tm for isolated CysK was ∼58 °C (Table S6), showing that the complex is more stable than the individual components. We also found that the CdiIEC536 and ImmRlac immunity proteins have a significant stabilizing effect, effectively increasing the Tm for each toxin (Table S6 and Fig. S4B). These data indicate that CdiA-CTEC536 is intrinsically less stable than Tox28Rlac but exhibits comparable thermostability when bound to CysK.
Table S6.
Melting temperatures of protein complexes determined by CD spectroscopy and differential scanning fluorimetry (DSF)
| Protein/complex | Tm, °C | |
| CD | DSF | |
| CdiA-CTEC536 | 54 ± 1 | ND |
| Tox28Rlac | 65 ± 2 | ND |
| CdiIEC536 | ND | 50 ± 2 |
| ImmRlac | ND | 66 ± 1 |
| CysK | ND | 58 ± 1 |
| CysK/CdiA-CTEC536 | 68 ± 2 | 64 ± 2 |
| CdiA-CT/CdiIEC536 | 62 ± 2 | 58 ± 2 |
| CysK/CdiA-CT/CdiIEC536 | ND | 73 ± 3 |
| Tox28/ImmRlac | 73 ± 1 | 74 ± 2 |
ND, not determined.
Fig. S4.
Thermal stability of Ntox28 toxins. (A) Thermal stability of toxin domains determined by circular dichroism (CD) spectroscopy. The fraction of secondary structure element content was calculated and used to determine melting temperatures (Tm). (B) Differential scanning fluorimetry (DSF) of toxin/immunity protein complexes.
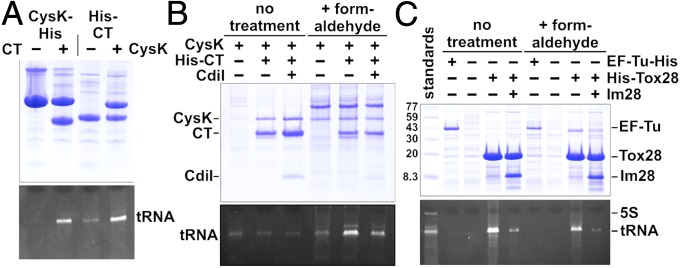
Finally, we tested whether CysK promotes the binding of tRNA substrates to CdiA-CTEC536. We expressed His6 epitope-tagged CdiA-CT(H178A)EC536 and/or CysK in E. coli and then treated the cell lysates with formaldehyde to cross-link tRNA to the proteins. Analysis of complexes isolated from these reactions revealed that tRNA was preferentially cross-linked when both CysK and CdiA-CTEC536 were present in the lysates (Fig. 6A). This interaction appears to reflect physiologically relevant substrate binding because coexpression of CdiIEC536 reduced tRNA cross-linking to the CysK/CdiA-CTEC536 complex (Fig. 6B). This latter result suggests that immunity protein blocks nuclease activity by occluding the tRNA-binding site. Because Tox28Rlac is an autonomous tRNase, we predicted that it should exhibit intrinsic tRNA-binding activity. We generated a His6-tagged version of Tox28Rlac that contains the His114Ala mutation to ablate nuclease activity and tested whether it binds substrate. Substantial amounts of tRNA copurified with the inactive Tox28Rlac domain, even without formaldehyde cross-linking (Fig. 6C). The interaction between tRNA and Tox28Rlac was effectively blocked by cognate ImmRlac immunity protein (Fig. 6C), again suggesting that the immunity protein binds over the nuclease active site. Together, these results indicate that CysK stabilizes CdiA-CTEC536, rendering the nuclease domain competent to bind substrate. By contrast, the Tox28Rlac domain is intrinsically stable and binds tRNA without assistance from CysK.
Fig. 6.
tRNA binding to Ntox28 nuclease domains. (A) Cross-linking of tRNA to CysK/CdiA-CTEC536 complexes. E. coli cell lysates containing CysK and/or CdiA-CT(H178A)EC536 were treated with formaldehyde, and cross-linked nucleoprotein complexes were purified by Ni2+-affinity chromatography. Purified samples were analyzed by SDS/PAGE (Top) and 50% urea PAGE (Bottom) to visualize proteins and nucleic acid, respectively. (B) CdiIEC536 blocks tRNA cross-linking to CysK/CdiA-CTEC536. E. coli cell lysates containing CysK-His6, CysK-His6/CdiA-CT(H178A)EC536, and CysK-His6/CdiA-CT(H178A)/CdiIEC536 were treated with formaldehyde where indicated, followed by Ni2+-affinity chromatography, and gel analysis as described in A. (C) Tox28Rlac interacts stably with tRNA substrate. Cell lysates containing His6-Tox28(H114A)Rlac and ImmRlac were treated as described above. To ascertain cross-linking specificity, EF-Tu-His6 was also purified and analyzed for tRNA binding.
Discussion
These results provide several insights into CdiA-CTEC536 toxin activation. As predicted from prior work (8), the crystal structures show that the toxin inserts its C-terminal GYGI tail into the CysK active site and anchors the complex. The toxin's C-terminal carboxylate forms important H-bond contacts with conserved CysK active-site residues in the substrate-binding loop, and these same interactions are observed in the structures of CysE peptides bound to CysK from Haemophilus influenzae and Arabidopsis thaliana (21, 22). The C-terminal Ile residues of CdiA-CTEC536 and CysE exploit the same hydrophobic and H-bond contacts as O-acetylserine to bind the CysK active site (19). Although C-terminal Ile residues are critical for binding, substitutions are tolerated at other positions within the peptide tail. Salsi et al. have shown that CysK binds CysE peptides altered at the penultimate and antepenultimate positions (13). This plasticity accommodates the natural variation in CysE tail sequences and accounts for the ability of E. coli CysK to bind both GDGI and GYGI motifs with high affinity. Structures are not available for the full cysteine synthase complex; thus, it is unclear whether CdiA-CTEC536 mimics other features of CysE. However, we note that the CysE structure differs markedly from CdiA-CTEC536. The CysE C-terminal domain forms a β-helix that terminates in a flexible tail (25). Further, the β-helical domain mediates trimerization, and two CysE trimers interact to form a larger homohexameric complex (25). If the β-helical domains of CysE interact with CysK, then the contacts are likely to be distinct from those observed with the CdiA-CTEC536 α-helical bundle. The same uncertainties apply to other “moonlighting” partners of CysK for which no structural information is available (15). It is intriguing that CysK has been repeatedly recruited as a binding partner by disparate proteins. Perhaps this phenomenon reflects the antiquity and immutability of cysteine synthase complexes, which remain remarkably similar in extant bacteria and plants. From the perspective of CDI, the ubiquity and conserved active-site architecture of CysK ensure that toxins can be activated in a broad range of bacteria.
The structure of the CdiA-CTEC536 nuclease domain is unique, and its catalytic mechanism seems to be distinct from other anticodon nucleases. Colicins E5 and D are the only other anticodon nucleases for which structures are available (26). The nuclease domains of colicins E5 and D share an α/β fold that characterizes the Barnase-EndoU-ColicinE5/D-RelE (BECR) family of RNases (4). Like barnase, colicins E5 and D are metal-independent nucleases that abstract a proton from the 2´-hydroxyl to initiate an intramolecular attack on the scissile phosphodiester bond (26). By contrast, CdiA-CTEC536 has divalent cation-dependent nuclease activity, which is usually associated with a hydrolytic mechanism. Mutational analyses support a role for CdiA-CTEC536 residues Asp155, Glu181, and His178 in catalysis. Asp155 and Glu181 are candidates to coordinate Mg2+, which could either activate water for hydrolysis or stabilize hydroxide ions generated by His178 acting as a general base (27). Another difference between colicins E5/D and CdiA-CTEC536 is the lack of a defined substrate-binding pocket in the latter nuclease. Several observations suggest that flexible loop L2 participates in tRNA binding. L2 is conserved among Ntox28 family members and always contains hydrophobic residues adjacent to His178. Our results show that Trp176 is important, but not strictly required, for CdiA-CTEC536 activity. We hypothesize that Trp176 stacks onto nucleobases within the tRNA anticodon loop. This mode of recognition is common among aminoacyl-tRNA synthetases, which bind cognate tRNAs using conserved hydrophobic/aromatic residues that stack onto the first and second nucleotides of the anticodon (28).
Finally, we propose that CysK promotes CdiA-CTEC536 nuclease activity by stabilizing the toxin's fold. The CdiA-CTEC536 helical bundle is relatively small and lacks an extensive hydrophobic core. Consequently, CdiA-CTEC536 has relatively low thermostability, raising the possibility that thermal fluctuations disrupt the active site by splaying the helices. CysK anchors toxin helices α2 and α3, thereby approximating Asp155 and Glu181 to coordinate Mg2+. The CysK scaffold also anchors the ends of loop L2, which we propose is important for substrate binding. In support of this model, we find that tRNA interacts with the CysK/CdiA-CTEC536 complex, but not with the individual components. CdiIEC536 blocks the interaction with substrate, strongly suggesting that the immunity protein occludes the nuclease active site. The extensive contact between loop L2 and CdiIEC536, together with the sequestration of Trp176 within the immunity protein, is consistent with this model. Although CysK is critical for CdiA-CTEC536 nuclease activity, related toxins from Gram-positive bacteria probably do not require extrinsic activation because purified Tox28 domain from R. lactaris has tRNase activity in vitro. Ntox28 domains are typically found at the C terminus of proteins that mediate interbacterial competition (4, 29–31). For example, the R. lactaris Tox28Rlac domain is part of a larger rearrangement hotspot (Rhs) repeat protein. Rhs and related YD-repeat proteins deliver toxic nuclease domains into both Gram-negative and Gram-positive bacteria (32, 33). Further, the Ntox28 homolog from Geobacillus sp. strain Y412MC10 (GYMC10_1092) is linked to an N-terminal ESAT-6–like domain, which is predicted to guide export through type VII secretion systems (4, 29). Given that all Ntox28 domains function in intercellular competition, perhaps the mechanism of toxin delivery into Gram-negative bacteria accounts for the relative instability of CdiA-CTEC536. We recently discovered that CDI toxins hijack a variety of inner-membrane proteins to enter the target-cell cytoplasm (9). If CdiA-CT domains must unfold during this translocation step, then there may be a selective pressure for toxins with low global stability.
Methods
Plasmid constructions (Table S7), site-directed mutagenesis, and protein purification procedures are described in SI Methods (8). Protein crystallization was previously described (6). Briefly, crystals were grown by the hanging-drop, vapor-diffusion method at room temperature against a reservoir containing 0.2 M NaSO4, 0.1 M Bis-Tris propane (pH 7.9), and 20% (wt/vol) PEG 3350, for the binary complex, and 0.1 M sodium cacodylate (pH 7.1), 0.2 M ammonium sulfate, and 17% (wt/vol) PEG-8000, for the ternary complex. Structural models were determined as described (6). Protein binding affinities were calculated using biolayer interferometry, and thermal stabilities were determined by DSF and CD spectroscopy as outlined in SI Methods. Nuclease activity assays were performed as described (8) with modifications outlined in SI Methods. Nucleoprotein cross-linking was performed as described in SI Methods.
Table S7.
Bacterial strains and plasmids
| Strain or plasmid | Description | Source |
| Strains | ||
| BL21-Gold(DE3) | E. coli B, F– ompT hsdS(rB– mB–) dcm+ gal λ(DE3) endA Hte, TetR | Stratagene |
| X90 | F´ lacIq lac´ pro´/ara ∆(lac-pro) nal1 argE(amb) rifr thi-1, RifR | (39) |
| CH1944 | X90 (DE3) ∆rna, RifR | (38) |
| CH2016 | X90 (DE3) ∆rna ∆slyD::kan, RifR KanR | (38) |
| CH7157 | X90 ∆clpX ∆clpA::kan, RifR KanR | (31) |
| CH8164 | X90 ∆cysK::kan, RifR KanR | (8) |
| CH8602 | X90 ∆cysK, RifR | (8) |
| CH8804 | X90 (DE3) ∆rna ∆slyD ∆cysK::kan, RifR KanR | This study |
| CH9648 | X90 (DE3) ∆rna ∆slyD ∆tufB tufA-his6-kan, RifR KanR | This study |
| CH10013 | Spontaneous rifampicin-resistant derivative of E. coli JCM158, RifR | (5) |
| CH10801 | CH10013 ∆cysK, RifR | This study |
| CH11002 | CH8804 pCH11014 pCH13129, RifR KanR TetR AmpR | This study |
| CH12810 | CH8804 pCH8649 pET21, RifR KanR TetR AmpR | This study |
| CH12811 | CH8804 pCH8649 pCH8501, RifR KanR TetR AmpR | This study |
| CH12812 | CH8804 pCH12817 pET21, RifR KanR TetR AmpR | This study |
| CH12813 | CH8804 pCH12817 pCH11027, RifR KanR TetR AmpR | This study |
| CH13101 | CH8804 pCH11014 pET21, RifR KanR TetR AmpR | This study |
| CH13102 | CH8804 pCH11014 pCH12515, RifR KanR TetR AmpR | This study |
| Plasmids | ||
| pTrc99a | IPTG-inducible expression plasmid, AmpR | GE Healthcare |
| pCH450 | pACYC184 derivative containing E. coli araC and the l-arabinose–inducible Para promoter, TetR | (38) |
| pET21 | T7 RNA polymerase-based overexpression vector, AmpR | (3) |
| pCH6190 | pET21::cdiA-CT/cdiIEC536, overproduces CdiA-CTEC536 and CdiIEC536-His6, AmpR | (3) |
| pCH6316 | pTrc99A::cdiA-CT/cdiIEC536(DAS), AmpR | (30) |
| pCH6938 | pET21::cdiA-CT(H178A)/cdiIEC536, overproduces CdiA-CT(H178A)EC536 and CdiIEC536-His6, AmpR | (8) |
| pCH7086 | pCH450::cdiA-CT(H178A)EC536, TetR | (8) |
| pCH7171 | pCH450::cdiA-CT/cdiIEC536(DAS), TetR | This study |
| pCH8215 | pET21::cysK(S2G), overproduces CysK-His6, AmpR | (8) |
| pCH8501 | pET21::cdiA-CT(H178A)EC536, AmpR | This study |
| pCH8649 | pCH450::cysK(S2G)-his6, TetR | (8) |
| pCH9320 | pCH450::cdiA-CTEC536, TetR | (8) |
| pCH9622 | pKAN-tufA(his6), AmpR KanR | This study |
| pCH10978 | pET21::cdiA-CT(D155A)/cdiIEC536, AmpR | This study |
| pCH10980 | pET21::cdiA-CT(W176A)/cdiIEC536, AmpR | This study |
| pCH10982 | pET21::cdiA-CT(E181A)/cdiIEC536, AmpR | This study |
| pCH11022 | pET21::cdiA-CT(T185I)/cdiIEC536, overproduces CdiA-CT(T185I) and CdiIEC536-His6, AmpR | This study |
| pCH11014 | pCH450::cysK(S2G), expresses untagged CysK, TetR | This study |
| pCH11016 | pCH450::cdiA-CT(D155A)EC536, TetR | This study |
| pCH11017 | pCH450::cdiA-CT(W176A)EC536, TetR | This study |
| pCH11019 | pCH450::cdiA-CT(E181A)EC536, TetR | This study |
| pCH11020 | pCH450::cdiA-CT(T185A)EC536, TetR | This study |
| pCH11022 | pET21P::cdiA-CT(T185A)/cdiIEC536, AmpR | This study |
| pCH11027 | pET21::cysK(S2G), overproduces untagged CysK, AmpR | This study |
| pCH12305 | pET21::tox/immRlac, overproduces Ntox28Rlac and ImmRlac-His6, AmpR | This study |
| pCH12371 | pCH450::cdiA-CT(N149D)EC536, TetR | This study |
| pCH12372 | pCH450::cdiA-CT(K152A)EC536, TetR | This study |
| pCH12373 | pCH450::cdiA-CT(R187A)EC536, TetR | This study |
| pCH12334 | pCH450::tox/immRlac(DAS), TetR | This study |
| pCH12515 | pET21::his6-cdiA-CT(H178A)/cdiIEC536; overproduces His6-CdiA-CT(H178A)EC536 and CdiIEC536, AmpR | This study |
| pCH12817 | pCH450::his6-cdiA-CT(H178A)EC536, TetR | This study |
| pCH13103 | pET21::his6-tox/immRlac, overproduces His6-Ntox28Rlac and ImmRlac, AmpR | This study |
| pCH13129 | pET21::his6-cdiA-CT(H178A)EC536, overproduces His6-CdiA-CTEC536, AmpR | This study |
| pCH13146 | pET21::his6-tox(H114A)/immRlac, overproduces His6-Ntox28(H114A)Rlac and ImmRlac, AmpR | This study |
| pCH13153 | pET21::his6-tox(H114A)Rlac, overproduces His6-Ntox28(H114A)Rlac, AmpR | This study |
AmpR, ampicillin-resistant; CmR, chloramphenicol-resistant; KanR, kanamycin-resistant; RifR, rifampicin-resistant; TetR, tetracycline-resistant.
SI Methods
Plasmid Constructs.
Plasmids used in this study are presented in Table S7. T7 RNA polymerase-based overexpression plasmids to produce CdiA-CT/CdiIEC536-His6 (pCH6190) and CysK-His6 (pCH8215) have been described (3, 8). The Escherichia coli cysK gene was amplified with primers CysK-S2G-Nco (5′-GGC CAT GGG TAA GAT TTT TGA AGA TAA CTC) and CysK-Xho-rev (5′-AAA CTC GAG TTA ATA ACG CTC ACC CGA TGA). The product was digested with NcoI/XhoI and ligated to pET21P and pCH450 to generate plasmids pCH11027 and pCH11014, respectively. These latter constructs produced untagged CysK. The cdiA-CT(H178A)EC536 coding sequence was amplified from pCH6938 (8) with primers 536-Nco-H6-Spe (5′-TTC CAT GGC AAA AAG TCA TCA TCA TCA TCA CCA CAC TAG TGT TGA GAA TAA TGC GCT GAG) and 536-CT-Xho-rev (5′-TTA CTC GAG GTA ATC ATA TTC CAT A). The product was digested with NcoI/XhoI and ligated to pET21 and pCH450 to generate His6-CdiA-CT(H178A)EC536 overproduction plasmids pCH11001 and pCH12817, respectively. Plasmid pCH6938 was also amplified with primers 536-Nco-H6-Spe and 536-cdiI-Xho-rev (5′-GGC CTC GAG TAG TTA TAC AAT TAT CTG), and the product was ligated to pET21 to generate a construct that overproduces His6-CdiA-CT(H178A) and CdiIEC536 (pCH12515). The cdiA-CT/cdiIEC536(DAS) module was excised from plasmid pCH6316 (30) with NcoI/PstI and ligated to pCH450 to generate plasmid pCH7171. His6-CdiA-CT(H178)EC536 and His6-CysK proteins with thrombin-cleavable His6 epitope tags were used for biolayer interferometry studies. The cysK gene from plasmid pCH11014 was amplified with primers CysK-Nde-for (5′-GAT CAT ATG ATG GGT AAG ATT TTT GAA GAT AAC TCG CTG ACT AT) and CysK-EcoR-rev (5′-GAT GAA TTC TCA CTC GAG ACT AGT CTG TTG CAA TTC TTT); and the cdiA-CT(H178)EC536 gene from plasmid pCH11001 was amplified with primers 536-Nde-for (5′-GAT CAT ATG ATG ACT AGT GTT GAG AAT AAT GCG CTG AGT) and 536-EcoR-rev (5′-GAT GAA TTC TCA TAT TCC ATA TCC TTT CAA GGC TGA TTC TAT TTT ATT AAT A). The resulting products were digested with NdeI/EcoRI and ligated to plasmid pET28a.
Missense mutations were introduced into the cdiA-CTEC536 coding sequence using megaprimer PCR (34). Megaprimers were amplified from template plasmid pCH6190 using 536-CT-Nco-for (5′-AGA CCA TGG TTG AGA ATA ATG CGC TGA G) in conjunction with mutagenic reverse primers 536-CT(D155A)-rev (5′-GAG AGT TCC AAT AAT AGC ATG ATC TTT CAG), 536-CT(W176A)-rev (5′-CCT GCA TAT GAT CCG CAT ATC CTC CAT TC), 536-CT(E181A)-rev (5′-GCG TAT TTT GCA TTG CCT GCA TAT GAT CC), 536-CT(T185A)-rev (5′-CTT AAT CCT CTG AGC GCA TTT TGC ATT TCC TGC), and 536-CT(T185I)-rev (5′-TTC TTA ATC CTC TGA GGA TAT TTT GCA TTT CCT GC). Megaprimers were then used in second reactions with 536-cdiI-Xho-rev (5′-GAT CTC GAG TAC AAT TAT CTG ATT GAT TTT T) to generate cdiA-CT/cdiIEC536 fragments for ligation to NcoI/XhoI-digested pET21. The resulting constructs were amplified with primers 536-CT-Nco-for and 536-CT-Xho-rev, and the products were ligated to pCH450 to generate arabinose-inducible CdiA-CTEC536 expression plasmids. Additional cdiA-CT mutations were made by megaprimer PCR using 536-CT-Xho-rev in conjunction with primers 536-N149D-for (5′-GAT AAC ACT ATA AAA GAT GCT CTG AAA GAT C), 536-K152A-for (5′-CTA TAA AAA ATG CTC TGG CAG ATC ATG ATA TTA T), and 536-R187A-for (5′-ATG CAA AAT ACG CTC GCA GGA TTA AGA AAT C). The resulting products were used with primer 536-CT-Nco-for to generate mutated cdiA-CTEC536 sequences for ligation into pCH450. All arabinose-inducible cdiA-CTEC536 expression constructs were initially cloned into E. coli X90 ∆cysK cells, which are resistant to CdiA-CTEC536 toxin activity (8).
The coding sequence for the Ruminococcus lactaris ATCC 29176 Ntox28 domain and immunity protein pair was custom synthesized by Integrated DNA Technologies. The synthetic DNA was digested with NcoI/SpeI and ligated to pET21P and pCH7171 to generate plasmids pCH12305 and pCH12334, respectively. pCH12305 overproduces the Ntox28/ImmRlac-His6 complex, and pCH12334 expresses Ntox28Rlac and ssrA(DAS)-tagged ImmRlac for in vivo toxicity studies. Plasmid pCH12305 was amplified with primers Rlac-his-tox-Nco-for (5′-TTT CCA TGG CAA AAA GTC ATC ATC ATC ATC ACC ACG AAA TAG CAA GCG TTG GTT CAT CC) and Rlac-imm-Xho-rev (5′-TTT CTC GAG ATC ACA TTA TTT TTT TGG ATA AAG TAT CTA TC), and the product was ligated to pET21 to generate plasmid pCH13103, which overproduces His6-Ntox28Rlac and ImmRlac. The His114Ala mutation was then introduced into the His6-Ntox28Rlac coding sequence using Rlac-H114A-for (5′-GTG GAT ATT TTG ATG CTT TAG GAG AAA TGC) and Rlac-imm-Xho-rev to generate a megaprimer, which was used with primer Rlac-his-tox-Nco-for to amplify the full toxin/immunity sequence. The final product was ligated to NcoI/XhoI-digested pET21 to generate pCH13146. Plasmid pCH13146 was amplified with primers Rlac-his-tox-Nco-for and Rlac-tox-Xho-rev (5′-TTT CTC GAG TCT TGC ATC AAT TTA TAC C), and the product was ligated to NcoI/XhoI-digested pET21 to generate the His6-Ntox28(H114A)Rlac overexpression construct pCH13153.
E. coli strain CH9648 was generated by Red-mediated recombination to introduce the coding sequence for a C-terminal His6 epitope to the tufA gene encoding EF-Tu. Homology regions were PCR-amplified using primer pairs tufA-Sac (5′-TCA GAG CTC TAC CTG TAC TGG CGT TG)/tufA(His6)-Bam (5′-TTT GGA TCC TTA ATG ATG GTG ATG ATG GTG GCC CAG AAC TTT AGC), and tufA-Eco (5′-GGG CGA ATT CCA CGT TAA TTA GTT TTG)/chiA-Hind (5′-CAT CAT AAG CTT TCG CTT TTC CCG). The resulting products were sequentially ligated into plasmid pKAN using SacI/BamHI and EcoRI/HindIII restriction sites to produce plasmid pCH9622. Plasmid pCH9622 was digested with SacI and HindIII, and the small fragment was electroporated into Red recombinase-expressing cells. Recombinants were selected on LB-agar supplemented with 50 µg/mL kanamycin.
Protein Expression and Purification.
CysK, His6-CdiA-CT(H178A)EC536, and the CdiA-CT/CdiIEC536-His6 complex were overproduced individually in E. coli BL21 (DE3) cells that carry pCH11027, pCH11001, and pCH6190 (respectively). Cells were grown aerobically at 37 °C in LB medium supplemented with 50 μg/mL ampicillin. Once the culture reached an optical density at 600 nm (OD600) of ∼0.8, protein production was induced with isopropyl-β-d-thiogalactosidase (IPTG) at 1 mM final concentration. Cultures were further incubated for 4 h, and then cells were collected by centrifugation and washed once with binding buffer [20 mM sodium phosphate (pH 7.0), 150 mM NaCl]. Cell pellets were resuspended in binding buffer and combined for the preparation of CysK/His6-CdiA-CT(H178A)EC536 binary and CysK/CdiA-CT/CdiIEC536-His6 ternary complexes. Mixed cell suspensions were supplemented with 10 mg/mL lysozyme and 1 mM phenylmethylsulfonyl fluoride (PMSF), and then the cells were disrupted by sonication on ice. Unbroken cells and debris were removed by centrifugation at 18,000 × g for 30 min, and the supernatant was passed through a 0.45-μm filter. Clarified lysates were applied to an Ni2+-charged HiTrap column and washed with binding buffer supplemented with 10 mM imidazole. Complexes were eluted with a linear gradient of imidazole (10–250 mM) in binding buffer. Fractions were collected, combined, and concentrated to ∼0.5 mL using a 10-kDa centrifugal concentrator. Concentrated samples were then passed over a Superdex 200 gel filtration column equilibrated with binding buffer. Protein complexes were concentrated to 20 mg/mL for crystallization trials. Selenomethionine (SeMet)-labeled proteins were produced in E. coli BL21 (DE3) Gold cells grown in M9 minimal medium supplemented with 50 mg/L leucine, isoleucine, and valine; 100 mg/L phenylalanine, lysine, and threonine; and 75 mg/L SeMet. The Ntox28/ImmRlac-His6 complex was overproduced as described for the CdiA-CT/CdiIEC536-His6 complex, but cells were induced with IPTG for 2 h. Because ImmRlac was produced in excess of the Ntox28Rlac toxin, concentrated samples were passed over a Superdex 200 gel filtration column equilibrated in binding buffer to separate Ntox28/ImmRlac-His6 complexes from free ImmRlac immunity protein.
Crystallization and Structure Determination.
CysK/His6-CdiA-CT(H178A)EC536 crystals were grown for 3 wk by hanging drop vapor-diffusion over a reservoir of 0.2 M NaSO4, 0.1 M Bis-Tris propane (pH 7.9), and 20% (wt/vol) PEG 3350. Hanging drops were prepared from a 1:1 mixture of 20 mg/mL protein to reservoir buffer. CysK/His6-CdiA-CT(H178A)EC536 crystallized in space group P41 with two complexes per asymmetric unit and dimensions of 64.01 Å × 64.01 Å × 365.37 Å. Crystals were mounted under cryo-conditions with the addition of 40% (vol/vol) glycerol as a cryoprotectant, and a dataset was collected at 70 K with wavelength 1.0 Å on beamline 7-1 at the Stanford Synchrotron Radiation Lightsource (SSRL). Images were indexed, integrated, and reduced using the HKL2000 suite (35), resulting in a 99.4% complete dataset up to 2.70 Å resolution. Initial phases were determined by molecular replacement with autoMR in PHENIX (36) using Salmonella Typhimurium CysK (PDB ID code 1OAS) as the search model (18). The initial Autobuild model contained only two CysK molecules, and therefore the CdiA-CTEC536 molecules were built through iterative manual building in Coot, followed by subsequent Autobuild cycles (36, 37). Phenix.refine (36) was used to refine the final model, which contains residues Lys127–Ile227 of CdiA-CTEC536 (numbered from Val1 of the Val-Glu-Asn-Asn peptide motif), residues Gly2–Ala314 for one chain, and Gly2–Ala315 for the other of CysK (where Asp314 and Leu315 are modeled as alanines), and 110 water molecules. Each CysK protomer contains pyridoxal 5′-phosphate (PLP) bound to Lys42 through Schiff-base linkage. CdiA-CTEC536 residues 168, 170–172, and 175 lack observable electron density and were modeled as alanine. The Ramachandran plot shows 97.08% and 2.92% of residues in the favorable allowed and allowed regions, respectively. Data collection and refinement statistics are presented in Table S1.
The CysK/CdiA-CT/CdiIEC536-His6 (where CdiA-CT/CdiIEC536-His6 is the SeMet derivative) ternary complex was crystallized by hanging drop-vapor diffusion over a reservoir containing 0.1 M sodium cacodylate (pH 7.1), 0.2 M ammonium sulfate, and 17% (wt/vol) PEG-8000. Hanging drops were prepared as a 1:1 mixture of 20 mg/mL protein to reservoir buffer. The complex crystallized in space group C2221 with unit cell dimensions as follows: 81.25 Å × 195.54 Å × 175.06 Å and two complexes per asymmetric unit. Crystals were collected by flash-freezing after soaking in a cryoprotectant solution containing a 1:1 mix of 40% glycerol (vol/vol) in crystallization buffer. A native dataset was collected at 70 K at 0.9795 Å on beamline 7-1 at SSRL. Data processing was conducted using the HKL2000 suite (35), resulting in a 100% complete dataset up to 2.75 Å resolution. Molecular replacement was performed with Phaser in PHENIX (36) using the CysK/CdiA-CT(H178A)EC536 complex structure as the search model. The initial Phaser-generated model was subjected to Autobuild and phenix.refine (36). The CdiIEC536 structure and final model were built through several cycles of manual building in Coot and structure refinement in phenix.refine. The final model includes residues Leu132–Ile227 of CdiA-CTEC536 and residues Gly2–Ala314 of CysK (where Asp314 is modeled as Ala), and CdiI includes residues Ile2–Ile127 of CdiIEC536 for one protomer and Ile2–Asn124 for the other. Each CysK protomer contains PLP bound to Lys42, as observed for the binary complex, and the final model includes 101 water molecules. The Ramachandran plots shows 95.83% of dihedral angles in favorable regions, 3.9% in allowed regions, and 0.27% (Asp111 of one CdiI protomer and Glu108 of both CdiI protomers) in the disallowed regions. Residues in the disallowed regions fit the electron density well. Data collection and refinement statistics are presented in Table S1. All molecular graphics were prepared using PyMol.
In Vitro Toxin-Binding and RNase Assays.
Purified complexes of toxins bound to His6-tagged immunity proteins were denatured in binding buffer supplemented with either 8 M urea or 6 M guanidine-HCl, and the toxins were isolated from the void volume during Ni2+-affinity chromatography (3). Toxins were refolded by dialysis against binding buffer. CysK-His6 and CdiIEC536-His6 were purified by Ni2+-affinity chromatography under nondenaturing conditions as described (7). ImmRlac-His6 was refolded by dialysis after Ni2+-affinity chromatography under denaturing conditions. All purified proteins were quantified by absorbance at 280 nm. Purified CdiA-CTEC536, CysK-His6, and CdiI-His6 were mixed at 10 µM final concentration in binding buffer, and protein–protein interactions were assessed by copurification during Ni2+-affinity chromatography as described (3, 7). Total RNA was isolated from E. coli X90 cells as described (38) and used as a substrate for in vitro nuclease assays. Total RNA (5 µg) was incubated with nuclease domains (1 µM) in reaction buffer [20 mM Tris⋅HCl (pH 7.5), 150 mM NaCl, and 1 mM MgCl2] for 1 h at 37 °C. Where indicated, tRNase reactions contained CysK and/or immunity proteins at 1 µM final concentration. Reactions were analyzed by denaturing electrophoresis on 50% urea–6% polyacrylamide gels in 1× Tris-borate-EDTA buffer. Gels were stained with ethidium bromide or transferred to a nylon membrane for Northern blot hybridization to 5′-radiolabeled oligonucleotide Ile1 probe, (5′-ACC GAC CTC ACC CTT ATC AG) as described (38).
In Vivo Toxicity Assays.
E. coli X90 and X90 ∆cysK cells were transformed with 100 ng of arabinose-inducible CdiA-CTEC536 expression plasmid expression constructs, followed by recovery for 1 h at 37 °C in LB media supplemented with 0.4% glucose. Stable transformants were selected on LB-agar supplemented with 12.5 µg/mL tetracycline and 0.4% d-glucose or l-arabinose. Ntox28Rlac toxicity was assessed using an arabinose-inducible construct (pCH12334) that produces Ntox28Rlac and ssrA(DAS)-tagged ImmRlac protein. Ntox28Rlac inhibition activity from this latter construct was compared with CysK-dependent toxicity of an analogous construct (pCH7171) that produces CdiA-CTEC536 and ssrA(DAS)-tagged CdiIEC536.
tRNA Cross-Linking.
To assess tRNA binding to CysK/CdiA-CTEC536 complexes, E. coli strains CH12810, CH12811, CH12812, and CH12813 were grown individually in 250 mL of LB media supplemented with 150 µg/mL ampicillin and 12.5 µg/mL tetracycline. Cultures were grown to midlog phase (OD600 ∼0.7) and then adjusted to 0.4% l-arabinose and 1 mM IPTG to induce CysK and CdiA-CT(H178A)EC536 production for 2.5 h. Cells were collected by centrifugation and resuspended in lysis buffer [20 mM Tris⋅HCl (pH 7.5), 150 mM NaCl, 1 mM MgCl2, and 0.01% Triton X-100]. Cells were broken by two passages through a French press at 18,000 psi, and unbroken cells were removed by centrifugation at 16,000 × g for 15 min. Clarified lysates were incubated with Ni2+-NTA agarose resin at 4 °C for 1 h and then treated with 0.1% formaldehyde for 10 min at ambient temperature. Reactions were quenched with l-glycine at 125 mM. The resins were washed with lysis buffer supplemented with 30 mM imidazole, and proteins were eluted in lysis buffer supplemented with 300 mM imidazole. Elutions were analyzed by SDS/PAGE to detect proteins and 50% urea-polyacrylamide gel electrophoresis and ethidium bromide staining to detect nucleic acids.
The effects of CdiIEC536 and ImmRlac immunity proteins on tRNA cross-linking were determined in a similar manner. E. coli strains CH11002 and CH13102 were grown individually in 150 mL of LB media supplemented with 150 µg/mL ampicillin, 12.5 µg/mL tetracycline, and 0.4% l-arabinose to induce CysK production. At midlog phase, the cultures were adjusted to 1 mM IPTG to induce the production of His6-CdiA-CT(H178A)EC536 or the His6-CdiA-CT(H178A)/CdiIEC536 complex for 30 min. For Ntox28/ImmRlac reactions, cultures of E. coli CH2016 carrying pET21, pCH13146, or pCH13153 were induced with 1.5 mM IPTG for 1 h, 30 min. E. coli strain CH9648, which expresses His6-tagged translation factor EF-Tu, was also tested as a specificity control. Cells were broken by French press as described above, and each lysate was preincubated with Ni2+-NTA agarose in 20 mM sodium phosphate (pH 8.0), 150 mM NaCl for 1 h at 4 °C. Each sample was split in two, and one-half was adjusted to 0.1% formaldehyde. After 10 min at ambient temperature, the reactions were quenched with 125 mM l-glycine. Resins were washed, and nucleoprotein complexes were eluted as described above.
Protein Thermal Stability Measurements.
Protein thermal stability was determined using differential scanning fluorimetry (DSF) and circular dichroism (CD) spectroscopy. For DSF measurements, proteins were incubated with 25 or 40 µM SYPRO orange dye in 20 mM sodium phosphate (pH 7.4), 150 mM NaCl. Samples were heated from 25 °C to 96 °C at 1 °C/min using an Mx3005P QPCR machine (Agilent Technologies). The dye was excited at 492 nm, and fluorescence emission was monitored at 610 nm. Melting curves were obtained in duplicate, and each experiment was conducted independently three times. Melting temperatures (Tms) were determined using nonlinear regression to determine melting-curve inflection points. Due to the high baseline fluorescence of SYPRO orange bound to CdiA-CTEC536 toxin at 25 °C, we were unable to determine Tm values for this toxin using DSF. Therefore, we monitored toxin thermal stability using a Jasco J-810 spectropolarimeter. CdiA-CTEC536 and Ntox28Rlac toxins concentrated to 0.5 mg/mL in 20 mM sodium phosphate (pH 7.4), and initial CD spectra (190–260 nm) were collected at 20 °C using a 1-mm cell to determine the secondary structure content. Proteins were heated from 20 °C to 80 °C, and ellipticity at 222 nm was measured every 2 °C. The percentage of secondary structure elements was calculated by measuring the change in ellipticity at 222 nm. Thermal melting curves were plotted, and Tm values were acquired from the inflection point of the curve. Tm values for CdiA-CT/CdiIEC536 and Ntox28/ImmRlac complexes were also determined by CD spectrometry to correlate with the values determined by DSF.
Acknowledgments
We thank the Stanford Synchrotron Radiation Lightsource (SSRL) and the Advanced Light Source (ALS) at Berkeley National Laboratory for invaluable help in data collection; and Xin Liu for technical support. This work was supported by National Institutes of Health Grant GM102318 (to C.W.G., C.S.H., and D.A.L.). P.M.J. was supported in part by University of California, Irvine Bridge Funding, and C.M.B. was supported in part by a University of California, Santa Barbara Dean’s Fellowship and the Chang Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 5J5V and 5J43).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1607112113/-/DCSupplemental.
References
- 1.Willett JL, Ruhe ZC, Goulding CW, Low DA, Hayes CS. Contact-dependent growth inhibition (CDI) and CdiB/CdiA two-partner secretion proteins. J Mol Biol. 2015;427(23):3754–3765. doi: 10.1016/j.jmb.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruhe ZC, Wallace AB, Low DA, Hayes CS. Receptor polymorphism restricts contact-dependent growth inhibition to members of the same species. MBio. 2013;4(4):e00480–13. doi: 10.1128/mBio.00480-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoki SK, et al. A widespread family of polymorphic contact-dependent toxin delivery systems in bacteria. Nature. 2010;468(7322):439–442. doi: 10.1038/nature09490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang D, de Souza RF, Anantharaman V, Iyer LM, Aravind L. Polymorphic toxin systems: Comprehensive characterization of trafficking modes, processing, mechanisms of action, immunity and ecology using comparative genomics. Biol Direct. 2012;7:18. doi: 10.1186/1745-6150-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck CM, et al. CdiA from Enterobacter cloacae delivers a toxic ribosomal RNase into target bacteria. Structure. 2014;22(5):707–718. doi: 10.1016/j.str.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morse RP, et al. Structural basis of toxicity and immunity in contact-dependent growth inhibition (CDI) systems. Proc Natl Acad Sci USA. 2012;109(52):21480–21485. doi: 10.1073/pnas.1216238110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikolakakis K, et al. The toxin/immunity network of Burkholderia pseudomallei contact-dependent growth inhibition (CDI) systems. Mol Microbiol. 2012;84(3):516–529. doi: 10.1111/j.1365-2958.2012.08039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diner EJ, Beck CM, Webb JS, Low DA, Hayes CS. Identification of a target cell permissive factor required for contact-dependent growth inhibition (CDI) Genes Dev. 2012;26(5):515–525. doi: 10.1101/gad.182345.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willett JL, Gucinski GC, Fatherree JP, Low DA, Hayes CS. Contact-dependent growth inhibition toxins exploit multiple independent cell-entry pathways. Proc Natl Acad Sci USA. 2015;112(36):11341–11346. doi: 10.1073/pnas.1512124112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kredich NM, Becker MA, Tomkins GM. Purification and characterization of cysteine synthetase, a bifunctional protein complex, from Salmonella typhimurium. J Biol Chem. 1969;244(9):2428–2439. [PubMed] [Google Scholar]
- 11.Zhao C, et al. On the interaction site of serine acetyltransferase in the cysteine synthase complex from Escherichia coli. Biochem Biophys Res Commun. 2006;341(4):911–916. doi: 10.1016/j.bbrc.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 12.Chattopadhyay A, et al. Structure, mechanism, and conformational dynamics of O-acetylserine sulfhydrylase from Salmonella typhimurium: Comparison of A and B isozymes. Biochemistry. 2007;46(28):8315–8330. doi: 10.1021/bi602603c. [DOI] [PubMed] [Google Scholar]
- 13.Salsi E, et al. Design of O-acetylserine sulfhydrylase inhibitors by mimicking nature. J Med Chem. 2010;53(1):345–356. doi: 10.1021/jm901325e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campanini B, et al. Interaction of serine acetyltransferase with O-acetylserine sulfhydrylase active site: Evidence from fluorescence spectroscopy. Protein Sci. 2005;14(8):2115–2124. doi: 10.1110/ps.051492805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campanini B, et al. Moonlighting O-acetylserine sulfhydrylase: New functions for an old protein. Biochim Biophys Acta. 2015;1854(9):1184–1193. doi: 10.1016/j.bbapap.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanous C, et al. The CymR regulator in complex with the enzyme CysK controls cysteine metabolism in Bacillus subtilis. J Biol Chem. 2008;283(51):35551–35560. doi: 10.1074/jbc.M805951200. [DOI] [PubMed] [Google Scholar]
- 17.Ma DK, Vozdek R, Bhatla N, Horvitz HR. CYSL-1 interacts with the O2-sensing hydroxylase EGL-9 to promote H2S-modulated hypoxia-induced behavioral plasticity in C. elegans. Neuron. 2012;73(5):925–940. doi: 10.1016/j.neuron.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burkhard P, et al. Three-dimensional structure of O-acetylserine sulfhydrylase from Salmonella typhimurium. J Mol Biol. 1998;283(1):121–133. doi: 10.1006/jmbi.1998.2037. [DOI] [PubMed] [Google Scholar]
- 19.Burkhard P, Tai CH, Ristroph CM, Cook PF, Jansonius JN. Ligand binding induces a large conformational change in O-acetylserine sulfhydrylase from Salmonella typhimurium. J Mol Biol. 1999;291(4):941–953. doi: 10.1006/jmbi.1999.3002. [DOI] [PubMed] [Google Scholar]
- 20.Morse RP, et al. Diversification of β-augmentation interactions between CDI toxin/immunity proteins. J Mol Biol. 2015;427(23):3766–3784. doi: 10.1016/j.jmb.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang B, Vetting MW, Roderick SL. The active site of O-acetylserine sulfhydrylase is the anchor point for bienzyme complex formation with serine acetyltransferase. J Bacteriol. 2005;187(9):3201–3205. doi: 10.1128/JB.187.9.3201-3205.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francois JA, Kumaran S, Jez JM. Structural basis for interaction of O-acetylserine sulfhydrylase and serine acetyltransferase in the Arabidopsis cysteine synthase complex. Plant Cell. 2006;18(12):3647–3655. doi: 10.1105/tpc.106.047316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Claus MT, Zocher GE, Maier TH, Schulz GE. Structure of the O-acetylserine sulfhydrylase isoenzyme CysM from Escherichia coli. Biochemistry. 2005;44(24):8620–8626. doi: 10.1021/bi050485+. [DOI] [PubMed] [Google Scholar]
- 24.Holm L, Rosenstrom P. Dali server: Conservation mapping in 3D. Nucleic Acids Res. 2010;38(Web Server issue):W545–W549. doi: 10.1093/nar/gkq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hindson VJ, Moody PC, Rowe AJ, Shaw WV. Serine acetyltransferase from Escherichia coli is a dimer of trimers. J Biol Chem. 2000;275(1):461–466. doi: 10.1074/jbc.275.1.461. [DOI] [PubMed] [Google Scholar]
- 26.Papadakos G, Wojdyla JA, Kleanthous C. Nuclease colicins and their immunity proteins. Q Rev Biophys. 2012;45(1):57–103. doi: 10.1017/S0033583511000114. [DOI] [PubMed] [Google Scholar]
- 27.Dupureur CM. Roles of metal ions in nucleases. Curr Opin Chem Biol. 2008;12(2):250–255. doi: 10.1016/j.cbpa.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Beuning PJ, Musier-Forsyth K. Transfer RNA recognition by aminoacyl-tRNA synthetases. Biopolymers. 1999;52(1):1–28. doi: 10.1002/(SICI)1097-0282(1999)52:1<1::AID-BIP1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 29.Zhang D, Iyer LM, Aravind L. A novel immunity system for bacterial nucleic acid degrading toxins and its recruitment in various eukaryotic and DNA viral systems. Nucleic Acids Res. 2011;39(11):4532–4552. doi: 10.1093/nar/gkr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poole SJ, et al. Identification of functional toxin/immunity genes linked to contact-dependent growth inhibition (CDI) and rearrangement hotspot (Rhs) systems. PLoS Genet. 2011;7(8):e1002217. doi: 10.1371/journal.pgen.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holberger LE, Garza-Sánchez F, Lamoureux J, Low DA, Hayes CS. A novel family of toxin/antitoxin proteins in Bacillus species. FEBS Lett. 2012;586(2):132–136. doi: 10.1016/j.febslet.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koskiniemi S, et al. Rhs proteins from diverse bacteria mediate intercellular competition. Proc Natl Acad Sci USA. 2013;110(17):7032–7037. doi: 10.1073/pnas.1300627110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitney JC, et al. Genetically distinct pathways guide effector export through the type VI secretion system. Mol Microbiol. 2014;92(3):529–542. doi: 10.1111/mmi.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aiyar A, Leis J. Modification of the megaprimer method of PCR mutagenesis: Improved amplification of the final product. Biotechniques. 1993;14(3):366–369. [PubMed] [Google Scholar]
- 35.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 36.Adams PD, et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 4):486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garza-Sánchez F, Janssen BD, Hayes CS. Prolyl-tRNA(Pro) in the A-site of SecM-arrested ribosomes inhibits the recruitment of transfer-messenger RNA. J Biol Chem. 2006;281(45):34258–34268. doi: 10.1074/jbc.M608052200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beckwith JR, Signer ER. Transposition of the lac region of Escherichia coli. I. Inversion of the lac operon and transduction of lac by phi80. J Mol Biol. 1966;19(2):254–265. doi: 10.1016/s0022-2836(66)80003-7. [DOI] [PubMed] [Google Scholar]