Abstract
A 55–200 CGG repeat expansion in the 5′-UTR of the fragile X mental retardation 1 (FMR1) gene is known as a premutation. Some carriers are affected by the neurodegenerative disorder fragile X-associated tremor/ataxia syndrome (FXTAS), primary ovarian insufficiency, and neurobehavioral impairments. Based on the mitochondrial dysfunction observed in fibroblasts and brain samples from carriers, as well as in neurons and brains from a mouse model of the premutation, we evaluated the presence of the Warburg effect in peripheral blood mononuclear cells (PBMCs) from 30 premutation carriers with either a rebalance of the metabolism [increasing glycolysis while decreasing oxidative phosphorylation (oxphos)] or a metabolic amplification (increasing glycolysis while maintaining/increasing oxphos). Deficits in oxphos—more pronounced in FXTAS-affected subjects—were accompanied by a shift toward glycolysis, suggesting increased glycolysis despite aerobic conditions. Differential proteomics extended these findings, unveiling a decreased antioxidant response, translation, and disrupted extracellular matrix and cytoskeleton organization with activation of prosenescence pathways. Lower bioenergetics segregated with increased incidence of low executive function, tremors, below-average IQ, and FXTAS. The combination of functional and proteomic data unveiled new mechanisms related to energy production in the premutation, showing the potential of being applicable to other psychiatric disorders to identify endophenotype-specific responses relevant to neurobiology.—Napoli, E., Song, G., Schneider, A., Hagerman, R., Eldeeb, M. A. A. A., Azarang, A., Tassone, F., Giulivi, C. Warburg effect linked to cognitive-executive deficits in FMR1 premutation.
Keywords: fragile X, mitochondria, proteomics, triplet nucleotide diseases, neurological disorder
Fragile X syndrome (FXS) affects individuals with more than 200 CGG repeats (full mutation) in the fragile X mental retardation 1 (FMR1) gene. These carriers experience cognitive and social impairments, developmental delays, and some features of autism spectrum disorders. Carriers of lower repeats (55–200 CGG repeats), also known as the premutation, are generally not severely affected early in life; however, they are at high risk of developing the late-onset neurodegenerative disorder, fragile X-associated tremor/ataxia syndrome [FXTAS; Online Mendelian Inheritance in Man (OMIM): 300623; http://www.ncbi.nlm.nih.gov/omim], fragile X-associated primary ovarian insufficiency, and other symptoms or neurobehavioral problems, including developmental delay, autism spectrum disorders (ASD), anxiety, depression, and immune-mediated disorders (1).
The possible role of mitochondria in the development of the morbidity of premutation carriers is mainly concerned with the efficiency of mitochondrial energy provision. The level of energetic metabolism is dependent on the amount and activities of respiratory chain complexes and ATP-synthase and on the level of proton leak through the mitochondrial membrane, which appears to be controlled by uncoupling proteins (2). The amount of ATP produced per cell and the balance between energy conservation in the molecule of ATP and energy dissipation in the form of heat loss represents a key metabolic control that may be crucial for possible progress of some of the neurologic and cognitive abnormalities observed in premutation carriers or with the development of FXTAS (3, 4). Oxidative phosphorylation (oxphos) at the mitochondrial inner membrane is the primary source of energy for aerobic cells, including neurons. Oxphos consists of 5 transport complexes. Complexes I–IV (NADH dehydrogenase, succinate dehydrogenase, ubiquinol/cytochrome c oxidoreductase and cytochrome c oxidase) constitute the electron transport chain (ETC), which transfers electrons to the final acceptor, molecular oxygen. During electron transport through the complexes, protons are pumped out of the mitochondrial matrix to the intermembrane space and the resulting electrochemical proton gradient is used by complex V (ATP synthase) to produce ATP. It has been shown that the activity of oxphos system can be affected by several factors including age or physical training (5).
More recently, it has been shown that mitochondria play a critical role in the coordination of metabolic pathways, leading not only to ATP production but also to the immune response (6), resulting in the activation and control of the innate immune response (7–9). In this regard, neuroinflammation and the associated increased oxidative stress damage has been implicated in the pathogenesis of several diseases, including Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis, and others (reviewed in refs. 10–15). Given the important role of peripheral blood mononuclear cells (PBMCs) in humoral and cell-mediated immunity (indicating their need for a readily available intracellular source of energy) and that FMR1 is highly expressed in PBMCs (16), it is tempting to propose that deficits recorded in PBMCs mirror the ones in neurons, which are more relevant to a disease with neurologic and emotional deficits. In this regard, the neuroimmune response is characterized by crosstalk between PBMCs and the CNS, and disruption of this process during early life may condition inflammatory responses, mitochondrial dysfunction (MD) and neurobehavioral changes that persist or emerge during adulthood (17–21).
Hence, PBMCs have been used in our study as a valuable and easily available biologic sample for the investigation of mitochondrial energy-providing systems. The overall capacity of energy metabolism depends primarily on the activity of the respiratory chain and Krebs cycle enzymes. Therefore, the purpose of our study was to characterize the oxphos status in PBMCs from control, asymptomatic, and symptomatic premutation carriers, with the idea of evaluating the Warburg effect with either a rebalance of the metabolism (increasing glycolysis while decreasing oxphos) or a metabolic amplification (increasing glycolysis while maintaining or increasing oxphos). To accomplish this goal, we used a thorough battery of functional mitochondrial studies accompanied by untargeted differential proteomics to evaluate the pathways underlying the energy balance in the premutation. We also sought to evaluate the segregation of mitochondrial outcomes with CGG repeat length, age, genotype, and neurological and emotional outcomes, including FXTAS, to elucidate their prognostic value.
MATERIALS AND METHODS
Subjects
Blood samples were obtained from 30 premutation carriers aged 8–75 yr who were recruited through the Fragile X Treatment and Research Center at the MIND Institute at the University of California, Davis, and who participated in our genotype–phenotype study of families with fragile X from 2013 through 2015. Blood samples were also obtained from 12 control subjects aged 23–57 yr. Blood samples were obtained by venipuncture with informed consent at the MIND Institute and approved by the institutional review board ethics committee at UC Davis Medical Center. Exclusion criteria were refusal to participate by the patient or guardian or the presence of infection or malignancy. FXTAS was diagnosed according to the criteria reported by Jacquemont et al. (22).
Genotyping of carriers
The number of CGG repeats in all individuals included in this study was measured from dried blood spots, by Southern blot and PCR analysis (23).
Lymphocyte preparation
Blood (5–7 ml) was collected in Vacutainer cell preparation tubes (CPTs; BD Biosciences, Franklin Lakes, NJ, USA), and lymphocytes were isolated according to the manufacturer’s instructions and analyzed within 2 h. Immediate processing of the samples yielded reproducible and accurate values. Upon collection, lymphocyte suspension was divided into 2 aliquots in Eppendorf tubes (Eppendorf North America, Hauppauge, NY, USA) and pelleted by centrifugation 1 min 2000 rpm in a microfuge at 4°C. The supernatant was removed and the pellet used immediately for mitochondrial outcomes, upon suspension in RPMI 1640 medium. An aliquot of PBMCs was suspended in 0.5 ml cold HEPES (20 mM) frozen at −70°C overnight and subsequently transferred into liquid nitrogen for extended storage. Genomic DNA was isolated from lymphocytes using Gentra Puregene Blood Kit (Qiagen, Valencia, CA, USA) (23).
Mitochondrial outcomes
All chemicals and biochemicals were of analytical grade or higher. Enzymatic activities of complexes I–V in digitonin-permeabilized lymphocytes were determined by polarography (24). In brief, an aliquot (0.5–1.0 × 106) of lymphocytes was added to the oxygen chamber in 0.3 ml of a buffer containing 0.22 M sucrose, 50 mM KCl, 1 mM EDTA, 10 mM KH2PO4, and 10 mM HEPES (pH 7.4). Oxygen consumption rates were evaluated in the presence of 1) 1 mM ADP plus 1 mM malate-10 mM glutamate, followed by the addition of 5 μM rotenone; 2) 10 mM succinate followed by the addition of 1 mM malonate; 3) 1 mM α-glycerophosphate followed by the addition of 3.6 μM antimycin A; and 4) 10 mM ascorbate and 0.2 mM N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD) followed by the addition of 1 mM KCN. The activities of mitochondrial succinate oxidase and cytochrome c oxidase were evaluated as the difference in oxygen uptake recorded before and after the addition of malonate and KCN, respectively, and normalized by the activity of citrate synthase. Citrate synthase activity was evaluated spectrophotometrically in the presence of acetylCoA and oxaloacetate by monitoring the production of CoASH coupled to dithiobis (2-nitrobenzoic) acid at 412 nm (25) in 2.0–5.0 × 104 cells. Spectrophotometric measurements were performed on an Infinite M200 microplate reader (Tecan, Männedorf, Switzerland) equipped with Magellan software (Tecan). Protein concentration was determined by the Pierce bicinchoninic acid protein assay method (Thermo Fisher Scientific, Grand Island, NY, USA) and calculated according to the bovine serum albumin standard curve. The respiratory control ratio (RCR) was calculated as the ratio between oxygen uptake rates of intact cells supplemented with 10 mM glucose (present in RPMI 1640 medium) in state 3u (with 2 µM carbonylcyanide-p-trifluoromethoxyphenylhydrazone, or FCCP) and state 4 (with 0.2 µM oligomycin) (21).
Proteomics
PBMCs were hand homogenized on ice in 20 mM HEPES (pH 6.8), 1× protease, and phosphatase inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). Protein concentration was determined with a BCA protein assay kit (Thermo Fisher Scientific, Sunnyvale, CA, USA). A total of 60 μg protein from 5 controls and 4 premutation and 5 FXTAS carriers was submitted to the UC Davis Mass Spectrometry Facility. The following processes refer to the method in Luckhart et al. (26), with minor modifications. Specifically, when Scaffold (ver. 3.5.1; Proteome Software Inc., Portland, OR, USA) was used to validate tandem mass spectrometry (MS/MS)–based peptide and protein identifications, peptide identifications were accepted if they could be established at greater than 95.0% probability, as specified by the Peptide Prophet algorithm (27). Protein identifications were accepted if they could be established at greater than 99.0% probability and contained at least 2 identified peptides. Protein probabilities were assigned by the Protein Prophet algorithm (28). Proteins that contained similar peptides and could not be differentiated by MS/MS analysis alone were grouped to satisfy the principles of parsimony. Using these parameters, the false-discovery rate was calculated to be 0.3% on the protein level and 0% on the peptide level for the Homo sapiens search set (29). Differential protein expression between premutation and controls or among controls, premutation carriers not affected with FXTAS, and those affected with FXTAS was determined by calculating the z score. To help focus our analysis on clinically relevant perturbations, we restricted our initial inspection of PBMCs proteomics to those proteins that that met a z-score cutoff of ≥0.4 or ≤−0.4 in at least 50% of the carriers. The cutoff that we set (z score ≥ |0.4|) does not guarantee statistical significance and instead was chosen to preserve important disease-related findings while allowing for significant reduction of data complexity. The probability that a protein met a z score ≥ |0.4| by chance was 0.3144. Pathway overrepresentation analysis was performed by using this feature within InnateDB (http://www.InnateDB.com) [using the hypergeometric algorithm with the Benjamini-Hochberg correction and REACTOME (http://www.reactome.org) as the database]. The P values shown were corrected by the false discovery rate, with a cutoff set at 0.05.
Lactate and pyruvate quantification
Levels of lactate and pyruvate were evaluated by automated liner exchange–cold injection system gas chromatography followed by time-of-flight MS (30). The MS analysis was performed at the Metabolomics Facility at UC Davis.
Statistics
Post hoc analysis to compute the achieved power, given the actual sample size used in this study (α = 0.05) indicated that it was >0.999 when performed with all mitochondrial outcomes (G*Power, v.3.1.92; http://gpower.software.informer.com/3.1/). A χ2 test with Yates’ correction was used to evaluate the incidence of depression, anxiety, attention-deficit/hyperactivity disorder (ADHD), ASD, migraines, tremors, executive function, and substance abuse in premutation carriers and controls. An unpaired, 2-tailed Student’s t test was used to compare outcomes from premutation-bearing (regardless of the diagnosis of FXTAS) and control individuals with significance set at P < 0.05. One-way ANOVA followed by Tukey’s post hoc test was used for the comparisons among controls, FXTAS-free premutation carriers and FXTAS-affected carriers. The statistical analysis between FXTAS-free and FXTAS-affected individuals, to evaluate the effect of the presence of the disease on the tested outcomes in individuals carrying >50 CGG repeats, was performed by unpaired Student’s t test.
RESULTS
Characteristics of the subjects enrolled in this study
The control study group consisted of 12 individuals (7 men and 5 women), with a mean age of 36 ± 4 yr with CGG repeats at the 5′-UTR of FMR1 of 28 ± 2 (means ± sem; Table 1). The premutation was composed of 30 premutation carriers, 16 men and 14 women, with a mean age of 42 ± 4 yr, with no significant differences in age (Student’s t test P = 0.377) or sex (χ2 test P = 0.490) compared to controls. Nine of the premutation carriers exhibited FXTAS at various stages of the disease ranging from 1 to 4 (symptomatic or FXTAS-affected group, as opposed to the asymptomatic or FXTAS-free group for carriers with no diagnosis of FXTAS). The average CGG repeats of the mutant allele in heterozygous carriers (females only, because FMR1 is an X-linked gene) was 84 ± 5 (means ± sem) whereas that of hemizygous carriers (males only) was significantly longer (111 ± 11; P = 0.025).
TABLE 1.
Clinical and demographic data of control and premutation carriers
| Clinical groups | Age (yr) | CGG repeats | Sex | Full-scale IQ | FXTAS stage | Structured clinical interview for diagnosis of mental disorders | ASD | ADHD | Fatigue | Migraines | Tremors | BDS-2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Depression | Gen anx dis | Anx panicDis | Anx dis; obsessive-compulsive | Anx dis; social phobia | Anx dis; specific phobia | Substance abuse | ||||||||||||||||||
| LP | C | LP | LP | C | LP | C | LP | C | LP | C | LP | C | ||||||||||||
| Control | ||||||||||||||||||||||||
| C1 | 26.0 | 30 | M | 119 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| C2 | 50.5 | 21 | M | 120 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 25 |
| C3 | 26.3 | 30,37 | F | 121 | 0 | 2 | 0 | 2 | 2 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| C4 | 33.7 | 23,30 | F | 104 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 24 |
| C5 | 41.5 | 20 | M | 117 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 23 |
| C6 | 41.2 | 43 | M | 113 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 |
| C7 | 23.0 | 29,30 | F | 115 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 18 |
| C8 | 28.8 | 20,33 | F | 106 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| C9 | 29.0 | 30 | M | 114 | 0 | 0 | 0 | 0 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 0 | 0 | 0 | 0 | 0 | 25 |
| C10 | 24.0 | 30 | M | 130 | 0 | 2 | 0 | 2 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 |
| C11 | 54.0 | 30 | M | 104 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 23 |
| C12 | 57.4 | 23,30 | F | 133 | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 2 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 25 |
| Premutation without FXTAS | ||||||||||||||||||||||||
| P1 | 9.1 | 160 | M | 86 | 0 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 1 | 1 | 0 | 0 | 0 | 11 |
| P2 | 24.0 | 30,79 | F | 125 | 0 | 2 | 0 | 2 | 2 | 1 | 2 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 24 |
| P3 | 25.0 | 67 | M | 57 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 8 |
| P4 | 53.0 | 16,67 | F | 108 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 22 |
| P5 | 45.3 | 69 | M | 50 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 15 |
| P6 | 8.4 | 150,180 | M | 99 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | NA |
| P7 | 55.4 | 30,69 | F | 114 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 26 |
| P8 | 49.3 | 31,86 | F | 118 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 25 |
| P9 | 24.0 | 31,93 | F | 96 | 0 | 2 | 0 | 2 | 2 | 1 | 2 | 1 | 1 | 0 | 2 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 19 |
| P10 | 9.7 | 31,63 | F | 102 | 0 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 0 | 1 | 0 | 0 | 0 | 15 |
| P11 | 38.4 | 33,60 | F | 112 | 0 | 2 | 0 | 1 | 2 | 1 | 0 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 24 |
| P12 | 19.7 | 177 | M | 99 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 23 |
| P13 | 43.2 | 30,106 | F | 98 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 22 |
| P14 | 17.3 | 16,67 | F | 95 | 0 | 0 | NA | 0 | 0 | NA | 0 | NA | NA | NA | NA | NA | 1 | NA | 0 | 1 | 0 | 0 | 0 | NA |
| P15 | 46.3 | 61 | M | 129 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 23 |
| P16 | 55.6 | 104 | M | 85 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 11 |
| P17 | 49.9 | 20, 98 | F | 123 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 26 |
| P18 | 52.0 | 29, 81 | F | 105 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 23 |
| P19 | 33.1 | 30,137 | F | 96 | 0 | 2 | 1 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 22 |
| P20 | 8.4 | 157,180 | M | 123 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | NA |
| P21 | 15.0 | 56 | M | 109 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 23 |
| Premutation with FXTAS | ||||||||||||||||||||||||
| F1 | 61.3 | 96 | M | 114 | 4 | 0 | NA | 0 | 0 | NA | 0 | NA | 0 | NA | 0 | NA | 0 | NA | 0 | 0 | 0 | 0 | 1 | 18 |
| F2 | 63.1 | 60 | M | 97 | 2 | 2 | 0 | 0 | 2 | 0 | 2 | 1 | 2 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 1 | 1 | 15 |
| F3 | 62.5 | 105 | M | 121 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 19 |
| F4 | 59.1 | 33,107 | F | 124 | 3 | 2 | 0 | 2 | 2 | 0 | 0 | 0 | 2 | 1 | 2 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 1 | 23 |
| F5 | 61.8 | 110-130 | M | 102 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 14 |
| F6 | 63.0 | 102 | F | 95 | 3 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 19 |
| F7 | 70.0 | 90 | M | 98 | 3 | 2 | 2 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 10 |
| F8 | 64.0 | 99 | M | 80 | 3 | 0 | 0 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 15 |
| F9 | 75.0 | 83 | M | NA | 3 | 1 | 2 | 0 | 0 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 18 |
For depression, generalized anxiety disorder (Gen anx dis), anxiety and panic disorders (Anx panic dis), obsessive-compulsive disorders, social phobia, specific phobia, substance abuse, ASD, ADHD, fatigue, migraines, and tremors: NA = missing data; 0 = absent; 1 = subthreshold; and 2 = meets criteria. ADHD, migraines, and tremors were self-reported; ASD was self-reported, but had been diagnosed. BDS-2 score ≤ 14 was considered an index of impaired executive function; full-scale IQ was diagnosed by WISC-IV in individuals 8–16 yr old and by WAIS-IV in individuals 17–80 yr old. Values <90 were considered below average; FXTAS stage refers to clinical evaluation determined by previous diagnosis made by authors Hagerman and Schneider: 0 = absent; 1 = stage 1; 2 = stage 2; 3 = stage 3; and 4 = stage 4. Italicized individuals denote samples used for proteomics analysis. LP, lifetime prevalence; C, current.
Both diagnostic groups (controls and premutation carriers) were characterized clinically by their full-scale intelligence quotient [obtained from Wechsler Adult Intelligence Scale, Fourth Edition (WAIS-IV) or Wechsler Intelligence Scale for Children, Fifth Edition (WISC-V), depending on the age group] and executive function [Behavioral Dyscontrol Scale II or BDS-2 (31)]. Through the Structured Clinical Interview for Diagnosis of Mental Disorders (SCID)-1 [Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV], subjects were tested for depression, generalized anxiety, panic disorders, obsessive-compulsive behavior, specific phobias, and substance abuse. Other symptoms or disorders were self-reported (ADHD, migraines, tremors, and decreased stamina), whereas ASD had already been diagnosed. The premutation group had an average BDS-2 and full-scale IQ lower than those of the controls (premutation vs. controls: 19 ± 5 vs. 24 ± 2, P = 0.003; 104 ± 5 vs. 117 ± 3, P = 0.019, respectively; Table 1). The incidences of depression, anxiety, panic, and obsessive-compulsive disorders, social and specific phobias, and substance abuse in the premutation group were not different from those in the controls. Conversely, the incidence of ADHD was significantly different in the 2 groups (11/30 in premutation vs. none in the control group; P = 0.015).
Assessment of the Warburg effect in PBMCs from carriers of the premutation
The Warburg effect consists of a process in which glucose is largely oxidized to lactate rather than via oxphos (32). This process is also associated with increased uptake of glucose, a common characteristic of cancers detectable in tumors in patients via [18F]-deoxyglucose-positron emission tomography (33). Given that a generalized MD that correlated with the CGG expansions has been observed in fibroblasts and postmortem brain samples from adult carriers (4, 34), as well as in neurons and brains from a mouse model of the premutation (35), we wanted to test whether a Warburg effect is present in cells from premutation carriers, with either a rebalance of the metabolism (increasing production of lactate from glucose while decreasing oxphos) or a metabolic amplification [increasing production of lactate from glucose while maintaining, or even increasing, oxphos (36, 37)]. To this end, a thorough evaluation of the oxphos capacity of PBMCs from controls and carriers of the premutation was undertaken, along with ATP level assessment.
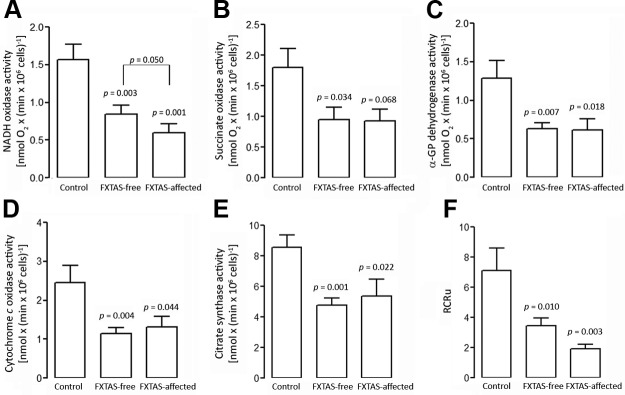
The oxphos capacity of PBMCs was tested upon permeabilization of the cell plasma membrane with digitonin, while leaving mitochondria, other organelles, and the cytoskeleton substantially intact. This approach allows testing mitochondrial function in the presence of a variety of respiratory substrates and inhibitors, to identify the oxphos steps affected in a particular disorder. In digitonin-permeabilized cells, the rotenone-sensitive, malate-glutamate–dependent oxygen uptake is driven by the activity of complexes I and III–V (NADH oxidase); the antimycin-sensitive succinate-dependent oxygen uptake is an index of the activity of complexes II–V (succinate oxidase); the α-glycerophosphate oxidoreductase (a mitochondrial internal membrane–associated enzyme providing electrons to complex III) evaluates the oxygen uptake from complexes III–V, whereas the cyanide-sensitive TMPD/ascorbate-dependent respiration depends only on complex IV (cytochrome c oxidase). Evaluation of the segments encompassing NADH oxidase, succinate oxidase, α-glycerophosphate oxidoreductase, and cytochrome c oxidase activities were significantly lower in carriers than in controls (∼50%, on average; Fig. 1). No statistically significant differences between FXTAS-affected and FXTAS-free carriers were observed for these activities with the exception of NADH oxidase (Fig. 1A–D). This segment showed a significant lower activity in PBMCs from symptomatic individuals relative to asymptomatic ones [0.6 ± 0.1 vs. 0.9 ± 0.1 nmol × (min × 106 cells)−1; P = 0.05; Fig. 1A]. Given that no other activity was different between these 2 groups, it is likely that the decrease in NADH oxidase observed in FXTAS-affected carriers is complex I specific.
Figure 1.
Oxygen consumption, citrate synthase activity, and RCR in PBMCs. A–E) oxphos measurements [NADH oxidase (A), succinate oxidase (B), α-glycerophosphate oxidoreductase (C), and cytochrome c oxidase activities (D)] were performed along with citrate synthase activity (E). Rates, normalized by citrate synthase, are shown in Table 2. F) The RCRu is reported as the ratio of oxygen uptake in the presence of FCCP and oligomycin in nonpermeabilized cells. Data were obtained from PBMCs from 12 controls, 21 asymptomatic premutation carriers, and 9 premutation carriers affected with FXTAS. Measurements were performed in duplicate for each sample (means ± sem). Statistical analysis was performed with ANOVA followed by Tukey’s post hoc test. P values are relative to controls.
When oxphos activities were normalized by that of citrate synthase (a ubiquitous mitochondrial matrix marker), the differences between controls and premutation were no longer observed (Table 2), as the average activity of citrate synthase was also decreased in carriers by a similar extent (by 40.5%; P = 0.004), with no significant difference between FXTAS-affected and -free carriers (Fig. 1E). These results are consistent with a lower mitochondrial mass in PBMCs from premutation carriers relative to that in controls. To evaluate whether the presence of FXTAS in carriers’ PBMCs segregated with a more impaired oxphos activity, normalized mitochondrial outcomes were compared between asymptomatic and symptomatic individuals. Only the normalized NADH oxidase activity was 1.6-fold lower (P = 0.027) in FXTAS-affected premutation individuals relative to FXTAS-free (Table 2).
TABLE 2.
Normalized mitochondrial outcomes in permeabilized PBMCs from control, FXTAS-free, and FXTAS-affected carriers
| Segment of the ETC | Control (n = 12) | FXTAS-free (n = 21) | FXTAS-affected (n = 6) | P |
|---|---|---|---|---|
| NADH oxidase | 0.18 ± 0.02 | 0.18 ± 0.02 | 0.11 ± 0.03 | 0.027 |
| Succinate oxidase | 0.21 ± 0.03 | 0.20 ± 0.03 | 0.17 ± 0.04 | 0.269 |
| α-GP oxidoreductase | 0.15 ± 0.02 | 0.13 ± 0.01 | 0.12 ± 0.03 | 0.368 |
| Cytochrome c oxidase | 0.29 ± 0.04 | 0.24 ± 0.04 | 0.24 ± 0.05 | 0.482 |
Activities are expressed as nanomoles × (minutes × 106 cells)−1 and normalized to citrate synthase activity (means ± sd). Averages of specific activities in controls and FXTAS-free and -affected individuals are shown in Fig. 1. P values were computed by Student’s t test for comparisons between FXTAS-free and -affected individuals. For comparisons involving the 3 groups, a 1-way ANOVA was performed as shown in Fig. 1.
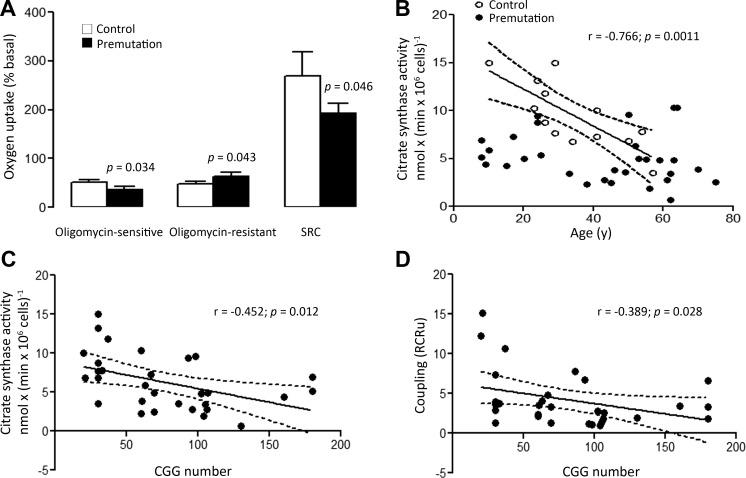
The coupling between electron transport harnessed to ATP production was evaluated in PBMCs by the RCR. This ratio was obtained by dividing the rate of oxygen uptake of intact cells supplemented with 10 mM glucose in the presence of the uncoupler FCCP (State 3u) by the rate of oxygen uptake obtained in the presence of the ATPase inhibitor oligomycin (oligomycin-induced state 4). This compound inhibits mitochondrial oxygen uptake only under phosphorylating conditions, making oligomycin a selective inhibitor of tightly coupled mitochondria while leaving the uncoupled unaffected. The mean RCR under uncoupling conditions (RCRu) in the premutation group was 2.2-fold lower (P = 0.006) than controls, attributed to a 1.34-fold higher state 4 oxygen uptake (Fig. 2A) and lower state 3u (1.41-fold; P = 0.03). The spare respiratory capacity (SRC; the capacity to produce extra ATP through oxphos in case of a sudden increase in energy demand) was 1.4-fold higher in the controls (P = 0.046). These results indicate that mitochondria from premutation carriers are loosely coupled or, in other words, show a low dependence of oxygen uptake on ATP production. The higher state 4 oxygen uptake indicates an increase in reactive oxygen species (ROS) production linked to MD, increased inner membrane leakiness, and processes not necessarily linked to mitochondrial function [e.g., superoxide-generating leukocyte NADPH oxidase (38)] whereas the lower state 3u oxygen uptake suggests a lower ETC activity encompassing complexes I, III, and IV (attributed mainly to FXTAS-affected, see below).
Figure 2.
Dependence of mitochondrial outcomes on donor’s age and CGG repeats. A) The oxygen uptake of intact PBMCs (12 controls and 30 premutation carriers) was evaluated with 10 mM glucose in PBS. Oxygen uptake was recorded under these conditions (total oxygen consumption): addition of 2 µM oligomycin allowed the evaluation of the oxygen uptake resistant to this complex V inhibitor; subsequent addition of 20 nM of the uncoupler FCCP allowed assessment of the maximum oxygen uptake by the ETC when not coupled to ATP production, used for the evaluation of the SRC. Values were expressed as percentages of basal rates (means ± sem). Statistical analysis was performed with Student’s t test. B, C) Citrate synthase was plotted vs. the age (B) and CGG repeats (C) of the donor. D) The RCR plotted against the CGG repeats. The corresponding correlation parameters (Pearson’s r and P values) are also shown. Dotted lines: 95% CI (B–D).
The higher uncoupling (Fig. 1F), the lower oligomycin-sensitive oxygen uptake (linked to ATP production; Fig. 2A) accompanied by a higher oligomycin-resistant oxygen uptake (not linked to ATP production), along with lower citrate synthase activity, indicates the presence of loosely coupled and dysfunctional mitochondria (lower oxphos capacity with increased inner membrane leakiness and ROS production) with significant decreases in the mitochondrial mass.
The effects of age, CGG repeat expansions, sex, and FXTAS diagnosis on mitochondrial outcomes were studied. The activity of citrate synthase decreased significantly with age in controls (P = 0.001; Fig. 2B), declining by almost half between 18 and 50 yr of age. This observation was consistent with the differences that have been reported for lymphocytes from individuals within the same age groups (39). No correlation between citrate synthase activity and age was observed in premutation carriers (P = 0.272), with average activity values lower than those of controls at all ages. Significant reciprocal correlations between the CGG expansion and both citrate synthase activity and mitochondrial uncoupling were noted, indicating that both mitochondrial outcomes in cells from carriers declined significantly with increasing CGG repeats (decreasing by 6–10% every 20 CGG repeats; Fig 2C, D).
Consistent with other studies (39, 40), no significant differences were observed in the control group between females and males in any of the mitochondrial outcomes tested. However, the mean RCRu was higher (1.6-fold) in heterozygous female carriers than in hemizygous male carriers (3.4 ± 0.7 vs. 2.1 ± 0.3, respectively; P = 0.064) suggesting either the influence of an X-linked gene other than FMR1 at modulating the mitochondrial coupling, or a compensatory effect provided by the remaining wild-type FMR1 allele.
As deficits in complex I were observed only in cells from FXTAS-affected carriers (Fig. 1A), a more severe uncoupling was also observed in lymphocytic mitochondria from symptomatic carriers relative to asymptomatic ones. This observation is supported by the statistically significant difference observed in the RCRu (3.9 ± 0.7 and 1.9 ± 0.2, respectively, for FXTAS-free and FXTAS-affected carriers, P = 0.007; Table 3) which was statistically attributable to a higher state 4 [rates of oxygen uptake under state 3u and state 4 were, respectively, 0.5-fold (P = 0.148), and 1.4-fold (P = 0.042), for FXTAS-affected vs. nonaffected PBMCs]. Although the difference is not statistically significant, we cannot exclude the biologic significance of a 2-fold lower state 3u oxygen uptake in FXTAS-affected vs. nonaffected carriers. Similarly, the spare respiratory capacity in PBMCs from symptomatic carriers was 50% of asymptomatic ones (P = 0.032).
TABLE 3.
Phosphorylating capacity and coupling and SRC in intact PBMCs from controls and premutation-bearing individuals
| Oxygen uptake rates normalized to basal oxygen uptake | Control (n = 11) | FXTAS-free (n = 21) | FXTAS-affected (n = 6) | P |
|---|---|---|---|---|
| Linked to ATP production | 0.52 ± 0.05 | 0.42 ± 0.05 | 0.2 ± 0.3 | 0.148 |
| Not linked to ATP production | 0.48 ± 0.05 | 0.58 ± 0.05 | 0.8 ± 0.3 | 0.042 |
| Spare respiratory capacity | 2.7 ± 0.5 | 2.1 ± 0.3 | 1.5 ± 0.1 | 0.032 |
| Coupling | ||||
| RCRu | 6.8 ± 1.5 | 3.9 ± 0.7 | 1.9 ± 0.2 | 0.007 |
Oxygen uptake rates of intact PBMCs are expressed as nanomoles O2 consumed × (minutes × 106 cells)−1 (means ± sem) supplemented with 10 mM glucose and normalized by the total (basal) respiration in glucose. RCRu is expressed as oxygen uptake in the presence of the uncoupler FCCP (state 3u, or maximum oxygen uptake by ETC) in relation to the rate of oxygen uptake obtained in the presence of the ATPase inhibitor oligomycin (oligomycin-induced state 4, or oxygen uptake not linked to ATP production). SRC is calculated as the ratio of oxygen uptake in the presence of FCCP and basal respiration. P values were computed by Student’s t test for comparisons between FXTAS-free and -affected individuals.
More severe mitochondrial functional deficits (uncoupling, higher state 4 oxygen uptake, lower NADH oxidase, and SRC) were observed in PBMCs from FXTAS-affected carriers. Although it could be argued that these differences are ascribable to the age disparity between these strata (31 ± 4 and 65 ± 2 yr; P < 0.0001), given the detrimental age-dependent effect on mitochondrial ATP synthesis (41), only 4 of the 9 mitochondrial outcomes tested were found to be significantly different between asymptomatic and symptomatic individuals, precluding this option.
In terms of energy production, levels of ATP from oxphos and glycolysis were evaluated in PBMCs from the 3 groups (Table 4). Energy deficits in PBMCs from carriers were apparent, as judged by the significant lower total ATP levels vs. controls (55% of controls, P = 0.001), more severe in FXTAS-affected than FXTAS-free carriers (49% and 61% of controls, respectively). Of the overall ATP produced in control cells, 67% was oxphos derived, whereas PBMCs from carriers of the premutation showed lower ATP from oxphos (∼33%) and higher ATP output from glycolysis (68%). These results confirmed a metabolic remodeling, leading to an increased glycolytic flux in PBMCs from premutation carriers accompanied by a lower mitochondrial capacity to produce ATP. This scenario provides support for a Warburg effect that included a rebalancing of metabolism (increasing production of lactate from glucose) in the presence of oxphos deficits. However, despite this rebalancing, energy deficits were significant (61 and 49% of total control ATP in asymptomatic and FXTAS-affected carriers, respectively) and most likely affected ATP-dependent cellular pathways (repair, biosynthesis, and sodium-potassium balance).
TABLE 4.
ATP levels in controls and premutation PBMCs
| Pathway | Control (n = 11) | FXTAS-free (n = 21) | FXTAS-affected (n = 9) | P |
|---|---|---|---|---|
| Oxphos | 15 ± 4 (67%) | 5.2 ± 0.6 (38%) | 2.9 ± 0.8 (27%) | 0.019 |
| Glycolysis | 7.3 ± 0.9 (33%) | 8.4 ± 0.4 (62%) | 8 ± 2 (73%) | 0.390 |
| Total ATP | 22.3 ± 1.2 | 13.6 ± 0.2 | 10.9 ± 0.2 | 0.0001 |
ATP levels from oxphos were calculated [as nanomoles ATP × (glucose × 106 cells)−1] (means ± sem) (%)] by the oligomycin-sensitive oxygen uptake of glucose-supplemented intact cells and normalized by glucose consumed per million cells. ATP from glycolysis was calculated from the lactate-to-glucose ratio obtained by metabolomics analysis and normalized as above. In parentheses, the percentage of total ATP produced by each pathway. P values were computed by Student’s t test for comparisons between FXTAS-free and -affected individuals.
Proteomics approach confirms the Warburg effect with a dysregulated cytoskeleton, antioxidant response, and RNA metabolism in carriers of the premutation
To gain a deeper understanding of the impact of the metabolic differences on the cells, we performed differential proteomics by liquid chromatography (LC)/MS/MS on a subset of randomly selected samples from each of the 3 groups (control, asymptomatic, and symptomatic premutation carriers; indicated in bold in Table 1). From the 923 proteins detected, 849 were unequivocally identified by using the UniProt database (http://www.uniprot.org/ reviewed entries only and species specific). To help focus our analysis to clinically relevant perturbations, we restricted our initial inspection of proteomics to those proteins that that met a z-score cutoff of ≥0.4 or ≤ −0.4 in at least 50% of the carriers. By using this score, we captured the population and technical variation for each protein and were able to employ biochemical pathway information to uncover diagnostic patterns of metabolic perturbations. Although the selected cutoff (z score ≥ |0.4|) does not guarantee statistical significance, it was chosen to preserve important disease-related findings, allowing for significant reduction of data complexity while considering that changes in enzyme abundance of ∼10% in near equilibrium, whereas nonequilibrium reactions can increase the flux by ∼2- and 1.11-fold, respectively (assuming no limitation of substrate). According to this criterion, 716 and 705 proteins were differentially expressed in the asymptomatic and FXTAS-affected groups, respectively, relative to controls, with 572 proteins (67.4%) shared by the 2 groups, and 144 and 133 unique to the asymptomatic and FXTAS groups, respectively. When the selected proteins were analyzed by cellular compartment [with cellular compartment feature of the gene ontology analysis (42, 43)], a significant percentage (at least 7%) were found to be associated with mitochondria (44, 45).
To evaluate the metabolic state of cells from carriers, proteins selected by their z score and clustered under the gene ontology term “metabolism” were separated according to the following categories: glycolysis, pentose phosphate shunt, Krebs cycle, and oxphos, proteins associated with tricarboxylic acid (e.g., anaplerotic reactions and transporters), fatty acid β-oxidation, mitochondrial redox/stress response, mitochondria morphology/dynamics and assembly, and others (mtDNA replication/transcription, protein biosynthesis, and methylglyoxal degradation; Table 5).
TABLE 5.
Overrepresented biological processes in PBMCs from premutation carriers and controls
| Intermediary metabolism and related proteins | Gene name |
Z score relative to controls |
|
|---|---|---|---|
| FXTAS-affected | FXTAS-free | ||
| Glycolysis | |||
| Hexokinase-1 | HK1 | −0.4a | 0.0 |
| ATP-dependent 6-phosphofructokinase, platelet type | PFKP | −0.6a | 0.5b |
| Fructose-bisphosphate aldolase A | ALDOA | 1.2b | 2.2b |
| Fructose-bisphosphate aldolase C | ALDOC | −1.0a | −0.8a |
| Triose phosphate isomerase | TPI1 | −0.1 | 1.8b |
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | 0.1 | −0.4a |
| Phosphoglycerate kinase 1 | PGK1 | −3.1a | −2.4a |
| Phosphoglycerate mutase 1 | PGAM1 | −2.1a | 1.5b |
| Pyruvate kinase | PKM | −1.2a | 2.4b |
| l-Lactate dehydrogenase A chain | LDHA | 0.0 | −0.1 |
| l-Lactate dehydrogenase B chain | LDHB | −2.9a | −1.2a |
| Na+/H+ exchange regulatory cofactor NHE-RF1 | SLC9A3R1 | −0.5a | 0.0 |
| Pentose phosphate shunt | |||
| Glucose-6-phosphate 1-dehydrogenase | G6PD | 0.7b | 1.2b |
| Krebs cycle and ETC | |||
| Pyruvate dehydrogenase E1 component subunit alpha, somatic form | PDHA1 | −0.6a | −0.6a |
| Dihydrolipoyl dehydrogenase | DLDH | −0.6a | −0.6a |
| Citrate synthase | CS | −0.5a | −0.2 |
| Aconitate hydratase | ACO2 | −0.6a | −0.4a |
| 2-Oxoglutarate dehydrogenase | OGDH | −0.6a | 2.2b |
| Dihydrolipoyllysine-residue succinyltransferase component of 2-OGDH complex | DLST | −0.6a | −0.1 |
| Succinyl-CoA ligase [ADP/GDP-forming] subunit alpha | SUCLG1 | −0.5a | −0.7a |
| Succinyl-CoA ligase [GDP-forming] subunit beta | SUCLG2 | −0.6a | −0.6a |
| Succinate dehydrogenase [ubiquinone] flavoprotein subunit | SDHA | −0.4a | −0.5a |
| Succinate dehydrogenase [ubiquinone] iron-sulfur subunit | SDHB | −0.8a | −0.5a |
| Malate dehydrogenase | MDH2 | 0.1 | 2.1b |
| NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 5 | NDUFA5 | 1.2b | 0.9b |
| NADH-ubiquinone oxidoreductase 75-kDa subunit | NDUFS1 | −0.6a | 0.4b |
| NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 6 | NDUFB6 | −0.6a | 0.9b |
| NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 10 | NDUFB10 | −0.6a | −0.6a |
| NADH dehydrogenase [ubiquinone] iron-sulfur protein 3 | NDUFS3 | −1.1a | 0.1 |
| Electron transfer flavoprotein subunit alpha | ETFA | −0.6a | −0.6a |
| Cytochrome b-c1 complex subunit 1 | UQCRC1 | 0.3 | 1.7b |
| Cytochrome b-c1 complex subunit 2 | UQCRC2 | 0.4b | 0.0 |
| Cytochrome b-c1 complex subunit 7 | UQCRB | −0.6a | −0.6a |
| Cytochrome b-c1 complex subunit 8 | UQCRQ | 0.8b | −0.1 |
| ATP synthase subunit alpha | ATP5A1 | −0.3 | −0.7a |
| ATP synthase subunit beta | ATP5B | −1.8a | −2.0a |
| ATP synthase subunit O | ATP5O | −0.7a | −0.7a |
| Associated with Krebs’ cycle and transport | |||
| Adenylate kinase 2 | AK2 | 0.1 | 2.2b |
| Glutamate dehydrogenase 1 | GLUD1 | −0.6a | 0.7b |
| Succinate-semialdehyde dehydrogenase | ALDH5A1 | −0.6a | −0.6a |
| Aspartate aminotransferase | GOT2 | −0.6a | −0.6a |
| Phosphate carrier protein | SLC25A3 | −0.6a | −0.6a |
| Mitochondrial 2-oxoglutarate/malate carrier protein | SLC25A11 | −0.6a | −0.6a |
| Voltage-dependent channel anion isoform 2 | VDAC2 | −0.6a | 0.3b |
| Voltage-dependent channel anion isoform 3 | VDAC3 | −0.3 | 1.6b |
| Adenine nucleotide translocator isoform 2 | ANT2 | −0.2 | −0.5a |
| Adenine nucleotide translocator isoform 3 | ANT3 | −0.6a | −0.6a |
| Delta-aminolevulinic dehydratase | ALAD | −0.6a | 3.7b |
| Fatty acid β-oxidation/ketone bodies | |||
| Carnitine O-palmitoyltransferase I | CPT1A | −0.6a | 0 |
| Very long-chain specific acyl-CoA dehydrogenase | ACADVL | −0.4a | −0.6a |
| Enoyl-CoA hydratase | ECHS1 | −0.9a | −0.9a |
| Trifunctional enzyme subunit alpha | HADHA | −0.6a | −0.6a |
| Trifunctional enzyme subunit beta | HADHB | −0.6a | −0.6a |
| Delta(3,5)-Delta(2,4)-dienoyl-CoA isomerase | ECH1 | −0.4a | −2.0a |
| Hydroxyacyl-coenzyme A dehydrogenase | HADH | −0.5a | −0.6a |
| 2,4-Dienoyl-CoA reductase | DECR1 | −0.1 | 0.9b |
| Acetyl-CoA acetyltransferase | ACAT1 | −0.4a | −0.6a |
| Acetyl-CoA acyltransferase | ACAA2 | −0.1 | 0.1 |
| Acylglycerol kinase, mitochondrial | AGK | −0.6a | −0.6a |
| Redox/stress/antioxidant response | |||
| 10-kDa heat shock protein | HSPE1 | −6.0a | −4.2a |
| 60-kDa heat shock protein | HSPD1 | −0.5a | −1.1a |
| Stress-70 protein (mortalin) | HSPA9 | −0.4a | 0.4b |
| Thioredoxin reductase 2 | TXNRD2 | −1.1a | −0.3 |
| Thioredoxin-dependent peroxide reductase | PRDX3 | −0.4a | 0.7b |
| Peroxiredoxin-5 | PRDX5 | −0.7a | −0.2 |
| NAD(P) transhydrogenase | NNT | −0.6a | −0.6a |
| Isocitrate dehydrogenase [NADP] | IDH2 | −0.6a | −0.6a |
| Sulfide/quinone oxidoreductase | SQRDL | −0.6a | −0.6a |
| Persulfide dioxygenase | ETHE1 | −0.2 | −0.9a |
| Superoxide dismutase | SOD2 | 0.8a | 0.7b |
| Mitochondria morphology/dynamics/assembly | |||
| Coiled-coil-helix-coiled-coil-helix domain-containing protein 3 | MICOS19 | −0.6a | −0.5a |
| Cytoplasmic dynein heavy chain 1 | DYNC1H1 | −0.6a | −0.1 |
| Conventional kinesin heavy chain | KIF5B | −0.6a | 0.5b |
| Protein deglycase DJ-1 | PARK7 | −0.7a | 0.1 |
| Others | |||
| mtDNA replication/transcription | |||
| Single-stranded DNA-binding protein | SSBP1 | −1.5a | −0.5a |
| Transcription factor A | TFAM | −0.6a | −0.6a |
| Leucine-rich PPR motif-containing protein | LRPPRC | −0.6a | −0.6a |
| Protein biosynthesis | |||
| Elongation factor Tu | TUFM | −0.4a | −0.7a |
| Methylglyoxal degradation | |||
| Hydroxyacylglutathione hydrolase | HAGH | −2.6a | −2.6a |
| Protein deglycase DJ-1 | PARK7 | −0.7a | 0.1 |
Proteins differentially expressed between cells from either asymptomatic or FXTAS-affected compared with controls were evaluated in terms of their z score. Cutoff values were ±0.4 [P(0.4 < Z < −0.4) = 0.311]. Shown are proteins that were differentially expressed in at least one of the two diagnostic groups and belonged to the gene ontology term metabolism. Proteins under glycolysis and pentose phosphate shunt were cytosolic, whereas all others had a mitochondrial location.
Down-regulated.
Up-regulated.
In cells from FXTAS-affected carriers, most mitochondrial proteins showed a lower abundance than control cells (54/67), including those belonging to the glycolytic pathway (8/11; under FXTAS compare up-regulated with down-regulated), with the exception of glucose-6-phosphate dehydrogenase, the pentose phosphate shunt entry point. Conversely, cells from asymptomatic carriers had about the same number of proteins, with levels similar to or above those of the controls (29/67 and 38/67, respectively), and most of the glycolytic proteins showed levels comparable or higher than controls (8/11). Among them, the key regulatory steps of glycolysis—namely hexokinase, phosphofructokinase, and pyruvate kinase. The similar abundance of lactate dehydrogenase (LDH)-A chain (or LDHM) in carriers (regardless of FXTAS) and controls and the significant lower LDHB (or LDHH) levels in carriers without (−1.2-fold) and with FXTAS (−2.9-fold) further supports the notion of a shift toward anaerobic metabolism. This finding is based on the biochemical evidence that LDH isoenzymes containing relatively larger proportions of LDHH tend to predominate in aerobic tissues (such as heart), whereas those containing mainly M subunits are found in tissues with considerable anaerobic metabolism (e.g., skeletal muscle and liver) (46, 47). Cells from asymptomatic carriers showed a more predominant role for the second half of the Krebs cycle (from α-ketoglutarate to oxaloacetate evidenced by higher abundance of α-ketoglutarate dehydrogenase and malate dehydrogenase) probably sustained by the anaplerosis of Glu/Gln (as judged by the higher abundance of glutamate dehydrogenase). In the case of the FXTAS affected, most of the Krebs cycle enzymes showed a lower abundance than controls. These results indicated that cells from FXTAS-affected carriers showed a decline in proteins belonging to both glycolysis and oxphos (70–80% of the identified proteins for each process) probably to direct most of glucose toward the pentose phosphate shunt, whereas in cells from asymptomatic carriers, an up-regulation of glycolysis (73% of the glycolytic proteins) seemed to occur, in an attempt to compensate for the lower oxphos (43% of mitochondrial proteins with lower abundance than controls). These results are consistent with the data on lower function (more uncoupling) and mitochondrial mass in cells from carriers.
Although mitochondrial function cannot be extrapolated from proteomic analysis, the fact that CHCHD3 (also known as MIC19), a component of the MICOS complex [a large protein complex of the mitochondrial inner membrane (48)], is lower in cells from carriers (with and without FXTAS) suggests an altered maintenance of cristae junctions, inner membrane architecture, and formation of contact sites to the outer membrane (49). Furthermore, the lower abundance of the Parkinson’s disease protein 7 (PARK-7) in FXTAS-affected carriers, protein required for correct mitochondrial morphology and function, autophagy of dysfunctional mitochondria (50, 51), and regulating expression or stability of mitochondrial uncoupling proteins (52), suggests not only lower mitochondrial function but also accumulation of damaged mitochondria via a disrupted mitophagy. Accumulation of mitochondria with aberrant morphology has also been observed in oocyte mitochondria in a mouse model of fragile X primary ovary insufficiency (53). Of interest, pathogenic mutations in PARK7 (loss of function) have been linked to autosomal recessive early-onset Parkinson’s disease (OMIM: 606324) connecting this information to the increased activation of cellular senescence-associated processes in PBMCs from FXTAS-affected carriers (i.e., DNA fragmentation, formation of senescence-associated heterochromatin foci, telomere stress-induced senescence, and increased amyloid formation). The lower abundance of 2 motor proteins, dynein, and kinesin, in PBMCs from FXTAS-affected carriers also points to issues relative to the normal distribution and networking of mitochondria, as well as cytoskeletal abnormalities, as we and others have reported before in fibroblasts from carriers (54) and neurons derived from a knock-in mouse model of the premutation (35, 55). Taken together, more severe deficits in mitochondria from FXTAS-affected carriers seem apparent from the proteomic data, in agreement with the observed decreased coupling and complex I activity, relative to asymptomatic carriers.
To elucidate overrepresented pathways in the control–premutation comparison, the proteins selected by their z scores were analyzed by using InnateDB, followed by pathway overrepresentation analysis (Table 6). Among the up-regulated pathways shared by both FXTAS-affected and nonaffected carriers were those related to glucose transport, activation of apoptosis, integrin signaling pathway, and extracellular matrix organization, including collagen formation (under “shared” indicated as up-regulated). The up-regulation of glucose transport supports the functional studies on energy metabolism, which pointed to an increased Warburg effect (see below). The only shared down-regulated pathway was translation elongation.
TABLE 6.
Differentially expressed proteins in PBMC from carriers vs. controls
| Premutation without FXTAS | Shared | Premutation with FXTAS |
|---|---|---|
| RNA regulation/transcription | ||
| Regulatory RNA pathwaysa | Transcriptional activation of mitochondrial biogenesisb | |
| Transcriptional regulation by small RNAsa | NMD, independent or enhanced by EHJb | |
| mRNA surveillance pathwayb | Gene Expressionb | |
| FOXA1 transcription factor networkb | ||
| Metabolism | ||
| Fat metabolism and transportb | Translocation of GLUT4 to the plasma membranea | Mitochondrial biogenesisb |
| Retinoid metabolism and transportb | Glucose, gluconeogensis and glycogen metabolismb | |
| Scavenging of heme from plasmab | Organelle biogenesis and maintenanceb | |
| Transport of vitamins, nucleosides, and related moleculesb | ||
| Transmembrane transport of small moleculesb | ||
| Apoptosis | ||
| Activation of BAD and translocation to mitochondriaa | Apoptotic execution phasea | |
| Activation of BH3-only proteinsa | Apoptosis induced DNA fragmentationa | |
| Intrinsic pathway for apoptosisa | ||
| Translation/protein synthesis/PTM | ||
| Sumoylationb | Eukaryotic translation elongationb | Eukaryotic translation initiation and terminationb |
| Viral mRNA transcription, Translation and replicationb | ||
| Peptide chain elongationb | ||
| Cap-dependent translation initiationb | ||
| SRP-dependent cotranslational protein targeting to membraneb | ||
| Ribosomal scanning and start codon recognitionb | ||
| Signaling | ||
| Mechanism of gene regulation by PPAR-αb | Integrin signaling pathwaya | IFN-α/β/γ signalingb |
| Scavenging by class A receptorsb | Gαi signaling eventsb | |
| Inositol phosphate metabolisma | Glucagon signaling in metabolic regulationb | |
| Fas signaling pathwaya | GPCR ligand bindingb | |
| Wnt signaling pathwaya | Response to elevated platelet cytosolic Ca2+a | |
| Insulin receptor signalinga | PI metabolisma | |
| Neurotrophin signaling pathwaya | ||
| GP1b-IX-V activation signalinga | ||
| Rap1 signalinga | ||
| IL3-mediated signaling eventsa | ||
| Role of calcineurin-dependent NFAT signaling in lymphocytesa | ||
| Syndecan interactionsa | ||
| Matrix organization/cytoskeleton | ||
| Post-chaperonin tubulin folding pathwaya | Extracellular matrix organizationa | Degradation of the extracellular matrixa |
| Regulation of actin cytoskeletona | Integrin cell surface interactionsa | Activation of matrix metalloproteinasesa |
| Collagen formationa | Membrane traffickinga | |
| Stress response/DNA damage/senescence | ||
| Detoxification of ROSa | Activation of DNA fragmentation factora | |
| Pentose phosphate pathway (hexose monophosphate shunt)a | Formation of senescence-associated heterochromatin focia | |
| Cellular responses to stressa | DNA damage/telomere sstress-induced senescencea | |
| Glutathione conjugationa | Amyloids | |
| Cellular senescencea | ||
| Immune response | ||
| Complement and coagulation cascadesb | Influenza infectionb | |
| Scavenging by class B receptorsb | ||
| Focal adhesiona | ||
| EGFR1a | ||
| ECM-receptor interactiona | ||
| Nectin adhesion pathwaya | ||
| C-MYB transcription factor networka | ||
| IL1a | ||
| Phagosomea | ||
| Adherens junctiona | ||
| Antigen presentation/folding, assembly and peptide loading of class I MHCa | ||
| CDC42 signaling eventsa | ||
| Cell cycle/proliferation | ||
| Cell cyclea | ||
| Mitotic prophasea | ||
| Others | ||
| Axon guidancea | ||
| Cell–cell communicationa | ||
Differentially expressed proteins as defined by their z score were imported into InnateDB to analyze the over-representation of pathways. The analysis considered a hypergeometric over-representation followed by the Benjamini-Hochberg analysis. Correction considering fold changes of ±1 and P < 0.1. The database used was Reactome. The results were then filtered by a −LOG10p value of 1.3.
Up-regulated.
Down-regulated.
Up-regulated pathways unique to cells from carriers without FXTAS were those involved in RNA metabolism, including transcriptional regulation by small RNAs, detoxification of ROS, and stress response and inflammation and immune responses (Table 6, up-regulated). Those up-regulated only in cells from FXTAS-affected carriers were mainly related to extracellular matrix degradation, including activation of metalloproteinases and cell stress response. Of interest, although the stress response in cells from asymptomatic carriers was mainly constituted by the antioxidant response, cells from FXTAS-affected carriers showed increased activation of cellular senescence-associated processes (i.e., DNA fragmentation, formation of senescence-associated heterochromatin foci, telomere stress-induced senescence, and increased amyloid formation) suggesting an “accelerated aging” process.
Down-regulated pathways unique to cells from asymptomatic carriers were mainly related to mRNA metabolism, intermediary metabolism (especially fat, heme, and transport of small molecules), and SUMOylation (Table 6, down-regulated), whereas those unique to cells from FXTAS-affected carriers included the down-regulation of intermediary metabolism (i.e., mitochondrial biogenesis and glucose, glycogen, and gluconeogenesis metabolism), nonsense-mediated decay of transcripts that are independent of or enhanced by exon junction complex, translation initiation and termination, peptide chain elongation, and cotranslational protein targeting.
In terms of signaling pathways that are consistent with these metabolic changes, FXTAS-affected and nonaffected individuals shared the up-regulation of integrin signaling. Cells from asymptomatic carriers showed mainly up-regulation of calcium-dependent pathways, such as inositol phosphate and calcineurin and Fas, Wnt, insulin, neurotropin, and Rap1 with a down-regulation of the PPARα-mediated gene regulation (Table 6). The lower representation of pathways mediated by PPARα activation is consistent with the lower protein abundance of components of the mitochondrial β-oxidation pathway, including various acyl-CoA dehydrogenases [(acyl-CoA dehydrogenase, step 1) and mitochondrial trifunctional enzyme (hydroxyacyl-coenzyme A dehydrogenase, steps 2–4 (56)]. Cells from FXTAS-affected carriers showed an up-regulation of the response to elevated calcium but also to phosphoinositide metabolism. In this regard, evidence for a role of phosphoinositides in pre-mRNA splicing, chromatin remodeling, and gene transcription has been provided (57), supported by the major nuclear localization of phosphoinositide-metabolizing enzymes. The potential role of phosphoinositides in mitochondria remains unexplored, although enzymes, such as a synaptojanin splice variant 2A, may have a role in mitochondrial division (58, 59). A down-regulation of signaling mediated by IFN, G proteins, glucagon, and GPCR was evident in cells from FXTAS-affected carriers, which may explain the lower glycolytic flux.
Taking these results together, we conclude that cells from premutation carriers exhibit increased glucose transport (translocation of GLUT4 to the membrane), apoptosis, and disrupted matrix/cytoskeleton organization, with decreased translation. These changes in PBMCs from asymptomatic carriers were accompanied by altered RNA and mRNA metabolism (splicing, processing, and transport) and increased response to stress, and, in the case of cells from FXTAS-affected individuals, by decreased intermediary metabolism, a negative regulation of DNA damage repair with enhanced senescence accompanied by a decrease in mitochondrial biogenesis and maintenance. Thus, the proteomic differences observed between asymptomatic and symptomatic carriers seem to point to a dynamic, progressive disease, given the presence of common pathways in both groups. However, once the FXTAS symptoms become overly evident, a significant shutdown of several critical pathways such as translation (or increased translational silencing), gene expression, DNA repair, increased senescence, mitochondrial maintenance and biogenesis, and glucose metabolism take place.
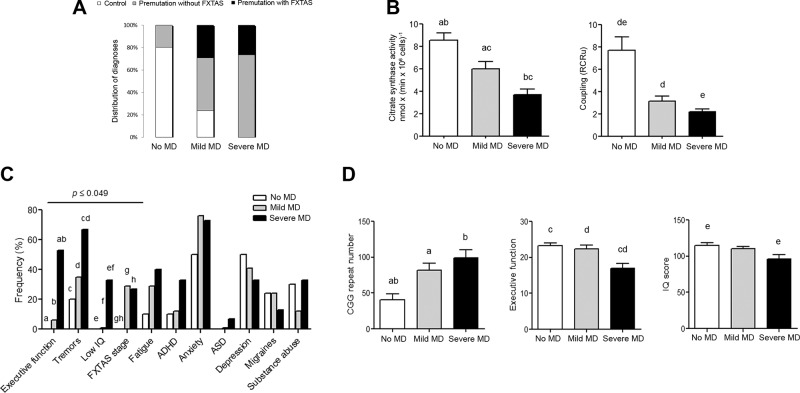
Segregation of MD with clinical symptoms
To discriminate which clinical symptoms segregate with lower mitochondrial capacity to synthesize ATP, mitochondrial mass and coupling (the 2 most affected mitochondrial outcomes in premutation-bearing individuals) were used for the analyses. By using the 95% confidence intervals (CIs) calculated for citrate synthase activity and RCRu with control values (n = 12), we grouped data from all subjects including controls, regardless of the diagnosis, into 3 classes: 1) those not having MD if both outcomes were within the 95% CI (which included 8/12 controls and 3/21 FXTAS-free premutation carriers; Fig. 3A); 2) those with mild MD, if at least 1 outcome was <95% CI (which included 4 controls, 8 asymptomatic carriers, and 5 FXTAS-affected); and 3) those with severe MD, if both mitochondrial outcomes were <95% CI (including 10 asymptomatic and 4 FXTAS symptomatic). The mean citrate synthase activity and RCRu for each of these groups correlated with the morbidity of the MD (Fig. 3B). The incidence of clinical outcomes (FXTAS stage, depression, anxiety, substance abuse, ASD, ADHD, migraines, fatigue, executive function, tremors, and IQ; Table 1) was counted in each group and a contingency table was built for each clinical outcome. A χ2 test was used to assess the incidence of biochemical outcomes and clinical manifestations within each group (Fig. 3C). The clinical outcomes that segregated with mild or severe MD were (in decreasing order of significance) low executive function (as judged by BDS-2 ≤ 14; P = 0.0008), low IQ (<90; P = 0.006), tremors (P = 0.045), and FXTAS diagnosis (P = 0.049). The segregation of other clinical outcomes, some of which have been linked to MD [i.e., migraines (60), executive ability (61), fatigue, ASD (24), ADHD (30, 62), and anxiety and depression (63, 64)], did not reach significance within this cohort of carriers, probably because of the relatively small sample in this study.
Figure 3.
Segregation of clinical symptoms with mitochondrial function. The 95% CIs were calculated with control values for each mitochondrial outcome significantly different between controls and carriers of the premutation (citrate synthase activity and coupling). A) According to these cutoffs, all subjects were classified into 3 clusters, defined as no MD, mild MD, and severe MD. B) Means ± sem of citrate synthase activity and RCRu for each group. The P values (obtained by ANOVA followed by Tukey’s post hoc test for multiple comparisons) are as follows: 0.032 (c); 0.016 (a); and <0.0001 (b, d, e). C) The frequency of each clinical symptom (shown in Table 1) was counted within each of the 3 clusters (no MD, with mild MD, or with severe MD; A) and shown as a percentage of symptomatic individuals within any given class. Low IQ was set at <90. Executive function was considered impaired if ≤14. Statistical analyses were performed by the 1-tailed χ2 test with Yates correction. Statistically significant clinical outcomes that segregated with MD were (in decreasing order of significance) low executive function (as judged by BDS-2; P = 0.0008), low IQ (P = 0.006), tremors (P = 0.045), and presence of FXTAS symptoms (P = 0.049). For these outcomes, the comparison of proportions between presence and absence of individual clinical symptoms was also computed. P values are as follows: 0.050 (d); 0.049 (g); 0.047 (e); 0.045 (h); 0.024 (c); 0.015 (f); 0.006 (a); and 0.0007 (b). D) Means ± sem of CGG repeats, IQ, and executive function scores (as judged by BDS-2) in the 3 groups. The P values (obtained by ANOVA followed by Tukey’s post hoc test for multiple comparisons) are as follows: 0.028 (a); 0.02 (e); 0.006 (d); 0.004 (c); and 0.002 (b).
Considering this classification based on mitochondrial function, continuous variables, such as CGG repeats and BDS-2 and IQ scores were averaged for each group and a statistical comparison was performed by 1-way ANOVA (Fig. 3D). Longer CGG repeats were observed in both the mild and severe MD groups relative to the no-MD one. Although a causal effect cannot be shown, severe MD was associated with executive function (BDS-2) and deficits and IQ scores below average, suggesting that these outcomes are linked to severe energy deficits, more so than either CGG repeats or an FXTAS diagnosis.
DISCUSSION
Mitochondria are at the center of ATP production in aerobic tissues, and their dysfunction is critical for initiating and propagating cognitive and behavioral abnormalities in neurodegenerative diseases (65). Besides their role as the cellular “powerhouse,” a direct link between mitochondrial function and immune response has been emerging (6, 66). In this regard, the study of lymphocytes (immune cells) in the context of FMR1 premutation brings a novel approach to understanding the interaction between bioenergetics and immunity, emphasizing the crosstalk between neuroinflammation and mitochondria and the CNS (67). Indeed, several reports have shown differential expression of genes in peripheral white blood cells in disorders of the CNS (68–72), and shared expression profiles between different CNS tissues and the blood (73) suggest the use of peripheral blood cells as surrogates for the brain. Moreover, individual gene expression variations of multiple brain regions correlate with those of blood in nonhuman primates (74).
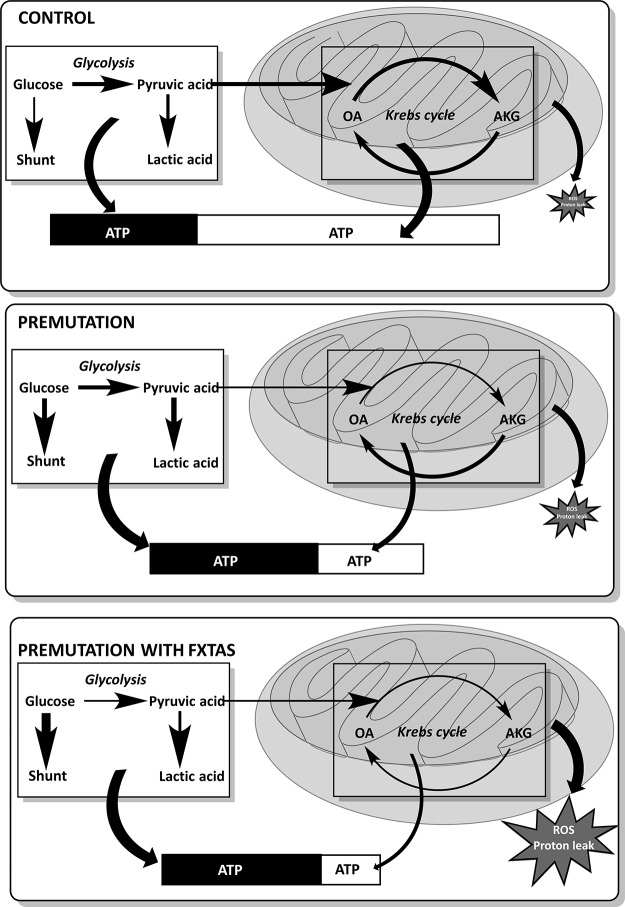
In this study, the regulation of energy metabolism in freshly isolated PBMCs from premutation carriers, as judged by their relatively lower overall oxphos capacity (lower coupling and mass) and increased glycolysis, differed significantly from that in age-matched controls (Fig. 4). Our study provided evidence of deficits (mass and phosphorylating capacity) in mitochondria-derived ATP in cells from carriers, which appears to be more evident in FXTAS-affected carriers, as judged by a more severe uncoupling, complex I activity deficits, higher proton leak, and ROS production (higher state 4 oxygen uptake rate) and lower SRC than FXTAS-free carriers (Fig. 4). The presence of loosely coupled mitochondria in PBMCs from premutation carriers without those carriers having a phenotype consistent with hypermetabolism (i.e., lower body mass index, polydipsia, polyphagia, increased perspiration, muscle wasting, or increased body temperature) can be explained by the tissue-specific protein expression of FMRP (highest in PBMCs, followed by lung and fetal ovary; GeneCards, http://www.genecards.org/), the role of FMRP in embryo development, and the energy threshold of the brain (75, 76). Although citrate synthase activity in PBMCs declined with age, as also observed by others (40), the lower levels observed in carriers regardless of age seem to signal a premature aging process, as it has been observed in skeletal muscle of elderly individuals compared with adults (77). Although the lower citrate synthase activity could be viewed as a strategy to shrink the matrix to gain inner membrane surface to increase ATP output to offset lower coupling between electron transport and ATP synthesis or lower ETC activity, the combination of both lower citrate synthase activity and lower ETC capacity in carriers point to a lower mass of functional mitochondria/cell. The lower oxphos was accompanied by a shift toward glycolysis favoring a Warburg effect (78), especially in cells from carriers not affected by FXTAS, whereas in those with FXTAS, the glucose flux seems to be redirected toward the pentose phosphate shunt (Fig. 4).
Figure 4.
Dysregulation of glycolysis and bioenergetic pathways in PBMCs from asymptomatic and FXTAS-symptomatic premutation-bearing individuals. In control cells, glucose is mainly used by oxphos, followed by glycolysis (as judged by ATP production) with almost negligible values shunted to the pentose phosphate pathway. The production of ATP is mainly derived from mitochondria compared to that of glycolysis (2-fold). In cells from premutation carriers, the glycolytic flux is enhanced with glucose also shunted to the pentose phosphate pathway to generate NADPH to provide reducing equivalents to the antioxidant defenses. Some pyruvate is directed to mitochondria in cells from FXTAS-free premutation but, because of higher uncoupling and lower citrate synthase of these cells compared to controls, the ratio of mitochondrial ATP to glycolytic is 0.6. Proteomic data supported a more predominant role for the second half of the Krebs cycle (from α-ketoglutarate to oxaloacetate) probably sustained by the anaplerosis of Glu/Gln (as judged by the higher abundance of glutamate dehydrogenase). The ensuing lower production of citrate may result in lower fatty acid and cholesterol syntheses as well as arachidonate-derived products (prostaglandins). Oxaloacetate will be then used to generate Asp, Ala, pyruvate, and others. In the case of cells from carriers affected with FXTAS, a more significant flux of glucose is shunted to the pentose phosphate pathway (higher abundance of glucose-6-phosphate-dehydrogenase than controls and FXTAS-free carriers) and glycolysis (higher levels of glycolytic ATP than controls but not than FXTAS-free carriers). The production of ATP is mainly glycolytic (ratio of mitochondrial ATP to glycolytic is 2.75) supported by the higher uncoupling (vs. controls and FXTAS-free carriers) and lower citrate synthase (vs. controls) of these cells. Based on proteomic data, lower overall Krebs cycle activity is expected with (based on functional data) increased production of mitochondrial ROS and increased proton leak. The total ATP levels are visualized as a bar in which the proportion of ATP derived from glycolysis is depicted in black and that of oxphos in white. The bars representing the ATP content in cells from carriers, are characterized as a percentage of that in control cells, to visualize the lower ATP content of these cells (61 and 49% of controls, for FXTAS-free and -affected carriers, respectively). Thicker and thinner arrows symbolize increased and decreased fluxes, respectively. Abbreviations: OA, oxaloacetate; AKG, α-ketoglutarate.
The strategy of switching to glycolysis may be perceived as having some beneficial effects: 1) lower citrate synthase activity—by lowering cytosolic citrate concentration—may control fatty acid and cholesterol syntheses but, more important, inflammatory responses via prostaglandin synthesis (79); and 2) mitochondrial uncoupling favors lower ROS production resulting in neuroprotective effects (80–83). However, the underexpression of pathways such as those involved in the detoxification of methylglyoxal and hydrogen peroxide (peroxiredoxins) clearly indicates an imbalance between oxidative damage (ROS-mediated or methylglyoxal-derived advanced glycation end products) and responses to oxidative stress, which may underlie the activation of prosenescence pathways. In addition, the control of the proinflammatory immune response, which depends on the balance between glycolysis and mitochondria-derived ATP (6–9, 84), leaves the Warburg effect to sustain a more proinflammatory response in premutation carriers (85, 86). Although the lower complex I activity observed in FXTAS-affected individuals compared to asymptomatic ones may be interpreted as an adaptive response to counteract the proinflammatory cytotoxic response favoring a preferential use of fatty acids over glucose [the “alternate activation pathway” (85, 86)], the lower content of mitochondrial fatty acid oxidation proteins undermines this attempt.
Along these lines, the presence of the premutation segregated with not only lower oxphos but also with a disrupted extracellular matrix and cytoskeleton organization and integrin signaling. In this regard, mitochondrial defects have been reported in carriers of lamin A pathogenic mutations along with increased oxidative stress, decreased DNA repair response, and premature cellular aging (87, 88), similar to the lamin A/C dysregulation reported in carriers of the premutation (89). Thus, it is possible that cellular stress derived from any of the current pathogenic mechanisms proposed for the premutation—higher expression of FMR1 with the sequestration of critical targets for cell growth (90–94), accumulation of toxic FMRP-related proteins generated via (repeat-associated non-AUG) translation (95), cotranscriptional processes of the R-loop promoted by the GC-rich FMR1 5′-UTR region (96), low FMRP expression during early stages of life (35), dysregulated Zn homeostasis with faulty import, and processing of mitochondrial proteins (3, 4, 35)—may result in a disrupted cytoskeleton and matrix organization that triggers integrin signaling, which in turn, may activate a hypoxic-like response, with enhanced tumorigenic-like activity perpetuating increased glycolysis and preventing a normal restoration of mitochondrial function.
Finally, genetically determined differences in the metabolic efficiency in PBMCs may affect not only the bioenergetics of immune cells but also, more important, those of other cells (such as neurons) that rely exclusively on oxphos for their ATP (97). Energy deficits, such as those observed in this study, have the potential to affect brain cognition and function, increasing the susceptibility of carriers to the development of neurologic or behavioral problems. The switch from oxphos to glycolysis may result, not only in neuronal energetic deficits but also in a broader context, in the dysfunction of mitochondria-centered immune response and neurotransmitter metabolism. This latter event may have a significant deleterious impact that contributes to the development of some of the clinical and neurologic problems observed in premutation carriers that segregate with MD (such as lower executive function and IQ and the presence of tremors) as well as the development of FXTAS later in life.
ACKNOWLEDGMENTS
The authors thank all families that provided the samples, making the study possible. The study was funded by U.S. National Institutes for Health (NIH) National Institute of Environmental Health Sciences Grants ES12691 and ES020392; the NIH Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant HD036071; Simons Foundation Grant 271406; MIND Institute Intellectual and Developmental Disabilities Research Center Grant U54 HD079125; and Health and Human Administration of Developmental Disabilities Grant 90DD0596. R.H. has received funding from Novartis, Roche/Genentech, Alcobra, and Neuren for treatment trials in FXS, autism, and Down syndrome, and has also consulted with Novartis and Roche/Genentech regarding treatment for FXS.
Glossary
- ADHD
attention-deficit/hyperactivity disorder
- ASD
autism spectrum disorder
- BDS2
Behavioral Dyscontrol Scale II, Second Edition
- CI
confidence interval
- ETC
electron transport chain
- FCCP
carbonylcyanide-p-trifluoromethoxyphenylhydrazone
- FMR1
fragile X mental retardation 1
- FXTAS
fragile X-associated tremor/ataxia syndrome
- LDH
lactate dehydrogenase
- MD
mitochondrial dysfunction
- MS/MS
tandem mass spectrometry
- OMIM
Online Mendelian Inheritance in Man
- oxphos
oxidative phosphorylation
- PARK
Parkinson’s disease protein
- PBMC
peripheral blood mononuclear cell
- PPAR
peroxisome proliferator-activated receptor
- RCR
respiratory control ratio
- RCRu
RCR under uncoupling conditions
- ROS
reactive oxygen species
- SRC
spare respiratory capacity
- TMPD
N,N,N′,N′-tetramethyl-p-phenylenediamine
- WAIS-IV
Wechsler Adult Intelligence Scale, Fourth Edition
- WISC-V
Wechsler Intelligence Scale for Children, Fifth Edition
AUTHOR CONTRIBUTIONS
E. Napoli carried out all polarographic measurements and data and statistical analyses, contributed to the writing of the manuscript, and revised and approved the final version as submitted; G. Song carried out citrate synthase activity measurements, revised the manuscript, and approved the final manuscript as submitted; R. Hagerman carried out clinical assessment of the carriers, wrote clinical findings, revised the manuscript, and approved the final manuscript as submitted; A. Schneider carried out cognitive and psychological testing on the patients, revised the manuscript, and approved the final manuscript; M.A.A.A. Eldeeb and A. Azarang carried out data analysis of clinical findings, revised the manuscript, and approved the final manuscript; F. Tassone provided molecular data on CGG repeat sizing, revised the manuscript, and approved the final manuscript as submitted; C. Giulivi conceptualized and designed the study, wrote most of the manuscript, and approved the final manuscript as submitted.
REFERENCES
- 1.Hagerman R., Hagerman P. (2013) Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurol. 12, 786–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klingenberg M., Echtay K. S. (2001) Uncoupling proteins: the issues from a biochemist point of view. Biochim. Biophys. Acta 1504, 128–143 [DOI] [PubMed] [Google Scholar]
- 3.Ross-Inta C., Omanska-Klusek A., Wong S., Barrow C., Garcia-Arocena D., Iwahashi C., Berry-Kravis E., Hagerman R. J., Hagerman P. J., Giulivi C. (2010) Evidence of mitochondrial dysfunction in fragile X-associated tremor/ataxia syndrome. Biochem. J. 429, 545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Napoli E., Ross-Inta C., Wong S., Omanska-Klusek A., Barrow C., Iwahashi C., Garcia-Arocena D., Sakaguchi D., Berry-Kravis E., Hagerman R., Hagerman P. J., Giulivi C. (2011) Altered zinc transport disrupts mitochondrial protein processing/import in fragile X-associated tremor/ataxia syndrome. Hum. Mol. Genet. 20, 3079–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chretien D., Rustin P., Bourgeron T., Rötig A., Saudubray J. M., Munnich A. (1994) Reference charts for respiratory chain activities in human tissues. Clin. Chim. Acta 228, 53–70 [DOI] [PubMed] [Google Scholar]
- 6.Arnoult D., Soares F., Tattoli I., Girardin S. E. (2011) Mitochondria in innate immunity. EMBO Rep. 12, 901–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cloonan S. M., Choi A. M. (2012) Mitochondria: commanders of innate immunity and disease? Curr. Opin. Immunol. 24, 32–40 [DOI] [PubMed] [Google Scholar]
- 8.Fossati G., Moulding D. A., Spiller D. G., Moots R. J., White M. R., Edwards S. W. (2003) The mitochondrial network of human neutrophils: role in chemotaxis, phagocytosis, respiratory burst activation, and commitment to apoptosis. J. Immunol. 170, 1964–1972 [DOI] [PubMed] [Google Scholar]
- 9.West A. P., Shadel G. S., Ghosh S. (2011) Mitochondria in innate immune responses. Nat. Rev. Immunol. 11, 389–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klegeris A., McGeer E. G., McGeer P. L. (2007) Therapeutic approaches to inflammation in neurodegenerative disease. Curr. Opin. Neurol. 20, 351–357 [DOI] [PubMed] [Google Scholar]
- 11.Lozano D., Gonzales-Portillo G. S., Acosta S., de la Pena I., Tajiri N., Kaneko Y., Borlongan C. V. (2015) Neuroinflammatory responses to traumatic brain injury: etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatr. Dis. Treat. 11, 97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harikrishnareddy D., Misra S., Upadhyay S., Modi M., Medhi B. (2015) Roots to start research in amyotrophic lateral sclerosis: molecular pathways and novel therapeutics for future. Rev. Neurosci. 26, 161–181 [DOI] [PubMed] [Google Scholar]
- 13.Floyd R. A. (1999) Antioxidants, oxidative stress, and degenerative neurological disorders. Proc. Soc. Exp. Biol. Med. 222, 236–245 [DOI] [PubMed] [Google Scholar]
- 14.Dhull D. K., Jindal A., Dhull R. K., Aggarwal S., Bhateja D., Padi S. S. (2012) Neuroprotective effect of cyclooxygenase inhibitors in ICV-STZ induced sporadic Alzheimer’s disease in rats. J. Mol. Neurosci. 46, 223–235 [DOI] [PubMed] [Google Scholar]
- 15.Blandini F. (2013) Neural and immune mechanisms in the pathogenesis of Parkinson’s disease. J. Neuroimmune Pharmacol. 8, 189–201 [DOI] [PubMed] [Google Scholar]
- 16.Hmadcha A., De Diego Y., Pintado E. (1998) Assessment of FMR1 expression by reverse transcriptase-polymerase chain reaction of KH domains. J. Lab. Clin. Med. 131, 170–173 [DOI] [PubMed] [Google Scholar]
- 17.Shanks N., Windle R. J., Perks P. A., Harbuz M. S., Jessop D. S., Ingram C. D., Lightman S. L. (2000) Early-life exposure to endotoxin alters hypothalamic-pituitary-adrenal function and predisposition to inflammation. Proc. Natl. Acad. Sci. USA 97, 5645–5650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boissé L., Mouihate A., Ellis S., Pittman Q. J. (2004) Long-term alterations in neuroimmune responses after neonatal exposure to lipopolysaccharide. J. Neurosci. 24, 4928–4934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellis S., Mouihate A., Pittman Q. J. (2005) Early life immune challenge alters innate immune responses to lipopolysaccharide: implications for host defense as adults. FASEB J. 19, 1519–1521 [DOI] [PubMed] [Google Scholar]
- 20.Spencer S. J., Heida J. G., Pittman Q. J. (2005) Early life immune challenge: effects on behavioural indices of adult rat fear and anxiety. Behav. Brain Res. 164, 231–238 [DOI] [PubMed] [Google Scholar]
- 21.Giulivi C., Napoli E., Schwartzer J., Careaga M., Ashwood P. (2013) Gestational exposure to a viral mimetic poly(i:C) results in long-lasting changes in mitochondrial function by leucocytes in the adult offspring. Mediators Inflamm. 2013, 609602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacquemont S., Hagerman R. J., Leehey M., Grigsby J., Zhang L., Brunberg J. A., Greco C., Des Portes V., Jardini T., Levine R., Berry-Kravis E., Brown W. T., Schaeffer S., Kissel J., Tassone F., Hagerman P. J. (2003) Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am. J. Hum. Genet. 72, 869–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tassone F., Hagerman R. J., Taylor A. K., Gane L. W., Godfrey T. E., Hagerman P. J. (2000) Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am. J. Hum. Genet. 66, 6–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giulivi C., Zhang Y. F., Omanska-Klusek A., Ross-Inta C., Wong S., Hertz-Picciotto I., Tassone F., Pessah I. N. (2010) Mitochondrial dysfunction in autism. JAMA 304, 2389–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Napoli E., Wong S., Hung C., Ross-Inta C., Bomdica P., Giulivi C. (2013) Defective mitochondrial disulfide relay system, altered mitochondrial morphology and function in Huntington’s disease. Hum. Mol. Genet. 22, 989–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luckhart S., Giulivi C., Drexler A. L., Antonova-Koch Y., Sakaguchi D., Napoli E., Wong S., Price M. S., Eigenheer R., Phinney B. S., Pakpour N., Pietri J. E., Cheung K., Georgis M., Riehle M. (2013) Sustained activation of Akt elicits mitochondrial dysfunction to block Plasmodium falciparum infection in the mosquito host. PLoS Pathog. 9, e1003180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keller A., Nesvizhskii A. I., Kolker E., Aebersold R. (2002) Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 74, 5383–5392 [DOI] [PubMed] [Google Scholar]
- 28.Nesvizhskii A. I., Keller A., Kolker E., Aebersold R. (2003) A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 75, 4646–4658 [DOI] [PubMed] [Google Scholar]
- 29.Tabb D. L. (2008) What’s driving false discovery rates? J. Proteome Res. 7, 45–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Napoli E., Tassone F., Wong S., Angkustsiri K., Simon T. J., Song G., Giulivi C. (2015) Mitochondrial citrate transporter-dependent metabolic signature in the 22q11.2 deletion syndrome. J. Biol. Chem. 290, 23240–23253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grigsby J., Kaye K., Eilertsen T. B., Kramer A. M. (2000) The behavioral dyscontrol scale and functional status among elderly medical and surgical rehabilitation patients. J. Clin. Geropsychol. 6, 259–268 [Google Scholar]
- 32.Warburg O. (1956) On respiratory impairment in cancer cells. Science 124, 269–270 [PubMed] [Google Scholar]
- 33.Gambhir S. S. (2002) Molecular imaging of cancer with positron emission tomography. Nat. Rev. Cancer 2, 683–693 [DOI] [PubMed] [Google Scholar]
- 34.Ross-Inta C., Omanska-Klusek A., Wong S., Barrow C., Garcia-Arocena D., Iwahashi C., Berry-Kravis E., Hagerman R. J., Hagerman P. J., Giulivi C. (2010) Evidence of mitochondrial dysfunction in fragile X-associated tremor/ataxia syndrome. Biochem. J. 429, 545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Napoli E., Ross-Inta C., Song G., Wong S., Hagerman R., Gane L. W., Smilowitz J. T., Tassone F., Giulivi C. (2016) Premutation in the fragile X mental retardation 1 (FMR1) gene affects maternal Zn-milk and perinatal brain bioenergetics and scaffolding. Front. Neurosci. 10, 159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koppenol W. H., Bounds P. L., Dang C. V. (2011) Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 11, 325–337 [DOI] [PubMed] [Google Scholar]
- 37.Porporato P. E., Dhup S., Dadhich R. K., Copetti T., Sonveaux P. (2011) Anticancer targets in the glycolytic metabolism of tumors: a comprehensive review. Front. Pharmacol. 2, 49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armstrong D. L., Eisenstein M., Zidovetzki R., Jacob C. O. (2015) Systemic lupus erythematosus-associated neutrophil cytosolic factor 2 mutation affects the structure of NADPH oxidase complex. J. Biol. Chem. 290, 12595–12602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Čapková M., Houstek J., Hansíková H., Hainer V., Kunesová M., Zeman J. (2002) Activities of cytochrome c oxidase and citrate synthase in lymphocytes of obese and normal-weight subjects. Int. J. Obes. Relat. Metab. Disord. 26, 1110–1117 [DOI] [PubMed] [Google Scholar]
- 40.Pastoris O., Boschi F., Verri M., Baiardi P., Felzani G., Vecchiet J., Dossena M., Catapano M. (2000) The effects of aging on enzyme activities and metabolite concentrations in skeletal muscle from sedentary male and female subjects. Exp. Gerontol. 35, 95–104 [DOI] [PubMed] [Google Scholar]
- 41.Short K. R., Bigelow M. L., Kahl J., Singh R., Coenen-Schimke J., Raghavakaimal S., Nair K. S. (2005) Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. USA 102, 5618–5623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang D. W., Sherman B. T., Lempicki, R. A. (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 [DOI] [PubMed] [Google Scholar]
- 43.Huang D. W., Sherman B. T., Lempicki, R. A. (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Catherman A. D., Durbin K. R., Ahlf D. R., Early B. P., Fellers R. T., Tran J. C., Thomas P. M., Kelleher N. L. (2013) Large-scale top-down proteomics of the human proteome: membrane proteins, mitochondria, and senescence. Mol. Cell. Proteomics 12, 3465–3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaca Jacome A. S., Rabilloud T., Schaeffer-Reiss C., Rompais M., Ayoub D., Lane L., Bairoch A., Van Dorsselaer A., Carapito C. (2015) N-terminome analysis of the human mitochondrial proteome. Proteomics 15, 2519–2524 [DOI] [PubMed] [Google Scholar]
- 46.Laughton J. D., Charnay Y., Belloir B., Pellerin L., Magistretti P. J., Bouras C. (2000) Differential messenger RNA distribution of lactate dehydrogenase LDH-1 and LDH-5 isoforms in the rat brain. Neuroscience 96, 619–625 [DOI] [PubMed] [Google Scholar]
- 47.Markert C. L., Shaklee J. B., Whitt G. S. (1975) Evolution of a gene: multiple genes for LDH isozymes provide a model of the evolution of gene structure, function and regulation. Science 189, 102–114 [DOI] [PubMed] [Google Scholar]
- 48.Friedman J. R., Mourier A., Yamada J., McCaffery J. M., Nunnari J. (2015) MICOS coordinates with respiratory complexes and lipids to establish mitochondrial inner membrane architecture. eLife 4, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Darshi M., Mendiola V. L., Mackey M. R., Murphy A. N., Koller A., Perkins G. A., Ellisman M. H., Taylor S. S. (2011) ChChd3, an inner mitochondrial membrane protein, is essential for maintaining crista integrity and mitochondrial function. J. Biol. Chem. 286, 2918–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiong H., Wang D., Chen L., Choo Y. S., Ma H., Tang C., Xia K., Jiang W., Ronai Z., Zhuang X., Zhang Z. (2009) Parkin, PINK1, and DJ-1 form a ubiquitin E3 ligase complex promoting unfolded protein degradation. J. Clin. Invest. 119, 650–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang B., Xiong H., Sun P., Zhang Y., Wang D., Hu Z., Zhu Z., Ma H., Pan Q., Xia J. H., Xia K., Zhang Z. (2006) Association of PINK1 and DJ-1 confers digenic inheritance of early-onset Parkinson’s disease. Hum. Mol. Genet. 15, 1816–1825 [DOI] [PubMed] [Google Scholar]
- 52.Junn E., Jang W. H., Zhao X., Jeong B. S., Mouradian M. M. (2009) Mitochondrial localization of DJ-1 leads to enhanced neuroprotection. J. Neurosci. Res. 87, 123–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conca Dioguardi C., Uslu B., Haynes M., Kurus M., Gul M., Miao D. Q., De Santis L., Ferrari M., Bellone S., Santin A., Giulivi C., Hoffman G., Usdin K., Johnson J. (2016) Granulosa cell and oocyte mitochondrial abnormalities in a mouse model of fragile X primary ovarian insufficiency. Mol. Hum. Reprod. 22, 384–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Napoli E., Song G., Wong S., Hagerman R., Giulivi C. (2016) Altered bioenergetics in primary dermal fibroblasts from adult carriers of the fmr1 premutation before the onset of the neurodegenerative disease fragile X-associated tremor/ataxia syndrome. [E-pub ahead of print] Cerebellum doi:10.1007/s12311-016-0779-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaplan E. S., Cao Z., Hulsizer S., Tassone F., Berman R. F., Hagerman P. J., Pessah I. N. (2012) Early mitochondrial abnormalities in hippocampal neurons cultured from Fmr1 pre-mutation mouse model. J. Neurochem. 123, 613–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rakhshandehroo M., Knoch B., Müller M., Kersten S. (2010) Peroxisome proliferator-activated receptor alpha target genes. PPAR Res. 2010, 612089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hammond G., Thomas C. L., Schiavo G. (2004) Nuclear phosphoinositides and their functions. Curr. Top. Microbiol. Immunol. 282, 177–206 [DOI] [PubMed] [Google Scholar]
- 58.De Matteis M. A., Di Campli A., Godi A. (2005) The role of the phosphoinositides at the Golgi complex. Biochim. Biophys. Acta 1744, 396–405 [DOI] [PubMed] [Google Scholar]
- 59.Roth M. G. (2004) Phosphoinositides in constitutive membrane traffic. Physiol. Rev. 84, 699–730 [DOI] [PubMed] [Google Scholar]
- 60.Sparaco M., Feleppa M., Lipton R. B., Rapoport A. M., Bigal M. E. (2006) Mitochondrial dysfunction and migraine: evidence and hypotheses. Cephalalgia 26, 361–372 [DOI] [PubMed] [Google Scholar]
- 61.Carvalho A. F., Miskowiak K. K., Hyphantis T. N., Kohler C. A., Alves G. S., Bortolato B., G Sales P. M., Machado-Vieira R., Berk M., McIntyre R. S. (2014) Cognitive dysfunction in depression - pathophysiology and novel targets. CNS Neurol. Disord. Drug Targets 13, 1819–1835 [DOI] [PubMed] [Google Scholar]
- 62.Meechan D. W., Maynard T. M., Tucker E. S., LaMantia A. S. (2011) Three phases of DiGeorge/22q11 deletion syndrome pathogenesis during brain development: patterning, proliferation, and mitochondrial functions of 22q11 genes. Int. J. Dev. Neurosci. 29, 283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khalifeh, S., Oryan, S., Khodagholi, F., Digaleh, H., Shaerzadeh, F., Maghsoudi, N., Zarrindast, M. R. (2015) Complexity of compensatory effects in Nrf1 knockdown: linking undeveloped anxiety-like behavior to prevented mitochondrial dysfunction and oxidative stress. Cell Mol. Neurobiol. 36, 553–563 [DOI] [PMC free article] [PubMed]
- 64.Da Pozzo E., Costa B., Martini C. (2012) Translocator protein (TSPO) and neurosteroids: implications in psychiatric disorders. Curr. Mol. Med. 12, 426–442 [DOI] [PubMed] [Google Scholar]
- 65.Gibson G. E., Starkov A., Blass J. P., Ratan R. R., Beal M. F. (2010) Cause and consequence: mitochondrial dysfunction initiates and propagates neuronal dysfunction, neuronal death and behavioral abnormalities in age-associated neurodegenerative diseases. Biochim. Biophys. Acta 1802, 122–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luckhart S., Pakpour N., Giulivi C. (2015) Host-pathogen interactions in malaria: cross-kingdom signaling and mitochondrial regulation. Curr. Opin. Immunol. 36, 73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sekar S., McDonald J., Cuyugan L., Aldrich J., Kurdoglu A., Adkins J., Serrano G., Beach T. G., Craig D. W., Valla J., Reiman E. M., Liang W. S. (2015) Alzheimer’s disease is associated with altered expression of genes involved in immune response and mitochondrial processes in astrocytes. Neurobiol. Aging 36, 583–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Washizuka S., Iwamoto K., Kakiuchi C., Bundo M., Kato T. (2009) Expression of mitochondrial complex I subunit gene NDUFV2 in the lymphoblastoid cells derived from patients with bipolar disorder and schizophrenia. Neurosci. Res. 63, 199–204 [DOI] [PubMed] [Google Scholar]
- 69.Padmos R. C., Hillegers M. H., Knijff E. M., Vonk R., Bouvy A., Staal F. J., de Ridder D., Kupka R. W., Nolen W. A., Drexhage H. A. (2008) A discriminating messenger RNA signature for bipolar disorder formed by an aberrant expression of inflammatory genes in monocytes. Arch. Gen. Psychiatry 65, 395–407 [DOI] [PubMed] [Google Scholar]
- 70.Scherzer C. R., Eklund A. C., Morse L. J., Liao Z., Locascio J. J., Fefer D., Schwarzschild M. A., Schlossmacher M. G., Hauser M. A., Vance J. M., Sudarsky L. R., Standaert D. G., Growdon J. H., Jensen R. V., Gullans S. R. (2007) Molecular markers of early Parkinson’s disease based on gene expression in blood. Proc. Natl. Acad. Sci. USA 104, 955–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kong S. W., Collins C. D., Shimizu-Motohashi Y., Holm I. A., Campbell M. G., Lee I. H., Brewster S. J., Hanson E., Harris H. K., Lowe K. R., Saada A., Mora A., Madison K., Hundley R., Egan J., McCarthy J., Eran A., Galdzicki M., Rappaport L., Kunkel L. M., Kohane I. S. (2012) Characteristics and predictive value of blood transcriptome signature in males with autism spectrum disorders. PLoS One 7, e49475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Glatt, S. J., Tsuang, M. T., Winn, M., Chandler, S. D., Collins, M., Lopez, L., Weinfeld, M., Carter, C., Schork, N., Pierce, K., Courchesne, E. (2012) Blood-based gene expression signatures of infants and toddlers with autism. J. Am. Acad. Child Adolesc. Psychiatry 51, 934–944.e932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sullivan P. F., Fan C., Perou C. M. (2006) Evaluating the comparability of gene expression in blood and brain. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 141, 261–268 [DOI] [PubMed] [Google Scholar]
- 74.Jasinska A. J., Service S., Choi O. W., DeYoung J., Grujic O., Kong S. Y., Jorgensen M. J., Bailey J., Breidenthal S., Fairbanks L. A., Woods R. P., Jentsch J. D., Freimer N. B. (2009) Identification of brain transcriptional variation reproduced in peripheral blood: an approach for mapping brain expression traits. Hum. Mol. Genet. 18, 4415–4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rossignol R., Faustin B., Rocher C., Malgat M., Mazat J. P., Letellier T. (2003) Mitochondrial threshold effects. Biochem. J. 370, 751–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fornuskova D., Brantova O., Tesarova M., Stiburek L., Honzik T., Wenchich L., Tietzeova E., Hansikova H., Zeman J. (2008) The impact of mitochondrial tRNA mutations on the amount of ATP synthase differs in the brain compared to other tissues. Biochim. Biophys. Acta 1782, 317–325 [DOI] [PubMed] [Google Scholar]
- 77.Conley K. E., Jubrias S. A., Esselman P. C. (2000) Oxidative capacity and ageing in human muscle. J. Physiol. 526, 203–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin C. C., Cheng T. L., Tsai W. H., Tsai H. J., Hu K. H., Chang H. C., Yeh C. W., Chen Y. C., Liao C. C., Chang W. T. (2012) Loss of the respiratory enzyme citrate synthase directly links the Warburg effect to tumor malignancy. Sci. Rep. 2, 785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Infantino V., Convertini P., Cucci L., Panaro M. A., Di Noia M. A., Calvello R., Palmieri F., Iacobazzi V. (2011) The mitochondrial citrate carrier: a new player in inflammation. Biochem. J. 438, 433–436 [DOI] [PubMed] [Google Scholar]
- 80.Andrews Z. B., Diano S., Horvath T. L. (2005) Mitochondrial uncoupling proteins in the CNS: in support of function and survival. Nat. Rev. Neurosci. 6, 829–840 [DOI] [PubMed] [Google Scholar]
- 81.Mao W., Yu X. X., Zhong A., Li W., Brush J., Sherwood S. W., Adams S. H., Pan G. (1999) UCP4, a novel brain-specific mitochondrial protein that reduces membrane potential in mammalian cells. FEBS Lett. 443, 326–330 [DOI] [PubMed] [Google Scholar]
- 82.Sanchis D., Fleury C., Chomiki N., Goubern M., Huang Q., Neverova M., Grégoire F., Easlick J., Raimbault S., Lévi-Meyrueis C., Miroux B., Collins S., Seldin M., Richard D., Warden C., Bouillaud F., Ricquier D. (1998) BMCP1, a novel mitochondrial carrier with high expression in the central nervous system of humans and rodents, and respiration uncoupling activity in recombinant yeast. J. Biol. Chem. 273, 34611–34615 [DOI] [PubMed] [Google Scholar]
- 83.Fleury C., Neverova M., Collins S., Raimbault S., Champigny O., Levi-Meyrueis C., Bouillaud F., Seldin M. F., Surwit R. S., Ricquier D., Warden C. H. (1997) Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat. Genet. 15, 269–272 [DOI] [PubMed] [Google Scholar]
- 84.Chen Y., Lu H., Liu Q., Huang G., Lim C. P., Zhang L., Hao A., Cao X. (2012) Function of GRIM-19, a mitochondrial respiratory chain complex I protein, in innate immunity. J. Biol. Chem. 287, 27227–27235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pellegrino M. W., Nargund A. M., Kirienko N. V., Gillis R., Fiorese C. J., Haynes C. M. (2014) Mitochondrial UPR-regulated innate immunity provides resistance to pathogen infection. Nature 516, 414–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vats D., Mukundan L., Odegaard J. I., Zhang L., Smith K. L., Morel C. R., Wagner R. A., Greaves D. R., Murray P. J., Chawla A. (2006) Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 4, 13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Caron M., Auclair M., Donadille B., Béréziat V., Guerci B., Laville M., Narbonne H., Bodemer C., Lascols O., Capeau J., Vigouroux C. (2007) Human lipodystrophies linked to mutations in A-type lamins and to HIV protease inhibitor therapy are both associated with prelamin A accumulation, oxidative stress and premature cellular senescence. Cell Death Differ. 14, 1759–1767 [DOI] [PubMed] [Google Scholar]
- 88.Liu B., Wang J., Chan K. M., Tjia W. M., Deng W., Guan X., Huang J. D., Li K. M., Chau P. Y., Chen D. J., Pei D., Pendas A. M., Cadiñanos J., López-Otín C., Tse H. F., Hutchison C., Chen J., Cao Y., Cheah K. S., Tryggvason K., Zhou Z. (2005) Genomic instability in laminopathy-based premature aging. Nat. Med. 11, 780–785 [DOI] [PubMed] [Google Scholar]
- 89.Arocena D. G., Iwahashi C. K., Won N., Beilina A., Ludwig A. L., Tassone F., Schwartz P. H., Hagerman P. J. (2005) Induction of inclusion formation and disruption of lamin A/C structure by premutation CGG-repeat RNA in human cultured neural cells. Hum. Mol. Genet. 14, 3661–3671 [DOI] [PubMed] [Google Scholar]
- 90.Garcia-Arocena D., Hagerman P. J. (2010) Advances in understanding the molecular basis of FXTAS. Hum. Mol. Genet. 19(R1), R83–R89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hagerman P. J., Hagerman R. J. (2015) Fragile X-associated tremor/ataxia syndrome. Ann. N. Y. Acad. Sci. 1338, 58–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jin P., Duan R., Qurashi A., Qin Y., Tian D., Rosser T. C., Liu H., Feng Y., Warren S. T. (2007) Pur alpha binds to rCGG repeats and modulates repeat-mediated neurodegeneration in a Drosophila model of fragile X tremor/ataxia syndrome. Neuron 55, 556–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sellier C., Rau F., Liu Y., Tassone F., Hukema R. K., Gattoni R., Schneider A., Richard S., Willemsen R., Elliott D. J., Hagerman P. J., Charlet-Berguerand N. (2010) Sam68 sequestration and partial loss of function are associated with splicing alterations in FXTAS patients. EMBO J. 29, 1248–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sellier C., Freyermuth F., Tabet R., Tran T., He F., Ruffenach F., Alunni V., Moine H., Thibault C., Page A., Tassone F., Willemsen R., Disney M. D., Hagerman P. J., Todd P. K., Charlet-Berguerand N. (2013) Sequestration of DROSHA and DGCR8 by expanded CGG RNA repeats alters microRNA processing in fragile X-associated tremor/ataxia syndrome. Cell Rep. 3, 869–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Todd P. K., Oh S. Y., Krans A., He F., Sellier C., Frazer M., Renoux A. J., Chen K. C., Scaglione K. M., Basrur V., Elenitoba-Johnson K., Vonsattel J. P., Louis E. D., Sutton M. A., Taylor J. P., Mills R. E., Charlet-Berguerand N., Paulson H. L. (2013) CGG repeat-associated translation mediates neurodegeneration in fragile X tremor ataxia syndrome. Neuron 78, 440–455; correction 79, 402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Loomis E. W., Sanz L. A., Chédin F., Hagerman P. J. (2014) Transcription-associated R-loop formation across the human FMR1 CGG-repeat region. PLoS Genet. 10, e1004294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nicholls D. G., Budd S. L. (2000) Mitochondria and neuronal survival. Physiol. Rev. 80, 315–360 [DOI] [PubMed] [Google Scholar]