Abstract
Accumulating evidence suggests that the α7 subtype of nicotinic acetylcholine receptors (nAChRs) plays a key role in inflammatory processes, thought to be involved in the pathophysiology of neuropsychiatric diseases, such as schizophrenia and Alzheimer’s disease. Preclinical and clinical studies showed that the diminished suppression of P50 auditory evoked potentials in patients with schizophrenia may be associated with a decreased density of α7 nAChRs in the brain. This points to a role for auditory sensory gating (P50) as a translational biomarker. A number of agonists and positive allosteric modulators (PAMs) for α7 nAChR promoted beneficial effects in animal models with sensory gating and cognitive deficits. Additionally, several clinical studies showed that α7 nAChR agonists could improve suppression in auditory P50 evoked potentials, as well as cognitive deficits, and negative symptoms in patients with schizophrenia. Taken together, α7 nAChR presents as an extremely attractive therapeutic target for schizophrenia. In this article, the author discusses recent findings on α7 nAChR agonists such as DMXB-A, RG3487, TC-5619, tropisetron, EVP-6124 (encenicline), ABT-126, AQW051 and α7 nAChR PAMs such as JNJ-39393406, PNU-120596 and AVL-3288 (also known as UCI-4083), and their potential as therapeutic drugs for neuropsychiatric diseases, such as schizophrenia.
Keywords: α7 nicotinic acetylcholine receptors, schizophrenia, auditory sensory gating, agonists, allosteric positive modulators, biomarker
Introduction
Epidemiological studies showed that patients with psychiatric diseases, including schizophrenia, smoke more heavily than the general population, thereby increasing their morbidity and mortality from smoking-related illnesses [1-5]. This elevated smoking rate in schizophrenia is thought to be a form of self-medication [6, 7]. There are various interpretations of the high smoking rates in schizophrenia, including the ideas that smoking reduces the side effects of antipsychotics [8] and that it alleviates symptoms, including those of depression, anxiety, anhedonia and amotivation [9-11].
Nicotine, the main psychoactive ingredient in tobacco smoke, binds to nicotinic acetylcholine receptors (nAChRs). The main subtypes of nAChRs in the central nervous system (CNS) are the α4β2 and α7 subtypes [12]. Multiple lines of evidence suggest that α7 nAChR plays a key role of the pathophysiology of neuropsychiatric diseases, including schizophrenia, making this receptor subtype one of the most attractive therapeutic targets for these diseases [13-23]. In this article, I will review the recent findings that highlight α7 nAChR as a potential therapeutic target for schizophrenia.
P50 sensory gating as a disease biomarker
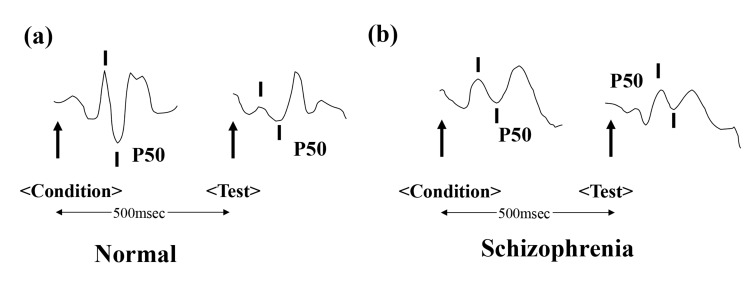
The P50 auditory evoked potential is a positive electroencephalography waveform that occurs 50 milliseconds (msec) after presentation of an auditory stimulus. When pairs of auditory stimuli are presented, with a 500 msec interstimulus interval, normal subjects show significantly reduced responses to the second stimulus. However, patients with schizophrenia fail to adequately inhibit their P50 response to the second stimulus (Fig. 1). This disability is deemed to be an underlying mechanism of cognitive impairment in schizophrenics, particularly attention deficits and lack of attention sustainment. A number of studies revealed abnormal suppression of P50, not only in schizophrenia patients [24-28], but also their unaffected relatives [29-32]. Recent meta-analyses support the findings of abnormal P50 suppression in patients with schizophrenia [33-36]. The P50 evoked potential has the highest effect size and is the most powerful and reliable neuroscience biomarker in schizophrenia [33]. Interestingly, diminished suppression of P50 was demonstrated in high-risk subjects and first-episode schizophrenic patients [37], although this finding was not replicated in other reports [38, 39]. Further detailed studies using large sample sizes will be needed to confirm the usefulness of auditory sensory gating P50 as an early biomarker for psychosis.
The use of translational biomarkers to validate animal models is a necessary process in the development of novel therapeutic drugs. Deficits in sensory information processing in schizophrenia are typically assessed in animal models using rodents. The hippocampal P20-N40 auditory evoked potential in rodents is thought to be analogous to the human P50 potential. Stevens et al. [40] reported a correlation between inhibitory gating (P20-N40) of the hippocampal auditory evoked response and hippocampal α7 nAChR density in inbred mouse strains. The auditory sensory gating deficits of DBA/2 mice with low density α7 nAChR have been utilized in an animal model of schizophrenia [41, 42]. The auditory sensory gating deficits of DBA/2 mice are improved by clozapine treatment and this effect is blocked by the α7 nAChR antagonist, α-bungarotoxin, implicating an α7 nAChR mediated mechanism in auditory sensory gating deficits [43].
Clozapine is reported to normalize P50 suppression in patients with schizophrenia unlike other antipsychotics [25, 44, 45], and this normalization corresponds with an improvement in clinical symptoms [44]. It is likely that clozapine blockade of 5-hydroxytrypta-mine-3 (5-HT3) receptors results in the release of acetylcholine from presynaptic terminals, which in turn stimulates α7 nAChR in the brain [46].
Postmortem studies of schizophrenia patients show decreased density of α7 nAChR in the dentate gyrus of the hippocampus, as well as area CA3, the reticular thalamic nucleus and the frontal and cingulate gyri [47-50]. Interestingly, this reduced expression of α7 nAChR has been linked to the degree of global cognitive deficits in patients with schizophrenia [51]. These results indicate that the P20-N40 auditory evoked potential in rodents and the P50 auditory evoked potential in humans could represent a translational biomarker from rodents to humans. This would make auditory evoked potentials an invaluable translational biomarker in the development of novel therapeutic drugs for schizophrenia [52].
Nicotine and auditory P50 deficits
Nicotine is known to restore P50 deficits in schizophrenic patients [53] and their first degree relatives [54]. However, there is no improvement in sensory gating deficits when mecamylamine, an α4β2 nAChR antagonist is administered in DBA/2 mice [55]. This led to the proposal that α7 nAChR, and not α4β2 nAChR, may be the major cholinergic receptor responsible for auditory P50 gating [56, 57].
Auditory P50 gating and the CHRNA7 gene
A genome-wide linkage analysis of nine multiplex families with schizophrenia revealed maximal linkage of the P50 deficit to chromosome 15q14, at a polymorphic marker, less than 120 kb from the α7 nAChR gene (CHRNA7), with a logarithm of odds (LOD) score of 5.3, Θ = 0.039 [58]. This linkage has been replicated in families from the National Institute of Mental Health (NIMH) Schizophrenia Genetics Initiative [59], but is not supported by all studies [60, 61]. A single nucleotide polymorphism in the 5’ core promoter region of the CHRNA7 gene is significantly associated with P50 suppression deficits [62, 63].
The Consortium on the Genetics of Schizophrenia (COGS), a 7-site study designed to eliminate site to site recording method differences, examined P50 suppression in 181 probands with schizophrenia, 429 of their first degree relatives, and 333 community comparison control subjects [64]. The results of this study led to the suggestion that auditory P50 deficits may be a possible schizophrenia endophenotype [64]. A recent analysis of 94 candidate genes and 12 endophenotypes for schizophrenia, obtained from COGS showed that the CHRNA7 gene is associated with schizophrenia [65].
It is reported that the CHRNA7 gene, located at chromosome 15q13.3 is partially duplicated [66]. The CHRFAM7A gene formed from the partial duplication of CHRNA7, acts as a dominant negative modulator of CNRNA7 function and is critical for receptor regulation in humans [67]. Furthermore, a 2-base pair deletion polymorphism in the partial duplication of CHRFAM7A shows association with schizophrenia [68].
α7 nAChR agonists
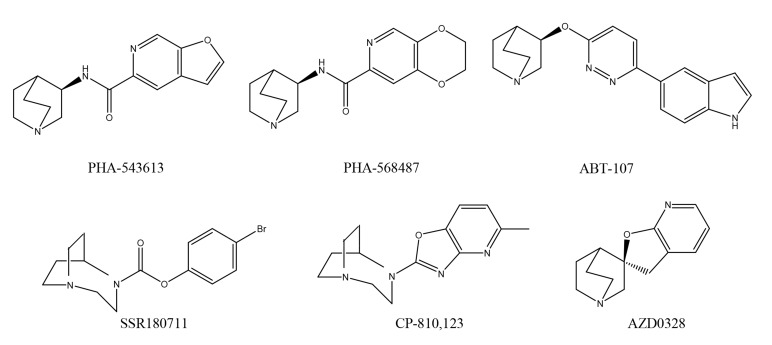
A number of α7 nAChR agonists have been developed as therapeutic drugs for the treatment of schizophrenia [13-23]. Agonists such as PHA-543613, N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]furo[2, 3-c]pyridine-5-carboxamide hydrochloride [69], PHA-568487, N-(3R)-1-azabicyclo[2.2.2]oct-3-yl-2, 3-dihydro-1, 4-benzodioxin-6-carboxamide fumarate [70], ABT-107, 5-(6-[(3R)-1-azabicyclo [2.2.2]oct-3-yloxy] pyridazin-3-yl)-1H-indole [71-73], SSR180711, 1, 4-diazabicyclo[3.2.2]nonane-4-carboxylic acid, 4-bromophenyl ester [74-76], CP-810123, 4-(5-methyloxazolo[4, 5-b]pyridin-2-yl)-1, 4-diazabicyclo[3.2.2]nonane [77], and AZD0328, (2′R)-spiro-[1-azabicyclo[2.2.2]octane-3, 2′(3′H)-furo[2, 3-b]pyridine [78, 79] (Fig. 2) were all discontinued after Phase I clinical trials, due to various adverse effects [80]. Next, I will discuss the drugs currently undergoing investigation in clinical trials.
DMXB-A
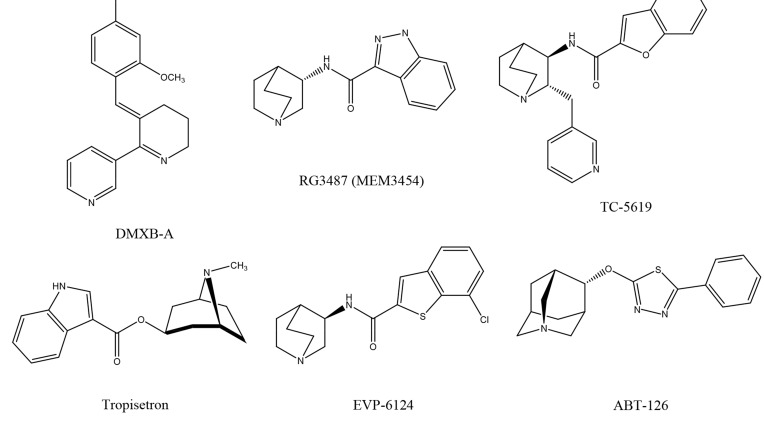
DMXB-A (formally called GTS-21), 3-(2, 4-dimethoxybenzy-lidene)-anabaseine (Fig. 3), is an analogue of anabaseine, a marine worm toxin, with a structure related to nicotine. DMXB-A is a partial agonist at the α7 nAChR and an antagonist at α4β2 nAChR. In addition, its major metabolite, 4-OH DMXB-A may be an α7 nAChR antagonist [22]. Reports suggest that DMXB-A improves the deficits in auditory sensory gating of DBA/2 mice [81-83] and isolation-reared rats [84].
A clinical study in healthy subjects showed positive effects for DMXB-A in attention, working memory and episodic memory [85]. A proof-of-concept study for DMXB-A in patients with schizophrenia demonstrated normalization of P50 deficits and cognitive improvement [86]. Although the Phase II study did not show improvements in the MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) Consensus Cognitive Battery, high doses (150 mg/day, b.i.d., 4-weeks) of DMXB-A improved negative symptoms [87].
A randomized, double-blind crossover study showed that DMXB-A administered at 150 mg/day, twice daily, for 4 weeks diminished hippocampal activity during pursuit eye movements [88]. These findings are consistent with the established function of α7 nAChR on inhibitory interneurons in the hippocampus [88]. A subsequent study showed that DMXB-A (75 and 150 mg/day) induced several alterations in the default network activity in schizophrenia, including a reduction in activity within the posterior cingulate, inferior parietal cortex, and medial frontal gyrus and an increase in precuneus activity [89]. Interestingly, the most robust difference, namely reduced activity in the posterior cingulate is affected by the CHRNA7 genotype. These findings suggest a dysfunction in α7 nAChR and the default network function in schizophrenia.
Because of low bioavailability and low half-life of DMXB-A in humans, a sustained-release DMXB-A-SR system has been developed. This is currently being investigated in a randomized, placebo-controlled study, of sustained-release DMXB-A-SR in patients with schizophrenia [90].
RG3487 (or MEM 3454)
RG3487 (formally called MEM3487), N-[(3S)-1-azabicyclo [2.2.2]oct-3-yl]-1H-indazole-3-carboxamide hydrochloride (Fig. 3), is an orally available, partial agonist at α7 nAChR (Ki = 6 nM for human α7 nAChR) and 5-HT3 antagonist, with no appreciable cross-reactivity with other nicotinic receptor subtypes or CNS targets [91]. RG3487 was effective at improving visual sustained attention, when given acutely in rats [92]. In another study, RG3487 improved object recognition memory in rats after acute or repeated administration. Furthermore, RG3487 improved apomorphine-induced prepulse inhibition (PPI) deficits and the N-methyl-D-aspartate (NMDA) receptor antagonist phencyclidine (PCP)-indu-ced impairments in an attentional set-shifting model [91]. These observations highlight RG3487 as a novel and potent drug capable of improving cognitive performance and sensorimotor gating.
A Phase 2 trial evaluating the safety and efficacy of RG3487 demonstrated statistically significant effects on cognition in patients with schizophrenia and Alzheimer’s disease [18, 20, 92]. However, there are currently no licensing plans from Roche for use of this drug in schizophrenia.
TC-5619
TC-5619, (2S, 3R)-N-[2-(pyridin-3-ylmethyl)-1-azabicyclo [2.2.2]oct-3-yl] benzo[b]-furan- 2-carboxamide (Fig. 3), is a novel partial agonist, with high affinity at α7 nAChR (Ki=1.40 nM for human α7 nAChR)[80, 93]. In the novel object recognition test in animals, TC-5619 improved short-term working memory. It also demonstrated therapeutic efficacy in the social withdrawal model in mice, suggesting an effectiveness in ameliorating the negative symptoms of schizophrenia [93].
A randomized, placebo-controlled study of TC-5619 in the USA and India, demonstrated that this drug improved executive function and negative symptoms in patients with schizophrenia [94]. Furthermore, it showed a statistically significant drug effect on working memory in tobacco users. TC-5619 was generally well tolerated with no clinically noteworthy safety findings. It showed statistically significant benefits on the Groton Maze Learning task of the CogState battery in patients, compared with the placebo group. This study pinpoints potential benefits for the use of TC-5619 in treating cognitive impairment and negative symptoms associated with schizophrenia. A 30-week, multi-center, double-blind, randomized, placebo-controlled study of TC-5619 (5 and 50 mg) in outpatients with schizophrenia is currently underway [95].
Tropisetron
Tropisetron (Fig. 3) is a potent 5-HT3 receptor antagonist, marketed outside of the United States for the treatment of patients with chemotherapy induced or postoperative nausea and vomiting [15, 18, 20]. Tropisetron is a partial, high affinity agonist at α7 nAChR, whereas ondansetron, another 5-HT3 receptor antagonist, shows weak affinity at α 7 nAChR [96, 97]. We previously reported that tropisetron improves deficient auditory inhibition processing in DBA/2 mice, and that this improvement could be antagonized by co-administration of methyllycaconitine (MLA) [98]. We also reported that PCP-induced cognitive deficits could be improved by subsequent subchronic administration of tropisetron, but not ondansetron, and that this improvement was also antagonized by co-administration of MLA [99]. Furthermore, tropisetron was capable of attenuating PPI deficits in rats after PCP or apomorphine dosing, and yet again, this effect was antagonized by MLA [100].
In addition, we reported that a single oral dose of tropisetron (10 mg) could improve P50 suppression deficits in non-smoking patients with schizophrenia [101]. In support of these findings, a randomized, placebo-controlled study of tropisetron found that administration of tropisetron (10 mg), but not placebo, significantly improved auditory sensory gating P50 deficits in non-smoking patients with schizophrenia [102]. Interestingly, we found that sustained visual attention in non-smoking patients was also significantly improved by tropisetron treatment [102]. Tropisetron was well tolerated in this trial, and was associated with no untoward effects.
Subsequent short-term treatment with tropisetron at 5, 10, or 20 mg/day for 10-days, showed an overall significant improvement in cognitive deficits in non-smoking patients with schizophrenia, at all doses, with 10 mg showing the greatest improvement in the immediate memory index score and 20 mg in the delayed memory index
score on the Repeatable Battery for the Assessment of Neuropsychological Status battery [103]. In addition, sensory gating P50 deficits showed recovery which correlated significantly with cognitive improvement. A recent, placebo-controlled study using tropisetron in conjunction with risperidone, showed that tropisetron (10 mg/day for 8-weeks) promoted greater enhancement in negative symptom scores and general psychopathology in patients with chronic stable schizophrenia [104].
These cumulative findings point towards tropisetron as a potential therapeutic drug for cognitive deficits and negative symptoms in schizophrenia. This drug is already in use worldwide for the treatment of various diseases, and further studies using large cohorts will be needed to confirm the efficacy of tropisetron in schizophrenia. To this end, a randomized, placebo-controlled study of tropisetron (10 mg/day for 12-weeks) plus risperidone (6 mg/day) in Chinese patients (n = 200) with early phase schizophrenia is currently underway [105].
EVP-6124 (Encenicline)
EVP-6124 (encenicline), (R)-7-chloro-N-quinuclidin-3-yl)benzo[b]thiophene-2-carboxamide (Fig. 3), is a potent and selective partial agonist at α7 nAChR (Ki = 9.98 nM for [3H]MLA binding, Ki = 4.33 nM for [125I]α-bungarotoxin binding) [106]. At a concentration of 10 μM, EVP-6124 lacked appreciable interaction with more than 60 molecular targets in a selectivity screening panel, which included receptors, ion channels, and amine transporters. Furthermore, EVP-6124 (0.3 mg/kg, orally) significantly restored memory function in scopolamine-treated rats, tested in the object recognition task, and this restorative effect was blocked by MLA [106].
A randomized, placebo-controlled Phase 2b study showed that when taken in combination with second-generation antipsychotics and using CogState battery tests, EVP-6124 exerted a clinically meaningful and statistically significant impact on patients’ overall cognition, the trial’s primary endpoint [107-109]. Additionally, EVP-6124 showed statistically significant clinical effects in key secondary endpoints, namely, improved clinical function, as assessed by the Schizophrenia Cognition Rating Scale, and reduced negative symptoms. Importantly, EVP-6124 was generally safe and well-tolerated during the three-month dosing period of the trial [110]. Double-blind, placebo-controlled, Phase 3 studies of EVP-6124 in patients with schizophrenia are currently underway [110]. In addition, two Phase III trials of EVP-6124 in patients with mild-to moderate Alzheimer’s disease are also underway [111].
ABT-126 and AQW-051
ABT-126 (AbbVie), (4s)-4-(5-phenyl-1, 3, 4-thiadiazol-2-yloxy)-1-azatricyclo[3.3.1.13, 7]decane (Fig. 3), is a novel partial agonist at α7 nAChR (Ki = 12 nM for human α7 nAChR, Ki = 1740 nM for α4β2 nAChR, Ki = 140 nM for 5-HT3 receptor) [112, 113]. A recent randomized, double-blind, placebo-controlled, multi-center Phase 2 study of ABT-126 in subjects (N=260) with mild-to-moderate Alzheimer’s related dementia, was presented at the Alzheimer’s Association International Conference [113]. High-dose ABT-126 improved Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog) scores in a manner similar to donepezil. This ADAS-cog improvement correlated with ABT-126 plasma levels. Several clinical studies of ABT-126 in patients with schizophrenia are underway [114-116].
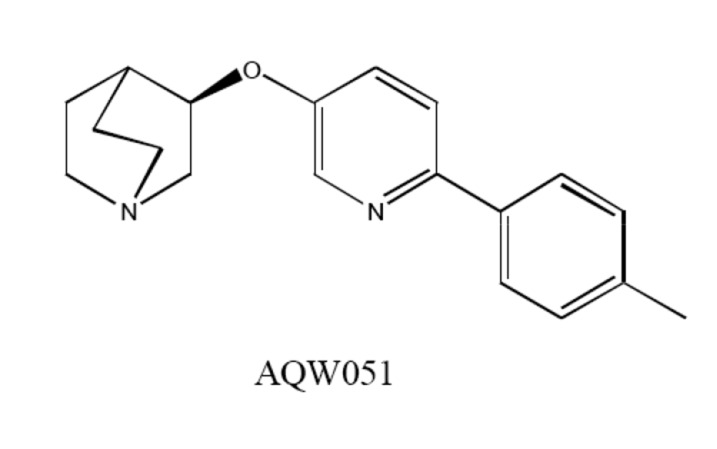
AQW051, (R)-3-(6-p-tolyl-pyridin-3-yloxy)-1-aza-bicyclo (2.2.2.)octane (Fig. 4), displayed high affinity for the human α7 nAChR with high selectivity towards other nAchRs [117]. AQW051 showed good oral bioavailability and rapid penetration into the rodent brain. Furthermore, AQW051 was effective in the animal models of object recognition, social recognition, and water maze [117]. Phase I study showed that AQW051 was well tolerated
in healthy young and elderly subjects. No serious adverse effects were shown [117]. Two such clinical studies in patients with chronic stable schizophrenia have been completed [118], while a subsequent, randomized, double-blind, placebo-controlled study of AQW051 (10 mg/day for 12-weeks) in patients with stable schizophrenia is underway [119].
α7 nAChR PAMs
Although many α7 nAChR full agonists protect against the deterioration of cognitive functions, the rapid desensitization of α7 nAChR, a process that occurs in milliseconds, raises concerns about the long-term administration of this class of compounds [120]. Nevertheless, PAMs of α7 nAChR have been the subject of much attention [18, 20, 121-123]. Based on electrophysiological characterization, at least two types of PAMs have been suggested: type I and type II. Both types increase the potency and efficacy of agonist-induced responses, which are observed as increases in peak agonist-evoked current responses, but type II has the additional ability to modify the desensitization profile of agonist responses [123].
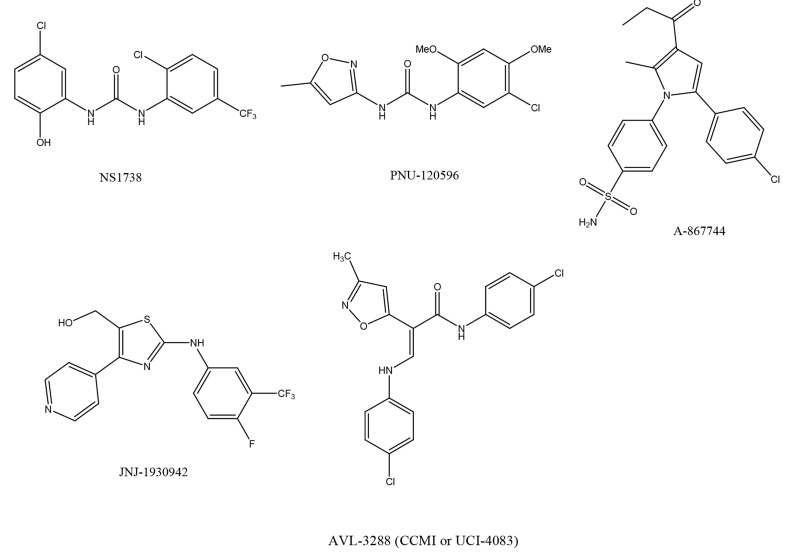
A number of new α7 nAChR PAMs, such as NS-1738, 1-(5-chloro-2-hydroxy-phenyl)-3-(2-chloro-5-trifluoromethyl-phenyl)-urea [124, 125], PNU-120596, 1-(5-chloro-2, 4-dimethoxy-phenyl)-3-(5-methyl-isoxazol-3-yl)-urea [126-129], A-867744, 4-(5-(4-chlorophenyl)-2-methyl-3-propionyl-1H-pyrrol-1-yl)benzenesulfo-namide [130, 131], and JNJ-1930942, 2-[[4-fluoro-3-(trifluoro-methyl)phenyl]amino]-4-(4-pyridinyl)-5-thiazolemethanol, [132], have been developed (Fig. 5). Below is a discussion of PAMs currently being tested in clinical trials.
AVL-3288 (or CCMI, UCI-4083)
N-(4-chlorophenyl)-α-[[(4-chlorophenyl)amino]methylene]-3-methyl-5-isoxazoleacet-amide (AVL-3288, CCMI, or UCI-4083) (Fig. 5) is a type I PAM. This is designated as the compound 6 in the literature [133]. AVL-3288 evokes robust, positive modulation of agonist-induced currents at α7 nAChR, while preserving the characteristic rapid desensitization kinetics. In addition, it has little to no efficacy at other ligand-gated ion channels. In rodent models, AVL-3288 promoted improvements in sensory gating and MK-801 evoked hyper-locomotion, as well as enhanced performance in the eight-arm radial maze [133]. A new Phase I study to evaluate the safety and pharmacokinetics of oral doses of AVL-3288 in healthy subjects is underway [134].
JNJ-39393406
JNJ-39393406, another α7 nAChR PAM, has been developed and used in clinical trials. Although the chemical structure of JNJ-39393406 has not been disclosed, it is known that it does not act on α4β2 nAChR, or the 5-HT3 receptor, nor does it interact with a panel of 62 receptors and enzymes [135], indicating a selectivity for 7 nAChR. JNJ-39393406 was shown to be effective in different animal models of cognition, such as the attentional set-shifting test and novel recognition test. In addition, this compound improved sensory gating deficits in DBA2 mice [135]. Unfortunately, a recent multicenter, double-blind, placebo-controlled, randomized study found that JNJ-39393406 showed no potential to reverse auditory sensory P50 deficits in patients with schizophrenia [135]. This finding led to the discontinuation of the trial. Despite this, a clinical study of JNJ-39393406 will be performed for smoking cessation under the National Institute of Health, USA [136].
Future directions
Cognitive deficits as seen in schizophrenia are frequently severe, leading to decreased functional outcome and quality of life. Although there are potential therapeutic drugs to treat the positive symptoms of schizophrenia, few drugs can effectively restore cognitive functions. Given the key role of α7 nAChR in auditory P50 deficits, as well as cognitive deficits, this receptor is an encouraging therapeutic target for schizophrenia, especially for the hard to treat cognitive disabilities.
A recent study showed that α7 nAChR stimulation is essential for excitation of NMDA receptor-mediated working memory [137], implying that α7 nAChR agonists may ameliorate key deficits in schizophrenia. Also, considering the NMDA receptor hypofunction hypothesis in schizophrenia [138-142], it is likely that stimulation by α7 nAChR agonists and PAMs could confer beneficial effects in patients with schizophrenia.
Accumulating evidence suggests a key role for α7 nAChR in inflammation [143-145], a process implicated in the pathophysiology of a number of neuropsychiatric diseases, including schizophrenia, Alzheimer’s disease and major depression [146-153]. Therefore, it is also highly likely that α7 nAChR could represent a therapeutic target for inflammation-related neuropsychiatric diseases.
Fig. (1).
P50 auditory evoked potentials as a biomarker for schizophrenia. Arrows show the onset of auditory stimuli. While normal healthy subjects show a lower response to the second stimulus (test), patients with schizophrenia fail to show this suppression.
Fig. (2).
Chemical structures of α7 nAChR agonists, including PHA-543613, PHA-568487, ABT-107, SSR180711, CP-810, 123, and AZD0328.
Fig. (3).
Chemical structures of α7 nAChR agonists, including DMXB-A, RG3487 (MEM3454), TC-5619, tropisetron, EVP-6124 (encenicline), and ABT-126.
Fig. (4).
Chemical structures of α7 nAChR agonist AQW051.
Fig. (5).
Chemical structure of α7 nAChR PAMs, including NS1738, PNU-120596, A-867744, JNJ-1930942, and AVL-3288 (CCMI or UCI-4083).
ACKNOWLEDGEMENTS
This study was supported by grants from the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation of Japan (Grant ID: 06-46), a Grant-in-Aid for Scientific Research on Innovative Areas of the Ministry of Education, Culture, Sports, Science and Technology, Japan (to K.H.), and Smoking Research Foundation, Tokyo, Japan. The author would also like to thank my collaborators who worked on some papers described in this review article, and who are listed as the co-authors of our papers in the reference list.
List of Abbreviations
- ACh
Acetylcholine
ADAS-cog = Alzheimer’s Disease Assessment Scale-cognitive subscale
- CNS
Central nervous system
- COGS
Consortium on the Genetics of Schizophrenia
- DMXB-A
3-(2, 4-dimethoxybenzylidene)-anabaseine
- 5-HT3
5-Hydroxytryptamine-3
- LOD
Logarithm of odds
- MATRICS
Measurement and Treatment Research to Improve Cognition in Schizophrenia
- MLA
Methyllycaconitine
- nAChR
Nicotinic acetylcholine receptor
- NIMH
National Institute of Mental Health
- NMDA
N-methyl D-aspartate
- PAM
Positive allosteric modulator
- PCP
Phencyclidine
- PPI
Prepulse inhibition
CONFLICT OF INTEREST
Dr. Kenji Hashimoto holds a patent for the use of tropisetron in neuropsychiatric diseases, including schizophrenia and Alzheimer’s disease.
REFERENCES
- 1.Lasser K., Boyd J.W., Woolhandler S., Himmelstein D.U., McCormick D., Bor D.H. Smoking and mental illness: A population-based prevalence study. JAMA. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 2.Uck A., Polat A., Bozkurt O., Meteris H. Cigarette smoking among patients with schizophrenia and bipolar disorders. Psychiatry Clin. Neurosci. 2004;58:434–437. doi: 10.1111/j.1440-1819.2004.01279.x. [DOI] [PubMed] [Google Scholar]
- 3.de Leon J., Diaz F.J. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr. Res. 2005;76:135–157. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Aubin H.J., Rollema H., Svensson T.H., Winterer G. Smoking, quitting, and psychiatric disease: a review. Neurosci. Biobehav. Rev. 2012;36:271–284. doi: 10.1016/j.neubiorev.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Tsoi D.T., Porwal M., Webster A.C. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst. Rev. 2013;2:CD007253. doi: 10.1002/14651858.CD007253.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumari V., Postma P. Nicotine use in schizophrenia: the self-medication hypotheses. Neurosci. Biobehav. Rev. 2005;29:1021–1034. doi: 10.1016/j.neubiorev.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Leonard S., Mexal S., Freedman R. Smoking, genetics and schizophrenia: evidence for self medication. J. Dual Diagn. 2007;3:43–59. doi: 10.1300/J374v03n03_05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goff D.C., Henderson D.C., Amico E. Cigarette smoking in schizophrenia: relationship to psychopathology and medication side effects. Am. J. Psychiatry. 1992;149:1189–1194. doi: 10.1176/ajp.149.9.1189. [DOI] [PubMed] [Google Scholar]
- 9.Tung C.S., Grenhoff J., Svensson T.H. Nicotine counteracts midbrain dopamine cell dysfunction induced by prefrontal cortex inactivation. Acta Physiol. Scand. 1990;138:427–428. doi: 10.1111/j.1748-1716.1990.tb08868.x. [DOI] [PubMed] [Google Scholar]
- 10.Glassman A.H. Cigarette smoking: implications for psychiatric illness. Am. J. Psychiatry. 1993;150:546–553. doi: 10.1176/ajp.150.4.546. [DOI] [PubMed] [Google Scholar]
- 11.Nisell M., Nomikos G.G., Svensson T.H. Nicotine dependence, midbrain dopamine systems and psychiatric disorders. Pharmacol. Toxicol. 1995;76:157–162. doi: 10.1111/j.1600-0773.1995.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 12.Dani J.A., Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- 13.Simosky J.K., Stevens K.E., Freedman R. Nicotinic agonists and psychosis. Curr. Drug Targets CNS Neurol. Disord. 2002;1:149–162. doi: 10.2174/1568007024606168. [DOI] [PubMed] [Google Scholar]
- 14.Martin L.F., Kem W.R., Freedman R. Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology (Berl.) 2004;174:54–64. doi: 10.1007/s00213-003-1750-1. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto K., Koike K., Shimizu E., Iyo M. α7 Nicotinic receptor agonists as potential therapeutic drugs for schizophrenia. Curr Med Chem CNS Agents. 2005;5:171–184. [Google Scholar]
- 16.Olincy A., Stevens K.E. Treating schizophrenia symptoms with an α7 nicotinic agonist, from mice to men. Biochem. Pharmacol. 2007;74:1192–1201. doi: 10.1016/j.bcp.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hajós M., Rogers B.N. Targeting α7 nicotinic acetylcholine receptors in the treatment of schizophrenia. Curr. Pharm. Des. 2010;16:538–554. doi: 10.2174/138161210790361434. [DOI] [PubMed] [Google Scholar]
- 18.Toyohara J., Hashimoto K. α7 Nicotinic receptor agonists: potential therapeutic drugs for treatment of cognitive impairments in schizophrenia and Alzheimer's disease. Open Med. Chem. J. 2010;4:37–56. doi: 10.2174/1874104501004010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomsen M.S., Hansen H.H., Timmerman D.B., Mikkelsen J.D. Cognitive improvement by activation of α7 nicotinic acetylcholine receptors: from animal models to human pathophysiology. Curr. Pharm. Des. 2010;16:323–343. doi: 10.2174/138161210790170094. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa M., Hashimoto K. α7 Nicotinic receptor as a potential therapeutic target for schizophrenia. Curr. Pharm. Des. 2011;17:121–129. doi: 10.2174/138161211795049561. [DOI] [PubMed] [Google Scholar]
- 21.Jones C.K., Byun N., Bubser M. Muscarinic and nicotinic acetylcholine receptor agonists and allosteric modulators for the treatment of schizophrenia. Neuropsychopharmacology. 2012;37:16–42. doi: 10.1038/npp.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurst R., Rollema H., Bertrand D. Nicotinic acetylcholine receptors: from basic science to therapeutics. Pharmacol. Ther. 2013;137:22–54. doi: 10.1016/j.pharmthera.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Young J.W., Geyer M.A. Evaluating the role of the alpha-7 nicotinic acetylcholine receptor in the pathophysiology and treatment of schizophrenia. Biochem. Pharmacol. 2013;86:1122–1132. doi: 10.1016/j.bcp.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adler L.E., Pachtman E., Franks R.D., Pecevich M., Waldo M.C., Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol. Psychiatry. 1982;17:639–654. [PubMed] [Google Scholar]
- 25.Freedman R., Adler L.E., Waldo M.C., Pachtman E., Franks R.D. Neurophysiological evidence for a defect in inhibitory pathways in schizophrenia: comparison of medicated and drug-free patients. Biol. Psychiatry. 1983;18:537–551. [PubMed] [Google Scholar]
- 26.Freedman R., Adler L.E., Gerhardt G.A., et al. Neurobiological studies of sensory gating in schizophrenia. Schizophr. Bull. 1987;13:669–678. doi: 10.1093/schbul/13.4.669. [DOI] [PubMed] [Google Scholar]
- 27.Judd L.L., McAdams L., Budnick B., Braff D.L. Sensory gating deficits in schizophrenia: new results. Am. J. Psychiatry. 1992;149:488–493. doi: 10.1176/ajp.149.4.488. [DOI] [PubMed] [Google Scholar]
- 28.Clementz B.A., Geyer M.A., Braff D.L. P50 suppression among schizophrenia and normal comparison subjects: a methodological analysis. Biol. Psychiatry. 1997;41:1035–1044. doi: 10.1016/S0006-3223(96)00208-9. [DOI] [PubMed] [Google Scholar]
- 29.Siegel C., Waldo M., Mizner G., Adler L.E., Freedman R. Deficits in sensory gating in schizophrenic patients and their relatives. Evidence obtained with auditory evoked responses. Arch. Gen. Psychiatry. 1984;41:607–612. doi: 10.1001/archpsyc.1984.01790170081009. [DOI] [PubMed] [Google Scholar]
- 30.Waldo M.C., Adler L.E., Freedman R. Defects in auditory sensory gating and their apparent compensation in relatives of schizophrenics. Schizophr. Res. 1988;1:19–24. doi: 10.1016/0920-9964(88)90035-7. [DOI] [PubMed] [Google Scholar]
- 31.Clementz B.A., Geyer M.A., Braff D.L. Poor P50 suppression among schizophrenia patients and their first-degree biological relatives. Am. J. Psychiatry. 1998;155:1691–1694. doi: 10.1176/ajp.155.12.1691. [DOI] [PubMed] [Google Scholar]
- 32.Ross R.G., Olincy A., Harris J.G., et al. Evidence for bilineal inheritance of physiological indicators of risk in childhood-onset schizophrenia. Am. J. Med. Genet. 1999;88:188–199. [PubMed] [Google Scholar]
- 33.Heinrichs R.W. Meta-analysis and the sciences of schizophrenia: variant evidence or evidence of variants? Neurosci. Biobehav. Rev. 2004;28:379–394. doi: 10.1016/j.neubiorev.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Potter D., Summerfelt A., Gold J., Buchanan R.W. Review of clinical correlates of P50 sensory gating abnormalities in patients with schizophrenia. Schizophr. Bull. 2006;32:692–700. doi: 10.1093/schbul/sbj050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bramon E., Rabe-Hesketh S., Sham P., Murray R.M., Frangou S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr. Res. 2004;70:315–329. doi: 10.1016/j.schres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Patterson J.V., Hetrick W.P., Boutros N.N., Jin Y., Sandman C., Potkin S., Bunney W.E. P50 sensory gating ratios in schizophrenia and controls: a review and data analysis. Psychiatry Res. 2008;158:226–247. doi: 10.1016/j.psychres.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 37.Brockhaus-Dumke A., Schultze-Lutter F., Mueller R., et al. Sensory gating in schizophrenia: P50 and N100 gating in antipsychotic-free subjects at risk, first-episode, and chronic patients. Biol. Psychiatry. 2008;64:376–384. [Google Scholar]
- 38.Hsieh M.H., Shan J.C., Huang W.L., et al. Auditory event-related potential of subjects with suspected pre-psychotic state and first-episode psychosis. Schizophr. Res. 2012;140:243–249. doi: 10.1016/j.schres.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 39.van Tricht M.J., Nieman D.H., Koelman J.T., et al. Sensory gating in subjects at ultra high risk for developing a psychosis before and after a first psychotic episode. World J. Biol. Psychiatry. 2015;16:12–21. doi: 10.3109/15622975.2012.680911. [DOI] [PubMed] [Google Scholar]
- 40.Stevens K.E., Freedman R., Collins A.C., Hall M., Leonard S. Genetic correlation of inhibitory gating of hippocampal auditory evoked response and α-bungarotoxin-binding nicotinic cholinergic receptors in inbred mouse strains. Neuropsychopharmacology. 1996;15:152–162. doi: 10.1016/0893-133X(95)00178-G. [DOI] [PubMed] [Google Scholar]
- 41.Adams C.E., Stevens K.E. Evidence for a role of nicotinic acetylcholine receptors in schizophrenia. Front. Biosci. 2007;12:4755–4772. doi: 10.2741/2424. [DOI] [PubMed] [Google Scholar]
- 42.Singer P., Feldon J., Yee B.K. Are DBA/2 mice associated with schizophrenia-like endophenotypes? A behavioural contrast with C57BL/6 mice. Psychopharmacology (Berl.) 2009;206:677–698. doi: 10.1007/s00213-009-1568-6. [DOI] [PubMed] [Google Scholar]
- 43.Simosky J.K., Stevens K.E., Adler L.E., Freedman R. Clozapine improves deficient inhibitory auditory processing in DBA/2 mice, via a nicotinic cholinergic mechanism. Psychopharmacology (Berl.) 2003;165:386–396. doi: 10.1007/s00213-002-1285-x. [DOI] [PubMed] [Google Scholar]
- 44.Nagamoto H.T., Adler L.E., McRae K.A., et al. Auditory P50 in schizophrenics on clozapine: improved gating parallels clinical improvement and changes in plasma 3-methoxy-4-hydroxyphenylglycol. Neuropsychobiology. 1999;39:10–17. doi: 10.1159/000026553. [DOI] [PubMed] [Google Scholar]
- 45.Light G.A., Geyer M.A., Clementz B.A., Cadenhead K.S., Braff D.L. Normal P50 suppression in schizophrenia patients treated with atypical antipsychotic medications. Am. J. Psychiatry. 2000;157:767–771. doi: 10.1176/appi.ajp.157.5.767. [DOI] [PubMed] [Google Scholar]
- 46.Shirazi-Southall S., Rodriguez D.E., Nomikos G.G. Effects of typical and atypical antipsychotics and receptor selective compounds on acetylcholine efflux in the hippocampus of the rat. Neuropsychopharmacology. 2002;26:583–594. doi: 10.1016/S0893-133X(01)00400-6. [DOI] [PubMed] [Google Scholar]
- 47.Freedman R., Hall M., Adler L.E., Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol. Psychiatry. 1995;38:22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- 48.Court J., Spurden D., Lloyd S., et al. Neuronal nicotinic receptors in dementia with Lewy bodies and schizophrenia: α-bungarotoxin and nicotine binding in the thalamus. J. Neurochem. 1999;73:1590–1597. doi: 10.1046/j.1471-4159.1999.0731590.x. [DOI] [PubMed] [Google Scholar]
- 49.Guan Z.Z., Zhang X., Blennow K., Nordberg A. Decreased protein level of nicotinic receptor α7 subunit in the frontal cortex from schizophrenic brain. Neuroreport. 1999;10:1779–1782. doi: 10.1097/00001756-199906030-00028. [DOI] [PubMed] [Google Scholar]
- 50.Marutle A., Zhang X., Court J., et al. Laminar distribution of nicotinic receptor subtypes in cortical regions in schizophrenia. J. Chem. Neuroanat. 2001;22:115–126. doi: 10.1016/s0891-0618(01)00117-x. [DOI] [PubMed] [Google Scholar]
- 51.Martin-Ruiz C.M., Haroutunian V.H., Long P., et al. Dementia rating and nicotinic receptor expression in the prefrontal cortex in schizophrenia. Biol. Psychiatry. 2003;54:1222–1233. doi: 10.1016/s0006-3223(03)00348-2. [DOI] [PubMed] [Google Scholar]
- 52.Freedman R., Ross R., Leonard S., et al. Early biomarkers of psychosis. Dialogues Clin. Neurosci. 2005;7:17–29. doi: 10.31887/DCNS.2005.7.1/frreedman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adler L.E., Hoffer L.D., Wiser A., Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am. J. Psychiatry. 1993;150:1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- 54.Adler L.E., Hoffer L.J., Griffith J., Waldo M.C., Freedman R. Normalization by nicotine of deficient auditory sensory gating in the relatives of schizophrenics. Biol. Psychiatry. 1992;32:607–616. doi: 10.1016/0006-3223(92)90073-9. [DOI] [PubMed] [Google Scholar]
- 55.Radek R.J., Miner H.M., Bratcher N.A., Decker M.W., Gopalakrishnan M., Bitner R.S. α4β2 nicotinic receptor stimulation contributes to the effects of nicotine in the DBA/2 mouse model of sensory gating. Psychopharmacology (Berl.) 2006;187:47–55. doi: 10.1007/s00213-006-0394-3. [DOI] [PubMed] [Google Scholar]
- 56.Freedman R., Adler L.E., Bickford P., et al. Schizophrenia and nicotinic receptors. Harv. Rev. Psychiatry. 1994;2:179–192. doi: 10.3109/10673229409017136. [DOI] [PubMed] [Google Scholar]
- 57.Olincy A., Freedman R. Nicotinic mechanisms in the treatment of psychiatric disorders: a focus on the α7 nicotinic receptor. Handbook Exp. Pharmacol. 2012;213:211–232. doi: 10.1007/978-3-642-25758-2_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Freedman R., Coon H., Myles-Worsley M., et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc. Natl. Acad. Sci. USA. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leonard S., Gault J., Moore T., et al. Further investigation of a chromosome 15 locus in schizophrenia: analysis of affected sibpairs from the NIMH Genetics Initiative. Am. J. Med. Genet. 1998;81:308–312. doi: 10.1002/(sici)1096-8628(19980710)81:4<308::aid-ajmg6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 60.Neves-Pereira M., Bassett A.S., Honer W.G., Lang D., King N.A., Kennedy J.L. No evidence for linkage of the CHRNA7 gene region in Canadian schizophrenia families. Am. J. Med. Genet. 1998;81:361–363. doi: 10.1002/(sici)1096-8628(19980907)81:5<361::aid-ajmg3>3.0.co;2-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Curtis L., Blouin J.L., Radhakrishna U., et al. No evidence for linkage between schizophrenia and markers at chromosome 15q13-14. Am. J. Med. Genet. 1999;88:109–112. doi: 10.1002/(sici)1096-8628(19990416)88:2<109::aid-ajmg1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 62.Leonard S., Gault J., Hopkins J., et al. Association of promoter variants in the α7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch. Gen. Psychiatry. 2002;59:1085–1096. doi: 10.1001/archpsyc.59.12.1085. [DOI] [PubMed] [Google Scholar]
- 63.Leonard S., Freedman R. Genetics of chromosome 15q13-q14 in schizophrenia. Biol. Psychiatry. 2006;60:115–122. doi: 10.1016/j.biopsych.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 64.Olincy A., Braff D.L., Adler L.E., et al. Inhibition of the P50 cerebral evoked response to repeated auditory stimuli: results from the Consortium on Genetics of Schizophrenia. Schizophr. Res. 2010;119:175–182. doi: 10.1016/j.schres.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Greenwood T.A., Lazzeroni L.C., Murray S.S., et al. Analysis of 94 candidate genes and 12 endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. Am. J. Psychiatry. 2011;168:930–946. doi: 10.1176/appi.ajp.2011.10050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gault J., Robinson M., Berger R., et al. Genomic organization and partial duplication of the human α7 neuronal nicotinic acetylcholine receptor gene (CHRNA7). Genomics. 1998;52:173–185. doi: 10.1006/geno.1998.5363. [DOI] [PubMed] [Google Scholar]
- 67.Araud T., Graw S., Berger R., et al. The chimeric gene CHRFAM7A, a partial duplication of the CHRNA7 gene, is a dominant negative regulator of α7 nAchR function. Biochem. Pharmacol. 2011;82:904–914. doi: 10.1016/j.bcp.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sinkus M.L., Lee M.J., Gault J., et al. A 2-base pair deletion polymorphism in the partial duplication of the α7 nicotinic acetylcholine gene (CHRFAM7A) on chromosome 15q14 is associated with schizophrenia. Brain Res. 2009;129:1–11. doi: 10.1016/j.brainres.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wishka D.G., Walker D.P., Yates K.M., et al. Discovery of N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]furo[2, 3-c]pyridine-5-carboxamide, an agonist of the α7 nicotinic acetylcholine receptor, for the potential treatment of cognitive deficits in schizophrenia: synthesis and structure--activity relationship. J. Med. Chem. 2006;49:4425–4436. [Google Scholar]
- 70.Walker D.P., Wishka D.G., Piotrowski D.W., et al. Design, synthesis, structure–activity relationship, and in vivo activity of azabicyclic aryl amides as α7 nicotinic acetylcholine receptor agonists. Bioorg. Med. Chem. 2006;14:8219–8248. doi: 10.1016/j.bmc.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 71.Malysz J., Anderson D.J., Grønlien J.H., et al. In vitro pharmacological characterization of a novel selective α7 neuronal nicotinic acetylcholine receptor agonist ABT-107. J. Pharmacol. Exp. Ther. 2010;334:863–874. doi: 10.1124/jpet.110.167072. [DOI] [PubMed] [Google Scholar]
- 72.Bitner R.S., Bunnelle W.H., Decker M.W., et al. In vivo pharmacological characterization of a novel selective α7 neuronal nicotinic acetylcholine receptor agonist ABT-107: preclinical considerations in Alzheimer's disease. J. Pharmacol. Exp. Ther. 2010;334:875–886. doi: 10.1124/jpet.110.167213. [DOI] [PubMed] [Google Scholar]
- 73.Othman A.A., Lenz R.A., Zhang J., et al. Single- and multiple-dose pharmacokinetics, safety, and tolerability of the selective α7 neuronal nicotinic receptor agonist, ABT-107, in healthy human volunteers. J. Clin. Pharmacol. 2011;51:512–526. doi: 10.1177/0091270010370460. [DOI] [PubMed] [Google Scholar]
- 74.Biton B., Bergis O.E., Galli F., et al. SSR180711, a novel selective alpha7 nicotinic receptor partial agonist: (1) binding and functional profile. Neuropsychopharmacology. 2007;32:1–16. doi: 10.1038/sj.npp.1301189. [DOI] [PubMed] [Google Scholar]
- 75.Pichat P., Bergis O.E., Terranova J.P., et al. SSR180711, a novel selective α7 nicotinic receptor partial agonist: (II) efficacy in experimental models predictive of activity against cognitive symptoms of schizophrenia. Neuropsychopharmacology. 2007;32:17–34. doi: 10.1038/sj.npp.1301188. [DOI] [PubMed] [Google Scholar]
- 76.Hashimoto K., Ishima T., Fujita Y., et al. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of the novel selective α7 nicotinic receptor agonist SSR180711. Biol. Psychiatry. 2008;63:92–97. doi: 10.1016/j.biopsych.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 77.O'Donnell C.J., Rogers B.N., Bronk B.S., et al. Discovery of 4-(5-methyloxazolo[4, 5-b]pyridin-2-yl)-1, 4-diazabicyclo[3.2.2]nonane (CP-810, 123), a novel α7 nicotinic acetylcholine receptor agonist for the treatment of cognitive disorders in schizophrenia: synthesis, SAR development, and in vivo efficacy in cognition models. J. Med. Chem. 2010;53:1222–1237. doi: 10.1021/jm9015075. [DOI] [PubMed] [Google Scholar]
- 78.Sydserff S., Sutton E.J., Song D., et al. Selective α7 nicotinic receptor activation by AZD0328 enhances cortical dopamine release and improves learning and attentional processes. Biochem. Pharmacol. 2009;78:880–888. doi: 10.1016/j.bcp.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 79.Castner S.A., Smagin G.N., Piser T.M., et al. Immediate and sustained improvements in working memory after selective stimulation of α7 nicotinic acetylcholine receptors. Biol. Psychiatry. 2011;69:12–18. doi: 10.1016/j.biopsych.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 80.Mazurov A.A., Kombo D.C., Hauser T.A., et al. Discovery of (2S, 3R)-N-[2-(pyridin-3-ylmethyl)-1-azabicyclo[2.2.2]oct-3-yl]benzo[b] furan-2-carboxamide (TC-5619), a selective α7 nicotinic acetylcholine receptor agonist, for the treatment of cognitive disorders. J. Med. Chem. 2012;55:9793–9809. doi: 10.1021/jm301048a. [DOI] [PubMed] [Google Scholar]
- 81.Stevens K.E., Kem W.R., Mahnir V.M., Freedman R. Selective α7-nicotinic agonists normalize inhibition of auditory response in DBA mice. Psychopharmacology (Berl.) 1998;136:320–327. doi: 10.1007/s002130050573. [DOI] [PubMed] [Google Scholar]
- 82.Simosky J.K., Stevens K.E., Kem W.R., Freedman R. Intragastric DMXB-A, an α7 nicotinic agonist, improves deficient sensory inhibition in DBA/2 mice. Biol. Psychiatry. 2001;50:493–500. doi: 10.1016/s0006-3223(01)01093-9. [DOI] [PubMed] [Google Scholar]
- 83.Stevens K.E., Cornejo B., Adams C.E., et al. Continuous administration of a selective α7 nicotinic partial agonist, DMXBA, improves sensory inhibition without causing tachyphylaxis or receptor upregulation in DBA/2 mice. Brain Res. 2010;1352:140–146. doi: 10.1016/j.brainres.2010.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.O'Neill H.C., Rieger K., Kem W.R., Stevens K.E. DMXB, an α7 nicotinic agonist, normalizes auditory gating in isolation-reared rats. Psychopharmacology (Berl.) 2003;169:332–339. doi: 10.1007/s00213-003-1482-2. [DOI] [PubMed] [Google Scholar]
- 85.Kitagawa H., Takenouchi T., Azuma R., et al. Safety, pharmacokinetics, and effects on cognitive function of multiple doses of GTS-21 in healthy, male volunteers. Neuropsychopharmacology. 2003;28:542–551. doi: 10.1038/sj.npp.1300028. [DOI] [PubMed] [Google Scholar]
- 86.Olincy A., Harris J., Johnson L., et al. Proof of concept trial of an α7 nicotinic agonist in schizophrenia. Arch. Gen. Psychiatry. 2006;63:630–638. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- 87.Freedman R., Olincy A., Buchanan R.W., et al. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am. J. Psychiatry. 2008;165:1040–1047. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tregellas J.R., Olincy A., Johnson L., et al. Functional magnetic resonance imaging of effects of a nicotinic agonist in schizophrenia. Neuropsychopharmacology. 2010;35:937–942. doi: 10.1038/npp.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tregellas J.R., Tanabe J., Rojas D.C., et al. Effects of an α7-nicotinic agonist on default network activity in schizophrenia. Biol. Psychiatry. 2011;69:7–11. doi: 10.1016/j.biopsych.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. NCT01400477 Nicotinic receptors and schizophrenia. Available athttp://clinicaltrials.gov/ct2/show/NCT01400477 .
- 91.Wallace T.L., Callahan P.M., Tehim A., et al. RG3487, a novel nicotinic α7 receptor partial agonist, improves cognition and sensorimotor gating in rodents. J. Pharmacol. Exp. Ther. 2011;336:242–253. doi: 10.1124/jpet.110.171892. [DOI] [PubMed] [Google Scholar]
- 92.Rezvani A.H., Kholdebarin E., Brucato F.H., Callahan P.M., Lowe D.A., Levin E.D. Effect of R3487/MEM3454, a novel nicotinic α7 receptor partial agonist and 5-HT3 antagonist on sustained attention in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33:269–275. doi: 10.1016/j.pnpbp.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 93.Hauser T.A., Kucinski A., Jordan K.G., et al. TC-5619: An α7 neuronal nicotinic receptor-selective agonist that demonstrates efficacy in animal models of the positive and negative symptoms and cognitive dysfunction of schizophrenia. Biochem. Pharmacol. 2009;78:803–812. doi: 10.1016/j.bcp.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lieberman J.A., Dunbar G., Segreti A.C., et al. A randomized exploratory trial of an α7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013;38:968–975. doi: 10.1038/npp.2012.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. NCT01488929 Efficacy, safety, and tolerability of TC-5619 as augmentation therapy to improve negative symptoms and cognition in outpatients with schizophrenia.
- 96.Macor J.E., Gurley D., Lanthorn T., et al. The 5-HT3 antagonist tropisetron (ICS 205-930) is a potent and selective α7 nicotinic receptor partial agonist. Bioorg. Med. Chem. Lett. 2001;11:319–321. doi: 10.1016/s0960-894x(00)00670-3. [DOI] [PubMed] [Google Scholar]
- 97.Papke R.L., Porter Papke J.K., Rose G.M. Activity of α7-selective agonists at nicotinic and serotonin 5HT3 receptors expressed in Xenopus oocytes. Bioorg. Med. Chem. Lett. 2004;14:1849–1853. doi: 10.1016/j.bmcl.2003.09.104. [DOI] [PubMed] [Google Scholar]
- 98.Hashimoto K., Iyo M., Freedman R., Stevens K.E. Tropisetron improves deficient inhibitory auditory processing in DBA/2 mice: role of α7 nicotinic acetylcholine receptors. Psychopharmacology (Berl.) 2005;183:13–19. doi: 10.1007/s00213-005-0142-0. [DOI] [PubMed] [Google Scholar]
- 99.Hashimoto K., Fujita Y., Ishima T., Hagiwara H., Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of tropisetron: role of α7 nicotinic receptors. Eur. J. Pharmacol. 2006;553:191–195. doi: 10.1016/j.ejphar.2006.09.055. [DOI] [PubMed] [Google Scholar]
- 100.Kohnomi S., Suemaru K., Goda M., et al. Ameliorating effects of tropisetron on dopaminergic disruption of prepulse inhibition via the α7 nicotinic acetylcholine receptor in Wistar rats. Brain Res. 2010;1353:152–158. doi: 10.1016/j.brainres.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 101.Koike K., Hashimoto K., Takai N., et al. Topisetron improves deficits in auditory P50 suppression in schizophrenia. Schizophr. Res. 2005;76:67–72. doi: 10.1016/j.schres.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 102.Shiina A., Shirayama Y., Niitsu T., et al. A randomised, double-blind, placebo-controlled trial of tropisetron in patients with schizophrenia. Ann. Gen. Psychiatry. 2010;9:27. doi: 10.1186/1744-859X-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang K.Y., Liu L., Liu S., et al. Short-term tropisetron treatment and cognitive and P50 auditory gating deficits in schizophrenia. Am. J. Psychiatry. 2012;169:974–981. doi: 10.1176/appi.ajp.2012.11081289. [DOI] [PubMed] [Google Scholar]
- 104.Noroozian M., Ghasemi S., Hosseini S.M., et al. A placebo-controlled study of tropisetron added to risperidone for the treatment of negative symptoms in chronic and stable schizophrenia. Psychopharmacology (Berl.) 2013;228:595–602. doi: 10.1007/s00213-013-3064-2. [DOI] [PubMed] [Google Scholar]
- 105. NCT00435370 Effectiveness of tropisetron plus risperidone for improving cognitive and perceptual disturbances in schizophrenia. http://clinicaltrials.gov/ct2/show/NCT00435370 http://clinicaltrials.gov/ct2/show/ NCT00435370.
- 106.Prickaerts J., van Goethem N.P., Chesworth R., et al. EVP-6124, a novel and selective α7 nicotinic acetylcholine receptor partial agonist, improves memory performance by potentiating the acetylcholine response of α7 nicotinic acetylcholine receptors. Neuropharmacology. 2012;62:1099–1110. doi: 10.1016/j.neuropharm.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 107.Preskorn S.H., Gawryl M., Dgetluck N., et al. Normalizing effects of EVP-6124, an α-7 nicotinic partial agonist, on event-related potentials and cognition: a proof of concept, randomized trial in patients with schizophrenia. J. Psychiatr. Pract. 2014;20:12–24. doi: 10.1097/01.pra.0000442935.15833.c5. [DOI] [PubMed] [Google Scholar]
- 108.Barbier A.J., Hilhorst M., Van Vliet A., et al. Pharmacodynamics, pharmacokinetics, safety, and tolerability of encenicline, a selective α7 nicotinic receptor partial agonist, in single ascending-dose and bioavailability studies. Clin. Ther. 2015;37:311–324. doi: 10.1016/j.clinthera.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 109.EnVivo Pharmaceutical Ltd. [homepage on the Internet]. EnVivo Pharmaceuticals announces initiation of Phase 3 clinical trial program of EVP-6124 in schizophrenia [February 28, 2013]. Available from:http://www.envivopharma.com/news-item.php?id=45 .
- 110. NCT01716975. Study of EVP-6124 (a7 nAChR) as an adjunctive pro-cognitive treatment in schizophrenia subjects receiving chronic stable atypical antipsychotic therapy. Available at:http://clinicaltrials.gov/ct2/show/NCT01716975.
- 111.Deardorff W.J., Shobassy A., Grossberg G.T. Safety and clinical effects of EVP-6124 in subjects with Alzheimer's disease currently or previously receiving an acetylcholinesterase inhibitor medication. Expert Rev. Neurother. 2015;15:7–17. doi: 10.1586/14737175.2015.995639. [DOI] [PubMed] [Google Scholar]
- 112.Chen S., Napier J.J., Zhang G.G., Brackenmeyer P.J. inventor; Abbott Laboratories, assignee. Novel monohydrate of azaadamantane derivatives. United States patent US 2012/0245195 A1. 2012 [Google Scholar]
- 113.Ritchie C., Robieson W., Pritchett Y., Othman A., Lenz R., Gault L. Efficacy and safety of the α7 agonist ABT-126 in mild-to-moderate Alzheimer’s dementia.; Alzheimer’s Association International Conference; July 13-18, 2013; Boston, MA, USA. [Google Scholar]
- 114. NCT01043458. Pharmacokinetic study of ABT-126 in stable subjects with schizophrenia. Available at:http://clinicaltrials.gov/ct2/show/ NCT01043458.
- 115. NCT01095562. Safety and efficacy study for cognitive deficits in adult subjects with schizophrenia. Available at:http://clinicaltrials.gov/ct2/show/NCT01095562. [Accessed August 1, 2013].
- 116. NCT01678755. A phase 2 study to evaluate ABT-126 for the treatment of cognitive deficits in schizophrenia. Available at:http://clinicaltrials.gov/ct2/show/ NCT01678755 .
- 117.Feuerbach D., Pezous N., Weiss M., et al. AQW051, a novel, potent and selective α7 nicotinic Ach receptor partial agonist: pharmacological characterization and phase I evaluation. Br. J. Pharmacol. 2015;172:1292–1304. doi: 10.1111/bph.13001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. NCT01163227. Effect of AQW051 on cognitive function in patients with schizophrenia. Available at:http://clinicaltrials.gov/ct2/show/NCT01163227.
- 119. NCT01730768. A study to evaluate AQW051 the effects of once daily doses of AQW051 on cognition, in stable schizophrenia patients. Available athttp://clinicaltrials.gov/ct2/show/ NCT001730768.
- 120.Harris J.G., Kongs S., Allensworth D., et al. Effects of nicotine on cognitive deficits in schizophrenia. Neuropsychopharmacology. 2004;29:1378–1385. doi: 10.1038/sj.npp.1300450. [DOI] [PubMed] [Google Scholar]
- 121.Bertrand D., Gopalakrishnan M. Allosteric modulation of nicotinic acetylcholine receptors. Biochem. Pharmacol. 2007;74:1155–1163. doi: 10.1016/j.bcp.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 122.Faghih R., Gopalakrishnan M., Briggs C.A. Allosteric modulators of the α7 nicotinic acetylcholine receptor. J. Med. Chem. 2008;51:701–712. doi: 10.1021/jm070256g. [DOI] [PubMed] [Google Scholar]
- 123.Williams D.K., Wang J., Papke R.L. Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: Advantages and limitations. Biochem. Pharmacol. 2011;82:915–930. doi: 10.1016/j.bcp.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Timmermann D.B., Grønlien J.H., Kohlhaas K.L., et al. An allosteric modulator of the α7 nicotinic acetylcholine receptor possessing cognition-enhancing properties in vivo. J. Pharmacol. Exp. Ther. 2007;323:294–307. doi: 10.1124/jpet.107.120436. [DOI] [PubMed] [Google Scholar]
- 125.Collins T., Young G.T., Millar N.S. Competitive binding at a nicotinic receptor transmembrane site of two α7-selective positive allosteric modulators with differing effects on agonist-evoked desensitization. Neuropharmacology. 2011;61:1306–1313. doi: 10.1016/j.neuropharm.2011.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hurst R.S., Hajós M., Raggenbass M., et al. A novel positive allosteric modulator of the α7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J. Neurosci. 2005;25:4396–4405. doi: 10.1523/JNEUROSCI.5269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barron S.C., McLaughlin J.T., See J.A., Richards V.L., Rosenberg R.L. An allosteric modulator of α7 nicotinic receptors, N-(5-Chloro-2, 4-dimethoxyphenyl)-N'-(5-methyl-3-isoxazolyl)-urea (PNU-120596), causes conformational changes in the extracellular ligand binding domain similar to those caused by acetylcholine. Mol. Pharmacol. 2009;76:253–263. doi: 10.1124/mol.109.056226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Munro G., Hansen R., Erichsen H., et al. The α7 nicotinic ACh receptor agonist compound B and positive allosteric modulator PNU-120596 both alleviate inflammatory hyperalgesia and cytokine release in the rat. Br. J. Pharmacol. 2012;167:421–435. doi: 10.1111/j.1476-5381.2012.02003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Callahan P.M., Hutchings E.J., Kille N.J., et al. Positive allosteric modulator of α7 nicotinic-acetylcholine receptors, PNU-120596 augments the effects of donepezil on learning and memory in aged rodents and non-human primates. Neuropharmacology. 2013;67:201–212. doi: 10.1016/j.neuropharm.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Faghih R., Gopalakrishnan S.M., Gronlien J.H., et al. Discovery of 4-(5-(4-chlorophenyl)-2-methyl-3-propionyl-1H-pyrrol-1-yl)benzenesulfonamide (A-867744) as a novel positive allosteric modulator of the alpha7 nicotinic acetylcholine receptor. J. Med. Chem. 2009;52:3377–3384. doi: 10.1021/jm9003818. [DOI] [PubMed] [Google Scholar]
- 131.Malysz J., Grønlien J.H., Anderson D.J., et al. In vitro pharmacological characterization of a novel allosteric modulator of α7 neuronal acetylcholine receptor, 4-(5-(4-chlorophenyl)-2-methyl-3-propionyl-1H-pyrrol-1-yl) benzenesulfonamide (A-867744), exhibiting unique pharmacological profile. J. Pharmacol. Exp. Ther. 2009;330:257–267. doi: 10.1124/jpet.109.151886. [DOI] [PubMed] [Google Scholar]
- 132.Dinklo T., Shaban H., Thuring J.W., et al. Characterization of 2-[[4-fluoro-3-(trifluoromethyl)phenyl]amino]-4-(4-pyridinyl)-5-thiazolemethanol (JNJ-1930942), a novel positive allosteric modulator of the α7 nicotinic acetylcholine receptor. J. Pharmacol. Exp. Ther. 2011;336:560–574. doi: 10.1124/jpet.110.173245. [DOI] [PubMed] [Google Scholar]
- 133.Ng H.J., Whittemore E.R., Tran M.B., et al. Nootropic α7 nicotinic receptor allosteric modulator derived from GABAA receptor modulators. Proc. Natl. Acad. Sci. USA. 2007;104:8059–8064. doi: 10.1073/pnas.0701321104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. NCT01851603. Phase I Study to Evaluate the Safety and Pharmacokinetics of Oral Doses of Anvylic-3288 in Healthy Subjects (AVL-3288). Available athttp://clinicaltrials.gov/ct2/show/NCT01851603.
- 135.Winterer G., Gallinant J., Brinkmyer J., et al. Allosteric α7 nicotinic receptor modulation and P50 sensory gating in schizophrenia: A proof-of-mechanism study. Neuropharmacol. 2013;64:197–204. doi: 10.1016/j.neuropharm.2012.06.040. [DOI] [PubMed] [Google Scholar]
- 136.Wadman M. NIH gambles on recycled drugs. Nature. 2013;499:263–264. doi: 10.1038/499263a. [DOI] [PubMed] [Google Scholar]
- 137.Yang Y., Paspalas C.D., Jin L.E., Picciotto M.R., Arnsten A.F., Wang M. Nicotinic α7 receptors enhance NMDA cognitive circuits in dorsolaternal prefrontal cortex. Proc. Natl. Acad. Sci. USA. 2013;110:12078–12083. doi: 10.1073/pnas.1307849110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Javitt D.C., Zukin S.R., Heresco-Levy U., Umbricht D. Has an angel shown the way? Etiological and therapeutic implications of the PCP/NMDA models of schizophrenia. Schizophr. Bull. 2012;38:958–966. doi: 10.1093/schbul/sbs069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Moghaddam B., Javitt D. From revolution to evolution: The gluatamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hashimoto K., Malchow B., Falkai P., Schmitt A. Glutamate modulators as potential therapeutic drugs in schizophrenia and affective disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2013;263:367–377. doi: 10.1007/s00406-013-0399-y. [DOI] [PubMed] [Google Scholar]
- 141.Hashimoto K. Targeting of NMDA receptors in new treatments for schizophrenia. Expert Opin. Ther. Targets. 2014;18:1049–1063. doi: 10.1517/14728222.2014.934225. [DOI] [PubMed] [Google Scholar]
- 142.Hashimoto K. Serine enantiomers as diagnostic biomarkers for schizophrenia and bipolar disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2015 doi: 10.1007/s00406-015-0602-4. [DOI] [PubMed] [Google Scholar]
- 143.Wang H., Yu M., Ochani M., et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 144.Shytle R.D., Mori T., Townsend K., et al. Cholinergic modulation of microglial activation by α7 nicotinic receptors. J. Neurochem. 2004;89:337–343. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- 145.Gallowitsch-Puerta M., Tracey K.J. Immunologic role of the cholinergic anti-inflammatory pathway and the nicotinic acetylcholine α7 receptor. Ann. N. Y. Acad. Sci. 2005;1062:209–219. doi: 10.1196/annals.1358.024. [DOI] [PubMed] [Google Scholar]
- 146.Hashimoto K. Microglial activation in schizophrenia and minocycline treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:1758–1759. doi: 10.1016/j.pnpbp.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 147.Monji A., Kato T., Kanba S. Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry Clin. Neurosci. 2009;63:257–265. doi: 10.1111/j.1440-1819.2009.01945.x. [DOI] [PubMed] [Google Scholar]
- 148.Hashimoto K. Can minocycline prevent the onset of Alzheimer’s disease? Ann. Neurol. 2011;69:739–740. doi: 10.1002/ana.22378. [DOI] [PubMed] [Google Scholar]
- 149.Meyer U. Anti-inflammatory signaling in schizophrenia. Brain Behav. Immun. 2011;25:1507–1518. doi: 10.1016/j.bbi.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 150.Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res. Brain Res. Rev. 2009;61:105–123. doi: 10.1016/j.brainresrev.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 151.Bredesen D.E., John V. Next generation therapeutics for Alzheimer’s disease. EMBO Mol. Med. 2013;5:795–798. doi: 10.1002/emmm.201202307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hashimoto K. Tropisetron and its targets in Alzheimer’s disease. Expert Opin. Ther. Targets. 2015;19:1–5. doi: 10.1517/14728222.2014.983901. [DOI] [PubMed] [Google Scholar]
- 153.Yang C., Ren Q., Zhang J.C., Hashimoto K. Tropisetron for postoperative cognitive decline. Aust. N. Z. J. Psychiatry. 2015;49:662–663. doi: 10.1177/0004867415579466. [DOI] [PubMed] [Google Scholar]