Abstract
Klebsiella pneumoniae is the predominant pathogen isolated from liver abscesses of diabetic patients in Asian countries. However, the effects of elevated blood glucose levels on the virulence of this pathogen remain largely unknown. Type 3 fimbriae, encoded by the mrkABCDF genes, are important virulence factors in K. pneumoniae pathogenesis. In this study, the effects of exogenous glucose and the intracellular cyclic AMP (cAMP) signaling pathway on type 3 fimbriae expression regulation were investigated. The production of MrkA, the major subunit of type 3 fimbriae, was increased in glucose-rich medium, whereas cAMP supplementation reversed the effect. MrkA production was markedly increased by cyaA or crp deletion, but slightly decreased by cpdA deletion. In addition, the mRNA levels of mrkABCDF genes and the activity of PmrkA were increased in Δcrp strain, as well as the mRNA levels of mrkHIJ genes that encode cyclic di-GMP (c-di-GMP)-related regulatory proteins that influence type 3 fimbriae expression. Moreover, the activities of PmrkHI and PmrkJ were decreased in ΔlacZΔcrp strain. These results indicate that CRP-cAMP down-regulates mrkABCDF and mrkHIJ at the transcriptional level. Further deletion of mrkH or mrkI in Δcrp strain diminished the production of MrkA, indicating that MrkH and MrkI are required for the CRP regulation of type 3 fimbriae expression. Furthermore, the high activity of PmrkHI in the ΔlacZΔcrp strain was diminished in ΔlacZΔcrpΔmrkHI, but increased in the ΔlacZΔcrpΔmrkJ strain. Deletion of crp increased the intracellular c-di-GMP concentration and reduced the phosphodiesterase activity. Moreover, we found that the mRNA levels of multiple genes related to c-di-GMP metabolism were altered in Δcrp strain. These indicate that CRP regulates type 3 fimbriae expression indirectly via the c-di-GMP signaling pathway. In conclusion, we found evidence of a coordinated regulation of type 3 fimbriae expression by the CRP-cAMP and c-di-GMP signaling pathways in K. pneumoniae.
Introduction
Cyclic AMP (cAMP) is a well-known second messenger that has a fundamental role in global gene regulation [1]. Its production has been demonstrated to be related to the exogenous glucose level, where bacteria grown in glucose-rich medium showed inhibited cAMP production, whereas bacteria grown in less-preferred carbon sources produced elevated levels of cAMP [1–3]. To balance the intracellular cAMP concentrations, the adenylate cyclase CyaA and the cAMP phosphodiesterase CpdA work in concert to carry out cAMP biosynthesis and degradation, respectively [1, 3–5]. The cellular target for cAMP signaling is the cAMP receptor protein (CRP), which forms a homodimer with cAMP (CRP-cAMP) that then binds to CRP binding sites (TGTGA-N6-TCACA and TGCGA-N6-TCGCA) in the promoter region of DNA in order to activate and control mRNA transcription [6–9]. In Escherichia coli, almost 200 operons expression is under CRP-cAMP regulation, which containing genes coding for carbon metabolism and various virulence factors [10–12]. Recently, we found that the biosynthesis of capsular polysaccharides (CPSs) in Klebsiella pneumoniae increases in response to exogenous glucose, a process that is regulated by CRP-cAMP [13]. Nevertheless, in K. pneumoniae pathogenesis, the targets regulated by CRP-cAMP remain largely unknown.
K. pneumoniae is a gram-negative facultative anaerobes that causes community-acquired diseases including pneumonia, bacteramia, septicamia, and urinary and respiratory tract infections in patients with underlying diseases [14]. In Asian countries, pyogenic liver abscess in diabetic patients is common caused by K. pneumoniae [15]. Recently, reports of Klebsiella liver abscess have also been found in western countries [16]. Pyogenic liver abscess isolated K. pneumoniae strains often carry heavy CPS that confer not only a mucoid phenotype to the bacterium but also protect the bacteria from phagocytosis and killing by serum factors. The degree of mucoidy in K. pneumoniae has also been highly related with the successful infection [17, 18]. Besides CPSs, fimbriae are considered as another crucial virulence factor in K. pneumoniae pathogenesis [19]. Most K. pneumoniae strains possess type 1 and type 3 fimbriae. In the heavily encapsulated K. pneumoniae strains, type 3 fimbriae are mainly expressed, whereas type 1 fimbriae are poorly expressed and phase-variable [20, 21]. Type 3 fimbriae, which are encoded by the mrkABCDF operon, play an important role in K. pneumoniae biofilm formation on biotic and abiotic surfaces [22–24]. Biofilm formation is considered to be a key factor in the development of nosocomial infections and increases the bacterial tolerance to antibiotics, which causes problems in medical treatments [25]. Although the diverse regulatory mechanisms of CRP-cAMP on type 1 fimbriae expression and/or biofilm formation have been demonstrated in several bacteria, including Escherichia coli [26], Vibrio cholerae [27], and Serratia marcescens [28], the effect of CRP-cAMP on type 3 fimbriae expression has not been characterized.
Recently, several reports have demonstrated that c-di-GMP is involved in mediating the expression of the two types of fimbriae in K. pneumoniae [24, 29–33]. Like cAMP, c-di-GMP is also a bacterial second messenger that modulates biofilm formation and controls the expression of virulence genes [34]. The intracellular concentration of c-di-GMP in bacteria is modulated through the activities of di-guanylate cyclases (DGCs) and phosphodiesterases (PDEs) [35, 36]. In K. pneumoniae, a gene cluster (mrkHIJ) adjacent to the type 3 fimbriae operon is involved in the modulation and sensing of c-di-GMP as well as regulation of type 3 fimbriae expression [24, 30–33]. MrkH is a PilZ-domain protein that is able to bind to c-di-GMP to activate type 3 fimbriae expression [32, 33]. In addition, MrkH has autoregulatory activity in response to a high intercellular concentration of c-di-GMP to further activate type 3 fimbriae expression [31]. MrkI has been shown to be a LuxR-type transcriptional regulator, activating its own operon and regulating type 3 fimbriae expression [24]. MrkJ possesses an EAL domain that functions as a PDE to hydrolyze c-di-GMP as well as to repress type 3 fimbriae expression [30]. Reverse-transcription PCR analysis revealed that mrkHIJ could be transcribed as a polycistronic mRNA, but mrkJ is activated independently [24]. Although the important roles of MrkH, MrkI, and MrkJ in regulating type 3 fimbriae expression is well established, the regulation of mrkHIJ is still unknown.
The coordination of various nucleotide second messenger signaling pathway to control physiological function and virulence factor expression in response to environmental stimulus is common [37–39]. In E. coli, c-di-GMP, cAMP, and (p)ppGpp signaling pathways are coordinated to regulate the bacterial mobility and expression of curli [40, 41]. In V. cholera, the regulation of the biofilm formation, toxin production, and fimbriae expression is connected by c-di-GMP and cAMP signaling pathways [27, 42, 43]. In Pseudomonas aeruginosa, the expression of acute virulence genes is regulated by the cross-talk between c-di-GMP and cAMP signaling pathways [44]. However, whether cAMP and c-di-GMP signaling pathways also coordinated to regulate the virulence gene expression in K. pneumoniae remains unknown.
Herein, we found that exogenous glucose could activate type 3 fimbriae expression via modulation of the cAMP level in K. pneumoniae CG43. CRP regulation of type 3 fimbriae expression is required for MrkH and MrkI activation. In addition, CRP affects the expression of c-di-GMP-related genes to influence the intracellular concentration of c-di-GMP and PDE activity. Taken together, we provide evidence of the coordination of the CRP-cAMP and c-di GMP-signaling pathways in modulating type 3 fimbriae expression in K. pneumoniae CG43.
Materials and Methods
Bacterial strains, plasmids, and media
Bacterial strains and plasmids used in this study are listed in S1 Table. Primers used in this study are list in S2 Table. Bacterial were routinely cultured at 37°C in Luria-Bertani (LB) medium supplemented with appropriate antibiotics. The antibiotics used include ampicillin (100 μg/ml), kanamycin (25 μg/ml), streptomycin (500 μg/ml), and tetracycline (12.5 μg/ml). Glucose and cAMP were dissolved by distilled water and filter sterilized. To prepare LB medium with various stimuli, autoclaved 2X LB medium was diluted by sterile water containing different concentrations of glucose and/or cAMP of the same volume.
Construction of the gene-deletion mutants
Specific gene deletion was introduced into K. pneumoniae CG43S3 or CG43S3-derived strains using an allelic exchange strategy as previously described [45]. The pKAS46 system was used in the selection of the mutants [46], and the mutations were respectively confirmed by PCR and Southern hybridization (data not shown).
Quantitative reverse-transcription PCR (qRT-PCR)
Total RNA extraction, reverse transcription of isolated mRNA to cDNA, qRT-PCR, and data analysis were performed as described in detail previously [47]. Primers and probes were designed for selected target sequences using Universal ProbeLibrary Assay Design Center (Roche-applied science) and shown in S2 Table. Relative gene expressions were quantified using the comparative threshold cycle 2-ΔΔCT method with 23S rRNA as the endogenous reference.
Measurement of promoter activity
The promoter region of mrkHI and mrkJ was PCR-amplified with primer pair GT288/GT289 and GT284/GT285, respectively, and the amplicons were then cloned into placZ15 to generate pmrkHIZ15 and pmrkJZ15. The promoter-reporter plasmids, pmrkAZ15, pmrkHZ15, and pmrkJZ15, were mobilized into K. pneumoniae strains by electroporation, respectively. The β-galactosidase activity of logarithmic phase bacteria was measured as previously described [48].
Western blotting
K. pneumoniae cultures were collected by centrifugation, re-suspended in PBS, and lysed by sonication. The bacterial total proteins were quantified by Bradford protein assay (Biorad), separated by SDS-PAGE (approximately 5 μg per lane), and transferred to PVDF membrane. After incubation with the corresponding antisera, membranes were visualized with an enhanced chemiluminescence ECL western blotting luminal reagent (PerkinElmer, Wellesley, MA, USA), and the signal was collected by ImageQuant LAS 4000 mini (GE Health, USA).
Intracellular concentration of c-di-GMP
As previous study, the intracellular concentration of c-di-GMP was extracted in K. pneumoniae using heat and ethanol precipitation [49]. The lyophilized samples were resuspended in distill water and further were measured the c-di-GMP level by a ELISA kit (Wuhan EIAab Science Co., Ltd). The c-di-GMP concentration was normalized by total protein concentration. The relative c-di-GMP content of Δcrp strain against WT strain was shown.
PDE activity
The PDE activity of the crude extracts in K. pneumoniae CG43S3 WT and mutant strains was performed as previous study by using bis-p-nitrophenyl phosphate (bis-pNPP) [30]. Briefly, 10 μg of total protein in assay buffer (50 mM Tris-HCl, 1 mM MnCl 2 [pH 8.5]) supplemented with 5 mM bis-pNPP at 37°C for 5 min. The PDE activity was determined by measuring the release of p-nitrophenol at 410 nm. Percentage of PDE activity was quantified by dividing the OD410 of WT strain.
Statistical method
The results of qRT-PCR analysis, and promoter activity, the intracellular concentration of c-di-GMP, and PDE activity measurement were performed in triplicate. The results are presented as the mean and standard deviation. Differences between groups were evaluated by an unpaired t-test. Values of P<0.05 and P<0.01 were considered statistically significant difference.
Results
Glucose and cAMP affect type 3 fimbriae expression
To analyze whether exogenous glucose and cAMP affect K. pneumoniae type 3 fimbriae expression, the bacterium was grown in LB broth supplemented with increasing amounts of glucose, and the level of MrkA (the major subunit of type 3 fimbriae) expression was quantified. As shown in Fig 1A, MrkA production increased with increasing amounts of glucose in the LB broth. Based on the intracellular cAMP concentration was reduced by the presence of glucose in the growth medium [1, 2], increasing amounts of exogenous cAMP were added to LB broth supplemented with 0.5% glucose to observe the production of MrkA. The addition of exogenous cAMP repressed the effect of glucose on MrkA production, suggesting that exogenous glucose can activate K. pneumoniae type 3 fimbriae expression through a reduction of the cAMP level. Besides, the addition of 0.5, 1, or 2 mM cAMP to LB broth did not apparently affect the production of MrkA (data not shown). To further investigate the role of the CRP-cAMP signaling pathway in type 3 fimbriae expression, gene-deletion mutants Δcrp, ΔcyaA, and ΔcpdA were generated and analyzed for the effect of the respective missing genes on MrkA production. As shown in Fig 1B, compared with the wild-type (WT) strain, we found that MrkA production increased in Δcrp and ΔcyaA strains, but slightly decreased in ΔcpdA strain. However, when the bacteria were grown in LB broth supplemented with 0.5% glucose, the deleting effect of crp or cyaA on the MrkA production was diminished (Fig 1C), which may due to the inactivated state of CRP-cAMP signaling pathway in glucose-rich conditions. Deletion of cpdA also caused a slight reduction of MrkA production (Fig 1C). These results suggested that the CRP-cAMP signaling pathway is downstream of glucose treatment to regulate the expression of type 3 fimbriae in K. pneumoniae.
Fig 1. Glucose and cAMP affects the type 3 fimbriae expression of K. pneumoniae CG43S3.
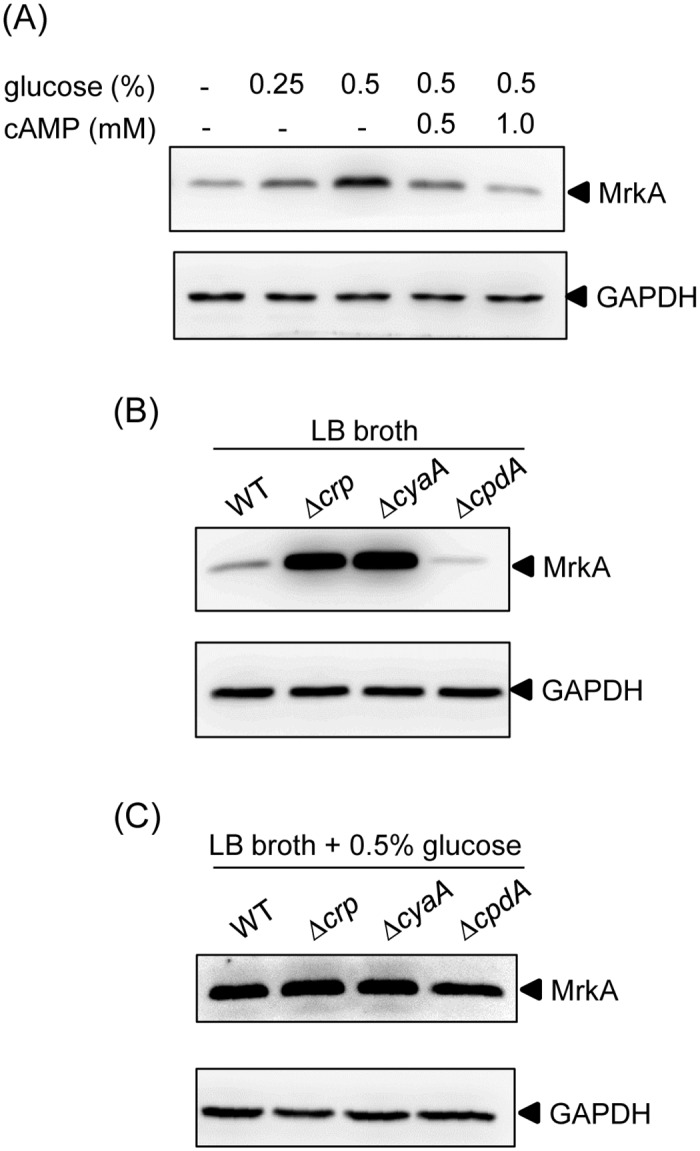
(A) K. pneumoniae CG43S3 was grown overnight at 37°C with agitation in LB broth supplemented with glucose and cAMP as indicated. K. pneumoniae CG43S3 WT, Δcrp, ΔcyaA, and ΔcpdA strains was grown overnight at 37°C with agitation in LB broth alone (B) or LB broth with 0.5% glucose (C) at 37°C with agitation to observe the type 3 fimbriae expression by Western blot analysis against MrkA (the upper panel) and GAPDH antiserum (the lower panel, for internal control). The MrkA and GAPDH proteins are indicated by an arrow, respectively.
CRP acts as a transcriptional repressor of type 3 fimbriae gene expression
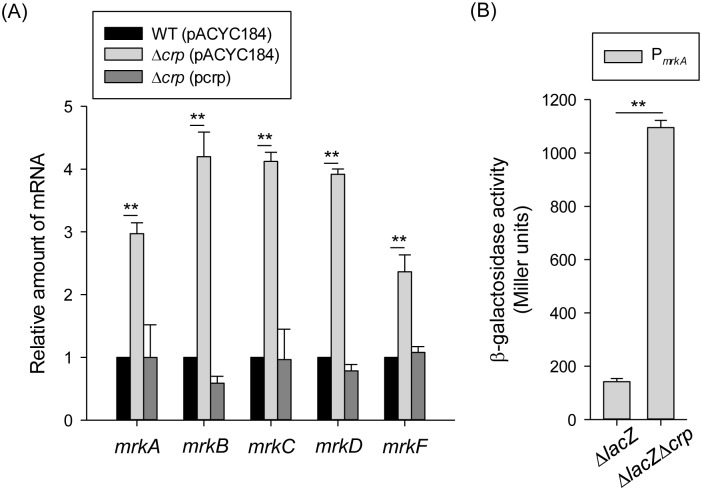
To study the regulatory role of CRP in type 3 fimbriae expression, the effect of crp deletion on the mRNA levels of the type 3 fimbriae gene cluster mrkABCDF was measured by qRT-PCR. As shown in Fig 2A, in the Δcrp strain carrying the empty vector control (pACYC184), the mRNA levels of mrkABCDF were increased relative to those of the WT strain. Introduction of the plasmid complement pcrp into Δcrp strain reversed the effect of the deletion. To further investigate whether CRP acts as a transcriptional repressor of mrkA, a plasmid carrying PmrkA fused with the lacZ reporter gene was constructed and introduced into the ΔlacZ and ΔlacZΔcrp strains, respectively. As shown in Fig 2B, the PmrkA activity was significantly higher in ΔlacZΔcrp stain than in ΔlacZ strain, suggesting that CRP represses type 3 fimbriae expression at the transcriptional level.
Fig 2. CRP-cAMP affects the type 3 fimbriae expression.
(A) qRT-PCR analyses of the mrkA, mrkB, mrkC, mrkD, and mrkF expressions for WT (pACYC184), Δcrp (pACYC184), and Δcrp (pcrp) strains in LB medium. (B) β-galactosidase activities of K. pneumoniae CG43S3ΔlacZ and the isogenic strain (ΔlacZΔcrp) carrying the reporter plasmid pmrkAZ15 (PmrkA::lacZ) were determined using log-phase cultures grown in LB medium. The results are representative of three independent experiments. Error bars indicate standard deviations. *P < 0.05 and ** P < 0.01 compared to WT (pACYC184) (A) and CG43S3ΔlacZ (B) strain.
MrkH, MrkI, and MrkJ are involved in the CRP regulation
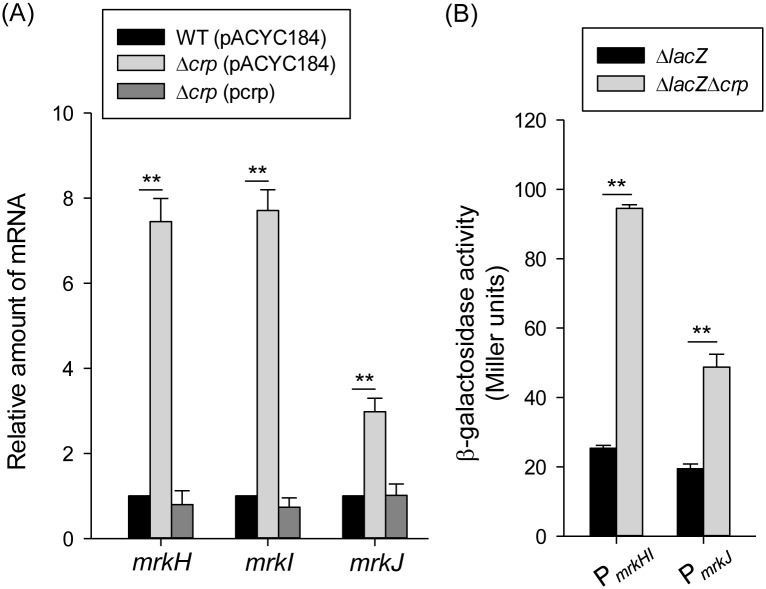
To further study the regulatory mechanism of CRP-cAMP in mrkA gene transcription, the sequences of the E. coli CRP binding sites (TGTGA-N6-TCACA and TGCGA-N6-TCGCA) were used to search for the promoter sequence of the mrkA gene. However, no typical CRP binding site was found in the promoter sequence of mrkA, suggesting that CRP regulates mrkA transcription indirectly. According to previous studies, a c-di-GMP-related gene cluster (i.e., mrkHIJ) adjacent to the type 3 fimbriae operon has been demonstrated to regulate type 3 fimbriae expression [24, 25]. To further investigate whether mrkHIJ are involved in the CRP regulon, the effect of crp deletion on the mRNA levels of these genes was determined by qRT-PCR. Compared with the WT[pACYC184] strain, the mRNA levels of mrkH, mrkI, and mrkJ were markedly increased in Δcrp[pACYC184] strain (Fig 3A). The introduction of pcrp into Δcrp restored the levels of mrkH, mrkI, and mrkJ to the same levels as observed in the WT[pACYC184] strain. This indicates that CRP represses the expression of mrkH, mrkI, and mrkJ. Furthermore, the activities of the promoters PmrkHI and PmrkJ were also repressed by CRP (Fig 3B), confirming that CRP acts a transcriptional repressor of mrkHIJ expression.
Fig 3. CRP represses the mrkHIJ expression.
(A) qRT-PCR analysis of mrkH, mrkI, and mrkJ expression was measured in WT (pACYC184), Δcrp (pACYC184), and Δcrp (pcrp) strains in LB medium. (B) β-galactosidase activities of K. pneumoniae CG43S3ΔlacZ and the isogenic strain (ΔlacZΔcrp) carrying the reporter plasmids pmrkHIZ15 (PmrkHI::lacZ) and pmrkJZ15 (PmrkJ::lacZ) were determined using log-phase cultures grown in LB medium. The results are representative of three independent experiments. Error bars indicate standard deviations. *P < 0.05 and ** P < 0.01 compared to WT (pACYC184) (A) and CG43S3ΔlacZ (B) strain.
Regulation of CRP in type 3 fimbriae expression is dependent on MrkH and MrkI
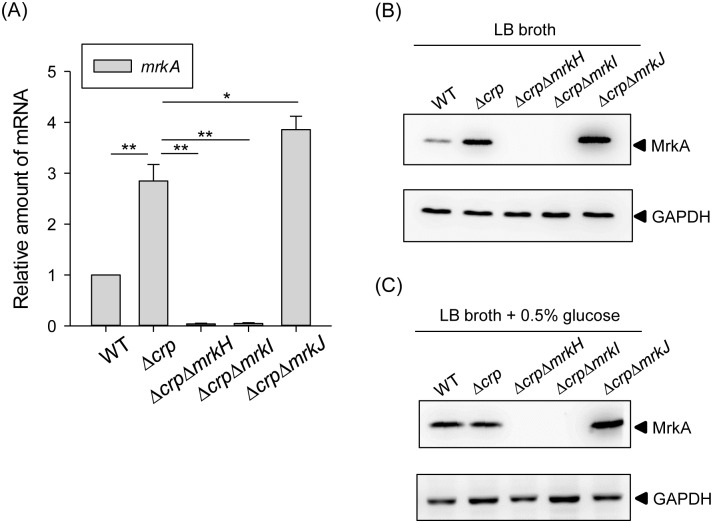
Several studies have demonstrated that MrkH and MrkI activate type 3 fimbriae expression in K. pneumoniae, whereas MrkJ represses type 3 fimbriae expression via the PDE degradation of c-di-GMP [24, 30, 32]. To verify the roles of MrkH, MrkI, and MrkJ in the CRP regulation of type 3 fimbriae expression, the mRNA level of mrkA was determined in the WT, Δcrp, ΔcrpΔmrkH, ΔcrpΔmrkI, and ΔcrpΔmrkJ strains. As shown in Fig 4A, compared with strain Δcrp, the mRNA level of mrkA was reduced in ΔcrpΔmrkH and ΔcrpΔmrkI strains, but increased in ΔcrpΔmrkJ strain, indicating that the deletion of mrkJ in Δcrp strain had a positive effect on mrkA expression. Furthermore, the MrkA production levels confirmed the effects of mrkH, mrkI, and mrkJ deletions in the Δcrp strain (Fig 4B), indicating that MrkH and MrkI are required for type 3 fimbriae expression and may therefore be repressed by CRP. Besides, similar results could be observed when the bacteria were grown in LB broth supplemented with 0.5% glucose (Fig 4C), confirming that MrkH and MrkI are required for the type 3 fimbriae expression in response to exogenous glucose stimuli. In addition, we suggest that CRP also affects the intracellular concentration of c-di-GMP to control type 3 fimbriae expression.
Fig 4. MrkH and MrkI are required for repression of CRP in type 3 fimbriae.
(A) qRT-PCR analysis of mrkA expression was measured in WT, Δcrp, ΔcrpΔmrkH, ΔcrpΔmrkI, and ΔcrpΔmrkJ strains in LB medium. The results are representative of three independent experiments. Error bars indicate standard deviations. *P < 0.05 and ** P < 0.01 compared to the indicated group. The MrkA expression of WT, Δcrp, ΔcrpΔmrkH, ΔcrpΔmrkI, and ΔcrpΔmrkJ strains was determined in LB broth without (B) or with 0.5% glucose (C) by Western blot analysis against MrkA (the upper panel) and GAPDH antiserum (the lower panel, for internal control). The MrkA and GAPDH proteins are indicated by an arrow, respectively.
CRP represses the auto-regulatory activities of MrkH and MrkI
Until now, the regulation of mrkHI in K. pneumoniae has remained largely unknown. As suggested above, the expression of mrkHI appears to be repressed by CRP. However, since no typical CRP binding site was found in the sequence upstream of mrkHI, the CRP repression of mrkHI transcription is likely to be indirect. According to previous studies, MrkH and MrkI could exert autoregulatory activity on their own promoter [24, 31]. Therefore, we postulated that the high activity of promoter PmrkHI in Δcrp strain is due to autoactivation by MrkH and MrkI. To demonstrate this possibility, the activity of PmrkHI was determined in ΔlacZ, ΔlacZΔcrp, and ΔlacZΔcrpΔmrkHI strains. As shown in Fig 5, the absence of mrkHI in ΔlacZΔcrpΔmrkHI strain resulted in lower PmrkHI activity than that in ΔlacZΔcrp strain. In addition, to confirm that the expression of mrkHI is c-di-GMP dependent, gene mrkJ was deleted in ΔlacZΔcrp strain and the activity of PmrkHI was observed. As expected, PmrkHI activity was increased in ΔlacZΔcrpΔmrkJ strain relative to ΔlacZΔcrp strain, confirming that the expression of mrkHI is c-di-GMP-dependent. Taken together, this indicates that CRP represses the autoactivation activity of MrkH and MrkI to further lead to the reduction of type 3 fimbriae expression.
Fig 5. CRP represses the auto-regulatory activities of MrkH and MrkI.
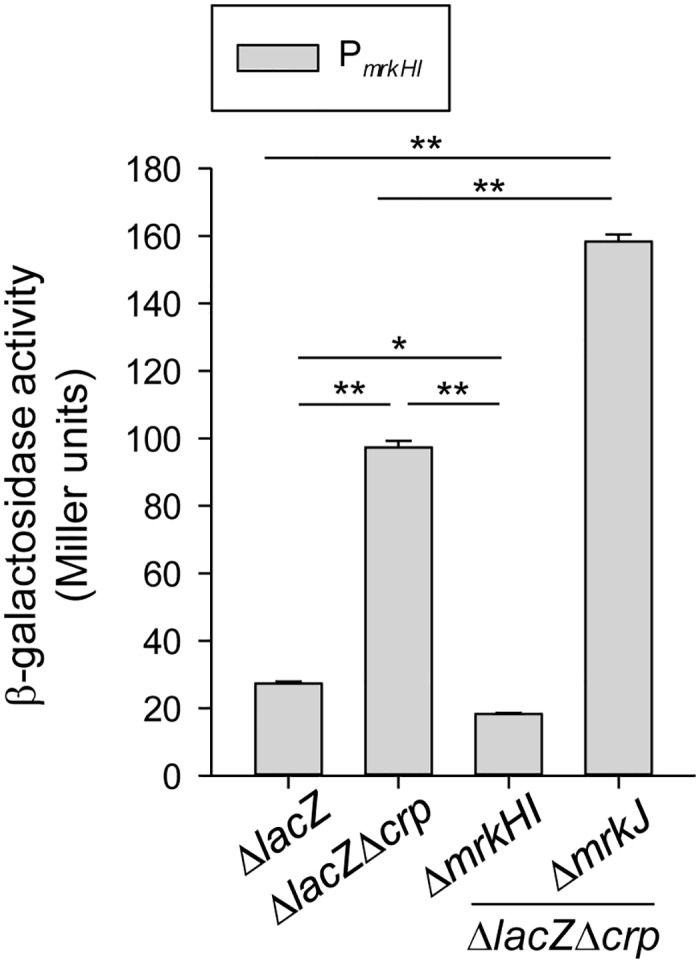
β-galactosidase activities of K. pneumoniae CG43S3ΔlacZ and the isogenic strains (ΔlacZΔcrp, ΔlacZΔcrpΔmrkHI, and ΔlacZΔcrpΔmrkJ) carrying the reporter plasmid pmrkHIZ15 (PmrkHI::lacZ) was determined using log-phase cultures grown in LB medium. The results are representative of three independent experiments. Error bars indicate standard deviations. *P < 0.05 and ** P < 0.01 compared to the indicated group.
CRP affects the intracellular c-di-GMP concentration and PDE activity
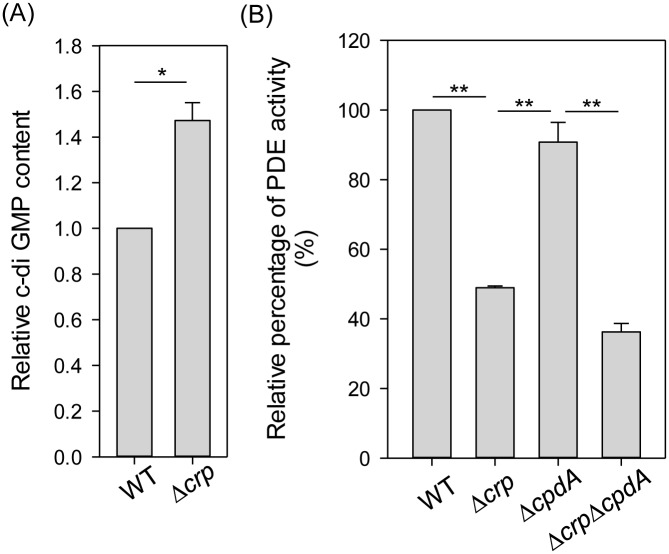
To further investigate the possibility that CRP affects the production of c-di-GMP to inhibit the autoregulatory activity of MrkH and MrkI, the intracellular concentration of c-di-GMP was measured in the WT and Δcrp strains. As shown in Fig 6A, the deletion of crp in K. pneumoniae resulted in a higher intracellular c-di-GMP concentration relative to that in the WT strain, confirming that CRP represses c-di-GMP production. In bacteria, the intracellular concentration of c-di-GMP is controlled by DGCs and PDEs [35, 36]. To verify this, the in vitro PDE activity in crude extracts of the WT and Δcrp strains was first evaluated, using the synthetic substrate bis-pNPP in a colorimetric assay. As shown in Fig 6B, the PDE activity in the Δcrp extract was significantly lower than that in the WT extract. To rule out the cAMP PDE effect, the PDE activity was further evaluated in ΔcpdA and ΔcrpΔcpdA strains. The absence of crp in ΔcrpΔcpdA strain caused a decrease in PDE activity relative to that in the ΔcpdA strain. This indicates that the deletion of crp decreases the PDE activity, thereby elevating the intracellular concentration of c-di-GMP and resulting in high expression levels of MrkH and MrkI and hence type 3 fimbriae.
Fig 6. CRP affects the intracellular c-di-GMP amount and the PDE activity.
(A) Relative c-di-GMP content of WT and Δcrp strains in LB medium was quantified by ELISA according to the manual (Wuhan EIAab Science). (B) The PDE activity of crude extracts of WT and Δcrp strains was determined by using bis-pNPP as substrate and measuring the absorbance at OD410. Relative percentage of PDE activity was calculated by the OD410 of crude extracts is relative to the OD410 of WT strain. The results are representative of three independent experiments. Error bars indicate standard deviations.
Effect of CRP on the expression of c-di-GMP-related genes
By analysis of the published genomic sequence of K. pneumoniae CG43 in the Ensembl Bacteria (http://bacteria.ensembl.org/index.html) database, 11 open reading frames (ORFs) containing the GGDEF domain, 10 ORFs containing the EAL domain, one ORF containing the HD-GYP domain, and six ORFs encoding proteins with both domains (GGDEF and EAL) were found (S1 Fig). In addition, three c-di-GMP receptor proteins harboring a PilZ domain were found in strain CG43; namely, MrkH and the cellulose synthases BcsA-1 and BcsA-2 (S1 Fig). Recently, a new c-di-GMP binding domain, GIL, was identified in enterobacteria [50]. In K. pneumoniae CG43, BcsE carrying GIL domain was also identified (S1 Fig). To further investigate the role of CRP in the c-di-GMP signaling pathway, the putative CRP binding site was used to search the promoter sequence of these c-di-GMP-related genes, with the maximum number of possible mismatched nucleotides set at 2. Using this criterion, the typical CRP binding site was found upstream of four ORFs containing the GGDEF domain, four ORFs containing the EAL domain, and one ORF containing the GIL domain (Table 1). It suggested that CRP affects the expression of these c-di-GMP-related genes to control the intracellular concentration of c-di-GMP in K. pneumoniae CG43. To confirm this possibility, the mRNA expression levels of these c-di-GMP-related genes were quantified in the WT and Δcrp strains. As shown in Table 1, the expression levels of the genes (locus tag number: D364_04720, D364_08130, D364_13295, D364_22720, and D364_19875) appeared to be increased by more than two-fold in the Δcrp strain, indicating that CRP may affect the expression of c-di-GMP-related genes to further influence the c-di-GMP signaling pathway.
Table 1. Analysis of CRP binding site in the upstream sequences of the c-di-GMP related ORFs in K. pneumoniae CG43 genome.
| Domain | Locus tag | Gene name | Typical CRP binding site TG(T/C)GA-N6-TC(A/G)CA | Positiona | Ratiob Δcrp/WT |
|---|---|---|---|---|---|
| GGDEF domain | |||||
| D364_04720 | - | TCAGA-TTGAAC-TCACA | -290 to -275 | 2.71±0.42 | |
| D364_06045 | - | TGCAA-GGAAAT-TCACG | -88 to -73 | 1.06±0.15 | |
| D364_09195 | - | TGCGC-GCAAAG-TCACC | -175 to -160 | 0.72±0.11 | |
| D364_15015 | GGTGA-GAATTG-TCCCA | -96 to -81 | 1.78±0.82 | ||
| EAL domain | |||||
| D364_06025 | - | TTTGA-TTTTTA-TTACA | -290 to -275 | 1.83±0.31 | |
| D364_08130 | - | AGTGG-AGGGAA-TCACA | -162 to -147 | 4.61±0.71 | |
| D364_13295 | - | TGCCA-CCTGGT-TCTCA | -112 to -97 | 2.45±0.23 | |
| D364_22720 | yjcC | TGTGA-ACTATA-TCACA | -382 to -367 | 2.85±0.24 | |
| GIL domain | |||||
| D364_19875 | bcsE | TGTCA-ATCAGG-GCGCA | -81 to -66 | 3.84±0.16 | |
aDistance to the translational start codon
bMean expression ratio of crp mutant relative to wild-type parental strain CG43S3
Discussion
Diabetic patients have been reported to have a higher susceptibility to infections [51, 52]. K. pneumoniae strains are more virulent in diabetic than in normal mice has been demonstrated [53]. We had previously found that exogenous glucose could stimulate CPS production in K. pneumoniae via regulation by the CRP-cAMP signaling pathway [13]. Likewise, this study has demonstrated that could increase type 3 fimbriae expression via the same signaling pathway (Figs 1 and 2). The negative regulation of CRP-cAMP on type 3 fimbriae expression correlated with reductions in the intracellular c-di-GMP concentration and MrkH and MrkI autoregulatory activity (Figs 3 to 5). These findings imply that in response to elevated blood glucose levels in diabetic patients, K. pneumoniae could increase the expression of virulence factors via the coordination of different regulatory mechanisms for a successful infection.
Several studies have reported that external glucose and the CRP-cAMP signaling pathway could influence fimbriae expression in bacteria [26, 54–56]. In E. coli, glucose could induce type 1 fimbriae expression and repress P fimbriae expression [26, 55]. Likewise, glucose induced type 1 fimbriae expression in Salmonella typhimurium and S. marcescens [54, 56]. Moreover, the deletion of crp in the uropathogenic E. coli isolate J96 caused a higher expression of type 1 fimbriae, as assessed by mannose-sensitive yeast agglutination (MSYA) assay [26]. However, in K. pneumoniae CG43, deletion of crp did not alter the bacterial MSYA activity (data not shown), suggesting that CRP does not regulate the expression of type 1 fimbriae in this bacterium, at least under this assay condition. Besides this, crosstalk regulation between type 1 and 3 fimbriae expression has been demonstrated in K. pneumoniae CG43 [29], and whether or not glucose is involved in this regulatory circuit awaits further investigations. On the other hand, deletion of crp from K. pneumoniae resulted in a reduced growth rate (S2A Fig). Colonies of the Δcrp strain appeared relatively translucent and small as compared to that of the WT strain (S2B Fig), which was probably due to the altered production of CPS, but not type 3 fimbriae. We considered that the expression of type 3 fimbriae is not a central factor that determines the morphology of K. pneumoniae colonies, since no obvious difference in colonial morphology could be found between the WT and ΔmrkA strain (data now shown).
Previous studies have demonstrated that, in different bacteria, the CRP-cAMP signaling pathway regulates the expression of fimbriae in different ways [26, 54, 56–61]. In E. coli J96, CRP-cAMP regulates the expression of type 1 fimbriae by an indirect mechanism that requires the activity of DNA gyrase and leucine-responsive protein (Lrp) [26]. In E. coli CFT073, CRP-cAMP must bind to the promoter upstream of Lrp site 4 to fully express P fimbriae [61]. In S. marcescens, CRP-cAMP acts as an indirect repressor of type 1 fimbriae expression [56]. These findings indicate that coordination between the CRP-cAMP signaling pathway and other regulatory mechanisms in the control of fimbriae expression is common. Likewise, we found that CRP-cAMP indirectly represses the expression of type 3 fimbriae in K. pneumoniae CG43. Deletion of crp in K. pneumoniae CG43 increased the expression of mrkHI, thereby activating the expression of type 3 fimbriae (Figs 3 and 4). In addition, previous studies have reported that the expression of mrkHI and type 3 fimbriae could be activated by intracellular c-di-GMP and the phosphorylation of MrkI [24, 31]. We also found that the deletion of crp increased the intracellular c-di-GMP level (Fig 6A). In addition, the absence of mrkJ in ΔlacZΔcrpΔmrkJ strain resulted in a higher activity of PmrkHI relative to that in ΔlacZΔcrp strain (Fig 5), confirming that the intracellular c-di-GMP level is critical for the mediation of mrkHI and type 3 fimbriae expression by the CRP-cAMP signaling pathway. Furthermore, whether the phosphorylated state of MrkI is also affected by the CRP-cAMP signaling pathway remains to be verified. On the other hand, the ferric uptake regulator (Fur) has been shown to bind directly to PmrkHI and PmrkA to mediate type 3 fimbriae expression [24]. However, we suggest that Fur is not involved in the CRP-cAMP regulation of type 3 fimbriae expression, since the expression of fur was not influenced by the CRP-cAMP signaling pathway in K. pneumoniae [47].
The interaction between the CRP-cAMP and c-di-GMP signaling pathways in the control of virulence gene expression has been demonstrated in several bacteria [27, 44]. In this study, we also found interplay of the CRP-cAMP and c-di-GMP signaling pathways in the control of type 3 fimbriae expression in K. pneumoniae CG43. Furthermore, the addition of glucose (0.5%) to LB broth decreased the intracellular concentration of cAMP (S3A Fig) and the promoter activity of Pcrp (S3B Fig) in K. pneumoniae; moreover, similar results have been reported in E. coli [62]. On the other hand, the intracellular concentration of c-di-GMP was found to be increased in response to glucose-rich environment (S3C Fig). Therefore, glucose availability is a critical environmental signal to affect CRP-cAMP and c-di-GMP signaling pathways in K. pneumoniae. In addition, the intracellular c-di-GMP level and PDE activity in K. pneumoniae were affected by the deletion of crp (Fig 6). In the genome of K. pneumoniae CG43, the search for typical CRP binding sites in the upstream region of ORFs encoding EAL domain proteins revealed three ORFs (D364_08130, D364_13295, and D364_22720) that may be directly repressed by CRP. Depending on various cellular conditions, dual activities or only single activity is present in hybrid proteins with both GGDEF and EAL domains [37, 63]. In K. pneumoniae, CRP may indirectly affect the expression of ORFs encoding GGDEF proteins or hybrid proteins with both GGDEF and EAL domains, to modulate the intracellular c-di-GMP level and PDE activity. In addition, we found that the mRNA level of D364_04720, encoding a GGDEF domain protein, was increased in the Δcrp strain (Table 1). However, whether CRP regulates the expression of D364_04720 to decrease the intracellular c-di-GMP level remains to be investigated.
In K. pneumoniae, CPS and adherence factors, including type 1 and type 3 fimbriae, have been demonstrated to play important roles in biofilm formation and pathogenesis, and the expression of these virulence genes is under coordinated regulation [24, 64]. Among the adherence factors, type 3 fimbriae have been described as the major determinant of biofilm formation. Furthermore, type 3 fimbriae also mediate the bacterial adherence to epithelial cells and extracellular matrix proteins [23, 65–67]. The ability of K. pneumoniae colonization and subsequent persistence in mice was reduced when the type 3 fimbriae expression was abolished [68]. In addition, immunization of mice with purified type 3 fimbriae confers protection against following challenge with virulent K. pneumoniae [69, 70]. These findings indicate the significance of type 3 fimbriae to the virulence of K. pneumoniae. In this study, we found that K. pneumoniae grown in LB broth supplemented with 0.5% glucose (Fig 1A) or with crp deleted (Fig 1B) resulted in an increased expression of type 3 fimbriae; however, the biofilm-forming activity was reduced (S4 Fig) and the CPS amount was increased [47]. A previous study also indicated that glucose could repress K. pneumoniae biofilm formation [71]. Since K. pneumoniae CPS has been shown to impede the assembly of fimbriae and the activity of biofilm formation [21, 72], we postulate that an increased CPS amount may hinder the adherence activity of type 3 fimbriae, resulting in decreased K. pneumoniae biofilm formation. It is known that biofilm formation is a complex process with several developmental stages in which bacteria express different genes. We suggested that type 3 fimbriae and CPS may act in different developmental stages to influence K. pneumoniae biofilm formation. However, how the CRP-cAMP signaling pathway orchestrate the expression of CPS and type 3 fimbriae, in response to exogenous glucose levels, to modulate the bacterial biofilm forming activity remains to be investigated. In addition, we noted that CRP represses the expression of bcsE, which encodes a GIL domain protein (Table 1). In enterobacteria, BcsE is required for maximal cellulose production and can bind to c-di-GMP via the GIL domain [50]. As cellulose is one of the extracellular polysaccharides involved in biofilm formation, it is possible that CRP modulates cellulose synthesis to influence biofilm formation through the regulation of bcsE expression.
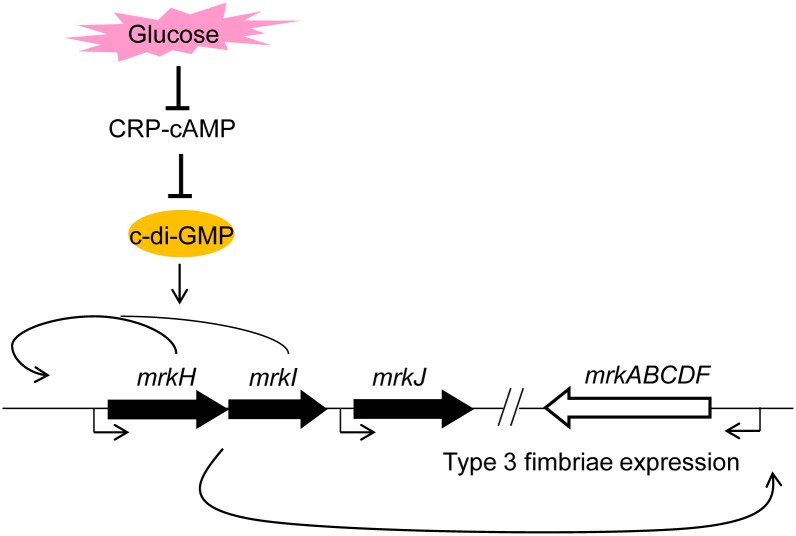
In this study, we have provided important evidence that the CRP-cAMP signaling pathway plays a profound regulatory role in type 3 fimbriae expression in response to an external glucose stimulus, and the regulatory mechanism is also required for MrkH and MrkI activity. In addition, we found that CRP increased the intracellular PDE activity in K. pneumoniae to further affect the intracellular c-di-GMP concentration. We also found that CRP affects the expression of several c-di-GMP-related genes to influence the c-di-GMP signaling pathway. According the results in this study, we proposed a working model as shown in Fig 7. In K. pneumoniae, external glucose stimulus repressed the function of CRP-cAMP to further elevate the intracellular concentration of c-di-GMP and the expression of MrkH and MrkI, resulting in a high expression of type 3 fimbriae. To the best of our knowledge, this is the first study to show the coordination between CRP-cAMP and c-di-GMP signaling in modulating type 3 fimbriae expression. Furthermore, we have previously demonstrated that CPS biosynthesis of K. pneumoniae was affected by CRP-cAMP signaling pathway [47] and a c-di-GMP related protein, YjcC [73]. The expression of yjcC may be directly repressed by CRP (Table 1). Therefore, we considered that K. pneumoniae modulates its virulence gene expression via the interplay of the CRP-cAMP and c-di-GMP signaling pathways in response to exogenous glucose, which may have a major impact on the bacterial virulence in diabetes mellitus patients.
Fig 7. A proposed model for CRP-cAMP signaling pathway on type 3 fimbriae and MrkHI expressions in K. pneumoniae.
Supporting Information
The locus tag (D364_number) of the genes encoding (A) GGDEF, (B) EAL, (C) HD-GYP, (D) GGDEF and EAL, (E) PilZ, and (F) GIL domain proteins were indicated.
(TIF)
(A) Growth rate of WT and Δcrp strain was determined in LB broth for 12 h at 37°C. (B) Colony morphology of WT and Δcrp strain was observed on LB plate after 16 h at 37°C.
(TIF)
(A) Relative cAMP content of K. pneumoniae CG43S3 in LB medium without or with 0.5% glucose was quantified by cAMP XP™ Assay Kit according to the manual (Cell Signaling Technology, Inc). (B) β-galactosidase activities of K. pneumoniae CG43S3ΔlacZ carrying the reporter plasmid pcrpZ15 (Pcrp::lacZ) was determined using log-phase cultures grown in LB medium without or with 0.5% glucose. (C) Relative c-di-GMP content of K. pneumoniae CG43S3 in LB medium without or with 0.5% glucose was quantified by ELISA according to the manual (Wuhan EIAab Science). The results are representative of three independent experiments. Error bars indicate standard deviations. ** P < 0.01 compared to the indicated group.
(TIF)
Biofilm formation of K. pneumoniae CG43S3 WT or Δcrp strain was determined in LB broth supplemented with the indicated glucose concentration using crystal violet staining as previously described [24]. The results are representative of three independent experiments. Error bars indicate standard deviations. ** P < 0.01 compared to the indicated group.
(TIF)
(DOCX)
(DOCX)
Acknowledgments
We are grateful to Mr. Jing-Ciao Lin for his technical assistance during the study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The work is supported by the grant from Ministry of Science and Technology (MOST 104-2320-B-039-037-) and Taichung Tzuchi Hospital, The Buddhist Tzu Chi Medical Foundation (TTCRD103-10). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Botsford JL, Harman JG. Cyclic AMP in prokaryotes. Microbiol Rev. 1992;56(1):100–22. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peterkofsky A, Gazdar C. Glucose and the metabolism of adenosine 3':5'-cyclic monophosphate in Escherichia coli. Proc Natl Acad Sci U S A. 1971;68(11):2794–8. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonough KA, Rodriguez A. The myriad roles of cyclic AMP in microbial pathogens: from signal to sword. Nat Rev Microbiol. 2011;10(1):27–38. Epub 2011/11/15. nrmicro2688 [pii] 10.1038/nrmicro2688 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imamura R, Yamanaka K, Ogura T, Hiraga S, Fujita N, Ishihama A, et al. Identification of the cpdA gene encoding cyclic 3',5'-adenosine monophosphate phosphodiesterase in Escherichia coli. J Biol Chem. 1996;271(41):25423–9. . [DOI] [PubMed] [Google Scholar]
- 5.Kim HS, Kim SM, Lee HJ, Park SJ, Lee KH. Expression of the cpdA gene, encoding a 3',5'-cyclic AMP (cAMP) phosphodiesterase, is positively regulated by the cAMP-cAMP receptor protein complex. J Bacteriol. 2009;191(3):922–30. 10.1128/JB.01350-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg OG, von Hippel PH. Selection of DNA binding sites by regulatory proteins. II. The binding specificity of cyclic AMP receptor protein to recognition sites. J Mol Biol. 1988;200(4):709–23. Epub 1988/04/20. 0022-2836(88)90482-2 [pii]. . [DOI] [PubMed] [Google Scholar]
- 7.Harman JG. Allosteric regulation of the cAMP receptor protein. Biochim Biophys Acta. 2001;1547(1):1–17. . [DOI] [PubMed] [Google Scholar]
- 8.Cameron AD, Redfield RJ. Non-canonical CRP sites control competence regulons in Escherichia coli and many other gamma-proteobacteria. Nucleic Acids Res. 2006;34(20):6001–14. Epub 2006/10/28. gkl734 [pii] 10.1093/nar/gkl734 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebright RH. Transcription activation at Class I CAP-dependent promoters. Mol Microbiol. 1993;8(5):797–802. Epub 1993/05/01. . [DOI] [PubMed] [Google Scholar]
- 10.Gosset G, Zhang Z, Nayyar S, Cuevas WA, Saier MH Jr. Transcriptome analysis of Crp-dependent catabolite control of gene expression in Escherichia coli. J Bacteriol. 2004;186(11):3516–24. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez-Antonio A, Collado-Vides J. Identifying global regulators in transcriptional regulatory networks in bacteria. Curr Opin Microbiol. 2003;6(5):482–9. . [DOI] [PubMed] [Google Scholar]
- 12.Zheng D, Constantinidou C, Hobman JL, Minchin SD. Identification of the CRP regulon using in vitro and in vivo transcriptional profiling. Nucleic Acids Res. 2004;32(19):5874–93. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin TH, Huang SH, Wu CC, Liu HH, Jinn TR, Chen Y, et al. Inhibition of Klebsiella pneumoniae Growth and Capsular Polysaccharide Biosynthesis by Fructus mume. Evid Based Complement Alternat Med. 2013;2013:621701 Epub 2013/09/26. 10.1155/2013/621701 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11(4):589–603. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang YS, Siu LK, Yeh KM, Fung CP, Huang SJ, Hung HC, et al. Recurrent Klebsiella pneumoniae liver abscess: clinical and microbiological characteristics. J Clin Microbiol. 2009;47(10):3336–9. Epub 2009/08/21. JCM.00918-09 [pii] 10.1128/JCM.00918-09 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lederman ER, Crum NF. Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics. Am J Gastroenterol. 2005;100(2):322–31. . [DOI] [PubMed] [Google Scholar]
- 17.Lin JC, Chang FY, Fung CP, Xu JZ, Cheng HP, Wang JJ, et al. High prevalence of phagocytic-resistant capsular serotypes of Klebsiella pneumoniae in liver abscess. Microbes Infect. 2004;6(13):1191–8. . [DOI] [PubMed] [Google Scholar]
- 18.Regueiro V, Campos MA, Pons J, Alberti S, Bengoechea JA. The uptake of a Klebsiella pneumoniae capsule polysaccharide mutant triggers an inflammatory response by human airway epithelial cells. Microbiology. 2006;152(Pt 2):555–66. . [DOI] [PubMed] [Google Scholar]
- 19.Livrelli V, De Champs C, Di Martino P, Darfeuille-Michaud A, Forestier C, Joly B. Adhesive properties and antibiotic resistance of Klebsiella, Enterobacter, and Serratia clinical isolates involved in nosocomial infections. J Clin Microbiol. 1996;34(8):1963–9. Epub 1996/08/01. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Struve C, Bojer M, Krogfelt KA. Characterization of Klebsiella pneumoniae type 1 fimbriae by detection of phase variation during colonization and infection and impact on virulence. Infect Immun. 2008;76(9):4055–65. Epub 2008/06/19. IAI.00494-08 [pii] 10.1128/IAI.00494-08 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schembri MA, Blom J, Krogfelt KA, Klemm P. Capsule and fimbria interaction in Klebsiella pneumoniae. Infect Immun. 2005;73(8):4626–33. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Martino P, Cafferini N, Joly B, Darfeuille-Michaud A. Klebsiella pneumoniae type 3 pili facilitate adherence and biofilm formation on abiotic surfaces. Res Microbiol. 2003;154(1):9–16. Epub 2003/02/11. S0923-2508(02)00004-9 [pii]. . [DOI] [PubMed] [Google Scholar]
- 23.Jagnow J, Clegg S. Klebsiella pneumoniae MrkD-mediated biofilm formation on extracellular matrix- and collagen-coated surfaces. Microbiology. 2003;149(Pt 9):2397–405. Epub 2003/09/02. 10.1099/mic.0.26434-0 . [DOI] [PubMed] [Google Scholar]
- 24.Wu CC, Lin CT, Cheng WY, Huang CJ, Wang ZC, Peng HL. Fur-dependent MrkHI regulation of type 3 fimbriae in Klebsiella pneumoniae CG43. Microbiology. 2012;158(Pt 4):1045–56. Epub 2012/01/21. mic.0.053801–0 [pii] 10.1099/mic.0.053801-0 . [DOI] [PubMed] [Google Scholar]
- 25.Murphy CN, Clegg S. Klebsiella pneumoniae and type 3 fimbriae: nosocomial infection, regulation and biofilm formation. Future Microbiol. 2012;7(8):991–1002. Epub 2012/08/24. 10.2217/fmb.12.74 . [DOI] [PubMed] [Google Scholar]
- 26.Muller CM, Aberg A, Straseviciene J, Emody L, Uhlin BE, Balsalobre C. Type 1 fimbriae, a colonization factor of uropathogenic Escherichia coli, are controlled by the metabolic sensor CRP-cAMP. PLoS Pathog. 2009;5(2):e1000303 Epub 2009/02/21. 10.1371/journal.ppat.1000303 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fong JC, Yildiz FH. Interplay between cyclic AMP-cyclic AMP receptor protein and cyclic di-GMP signaling in Vibrio cholerae biofilm formation. J Bacteriol. 2008;190(20):6646–59. Epub 2008/08/19. JB.00466-08 [pii] 10.1128/JB.00466-08 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalivoda EJ, Brothers KM, Stella NA, Schmitt MJ, Shanks RM. Bacterial cyclic AMP-phosphodiesterase activity coordinates biofilm formation. PLoS One. 2013;8(7):e71267 Epub 2013/08/08. 10.1371/journal.pone.0071267 PONE-D-13-10896 [pii]. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang ZC, Huang CJ, Huang YJ, Wu CC, Peng HL. FimK regulation on the expression of type 1 fimbriae in Klebsiella pneumoniae CG43S3. Microbiology. 2013;159(Pt 7):1402–15. Epub 2013/05/25. mic.0.067793–0 [pii] 10.1099/mic.0.067793-0 . [DOI] [PubMed] [Google Scholar]
- 30.Johnson JG, Clegg S. Role of MrkJ, a phosphodiesterase, in type 3 fimbrial expression and biofilm formation in Klebsiella pneumoniae. J Bacteriol. 2010;192(15):3944–50. Epub 2010/06/01. JB.00304-10 [pii] 10.1128/JB.00304-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan JW, Wilksch JJ, Hocking DM, Wang N, Srikhanta YN, Tauschek M, et al. Positive auto-regulation of mrkHI by the c-di-GMP-dependent MrkH protein in the biofilm regulatory circuit of Klebsiella pneumoniae. J Bacteriol. 2015. Epub 2015/03/04. JB.02615-14 [pii] 10.1128/JB.02615-14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilksch JJ, Yang J, Clements A, Gabbe JL, Short KR, Cao H, et al. MrkH, a novel c-di-GMP-dependent transcriptional activator, controls Klebsiella pneumoniae biofilm formation by regulating type 3 fimbriae expression. PLoS Pathog. 2011;7(8):e1002204 Epub 2011/09/09. 10.1371/journal.ppat.1002204 PPATHOGENS-D-10-00590 [pii]. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang J, Wilksch JJ, Tan JW, Hocking DM, Webb CT, Lithgow T, et al. Transcriptional activation of the mrkA promoter of the Klebsiella pneumoniae type 3 fimbrial operon by the c-di-GMP-dependent MrkH protein. PLoS One. 2013;8(11):e79038 Epub 2013/11/19. 10.1371/journal.pone.0079038 PONE-D-13-17623 [pii]. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamayo R, Pratt JT, Camilli A. Roles of cyclic diguanylate in the regulation of bacterial pathogenesis. Annu Rev Microbiol. 2007;61:131–48. Epub 2007/05/08. 10.1146/annurev.micro.61.080706.093426 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hengge R. Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol. 2009;7(4):263–73. Epub 2009/03/17. nrmicro2109 [pii] 10.1038/nrmicro2109 . [DOI] [PubMed] [Google Scholar]
- 36.Simm R, Morr M, Kader A, Nimtz M, Romling U. GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol Microbiol. 2004;53(4):1123–34. Epub 2004/08/13. 10.1111/j.1365-2958.2004.04206.x MMI4206 [pii]. . [DOI] [PubMed] [Google Scholar]
- 37.Kalia D, Merey G, Nakayama S, Zheng Y, Zhou J, Luo Y, et al. Nucleotide, c-di-GMP, c-di-AMP, cGMP, cAMP, (p)ppGpp signaling in bacteria and implications in pathogenesis. Chem Soc Rev. 2012;42(1):305–41. Epub 2012/10/02. 10.1039/c2cs35206k . [DOI] [PubMed] [Google Scholar]
- 38.Pesavento C, Hengge R. Bacterial nucleotide-based second messengers. Curr Opin Microbiol. 2009;12(2):170–6. Epub 2009/03/26. S1369-5274(09)00006-X [pii] 10.1016/j.mib.2009.01.007 . [DOI] [PubMed] [Google Scholar]
- 39.Hengge R, Grundling A, Jenal U, Ryan R, Yildiz F. Bacterial Signal Transduction by Cyclic Di-GMP and Other Nucleotide Second Messengers. J Bacteriol. 2016;198(1):15–26. Epub 2015/06/10. JB.00331-15 [pii] 10.1128/JB.00331-15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durfee T, Hansen AM, Zhi H, Blattner FR, Jin DJ. Transcription profiling of the stringent response in Escherichia coli. J Bacteriol. 2008;190(3):1084–96. Epub 2007/11/28. JB.01092-07 [pii] 10.1128/JB.01092-07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Magnusson LU, Gummesson B, Joksimovic P, Farewell A, Nystrom T. Identical, independent, and opposing roles of ppGpp and DksA in Escherichia coli. J Bacteriol. 2007;189(14):5193–202. Epub 2007/05/15. JB.00330-07 [pii] 10.1128/JB.00330-07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cotter PA, Stibitz S. c-di-GMP-mediated regulation of virulence and biofilm formation. Curr Opin Microbiol. 2007;10(1):17–23. Epub 2007/01/09. S1369-5274(06)00192-5 [pii] 10.1016/j.mib.2006.12.006 . [DOI] [PubMed] [Google Scholar]
- 43.Lim B, Beyhan S, Yildiz FH. Regulation of Vibrio polysaccharide synthesis and virulence factor production by CdgC, a GGDEF-EAL domain protein, in Vibrio cholerae. J Bacteriol. 2007;189(3):717–29. Epub 2006/11/24. JB.00834-06 [pii] 10.1128/JB.00834-06 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Almblad H, Harrison JJ, Rybtke M, Groizeleau J, Givskov M, Parsek MR, et al. The Cyclic AMP-Vfr Signaling Pathway in Pseudomonas aeruginosa Is Inhibited by Cyclic Di-GMP. J Bacteriol. 2015;197(13):2190–200. Epub 2015/04/22. JB.00193-15 [pii] 10.1128/JB.00193-15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lai YC, Peng HL, Chang HY. RmpA2, an activator of capsule biosynthesis in Klebsiella pneumoniae CG43, regulates K2 cps gene expression at the transcriptional level. J Bacteriol. 2003;185(3):788–800. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skorupski K, Taylor RK. Positive selection vectors for allelic exchange. Gene. 1996;169(1):47–52. . [DOI] [PubMed] [Google Scholar]
- 47.Lin CT, Chen YC, Jinn TR, Wu CC, Hong YM, Wu WH. Role of the cAMP-Dependent Carbon Catabolite Repression in Capsular Polysaccharide Biosynthesis in Klebsiella pneumoniae. PLoS One. 2013;8(2):e54430 Epub 2013/02/15. 10.1371/journal.pone.0054430 PONE-D-12-27906 [pii]. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin CT, Huang TY, Liang WC, Peng HL. Homologous response regulators KvgA, KvhA and KvhR regulate the synthesis of capsular polysaccharide in Klebsiella pneumoniae CG43 in a coordinated manner. J Biochem (Tokyo). 2006;140(3):429–38. . [DOI] [PubMed] [Google Scholar]
- 49.Morgan R, Kohn S, Hwang SH, Hassett DJ, Sauer K. BdlA, a chemotaxis regulator essential for biofilm dispersion in Pseudomonas aeruginosa. J Bacteriol. 2006;188(21):7335–43. Epub 2006/10/20. 188/21/7335 [pii] 10.1128/JB.00599-06 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fang X, Ahmad I, Blanka A, Schottkowski M, Cimdins A, Galperin MY, et al. GIL, a new c-di-GMP-binding protein domain involved in regulation of cellulose synthesis in enterobacteria. Mol Microbiol. 2014;93(3):439–52. Epub 2014/06/20. 10.1111/mmi.12672 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Geerlings SE, Stolk RP, Camps MJ, Netten PM, Hoekstra JB, Bouter PK, et al. Asymptomatic bacteriuria can be considered a diabetic complication in women with diabetes mellitus. Adv Exp Med Biol. 2000;485:309–14. . [DOI] [PubMed] [Google Scholar]
- 52.Patterson JE, Andriole VT. Bacterial urinary tract infections in diabetes. Infect Dis Clin North Am. 1997;11(3):735–50. . [DOI] [PubMed] [Google Scholar]
- 53.Wu JH, Tsai CG. Infectivity of hepatic strain Klebsiella pneumoniae in diabetic mice. Exp Biol Med (Maywood). 2005;230(10):757–61. . [DOI] [PubMed] [Google Scholar]
- 54.Saier MH Jr., Schmidt MR, Leibowitz M. Cyclic AMP-dependent synthesis of fimbriae in Salmonella typhimurium: effects of cya and pts mutations. J Bacteriol. 1978;134(1):356–8. Epub 1978/04/01. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.White-Ziegler CA, Villapakkam A, Ronaszeki K, Young S. H-NS controls pap and daa fimbrial transcription in Escherichia coli in response to multiple environmental cues. J Bacteriol. 2000;182(22):6391–400. Epub 2000/10/29. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalivoda EJ, Stella NA, O'Dee DM, Nau GJ, Shanks RM. The cyclic AMP-dependent catabolite repression system of Serratia marcescens mediates biofilm formation through regulation of type 1 fimbriae. Appl Environ Microbiol. 2008;74(11):3461–70. Epub 2008/04/22. AEM.02733-07 [pii] 10.1128/AEM.02733-07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spurbeck RR, Alteri CJ, Himpsl SD, Mobley HL. The multifunctional protein YdiV represses P fimbria-mediated adherence in uropathogenic Escherichia coli. J Bacteriol. 2013;195(14):3156–64. Epub 2013/05/15. JB.02254-12 [pii] 10.1128/JB.02254-12. 10.1128/JB.02254-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eisenstein BI, Beachey EH, Solomon SS. Divergent effects of cyclic adenosine 3',5'-monophosphate on formation of type 1 fimbriae in different K-12 strains of Escherichia coli. J Bacteriol. 1981;145(1):620–3. Epub 1981/01/01. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beatson SA, Whitchurch CB, Sargent JL, Levesque RC, Mattick JS. Differential regulation of twitching motility and elastase production by Vfr in Pseudomonas aeruginosa. J Bacteriol. 2002;184(13):3605–13. Epub 2002/06/12. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stella NA, Shanks RM. Cyclic-AMP inhibition of fimbriae and prodigiosin production by Serratia marcescens is strain-dependent. Arch Microbiol. 2014;196(5):323–30. Epub 2014/03/13. 10.1007/s00203-014-0970-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weyand NJ, Braaten BA, van der Woude M, Tucker J, Low DA. The essential role of the promoter-proximal subunit of CAP in pap phase variation: Lrp- and helical phase-dependent activation of papBA transcription by CAP from -215. Mol Microbiol. 2001;39(6):1504–22. Epub 2001/03/22. mmi2338 [pii]. . [DOI] [PubMed] [Google Scholar]
- 62.Ishizuka H, Hanamura A, Kunimura T, Aiba H. A lowered concentration of cAMP receptor protein caused by glucose is an important determinant for catabolite repression in Escherichia coli. Mol Microbiol. 1993;10(2):341–50. Epub 1993/10/01. . [DOI] [PubMed] [Google Scholar]
- 63.Ferreira RB, Antunes LC, Greenberg EP, McCarter LL. Vibrio parahaemolyticus ScrC modulates cyclic dimeric GMP regulation of gene expression relevant to growth on surfaces. J Bacteriol. 2008;190(3):851–60. Epub 2007/11/13. JB.01462-07 [pii] 10.1128/JB.01462-07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vuotto C, Longo F, Balice MP, Donelli G, Varaldo PE. Antibiotic Resistance Related to Biofilm Formation in Klebsiella pneumoniae. Pathogens. 2014;3(3):743–58. Epub 2014/12/02. pathogens3030743 [pii] 10.3390/pathogens3030743 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hornick DB, Allen BL, Horn MA, Clegg S. Adherence to respiratory epithelia by recombinant Escherichia coli expressing Klebsiella pneumoniae type 3 fimbrial gene products. Infect Immun. 1992;60(4):1577–88. Epub 1992/04/01. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sebghati TA, Clegg S. Construction and characterization of mutations within the Klebsiella mrkD1P gene that affect binding to collagen type V. Infect Immun. 1999;67(4):1672–6. Epub 1999/03/20. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tarkkanen AM, Virkola R, Clegg S, Korhonen TK. Binding of the type 3 fimbriae of Klebsiella pneumoniae to human endothelial and urinary bladder cells. Infect Immun. 1997;65(4):1546–9. Epub 1997/04/01. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murphy CN, Mortensen MS, Krogfelt KA, Clegg S. Role of Klebsiella pneumoniae type 1 and type 3 fimbriae in colonizing silicone tubes implanted into the bladders of mice as a model of catheter-associated urinary tract infections. Infect Immun. 2013;81(8):3009–17. Epub 2013/06/12. IAI.00348-13 [pii] 10.1128/IAI.00348-13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lavender H, Jagnow JJ, Clegg S. Klebsiella pneumoniae type 3 fimbria-mediated immunity to infection in the murine model of respiratory disease. Int J Med Microbiol. 2005;295(3):153–9. Epub 2005/07/29. S1438-4221(05)00052-4 [pii] 10.1016/j.ijmm.2005.04.001 . [DOI] [PubMed] [Google Scholar]
- 70.Wang Q, Chang CS, Pennini M, Pelletier M, Rajan S, Zha J, et al. Target-Agnostic Identification of Functional Monoclonal Antibodies Against Klebsiella pneumoniae Multimeric MrkA Fimbrial Subunit. J Infect Dis. 2016;213(11):1800–8. Epub 2016/01/16. jiw021 [pii] 10.1093/infdis/jiw021 . [DOI] [PubMed] [Google Scholar]
- 71.Jackson DW, Simecka JW, Romeo T. Catabolite repression of Escherichia coli biofilm formation. J Bacteriol. 2002;184(12):3406–10. Epub 2002/05/25. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goncalves Mdos S, Delattre C, Balestrino D, Charbonnel N, Elboutachfaiti R, Wadouachi A, et al. Anti-biofilm activity: a function of Klebsiella pneumoniae capsular polysaccharide. PLoS One. 2014;9(6):e99995 Epub 2014/06/17. 10.1371/journal.pone.0099995 PONE-D-14-05673 [pii]. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang CJ, Wang ZC, Huang HY, Huang HD, Peng HL. YjcC, a c-di-GMP phosphodiesterase protein, regulates the oxidative stress response and virulence of Klebsiella pneumoniae CG43. PLoS One. 2013;8(7):e66740 Epub 2013/08/13. 10.1371/journal.pone.0066740 PONE-D-13-04125 [pii]. . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The locus tag (D364_number) of the genes encoding (A) GGDEF, (B) EAL, (C) HD-GYP, (D) GGDEF and EAL, (E) PilZ, and (F) GIL domain proteins were indicated.
(TIF)
(A) Growth rate of WT and Δcrp strain was determined in LB broth for 12 h at 37°C. (B) Colony morphology of WT and Δcrp strain was observed on LB plate after 16 h at 37°C.
(TIF)
(A) Relative cAMP content of K. pneumoniae CG43S3 in LB medium without or with 0.5% glucose was quantified by cAMP XP™ Assay Kit according to the manual (Cell Signaling Technology, Inc). (B) β-galactosidase activities of K. pneumoniae CG43S3ΔlacZ carrying the reporter plasmid pcrpZ15 (Pcrp::lacZ) was determined using log-phase cultures grown in LB medium without or with 0.5% glucose. (C) Relative c-di-GMP content of K. pneumoniae CG43S3 in LB medium without or with 0.5% glucose was quantified by ELISA according to the manual (Wuhan EIAab Science). The results are representative of three independent experiments. Error bars indicate standard deviations. ** P < 0.01 compared to the indicated group.
(TIF)
Biofilm formation of K. pneumoniae CG43S3 WT or Δcrp strain was determined in LB broth supplemented with the indicated glucose concentration using crystal violet staining as previously described [24]. The results are representative of three independent experiments. Error bars indicate standard deviations. ** P < 0.01 compared to the indicated group.
(TIF)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.