Abstract
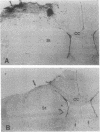
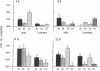
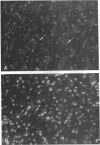
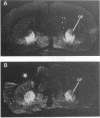
Ischemic lesions of the cerebral cortex occur frequently in humans as a result of stroke. One major consequence of the death of cortical neurons is the loss of excitatory cortical projections to subcortical regions. Little is known, however, about the transsynaptic effect of such lesions on neurotransmitter expression in subcortical structures. We have examined the effects of ischemic cortical lesions on the peptidergic neurotransmitters enkephalin and tachykinins in the striatum, a brain region massively innervated by glutamatergic cortical inputs. The levels of enkephalin and tachykinin mRNAs increased in the striatum of adult rats after thermocoagulation of pial vessels. The effects were more pronounced in the striatal region most heavily innervated by the lesioned cortex but were also observed in other striatal regions and on the contralateral side. Increased gene expression was accompanied by increased immunoreactivity for the two peptides. Elevated levels of enkephalin mRNA were observed up to 3 months after surgery in the ipsilateral striatum. Whereas results of previous studies of acute cortical ablations suggested that excitatory corticostriatal neurons were necessary to maintain normal peptide levels in striatal efferent neurons, the present data indicate that lesions of the same corticostriatal neurons secondary to local ischemia result in a paradoxical transsynaptic activation of neuropeptide synthesis in subcortical structures. This effect may play a role in the functional consequences of cortical strokes and progressive cortical atrophy in humans and may have critical bearing for their treatment and prognosis.
Full text
PDF
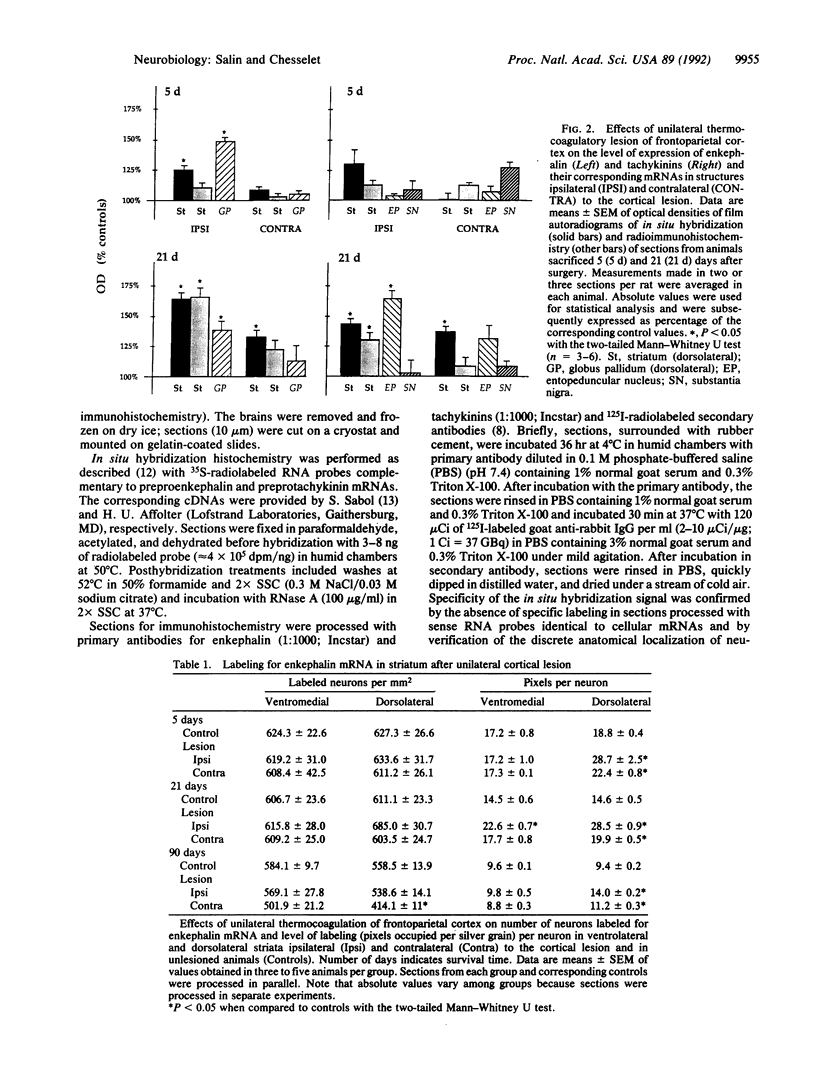

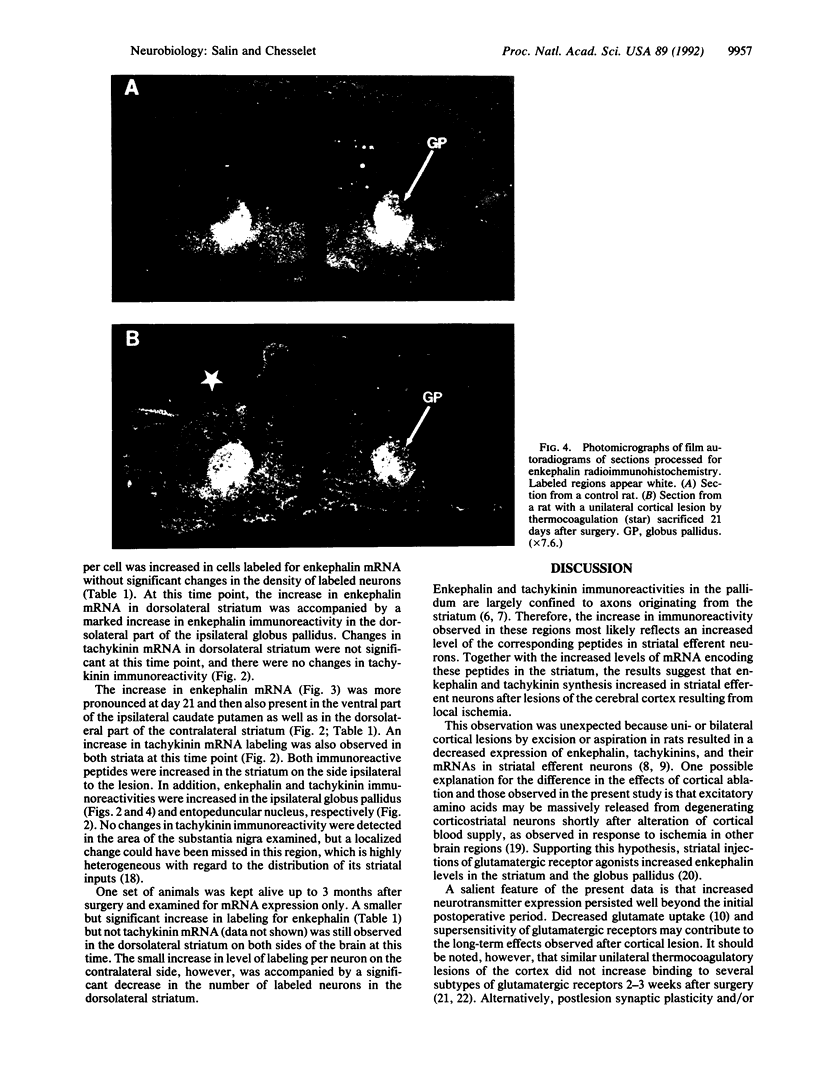

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander G. E., Crutcher M. D. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990 Jul;13(7):266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Benveniste H., Drejer J., Schousboe A., Diemer N. H. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem. 1984 Nov;43(5):1369–1374. doi: 10.1111/j.1471-4159.1984.tb05396.x. [DOI] [PubMed] [Google Scholar]
- Bouras C., Vallet P. G., Hof P. R., Charnay Y., Golaz J., Constantinidis J. Substance P immunoreactivity in Alzheimer disease: a study in cases presenting symmetric or asymmetric cortical atrophy. Alzheimer Dis Assoc Disord. 1990;4(1):24–34. [PubMed] [Google Scholar]
- Chen S., Hillman D. E. Robust synaptic plasticity of striatal cells following partial deafferentation. Brain Res. 1990 Jun 18;520(1-2):103–114. doi: 10.1016/0006-8993(90)91695-d. [DOI] [PubMed] [Google Scholar]
- Chesselet M. F., Weiss L., Wuenschell C., Tobin A. J., Affolter H. U. Comparative distribution of mRNAs for glutamic acid decarboxylase, tyrosine hydroxylase, and tachykinins in the basal ganglia: an in situ hybridization study in the rodent brain. J Comp Neurol. 1987 Aug 1;262(1):125–140. doi: 10.1002/cne.902620110. [DOI] [PubMed] [Google Scholar]
- Correa F. M., Guilhaume S. G., Saavedra J. M. Quantitative radioimmunohistochemical method using [125I]-protein A to measure the content of methionine enkephalin in discrete rat brain areas. J Histochem Cytochem. 1988 Nov;36(11):1379–1386. doi: 10.1177/36.11.3171164. [DOI] [PubMed] [Google Scholar]
- Errami M., Nieoullon A. Development of a micromethod to study the Na+-independent L-[3H]glutamic acid binding to rat striatal membranes. II. Effects of selective striatal lesions and deafferentations. Brain Res. 1986 Feb 26;366(1-2):178–186. doi: 10.1016/0006-8993(86)91293-x. [DOI] [PubMed] [Google Scholar]
- Errami M., Nieoullon A. alpha-[3H]Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid binding to rat striatal membranes: effects of selective brain lesions. J Neurochem. 1988 Aug;51(2):579–586. doi: 10.1111/j.1471-4159.1988.tb01078.x. [DOI] [PubMed] [Google Scholar]
- Hassler R., Haug P., Nitsch C., Kim J. S., Paik K. Effect of motor and premotor cortex ablation on concentrations of amino acids, monoamines, and acetylcholine and on the ultrastructure in rat striatum. A confirmation of glutamate as the specific cortico-striatal transmitter. J Neurochem. 1982 Apr;38(4):1087–1098. doi: 10.1111/j.1471-4159.1982.tb05352.x. [DOI] [PubMed] [Google Scholar]
- Kemp J. M., Powell T. P. The termination of fibres from the cerebral cortex and thalamus upon dendritic spines in the caudate nucleus: a study with the Golgi method. Philos Trans R Soc Lond B Biol Sci. 1971 Sep 30;262(845):429–439. doi: 10.1098/rstb.1971.0105. [DOI] [PubMed] [Google Scholar]
- McGeorge A. J., Faull R. L. The organization and collateralization of corticostriate neurones in the motor and sensory cortex of the rat brain. Brain Res. 1987 Oct 13;423(1-2):318–324. doi: 10.1016/0006-8993(87)90855-9. [DOI] [PubMed] [Google Scholar]
- McGeorge A. J., Faull R. L. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29(3):503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- McLean S., Skirboll L. R., Pert C. B. Comparison of substance P and enkephalin distribution in rat brain: an overview using radioimmunocytochemistry. Neuroscience. 1985 Mar;14(3):837–852. doi: 10.1016/0306-4522(85)90147-2. [DOI] [PubMed] [Google Scholar]
- Reiner A., Anderson K. D. The patterns of neurotransmitter and neuropeptide co-occurrence among striatal projection neurons: conclusions based on recent findings. Brain Res Brain Res Rev. 1990 Sep-Dec;15(3):251–265. doi: 10.1016/0165-0173(90)90003-7. [DOI] [PubMed] [Google Scholar]
- Ruzicka B. B., Jhamandas K. Elevation of Met-enkephalin-like immunoreactivity in the rat striatum and globus pallidus following the focal injection of excitotoxins. Brain Res. 1990 Dec 17;536(1-2):227–239. doi: 10.1016/0006-8993(90)90029-b. [DOI] [PubMed] [Google Scholar]
- Salin P., Kerkerian-Le Goff L., Heidet V., Epelbaum J., Nieoullon A. Somatostatin-immunoreactive neurons in the rat striatum: effects of corticostriatal and nigrostriatal dopaminergic lesions. Brain Res. 1990 Jun 25;521(1-2):23–32. doi: 10.1016/0006-8993(90)91520-q. [DOI] [PubMed] [Google Scholar]
- Salin P., Mercugliano M., Chesselet M. F. Differential effects of chronic treatment with haloperidol and clozapine on the level of preprosomatostatin mRNA in the striatum, nucleus accumbens, and frontal cortex of the rat. Cell Mol Neurobiol. 1990 Mar;10(1):127–144. doi: 10.1007/BF00733640. [DOI] [PubMed] [Google Scholar]
- Samuel D., Errami M., Nieoullon A. Localization of N-methyl-D-aspartate receptors in the rat striatum: effects of specific lesions on the [3H]3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid binding. J Neurochem. 1990 Jun;54(6):1926–1933. doi: 10.1111/j.1471-4159.1990.tb04893.x. [DOI] [PubMed] [Google Scholar]
- Selemon L. D., Goldman-Rakic P. S. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci. 1985 Mar;5(3):776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman T. G., McKelvy J. F., Watson S. J. Vasopressin mRNA regulation in individual hypothalamic nuclei: a northern and in situ hybridization analysis. J Neurosci. 1986 Jun;6(6):1685–1694. doi: 10.1523/JNEUROSCI.06-06-01685.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers D. L., Beckstead R. M. Striatal preprotachykinin and preproenkephalin mRNA levels and the levels of nigral substance P and pallidal Met5-enkephalin depend on corticostriatal axons that use the excitatory amino acid neurotransmitters aspartate and glutamate: quantitative radioimmunocytochemical and in situ hybridization evidence. Brain Res Mol Brain Res. 1990 Jul;8(2):143–158. doi: 10.1016/0169-328x(90)90059-m. [DOI] [PubMed] [Google Scholar]
- Somogyi P., Bolam J. P., Smith A. D. Monosynaptic cortical input and local axon collaterals of identified striatonigral neurons. A light and electron microscopic study using the Golgi-peroxidase transport-degeneration procedure. J Comp Neurol. 1981 Feb 1;195(4):567–584. doi: 10.1002/cne.901950403. [DOI] [PubMed] [Google Scholar]
- Uhl G. R., Navia B., Douglas J. Differential expression of preproenkephalin and preprodynorphin mRNAs in striatal neurons: high levels of preproenkephalin expression depend on cerebral cortical afferents. J Neurosci. 1988 Dec;8(12):4755–4764. doi: 10.1523/JNEUROSCI.08-12-04755.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. J., Phelan K. D. Dual topographic representation of neostriatum in the globus pallidus of rats. Brain Res. 1982 Jul 15;243(2):354–359. doi: 10.1016/0006-8993(82)90260-8. [DOI] [PubMed] [Google Scholar]