Abstract
Gastric diseases cause considerable worldwide burden. However, the stomach is still poorly understood in terms of the molecular–cellular processes that govern its development and homeostasis. In particular, the complex relationship between the differentiated cell types located within the stomach and the stem and progenitor cells that give rise to them is significantly understudied relative to other organs. In this review, we highlight the current state of the literature relating to specification of gastric cell lineages from embryogenesis to adulthood. Special emphasis is placed on substantial gaps in knowledge about stomach specification that we think should be tackled to advance the field. For example, it has long been assumed that adult gastric units have a granule-free stem cell that gives rise to all differentiated lineages. Here, we point out that there are also other models that fit all extant data, such as long-lived, lineage-committed progenitors that might serve as a source of new cells during homeostasis.
Keywords: Granule-Free, Lineage Tracing, Metaplasia
Abbreviations used in this paper: BMP, bone morphogenetic protein; ECL, enterochromaffin-like; FGF, fibroblast growth factor; RA, retinoic acid; Shh, sonic hedgehog
Summary.
This review details the current understanding of gastric specification during development and adult homeostasis.
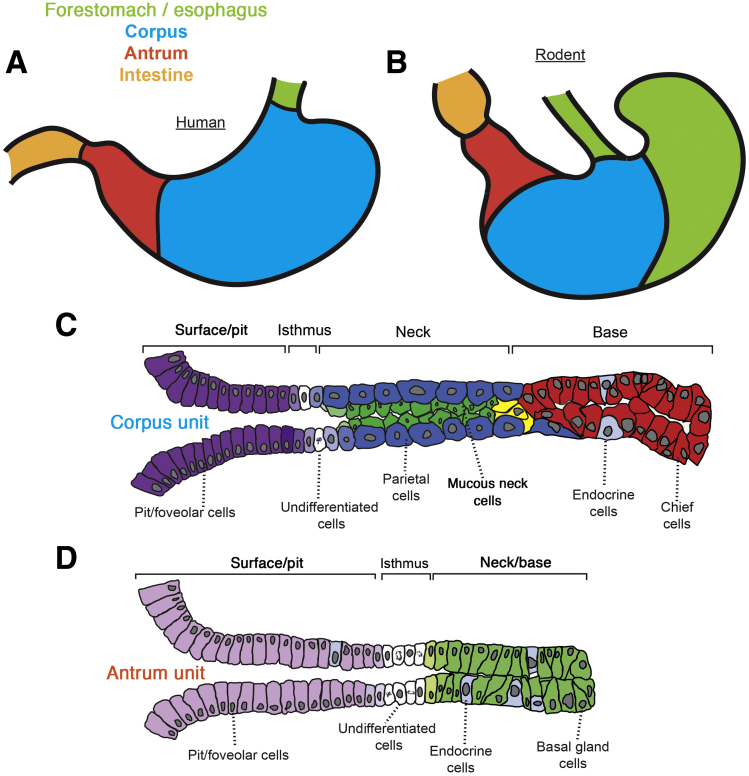
The adult stomach produces acid and enzymes that aid in food digestion and kill microbes, and it regulates delivery of food to the small intestine. The stomach also works remotely via its endocrine cells, which send distal signals to help coordinate hunger/satiety and Ca++ homeostasis.1 The stomach comprises tissues originating from all 3 embryonic germ layers including the ectodermally derived enteric nerves, mesodermally derived smooth muscle and mesenchymal cells, and the endodermally derived epithelium lining the lumen of the stomach. In this review, we largely focus on the processes governing epithelial development and homeostasis. The glandular epithelium in most mammals is arranged into 2 principal compartments: corpus and antrum (Figure 1). Both compartments are composed of a single layer of epithelial cells arranged into invaginated units. The principal cellular constituents of corpus units include the surface mucous (pit/foveolar) cells, acid-secreting parietal cells, mucous neck cells, digestive-enzyme secreting (zymogenic) chief cells, endocrine cells, and isthmal cells with undifferentiated features that likely serve as multipotent stem cells. The antral units can contain some chief and parietal cells depending on the species, but primarily are composed of pit/foveolar cells on the surface and deep glandular cells that express markers of both mucous neck cells and chief cells (Figure 1). Scattered throughout the corpus and antrum are the rarer endocrine cells, each type named for the predominant hormone they secrete (eg, gastrin-secreting G cells of the antrum).
Figure 1.
Architecture of the adult stomach and the organization of corpus and antral units. (A) The adult human stomach is composed entirely of glandular epithelium (blue, red), whereas (B) the adult rodent stomach contains a squamous-epithelium–lined forestomach (green), in addition to a glandular stomach. (C) Adult corpus units contain pit/foveolar cells (purple), isthmal stem cells (white), parietal cells (blue), mucous neck cells (green), endocrine cells (light blue), and chief cells (red). Cells transitioning from neck to chief cells are indicated in yellow. (D) Antral units primarily contain pit/fovelar cells (light purple), proliferative isthmal stem cells (white), basal gland cells (light green) similar to mucous neck cells with a hint of chief cell differentiation, and endocrine cells (grey). Note that up to half of human antral gastric units also contain parietal cells (not shown).
Understanding cellular development in the normal stomach should help us better understand the origins of gastric cancer, one of the most common causes of cancer death worldwide.2 Most gastric cancer is initiated in the setting of chronic infection with the bacterium Helicobacter pylori, which is estimated to infect more than half the world’s population.3 In addition to increasing the risk for gastric cancer, it is also the cause of most ulcers of the stomach and duodenum. Those patients at risk for gastric cancer show a response to infection with H pylori characterized by an overall loss of specific differentiated cell lineages, a condition known pathologically as chronic atrophic gastritis. Molecular and cellular mechanistic studies have shown that chronic atrophic gastritis is not characterized simply by a chronic inflammatory infiltrate (gastritis) and the loss of acid-secreting parietal cells (oxyntic atrophy), but also by changes in differentiation of the chief cells (metaplasia).4, 5, 6 A thorough understanding of the processes that control the specification of cells within the gastric epithelium during development and adult homeostasis could be crucial to deciphering the disease etiology, particularly the metaplastic changes that arise after H pylori infection. However, currently in the stomach, in both the adult and embryonic state, there is a rudimentary understanding of the cell lineage relationships. Furthermore, there is also a marked lack of lineage-specific markers and genetic tools for studying development and differentiation. In this review, we highlight the relatively limited information we have about stomach specification, starting with the embryo and continuing through adulthood.
One caveat is that most of the work on mammalian gastric development has been in rodents. Much work also has been performed in nonmammalian model organisms such as in chicks. The degree to which human gastric development follows the same rules as rodents—let alone nonmammalian vertebrates—is not known in most cases. Because of our relatively close ancestry, it is likely that most developmental patterns will be similar between human beings and these model organisms. However, there are some known differences. For example, the human stomach is lined entirely by glandular units while the rodent stomach contains an additional anatomic compartment known as the forestomach, which is not glandular at all, but rather is lined with squamous epithelium (Figure 1). In the human stomach, up to half of antral units harbor parietal cells, whereas they are absent from antral units in the rodent.7 In addition, chief cells in the rodent express gastric intrinsic factor, whereas intrinsic factor is expressed by parietal cells in human beings.8
Early Specification
Gastric specification in the mouse begins during gastrulation with derivation of the endodermal germ layer that eventually will seed the epithelial lining of the digestive, respiratory, and urogenital systems. The endoderm germ layer is formed by the ingression of epiblast cells through the primitive streak. As the cells exit the primitive streak, they arrange into a single-layered epithelial sheet on the outside of the embryo (embryonic day [E]6–E7.5). This sheet forms pockets at the anterior (future foregut) and posterior (future hindgut) end of the embryo and progressively zippers into a complete gut tube. Zippering of the gut tube, mesodermal growth, and embryonic turning transform the endodermal sheet on the outside of the embryo into an internal tube consisting of 3 major regions: foregut, midgut, and hindgut (E7.5–E9).9 Regional and subsequent organ identity is assembled within the naive, as yet unspecified, gut tube through the integration of signaling inputs from mesodermal tissues located apposed to the endoderm and the endodermal progenitors themselves.10 One recognizable output of the stage when regional identity is acquired is a pattern of expression of overlapping transcription factor domains that facilitate subsequent organ-specific differentiation programs.
Stomach epithelial progenitors derive from the foregut region of the endoderm, which also gives rise to liver, pancreas, lungs, and the luminal gastrointestinal organs from the pharynx to the anterior duodenum. Signaling pathways and transcription factors that drive specification of pregastric endodermal progenitors from other emerging organs within the foregut have not been well characterized.11 However, a number of signaling pathways that promote or restrict foregut identity by patterning the anterior/posterior axis of the endoderm are known. Retinoic acid (RA), for example, has a complex spatiotemporal role patterning the anterior–posterior axis of the endoderm. During late gastrulation, RA signaling promotes the specification of posterior endodermal fates over anterior endodermal fates, particularly at the foregut–midgut boundary.12, 13 Subsequently, RA signaling is required to promote the development of a number of foregut tissues. Animals with defective RA signaling have abnormal stomach development, but a specific consequence to gastric specification is unclear.14 WNT and fibroblast growth factor (FGF) signals produced by the mesoderm promote expression of posterior endodermal markers such as Cdx2 over anterior endodermal markers.15, 16, 17 Studies in zebrafish also have shown that bone morphogenetic protein (BMP) signaling drives posterior over anterior endodermal fates.18
Through the study of other endodermal organs, a number of tissues have been shown to produce important signaling molecules to promote foregut organ specification. For example, the dorsal aorta and notochord produce several key signaling molecules involved in dorsal pancreatic specification.19, 20 These same tissues also could impact pregastric gene expression given the proximity of gastric and dorsal pancreatic progenitors. Ventral tissues, including cardiac mesoderm, also could impact gastric specification from other ventral organs such as the liver and lung.21 Other signaling pathways such as sonic hedgehog (Shh) have been implicated in gastric growth through epithelial to mesenchyme signaling, although Shh does not appear to be involved in gastric specification.22
During endodermal specification, a highly conserved core transcription network (including FoxA, Gata, Sox17, and Mixl1 transcription factors) is activated and guides the growth and survival of endodermal cells before regionalization.23 Expression of these transcription factors in early endoderm is necessary to generate foregut progenitors that give rise to the stomach. As the endoderm regionalizes, a number of transcription factors are expressed either throughout the foregut endoderm or regionally in the pregastric domain. Broadly expressed transcription factors such as Foxa1/2/3, Gata4/6, Hnf1β, and Sox2 all could play an important role in gastric specification (Figure 2). For example, the FoxA family is expressed throughout the early endoderm and is important in the development of a number of organs including the liver, pancreas, and intestine.24, 25, 26 The specific role of this family in the stomach has yet to be determined, however, FoxAs are known to be involved in promoting Pdx1 expression in the foregut (Figure 2). Because Pdx1 is expressed only in the gastric antrum and not the more proximal corpus,27 FoxA factors thus could be involved in regionalizing the stomach.25
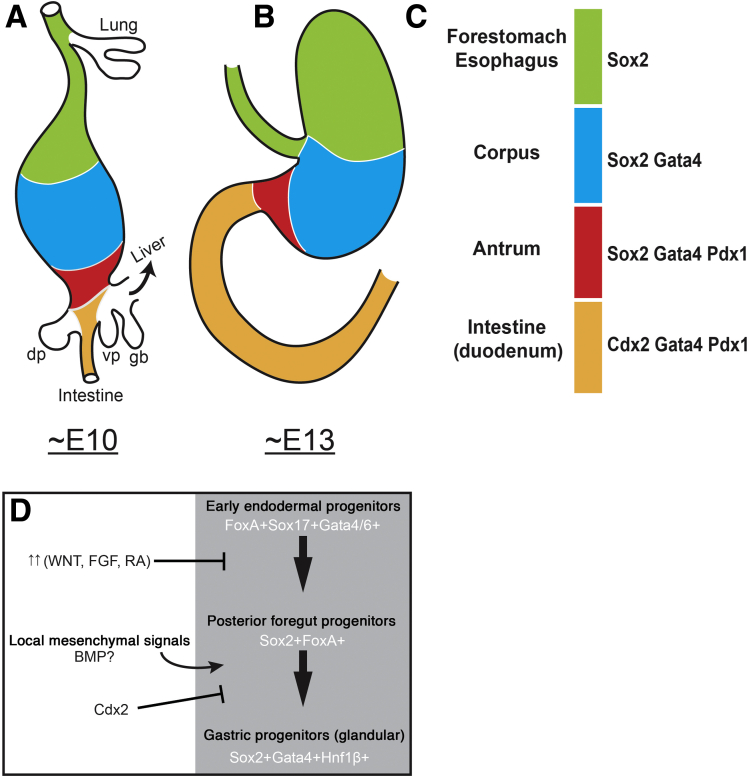
Figure 2.
Transcription factor domains in the development of the gastric region. Representation of the mouse developing posterior foregut at (A) approximately E10 and at (B) approximately E13. (A and B) Color codes correspond to specific transcription factor signatures in panel C. The future forestomach and esophagus (green) expresses Sox2, but not other glandular markers such as Gata4 and Pdx1. The future corpus (blue) expresses Sox2 and Gata4, but not the more posterior regional markers such as Pdx1. The future antrum (red) expresses Sox2, Gata4, and Pdx1, but not the intestinal marker Cdx2. The future anterior small intestine expresses Cdx2, Gata4, and Pdx1, but not the anterior endodermal marker Sox2. The anterior boundary of Gata4 (blue/green border) is expressed in the glandular stomach but not the forestomach (green). (D) Speculative model of glandular stomach specification during development. Based on developmental studies, early foregut progenitors express the important transcription factors of the FoxA family, Sox17, and Gata4/6. Around this time, an appropriate balance of WNT, FGF, and RA signaling is needed to specify the region of the gut that gives rise to gastric progenitors. These pathways actively posteriorize the endoderm—too little or too much signaling could drive the endoderm to a more anterior or posterior fate, respectively. Future gastric progenitors need to acquire Sox2 expression and not the intestine determinant Cdx2, which is expressed in more posterior endoderm. Once organ budding begins, local mesenchymal signals are crucial to enforce glandular identity and repress adjacent nonglandular stomach organ fates such as the esophagus/forestomach and intestine. Potentially, these signals act through driving expression of potential gastric specification transcription factors such as Gata4 and Hnf1β.
Gata4 and Gata6 are involved in the specification of the extraembryonic endoderm28, 29, 30 and are expressed throughout the early definitive endoderm. During endodermal regionalization, both genes are expressed in the foregut. The expression domain of Gata4 is particularly interesting because its anterior boundary resides at the future forestomach/glandular stomach boundary. Potentially, Gata4 may have an important role in specifying the glandular stomach or specifying the forestomach vs the glandular stomach (Figure 2). Consistent with the idea that Gata4 is important for glandular stomach specification, Gata4 null cells do not appear to be able to adopt gastric identity in chimeric embryos when they are competing with wild-type cells.31
Sox2 is expressed broadly throughout the foregut from the most anterior pharyngeal endoderm to the future boundary of gastric antrum and duodenum. Studies wherein expression of Sox2 is reduced in developing endoderm have shown that it helps govern the development of a number of foregut organs including the stomach, esophagus, trachea, and lung.32, 33 Such experiments involved hypomorphic animals, so it will be interesting to know what the effects of complete loss of SOX2 from early endoderm might be. Perhaps SOX2 has an even more critical role in anterior foregut and stomach specification than currently thought. The border between Sox2 and Cdx2 expression during development (Figure 2) resides at the prospective gastrointestinal junction and suggests that Sox2 could define this boundary. Misexpressing Sox2 in Cdx2-positive progenitors in the developing intestine increases expression of gastric-specific differentiation markers.34 Interestingly, loss of Cdx2 during early development causes a dramatic transformation of the prospective intestine into Sox2-expressing esophageal-like progenitors and not gastric progenitors,35 indicating that SOX2 is not a simple progastric, anti-intestine transcription factor. Indeed, SOX2 levels are high in both adult esophagus and adult stomach.36
Pdx1 is expressed regionally within the posterior foregut in the areas that give rise to the posterior stomach (antrum/pylorus), anterior duodenum, dorsal and ventral pancreatic buds, and proximal extrahepatic biliary system.27, 37 Pdx1 expression can be used during development to distinguish antral gastric progenitors (SOX2+GATA4+PDX1+) from corpus progenitors (SOX2+GATA4+PDX1-). Loss of Pdx1 causes aberrant antral stomach progenitors including pyloric defects27 and loss of gastrin-producing endocrine cells.38 Hnf1β is expressed in the early endoderm and implicated in stomach specification. Definitive endoderm-specific knockout of Hnf1β alters gene expression within caudal stomach progenitors, including causing loss of Pdx1 and Indian hedgehog (Ihh).39 The impact on gastric specification in these knockouts remains unclear, but recent in vitro studies intriguingly have implicated Hnf1β in promoting antral stomach specification in organoid culture.40
To date, no specific gene has been shown to have expression restricted only to early gastric progenitors; thus, it remains difficult to examine directly how the stomach is specified from other organs, the way, for example, Cdx1 and Cdx2 have been studied in intestinal specification. Instead, investigators rely on more broadly expressed genes (ie, expressed concomitantly in other organs besides the stomach) such as Sox2, Gata4, and Pdx1 to identify the factors defining the prospective gastric regions. Further identification of transcripts that may have more restricted or specific expression to gastric progenitors (particularly to the glandular stomach) during early development could lead to the generation of new genetic tools to explore and characterize gastric specification or even to perform stomach-specific epithelial cell gene deletion because intestinal epithelial-specific deletion can be driven by Villin-Cre. However, there could be marked improvement in our understanding of stomach specification simply by manipulating gene expression in early endoderm with tools that already exist. For example, signaling pathways and transcription factors suspected of being involved in gastric development could be deleted via crosses to well-characterized mouse pedigrees that express Foxa3-, Sox17-, or Shh- Cre.41, 42, 43
Summing up all that currently is known and can be inferred from published studies, we have proposed one possible signaling and epistasis model for specification of glandular stomach (Figure 2).
Mesoderm
Regionalization throughout the luminal gastrointestinal tract depends in large part on epithelial–mesenchymal cross-talk, and the stomach does not seem to be an exception. For example, foundational experiments in chicks have shown that placing proventricular (stomach region in chicks similar to the mammalian glandular stomach) mesenchyme with gizzard (anterior chicken stomach) or esophageal endoderm induces proventricular gene expression and causes gland development in these normally nonglandular tissues.44, 45 Similarly, gizzard or esophageal mesenchyme can suppress proventricular gene expression and gland development in proventricular endoderm and promote squamous fates.46 Interestingly, proventricular mesenchyme could not induce proventricular gene expression in intestinal endoderm47; hence, overlying mesoderm can instruct endoderm identity but only within restricted regions. BMP factors have been implicated in promoting proventricular identity.48
Although BMP signaling, principally deriving from the mesenchyme, influences gastric epithelial development, Hedgehog signaling derived from the epithelium influences the mesenchyme. For example, in addition to their early role in foregut growth, Hedgehog (Shh/Ihh) signals are produced by the gastric endoderm to support mesenchymal growth and differentiation, a pattern that is maintained in the adult.49, 50 Another example of a factor that originates from the mesenchyme and regulates the epithelium is FGF10, likely via the FGF receptor 2B.51 FGF10 promotes epithelial proliferation and gland development.51, 52 Although it may not be required for adult homeostasis, it has been shown to inhibit parietal and chief cell differentiation in favor of the mucous neck cell type.53
In addition to the themes of epithelial Hedgehog and mesenchymal BMP signaling that occur throughout the gastrointestinal tract, there have been some descriptions of signals more specific to gastric development vs other regions. For example, Barx1 is a transcription factor that is restricted to the prospective esophageal and gastric mesoderm. Barx1 null mice have significantly altered stomach morphology with disrupted patterning of the stomach. The stomach–intestinal boundary is disturbed such that ectopic CDX2+ intestinal epithelial cells can be found in the posterior stomach.54, 55 In addition to the disrupted interorgan patterning, the division of intrastomach domains is altered. For example, H+/K+–adenosine triphosphatase–expressing cells are seen intermingled with PDX1+ cells, which in mice are normally exclusive to the corpus and antrum, respectively. Bapx1 (Nkx3-2), Nkx2-5, Gata3, Six2, Nr2f2 (Chicken Ovalbumine Upstream Promoter-Transcription Factor II), and Sox9 are other known transcription factors expressed in the posterior stomach mesenchyme and involved in specifying the pylorus.56, 57, 58 In the absence of those transcription factors, there is aberrant neuromuscular regulation of the pyloric sphincter, which in human beings can manifest as the relatively common condition known as pyloric stenosis.58, 59
Cell Lineage Specification
Between the stage of endodermal specification and the stage of specific cell lineage commitment in the stomach, the gastric epithelium remains a simple epithelium with no obvious differentiation. At approximately E14.5–E16.5, markers representative of cell types such as endocrine, parietal, and chief cells begin to be expressed, and small glands begin to invaginate into the mesenchyme from the simple epithelium lining the lumen.52 Between E16.5 and 2 weeks of postnatal development, most of the major cell types arise within the stomach, and the glandular stomach mostly becomes organized into its adult form. However, the murine stomach does not reach adult organization with full chief cell and endocrine cell lineage specification until 6–8 weeks postnatally.60 For most cell lineages in the stomach we have a poor understanding of pathways and factors involved in their specification and the progenitors from which they directly derive. For example, and this is truly remarkable when contrasted to the state of our understanding in the intestines, there is no specific factor that is known to be necessary or sufficient for specification of chief, parietal, pit, mucous neck, or isthmal cells. The markers used in gastric biology represent the terminal differentiation of those cells (eg, Atp4b, Tff1, Pgc, and Gif). The lack of this basic specification knowledge greatly hinders deciphering the molecular mechanisms underlying how gastric disease causes the loss or increase of any particular cell lineage. The developmental sequence between gastric epithelial progenitors in an adult gastric unit and the differentiated progeny that arise continuously throughout life also is unknown. It is entirely possible that all the mature cell types are specified from a single multipotent progenitor that persists throughout life,61 or, in turn, there might be numerous long-lived lineage-restricted progenitors62, 63 (Figures 3 and 4, and see detailed discussion later).
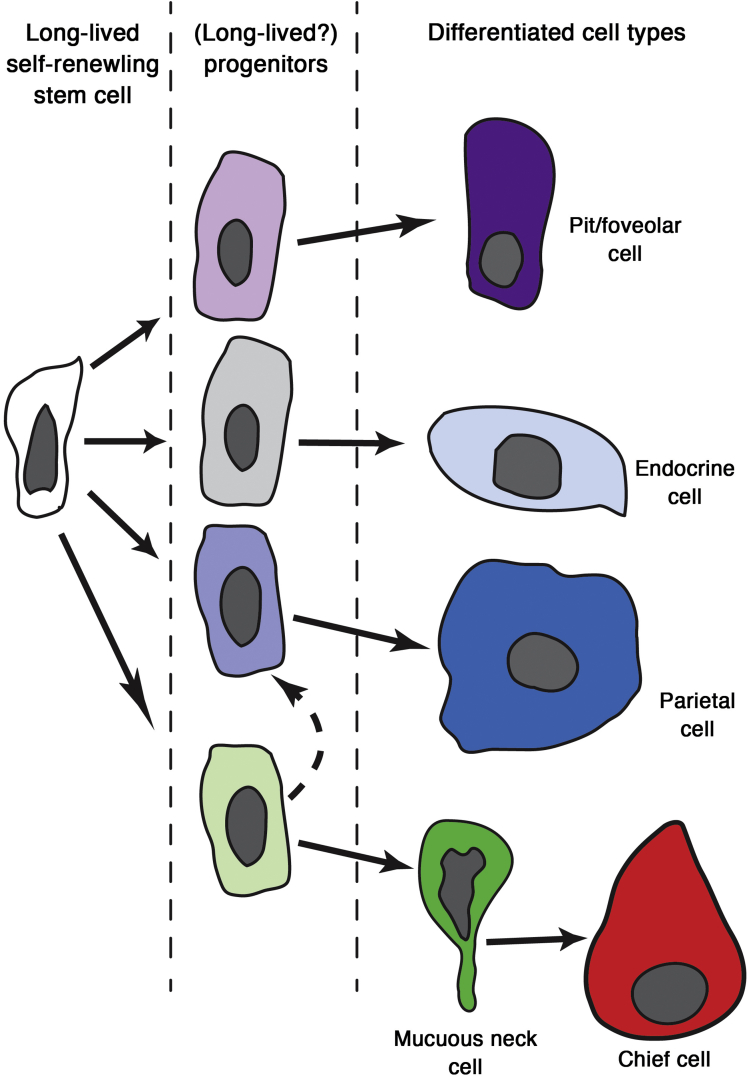
Figure 3.
Putative lineage tree of the adult corpus stem cell. Based on the labeling and ultrastructural studies of Karam and Leblond, the isthmus contains a granule-free stem cell that enters the cell cycle to give rise to progenitors that migrate up and down the corpus unit.61 Cells that migrate up the unit adopt a prepit phenotype (light purple) and eventually turn into mature pit cells (purple). Cells that migrate down the unit appear to adopt a preneck (light green), preparietal (light blue), or pre-endocrine/endocrine phenotype (grey). Neck cells (green) appear to undergo a further transition at the bottom of the unit and eventually become transitional cells with both neck and chief cell characteristics, and finally fully mature chief cells. It is clear that the granule-free cell is long-lived and self-renewing, but each of the progenitors committed to more specific lineage(s) also might be long-lived and self-renewing as well.
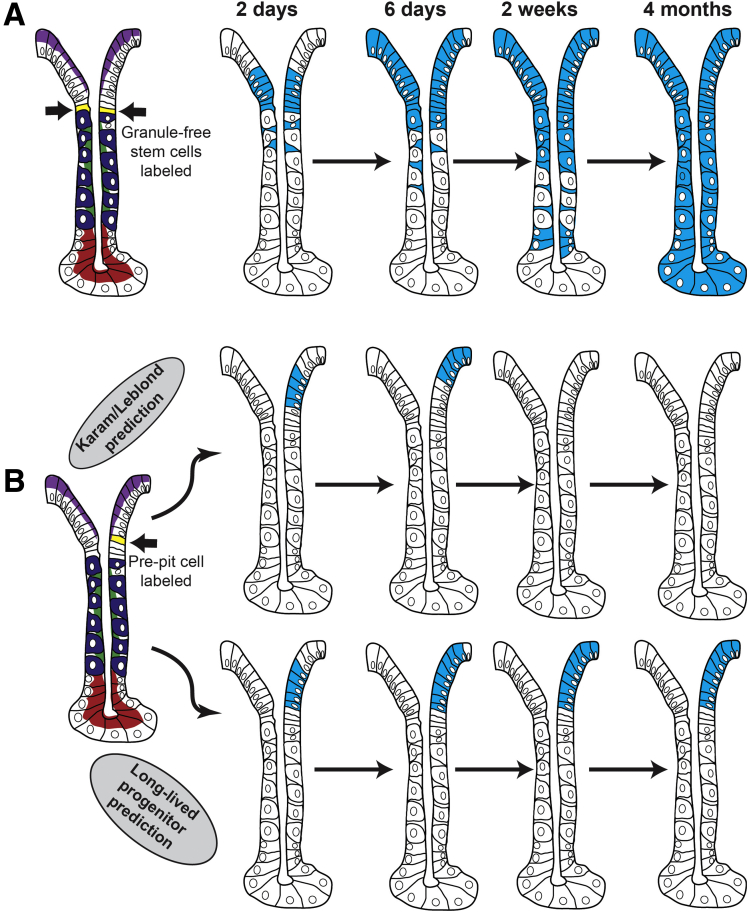
Figure 4.
Potential behavior of the adult stem cell and lineage-committed progenitors in the adult corpus. (A) If the prediction by Karam and Leblond61 that there is a single adult stem cell in the corpus holds true, then labeling that cell eventually will result in the long-term maintenance of label as well as labeling of all corpus cell types. (B) It remains possible that the corpus contains long-lived lineage–restricted progenitors as well. Such cells would have early characteristics of pit cells or neck cells, they would be self-renewing and long-lived but give rise only to differentiated pit or neck/chief cells, respectively. Labeled-nucleotide pulse-chase experiments performed by Karam and Leblond to understand how stem cells behave in the stomach would not be able to distinguish between the 2 possibilities (ie, a long-lived multipotent stem cell vs long-lived committed progenitors). Lineage tracing experiments with an appropriate promoter (eg, similar to Lgr5 in the intestine) should be able to distinguish how stem cell hierarchies are arranged. Examples of different lineage tracing patterns with hypothetical, appropriate promoters are shown. If a promoter that is pit-cell lineage-specific could be induced and traced, then the Karam and Leblond model (all cells rapidly arise from a long-lived, self-renewing, multipotent stem cell) would result in temporary labeling of the pit lineage with eventual loss of the label because the stem cell would not be labeled, and pit cell progenitors are not long-lived. However, if long-lived lineage-restricted progenitors exist (contrary to Karam and Leblond), then labeling the prepit cell will result in maintenance of the label throughout the pit cell lineage because the prepit cells will self-renew and not die, and they will continue to label all their progeny. Similar predictions would hold to other cell lineages in the corpus.
The only stomach lineages with known genetic determinants and known progenitor markers are endocrine cells, which are controlled by the master regulators Ascl164 and Ngn3.65, 66 Ngn3 marks endocrine progenitor cells but not mature forms. Ngn3 null embryos lack gastrin, somatostatin, and glucagon endocrine cell types, with largely reduced census of serotonin-positive cells, but enterochromaffin-like (ECL) and ghrelin populations still are present.65, 66 Ascl1 null embryos wholly lack gastrin, somatostatin, and glucagon-secreting endocrine cell types (the former 2 missing from their usual niches in the stomach), and gastric serotonin and ghrelin endocrine cells are decreased in number. Ascl1 null embryos die before ECL cells emerge developmentally64; however, it was noted that the vast majority of chromogranin A–positive cells (chromogranin A is a general marker of endocrine cells) are missing in Ascl1 null embryos, and ECL cells represent the majority of chromogranin-positive cells in the corpus. Thus, if Ascl1 is required for all chromogranin A–positive cells to emerge, a conditional deletion in the adult also might show that ECL cells are Ascl1-dependent, although this only can be speculated with current data.
Taken together, the data show that Ascl1 and Ngn3 are each required to specify gastrin, somatostatin, and glucagon-positive endocrine cells. The eventual emergence of ECL cells may be dependent on Ascl1 but not Ngn3. Serotonin-positive endocrine cells in the antrum also largely are lost in Ascl1 and Ngn3 mutants. A recent study showed that serotonin-positive cells in the corpus are bone marrow–derived, mucosal-associated mast cells and not descendants from endodermal progenitors, an Ngn3 lineage, or epithelial cells at all.67 If corpus serotonin-positive cells are not derived from the endoderm, those cells likely account for the presence of nonantral serotonin-positive cells in Ascl1 mutants mentioned earlier. It is unclear how specific mature endocrine cell types arise from endocrine-committed progenitors during embryogenesis and adult homeostasis; however, endocrine specification in the pancreas and intestine may serve as an illustrative model.68, 69 Endocrine-committed progenitors derived from Ascl1- or Ngn3-positive populations differentiate into individual endocrine cell types depending on the specific downstream transcription program that is enacted. There is indication that similar programs exist in the stomach because mice null for various transcription factors have defects in specific endocrine lineages. For example, Pdx1, Nkx6.3, Pax4/6, and Arx all have been implicated in controlling differentiation of mature endocrine cell types in the stomach.38, 70, 71, 72
Although it is mostly not clear what controls the specification of nonendocrine cell lineages within the stomach, some factors have been implicated in maturation of those cell types. The transcription factor Spdef has been shown to be crucial for antral deep mucous cell maturation.73 Foxq1 is necessary for the expression of Muc5ac in pit cells (MUC5AC is the key mucin protein secreted by these cells).74 Xbp1 and Mist1 (Bhlha15) are important for the ultrastructural maturation of chief cells.75, 76 Specifically, they coordinate the architectural changes necessary for these cells to become regulated secretory factories. In their absence, chief cells fail to generate a dense rough endoplasmic reticulum network and do not make large zymogen-containing vesicles. Mucous neck cells, the progenitors for chief cells, emerge in rodents around the time of weaning in a process that depends in part on transforming growth factor α and the epidermal growth factor receptor,77, 78 although whether these play a role in maturation or specification is not known.
Adult Homeostasis
Stem Cells
The isthmus of the corpus epithelium contains a continuously proliferating cell population that lacks any differentiated nuclear and cytoplasmic features (eg, secretory granules or specialized organelles). Nucleotide analog labeling studies (eg, 3H-thymidine or bromodeoxyuridine) show that labeled nucleotides are incorporated most frequently in isthmal cells with those morphologically immature characteristics, and this cell lineage has been termed the granule-free stem cell.61 Pulse-chase experiments with such analogs show that the labeled nucleotides spread bidirectionally from the isthmus. Karam and Leblond hypothesized that the granule-free stem cell directly gave rise to progenitors that were immature versions of each of the mature corpus lineages.61 Some of the earliest cells to incorporate label were cells characterized by ultrastructural features of immature pit cells (eg, scant but distinctive pit cell mucous granules79). Label spread more slowly in the other direction (ie, toward the base and away from the gastric lumen). The cells that showed early incorporation of label in that direction commonly have early/immature mucous neck cell features.80 At 1–2 weeks after injection of labeled nucleotides, label appears in the prezymogenic chief cells at the top of the base, those with features characteristic of both mucous neck cells and zymogenic cells.76, 80 It appears eventually, also in parietal cells and endocrine cells, first in the isthmus area.81, 82 In pulse-chase experiments wherein the nucleotides are given only once—as opposed to continuously—label typically is not retained for longer than a few days in either the immature (presumptive progenitor) cells or in the granule-free cells. Rather, label is retained long-term only in mature parietal, chief, and endocrine cells. The simplest interpretation of these observations is that the undifferentiated, granule-free isthmal cell is a constitutively active multipotent stem cell that can give rise to and replenish all the mature cell lineages (Figure 3). However, this has not been proven formally.
Other than such studies, wherein lineage relationships are inferred from morphology and labeled nucleotide migration patterns, there is little else known about transitions from the stem cell to progenitors and lineage-committed cells in the corpus. The limited state of understanding in the gastric corpus is in marked contrast to that in the small intestine, where numerous markers of crypt-based cells with stem cell potential have been identified over the past 10 years.83, 84, 85, 86, 87 Strikingly, there is still neither a specific molecular marker nor a specific promoter whose expression is restricted to an undifferentiated isthmal cell in the corpus that has yet been identified.
Several studies using chimeric mice and mosaic silencing of an x-linked transgene in female mice suggest that stomach glands start off polyclonal but become monoclonal over time.62, 88, 89, 90 These results thus support the single gastric unit stem cell hypothesis. However, Bjerknes and Cheng62 found patterns of mutant clones that showed that, as mice age, there might be other progenitor-progeny relationships outside the dogma of the single, long-lived, multipotent stem cell proposed by Karam and Leblond.61 In adult mice, Bjerknes and Cheng62 saw units that seemingly had stable labeling restricted to specific single lineages, suggesting that there also might be long-lived, lineage-committed progenitor cells rather than the transient ones hypothesized by Karam and Leblond.61 We have provided a cartoon to distinguish the 2 models (ie, a multipotent, undifferentiated stem cell giving rise to all lineages vs multiple long-lived, lineage-committed progenitors, each fueling only their specific lineage) (Figure 4).
Genetic lineage tracing experiments that have been attempted in the stomach suffer from the caveat that the promoters used to drive lineage tracing also are expressed (usually much more strongly) in differentiated cells. For example, long-term lineage tracing in adult animals using the Sox2 promoter suggested that some Sox2-promoter-expressing cells have stem cell function with the capacity for self-renewal and differentiation into all lineages in the corpus. Rare, highly Sox2+ cells were suggested to be the most stem-like. Interestingly, those cells localized to the base of corpus units, not the isthmus.91 SOX2 protein can be found in many cell lineages throughout the corpus at mid-to-low levels and even can be used as a marker in human beings of gastric differentiation relative to intestinal.92, 93 Other lineage tracing studies have focused on marking mature chief cells using the Tumor necrosis factor receptor superfamily member 19 (Tnfrsf19 or Troy) or Muscle, intestine and stomach expression 1 (Mist1) promoters.94 Long-term lineage tracing using those promoters suggested that chief cells also can act as stem cells and give rise to all the cell lineages within the corpus. MIST1 protein and RNA are almost exclusive to mature chief cells, and TROY is restricted to a handful of chief and parietal cells. These results indicate that differentiated chief cells have the potential to serve as stem cells in some situations, albeit such functional stem cell activity in chief cells seems relatively rare, at least during homeostasis.95 Recent studies have indicated that rare cells labeled with a Mist1CreER knock-in allele also can be found in the isthmus of the corpus.96 These occasional, isthmus-localized cells that express the Mist1 promoter could be another source of stemness in the corpus. However, the molecular/cellular identity of those cells is defined only by this spurious Mist1CreER expression, given that neither the endogenous Mist1 transcript nor the MIST1 protein has been shown to be expressed outside of chief cells in wild-type mice.
Definitive lineage tracing studies in the stomach also have been hampered by a technical problem that does not affect other gastrointestinal organs to the same degree, such as small intestine and pancreas, where lineage tracing has been used to great effect. The vast majority of genetic lineage tracing tools use a modified Cre recombinase that requires binding tamoxifen to be transported to the nucleus where it can activate reporter genes or other genetic tools (CreER). Unfortunately, for gastric researchers, tamoxifen induces parietal cell death and chief cell metaplasia when delivered above a threshold dose.97, 98, 99 Thus, inducible lineage tracing using CreER with tamoxifen can be confounding in the stomach because it may induce nonhomeostatic patterns of differentiation with increased cellular plasticity.95 The stomach is also particularly sensitive to high doses of Cre itself.100 It would be ideal to develop more stomach-lineage–specific promoters and induce lineage tracing with methods such as tetracycline-inducible systems or estrogen receptor agonists that do not induce injury. Furthermore, any lineage tracing in the stomach should be performed with proper controls: mice homozygous for floxed alleles but lacking Cre recombinase expression and mice with Cre recombinase but with a nonfloxed allele of the gene of interest.
To highlight how our understanding of stem cell dynamics in the intestine is more advanced than in the corpus, we point out how CreER driven by the Lgr5 promoter is an efficient marker of functional stem cell activity in the intestine. Lgr5CreER is expressed at higher levels in the presumptive stem cells in the small intestine than in differentiated progeny. Importantly, both the endogenous Lgr5 transcript and LGR5 protein also are expressed preferentially in the presumptive stem cell.83 Lgr5CreER can be used during homeostasis to trace labeled cells, and all cell lineages can be seen eventually to derive from Lgr5-promoter–expressing, crypt-resident (presumptive stem) cells with undifferentiated features. Lgr5 promoter-based studies corroborate other studies that, together, make it seem incontrovertible that there is a population of constitutively active, long-lived, multipotent stem cells in the small intestine.
Cellular Differentiation and Maturation in the Corpus Epithelium
Although the gastric epithelial stem cell in the adult corpus remains unidentified, there has been some beginning characterization of the patterns of molecular and cellular differentiation of the various mature cell lineages deriving from that stem cell. One strong line of evidence supports an interesting differentiation pattern wherein mucous neck cells give rise to chief cells, a differentiation step that has been termed a transdifferentiation to chief cells.76, 78, 101 Evidence supporting the lineage relationship between neck and chief cells is circumstantial, but varied and relatively abundant. For one, there are situations in which neck and chief cell markers are co-expressed. During postnatal maturation, gastric units contain cells with characteristics of both neck and chief cells.60 After maturation, units in the corpus harbor similar transitional cells with characteristics of both cell populations by ultrastructural and gene expression analysis.76, 80, 101, 102 When the stomach is injured, metaplastic cells arising from the chief cell lineage express both neck and chief cell markers.6, 95, 101 Furthermore, lineage tracing studies using the Tff2 promoter have suggested that parietal cells and mucous neck/chief cells are derived from a common progenitor pool.103 Finally, slowing maturation of chief cells by deleting either Mist1 or Xbp1 leads to increased cells with neck-chief transitional characteristics.75, 76, 101
The surface mucous (pit/foveolar) cells clearly arise rapidly from a progenitor in the isthmus. The nature of the progenitor has not been established but must be either a committed pit-specific, long-lived progenitor or the canonical multipotent stem cell (or both). Interestingly, we have observed that decreased proliferation in the isthmal progenitor zone tends to have effects on pit cells more than the deeper glandular cells,104 indicating that much of the proliferation in the isthmus, at least under normal conditions, is directed toward surface mucous cell replenishment. As mentioned earlier, the transcription factor FOXQ1 is involved in pit cell differentiation because it is required for expression of the key component of the pit cell mucous granules (MUC5AC), although it is not required for specification of the lineage itself.74
A number of signaling pathways have been shown to be active during stomach homeostasis and affect cell behavior. Notch signaling is active in the isthmus of the corpus and promotes proliferation within this region.105 Ectopic Notch signaling driven by a parietal cell-lineage–specific promoter blocked differentiation and maintained progenitor characteristics in differentiating parietal cells.105 Inhibition of the BMP signaling pathway promotes increased cell proliferation in the adult stomach: glands contained fewer parietal cells and more transitional cells (cells with both neck and zymogenic chief cell characteristics – similar to the metaplastic or transitional cells mentioned earlier) at the base of the unit,106 indicating that BMP signals regulate progenitor proliferation and cell maturation in the corpus.
Gastrin is a hormone produced by G cells in the antrum. The primary physiological role of gastrin is to promote acid secretion by activating ECL and parietal cells. Absence of gastrin causes decreased cellular proliferation in the corpus and leads to generation of immature parietal and ECL cells,107, 108, 109, 110 whereas overexpression of gastrin causes increased proliferation of those cell populations.111 Parietal cell production of Shh is an important regulator of gastrin production. In the absence of this source of Shh, excess gastrin is produced by G cells, and pit cells in the corpus have increased proliferation.112
Antral Homeostasis
The antrum is considerably less complex then the corpus (in organization and number of cell types). The cell lineages also turn over faster. Continuously proliferating cells in the antrum are located at the isthmus of the unit. In the isthmus, Lee and Leblond113 identified the most actively proliferating cells as also being the least differentiated ultrastructurally (like the granule-free presumptive stem cell in the isthmus of the corpus). In the antrum, the isthmus is much nearer the base than in the corpus, in a pattern more resembling the large intestine. In pulse-chase experiments, labeled DNA spreads both upward to the lumen and further down into the base from this isthmus zone113 (Figure 1).
In contrast to the corpus, markers and gene promoters have been shown to efficiently label cells with multipotent progenitor capacity. For example, as in the small intestine, Lgr5 shows a pattern of homeostatic expression that is confined to a specific cell population that frequently and efficiently can be traced into progeny that include all the cell lineages in the antrum and cardia, but not the corpus.114 Similarly, Cck2r-based lineage tracing labeled as +4 (the designation of +4 is borrowed from the intestine, wherein cells traditionally have been numbered from the most basal cell upward to the lumen) antral cells that also has stem cell potential and was shown to give rise to Lgr5+ antral cells.115 In addition, Villin- and Sox2-promoter–based lineage tracing also label rare cells in antral glands that show functional stem cell characteristics.91, 116 It is not yet clear what the relationship among all of these cell populations with stem cell capacity is yet. The LGR5+ cells clearly are not the granule-free, isthmal antral cells113 because they commonly are located at the very base of the antral unit, not the isthmus, and they show ultrastructural features of differentiation.113, 114 The CCK2R and LGR5 populations seem to be overlapping, at least functionally, but are distinct from each other. Cells labeled by Villin are rare and activated only by inflammation.116 Sox2, on the other hand, is expressed in many cells, therefore it likely is not specific to a defined stem cell.91 It is possible that cells in the antrum are plastic, so that many cells can serve as stem cells even homeostatically. Antral glands also undergo relatively frequent fission events, in which one gland gives rise to another,62, 89, 117 so perhaps some of the markers label cells that are not constitutive stem cells but that drive budding off of new glands. In sum, Sox2, Lgr5, and Villin are not principally expressed in the zone where the least differentiated, most proliferative cells are. That is in contrast to the intestine where the Lgr5-expressing (crypt-base-columnar) cells are the most proliferative and the least ultrastructurally differentiated cells. Perhaps, thus, a marker of isthmal, antral stem cells that is equivalent to LGR5 in the intestine has yet to be identified in the stomach.
In the antrum, Notch signaling regulates the behavior of Lgr5+ antral stem cells. Inhibition of Notch signaling promotes mucous and endocrine cell differentiation, whereas activating the pathway stops differentiation.118 BMP signaling, through BMPR1A, regulates the proliferation and differentiation of mucous cells in the antrum.100, 119 In the absence of BMPR1A, antral mucous cells hyperproliferate and fail to express the mucin MUC5AC.119 Mutations in the BMP family are known to cause juvenile polyposis syndrome, which presents with polyps throughout the gastrointestinal tract, including the antrum.120 Finally, given that LGR5 is expressed and may regulate stem cell activity in the antrum, there may be a role for WNT signaling in regulating antral homeostasis because LGR5 is a co-receptor for canonical WNT signals.121
Conclusions
Many facets of stomach specification remain understudied or unexplored. Although it is clear that tissue interactions between the gastric endoderm and mesoderm are important for gastric development, there is still scant knowledge about how naive endodermal progenitors become specified to the gastric progenitor state. There is equally poor understanding about the factors that control the specification of gastric lineages during development and adult homeostasis, other than some initial inroads into outlining the origins of endocrine lineages. Many more studies are needed to determine which cell types have stem cell properties in the adult stomach (in particular the corpus), and their relative contribution during homeostasis and disease/injury conditions. Such studies will depend on the development of promoter-based tools that, similar to the intestine, are specific for stem cells and not expressed in differentiated cells. Preferably, such tools would not depend on the possibly confounding agent tamoxifen. Other new potential tools to help sort out gastric specification are being developed. Recent reports have described the derivation of mouse and human gastric organoids derived either from adult stomach94, 122, 123, 124, 125, 126 or via differentiation from induced pluripotent stem cells.40 Potentially through the manipulation of these cells, the field might have a new approach to better understand the pathways and factors controlling stemness and specification of gastric lineages. The generation of such new tools to study these processes is an important first step to exploring the mechanisms that control gastric specification.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Supported by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases grants DK094989, DK052574, and DK105129; Washington University Digestive Disease Research Core Center grant P30DK052574; the Washington University Alvin J. Siteman Cancer Center (supported by National Cancer Institute P30 CA91842); a pre-Program Project grant (J.C.M.); and National Cancer Institute CA009547 (S.G.W.).
References
- 1.Hunt R.H., Camilleri M., Crowe S.E. The stomach in health and disease. Gut. 2015;64:1650–1668. doi: 10.1136/gutjnl-2014-307595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stewart B.W., Wild C., International Agency for Research on Cancer . International Agency for Research on Cancer WHO Press; Lyon: 2014. World cancer report 2014. [Google Scholar]
- 3.Hatakeyama M. Helicobacter pylori CagA and gastric cancer: a paradigm for hit-and-run carcinogenesis. Cell Host Microbe. 2014;15:306–316. doi: 10.1016/j.chom.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Lennerz J.K., Kim S.H., Oates E.L. The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia, and carcinoma. Am J Pathol. 2010;177:1514–1533. doi: 10.2353/ajpath.2010.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt P.H., Lee J.R., Joshi V. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest. 1999;79:639–646. [PMC free article] [PubMed] [Google Scholar]
- 6.Nam K.T., Lee H.J., Sousa J.F. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139:2028–2037 e9. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi E., Roland J.T., Barlow B.J. Cell lineage distribution atlas of the human stomach reveals heterogeneous gland populations in the gastric antrum. Gut. 2014;63:1711–1720. doi: 10.1136/gutjnl-2013-305964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine J.S., Nakane P.K., Allen R.H. Immunocytochemical localization of human intrinsic factor: the nonstimulated stomach. Gastroenterology. 1980;79:493–502. [PubMed] [Google Scholar]
- 9.Zorn A.M., Wells J.M. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol. 2009;25:221–251. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLin V.A., Henning S.J., Jamrich M. The role of the visceral mesoderm in the development of the gastrointestinal tract. Gastroenterology. 2009;136:2074–2091. doi: 10.1053/j.gastro.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Khurana S., Mills J.C. The gastric mucosa development and differentiation. Prog Mol Biol Transl Sci. 2010;96:93–115. doi: 10.1016/B978-0-12-381280-3.00004-X. [DOI] [PubMed] [Google Scholar]
- 12.Stafford D., Prince V.E. Retinoic acid signaling is required for a critical early step in zebrafish pancreatic development. Curr Biol. 2002;12:1215–1220. doi: 10.1016/s0960-9822(02)00929-6. [DOI] [PubMed] [Google Scholar]
- 13.Bayha E., Jorgensen M.C., Serup P. Retinoic acid signaling organizes endodermal organ specification along the entire antero-posterior axis. PLoS One. 2009;4:e5845. doi: 10.1371/journal.pone.0005845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z., Dolle P., Cardoso W.V. Retinoic acid regulates morphogenesis and patterning of posterior foregut derivatives. Dev Biol. 2006;297:433–445. doi: 10.1016/j.ydbio.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 15.Wells J.M., Melton D.A. Early mouse endoderm is patterned by soluble factors from adjacent germ layers. Development. 2000;127:1563–1572. doi: 10.1242/dev.127.8.1563. [DOI] [PubMed] [Google Scholar]
- 16.Dessimoz J., Opoka R., Kordich J.J. FGF signaling is necessary for establishing gut tube domains along the anterior-posterior axis in vivo. Mech Dev. 2006;123:42–55. doi: 10.1016/j.mod.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Sherwood R.I., Maehr R., Mazzoni E.O. Wnt signaling specifies and patterns intestinal endoderm. Mech Dev. 2011;128:387–400. doi: 10.1016/j.mod.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiso N., Filippi A., Pauls S. BMP signalling regulates anteroposterior endoderm patterning in zebrafish. Mech Dev. 2002;118:29–37. doi: 10.1016/s0925-4773(02)00252-6. [DOI] [PubMed] [Google Scholar]
- 19.Hebrok M., Kim S.K., Melton D.A. Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes Dev. 1998;12:1705–1713. doi: 10.1101/gad.12.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S.K., Hebrok M., Melton D.A. Notochord to endoderm signaling is required for pancreas development. Development. 1997;124:4243–4252. doi: 10.1242/dev.124.21.4243. [DOI] [PubMed] [Google Scholar]
- 21.Bort R., Martinez-Barbera J.P., Beddington R.S. Hex homeobox gene-dependent tissue positioning is required for organogenesis of the ventral pancreas. Development. 2004;131:797–806. doi: 10.1242/dev.00965. [DOI] [PubMed] [Google Scholar]
- 22.Mao J., Kim B.M., Rajurkar M. Hedgehog signaling controls mesenchymal growth in the developing mammalian digestive tract. Development. 2010;137:1721–1729. doi: 10.1242/dev.044586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grapin-Botton A., Constam D. Evolution of the mechanisms and molecular control of endoderm formation. Mech Dev. 2007;124:253–278. doi: 10.1016/j.mod.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Lee C.S., Friedman J.R., Fulmer J.T. The initiation of liver development is dependent on Foxa transcription factors. Nature. 2005;435:944–947. doi: 10.1038/nature03649. [DOI] [PubMed] [Google Scholar]
- 25.Gao N., LeLay J., Vatamaniuk M.Z. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev. 2008;22:3435–3448. doi: 10.1101/gad.1752608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye D.Z., Kaestner K.H. Foxa1 and Foxa2 control the differentiation of goblet and enteroendocrine L- and D-cells in mice. Gastroenterology. 2009;137:2052–2062. doi: 10.1053/j.gastro.2009.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Offield M.F., Jetton T.L., Labosky P.A. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- 28.Kuo C.T., Morrisey E.E., Anandappa R. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997;11:1048–1060. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- 29.Molkentin J.D., Lin Q., Duncan S.A. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11:1061–1072. doi: 10.1101/gad.11.8.1061. [DOI] [PubMed] [Google Scholar]
- 30.Koutsourakis M., Langeveld A., Patient R. The transcription factor GATA6 is essential for early extraembryonic development. Development. 1999;126:723–732. [PubMed] [Google Scholar]
- 31.Jacobsen C.M., Narita N., Bielinska M. Genetic mosaic analysis reveals that GATA-4 is required for proper differentiation of mouse gastric epithelium. Dev Biol. 2002;241:34–46. doi: 10.1006/dbio.2001.0424. [DOI] [PubMed] [Google Scholar]
- 32.Que J., Luo X., Schwartz R.J. Multiple roles for Sox2 in the developing and adult mouse trachea. Development. 2009;136:1899–1907. doi: 10.1242/dev.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Que J., Okubo T., Goldenring J.R. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development. 2007;134:2521–2531. doi: 10.1242/dev.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raghoebir L., Bakker E.R., Mills J.C. SOX2 redirects the developmental fate of the intestinal epithelium toward a premature gastric phenotype. J Mol Cell Biol. 2012;4:377–385. doi: 10.1093/jmcb/mjs030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao N., White P., Kaestner K.H. Establishment of intestinal identity and epithelial-mesenchymal signaling by Cdx2. Dev Cell. 2009;16:588–599. doi: 10.1016/j.devcel.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X., Qin R., Liu B. Multilayered epithelium in a rat model and human Barrett's esophagus: similar expression patterns of transcription factors and differentiation markers. BMC Gastroenterol. 2008;8:1. doi: 10.1186/1471-230X-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nomura S., Settle S.H., Leys C.M. Evidence for repatterning of the gastric fundic epithelium associated with Menetrier's disease and TGFalpha overexpression. Gastroenterology. 2005;128:1292–1305. doi: 10.1053/j.gastro.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 38.Larsson L.I., Madsen O.D., Serup P. Pancreatic-duodenal homeobox 1-role in gastric endocrine patterning. Mech Dev. 1996;60:175–184. doi: 10.1016/s0925-4773(96)00609-0. [DOI] [PubMed] [Google Scholar]
- 39.Haumaitre C., Barbacci E., Jenny M. Lack of TCF2/vHNF1 in mice leads to pancreas agenesis. Proc Natl Acad Sci U S A. 2005;102:1490–1495. doi: 10.1073/pnas.0405776102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCracken K.W., Cata E.M., Crawford C.M. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature. 2014;516:400–404. doi: 10.1038/nature13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee C.S., Sund N.J., Behr R. Foxa2 is required for the differentiation of pancreatic alpha-cells. Dev Biol. 2005;278:484–495. doi: 10.1016/j.ydbio.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 42.Choi E., Kraus M.R., Lemaire L.A. Dual lineage-specific expression of Sox17 during mouse embryogenesis. Stem Cells. 2012;30:2297–2308. doi: 10.1002/stem.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harfe B.D., Scherz P.J., Nissim S. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 44.Hayashi K., Yasugi S., Mizuno T. Pepsinogen gene transcription induced in heterologous epithelial-mesenchymal recombinations of chicken endoderms and glandular stomach mesenchyme. Development. 1988;103:725–731. doi: 10.1242/dev.103.4.725. [DOI] [PubMed] [Google Scholar]
- 45.Urase K., Fukuda K., Ishii Y. Analysis of mesenchymal influence on the pepsinogen gene expression in the epithelium of chicken embryonic digestive tract. Rouxs Arch Dev Biol. 1996;205:382–390. doi: 10.1007/BF00377218. [DOI] [PubMed] [Google Scholar]
- 46.Takiguchi K., Yasugi S., Mizuno T. Gizzard epithelium of chick-embryos can express embryonic pepsinogen antigen, a marker protein of proventriculus. Rouxs Arch Dev Biol. 1986;195:475–483. doi: 10.1007/BF00375887. [DOI] [PubMed] [Google Scholar]
- 47.Yasugi S., Matsushita S., Mizuno T. Gland formation induced in the allantoic and small intestinal endoderm by the proventricular mesenchyme is not coupled with pepsinogen expression. Differentiation. 1985;30:47–52. [Google Scholar]
- 48.Narita T., Saitoh K., Kameda T. BMPs are necessary for stomach gland formation in the chicken embryo: a study using virally induced BMP-2 and Noggin expression. Development. 2000;127:981–988. doi: 10.1242/dev.127.5.981. [DOI] [PubMed] [Google Scholar]
- 49.Ramalho-Santos M., Melton D.A., McMahon A.P. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–2772. doi: 10.1242/dev.127.12.2763. [DOI] [PubMed] [Google Scholar]
- 50.El-Zaatari M., Saqui-Salces M., Waghray M. Sonic hedgehog in gastric physiology and neoplastic transformation: friend or foe? Curr Opin Endocrinol Diabetes Obes. 2009;16:60–65. doi: 10.1097/MED.0b013e328320a821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spencer-Dene B., Sala F.G., Bellusci S. Stomach development is dependent on fibroblast growth factor 10/fibroblast growth factor receptor 2b-mediated signaling. Gastroenterology. 2006;130:1233–1244. doi: 10.1053/j.gastro.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 52.Nyeng P., Norgaard G.A., Kobberup S. FGF10 signaling controls stomach morphogenesis. Dev Biol. 2007;303:295–310. doi: 10.1016/j.ydbio.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Speer A.L., Al Alam D., Sala F.G. Fibroblast growth factor 10-fibroblast growth factor receptor 2b mediated signaling is not required for adult glandular stomach homeostasis. PLoS One. 2012;7:e49127. doi: 10.1371/journal.pone.0049127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim B.M., Buchner G., Miletich I. The stomach mesenchymal transcription factor Barx1 specifies gastric epithelial identity through inhibition of transient Wnt signaling. Dev Cell. 2005;8:611–622. doi: 10.1016/j.devcel.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 55.Kim B.M., Miletich I., Mao J. Independent functions and mechanisms for homeobox gene Barx1 in patterning mouse stomach and spleen. Development. 2007;134:3603–3613. doi: 10.1242/dev.009308. [DOI] [PubMed] [Google Scholar]
- 56.Verzi M.P., Stanfel M.N., Moses K.A. Role of the homeodomain transcription factor Bapx1 in mouse distal stomach development. Gastroenterology. 2009;136:1701–1710. doi: 10.1053/j.gastro.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Self M., Geng X., Oliver G. Six2 activity is required for the formation of the mammalian pyloric sphincter. Dev Biol. 2009;334:409–417. doi: 10.1016/j.ydbio.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Udager A.M., Prakash A., Saenz D.A. Proper development of the outer longitudinal smooth muscle of the mouse pylorus requires Nkx2-5 and Gata3. Gastroenterology. 2014;146:157–165 e10. doi: 10.1053/j.gastro.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prakash A., Udager A.M., Saenz D.A. Roles for Nkx2-5 and Gata3 in the ontogeny of the murine smooth muscle gastric ligaments. Am J Physiol Gastrointest Liver Physiol. 2014;307:G430–G436. doi: 10.1152/ajpgi.00360.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keeley T.M., Samuelson L.C. Cytodifferentiation of the postnatal mouse stomach in normal and Huntingtin-interacting protein 1-related-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1241–G1251. doi: 10.1152/ajpgi.00239.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karam S.M., Leblond C.P. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec. 1993;236:259–279. doi: 10.1002/ar.1092360202. [DOI] [PubMed] [Google Scholar]
- 62.Bjerknes M., Cheng H. Multipotential stem cells in adult mouse gastric epithelium. Am J Physiol Gastrointest Liver Physiol. 2002;283:G767–G777. doi: 10.1152/ajpgi.00415.2001. [DOI] [PubMed] [Google Scholar]
- 63.McDonald S.A., Greaves L.C., Gutierrez-Gonzalez L. Mechanisms of field cancerization in the human stomach: the expansion and spread of mutated gastric stem cells. Gastroenterology. 2008;134:500–510. doi: 10.1053/j.gastro.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 64.Kokubu H., Ohtsuka T., Kageyama R. Mash1 is required for neuroendocrine cell development in the glandular stomach. Genes Cells. 2008;13:41–51. doi: 10.1111/j.1365-2443.2007.01146.x. [DOI] [PubMed] [Google Scholar]
- 65.Jenny M., Uhl C., Roche C. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 2002;21:6338–6347. doi: 10.1093/emboj/cdf649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee C.S., Perreault N., Brestelli J.E. Neurogenin 3 is essential for the proper specification of gastric enteroendocrine cells and the maintenance of gastric epithelial cell identity. Genes Dev. 2002;16:1488–1497. doi: 10.1101/gad.985002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li H.J., Johnston B., Aiello D. Distinct cellular origins for serotonin-expressing and enterochromaffin-like cells in the gastric corpus. Gastroenterology. 2014;146:754–764 e3. doi: 10.1053/j.gastro.2013.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mastracci T.L., Sussel L. The endocrine pancreas: insights into development, differentiation, and diabetes. Wiley Interdiscip Rev Dev Biol. 2012;1:609–628. doi: 10.1002/wdev.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li H.J., Ray S.K., Singh N.K. Basic helix-loop-helix transcription factors and enteroendocrine cell differentiation. Diabetes Obes Metab. 2011;13(Suppl 1):5–12. doi: 10.1111/j.1463-1326.2011.01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Larsson L.I., St-Onge L., Hougaard D.M. Pax 4 and 6 regulate gastrointestinal endocrine cell development. Mech Dev. 1998;79:153–159. doi: 10.1016/s0925-4773(98)00182-8. [DOI] [PubMed] [Google Scholar]
- 71.Choi M.Y., Romer A.I., Wang Y. Requirement of the tissue-restricted homeodomain transcription factor Nkx6.3 in differentiation of gastrin-producing G cells in the stomach antrum. Mol Cell Biol. 2008;28:3208–3218. doi: 10.1128/MCB.01737-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Du A., McCracken K.W., Walp E.R. Arx is required for normal enteroendocrine cell development in mice and humans. Dev Biol. 2012;365:175–188. doi: 10.1016/j.ydbio.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Horst D., Gu X., Bhasin M. Requirement of the epithelium-specific Ets transcription factor Spdef for mucous gland cell function in the gastric antrum. J Biol Chem. 2010;285:35047–35055. doi: 10.1074/jbc.M110.164541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Verzi M.P., Khan A.H., Ito S. Transcription factor foxq1 controls mucin gene expression and granule content in mouse stomach surface mucous cells. Gastroenterology. 2008;135:591–600. doi: 10.1053/j.gastro.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huh W.J., Esen E., Geahlen J.H. XBP1 controls maturation of gastric zymogenic cells by induction of MIST1 and expansion of the rough endoplasmic reticulum. Gastroenterology. 2010;139:2038–2049. doi: 10.1053/j.gastro.2010.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ramsey V.G., Doherty J.M., Chen C.C. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development. 2007;134:211–222. doi: 10.1242/dev.02700. [DOI] [PubMed] [Google Scholar]
- 77.Osaki L.H., Curi M.A., Alvares E.P. Early weaning accelerates the differentiation of mucous neck cells in rat gastric mucosa: possible role of TGFalpha/EGFR. Differentiation. 2010;79:48–56. doi: 10.1016/j.diff.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 78.Hanby A.M., Poulsom R., Playford R.J. The mucous neck cell in the human gastric corpus: a distinctive, functional cell lineage. J Pathol. 1999;187:331–337. doi: 10.1002/(SICI)1096-9896(199902)187:3<331::AID-PATH241>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 79.Karam S.M., Leblond C.P. Dynamics of epithelial cells in the corpus of the mouse stomach. II. Outward migration of pit cells. Anat Rec. 1993;236:280–296. doi: 10.1002/ar.1092360203. [DOI] [PubMed] [Google Scholar]
- 80.Karam S.M., Leblond C.P. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat Rec. 1993;236:297–313. doi: 10.1002/ar.1092360204. [DOI] [PubMed] [Google Scholar]
- 81.Karam S.M. Dynamics of epithelial cells in the corpus of the mouse stomach. IV. Bidirectional migration of parietal cells ending in their gradual degeneration and loss. Anat Rec. 1993;236:314–332. doi: 10.1002/ar.1092360205. [DOI] [PubMed] [Google Scholar]
- 82.Karam S.M., Leblond C.P. Dynamics of epithelial cells in the corpus of the mouse stomach. V. Behavior of entero-endocrine and caveolated cells: general conclusions on cell kinetics in the oxyntic epithelium. Anat Rec. 1993;236:333–340. doi: 10.1002/ar.1092360206. [DOI] [PubMed] [Google Scholar]
- 83.Barker N., van Es J.H., Kuipers J. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 84.Montgomery R.K., Carlone D.L., Richmond C.A. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci U S A. 2011;108:179–184. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Powell A.E., Wang Y., Li Y. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149:146–158. doi: 10.1016/j.cell.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tian H., Biehs B., Warming S. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Takeda N., Jain R., LeBoeuf M.R. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420–1424. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tatematsu M., Fukami H., Yamamoto M. Clonal analysis of glandular stomach carcinogenesis in C3H/HeN<==>BALB/c chimeric mice treated with N-methyl-N-nitrosourea. Cancer Lett. 1994;83:37–42. doi: 10.1016/0304-3835(94)90296-8. [DOI] [PubMed] [Google Scholar]
- 89.Nomura S., Esumi H., Job C. Lineage and clonal development of gastric glands. Dev Biol. 1998;204:124–135. doi: 10.1006/dbio.1998.9055. [DOI] [PubMed] [Google Scholar]
- 90.Thompson M., Fleming K.A., Evans D.J. Gastric endocrine cells share a clonal origin with other gut cell lineages. Development. 1990;110:477–481. doi: 10.1242/dev.110.2.477. [DOI] [PubMed] [Google Scholar]
- 91.Arnold K., Sarkar A., Yram M.A. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317–329. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Khalili M., Vasei M., Khalili D. Downregulation of the genes involved in reprogramming (SOX2, c-MYC, miR-302, miR-145, and P21) in gastric adenocarcinoma. J Gastrointest Cancer. 2015;46:251–258. doi: 10.1007/s12029-015-9695-2. [DOI] [PubMed] [Google Scholar]
- 93.van Olphen S., Biermann K., Spaander M.C. SOX2 as a novel marker to predict neoplastic progression in Barrett's esophagus. Am J Gastroenterol. 2015;110:1420–1428. doi: 10.1038/ajg.2015.260. [DOI] [PubMed] [Google Scholar]
- 94.Stange D.E., Koo B.K., Huch M. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 2013;155:357–368. doi: 10.1016/j.cell.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mills J.C., Sansom O.J. Reserve stem cells: differentiated cells reprogram to fuel repair, metaplasia, and neoplasia in the adult gastrointestinal tract. Sci Signal. 2015;8:re8. doi: 10.1126/scisignal.aaa7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hayakawa Y., Ariyama H., Stancikova J. Mist1 expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell niche. Cancer Cell. 2015;28:800–814. doi: 10.1016/j.ccell.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huh W.J., Khurana S.S., Geahlen J.H. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology. 2012;142:21–24 e7. doi: 10.1053/j.gastro.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Saenz J.B., Burclaff J., Mills J.C. Modeling murine gastric metaplasia through tamoxifen-induced acute parietal cell loss. Methods Mol Biol. 2016;1422:329–339. doi: 10.1007/978-1-4939-3603-8_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maeda Y., Echizen K., Oshima H. Myeloid differentiation factor 88 signaling in bone marrow-derived cells promotes gastric tumorigenesis by generation of inflammatory microenvironment. Cancer Prev Res (Phila) 2016;9:253–263. doi: 10.1158/1940-6207.CAPR-15-0315. [DOI] [PubMed] [Google Scholar]
- 100.Huh W.J., Mysorekar I.U., Mills J.C. Inducible activation of Cre recombinase in adult mice causes gastric epithelial atrophy, metaplasia, and regenerative changes in the absence of “floxed” alleles. Am J Physiol Gastrointest Liver Physiol. 2010;299:G368–G380. doi: 10.1152/ajpgi.00021.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bredemeyer A.J., Geahlen J.H., Weis V.G. The gastric epithelial progenitor cell niche and differentiation of the zymogenic (chief) cell lineage. Dev Biol. 2009;325:211–224. doi: 10.1016/j.ydbio.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Suzuki S., Tsuyama S., Murata F. Cells intermediate between mucous neck cells and chief cells in rat stomach. Cell Tissue Res. 1983;233:475–484. doi: 10.1007/BF00212218. [DOI] [PubMed] [Google Scholar]
- 103.Quante M., Marrache F., Goldenring J.R. TFF2 mRNA transcript expression marks a gland progenitor cell of the gastric oxyntic mucosa. Gastroenterology. 2010;139:2018–2027 e2. doi: 10.1053/j.gastro.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khurana S.S., Riehl T.E., Moore B.D. The hyaluronic acid receptor CD44 coordinates normal and metaplastic gastric epithelial progenitor cell proliferation. J Biol Chem. 2013;288:16085–16097. doi: 10.1074/jbc.M112.445551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim T.H., Shivdasani R.A. Notch signaling in stomach epithelial stem cell homeostasis. J Exp Med. 2011;208:677–688. doi: 10.1084/jem.20101737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shinohara M., Mao M., Keeley T.M. Bone morphogenetic protein signaling regulates gastric epithelial cell development and proliferation in mice. Gastroenterology. 2010;139:2050–2060 e2. doi: 10.1053/j.gastro.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Friis-Hansen L., Sundler F., Li Y. Impaired gastric acid secretion in gastrin-deficient mice. Am J Physiol. 1998;274:G561–G568. doi: 10.1152/ajpgi.1998.274.3.G561. [DOI] [PubMed] [Google Scholar]
- 108.Koh T.J., Goldenring J.R., Ito S. Gastrin deficiency results in altered gastric differentiation and decreased colonic proliferation in mice. Gastroenterology. 1997;113:1015–1025. doi: 10.1016/s0016-5085(97)70199-9. [DOI] [PubMed] [Google Scholar]
- 109.Ohning G.V., Wong H.C., Lloyd K.C. Gastrin mediates the gastric mucosal proliferative response to feeding. Am J Physiol. 1996;271:G470–G476. doi: 10.1152/ajpgi.1996.271.3.G470. [DOI] [PubMed] [Google Scholar]
- 110.Hinkle K.L., Bane G.C., Jazayeri A. Enhanced calcium signaling and acid secretion in parietal cells isolated from gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2003;284:G145–G153. doi: 10.1152/ajpgi.00283.2002. [DOI] [PubMed] [Google Scholar]
- 111.Wang T.C., Koh T.J., Varro A. Processing and proliferative effects of human progastrin in transgenic mice. J Clin Invest. 1996;98:1918–1929. doi: 10.1172/JCI118993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xiao C., Ogle S.A., Schumacher M.A. Loss of parietal cell expression of Sonic hedgehog induces hypergastrinemia and hyperproliferation of surface mucous cells. Gastroenterology. 2010;138:550–561. doi: 10.1053/j.gastro.2009.11.002. 561 e1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee E.R., Leblond C.P. Dynamic histology of the antral epithelium in the mouse stomach: II. Ultrastructure and renewal of isthmal cells. Am J Anat. 1985;172:205–224. doi: 10.1002/aja.1001720304. [DOI] [PubMed] [Google Scholar]
- 114.Barker N., Huch M., Kujala P. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 115.Hayakawa Y., Jin G., Wang H. CCK2R identifies and regulates gastric antral stem cell states and carcinogenesis. Gut. 2015;64:544–553. doi: 10.1136/gutjnl-2014-307190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Qiao X.T., Ziel J.W., McKimpson W. Prospective identification of a multilineage progenitor in murine stomach epithelium. Gastroenterology. 2007;133:1989–1998. doi: 10.1053/j.gastro.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee E.R. Dynamic histology of the antral epithelium in the mouse stomach: I. Architecture of antral units. Am J Anat. 1985;172:187–204. doi: 10.1002/aja.1001720303. [DOI] [PubMed] [Google Scholar]
- 118.Demitrack E.S., Gifford G.B., Keeley T.M. Notch signaling regulates gastric antral LGR5 stem cell function. EMBO J. 2015;34:2522–2536. doi: 10.15252/embj.201490583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bleuming S.A., He X.C., Kodach L.L. Bone morphogenetic protein signaling suppresses tumorigenesis at gastric epithelial transition zones in mice. Cancer Res. 2007;67:8149–8155. doi: 10.1158/0008-5472.CAN-06-4659. [DOI] [PubMed] [Google Scholar]
- 120.Brosens L.A., Langeveld D., van Hattem W.A. Juvenile polyposis syndrome. World J Gastroenterol. 2011;17:4839–4844. doi: 10.3748/wjg.v17.i44.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.de Lau W., Barker N., Low T.Y. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476:293–297. doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- 122.Gifford G.B., Demitrack E.S., Keeley T.M. Notch1 and Notch2 receptors regulate mouse and human gastric antral epithelial cell homoeostasis. Gut. 2016 doi: 10.1136/gutjnl-2015-310811. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schumacher M.A., Aihara E., Feng R. The use of murine-derived fundic organoids in studies of gastric physiology. J Physiol. 2015;593:1809–1827. doi: 10.1113/jphysiol.2014.283028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li X., Nadauld L., Ootani A. Oncogenic transformation of diverse gastrointestinal tissues in primary organoid culture. Nat Med. 2014;20:769–777. doi: 10.1038/nm.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moore B.D., Jin R.U., Osaki L. Identification of alanyl aminopeptidase (CD13) as a surface marker for isolation of mature gastric zymogenic chief cells. Am J Physiol Gastrointest Liver Physiol. 2015;309:G955–G964. doi: 10.1152/ajpgi.00261.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schlaermann P., Toelle B., Berger H. A novel human gastric primary cell culture system for modelling Helicobacter pylori infection in vitro. Gut. 2016;65:202–213. doi: 10.1136/gutjnl-2014-307949. [DOI] [PMC free article] [PubMed] [Google Scholar]