Abstract
The oncofetal protein sine oculis-related homeobox 1 (SIX1) is a developmental transcription factor associated with carcinogenesis in several human cancer types, but has not been investigated in human endometrial cancer. In a model of hormonal carcinogenesis, mice neonatally exposed to the soy phytoestrogen genistein (GEN) or the synthetic estrogen diethylstilbestrol (DES) develop endometrial cancer as adults. Previously, we demonstrated that SIX1 becomes aberrantly expressed in the uteri of these mice. Here we used this mouse model to investigate the role of SIX1 expression in endometrial carcinoma development and used human tissue microarrays to explore the utility of SIX1 as a biomarker in human endometrial cancer. In mice neonatally exposed to GEN or DES, the Six1 transcript level increased dramatically over time in uteri at 6, 12, and 18 months of age and was associated with development of endometrial carcinoma. SIX1 protein localized within abnormal basal cells and all atypical hyperplastic and neoplastic lesions. These findings indicate that developmental estrogenic chemical exposure induces persistent endometrial SIX1 expression that is strongly associated with abnormal cell differentiation and cancer development. In human endometrial tissue specimens, SIX1 was not present in normal endometrium but was expressed in a subset of endometrial cancers in patients who were also more likely to have late-stage disease. These findings identify SIX1 as a disease biomarker in a model of hormonal carcinogenesis and suggest that SIX1 plays a role in endometrial cancer development in both mice and women.
Implications
The SIX1 oncoprotein is aberrantly expressed in the uterus following developmental exposure to estrogenic chemicals, correlates with uterine cancer, and is a biomarker in human endometrial cancers.
Keywords: biomarker, oncofetal, diethylstilbestrol, estrogenic chemicals
INTRODUCTION
There is now widespread acceptance of the idea that environmental and stochastic factors that modify gene expression early in life may determine disease susceptibility later in life through epigenomic alterations. However, the molecular mechanisms that define the relationships between early environmental cues and disease phenotypes are poorly understood, in large part because these interactions are complex, difficult to quantify accurately, and often occur over long periods of time.
In human studies and animal models, environmental exposures during critical stages of female reproductive tract (FRT) differentiation can establish permanent epigenomic alterations that contribute to adverse adult phenotypes, including infertility and cancer (1–4). Chemicals with estrogenic activity are of particular interest due to their widespread presence in the environment and their association with adverse human health outcomes (4–7). For example, human gestational exposure to the potent synthetic estrogen diethylstilbestrol (DES) results in a high incidence of reproductive tract abnormalities and is associated with vaginal clear cell adenocarcinoma among women exposed in utero (5–8). Although DES is no longer used clinically (9, 10), approximately 13% of newborns in the U.S. are exposed to the phytoestrogen genistein (GEN) through consumption of soy-based infant formulas at dietary levels that may be able to exert a biological estrogenic effect (11–14). It is unknown whether GEN and DES work through parallel estrogen receptor (ER)-mediated mechanisms, but the close similarities between GEN and DES-induced phenotypes in animal models of hormonal carcinogenesis clearly indicate that other environmental estrogens may have important biological effects, including increased cancer risk.
In the mouse, the FRT undergoes cellular differentiation and gland formation during neonatal life, making this time period particularly sensitive to disruption (2, 15). Indeed, neonatal exposure to either GEN or DES results in several nonneoplastic pathologies and a high incidence of endometrial carcinoma (16, 17). Interestingly, ovariectomy prior to puberty prevents endometrial carcinoma development in this model, likely due to the absence of endogenous estrogens (16, 18). These findings indicate that carcinogenesis in this model of developmental estrogen exposure follows the two-hit cancer hypothesis, where the first hit occurs during early developmental estrogen exposure and the second hit occurs through promotion by endogenous estrogens.
Despite many studies having reported latent effects of developmental GEN or DES exposures, the biological mechanisms driving these pathological changes are unknown. We previously showed that neonatal estrogenic chemical exposure causes epigenomic alterations and fundamentally alters developmental patterning of the mouse FRT (19, 20). One of the altered proteins is sine oculis-related homeobox 1 (SIX1), which becomes aberrantly expressed in the uteri of mice exposed neonatally to GEN or DES, likely as a result of permanent alterations in Six1 gene locus-specific epigenetic marks (19, 20). SIX1 is a homeodomain-containing transcription factor that plays essential roles in mouse organogenesis by regulating cell proliferation, survival, migration, and invasion (21, 22). Indeed, it is considered an oncofetal protein because dysregulation and inappropriate re-expression result in genomic instability, malignant transformation, and metastasis in animal models and humans (21–23). Neonatal exposure to estrogenic chemicals not only causes a dramatic increase in Six1 transcript expression in the mouse uterus, but it also causes Six1 expression to become estrogen-responsive (19, 20). These findings suggest that aberrant endometrial expression of SIX1 following neonatal GEN or DES exposure could drive the endometrial carcinoma phenotype in these models of hormonal carcinogenesis.
Here we evaluated endometrial SIX1 expression during the development of endometrial carcinoma in mice following neonatal GEN or DES exposure. SIX1 expression following both exposures was highly associated with endometrial carcinoma development and SIX1 was prominently expressed in an abnormal basal cell population and all preneoplastic and neoplastic lesions. We also surveyed a large number of human endometrial cancer tissues for the presence of SIX1 to determine whether it might contribute to endometrial cancer pathophysiology in women. SIX1 was expressed in a subset of human endometrial cancer patients who were more likely to have late-stage disease. These findings indicate that SIX1 expression may serve as a useful biomarker of endometrial carcinogenesis.
MATERIALS AND METHODS
Animals
Care and use of animals complied with the NIEHS/NIH animal care guidelines and followed an approved institutional animal care and use protocol. The estrogenic chemical exposure model has been described previously (19, 20). Key details include daily subcutaneous injection (0.02 mL) of female CD-1 pups beginning on the day of birth (postnatal day 1 [PND1]) through PND5 with vehicle alone (corn oil), genistein (GEN; 50 mg/kg/day), or diethylstilbestrol (DES; 1 mg/kg/day). Mice were euthanized by CO2 asphyxiation at their respective endpoints and the reproductive tracts were collected. A cranial segment of the right uterine horn was removed, snap-frozen on dry ice, and stored at −80°C until use. The remaining FRT was formalin-fixed, processed using standard histologic procedures, sectioned longitudinally at 6 µm, and stained with hematoxylin and eosin (H&E) or left unstained for immunohistochemistry.
Human Endometrial Tissue Samples
After receiving Institutional Review Board (IRB) approval, women undergoing hysterectomy for endometrial hyperplasia, endometrial cancer, and benign indications were identified, and their hysterectomy specimens obtained from the University of North Carolina at Chapel Hill (UNC-CH) Department of Pathology. Tissue microarrays (TMAs) were constructed from the formalin-fixed, paraffin-embedded hysterectomy specimens. Additional endometrial TMAs were purchased from a commercial vendor (U.S. Biomax, Inc., Rockville, MD; TMA UT501, UT801, UT803, UT1501, EMC1021). All TMA specimens were obtained with informed consent according to U.S. federal law. Patient diagnoses and pathological descriptions were provided with the TMAs. For comparison, TNM scores were converted to FIGO stage based on previously described guidelines (24). Serial sections of TMAs were freshly cut for immunohistochemical (IHC) staining.
Mouse and Human Immunohistochemistry
Serial sections of mouse FRT or human endometrial TMAs were deparaffinized in xylene and rehydrated with gradient ethanol. Heat-induced epitope retrieval was performed using respective antigen retrieval solutions (SIX1, Ki67: citrate buffer, pH 6.0; CK18: Nuclear Decloaker, pH 9.5, Biocare, Concord, CA) in the Decloaker® pressure chamber for 5 minutes at 120°C, followed by 3% H2O2 for 15 minutes to quench endogenous peroxidase activity. Non-specific binding was blocked using respective blocking solutions and serum (SIX1: Avidin/biotin blocking kit, Vector, Burlingame, CA, with 10% donkey serum, Jackson Immunoresearch, West Grove, PA; Ki67: Rodent Block M, Biocare; keratin 18, type I (CK18): Avidin/biotin blocking kit, Vector, with 10% horse serum, Jackson Immunoresearch). Sections were incubated with respective primary and secondary antibodies (SIX1: 0.2–0.4 µg/ml anti-SIX1 antibody, HPA001893, Sigma-Aldrich, St. Louis, MO, and 2.2 µg/ml biotinylated donkey anti-rabbit IgG, Jackson Immunoresearch; Ki67: 1.1 µg/ml anti-Ki-67 antibody, CRM325C, Biocare; CK18: 8 µg/ml anti-cytokeratin 18 antibody, sc-51582, Santa Cruz, Dallas, TX, and 0.5 µg/ml biotinylated horse anti-mouse IgG, Vector) and visualized using respective detection systems (SIX1 and CK18: Vectastain Elite ABC R.T.U. label, Vector) and 3,3-diaminobenzidine, Dako, Carpinteria, CA; Ki67: Rabbit on Rodent HRP Polymer, Biocare, and 3,3-diaminobenzidine, Dako). Slides were counterstained with hematoxylin. Mouse sections were stained via the Intellipath FLX autostainer (Biocare). Human endometrial TMAs were stained manually. Appropriate positive and negative control tissues were stained for all IHC experiments.
Mouse Histopathologic Analysis
A single H&E section of the FRT including uterine horns/body, cervix, and anterior vaginal canal was evaluated for each mouse via light microscopy by a certified study pathologist (CEW). Histopathologic diagnoses were based on standard criteria and nomenclature for nonneoplastic and neoplastic lesions (16, 25). Images were captured using a Lumenera Infinity 2-3C digital camera (Ottawa, Ontario).
Mouse and Human Immunohistochemical Analysis
Mouse FRT sections were stained for SIX1 and Ki67. Overall abundance of SIX1 immunolabeling was evaluated by light microscopy and assigned a qualitative labeling score from 0–4 (0=absent, 1=minimal, 2=mild, 3=moderate, 4=severe) based on staining intensity and the estimated percentage of labeled cells within a section. Lesion-specific localization of SIX1 immunolabeling was confirmed as needed by comparison with corresponding adjacent H&E-stained sections.
Human TMA serial sections were stained for SIX1 and CK18 and evaluated by a certified pathologist (CEW) blinded to all clinical and pathological information. Core biopsy sections lacking epithelium (confirmed by absence of CK18 staining) were unable to be evaluated and removed from the study. For core biopsy sections containing epithelial tissue, SIX1 staining was categorized as positive or negative based on the presence of brown labeling in at least 1% of epithelial nuclei. Nuclear labeling that was low intensity but readily discernible at 20x objective magnification and clearly distinguished from nearby unlabeled nuclei was called positive. A limited number of sections had weak cytoplasmic staining for SIX1 diffusely or along tissue edges, which was considered background. Cytoplasmic or nuclear staining of non-epithelial “off-target” cells (myometrial, stromal, or immune) observed in a subset of sections was not considered in SIX1 classification, which was based strictly on nuclear staining in epithelial cells.
Approximately 10% of core biopsies (63/643) had equivocal SIX1 labeling that could not be clearly identified as positive or negative. Equivocal SIX1 labeled cores presented with weak, light brown nuclear speckling in epithelial cells. As a result, 20 patients with only equivocal core biopsies were removed from analysis, leaving 369 patients with clearly identifiable core staining.
Real time RT-PCR
Total RNA was extracted from whole tissue homogenate of uterine horns and real-time RT-PCR was performed using Six1 primers and normalized to cyclophilin A (Ppia) as previously described (20). Expression levels were calculated using the delta Ct method (20, 26).
Immunoblots
Following the manufacturer’s instructions, nuclear protein was extracted from whole uterine tissue using the NE-PER nuclear and cytoplasmic extraction kit and protein concentration was measured using the BCA kit (Thermo Scientific, Rockford, IL). Nuclear proteins (10 µg) were separated on Novex 16% Tris-glycine gels and transferred to PVDF membrane (Invitrogen, Carlsbad, CA). Blots were blocked with 5% milk in Tris-buffered saline containing 0.1% Tween-20 (TBST) and then incubated overnight at 4°C with 0.2 µg/ml anti-SIX1 antibody (HPA001893, Sigma-Aldrich) or 2 ng/ml anti-β-actin antibody (A1978, Sigma-Aldrich) in 5% milk in TBST and followed by application of the appropriate secondary antibody. Blots were incubated with SuperSignal West Femto Chemiluminescent Substrate (Thermo Scientific) and then exposed to film. Blots were scanned using the HP Scanjet 7650 (Hewlett-Packard, Palo Alto, CA). Images were desaturated in Adobe Photoshop Elements (Adobe, San Jose, CA) to remove color without altering the brightness value of the pixels.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism, version 5.0c (La Jolla, CA). Data were analyzed using 2-way ANOVA, one- or two-tailed Fisher’s exact test, or Chi-square test, and appropriate post-hoc tests for multiple comparisons. Tests used are indicated in Figure Legends. Error bars show S.E.M. for all graphs.
RESULTS
Progression of SIX1 immunolabeled endometrial cells following neonatal GEN or DES exposure
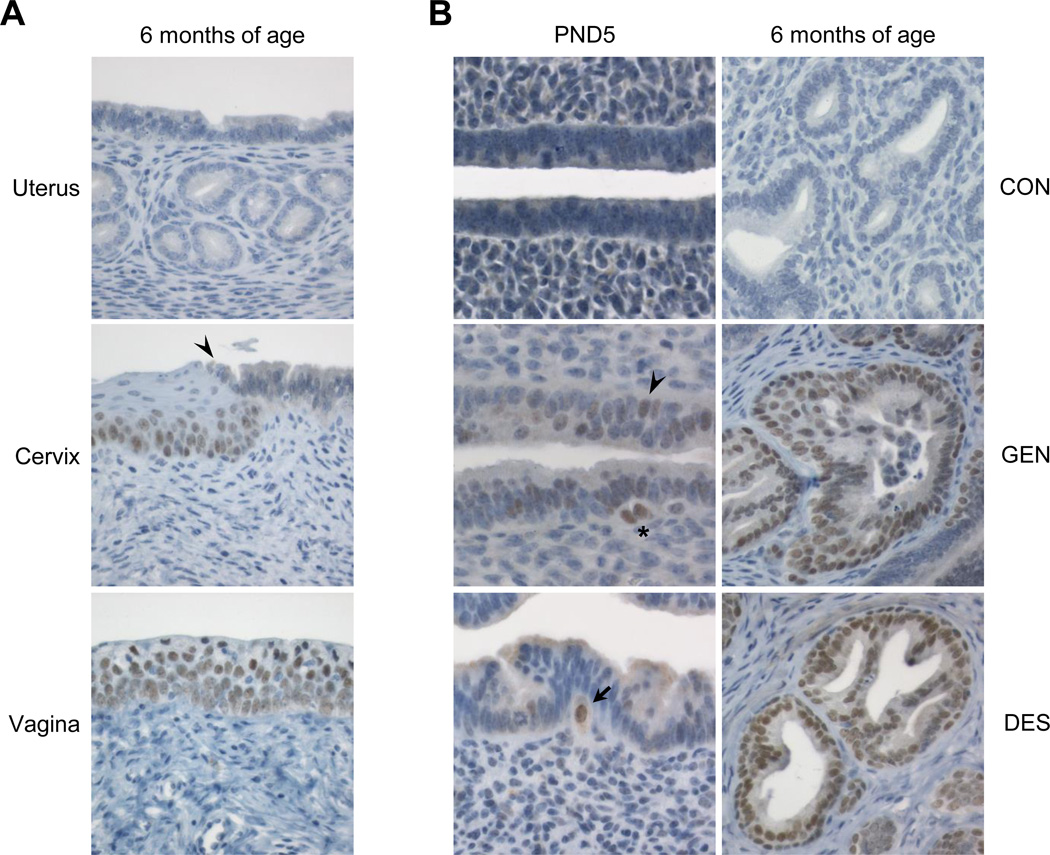
We previously reported that in untreated mice on PND1 through adulthood, Six1 transcripts are expressed in the vagina and cervix but not in the uterine body/horn or oviduct (19). To determine where SIX1 protein normally localizes along the female reproductive tract in adult mice, we performed SIX1 IHC at 6, 12, or 18 months of age. Consistent with the previously observed transcript expression differences (19), there were distinct cell-type specific differences in SIX1 expression (Fig. 1A). In the vaginal and cervical epithelium, SIX1 localized to the stratified squamous epithelium, with highest expression in the basal and suprabasal layers. In the endocervix, nuclear SIX1 immunolabeling was observed in simple columnar glandular epithelial cells only when there was a layer of progenitor-like basal cells directly subjacent to the luminal cells. SIX1 expression was not observed in endometrial luminal epithelium or morphologically normal glands (Fig. 1A; Table 1). SIX1 was present in the uteri of a few control mice but was limited to small focal areas of squamous metaplasia in the uterine body (Table 1).
Figure 1.
SIX1 localization in controls and following neonatal estrogenic chemical exposure. A. Representative SIX1 immunolabeling in a control adult female mouse reproductive tract at 6 months of age. Arrowhead indicates squamocolumnar junction (SCJ). B. Appearance and expansion of SIX1 immunolabeled cells in mouse endometrium following neonatal GEN or DES exposure at PND5 or 6 months of age. Arrowhead indicates SIX1-positive columnar cells and asterisk indicates SIX1-positive basal-type cells underlying the glandular epithelium. Arrow indicates large SIX1-positive basal-type cell that appears to be traversing the basement membrane. Representative images were taken at an objective magnification of 60× (PND 5) or 40× (6 months of age).
Table 1.
Incidence of reproductive tract abnormalities and SIX1 expression in mice exposed neonatally to GEN or DES1
| Age (months)2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6 | 12 | 18 | ||||||||
| Site | Pathology | CON | GEN | DES | CON | GEN | DES | CON | GEN | DES |
| Vagina | Adenosis | 0/33 (0%) |
0/30 (0%) |
2/31 (6%) |
0/29 (0)% |
0/30 (0%) |
2/26 (8%) |
0/30 (0%) |
1/30 (3%) |
2/30 (7%) |
| Uterus | Cystic Change | 2/33 (6%) |
12/30* (40%) |
4/31 (13%) |
7/29 (24%) |
23/30* (77%) |
10/26 (38%) |
15/30 (50%) |
27/30* (90%) |
23/30 (77%) |
| Adenomyosis | 0/33 (0%) |
6/30* (20%) |
13/31* (42%) |
3/29 (10%) |
10/30 (33%) |
13/26* (50%) |
4/30 (13%) |
13/30* (43%) |
18/30* (60%) |
|
| Basal Cell Metaplasia |
3/33 (9%) |
19/30* (63%) |
30/31* (97%) |
2/29 (7%) |
26/30* (87%) |
25/26* (96%) |
0/30 (0%) |
30/30* (100%) |
29/30* (97%) |
|
| Squamous Metaplasia3 |
1/33 (3%) |
11/30* (37%) |
7/31* (23%) |
0/29 (0%) |
16/30* (53%) |
12/26* (46%) |
0/30 (0%) |
24/30* (80%) |
14/30* (47%) |
|
| Atypical Hyperplasia |
0/33 (0%) |
3/30 (10%) |
14/31* (45%) |
0/29 (0%) |
9/30* (30%) |
14/26* (54%) |
0/30 (0%) |
15/30* (50%) |
18/30* (60%) |
|
| Carcinoma | 0/33 (0%) |
2/30 (7%) |
5/31 (16%) |
0/29 (0%) |
7/30* (23%) |
9/26* (35%) |
0/30 (0%) |
10/30* (33%) |
12/30* (40%) |
|
| Sarcoma | 0/33 (0%) |
0/30 (0%) |
1/31 (3%) |
0/29 (0%) |
1/30 (3%) |
0/26 (0%) |
0/30 (0%) |
2/30 (7%) |
0/30 (0%) |
|
| SIX1 IHC | ||||||||||
| Vagina | Epithelium | 33/33 (100%) |
30/30 (100%) |
31/31 (100%) |
29/29 (100%) |
30/30 (100%) |
26/26 (100%) |
30/30 (100%) |
30/30 (100%) |
30/30 (100%) |
| Average SIX1 severity index4 |
2.2 | 1.8 | 1.5 | 1.6 | 1.1 | 1.2 | 1.9 | 1.7 | 1.7 | |
| Uterus | Nonneoplastic Glands5 |
3/33 (9%) |
21/30* (70%) |
30/31* (97%) |
2/29 (7%) |
26/30* (87%) |
25/26* (96%) |
0/30 (0%) |
30/30* (100%) |
30/30* (100%) |
| Average SIX1 severity index4 |
0.1 | 0.9 | 1.5 | 0.1 | 1.5 | 2.0 | 0.0 | 2.0 | 2.6 | |
| Neoplastic Glands6 |
na | 2/2 (100%) |
5/5 (100%) |
na | 7/7 (100%) |
9/9 (100%) |
na | 10/10 (100%) |
12/12 (100%) |
|
| Average SIX1 severity index4 |
na | 2.5 | 2.8 | na | 2.0 | 2.7 | na | 2.1 | 2.4 | |
Statistically significant using one-tailed Fisher’s Exact Test p<0.05 compared to corresponding age-matched CON mice.
n=26–33 mice per treatment and age group as indicated in the table.
Mice were given injections of GEN (50 mg/kg/day) or DES (1 mg/kg/day) on days 1–5 of life.
Mice were necropsied at indicated ages.
Stratified squamous cells in place of glandular epithelium; distinct from single layer of basal cells underlying luminal glandular cells.
Average qualitative severity score from 0 to 4+ for SIX1 immunolabeling across the group.
Number of animals with SIX1-positive immunolabeling out of all animals within that group.
Number of animals with SIX1-positive immunolabeling in neoplastic lesions out of animals with carcinoma.
Following a 5-day neonatal exposure to GEN or DES, uterine Six1 transcript expression is increased at PND5, PND22, and 2 months of age, when there is also a dramatic increase in SIX1 protein expression in the uterus (19, 20). To assess SIX1 localization during its initial appearance and progression, we performed SIX1 IHC on uteri collected on the final day of treatment (PND5) and at 6 months of age, when endometrial carcinoma was first observed. In controls, SIX1 was not present at either PND5 or in the vast majority of mice at 6 months of age (Fig. 1B). In both neonatally GEN- and DES-exposed groups, nuclear SIX1 was present in low numbers of scattered luminal and basal-type epithelial cells on PND5 (Fig. 1B). In some cases these SIX1 positive cells appeared to be traversing the basement membrane. Uteri from GEN- and DES-exposed mice on PND5 exhibited classical responses to estrogen similar to those previously reported, including increased columnar cell height, an overall increase in cellularity, and edema (27). However, there was no evidence of basal cell metaplasia, squamous metaplasia, or other proliferative lesions at PND5. At 6 months of age, SIX1 localized to basal cell and squamous metaplasia in nonneoplastic endometrial glands of most mice neonatally exposed to GEN or DES (Fig. 1B, Table 1). SIX1 immunolabeling was often present in a patchy or “hot spot” distribution (i.e. abundantly expressed throughout a given metaplastic gland but not in surrounding glands), suggesting a differentiation-specific expression pattern that may have developed from scattered SIX1-positive founder cells present in early development. SIX1 was most prominent in glands with basal-type cells underlying luminal columnar cells. These findings indicated that endometrial SIX1 expression and epithelial morphology was persistently altered following neonatal exposure to GEN or DES.
Reproductive tract changes resulting from neonatal GEN or DES exposure
To determine how SIX1 expression correlated with uterine histopathological changes, we first characterized the reproductive tract changes at 6, 12, and 18 months. Neonatal exposure to either GEN or DES resulted in histopathological changes consistent with previous reports in this model (16, 17). A description and representative images of these diagnoses are included in Supplementary Figure S1. Neonatal exposure to GEN or DES resulted in a similar spectrum of nonneoplastic uterine abnormalities, including cystic change, adenomyosis, squamous metaplasia, and basal cell metaplasia. Atypical hyperplasia and carcinomas of endometrial glands were also observed in these groups. All of these findings increased with age in GEN- or DES-exposed groups and had a significantly higher incidence compared to control mice by 18 months of age (Table 1). There was also a low incidence of vaginal adenosis in DES-treated mice at each time point.
Basal cell metaplasia was a prominent feature in the endometrium of GEN- or DES-exposed mice at all time points. This change was characterized by the presence of cuboidal basal epithelial cells underlying columnar glandular epithelial cells (beyond the endocervix), which gave these endometrial glands a distinctive bilaminar appearance (Supplementary Fig. S1E). Metaplastic basal cells did not have a high proliferation rate as compared to normal endometrial glandular epithelium based on labeling with the proliferation marker Ki67. Basal cell metaplasia was distinguished from squamous metaplasia by the lack of a clear maturation lineage of stratified squamous cells but appeared to be the morphologic precursor of squamous metaplasia. Notably, both basal cell and squamous metaplasia were prominent features of atypical hyperplasias and carcinomas in GEN- or DES-exposed mice. Previously, these neoplastic lesions were diagnosed as adenocarcinomas because neoplastic cells consistently form rudimentary gland-like structures with central lumens (16, 17). However, these cancers often showed pleomorphic differentiation patterns, including distinct squamous-like features and, less commonly, mucous cell populations. According to current nomenclature, a subset of more squamous neoplastic lesions would qualify as adenosquamous carcinomas.
Uterine SIX1 expression is associated with development of endometrial carcinoma and localized to neoplastic lesions
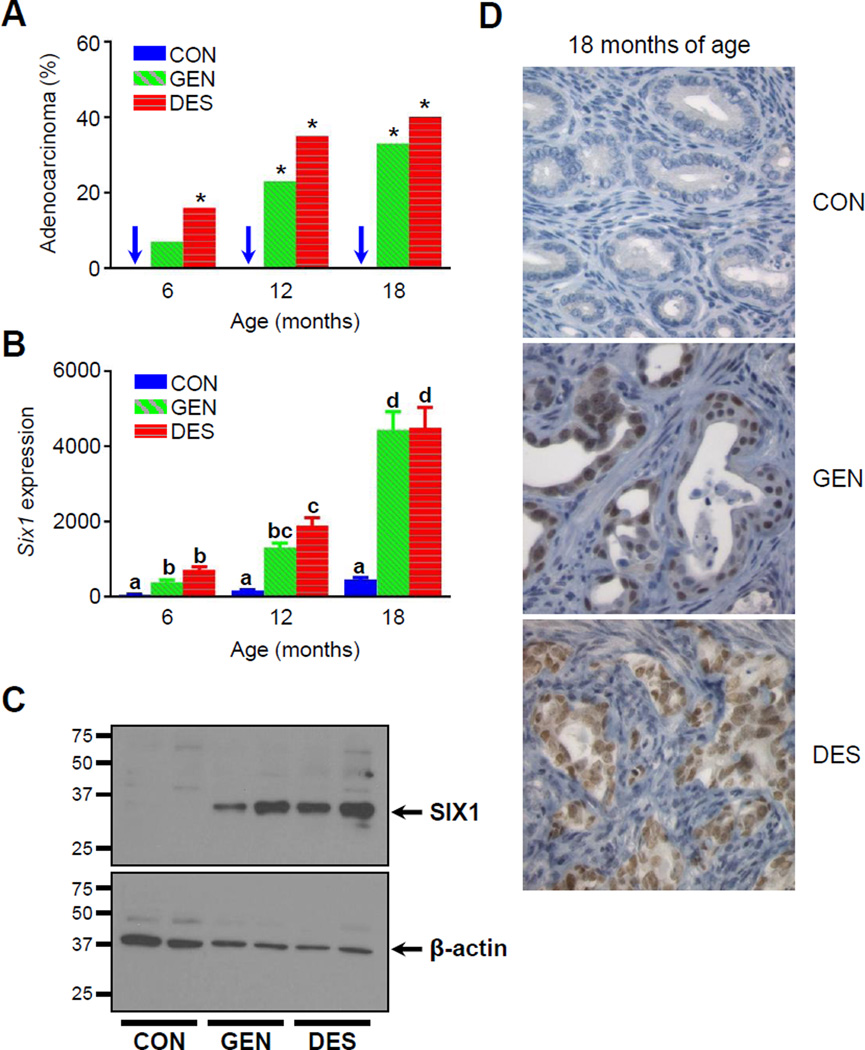
To investigate if Six1 expression correlated with increasing cancer incidence in mice neonatally exposed to GEN or DES, we measured Six1 transcript levels by quantitative PCR at 6, 12, and 18 months of age, and confirmed protein expression by immunoblot at 6 months of age. Endometrial carcinomas were not observed in control mice at any time point, while marked increases in carcinoma incidence were observed in GEN- or DES-exposed groups at 12 and 18 months of age (Fig. 2A; Table 1). Consistent with our previous results, uteri from control mice expressed low levels of Six1 transcript at all time points (20), likely due to normal expression in the myometrium (22, 28). Six1 transcript levels increased with age in GEN- or DES-exposed groups, with >9-fold increases at 18 months of age (Fig. 2B). Uterine SIX1 protein expression was also observed in mice exposed neonatally with GEN or DES but not in control mice (Fig. 2C).
Figure 2.
Association between development of endometrial carcinoma and SIX1 expression in mice neonatally exposed to GEN or DES. A, Incidence of endometrial carcinoma over time in aged CON, GEN, or DES groups. n=26–31 mice per treatment and age group. One-tailed Fisher’s Exact Test, *p<0.05 compared to corresponding age-matched CON group. B, Six1 transcript expression in aged CON, GEN, and DES groups. n=27–33 mice per treatment and age group; mean ± S.E.M. is plotted. Two-way ANOVA with Tukey’s test for multiple comparison (a–d), p=0.0001. C, SIX1 immunoblotting of whole uterine horn tissue from two individual CON, GEN, and DES mice at 6 months of age; protein from one mouse per lane; n=4 mice per group in two blots. D, SIX1 immunolabeling in endometrial carcinoma lesions from neonatally GEN- or DES-exposed mice at 18 months of age. Normal endometrium from CON mouse at 18 months of age shown for comparison. Images were taken at an objective magnification of 40×.
We next evaluated SIX1 protein localization over time in GEN- or DES-exposed mice. By 18 months of age, all mice neonatally exposed to GEN or DES had SIX1 immunolabeling specifically within nonneoplastic glands that exhibited basal cell or squamous metaplasia (Table 1). SIX1 expression was present in both basal and luminal cells; however, luminal expression was typically present only when there were subjacent SIX1-positive basal cells. All hyperplastic and neoplastic lesions showed positive nuclear labeling for SIX1, which was present in areas of glandular, basal cell, and squamous differentiation (Fig. 2D, Table 1). In sum, SIX1 expression localized to regions of abnormal differentiation (metaplastic basal cells), luminal cells adjacent to aberrant basal cells, hyperplastic lesions, and carcinomas, findings that are consistent with a role for SIX1 in malignant transformation.
SIX1 is expressed in human endometrial cancers and correlates with late-stage cancer
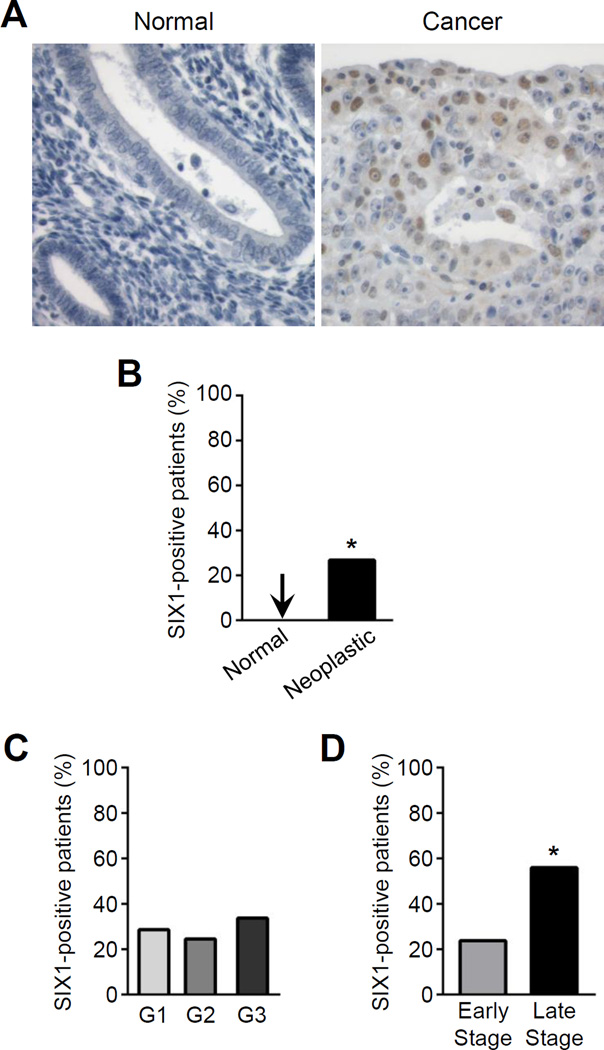
SIX1 overexpression is observed in a number of primary human cancers, where it is associated with recurrence, metastasis, resistance to standard chemotherapeutic agents, and decreased patient survival (22, 23, 29–33). Our findings in the mouse suggested that aberrant SIX1 expression could have a role in human endometrial cancer, but to date there are no published reports that have addressed this question. To test if SIX1 expression is a feature of human endometrial cancers, we evaluated SIX1 immunolabeling in human endometrial tissue microarrays containing biopsies from patients with normal, pathologically abnormal but nonneoplastic, preneoplastic, and neoplastic endometrial tissue (Fig. 3; Table 2). A total of 580 core biopsies from 369 patients (1–4 core biopsies per patient) were evaluated. All patients had at least one core containing glandular epithelium identifiable by morphology and positive CK18 immunolabeling. Patients with at least one core biopsy with SIX1 immunolabeling were considered SIX1-positive patients; an example is shown in Figure 3A. Patients lacking SIX1 immunolabeling in all of their core biopsies were considered SIX1-negative patients. Twenty-two percent (81/369) of patients were classified as SIX1-positive (Table 2). Of the 81 SIX1-positive patients, 63 had 100% SIX1 positive cores and 18 had 50–75% SIX1 positive cores. These findings indicate that patients with fewer core biopsies were not more likely to be falsely assigned as SIX1-negative. SIX1 immunolabeling was generally low-intensity with pockets of moderate- to high-intensity positive cells. SIX1 immunolabeling was only detected in preneoplastic and neoplastic tissue from endometrial cancer patients and was not observed in any other types of endometrial tissue (Fig. 3B; Table 2). SIX1 labeling was not specific to a particular morphologic subtype of endometrial cancer analyzed (Table 2). There was no difference in average age or BMI between SIX1-positive and SIX1-negative patients (data not shown). Together, these data indicate that SIX1 expression is specific to a molecular subset of endometrial cancers.
Figure 3.
SIX1 immunolabeling in human endometrial cancers. A, Normal endometrial tissue (left) and endometrial carcinoma (right). Images were taken at an objective magnification of 40×. B, Percentage of patients with SIX1 immunolabeling; n=28 normal patients and n=299 cancer patients. Two-tailed Fisher's Exact Test, *p=0.0025. C,D, Percentage of patients with SIX1 immunolabeling separated by C, grade; n=84 for G1, n=110 for G2, and n=83 for G3 and D, stage; n=248 for early and n=34 for late. Two-tailed Fisher's Exact Test, *p=0.0003.
Table 2.
Incidence of endometrial SIX1 expression in patients with different histopathological diagnoses
| Category | Diagnosis | # Patients | # SIX1- Positive |
# SIX1- Negative |
%SIX1- Positive |
|---|---|---|---|---|---|
| Normal | Normal | 28 | 0 | 28 | 0% |
| Nonneoplastic | Normal cancer adjacent tissue | 13 | 0 | 13 | 0% |
| Hyperplasia | 21 | 0 | 21 | 0% | |
| Inflammation | 5 | 0 | 5 | 0% | |
| Preneoplastic | Atypical hyperplasia | 3 | 1 | 2 | 33% |
| Neoplastic | Endometrioid adenocarcinoma | 171 | 43 | 128 | 25% |
| Unspecified adenocarcinoma | 87 | 28 | 59 | 32% | |
| Serous carcinoma | 11 | 4 | 7 | 36% | |
| Clear cell carcinoma | 4 | 0 | 4 | 0% | |
| Mucinous carcinoma | 1 | 1 | 0 | 100% | |
| Adenocarcinoma metastasis | 5 | 0 | 5 | 0% | |
| Adenosquamous carcinoma | 7 | 1 | 6 | 14% | |
| Squamous carcinoma | 8 | 3 | 5 | 38% | |
| Undifferentiated carcinoma | 2 | 0 | 2 | 0% | |
| Stromal sarcoma | 1 | 0 | 1 | 0% | |
| Chorionic carcinoma | 2 | 0 | 2 | 0% |
To test if SIX1 immunolabeling was associated with cancer progression and metastasis, we grouped cancer patients by grade and stage. Cancer grade, ranging from grade 1–3 (G1–3), was provided for 277/299 endometrial cancer patients (79/80 SIX1-positive and 198/219 SIX1-negative patients). When cancer patients were grouped by their defined grade, the percentage of cancer patients with SIX1 immunolabeling was similar between cancer grades (Fig. 3C). Cancer stage information was provided for 282/299 endometrial cancer patients (78/80 SIX1-positive and 204/219 SIX1-negative patients). SIX1-positive cancer patients with defined stages were separated into two stage categories for analysis, early-stage (I–II) and late-stage (III–IV), because there were few patients with either stage II or IV cancers. The percentage of cancer patients with SIX1 immunolabeling was higher in late-stage as compared to early-stage patients (Fig. 3D). These findings indicate that patients with SIX1-positive cancers were also more likely to have late-stage disease.
Discussion
Here we demonstrated that neonatally exposing mice to GEN or DES induces permanent uterine expression of the oncofetal protein SIX1. Transcript and protein levels of SIX1 increased with age and were associated with endometrial carcinoma incidence. SIX1 expression was localized to an abnormal population of basal epithelial cells within metaplastic endometrial glands and to glandular, basal, and squamous cells within all endometrial hyperplastic lesions and carcinomas. These findings indicate that neonatal estrogenic chemical exposure initiates aberrant expression of SIX1 within the endometrium, which over time may promote malignant transformation.
Previous work indicates that neonatal exposure to an estrogenic chemical alone does not cause uterine cancer in this mouse model; instead, subsequent exposure to ovarian hormones is required (16). These findings indicate that endogenous ovarian hormones secreted following puberty play an essential role in cancer development, likely a result of estrogen action (18). We previously showed that pre-treatment with an ER antagonist blocks neonatal GEN-induced Six1 transcript expression (19). Additionally, exposing adult control mice to estradiol or DES does not induce uterine Six1 expression (20). Taken together, these data suggest that neonatal estrogenic chemical exposure induces Six1 expression and reprograms Six1 to be responsive to later estrogen exposure. This estrogen sensitivity is likely mediated by epigenetic changes induced by neonatal estrogen exposure at the Six1 gene locus (20). The timeline of SIX1 expression and cancer development thus fits with a multi-hit cancer hypothesis.
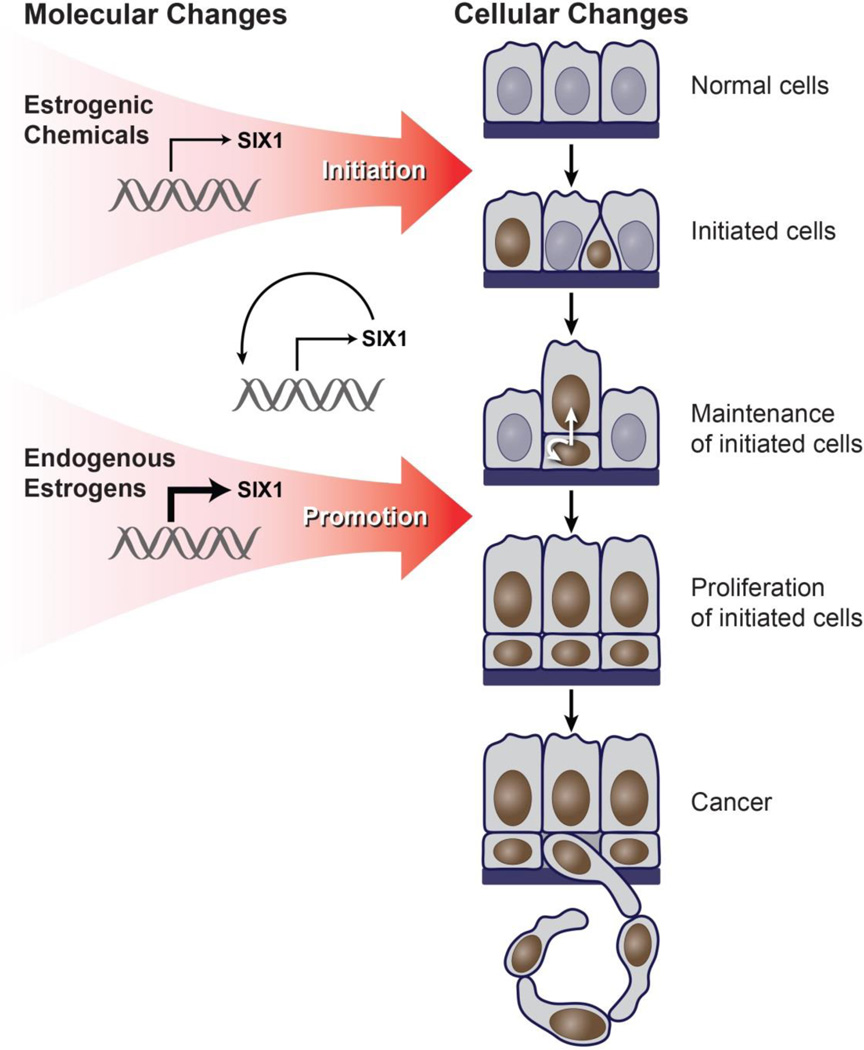
To explain neonatal estrogen-induced malignant transformation of the endometrium, we propose a model outlining the developmental origins and progression of hormonal carcinogenesis (Fig. 4). A critical component of the model is that exposure during a sensitive developmental period is essential to establish aberrant constitutive expression of SIX1 and serves as a molecular initiating event. Neonatal estrogenic chemical exposure initiates aberrant SIX1 expression in cells that continue to express SIX1 as a result of positive autoregulation (34, 35). Persistent SIX1 expression causes the establishment of a basal cell population that gives the endometrial epithelium a distinct bilaminar morphology. SIX1 localization in luminal cells appears to be dependent upon the abnormal presence of underlying SIX1-positive basal cells, suggesting the basal cells either differentiate into SIX1-positive luminal cells or facilitate luminal SIX1 expression via cell-to-cell communication. Subsequent cyclic exposure to endogenous estrogen, beginning at puberty, promotes SIX1 expression in these cells through its acquired estrogen responsiveness. SIX1 then causes proliferation of the basal cells through its effects on cell cycle-regulatory proteins and resistance to apoptosis (30, 31, 36–38). These cells can undergo further transformation (39–46). This neoplastic process occurs specifically in the endometrial epithelium, which lacks normal location-specific growth restraints that may be present in the stratified squamous epithelium of the lower reproductive tract. Future studies are needed to investigate factors that drive malignant transformation specifically in the endometrium but not in other sites like cervical and vaginal epithelium, where SIX1 is expressed but does not promote cancer in the neonatal mouse model.
Figure 4.
Multi-hit model outlining the developmental origins and progression of estrogen-induced hormonal carcinogenesis. The model is described in detail in the text. Blue nuclei indicate cells that do not express SIX1 and brown nuclei indicate cells that express SIX1.
Recent studies across different cancer types have implicated SIX1 in several key carcinogenic processes. These hallmarks include sustained proliferative cell signaling, invasion and metastasis, evasion of growth suppressors, induction of genomic instability, and resistance to cell death (23, 47). In pancreatic, rhabdomyosarcoma, and breast cancer cell lines, SIX1 modulates cell cycle progression by direct transcriptional regulation of cyclins (36–38). SIX1 also indirectly downregulates p53 in breast cancer cell lines (30). Furthermore, mammary gland-specific SIX1 overexpression in a transgenic mouse model induces mammary gland tumors (40). The potential pleiotropic roles of SIX1 in the uterus following neonatal estrogenic chemical exposure are unknown, but SIX1 binding partners, including eyes absent 1–4, dachshund 1 and 2, and CREB binding protein, are expressed in the mouse FRT based on microarray data, suggesting that SIX1 is regulating transcriptional activity in the uterus (19, 23). Determining if SIX1 is necessary or sufficient for endometrial carcinoma development will require additional studies using mouse genetic models.
We showed that SIX1 was expressed in a subset of human endometrial cancers, suggesting that this biomarker may define a molecular subtype. A similar incidence of aberrant upregulated SIX1 expression has been reported in cervix cancer (48). We found that SIX1 was not differentially expressed in endometrial cancers based on their specific histological classifications, similar to previous observations in breast and cervix cancers (32, 49). Because SIX1 overexpression in human endometrial and other cancers may represent a loss of differentiation or reversion to a developmental phenotype, SIX1 may be acting as an oncofetal protein (37, 38). The diverse morphologic features represented in neoplastic endometrial glands in our mouse model, which included glandular, squamous, and mucous cell differentiation, support this idea. Similarly, histologically diverse neoplastic lesions were observed in a mouse model of mammary gland-specific transgenic SIX1 expression (40), suggesting multipotency of aberrant SIX1-positive cells. We did not observe a correlation between SIX1 expression and cancer grade, which has been observed in previous studies of prostate cancer (33), but did find that SIX1 was expressed more often in endometrial cancers from patients with later stage disease. This finding is consistent with studies showing that SIX1 expression correlates with advanced cancer stage in prostate, ovarian, cervix, and breast cancers (33, 48, 50, 51) and with effects of SIX1 in promoting cell migration, invasion, and metastasis by upregulating pro-tumorigenic genes including tumor growth factor beta, vascular endothelial growth factor-c, and ezrin (32, 36, 41, 44).
Findings from this study indicate that in a mouse model of hormonal carcinogenesis, exposure to estrogenic chemicals during a key period of reproductive tract cell differentiation results in the establishment of a new cell type within the endometrial glands that aberrantly expresses SIX1 and is predisposed to endometrial carcinoma development. Additionally, SIX1 is specifically expressed in a subset of human endometrial cancers. Together, these findings indicate that SIX1 may play a role in endometrial carcinogenesis in mice and women and that SIX1 may serve as a biomarker of aberrant response to estrogen and a molecular subtype of endometrial cancer.
Supplementary Material
Acknowledgments
We thank Dominique Chevalier, Heather Jensen, and Sara Wobker for technical assistance, and Yin Li and Harriet Kinyamu for critical review of the manuscript. This work was supported by the Intramural Research Program of the National Institutes of Health, National Institutes of Environmental Health Sciences, 1ZIAES102985 (CJW), the Steelman Fund (VLBJ), and the NIH/NCI K23 Mentored Patient-Oriented Research Career Development Grant, 1K23CA143154-01A1 (VLBJ). Alisa Suen was supported in part by the UNC Environmental Health Sciences Toxicology Training Grant, T32-ES007126. The research described in this article has been reviewed by the U.S. EPA and approved for publication. Approval does not signify that the contents necessarily reflect the views or the policies of the Agency. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Footnotes
Conflict of interest statement: The authors disclose no potential conflicts of interest.
References
- 1.Masse J, Watrin T, Laurent A, Deschamps S, Guerrier D, Pellerin I. The developing female genital tract: from genetics to epigenetics. Int J Dev Biol. 2009;53(2–3):411–424. doi: 10.1387/ijdb.082680jm. [DOI] [PubMed] [Google Scholar]
- 2.Kurita T. Normal and abnormal epithelial differentiation in the female reproductive tract. Differentiation. 2011;82(3):117–126. doi: 10.1016/j.diff.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker CL, Ho SM. Developmental reprogramming of cancer susceptibility. Nature reviews Cancer. 2012;12(7):479–486. doi: 10.1038/nrc3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schug TT, Janesick A, Blumberg B, Heindel JJ. Endocrine disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol. 2011;127(3–5):204–215. doi: 10.1016/j.jsbmb.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. The New England journal of medicine. 1971;284(15):878–881. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- 6.Giusti RM, Iwamoto K, Hatch EE. Diethylstilbestrol revisited: a review of the long-term health effects. Annals of internal medicine. 1995;122(10):778–788. doi: 10.7326/0003-4819-122-10-199505150-00008. [DOI] [PubMed] [Google Scholar]
- 7.Reed CE, Fenton SE. Exposure to diethylstilbestrol during sensitive life stages: a legacy of heritable health effects. Birth defects research Part C, Embryo today : reviews. 2013;99(2):134–146. doi: 10.1002/bdrc.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Troisi R, Hatch EE, Titus-Ernstoff L, Hyer M, Palmer JR, Robboy SJ, et al. Cancer risk in women prenatally exposed to diethylstilbestrol. International journal of cancer Journal international du cancer. 2007;121(2):356–360. doi: 10.1002/ijc.22631. [DOI] [PubMed] [Google Scholar]
- 9.IARC. Overall evaluations of carcinogenicity: an updating of IARC Monographs volumes 1 to 42. IARC monographs on the evaluation of carcinogenic risks to humans Supplement / World Health Organization, International Agency for Research on Cancer. 1987;7:1–440. [PubMed] [Google Scholar]
- 10.IARC. Pharmaceuticals Volume 100 A. A review of human carcinogens. IARC monographs on the evaluation of carcinogenic risks to humans / World Health Organization, International Agency for Research on Cancer. 2012;100(Pt A):1–401. [Google Scholar]
- 11.Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet. 1997;350(9070):23–27. doi: 10.1016/S0140-6736(96)09480-9. [DOI] [PubMed] [Google Scholar]
- 12.Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Isoflavone content of infant formulas and the metabolic fate of these phytoestrogens in early life. The American journal of clinical nutrition. 1998;68(6 Suppl):1453s–1461s. doi: 10.1093/ajcn/68.6.1453S. [DOI] [PubMed] [Google Scholar]
- 13.McCarver G, Bhatia J, Chambers C, Clarke R, Etzel R, Foster W, et al. NTP-CERHR expert panel report on the developmental toxicity of soy infant formula. Birth defects research Part B, Developmental and reproductive toxicology. 2011;92(5):421–468. doi: 10.1002/bdrb.20314. [DOI] [PubMed] [Google Scholar]
- 14.Jefferson WN, Williams CJ. Circulating levels of genistein in the neonate, apart from dose and route, predict future adverse female reproductive outcomes. Reprod Toxicol. 2011;31(3):272–279. doi: 10.1016/j.reprotox.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooke PS, Spencer TE, Bartol FF, Hayashi K. Uterine glands: development, function and experimental model systems. Mol Hum Reprod. 2013;19(9):547–558. doi: 10.1093/molehr/gat031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newbold RR, Bullock BC, McLachlan JA. Uterine adenocarcinoma in mice following developmental treatment with estrogens: a model for hormonal carcinogenesis. Cancer research. 1990;50(23):7677–7681. [PubMed] [Google Scholar]
- 17.Newbold RR, Banks EP, Bullock B, Jefferson WN. Uterine adenocarcinoma in mice treated neonatally with genistein. Cancer research. 2001;61(11):4325–4328. [PubMed] [Google Scholar]
- 18.Ostrander PL, Mills KT, Bern HA. Long-term responses of the mouse uterus to neonatal diethylstilbestrol treatment and to later sex hormone exposure. Journal of the National Cancer Institute. 1985;74(1):121–135. [PubMed] [Google Scholar]
- 19.Jefferson WN, Padilla-Banks E, Phelps JY, Gerrish KE, Williams CJ. Permanent oviduct posteriorization after neonatal exposure to the phytoestrogen genistein. Environmental health perspectives. 2011;119(11):1575–1582. doi: 10.1289/ehp.1104018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jefferson WN, Chevalier DM, Phelps JY, Cantor AM, Padilla-Banks E, Newbold RR, et al. Persistently altered epigenetic marks in the mouse uterus after neonatal estrogen exposure. Molecular endocrinology (Baltimore, Md) 2013;27(10):1666–1677. doi: 10.1210/me.2013-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christensen KL, Patrick AN, McCoy EL, Ford HL. The six family of homeobox genes in development and cancer. Advances in cancer research. 2008;101:93–126. doi: 10.1016/S0065-230X(08)00405-3. [DOI] [PubMed] [Google Scholar]
- 22.Wu W, Ren Z, Li P, Yu D, Chen J, Huang R, et al. Six1: a critical transcription factor in tumorigenesis. International journal of cancer Journal international du cancer. 2015;136(6):1245–1253. doi: 10.1002/ijc.28755. [DOI] [PubMed] [Google Scholar]
- 23.Blevins MA, Towers CG, Patrick AN, Zhao R, Ford HL. The SIX1-EYA transcriptional complex as a therapeutic target in cancer. Expert opinion on therapeutic targets. 2015;19(2):213–225. doi: 10.1517/14728222.2014.978860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Creasman WT. 2015 Endometrial Cancer Staging. < http://emedicinemedscapecom/article/2006791-overview>. [Google Scholar]
- 25.Dixon D, Alison R, Bach U, Colman K, Foley GL, Harleman JH, et al. Nonproliferative and proliferative lesions of the rat and mouse female reproductive system. Journal of toxicologic pathology. 2014;27(3–4 Suppl):1s–107s. doi: 10.1293/tox.27.1S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic acids research. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida A, Newbold RR, Dixon D. Effects of neonatal diethylstilbestrol (DES) exposure on morphology and growth patterns of endometrial epithelial cells in CD-1 mice. Toxicol Pathol. 1999;27(3):325–333. doi: 10.1177/019262339902700308. [DOI] [PubMed] [Google Scholar]
- 28.El-Hashash AH, Al Alam D, Turcatel G, Rogers O, Li X, Bellusci S, et al. Six1 transcription factor is critical for coordination of epithelial, mesenchymal and vascular morphogenesis in the mammalian lung. Developmental biology. 2011;353(2):242–258. doi: 10.1016/j.ydbio.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z, Tian T, Hu X, Zhang X, Nan F, Chang Y, et al. Six1 mediates resistance to paclitaxel in breast cancer cells. Biochemical and biophysical research communications. 2013;441(3):538–543. doi: 10.1016/j.bbrc.2013.10.131. [DOI] [PubMed] [Google Scholar]
- 30.Towers CG, Guarnieri AL, Micalizzi DS, Harrell JC, Gillen AE, Kim J, et al. The Six1 oncoprotein downregulates p53 via concomitant regulation of RPL26 and microRNA-27a-3p. Nat Commun. 2015;6:10077. doi: 10.1038/ncomms10077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armat M, Oghabi Bakhshaiesh T, Sabzichi M, Shanehbandi D, Sharifi S, Molavi O, et al. The role of Six1 signaling in paclitaxel-dependent apoptosis in MCF-7 cell line. Bosnian journal of basic medical sciences / Udruzenje basicnih mediciniskih znanosti = Association of Basic Medical Sciences. 2016 doi: 10.17305/bjbms.2016.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwanaga R, Wang CA, Micalizzi DS, Harrell JC, Jedlicka P, Sartorius CA, et al. Expression of Six1 in luminal breast cancers predicts poor prognosis and promotes increases in tumor initiating cells by activation of extracellular signal-regulated kinase and transforming growth factor-beta signaling pathways. Breast Cancer Res. 2012;14(4):R100. doi: 10.1186/bcr3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng J, Shi R, Cai CX, Liu XR, Song YB, Wei M, et al. Increased expression of Six1 correlates with progression and prognosis of prostate cancer. Cancer cell international. 2015;15:63. doi: 10.1186/s12935-015-0215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato S, Ikeda K, Shioi G, Nakao K, Yajima H, Kawakami K. Regulation of Six1 expression by evolutionarily conserved enhancers in tetrapods. Developmental biology. 2012;368(1):95–108. doi: 10.1016/j.ydbio.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 35.Grifone R, Demignon J, Houbron C, Souil E, Niro C, Seller MJ, et al. Six1 and Six4 homeoproteins are required for Pax3 and Mrf expression during myogenesis in the mouse embryo. Development (Cambridge, England) 2005;132(9):2235–2249. doi: 10.1242/dev.01773. [DOI] [PubMed] [Google Scholar]
- 36.Yu Y, Davicioni E, Triche TJ, Merlino G. The homeoprotein six1 transcriptionally activates multiple protumorigenic genes but requires ezrin to promote metastasis. Cancer research. 2006;66(4):1982–1989. doi: 10.1158/0008-5472.CAN-05-2360. [DOI] [PubMed] [Google Scholar]
- 37.Li Z, Tian T, Lv F, Chang Y, Wang X, Zhang L, et al. Six1 promotes proliferation of pancreatic cancer cells via upregulation of cyclin D1 expression. PloS one. 2013;8(3):e59203. doi: 10.1371/journal.pone.0059203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coletta RD, Christensen K, Reichenberger KJ, Lamb J, Micomonaco D, Huang L, et al. The Six1 homeoprotein stimulates tumorigenesis by reactivation of cyclin A1. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(17):6478–6483. doi: 10.1073/pnas.0401139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coletta RD, Christensen KL, Micalizzi DS, Jedlicka P, Varella-Garcia M, Ford HL. Six1 overexpression in mammary cells induces genomic instability and is sufficient for malignant transformation. Cancer research. 2008;68(7):2204–2213. doi: 10.1158/0008-5472.CAN-07-3141. [DOI] [PubMed] [Google Scholar]
- 40.McCoy EL, Iwanaga R, Jedlicka P, Abbey NS, Chodosh LA, Heichman KA, et al. Six1 expands the mouse mammary epithelial stem/progenitor cell pool and induces mammary tumors that undergo epithelial-mesenchymal transition. The Journal of clinical investigation. 2009;119(9):2663–2677. doi: 10.1172/JCI37691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Micalizzi DS, Christensen KL, Jedlicka P, Coletta RD, Baron AE, Harrell JC, et al. The Six1 homeoprotein induces human mammary carcinoma cells to undergo epithelial-mesenchymal transition and metastasis in mice through increasing TGF-beta signaling. The Journal of clinical investigation. 2009;119(9):2678–2690. doi: 10.1172/JCI37815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ono H, Imoto I, Kozaki K, Tsuda H, Matsui T, Kurasawa Y, et al. SIX1 promotes epithelial-mesenchymal transition in colorectal cancer through ZEB1 activation. Oncogene. 2012;31(47):4923–4934. doi: 10.1038/onc.2011.646. [DOI] [PubMed] [Google Scholar]
- 43.Radisky DC. Defining a role for the homeoprotein Six1 in EMT and mammary tumorigenesis. The Journal of clinical investigation. 2009;119(9):2528–2531. doi: 10.1172/JCI40555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang CA, Jedlicka P, Patrick AN, Micalizzi DS, Lemmer KC, Deitsch E, et al. SIX1 induces lymphangiogenesis and metastasis via upregulation of VEGF-C in mouse models of breast cancer. The Journal of clinical investigation. 2012;122(5):1895–1906. doi: 10.1172/JCI59858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeng J, Wei M, Shi R, Cai C, Liu X, Li T, et al. MiR-204-5p/Six1 feedback loop promotes epithelial-mesenchymal transition in breast cancer. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2015 doi: 10.1007/s13277-015-4039-1. [DOI] [PubMed] [Google Scholar]
- 46.Cieply B, Farris J, Denvir J, Ford HL, Frisch SM. Epithelial-mesenchymal transition and tumor suppression are controlled by a reciprocal feedback loop between ZEB1 and Grainyhead-like-2. Cancer research. 2013;73(20):6299–6309. doi: 10.1158/0008-5472.CAN-12-4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 48.Wan F, Miao X, Quraishi I, Kennedy V, Creek KE, Pirisi L. Gene expression changes during HPV-mediated carcinogenesis: a comparison between an in vitro cell model and cervical cancer. International journal of cancer Journal international du cancer. 2008;123(1):32–40. doi: 10.1002/ijc.23463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng XH, Liang PH, Guo JX, Zheng YR, Han J, Yu LL, et al. Expression and clinical implications of homeobox gene Six1 in cervical cancer cell lines and cervical epithelial tissues. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2010;20(9):1587–1592. [PubMed] [Google Scholar]
- 50.Behbakht K, Qamar L, Aldridge CS, Coletta RD, Davidson SA, Thorburn A, et al. Six1 overexpression in ovarian carcinoma causes resistance to TRAIL-mediated apoptosis and is associated with poor survival. Cancer research. 2007;67(7):3036–3042. doi: 10.1158/0008-5472.CAN-06-3755. [DOI] [PubMed] [Google Scholar]
- 51.Reichenberger KJ, Coletta RD, Schulte AP, Varella-Garcia M, Ford HL. Gene amplification is a mechanism of Six1 overexpression in breast cancer. Cancer research. 2005;65(7):2668–2675. doi: 10.1158/0008-5472.CAN-04-4286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.