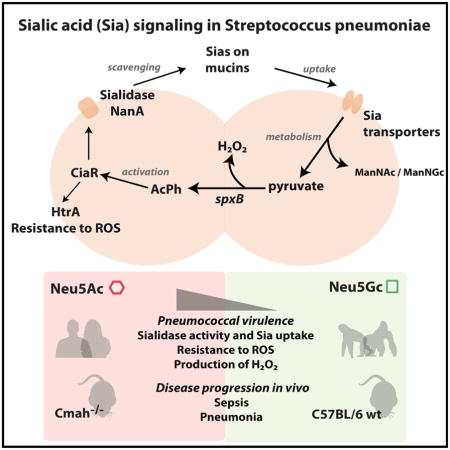
Summary
Streptococcus pneumoniae is a human-adapted pathogen that encounters terminally sialylated glycoconjugates and free sialic acid (Sia) in the airways. Upon scavenging by the bacterial sialidase NanA, Sia products serve as carbon sources for the bacteria. Unlike most animals in which cytidine-monophosphate-N-acetylneuraminic acid hydroxylase (CMAH) converts Sia N-acetylneuraminic acid (Neu5Ac) into N-glycolylneuraminic acid (Neu5Gc), humans have an inactive CMAH, causing an absence of Neu5Gc and excess Neu5Ac. We find that pneumococcal challenge in Cmah−/− mice leads to heightened bacterial loads, virulence, and NanA expression. In vitro, NanA is upregulated in response to Neu5Ac compared to Neu5Gc, a process controlled by two-component response regulator CiaR and requiring Sia uptake by the transporter SatABC. Additionally, compared to Neu5Gc, Neu5Ac increases pneumococcal resistance to antimicrobial reactive oxygen species in a CiaR-dependant manner. Thus, S. pneumoniae senses and responds to Neu5Ac, leading to CiaR activation and increased virulence and potentially explaining greater susceptibility in humans.
eTOC
S. pneumoniae infects human lungs, which have abundant levels of sialic acid Neu5Ac due to inactivation of a hydroxylase. Hentrich et al. show that the pneumococcal response regulator CiaR responds to Neu5Ac, resulting in the upregulation of genes involved in sialic acid metabolism and signaling and leading to increased virulence.
Introduction
Streptococcus pneumoniae (the pneumococcus) is a Gram-positive bacterium adapted to the human upper respiratory tract, especially predominant in pre-school age children and those attending day care centers. S. pneumoniae causes diseases ranging from milder respiratory tract infections such as sinusitis and otitis media, to severe diseases like pneumonia, septicemia and meningitis. Pneumococcal infections are a major threat to human health, causing about 11% of all deaths among children below the age of five (O’Brien et al., 2009).
When entering the human nasopharynx, S. pneumoniae encounters mucus, which contains glycoconjugates displaying sialic acids (Sias) as terminal monosaccharides. In the nasopharynx, glucose is scarce as carbon source. Therefore, colonizing pneumococci need to acquire carbohydrates from glycoconjugates, a process that involves removal of terminal Sias, which themselves can be used as a carbon source for the bacteria (Burnaugh et al., 2008; King et al., 2004; Traving and Schauer, 1998). In S. pneumoniae, the genes involved in Sia retrieval, uptake and metabolism, are encoded in the nanAB locus (King et al., 2004; Vimr et al., 2004), and in 51% of all pneumococci also in the nanC locus (Pettigrew et al., 2006). The nanAB locus contains genes encoding sialidases, such as NanA, and the main Sia transporter SatABC (King et al., 2004; Marion et al., 2011). Furthermore, the locus contains catabolite repression elements (cre), leading to transcriptional inhibition of genes within the locus in the presence of glucose (Afzal et al., 2015; Gualdi et al., 2012). NanA scavenges Sias from host glycoconjugates (King et al., 2006; Tong et al., 2002), and is a known pneumococcal virulence factor promoting colonization of the nasopharynx and lungs (Orihuela et al., 2004), invasion of the brain endothelium (Uchiyama et al., 2009), cytokine release in human monocytes, and NET formation of human neutrophils (Chang et al., 2012). Thus, NanA plays an important role in the activation of the innate immune response. Besides Sia removal, Sia uptake by SatABC promotes pneumococcal virulence (Marion et al., 2011). The most abundant Sias in mammals are Neu5Ac (N-acetylneuraminic acid) and Neu5Gc (N-glycolylneuraminic acid). The enzyme CMP-Neu5Ac hydroxylase (CMAH) converts CMP-Neu5Ac to CMP-Neu5Gc in most mammals, by addition of a single oxygen atom (Shaw and Schauer, 1989). While humans have a fixed exon deletion in the gene encoding CMAH, and therefore only synthesize Neu5Ac, most other mammals including old world primates tend to have a predominance of Neu5Gc on their cell surfaces and secreted glycoconjugates (Chou et al., 1998; Muchmore et al., 1998). Recently, ferrets and New World Monkeys have been shown to have independent inactivation of CMAH in their evolution (Ng et al., 2014; Springer et al., 2014). Microbes adhere to Sias during colonization and infection (Traving and Schauer, 1998), and many virulence factors are adapted to preferentially engage Neu5Ac or Neu5Gc, depending on the host species. Plasmodium falciparum and Salmonella Typhi, causative agents of malaria and typhoid fever respectively, were shown to optimize specific virulence mechanisms to the human Sia condition. The toxin of S. Typhi as well as the merozoite of P. falciparum each recognize Neu5Ac, with absent or weak binding to Neu5Gc (Deng et al., 2014; Martin et al., 2005).
Bacteria sense and respond to environmental changes with the help of two-component systems (TCS) consisting of a membrane-bound histidine kinase (HK), and a cytoplasmic response regulator (RR). The HK senses the signal, is autophosphorylated and transfers phosphate to the RR which, in turn, changes its conformation and acts as a transcriptional regulator (Stock et al., 1989). To date, 13 TCS and a single RR have been identified in S. pneumoniae, of which several have been associated with virulence regulation (Throup et al., 2000). TCS05, also known as CiaRH, was identified in a screen for spontaneous cefotaxime-resistant mutants (Guenzi et al., 1994). CiaRH is involved in competence, cefotaxime susceptibility, autolysis, bacteriocin production, resistance to oxidative stress and plays a role in pneumococcal virulence (Dagkessamanskaia et al., 2004; Ibrahim et al., 2004a; Mascher et al., 2006; Mascher et al., 2003; Throup et al., 2000). The RR CiaR directly controls a number of promoters and genes, including the high-temperature requirement A gene htrA, enhancing resistance to oxidative stress, and has been shown to affect transcription of several small non-coding RNAs with different down-stream effects (Halfmann et al., 2007; Marx et al., 2010). Phosphorylation of CiaR is required for its regulatory activity, but internal acetyl phosphate (AcPh) may serve as phosphor-donor besides CiaH (Marx et al., 2014).
In this study, we show that TIGR4 exhibits a differential response to the human form of Sia, Neu5Ac, compared to Neu5Gc, by upregulating sialidase NanA, the main sialic acid transporter SatABC and HtrA in response to Neu5Ac, in contrast to Neu5Gc. This response is dependent on the RR CiaR and leads to bacterial resistance to oxidative stress. Furthermore, mice with a human-like defect in the Cmah gene showed a faster pneumococcal disease progression than C57BL/6 wt mice after intranasal, but not after intravenous, challenge.
Results
Pneumococcal disease progresses faster in human-like Cmah−/− compared to C57BL/6 wild-type mice after intranasal challenge with S. pneumoniae TIGR4
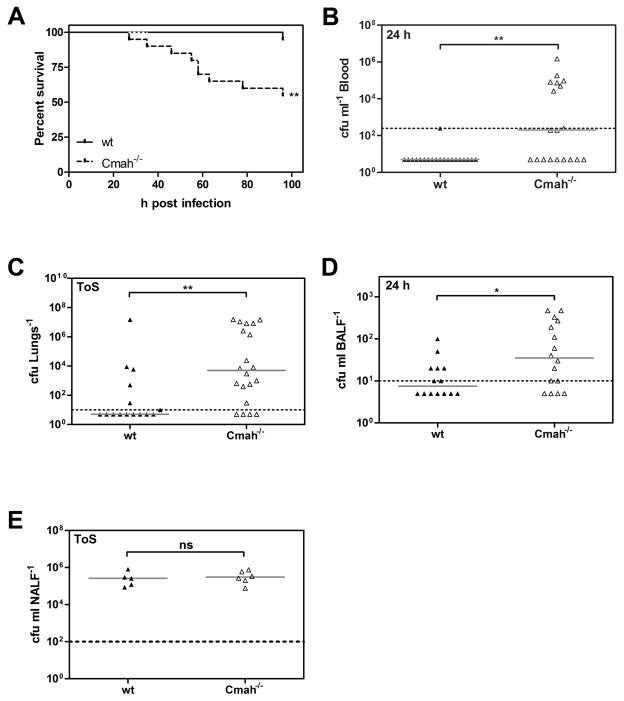
To determine whether the human-adapted pathogen S. pneumoniae is more virulent in response to Neu5Ac than Neu5Gc, we made use of Neu5Gc-deficient Cmah−/− mice, which have a human-like excess of Neu5Ac on the surface of their cells. Using an intranasal challenge with the pneumococcal strain TIGR4 of 3×106 colony forming units (cfu) per animal, disease progressed significantly faster in Cmah−/− compared to wt mice. We observed a drastically lower survival rate (Fig 1A), and a significantly higher rate of septicemia in Cmah−/− compared to wt mice at 24 h post-infection (p.i.) (Fig 1B). Moreover, at 96 h p.i. or at time of sacrifice (ToS) the bacterial burden was higher in the lungs of Cmah−/− compared to wt mice (Fig 1C). Already at 24 h p.i. we observed higher bacterial numbers in the bronchial lavage (BALF) of Cmah−/− compared to wt mice (Fig 1D), even though the nasopharyngeal colonization (NALF) was similar between the mice at 96 h p.i. or at ToS (Fig 1E).
Figure 1. Pneumococcal disease progresses faster in human-like Cmah−/− compared to C57BL/6 wild-type mice after intranasal challenge.
Mice were infected intranasally with S. pneumoniae TIGR4 in 20 μl PBS.
(A) Survival rates were monitored over the course of the experiments.
(B) Bacterial counts in the blood of wt and Cmah−/− mice were measured at 24h post-infection (p.i.). by plating.
(C) Bacterial numbers in the lungs of mice were determined by plating lung homogenates at 96 h p.i. or at time of sacrifice (ToS).
(D) Bacterial counts in bronchial lavages (BALF) of wt and Cmah−/− mice were measured at 24 h p.i. by plating.
(E) Bacterial counts in nasopharyngeal lavages (NALF) of wt and Cmah−/− mice were measured at 96 h p.i. or at ToS by plating (A–E) Dotted lines indicate the detection limits. Horizontal bars represent medians. For survival rates the Log-Rank Mantel-Cox Test and for cfu counts the Mann-Whitney Test were used for statistical analysis. *, p<0.05, **; p<0.01; ***, p<0.001; ns, non-significant. The graph represents 2 independent experiments with at least 10 mice per group. See also related Figure S1.
We infected C57BL/6 wt and Cmah−/− mice intravenously with TIGR4 and found no differences in disease progression between the mice (Supplemental Figure S1), excluding a general immune-deficiency of the Cmah−/− mice.
S. pneumoniae senses sialic acid, leading to an upregulation of sialidase NanA, and the main sialic acid transporter SatABC, in response to the sialic acid Neu5Ac as compared to Neu5Gc
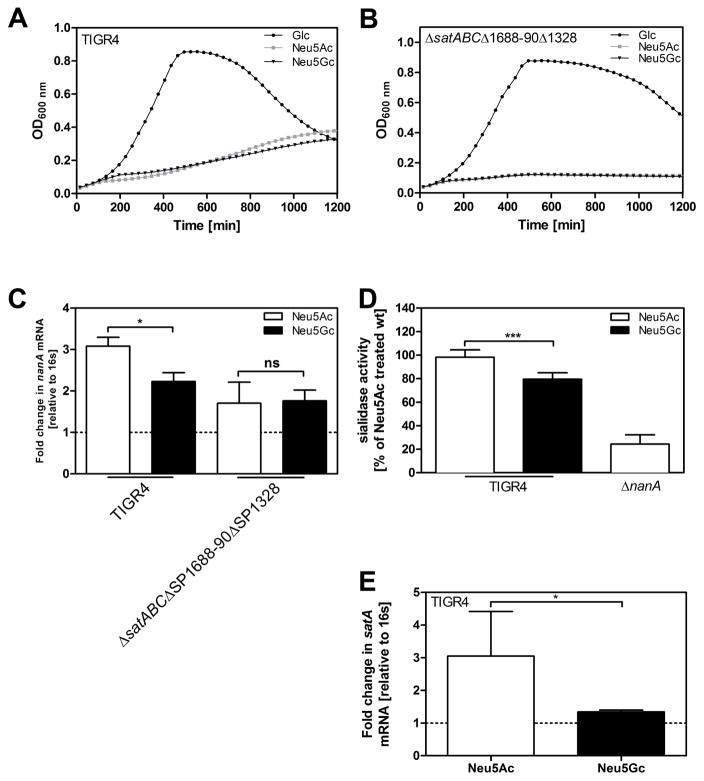
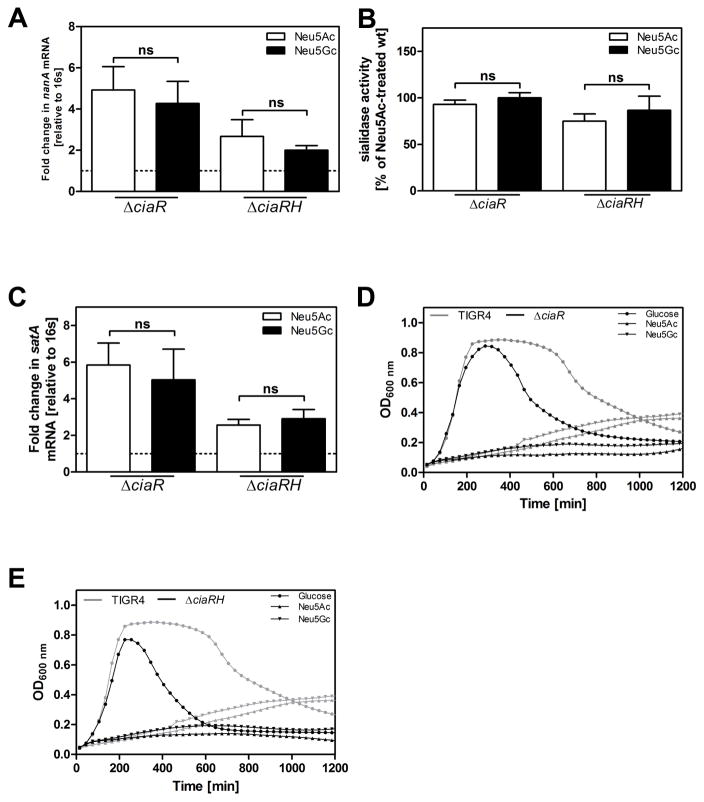
Next we studied whether there are differences in gene regulation between bacteria grown on Neu5Ac or Neu5Gc. We hypothesized that Neu5Ac preferentially activates the established virulence factor NanA, given the higher pneumococcal virulence in Cmah−/− compared to wt mice. We cultured pneumococcal strain TIGR4 in C+Y medium supplemented with either 12mM glucose, Neu5Ac or Neu5Gc. Besides being able to grow on Neu5Ac (Marion et al., 2011), TIGR4 (as well as in its streptomycin resistant derivative TIGR4 Smr) could use Neu5Gc as sole carbon source. Pneumococcal growth rates on either Sia were lower than on glucose, but similar to one another (Fig 2A). Bacterial growth required Sia transport since a mutant lacking all three known Sia transporters, SatABC, SP1688-90 and SP1328, showed strongly reduced growth on both Neu5Ac and Neu5Gc (Fig 2B). Transcription of sialidase NanA was increased in TIGR4 (and in TIGR4 Smr), after incubation with Neu5Ac compared to Neu5Gc. The mutant deficient in sialic transport, TIGR4ΔsatABCΔSP1688-90ΔSP1328, showed no differential transcription of NanA in response to Neu5Ac or Neu5Gc (Fig 2C). In coherence with these results, total sialidase activity was higher in TIGR4 cultured on Neu5Ac than on Neu5Gc. A mutant lacking NanA had residual sialidase activity, probably due to the activity of the sialidases NanB and NanC (Fig 2D). Furthermore, transcription of the substrate binding protein satA of the main sialic acid transporter SatABC was upregulated in response to Neu5Ac compared to Neu5Gc (Fig 2E). Due to catabolite repression (Afzal et al., 2015; Gualdi et al., 2012), nanA and satA were more upregulated in response to Sia after normalization to glucose-treatment compared to treatment without monosaccharide (Supplemental Fig S2A–B).
Figure 2. S. pneumoniae senses sialic acid, leading to an upregulation of sialidase NanA, and the main sialic acid transporter SatABC, in response to the sialic acid Neu5Ac as compared to Neu5Gc.
(A) TIGR4 and (B) TIGR4ΔsatABΔSP1688-90ΔSP1328 were grown on glucose, Neu5Ac or Neu5Gc (12 mM). Optical density at 600 nm was monitored over time.
(C) TIGR4 and TIGR4ΔsatABCΔSP1688-90ΔSP1328 were grown on glucose until OD620nm=0.45. Cells were washed with PBS and resuspended in C+Y medium containing either 12 mM Neu5Ac or Neu5Gc or no supplemented carbohydrate. After incubation for 1.5 h, RNA was isolated, cDNA was synthetized and qPCR was performed to determine mRNA levels of the pneumococcal sialidase A (NanA). 16s was used as endogenous control. Fold change of transcription was normalized to bacteria incubated in monosaccharide-free C+Y (dotted line).
(D) Sialidase activity of TIGR4 incubated in C+Y medium containing either 12 mM Neu5Ac or Neu5Gc was determined using 2-O-(p-nitrophenyl)-α-d-N acetylneuraminic acid (pNANA): Values were normalized to Neu5Ac-treated TIGR4.
(E) TIGR4 was incubated as described above. RNA was isolated, cDNA was synthetized and qPCR was performed to measure mRNA levels of SatA, the substrate binding protein of the main sia transporter, SatABC. 16s was used as endogenous control. Fold change of transcription was normalized to bacteria incubated in monosaccharide-free C+Y (dotted line). Data in (A–B) are represented as mean of 2 independent experiments and in (C–E) as mean ± SEM from at least 3 independent experiments. The Mann-Whitney Test was used for statistical analysis. *, p<0.05; **, p<0.01; ***, p<0.001; ns, non-significant. See also related Figure S2.
Streptococcus pneumoniae senses sialic acid in vivo in the nasopharynx of mice
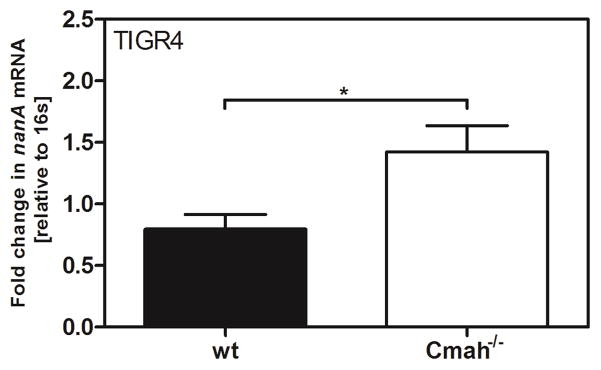
To study whether Sia sensing also occurs in vivo, we isolated pneumococci from the nasopharynx of mice 6 h p.i., and nanA mRNA levels were determined from these bacteria. We observed an upregulation of nanA transcription in Cmah−/− compared to wild-type (wt) mice after challenge with TIGR4 (Fig 3). In order to exclude unspecific primer binding to host RNA, we performed the same experiment with TIGR4ΔnanA and did not detect any nanA transcription in these samples (data not shown).
Figure 3. Streptococcus pneumoniae senses sialic acid in vivo in the nasopharynx of mice.
Mice were infected intranasally with S. pneumoniae TIGR4 in 20 μl PBS. Nasopharyngeal lavages in PBS were obtained at 6 h p.i.. Bacteria were spun down and resuspended in RLT buffer. RNA was isolated and cDNA was synthetized. The transcription of the sialidase nanA was monitored by qPCR. 16s was used as an endogenous control. The values are presented relative to bacteria isolated from the nasopharynx of Cmah−/− mice. The Mann-Whitney Test was used for statistical analysis. *, p<0.05.
Intracellular signaling of sialic acid leads to higher transcription of the two-component-system CiaRH in response to Neu5Ac as compared to Neu5Gc
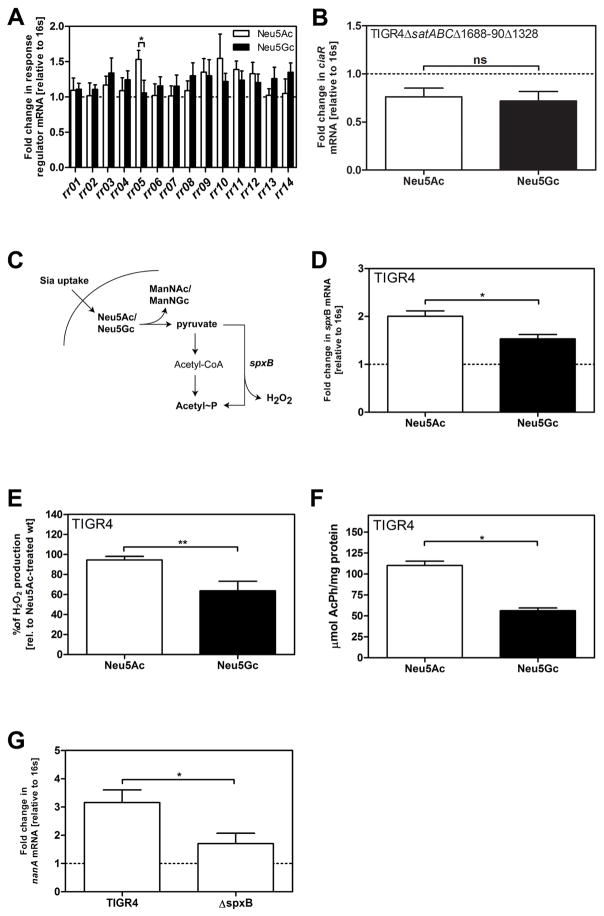
As strain TIGR4 signals differentially in response to Neu5Ac and Neu5Gc, we performed qPCR to compare transcription rates of all pneumococcal RR in response to Neu5Ac or Neu5Gc, in relation to no carbohydrates added. Out of all known RR, only CiaR was upregulated significantly higher in response to Neu5Ac compared to Neu5Gc (Fig 4A). CiaR is co-transcribed with its cognate HK CiaH, which showed similar transcriptional response patterns to the different sugars (data not shown). In TIGR4ΔsatABCΔSP1688-90ΔSP1328, lacking all three known Sia transporters, the ciaR transcription was downregulated in comparison to incubation without sugar. Moreover, we did not observe any difference in ciaR mRNA levels after treatment with either of the Sias (Fig 4B), suggesting that ciaR upregulation in response to the two Sias requires transport. It has been demonstrated that phosphorylation of CiaR is required for its regulatory effects (Halfmann et al., 2011). The experiments described above therefore suggest that the level of CiaR phosphorylation might be higher after exposure to Neu5Ac compared to Neu5Gc, and that this increased phosphorylation requires Sia transport. It has been shown that after Sia uptake, Neu5Ac and Neu5Gc are metabolized to ManNAc or ManNGc respectively and pyruvate (Hopkins et al., 2013; Vimr and Troy, 1985). Subsequently, pyruvate is catabolized to AcPh and hydrogen peroxide (H2O2) by the SpxB pyruvate oxidase (Spellerberg et al., 1996) (Fig 4C). As AcPh can act as a phosphor-donor to activate CiaR we measured the transcriptional response of spxB for TIGR4 growing on either of the two Sias. We found a higher expression of spxB, and also higher amounts of H2O2, and AcPh, with Neu5Ac than Neu5Gc (Fig 4D–F). Furthermore, we found the transcriptional level of nanA to be decreased in a spxB-deficient mutant compared to TIGR4 wt (Fig 4G), suggesting that spxB transcription and internal AcPh affect transcription of nanA.
Figure 4. Intracellular signaling of sialic acid leads to higher transcription of the two-component-system CiaRH in response to Neu5Ac as compared to Neu5Gc.
(A) RNA samples of TIGR4 incubated either on no supplemented carbohydrate or Neu5Ac or Neu5Gc (12 mM) for 1.5 h were isolated and cDNA was synthetized. The transcription rates of the response regulators RR01, RR02, RR03, RR04, RR05 (CiaR), RR06, RR07; RR08, RR09, RR10, RR11, RR12, RR13 and RR14 were determined.
(B) RNA samples of TIGR4ΔsatABCΔSP1688-90ΔSP1328 incubated as described above were isolated and cDNA was synthetized. The transcription rates of RR05 (CiaR) were determined.
(C) A schematic representation of the uptake and catabolism of Neu5Ac and Neu5Gc and acetyl phosphate production in S. pneumoniae is displayed.
(D) RNA samples of TIGR4 incubated as described before were isolated and cDNA was synthetized. Transcription rates of spxB were determined.
(E) Bacteria were incubated as described before. After an incubation with 2,2′-azino-bis (3-ethylbenzthiazoline-6sulfonic acid) and horseradish peroxidase, optical densities at 560 nm were measured and hydrogen peroxide concentrations were calculated using a standard curve using known concentrations. Values are normalized to amounts of H2O2 produced by TIGR4.
(F) TIGR4 was grown as described above and production of AcPh was assayed by converting ADP and AcPh to ATP by the acetate kinase of B. stearothermophilus. Total ATP was finally measured using an ATP Bioluminescence Kit and normalized to the protein content of the sample.
(G) RNA samples of TIGR4 and TIGR4ΔspxB were incubated on Neu5Ac as described above, isolated and cDNA was synthetized. Transcription rates of nanA were determined.
(A–B, D, G) qPCR were performed with 16s as endogenous control. mRNA values were normalized to bacteria incubated in C+Y medium without supplemented sugar (dotted lines).
(A–B, D–G) Data are represented as mean ± SEM of at least 3 independent experiments. The Mann-Whitney Test was used for statistical analysis. ns, non-significant.*, p<0.05, **, p<0.01. See also related Figure S3.
The response regulator CiaR controls transcription of sialidase NanA and is required for normal pneumococcal growth on sialic acid
To determine whether CiaR regulates transcription of genes involved in Sia metabolism, we performed qPCR to measure nanA and satA transcription in the TIGR4ΔciaR and TIGR4ΔciaRH mutants. Both mutant strains failed to significantly upregulate nanA or satA in response to Neu5Ac relative Neu5Gc. Thus, transcription of the sialidase gene nanA and total sialidase activity were similar between the two Sia conditions, and upregulated in contrast to bacteria treated without supplemented carbohydrate (Fig 5A–C). Consequently, the preferential upregulation of nanA in TIGR4 bacteria exposed to Neu5Ac compared to Neu5Gc is CiaR dependent. To test whether the RR CiaR affects the growth of TIGR4 on Sia we cultured TIGR4ΔciaR or TIGR4ΔciaRH on glucose, Neu5Ac or Neu5Gc. We observed growth defects of TIGR4ΔciaR and TIGR4ΔciaRH on either Sia, compared to wild-type TIGR4 (Fig 5D–E). Noteworthy, the growth rate of TIGR4ΔciaR on galactose was also significantly reduced in comparison to wt TIGR4 (data not shown). In contrast, the growth rate on glucose did not differ between TIGR4 and the two mutant strains (Fig 5D–E). Deletion mutations of other RR did not affect growth on glucose or Sia (Supplemental Figure S3).
Figure 5. The response regulator CiaR controls transcription of sialidase NanA and is required for normal pneumococcal growth on sialic acid.
(A) TIGR4ΔciaR and TIGR4ΔciaRH were grown on glucose until OD620nm=0.45, cells were washed with PBS and resuspended in C+Y containing either no supplemented carbohydrate or 12 mM Neu5Ac or Neu5Gc. After incubation for 1.5 h, RNA was isolated and cDNA was synthetized. The transcription of the sialidase nanA was monitored. 16s was used as an endogenous control. Values are presented relative to bacteria incubated in C+Y medium without supplemented carbohydrates (dotted line).
(B) The neuraminidase activities of TIGR4ΔciaR and TIGR4ΔciaRH incubated in C+Y medium containing either 12 mM Neu5Ac or Neu5Gc were determined using 2-O-(p-nitrophenyl)-α-d-N-acetylneuraminic acid (pNANA). Values were plotted in relation to Neu5Ac-incubated wildtype bacteria.
(C) TIGR4ΔciaR and TIGR4ΔciaRH were grown on glucose until OD620nm=0.45, cells were washed with PBS and resuspended in C+Y containing either no supplemented carbohydrate or 12 mM Neu5Ac or Neu5Gc. After incubation for 1.5 h, RNA was isolated and cDNA was synthetized. The transcription of the substrate binding protein satA of main sialic acid transporter SatABC was monitored. 16s was used as an endogenous control. Values are presented relative to bacteria incubated in C+Y medium without supplemented carbohydrates (dotted line).
(A–C) Data are represented as mean ± SEM of 3 independent experiments. The Mann-Whitney Test was used for statistical analysis. ns, non-significant.
(D) Mutant strainTIGR4ΔciaR and (E) mutant strain TIGR4ΔciaRH, were grown on 12 mM glucose (Glc), Neu5Ac or Neu5Gc. Optical density at 600 nm was monitored over time. Growth curves of TIGR4 on 12 mM glucose, Neu5Ac or Neu5Gc were plotted as a reference in each graph. Data are represented as mean of at least 2 independent experiments.
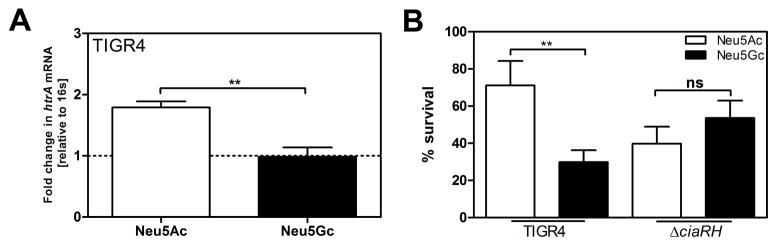
Upregulation of ciaRH and htrA in response to Neu5Ac mediates higher resistance to oxidative stress, compared to incubation with Neu5Gc
CiaRH is known to mediate resistance to oxidative stress via the high-temperature requirement A, HtrA (Ibrahim et al., 2004a, b). Therefore, we determined the transcriptional level of htrA and the survival rate of TIGR4 in response to H2O2. Treatment with Neu5Ac resulted in significantly increased transcription of htrA, and higher numbers of recovered bacteria in response to H2O2 than treatment with Neu5Gc (Fig 6A–B). In contrast, survival rates were similar after treatment with Neu5Ac or Neu5Gc of the deletion mutant of the TCS CiaRH (TIGR4ΔciaRH).
Figure 6. Upregulation of ciaRH and htrA in response to Neu5Ac mediates higher resistance to oxidative stress, compared to incubation with Neu5Gc.
(A) TIGR4 was grown on glucose until OD620nm=0.45, cells were washed with PBS and resuspended in C+Y containing either no supplemented carbohydrate or 12 mM Neu5Ac or Neu5Gc. After incubation for 1.5 h, RNA was isolated and cDNA was synthetized. The transcription of htrA was monitored. 16s was used as an endogenous control. Values are presented relative to bacteria incubated in C+Y medium without supplemented carbohydrates (dotted line).
(B) TIGR4 and TIGR4ΔciaRH were grown on glucose until OD620nm=0.45. Cells were washed with PBS and resuspended in C+Y medium containing either 12 mM Neu5Ac or Neu5Gc. After incubation for 1.5 h, 40 mM H2O2 was added and cells were incubated at 37°C for 10 min. Viable bacterial numbers were determined before and after exposure to H2O2. The graph represents the percentage of surviving cells. (A–B) Values are represented as mean from 3 independent experiments. The Mann-Whitney Test was used for statistical analysis. *, p<0.05.
Sialidase NanA, the main sialic acid transporter SatABC and the response regulator CiaR contribute to the differential increase in pneumococcal virulence between Cmah−/− and wt mice
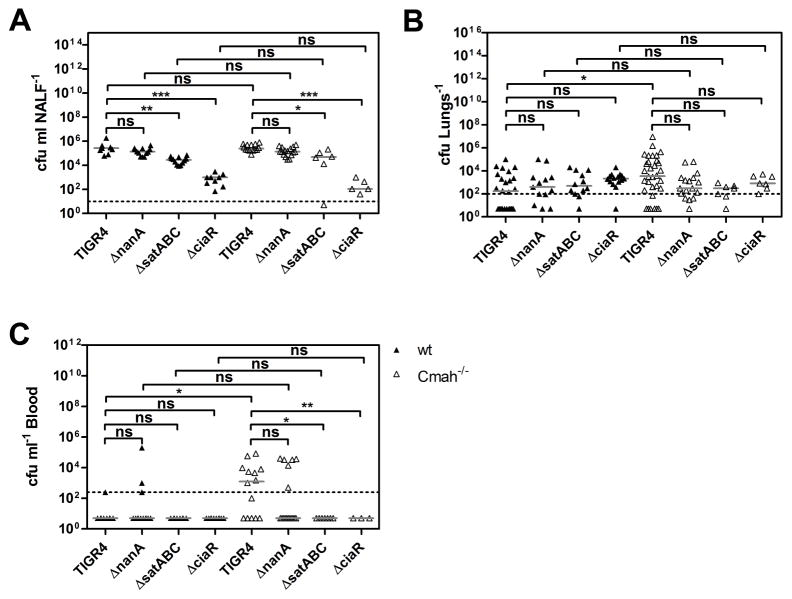
Since we observed a more severe pneumococcal disease outcome in Cmah−/− compared to wt mice, we asked whether NanA, SatABC or CiaR are required for this differential increase in pneumococcal virulence in vivo. We infected wt and Cmah−/− mice with TIGR4 or its isogenic mutants lacking NanA, SatABC or CiaR. At 24 h p.i., nasopharyngeal colonization did not differ between wt and Cmah−/− mice challenged with TIGR4, TIGR4ΔnanA, TIGR4ΔsatABC or TIGR4ΔciaR (Fig 7A). However, we found that TIGR4ΔsatABC and especially TIGR4ΔciaR exhibited a marked decrease in nasopharyngeal colonization in both wt and Cmah−/− mice. Challenge with wild-type TIGR4 resulted in higher bacterial numbers in the lungs of Cmah−/− compared to wt mice, whereas infections with TIGR4ΔnanA, TIGR4ΔsatABC or TIGR4ΔciaR led to similar numbers in the lungs of wt and Cmah−/− mice (Fig 7B). Infection with TIGR4 led to sepsis in Cmah−/−, but not in wt mice, whereas challenge with TIGR4ΔsatABC and TIGR4ΔciaR did not result in bacterial invasion into the blood and TIGR4ΔnanA showed low bacteremia levels (Fig 7C). Hence, NanA, SatABC and CiaR each contribute to the higher pneumococcal virulence in Cmah−/− as compared to wild-type mice.
Figure 7. Sialidase NanA, the main sialic acid transporter SatABC and the response regulator CiaR contribute to the differential increase in pneumococcal virulence between Cmah−/− and wt mice.
Wt and Cmah−/− mice were challenged intranasally with S. pneumoniae TIGR4, TIGR4ΔnanA, TIGR4ΔsatABC or TIGR4ΔciaR. Samples were taken at 24 h p.i.
(A) Nasopharyngeal lavages were obtained and bacterial numbers were determined by plating.
(B) Bacterial counts in the lungs were measured by plating lung homogenates.
(C) Bacterial numbers in the blood were determined by plating. (A–C) Dotted lines indicate the detection limits. Horizontal bars represent medians. Kruskal-Wallis with Dunn’s Multiple Comparison Test was used for statistical analysis. *, p<0.05, **, p<0.01, ***, p<0.001; ns, non-significant. 3 different experiments were performed with group sizes between 3 and 10 mice.
Discussion
S. pneumoniae, like other respiratory pathogens, closely interacts with Sias in mucins during infection (Feldman et al., 1992; Traving and Schauer, 1998). Since humans are its natural host (Pan et al., 2004), we hypothesized that S. pneumoniae would respond differently upon encounter of Neu5Ac, abundant in humans, compared to Neu5Gc, which is mostly present in other animal species. Importantly, we show an upregulation of pneumococcal sialidase NanA and the main sialic acid transporter SatABC in response to Neu5Ac compared to Neu5Gc, and found that the RR CiaR was involved in the response to Sias. Sia scavenging, uptake and signaling mediated increased pneumococcal virulence in response to Neu5Ac compared to Neu5Gc after intranasal, but not after intravenous challenge.
To determine whether S. pneumoniae exhibits higher virulence in response to Neu5Ac compared to Neu5Gc in vivo, we infected C57BL/6 wt, predominantly having Neu5Gc, and Cmah−/− mice, lacking Neu5Gc, intranasally with TIGR4 (Hedlund et al., 2007; Makatsori et al., 1998). Cmah−/− mice had significantly more bacteria in the lungs and blood than wt mice and a significantly lower survival rate. Already at 24 h p.i. we observed higher bacterial numbers in the BALF of Cmah−/− compared to wt mice, suggesting that pneumococcal growth in the lower airways is higher in a Neu5Ac than in a Neu5Gc environment. This could be due to both enhanced bacterial replication, because of a more efficient retrieval and uptake of carbohydrates from glycoconjugates, and to increased resistance to host mediated clearance in this compartment through upregulation of virulence traits in response to Neu5Ac relative to Neu5Gc. These results are in line with earlier studies showing an increased bacterial burden in the lungs of mice after intranasal administration of Neu5Ac compared to Neu5Gc (Trappetti et al., 2009).
We hypothesized that the increased virulence in Cmah−/− compared to wild-type mice is caused by differential affinity of pneumococcal factors affecting growth and virulence in the glucose poor lower airways favoring Neu5Ac compared to Neu5Gc. In general, sialidases are shown to cleave Neu5Ac from glyconconjugates more efficiently than Neu5Gc (Corfield et al., 1981), and species-specific interaction of pathogens and host-structures has been described before, e.g. sialidases of Salmonella spp., and also the pneumococcal sialidase NanC bind stronger to Neu5Ac than to Neu5Gc (Minami et al., 2013; Parker et al., 2012). S. pneumoniae de-glycosylates structures on the surface of host cells with the help of the sialidase NanA (King et al., 2004), and can use Sias as carbon source (Burnaugh et al., 2008; Marion et al., 2011). In TIGR4, genes encoding NanA and other factors involved in Sia uptake and metabolism are encoded in two loci, the nanAB and the nanC locus (King et al., 2004; Vimr et al., 2004). Our in vitro data reveals that S. pneumoniae not only grows on Neu5Ac as a sole carbon source (Burnaugh et al., 2008), but can also utilize Neu5Gc in the same manner. The three known Sia transporters SatABC, SP1688-90 and SP1328 are required for growth on both Sias. Genes within the nanAB locus are upregulated in the presence of Neu5Ac (Afzal et al., 2015; Gualdi et al., 2012). Trappetti and colleagues suggested that the initial encounter between pneumococci and free Neu5Ac triggers an increased expression of sialidases, e.g. NanA, resulting in further release of free Neu5Ac, therefore leading to a positive feedback-loop (Trappetti et al., 2009). Our data reveal an upregulation of NanA and the substrate binding protein SatA of the main sialic acid transporter SatABC in response to Neu5Ac compared to Neu5Gc after Sia uptake in vitro, as well as an increased pneumococcal sialidase expression in the nasopharynx of Cmah−/− compared to wt mice. Subsequently, there are higher amounts of free Sia available which are taken up into the bacteria to a higher extend in Cmah−/− compared to wt mice. Also, Siegel et al. showed that increased desialyation rates of host glycoconjugates promote bacterial growth which, in turn, enhances translocation of bacteria from the nasopharynx to the lower respiratory tract (Siegel et al., 2014).
TCS-mediated carbohydrate signaling in bacteria has been described for Group B streptococci. TCS16 in this species controls sugar phosphotransferase systems, and a deficient mutant failed to grow on fructose-6-phosphate (Faralla et al., 2014). In our study, several of the 14 known pneumococcal RR were upregulated in response to Neu5Ac and Neu5Gc in comparison to incubation without supplemented carbohydrate. Among all known RR in S. pneumoniae, RR05, called CiaR, was transcribed significantly higher when TIGR4 was exposed to Neu5Ac relative to Neu5Gc. This differential effect on ciaR transcription was not observed in a mutant lacking all three Sia transporters. Instead, we observed a downregulation of ciaR in this mutant when exposed to either Neu5Ac or Neu5Gc relative to no carbohydrates added. Subsequently, CiaR might be involved in signaling in response to intracellular Sia or its metabolites, although we cannot exclude that other RR play a role in the pneumococcal response to sialic acids without being differentially regulated in response to Neu5Ac compared to Neu5Gc
The TCS CiaRH is well studied, and has been shown to affect pneumococcal virulence and competence (Dagkessamanskaia et al., 2004; Throup et al., 2000). Deletion of the RR CiaR led to similar nanA and satA mRNA levels in response to Neu5Ac or Neu5Gc. Hence, CiaR is required for the differential response to Neu5Ac and Neu5Gc, possibly due to CiaR-regulated small RNAs mediating virulence (Halfmann et al., 2007; Marx et al., 2010). CiaR mediated effects on gene expression require its phosphorylation (Halfmann et al., 2011). However, not only the cognate sensor CiaH and other TCS sensors may mediate phosphorylation of CiaR. The internal pool of AcPh, the high-energy intermediate of the acetate kinase phosphor transacetylase pathway, can also act as phosphor-donor. Intracellular AcPh levels are affected by the pyruvate oxidase SpxB (Marx et al., 2014). It is interesting to note that pyruvate, the source of AcPh in this pathway, is also a metabolite generated in the pneumococcal catabolism of Neu5Ac (Vimr and Troy, 1985), and probably also of Neu5Gc (Hopkins et al., 2013). Subsequently, SpxB oxidizes pyruvate to H2O2 and AcPh. Interestingly we found that the gene encoding pyruvate oxidase, spxB, as well levels of H2O2, and AcPh, were upregulated in response to Neu5Ac relative to Neu5Gc. Since SpxB produces AcPh (Pericone et al., 2003), we hypothesized that increased intracellular AcPh, because of upregulated SpxB, lead to increased phosphorylation and activation of CiaR and probably also other response regulators in the bacterial cell. Marx et al. showed that a deletion of the spxB gene leads to a 2-fold reduction of ciaR transcription and its target genes (Marx et al., 2014). We found that not only ciaR was downregulated (data not shown), but also nanA transcription was reduced in a spxB-deletion mutant, supporting our hypothesis that CiaR (indirectly) controls transcription of the sialidase NanA.
After phagocytosis of microbes, immune cells like macrophages or neutrophils are known to produce reactive oxygen species (ROS) (Gee et al., 1970; Rossi and Zatti, 1964), exerting antimicrobial activity (Babior, 1978a, b). Bacteria have evolved different evasion strategies in order to fight oxidative stress by immune cells. Pneumococcal CiaRH has been shown to regulate the expression of the high-temperature requirement A (HtrA), which mediates resistance to oxidative stress (Ibrahim et al., 2004b). Hence, we studied htrA transcription and bacterial survival in response to oxidative stress after incubation with the two Sias and found an increased transcription of htrA, as well as higher survival rates when bacteria were incubated with Neu5Ac as compared to Neu5Gc.
The RR CiaR, the main sialic acid transporter SatABC and the sialidase NanA contribute to pneumococcal virulence (Manco et al., 2006; Marion et al., 2011; Marra et al., 2002; Orihuela et al., 2004; Throup et al., 2000). Consistent with this, TIGR4ΔsatABC and TIGR4ΔciaR did not cause and TIGR4ΔnanA reduced sepsis in wt or Cmah−/− mice in our infection model in contrast to infections with wild-type TIGR4. Challenge with TIGR4 led to a higher bacterial burden in the lungs of Cmah−/− compared to wild-type mice at 24 h p.i.. After infection with TIGR4ΔciaR, TIGR4ΔsatABC or TIGR4ΔnanA, we did not detect any differences in bacterial loads of the lungs between wt and Cmah−/− mice 24 h p.i., suggesting that these genes contribute to the increased pneumococcal virulence observed in response to Neu5Ac compared to Neu5Gc. Bacterial counts in the nasopharynx of wt and Cmah−/− were similar after infection with TIGR4 or TIGR4ΔnanA, while a deletion of SatABC or the RR CiaR, previously associated with nasopharyngeal colonization (Sebert et al., 2002), led to lower bacterial numbers in the nasopharynx of both wt and Cmah−/− mice. TIGR4ΔsatABC and TIGR4ΔciaR had significant growth defects or failed to grow on Sia and TIGR4ΔciaR additionally did not grow on galactose (data not shown), which like Sia is known to be part of the glycan chain. These data suggest a retained catabolite repression in the ciaR mutant.
After intravenous challenge with TIGR4, no differences in disease progression were observed between wt and Cmah−/− mice. Since the nanAB locus contains cre (Afzal et al., 2015; Gualdi et al., 2012), and ciaR is known to be repressed in the presence of glucose (Paixao et al., 2015), we hypothesized that these genes specifically mediate increased virulence in response to Neu5Ac in the glucose-free respiratory tract (Kingston et al., 1991), but not in the glucose rich blood stream (Han et al., 2008), which possesses very low levels of free Sias due to rapid renal clearance (Banda et al., 2012; Tangvoranuntakul et al., 2003).
In summary, we present findings in pneumococcal host specificity and pathogenesis. We demonstrate that pneumococci are able to sense the human type Sia Neu5Ac in a host-specific manner involving the RR CiaR. Our data shows an upregulation of nanA, satA and ciaR and subsequent enhanced bacterial burden in Cmah−/− compared to wt mice. The increased expression of CiaR led to upregulation of htrA and higher resistance to reactive oxygen species and therefore decreased bacterial killing by host immune cells. Together these factors mediate increased virulence in response to Neu5Ac, mainly abundant in humans, as compared to Neu5Gc.
Experimental Procedures
Bacterial strains used and growth conditions
Streptococcus pneumoniae TIGR4 (TIGR4, ATCC BAA-334) and its isogenic mutants (listed in Supplemental Table S1) were grown in C+Y medium until OD620nm=0.45, washed with PBS and resuspended in monosaccharide-free C+Y medium supplemented with either 12 mM glucose (Sigma-Aldrich), Neu5Ac (Nacalai) or Neu5Gc (Inalco) or no cabohydrate. The cultures were grown at 37°C in a water bath or in multiwell plates using a Bioscreen instrument (Growth curves OY) in order to follow the growth.
Mutant construction
Bacterial mutants used in this study were constructed by fusion PCR mutagenesis (Karreman, 1998). Upstream and downstream fragments of the target gene, as well as the erythromycin cassette were amplified with overlapping regions using primers listed in Supplemental Table S2. The erythromycin cassette was fused to the upstream region of the target gene and afterwards the downstream region was added by PCR. The correct fragment was gel-purified and transformed into S. pneumoniae. Transformation was carried out by culturing S. pneumoniae in C+Y medium until the bacteria reached OD620nm=0.15. To 400 μl of the culture 100 ng μl−1 of competence-stimulating peptide CSP-2 was added. After incubation at 37°C for 15 minutes, the fusion PCR product was added. The cells were grown at 37°C for 90 minutes and plated onto blood agar plates containing appropriate antibiotics. Mutants were confirmed by PCR and sequencing.
RNA isolation, cDNA synthesis and real-time PCR (qPCR)
RNA from bacteria grown under in vitro conditions or from nasopharyngeal lavages of mice was isolated using the RNeasy MiniKit (QIAGEN) as described in Supplemental Methods.
Sialidase activity assay
Sialidase activity was determined as described previously (Manco et al., 2006), see Supplemental Methods.
H2O2 production and sensitivity assay
Pneumococcal production of and sensitivity to H2O2 was tested as described previously (Ibrahim et al., 2004a; Pericone et al., 2003), see Supplemental Methods.
Acetyl phosphate production assay
Pneumococcal production of acetyl phosphate (AcPh) was measured as described before (Pruss and Wolfe, 1994), for detailed description see Supplemental Methods.
Mouse challenge
All experiments were performed in accordance with the local ethical committee (Stockholms Norra djurförsöksetiska nämnd). Six to eight weeks old male C57BL/6 wild-type and Cmah−/−mice with a human-like deletion in the exon 6 of the Cmah gene (Hedlund et al., 2007) were used. The Cmah−/− mice were negatively tested for a dock2 mutation, recently shown to be present in some of these mice due to backcrossing into commercially available C57BL/6 mice (Mahajan et al., 2016). Sedation was performed by inhalation of 4% isoflurane or by intraperitoneal injection of 80 mg kg−1 Ketamin and 5 mg kg−1 Rompun (Bayer AG) according to their body weight. Mice were infected intranasally or intravenously with 106 cfu per mouse in 20 μl or 105 cfu per mouse in 100 μl PBS, respectively. The health status of the mice was controlled regularly and clinical scores were given. In order to obtain BALF, lungs were lavaged twice with PBS. To determine bacterial numbers in the BALF, NALF, blood and lungs (homogenized), samples were plated onto blood agar plates.
Statistical analysis
Statistical analysis was performed using Graphpad Prism. Mann-Whitney test or Kruskal-Wallis with Dunn’s Multiple Comparison Test were performed to compare two groups or more than two groups, respectively.
Supplementary Material
Highlights.
Intranasal infection with pneumococci progresses faster in Cmah−/− than wildtype mice
Sialidase NanA and transporter SatABC are upregulated by Neu5Ac compared to Neu5Gc
Activation of response regulator CiaR controls expression of NanA and SatABC
Uptake and metabolic signaling mediate increased virulence by Neu5Ac versus Neu5Gc
Acknowledgments
This work was supported by grants from the Knut and Alice Wallenberg foundation, the Swedish Research Council, the Foundation for Strategic research (SSF), ALF grant from Stockholm County Council and NIH grant R01GM32373. We thank Dr. Samantha King (Nationwide Children’s Hospital) for providing bacterial strains, Ezgi Tasköprü for help with some experiments and Prof. Staffan Normark and Dr. Peter Mellroth for fruitful discussions.
Footnotes
AUTHOR CONTRIBUTIONS
K.H., J.L. and B.H.N. designed the study; K.H., J.L. and A.P. performed research experiments; K.H., J.L., A.P. and B.H.N. analyzed data; and K.H., V.N., A.V. and B.H.N. wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afzal M, Shafeeq S, Ahmed H, Kuipers OP. Sialic acid-mediated gene expression in Streptococcus pneumoniae and role of NanR as a transcriptional activator of the nan gene cluster. Applied and environmental microbiology. 2015;81:3121–3131. doi: 10.1128/AEM.00499-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior BM. Oxygen-dependent microbial killing by phagocytes (first of two parts) The New England journal of medicine. 1978a;298:659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Babior BM. Oxygen-dependent microbial killing by phagocytes (second of two parts) The New England journal of medicine. 1978b;298:721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Banda K, Gregg CJ, Chow R, Varki NM, Varki A. Metabolism of vertebrate amino sugars with N-glycolyl groups: mechanisms underlying gastrointestinal incorporation of the non-human sialic acid xeno-autoantigen N-glycolylneuraminic acid. The Journal of biological chemistry. 2012;287:28852–28864. doi: 10.1074/jbc.M112.364182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnaugh AM, Frantz LJ, King SJ. Growth of Streptococcus pneumoniae on human glycoconjugates is dependent upon the sequential activity of bacterial exoglycosidases. J Bacteriol. 2008;190:221–230. doi: 10.1128/JB.01251-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Uchiyama S, Varki A, Nizet V. Leukocyte inflammatory responses provoked by pneumococcal sialidase. MBio. 2012;3 doi: 10.1128/mBio.00220-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, Muchmore EA, Nelson DL, Warren ST, Varki A. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci U S A. 1998;95:11751–11756. doi: 10.1073/pnas.95.20.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfield AP, Veh RW, Wember M, Michalski JC, Schauer R. The release of Nacetyl- and N-glycolloyl-neuraminic acid from soluble complex carbohydrates and erythrocytes by bacterial, viral and mammalian sialidases. The Biochemical journal. 1981;197:293–299. doi: 10.1042/bj1970293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagkessamanskaia A, Moscoso M, Henard V, Guiral S, Overweg K, Reuter M, Martin B, Wells J, Claverys JP. Interconnection of competence, stress and CiaR regulons in Streptococcus pneumoniae: competence triggers stationary phase autolysis of ciaR mutant cells. Molecular microbiology. 2004;51:1071–1086. doi: 10.1111/j.1365-2958.2003.03892.x. [DOI] [PubMed] [Google Scholar]
- Deng L, Song J, Gao X, Wang J, Yu H, Chen X, Varki N, Naito-Matsui Y, Galan JE, Varki A. Host adaptation of a bacterial toxin from the human pathogen Salmonella Typhi. Cell. 2014;159:1290–1299. doi: 10.1016/j.cell.2014.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faralla C, Metruccio MM, De Chiara M, Mu R, Patras KA, Muzzi A, Grandi G, Margarit I, Doran KS, Janulczyk R. Analysis of two-component systems in group B Streptococcus shows that RgfAC and the novel FspSR modulate virulence and bacterial fitness. MBio. 2014;5:e00870–00814. doi: 10.1128/mBio.00870-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman C, Read R, Rutman A, Jeffery PK, Brain A, Lund V, Mitchell TJ, Andrew PW, Boulnois GJ, Todd HC, et al. The interaction of Streptococcus pneumoniae with intact human respiratory mucosa in vitro. The European respiratory journal. 1992;5:576–583. [PubMed] [Google Scholar]
- Gee JB, Vassallo CL, Bell P, Kaskin J, Basford RE, Field JB. Catalase-dependent peroxidative metabolism in the alveolar macrophage during phagocytosis. The Journal of clinical investigation. 1970;49:1280–1287. doi: 10.1172/JCI106340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualdi L, Hayre JK, Gerlini A, Bidossi A, Colomba L, Trappetti C, Pozzi G, Docquier JD, Andrew P, Ricci S, et al. Regulation of neuraminidase expression in Streptococcus pneumoniae. BMC microbiology. 2012;12:200. doi: 10.1186/1471-2180-12-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenzi E, Gasc AM, Sicard MA, Hakenbeck R. A two-component signaltransducing system is involved in competence and penicillin susceptibility in laboratory mutants of Streptococcus pneumoniae. Molecular microbiology. 1994;12:505–515. doi: 10.1111/j.1365-2958.1994.tb01038.x. [DOI] [PubMed] [Google Scholar]
- Halfmann A, Kovacs M, Hakenbeck R, Bruckner R. Identification of the genes directly controlled by the response regulator CiaR in Streptococcus pneumoniae: five out of 15 promoters drive expression of small non-coding RNAs. Molecular microbiology. 2007;66:110–126. doi: 10.1111/j.1365-2958.2007.05900.x. [DOI] [PubMed] [Google Scholar]
- Halfmann A, Schnorpfeil A, Muller M, Marx P, Gunzler U, Hakenbeck R, Bruckner R. Activity of the two-component regulatory system CiaRH in Streptococcus pneumoniae R6. J Mol Microbiol Biotechnol. 2011;20:96–104. doi: 10.1159/000324893. [DOI] [PubMed] [Google Scholar]
- Han BG, Hao CM, Tchekneva EE, Wang YY, Lee CA, Ebrahim B, Harris RC, Kern TS, Wasserman DH, Breyer MD, et al. Markers of glycemic control in the mouse: comparisons of 6-h- and overnight-fasted blood glucoses to Hb A1c. Am J Physiol Endocrinol Metab. 2008;295:E981–986. doi: 10.1152/ajpendo.90283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund M, Tangvoranuntakul P, Takematsu H, Long JM, Housley GD, Kozutsumi Y, Suzuki A, Wynshaw-Boris A, Ryan AF, Gallo RL, et al. N-glycolylneuraminic acid deficiency in mice: implications for human biology and evolution. Mol Cell Biol. 2007;27:4340–4346. doi: 10.1128/MCB.00379-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins AP, Hawkhead JA, Thomas GH. Transport and catabolism of the sialic acids N-glycolylneuraminic acid and 3-keto-3-deoxy-D-glycero-D-galactonononic acid by Escherichia coli K-12. FEMS microbiology letters. 2013;347:14–22. doi: 10.1111/1574-6968.12213. [DOI] [PubMed] [Google Scholar]
- Ibrahim YM, Kerr AR, McCluskey J, Mitchell TJ. Control of virulence by the twocomponent system CiaR/H is mediated via HtrA, a major virulence factor of Streptococcus pneumoniae. J Bacteriol. 2004a;186:5258–5266. doi: 10.1128/JB.186.16.5258-5266.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim YM, Kerr AR, McCluskey J, Mitchell TJ. Role of HtrA in the virulence and competence of Streptococcus pneumoniae. Infect Immun. 2004b;72:3584–3591. doi: 10.1128/IAI.72.6.3584-3591.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karreman C. Fusion PCR, a one-step variant of the “megaprimer” method of mutagenesis. Biotechniques. 1998;24:736, 740, 742. doi: 10.2144/98245bm08. [DOI] [PubMed] [Google Scholar]
- King SJ, Hippe KR, Gould JM, Bae D, Peterson S, Cline RT, Fasching C, Janoff EN, Weiser JN. Phase variable desialylation of host proteins that bind to Streptococcus pneumoniae in vivo and protect the airway. Molecular microbiology. 2004;54:159–171. doi: 10.1111/j.1365-2958.2004.04252.x. [DOI] [PubMed] [Google Scholar]
- King SJ, Hippe KR, Weiser JN. Deglycosylation of human glycoconjugates by the sequential activities of exoglycosidases expressed by Streptococcus pneumoniae. Molecular microbiology. 2006;59:961–974. doi: 10.1111/j.1365-2958.2005.04984.x. [DOI] [PubMed] [Google Scholar]
- Kingston GW, Phang PT, Leathley MJ. Increased incidence of nosocomial pneumonia in mechanically ventilated patients with subclinical aspiration. American journal of surgery. 1991;161:589–592. doi: 10.1016/0002-9610(91)90906-t. [DOI] [PubMed] [Google Scholar]
- Mahajan VS, Demissie E, Mattoo H, Viswanadham V, Varki A, Morris R, Pillai S. Striking Immune Phenotypes in Gene-Targeted Mice Are Driven by a Copy-Number Variant Originating from a Commercially Available C57BL/6 Strain. Cell Rep. 2016;15:1901–1909. doi: 10.1016/j.celrep.2016.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makatsori E, Fermani K, Aletras A, Karamanos NK, Tsegenidis T. Screening of Nacylneuraminic acids in serum and tissue specimens of mouse C57BI with Lewis’ lung cancer by high-performance liquid chromatography. Journal of chromatography B, Biomedical sciences and applications. 1998;712:23–29. doi: 10.1016/s0378-4347(98)00150-9. [DOI] [PubMed] [Google Scholar]
- Manco S, Hernon F, Yesilkaya H, Paton JC, Andrew PW, Kadioglu A. Pneumococcal neuraminidases A and B both have essential roles during infection of the respiratory tract and sepsis. Infect Immun. 2006;74:4014–4020. doi: 10.1128/IAI.01237-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion C, Burnaugh AM, Woodiga SA, King SJ. Sialic acid transport contributes to pneumococcal colonization. Infect Immun. 2011;79:1262–1269. doi: 10.1128/IAI.00832-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra A, Asundi J, Bartilson M, Lawson S, Fang F, Christine J, Wiesner C, Brigham D, Schneider WP, Hromockyj AE. Differential fluorescence induction analysis of Streptococcus pneumoniae identifies genes involved in pathogenesis. Infect Immun. 2002;70:1422–1433. doi: 10.1128/IAI.70.3.1422-1433.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MJ, Rayner JC, Gagneux P, Barnwell JW, Varki A. Evolution of humanchimpanzee differences in malaria susceptibility: relationship to human genetic loss of Nglycolylneuraminic acid. Proc Natl Acad Sci U S A. 2005;102:12819–12824. doi: 10.1073/pnas.0503819102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx P, Meiers M, Bruckner R. Activity of the response regulator CiaR in mutants of Streptococcus pneumoniae R6 altered in acetyl phosphate production. Front Microbiol. 2014;5:772. doi: 10.3389/fmicb.2014.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx P, Nuhn M, Kovacs M, Hakenbeck R, Bruckner R. Identification of genes for small non-coding RNAs that belong to the regulon of the two-component regulatory system CiaRH in Streptococcus. BMC Genomics. 2010;11:661. doi: 10.1186/1471-2164-11-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascher T, Heintz M, Zahner D, Merai M, Hakenbeck R. The CiaRH system of Streptococcus pneumoniae prevents lysis during stress induced by treatment with cell wall inhibitors and by mutations in pbp2x involved in beta-lactam resistance. J Bacteriol. 2006;188:1959–1968. doi: 10.1128/JB.188.5.1959-1968.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascher T, Zahner D, Merai M, Balmelle N, de Saizieu AB, Hakenbeck R. The Streptococcus pneumoniae cia regulon: CiaR target sites and transcription profile analysis. J Bacteriol. 2003;185:60–70. doi: 10.1128/JB.185.1.60-70.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami A, Ishibashi S, Ikeda K, Ishitsubo E, Hori T, Tokiwa H, Taguchi R, Ieno D, Otsubo T, Matsuda Y, et al. Catalytic preference of Salmonella typhimurium LT2 sialidase for Nacetylneuraminic acid residues over N-glycolylneuraminic acid residues. FEBS Open Bio. 2013;3:231–236. doi: 10.1016/j.fob.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchmore EA, Diaz S, Varki A. A structural difference between the cell surfaces of humans and the great apes. American journal of physical anthropology. 1998;107:187–198. doi: 10.1002/(SICI)1096-8644(199810)107:2<187::AID-AJPA5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Ng PS, Bohm R, Hartley-Tassell LE, Steen JA, Wang H, Lukowski SW, Hawthorne PL, Trezise AE, Coloe PJ, Grimmond SM, et al. Ferrets exclusively synthesize Neu5Ac and express naturally humanized influenza A virus receptors. Nat Commun. 2014;5:5750. doi: 10.1038/ncomms6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- Orihuela CJ, Gao G, Francis KP, Yu J, Tuomanen EI. Tissue-specific contributions of pneumococcal virulence factors to pathogenesis. The Journal of infectious diseases. 2004;190:1661–1669. doi: 10.1086/424596. [DOI] [PubMed] [Google Scholar]
- Paixao L, Caldas J, Kloosterman TG, Kuipers OP, Vinga S, Neves AR. Transcriptional and metabolic effects of glucose on Streptococcus pneumoniae sugar metabolism. Front Microbiol. 2015;6:1041. doi: 10.3389/fmicb.2015.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker RB, McCombs JE, Kohler JJ. Sialidase specificity determined by chemoselective modification of complex sialylated glycans. ACS chemical biology. 2012;7:1509–1514. doi: 10.1021/cb300241v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pericone CD, Park S, Imlay JA, Weiser JN. Factors contributing to hydrogen peroxide resistance in Streptococcus pneumoniae include pyruvate oxidase (SpxB) and avoidance of the toxic effects of the fenton reaction. J Bacteriol. 2003;185:6815–6825. doi: 10.1128/JB.185.23.6815-6825.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew MM, Fennie KP, York MP, Daniels J, Ghaffar F. Variation in the presence of neuraminidase genes among Streptococcus pneumoniae isolates with identical sequence types. Infect Immun. 2006;74:3360–3365. doi: 10.1128/IAI.01442-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss BM, Wolfe AJ. Regulation of acetyl phosphate synthesis and degradation, and the control of flagellar expression in Escherichia coli. Molecular microbiology. 1994;12:973–984. doi: 10.1111/j.1365-2958.1994.tb01085.x. [DOI] [PubMed] [Google Scholar]
- Rossi F, Zatti M. Biochemical aspects of phagocytosis in polymorphonuclear leucocytes. NADH and NADPH oxidation by the granules of resting and phagocytizing cells. Experientia. 1964;20:21–23. doi: 10.1007/BF02146019. [DOI] [PubMed] [Google Scholar]
- Sebert ME, Palmer LM, Rosenberg M, Weiser JN. Microarray-based identification of htrA, a Streptococcus pneumoniae gene that is regulated by the CiaRH twocomponent system and contributes to nasopharyngeal colonization. Infect Immun. 2002;70:4059–4067. doi: 10.1128/IAI.70.8.4059-4067.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw L, Schauer R. Detection of CMP-N-acetylneuraminic acid hydroxylase activity in fractionated mouse liver. The Biochemical journal. 1989;263:355–363. doi: 10.1042/bj2630355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel SJ, Roche AM, Weiser JN. Influenza Promotes Pneumococcal Growth during Coinfection by Providing Host Sialylated Substrates as a Nutrient Source. Cell Host Microbe. 2014;16:55–67. doi: 10.1016/j.chom.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellerberg B, Cundell DR, Sandros J, Pearce BJ, Idanpaan-Heikkila I, Rosenow C, Masure HR. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Molecular microbiology. 1996;19:803–813. doi: 10.1046/j.1365-2958.1996.425954.x. [DOI] [PubMed] [Google Scholar]
- Springer SA, Diaz SL, Gagneux P. Parallel evolution of a self-signal: humans and new world monkeys independently lost the cell surface sugar Neu5Gc. Immunogenetics. 2014;66:671–674. doi: 10.1007/s00251-014-0795-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock JB, Ninfa AJ, Stock AM. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989;53:450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangvoranuntakul P, Gagneux P, Diaz S, Bardor M, Varki N, Varki A, Muchmore E. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci U S A. 2003;100:12045–12050. doi: 10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Throup JP, Koretke KK, Bryant AP, Ingraham KA, Chalker AF, Ge Y, Marra A, Wallis NG, Brown JR, Holmes DJ, et al. A genomic analysis of two-component signal transduction in Streptococcus pneumoniae. Molecular microbiology. 2000;35:566–576. doi: 10.1046/j.1365-2958.2000.01725.x. [DOI] [PubMed] [Google Scholar]
- Tong HH, Liu X, Chen Y, James M, Demaria T. Effect of neuraminidase on receptor-mediated adherence of Streptococcus pneumoniae to chinchilla tracheal epithelium. Acta oto-laryngologica. 2002;122:413–419. doi: 10.1080/00016480260000111. [DOI] [PubMed] [Google Scholar]
- Trappetti C, Kadioglu A, Carter M, Hayre J, Iannelli F, Pozzi G, Andrew PW, Oggioni MR. Sialic acid: a preventable signal for pneumococcal biofilm formation, colonization, and invasion of the host. J Infect Dis. 2009;199:1497–1505. doi: 10.1086/598483. [DOI] [PubMed] [Google Scholar]
- Traving C, Schauer R. Structure, function and metabolism of sialic acids. Cellular and molecular life sciences : CMLS. 1998;54:1330–1349. doi: 10.1007/s000180050258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama S, Carlin AF, Khosravi A, Weiman S, Banerjee A, Quach D, Hightower G, Mitchell TJ, Doran KS, Nizet V. The surface-anchored NanA protein promotes pneumococcal brain endothelial cell invasion. The Journal of experimental medicine. 2009;206:1845–1852. doi: 10.1084/jem.20090386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimr ER, Kalivoda KA, Deszo EL, Steenbergen SM. Diversity of microbial sialic acid metabolism. Microbiology and molecular biology reviews : MMBR. 2004;68:132–153. doi: 10.1128/MMBR.68.1.132-153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimr ER, Troy FA. Identification of an inducible catabolic system for sialic acids (nan) in Escherichia coli. J Bacteriol. 1985;164:845–853. doi: 10.1128/jb.164.2.845-853.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.