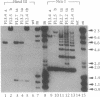
Abstract
Foreign DNA was inserted into unique restriction endonuclease cleavage sites (Sma I or Not I) of the 200,000-base-pair vaccinia virus genome by direct molecular cloning. The modified vaccinia virus DNA was packaged in fowlpox virus-infected avian cells, and chimeric vaccinia virus was isolated from mammalian cells not supporting the growth of the fowlpox helper virus. In contrast to the classical "in vivo" recombination technique, chimeric viruses with inserts in both possible orientations and families of chimeras with multiple inserts were obtained. The different genomic configurations of chimeric viruses provide a broader basis for screening of optimal viruses. In addition to packaging in avian cells, a second packaging procedure for vaccinia DNA, based on the abortive infection of mammalian cells with the fowlpox helper virus, was developed. This procedure permits simultaneous packaging and host-range selection for the packaged virus. The cloning/packaging procedure allows the direct insertion of foreign DNA without the need for plasmids having flanking regions homologous to viral nonessential regions and is independent of inefficient in vivo recombination events. By direct cloning and packaging, about 5-10% of the total vaccinia virus yield consisted of chimeras. The procedure is, therefore, a useful tool in molecular virology.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bostock C. J. Parameters of field inversion gel electrophoresis for the analysis of pox virus genomes. Nucleic Acids Res. 1988 May 25;16(10):4239–4252. doi: 10.1093/nar/16.10.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle D. B., Coupar B. E. A dominant selectable marker for the construction of recombinant poxviruses. Gene. 1988 May 15;65(1):123–128. doi: 10.1016/0378-1119(88)90424-6. [DOI] [PubMed] [Google Scholar]
- Cochran M. A., Puckett C., Moss B. In vitro mutagenesis of the promoter region for a vaccinia virus gene: evidence for tandem early and late regulatory signals. J Virol. 1985 Apr;54(1):30–37. doi: 10.1128/jvi.54.1.30-37.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENNER F., WOODROOFE G. M. The reactivation of poxviruses. II. The range of reactivating viruses. Virology. 1960 May;11:185–201. doi: 10.1016/0042-6822(60)90061-1. [DOI] [PubMed] [Google Scholar]
- Falkner F. G., Moss B. Escherichia coli gpt gene provides dominant selection for vaccinia virus open reading frame expression vectors. J Virol. 1988 Jun;62(6):1849–1854. doi: 10.1128/jvi.62.6.1849-1854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel S. J., Johnson G. P., Perkus M. E., Davis S. W., Winslow J. P., Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990 Nov;179(1):247-66, 517-63. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gritz L., Destree A., Cormier N., Day E., Stallard V., Caiazzo T., Mazzara G., Panicali D. Generation of hybrid genes and proteins by vaccinia virus-mediated recombination: application to human immunodeficiency virus type 1 env. J Virol. 1990 Dec;64(12):5948–5957. doi: 10.1128/jvi.64.12.5948-5957.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Hruby D. E., Schneewind O., Wilson E. M., Fischetti V. A. Assembly and analysis of a functional vaccinia virus "amplicon" containing the C-repeat region from the M protein of Streptococcus pyogenes. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3190–3194. doi: 10.1073/pnas.88.8.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ink B. S., Pickup D. J. Transcription of a poxvirus early gene is regulated both by a short promoter element and by a transcriptional termination signal controlling transcriptional interference. J Virol. 1989 Nov;63(11):4632–4644. doi: 10.1128/jvi.63.11.4632-4644.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOKLIK W. K., HOLMES I. H., BRIGGS M. J. The reactivation of poxviruses. III. Properties of reactivable particles. Virology. 1960 May;11:202–218. doi: 10.1016/0042-6822(60)90062-3. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The purification fo four strains of poxvirus. Virology. 1962 Sep;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K., WOODROOFE G. M., HOLMES I. H., FENNER F. The reactivation of poxviruses. I. Demonstration of the phenomenon and techniques of assay. Virology. 1960 May;11:168–184. doi: 10.1016/0042-6822(60)90060-x. [DOI] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7415–7419. doi: 10.1073/pnas.79.23.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr A., Malicki K. Attenuierung von virulentem Hühnerpockenvirus in Zellkulturen und Eigneschaften des attenuierten Virus. Zentralbl Veterinarmed B. 1966 Feb;13(1):1–13. [PubMed] [Google Scholar]
- Moss B. Regulation of vaccinia virus transcription. Annu Rev Biochem. 1990;59:661–688. doi: 10.1146/annurev.bi.59.070190.003305. [DOI] [PubMed] [Google Scholar]
- Panicali D., Paoletti E. Construction of poxviruses as cloning vectors: insertion of the thymidine kinase gene from herpes simplex virus into the DNA of infectious vaccinia virus. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4927–4931. doi: 10.1073/pnas.79.16.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkus M. E., Limbach K., Paoletti E. Cloning and expression of foreign genes in vaccinia virus, using a host range selection system. J Virol. 1989 Sep;63(9):3829–3836. doi: 10.1128/jvi.63.9.3829-3836.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J., Weinberg R., Languet B., Desmettre P., Paoletti E. Recombinant fowlpox virus inducing protective immunity in non-avian species. Vaccine. 1988 Dec;6(6):497–503. doi: 10.1016/0264-410x(88)90100-4. [DOI] [PubMed] [Google Scholar]
- Venkatesan S., Baroudy B. M., Moss B. Distinctive nucleotide sequences adjacent to multiple initiation and termination sites of an early vaccinia virus gene. Cell. 1981 Sep;25(3):805–813. doi: 10.1016/0092-8674(81)90188-4. [DOI] [PubMed] [Google Scholar]