Abstract
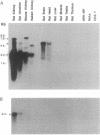
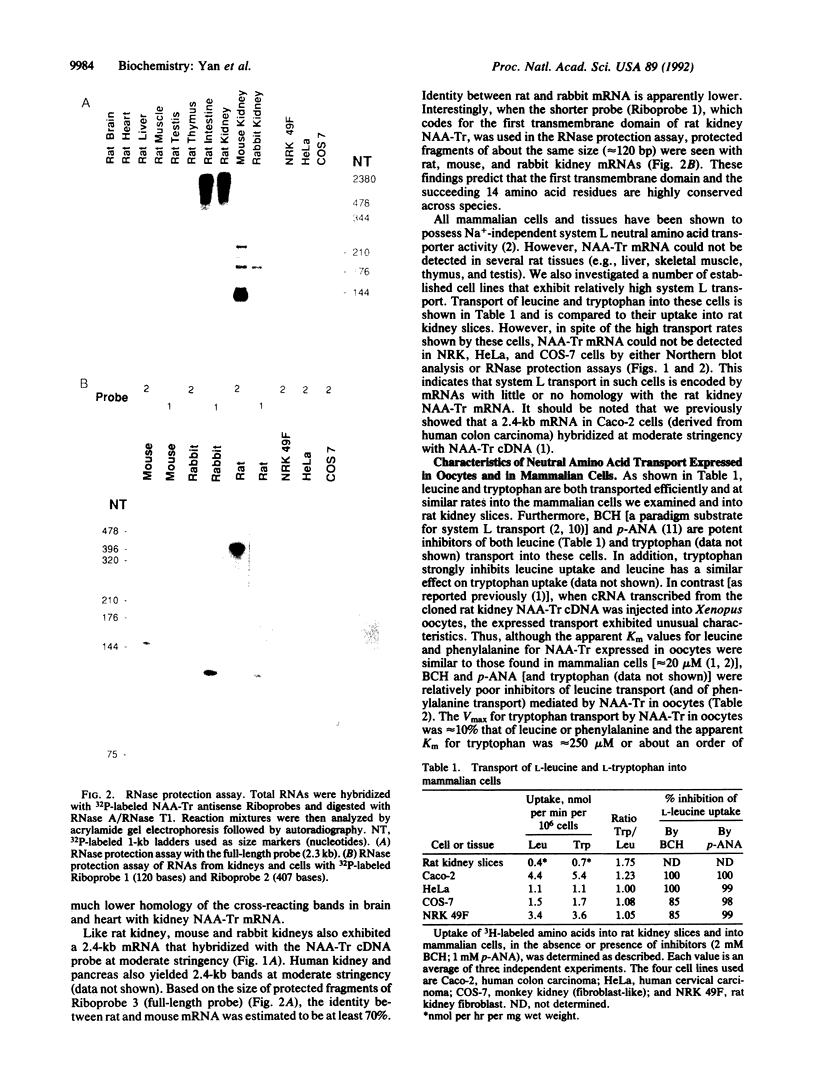
The Na(+)-independent neutral amino acid transporter (NAA-Tr) that we had previously cloned from rat kidney has been investigated with respect to its distribution in mammalian tissues and cells. By Northern blot analysis and RNase protection assay, a 2.4-kilobase (kb) mRNA in rat intestine was found to be identical to that in rat kidney. Of the other rat tissues examined, only brain and heart were found to contain mRNAs related to kidney NAA-Tr by Northern assay. However, these were larger (approximately 5 and approximately 7 kb). Mouse and rabbit kidney also contain mRNAs of 2.4 kb that exhibited a high degree of homology with rat kidney NAA-Tr. Of the several cultured cells investigated that demonstrated considerable Na(+)-independent neutral amino acid transport activity, only human colon carcinoma (Caco) cells were positive by Northern assay. The failure to detect NAA-Tr mRNA in many cells and tissues that carry out Na(+)-independent transport indicates that unrelated transporters must also exist. Cells and tissues that were negative with respect to rat kidney NAA-Tr as well as those that were positive transported leucine and tryptophan equally well. However, when mRNA from the same cells and tissues was expressed in oocytes, in all cases tryptophan was transported far less efficiently than leucine. This defect in tryptophan transport is apparently due to aberrant expression of neutral amino acid transporters in general in Xenopus oocytes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker G. A., Ellory J. C. The identification of neutral amino acid transport systems. Exp Physiol. 1990 Jan;75(1):3–26. doi: 10.1113/expphysiol.1990.sp003382. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Christensen H. N. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev. 1990 Jan;70(1):43–77. doi: 10.1152/physrev.1990.70.1.43. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- Goulding E. H., Ngai J., Kramer R. H., Colicos S., Axel R., Siegelbaum S. A., Chess A. Molecular cloning and single-channel properties of the cyclic nucleotide-gated channel from catfish olfactory neurons. Neuron. 1992 Jan;8(1):45–58. doi: 10.1016/0896-6273(92)90107-o. [DOI] [PubMed] [Google Scholar]
- Lee J. J., Costlow N. A. A molecular titration assay to measure transcript prevalence levels. Methods Enzymol. 1987;152:633–648. doi: 10.1016/0076-6879(87)52070-5. [DOI] [PubMed] [Google Scholar]
- Segel G. B., Lichtman M. A. Amino acid transport in human lymphocytes: distinctions in the enhanced uptake with PHA treatment or amino acid deprivation. J Cell Physiol. 1981 Feb;106(2):303–308. doi: 10.1002/jcp.1041060217. [DOI] [PubMed] [Google Scholar]
- Smith K. E., Borden L. A., Hartig P. R., Branchek T., Weinshank R. L. Cloning and expression of a glycine transporter reveal colocalization with NMDA receptors. Neuron. 1992 May;8(5):927–935. doi: 10.1016/0896-6273(92)90207-t. [DOI] [PubMed] [Google Scholar]
- Snutch T. P. The use of Xenopus oocytes to probe synaptic communication. Trends Neurosci. 1988 Jun;11(6):250–256. doi: 10.1016/0166-2236(88)90102-6. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Yan N., Udenfriend S. Expression cloning of a Na(+)-independent neutral amino acid transporter from rat kidney. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):1–5. doi: 10.1073/pnas.89.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udenfriend S., Zaltzman-Nirenburg P., Guroff G. A study of cellular transport with the fluorescent amino acid, aminonaphthylalanine. Arch Biochem Biophys. 1966 Sep 26;116(1):261–270. doi: 10.1016/0003-9861(66)90032-4. [DOI] [PubMed] [Google Scholar]
- Wells R. G., Hediger M. A. Cloning of a rat kidney cDNA that stimulates dibasic and neutral amino acid transport and has sequence similarity to glucosidases. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5596–5600. doi: 10.1073/pnas.89.12.5596. [DOI] [PMC free article] [PubMed] [Google Scholar]