Abstract
The ability to assess the complex motor functions of the gastrointestinal tract accurately has been of tremendous value to understanding and treating digestive diseases. Unlike other smooth or cardiac muscle organ systems with relatively more rhythmic and patterned motor behavior, the complexity of the diverse motor behaviors of the alimentary canal have made the development and use of clinical and preclinical tests of gastrointestinal motor function a great challenge. It is perhaps this complexity, as well as the importance of gastrointestinal function to overall health and well-being, that have fascinated early physiologists and continue to push modern physiologists and clinical diagnosticians to develop new and more accurate measurements of motility. It is also because of this complexity that the standardization of these measures presents hurdles to broad adoption and that the measurements of the more complex motility functions remain restricted mainly to tertiary referral centers.
Keywords: Acute Pancreatitis, Biliary Pancreatitis, Necroptosis, Apoptosis, Pancreatic Cell Death
Abbreviations used in this paper: AC, ascending colon; CF6, filling the colon at 6 hours; CT, computed tomography; GEBT, gastric emptying breath test; HDAM, high-definition anorectal pressure manometry/topography; HRAM, high-resolution anorectal manometry; HT, hydroxytryptophan; IQR, interquartile range; MMC, migrating motor complex; MRI, magnetic resonance imaging; 99mTc, technetium-99m; SPECT, single-photon emission computed tomography; 13C, carbon-13; 3-D, 3-dimensional; WMC, wireless motility capsule
Summary.
Accurately measuring the complex motor behaviors of the gastrointestinal tract has tremendous value for the understanding, diagnosis, and treatment of digestive diseases. This review synthesizes the literature regarding current tests that are used in both human beings and animals. Further opportunity remains to enhance such tests, especially when such tests are able to provide value in both the preclinical and the clinical settings.
Assessing motility in human beings has 3 obvious values. First, standardized clinical tests have diagnostic value in stratifying patients who present with a relatively limited repertoire of symptoms in the complex multifactorial digestive diseases into more manageable subsets and in the identification of underlying pathophysiology. Second, these clinical tests provide measures that can be used to objectively determine the efficacy of therapies for digestive diseases in the clinic and during drug and device development in clinical trials. Third, motility measurements in human beings have value in broadening our understanding of the physiology and pathophysiology of the gastrointestinal tract to generate new hypotheses and new drug targets to understand and treat digestive diseases.
Motility tests in nonhuman animals also have value that parallels the value of tests for human beings. First, animals serving as companions, or in labor, sports, and food production industries, benefit from the diagnostic value of accurate motility tests in veterinary medicine. Second, motility tests provide the basis for objective measures to assess the efficacy and dosing guidelines of new therapies in preclinical drug and device development. Third, animal models provide the basis for understanding the physiology and pathophysiology of the gastrointestinal tract. This latter value historically has been greater in nonhuman animals because of the ability to conduct terminal or ex vivo experiments followed by anatomic or biochemical assessments that are not possible in human beings.
The purpose of this review is to critically assess the current state of motility tests (listed in Table 1, and examples given in Figure 1), based on these values in both human beings and non–human beings. It is organized by region of the alimentary canal in an oral to anal direction, followed by measures of whole-gut transit. The hope is that such juxtaposition of human and nonhuman tests will enlighten both the benefit and deficiencies in each to aid in the de novo or cross-development of new motility tests.
Table 1.
Tests Currently Available for Measuring Gastrointestinal and Colonic Motility
| Function | Tests available |
|---|---|
| Gastric capacity or accommodation | Barostat balloon measurements |
| Nutrient drink test | |
| SPECT | |
| Ultrasonography | |
| MRI | |
| High-resolution intragastric manometry | |
| Gastric emptying | Scintigraphy |
| Wireless pH and motility capsule | |
| Stable isotope breath tests | |
| Gastric transit in preclinical studies | Analysis of gastric contents |
| Stable isotope breath tests | |
| Scintigraphy | |
| Small-bowel transit | Breath hydrogen tests |
| Stable isotope breath tests | |
| Scintigraphy | |
| Wireless pH and motility capsule | |
| Whole-gut transit in preclinical studies | Nonabsorbable marker such as carmine red |
| Scintigraphy using steel beads and barium in mice | |
| Colonic transit | Radiopaque markers |
| Scintigraphy | |
| Wireless pH and motility capsule | |
| Gastrointestinal, colonic, and anorectal contractility | Antropyloroduodenal manometry |
| Wireless pH and motility capsule | |
| Colonic phasic contractility (including high-resolution manometry) and tone | |
| Anorectal manometry | |
| Colonic motility and transit in preclinical studies | Bead expulsion |
| Colonic manometry (including high-resolution manometry) | |
| Scintigraphy | |
| New MRI applications | All the earlier-described functions as well as anorectal and pelvic floor motion and anatomic integrity |
NOTE. Tests with the strongest validation or most widely available and used are indicated in italics.
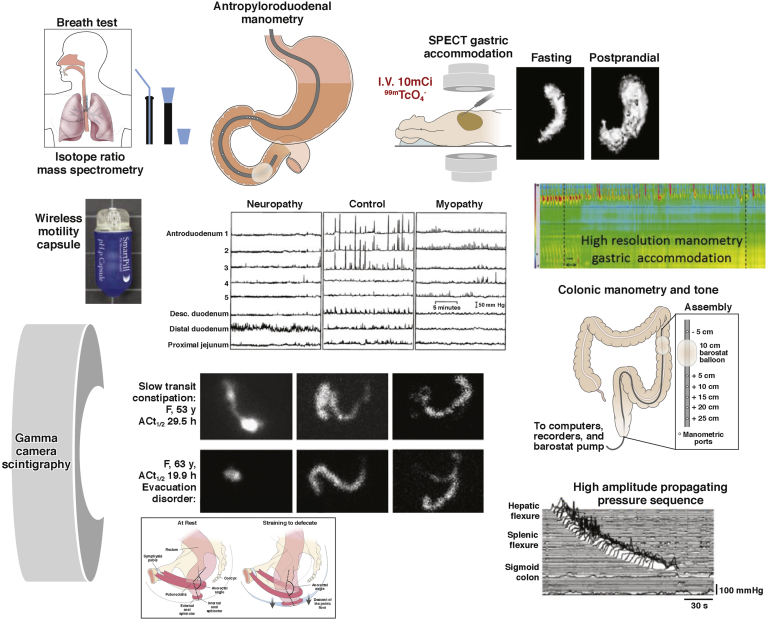
Figure 1.
Examples of the wide range of motility measurements available for human studies: stable isotope breath test, scintigraphic transit, intraluminal manometry by perfused manometers or strain gauges on tubes or wireless capsules, and measurement of gastric capacity and accommodation by SPECT or high-resolution manometry.
In the interest of brevity, we will not describe tests of esophageal motility here. High-resolution manometry has become the diagnostic tool of choice, about which many recent reviews have been published.1, 2, 3
Tests to Evaluate Gastric Capacity and Accommodation
One of the principal functions of the proximal stomach is the storage of ingested food. The gastric fundus and body are able to accommodate large volume changes, while maintaining a relatively low intragastric pressure. Altered gastric tone and distensibility may occur in several disease states, including tumor infiltration, vagal dysfunction, and post–gastric surgery status, and in up to 40% of patients with functional dyspepsia.4
Barostat Balloon Measurements
The gold standard for the measurement of tone in hollow organs was the barostat,5 which estimates changes in tone by the change of volume of air in an infinitely (typically polyethylene) compliant balloon maintained at a constant pressure to maintain the balloon in apposition with the stomach lining. The barostat maintains the constant pressure by infusion or aspiration of air in response to relaxation or contraction of stomach tone. This method is not used extensively in clinical practice because it requires intubation and results in stress and discomfort during the tests, which may last 3 hours or longer.6
Development and validation studies of the barostat to measure compliance, tone, and postprandial accommodation in the dog were performed by Azpiroz and Malagelada.5 Since then, the barostat has been used extensively in animals including cats,7 rabbits,8 pigs,9 horses,10 rats,8, 11 and mice.12
Satiation or Nutrient Drink Test
The nutrient drink test has been proposed as a surrogate method for estimating gastric volumes. In this test, a standardized liquid nutrient drink, such as Ensure (1 kcal/mL; Ross Products, Division of Abbott Laboratories, Columbus, OH), is ingested at a standard rate of 30 mL/min, and the volume to normal fullness and the maximum tolerated volume are recorded as measures of satiation. Postprandial symptoms of nausea, fullness, bloating, and pain are measured 30 minutes after the meal.13 Tack et al14 suggested that a high-caloric, slowly administered drinking test compared favorably with the barostat in predicting impaired gastric accommodation, especially in patients with a maximum tolerated volume less than 750 kcal. Because of the obvious limitations of feedback regarding sensory experiences, there are no reports of the use of nutrient drink tests in nonhuman animals.
Single-Photon Emission Computed Tomography
Single-photon emission computed tomography (SPECT) imaging has been validated extensively in vitro and in vivo for the measurement of gastric volumes during fasting and postprandially in human beings, including comparison with the barostat.15, 16, 17 After intravenous administration of 10 mCi technetium-99m (99mTc)-pertechnetate, a substrate for the sodium/iodide symporter that is accumulated and secreted into the lumen by parietal and mucin-secreting cells of the gastric mucosa, tomographic images of the stomach are acquired using a large field-of-view, dual-headed gamma camera, with the patient in a supine position. From the transaxial images of the stomach, 3-dimensional images are reconstructed using a commercially available software analysis program that is used for other 3-dimensional volume rendering with transaxial imaging (eg, computed tomography [CT], magnetic resonance imaging [MRI]) and total gastric volume is measured during fasting and during the first 10 minutes after a standard liquid nutrient meal (300 mL Ensure). This allows reconstruction of the stomach based on the location of the mucosal layer, and the estimated volume serves as a surrogate for the internal volume of the stomach. SPECT shows the effects of disease on post-meal gastric accommodation and effects of medications such as nitrates, erythromycin, glucagon-like peptide-1, and octreotide18, 19 in health and diseases such as diabetes, fundoplication, and functional dyspepsia.20, 21
Intraindividual and interindividual coefficients of variance in postprandial and accommodation volumes by SPECT were not significantly different and ranged from 16% to 22%.22 The effects of liquid and solid equicaloric meals on gastric volumes have been described, and measurements of gastric volume with the same caloric liquid meal an average of 9 months apart showed a coefficient of variation of 10%.23
It also is possible to measure gastric emptying and volume simultaneously.24, 25 The noninvasive nature of the method is attractive and is used extensively at the Mayo Clinic in research and practice, especially in suspected disorders of gastric accommodation such as dyspepsia. However, the test involves radiation exposure, and SPECT equipment and the 3-dimensional reconstruction and volume rendering are not widely available. Another potential pitfall is that the resolution of the imaging does not equal that of CT or MRI.
Application in animal studies
Although NanoSPECT-CT (Mediso Medical Imaging Systems, Budapest, Hungary) of gavaged technetium-labeled activated charcoal diethylene triaminepentaacetic acid has been used to assess gastrointestinal transit in mice,26 to our knowledge SPECT imaging has not been used to assess gastric accommodation specifically in nonhuman animals. Given the rapidly advancing use of 99mTc-pertechnetate and other sodium/iodide symporter substrates in numerous animal models27, 28 as well as descriptions of methods to circumvent the high stomach signal that confounds such studies,29 it is reasonable to assume that this well-validated approach to assess gastric accommodation in human beings can be reverse-translated easily for use in preclinical studies.
Ultrasonography
Imaging-based methods to measure gastric volume include 3-dimensional reconstruction of images acquired by ordinary ultrasonography assisted by magnetic scan-head tracking.30, 31 Thus, an outline of the total stomach volume visualized after ingestion of a liquid meal (that serves as a contrast medium)32 has been applied in adolescents and compared with simultaneously measured gastric volumes by SPECT.33
Magnetic Resonance Imaging
The first application of MRI using a spin-echo T1-weighted imaging sequence addressed the volume of the stomach during fasting, but not in the postprandial period.34 Volumes measured with MRI and barostat differ significantly because the barostat measures only the proximal stomach, whereas MRI records the entire stomach volume; however, there was a statistically significant correlation between the 2 methods, and MRI also was able to show volume effects induced by glucagon (increase) and erythromycin (decrease).
MRI, using 3-dimensional gradient-echo and 2-dimensional half-Fourier acquisition single-shot turbo spin echo sequences,35 has been used to measure postprandial gastric volume change, which exceeded the ingested meal volume by 106 ± 12 mL (SEM). The advantage of MRI over SPECT is the ability to distinguish air from fluid under fasting and postprandial conditions, respectively. MRI also has shown that the postprandial volume excess mainly comprised air (61 ± 5 mL), which was not significantly different when the volume ingested was ingested in 30- or 150-mL aliquots. Fasting and postprandial gastric volumes measured by MRI generally were reproducible within subjects. Gastric volumes measured by SPECT were higher than MRI, reflecting the fact that SPECT reconstruction includes the volume occupied by the imaged gastric wall. Although MRI has many advantages, including a lack of radiation exposure, it is not widely used to measure gastric accommodation in clinical practice or research.
High-Resolution Intragastric Manometry
By using a high-resolution manometry catheter, which typically is used for esophageal motility measurements, Janssen et al36 showed that, during nutrient drink ingestion, there is a reduction in intraluminal pressure that provides a less-invasive alternative to the barostat for the assessment of gastric accommodation. The method also has been used to show pharmacologic effects, such as with peppermint oil37 and liraglutide.38
Gastrointestinal and Colonic Transit
Gastric Emptying
Scintigraphy
Gamma camera scintigraphy is the most widely used test for the assessment of gastric motility; it provides a direct, noninvasive quantification of gastric emptying.39 A simplified protocol with imaging at 1, 2, and 4 hours with a standard meal was first proposed at the Mayo Clinic,40 and a variation subsequently was validated in a large multinational study in 123 subjects41 and was adopted by the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine.42 A standard, 2% fat meal consisted of 4 ounces of Eggbeaters (Conagra Foods, Omaha, NE) or equivalent egg white substitute, 2 slices of bread, strawberry jam (30 g), and 120 mL water (total 240 kcal) and was radiolabeled with 0.5–1 mCi 99mTc-sulfur colloid. This is a relatively small meal, which may not reliably induce symptoms in patients with functional dyspepsia, although it is useful for diagnosing gastroparesis.
The Mayo Clinic method uses 2 natural eggs and contains 30% of the calories as fat (total, 320 kcal). The test meal determines the rate of emptying,43 and normal values are essential for interpretation of the test when performed clinically; thus, Mayo Clinic published data from 319 healthy controls.44 There is significant intraindividual variation in gastric emptying rates of 12%–15%, even in healthy individuals.44, 45 The performance characteristics of the 30% fat, 320-kcal meal have been documented.44
The main indications for use of this test are the investigation of unexplained nausea, vomiting, and dyspeptic symptoms; screening for impaired gastric emptying in diabetic patients being considered for incretin treatment to enhance glycemic control (eg, pramlintide and GLP-1 agonists); and assessment of patients with suspected diffuse gastrointestinal motility disorder in combination with small-bowel and colonic transit.
In human research, the gastric emptying test is used to understand the pathophysiology of symptoms or in the development of pharmacologic agents. There is a vast amount of literature on the use of radioscintigraphy to measure gastric emptying in large animals. The first application documented the effects of vagotomy and carbachol on gastric emptying in dogs.46
Wireless pH and motility capsule
These nondigestible wireless capsules can measure pH, pressure, and temperature throughout the gastrointestinal tract. The abrupt change in pH from the gastric acidic milieu to the almost alkaline duodenum usually is associated with antral phasic contractions of the migrating motor complex (MMC), and it signals that the capsule has left the stomach.47 When taken with a meal, the capsule generally empties from the stomach after liquids and triturable solids have emptied, usually with phase III of the MMC or, in approximately one third of cases, with high-amplitude antral contractions.48
Patients ingest the capsule with a standard meal and, from 6 hours after capsule ingestion, patients can engage in normal daily activity, including ad libitum feeding. The wireless capsule acquires data continuously for up to 5 days, and this permits calculation of gastric, small-bowel, colon, and whole-gut transit. These wireless capsules also measure intraluminal pressure. In validation studies conducted with simultaneous gastric emptying by scintigraphy in healthy subjects and patients with gastroparesis,49 the gastric emptying time for the capsule and the scintigraphic gastric emptying time at 4 hours were correlated significantly (r = 0.73), and the capsule discriminated between normal or delayed gastric emptying with a sensitivity of 0.87 at a specificity of 0.92.49 The advantages of the motility capsule are that the study can be conducted anywhere, there is a lack of radioactivity, and it has an ability to determine small-bowel, colon, and whole-gut transit times, as well as contractility.50, 51, 52 The wireless motility capsule also is capable of identifying the effects of pharmacologic agents on gastric contractility.53
Stable isotope breath tests
These tests evaluate gastric emptying noninvasively and without radiation hazard. The carbon-13 (13C) isotope can be incorporated into components of a solid meal (eg, the medium-chain fatty acid, octanoic acid, or the blue-green algae, Spirulina platensis) or in a liquid meal (13C-acetate) to assess gastric emptying of liquids. After ingestion, the solid meal is triturated and emptied by the stomach, digested, and absorbed in the proximal small intestine, metabolized by the liver, and 13CO2 is excreted by the lungs, resulting in an increase in expired 13CO2 over baseline. The test and breath sample collection can occur at the point of care. The rate-limiting step in 13CO2 excretion is gastric emptying of the meal.
In studies comparing the 13C gastric emptying breath test (GEBT) performed simultaneously with scintigraphy, the GEBT provided accurate assessment of the gastric emptying of solids with a coefficient of variation comparable with scintigraphy.45 In addition, the 13C-spirulina GEBT documented accelerated or delayed gastric emptying induced with erythromycin or atropine, respectively.54 The 13C-spirulina GEBT was approved by the Food and Drug Administration in 201555 based on a clinical study using data from 115 participants who typically would have undergone a gastric emptying test, showing that the GEBT results agreed with the scintigraphy results 73%–97% of the time when measured at various time points during the test.56 Pitfalls included a potential loss of accuracy in patients with diseases involving the intestinal mucosa, pancreas, liver, and respiratory system.
Gastric Emptying Studies in Nonhuman Animals
Acute gastric emptying study by analysis of gastric contents
The long history and vast number of physiological and preclinical drug development studies using timed end point analysis of gastric contents after gavage or ingestion of a labeled or visually detectable and quantifiable meal to study gastric emptying are too numerous to reproduce in this short review. The approach is precise and inexpensive because, rather than relying on indirect measurements, the percentage of the meal remaining in the stomach can be calculated directly. Therefore, the approach remains the standard by which preclinical validation studies of novel tests of gastric emptying are compared. Relatively recent advancements in this approach have been the development of new tracers to increase the sensitivity of labeled meal detection as well as the detection of the labeled meal throughout the gastrointestinal tract to assess overall transit as measured by the geometric center,57, 58 rather than the percentage emptied or leading edge. These new tests that define the geometric center and segment distribution also can be used to study whole-gut transit.
Longitudinal gastric emptying studies
The advent of longitudinal studies in mice that required repeated measures of gastric emptying during disease development or therapy has led to the use of gastric emptying tests developed for human beings in preclinical models. In these studies, the need for noninvasive repeatable measures outweighs the relatively low interindividual variability and cost of end point studies. These tests include scintigraphy59, 60 and the 13C-octanoic acid GEBT.61, 62 The 13C-octanoic acid GEBT also has been validated for measurement of gastric emptying in horses,63, 64 cattle,65 dogs,66, 67 and rats.68 In addition, in nonobese diabetic mice, a model of type 1 diabetes, the expected effects of bethanechol and atropine were shown.62 These gastric emptying tests developed in human beings and now used routinely in preclinical studies, which can be translated directly into clinical trials, are the clearest example of the benefit of the bedside-to-bench-to-bedside approach, and should serve as a model for other motility tests that are presented in this review.
Gamma camera scintigraphy has been validated for use in small animals; thus, awake mice were accustomed to light restraint and to eating cooked egg white (0 g fat), whole egg (0.10 g fat/g), or egg yolk (0.31 g fat/g). Gastric emptying of each diet was measured by labeling the test meals with 99mTc-mebrofenin and using a conventional gamma camera equipped with a high-resolution, parallel-hole collimator. This method has been used to document the effects of devazepide (a cholecystokinin-A–receptor antagonist)60 botulinum toxin injection into the antral wall in knockout animals and in pharmacologic modulation,69, 70 and the role of inflammation and novel therapies for postoperative ileus in animal models.71, 72
Recent advances in ultrasound research in nonhuman animals have suggested that clinical tests using this noninvasive approach soon may be in development.73, 74
Orocecal or Small-Bowel Transit
Breath Hydrogen Tests
The breath test is based on the presumption that the hydrogen excreted in the breath is the product of colonic bacterial fermentation of unabsorbed carbohydrates ingested orally. Lactulose is the most widely used carbohydrate substrate for orocecal transit time determination because the time lag between ingestion of lactulose and the increase in breath hydrogen is at least 3, 5, or 10 parts per million above baseline. There is a high degree of correlation with simultaneous liquid transit by scintigraphy,75, 76, 77, 78 and the test documents pharmacologic effects on gut motility.79, 80, 81, 82, 83 Unfortunately, lactulose itself markedly accelerates transit, presumably owing to its osmotic activity. In addition, these tests usually are conducted during fasting, and this may not accurately reflect postprandial small-bowel transit, and, usually, symptoms occur postprandially.
Stable Isotope Breath Test
A stable isotope (lactose 13C-ureide) was proposed with a very small dose of the substrate (0.5–1.2 g), which cannot be cleaved at the human intestinal brush border and requires the colonic bacterial flora to free 13C-ureide, which subsequently undergoes hydrolysis with release of 13CO2 and excretion by the lungs. The ratio of breath 13CO2/12CO2 is determined by isotope ratio mass spectrometry, and the first increase in breath at 2.5 SDs greater than the running average indicates the orocecal transit time.84 The lactose 13C-ureide test has been validated by comparison with scintigraphy84 and has been used to evaluate the effects of pharmacologic agents.85, 86, 87
Small-Intestine Scintigraphy
Small-bowel scintigraphy is not used commonly outside of research as part of whole-gut transit tests.88 Small-bowel transit time can be calculated as the time for 10% or 50% of the radiolabel to arrive at the terminal ileum or cecum, after correcting for gastric emptying by subtracting the time for the equivalent proportion emptied from the stomach.89 A valid surrogate for the 10% scintigraphic small-bowel transit time is the percentage of the meal filling the colon at 6 hours (CF6), which reflects orocecal transit.40 Because the radiolabel is ingested with the meal, the result is impacted significantly by the rate of gastric emptying. The range of normal values for colonic filling at 6 hours is 11%–70% with radiolabeled nondigestible particles,90, 91 or 43%–95% for radiolabeled digestible solids.92 However, more recent studies conducted in more than 200 healthy controls have shown that variation in CF6 in healthy subjects is 0%–100% and, therefore, it is not possible to definitely diagnose abnormal small-bowel motility using CF6 (Camilleri, unpublished data). In clinical practice, a low value (eg, <20%) of CF6 often is associated with slow colonic transit, particularly in the right colon, and it is unclear whether this is a reflection of significant pathology in the small intestine or is simply a consequence of failed ascending colon emptying preventing the flow of the ileal content into the colon.
In research, these measurements of small-bowel transit time have shown the effects of treatment, such as cisapride in gastroparesis and chronic intestinal dysmotility,93, 94 tegaserod in irritable bowel syndrome with constipation,95 or prucalopride and YKP10811 in functional constipation.96, 97
First CT enterography and now MR enterography have become routine diagnostic tools for inflammatory bowel disease,98, 99 and it is of great interest that these tests include dynamic image sets to assess motility in affected bowel segments.100 Perhaps the routine collection of large data sets can be used to develop better automated image analyses and more robust small-bowel imaging for motility disorders independent of organic disease. Indeed, a very recent study used the automated motility assessment of MR enterography developed for patients with Crohn’s disease to assess small-bowel motility in patients with chronic intestinal pseudo-obstruction,101 showing the potential of such an approach.
Use of Wireless pH and Motility Capsule to Measure Small-Bowel Transit
Small-bowel transit time also can be measured with a wireless motility capsule (WMC).49 The transit of large indigestible solids depends on the phase II and phase III activity of the interdigestive motor complex,102 as well as fed intestinal motility, but the evidence is limited. The procedure is similar to that for WMC measurement of gastric emptying. The WMC small-bowel transit time is defined as the interval between this increase in pH and the time when the pH suddenly decreases by more than 1 U for at least 5 minutes as the WMC enters the cecum.
In separate studies in 9 healthy subjects, the median WMC small-bowel transit time was 350 minutes (interquartile range [IQR], 169–676 min),103 276 minutes (IQR, 240–354 min),104 and 234 minutes (IQR, 201–293 min).105 A practical disadvantage of the test is occasional difficulty in identifying the 1-U pH decrease signifying passage into the cecum, especially in patients with postsurgical changes (eg, right hemicolectomy) or incompetent ileocecal valve resulting in bacterial colonization of the distal ileum. The nondigestible, 26 × 13 mm WMC may become impacted in the gastrointestinal tract; therefore, contraindications include suspected mechanical obstruction, recent gastrointestinal surgery (within 3 months), and Crohn’s disease.
Whole-Gut Transit in Preclinical Models
Whole-gut transit in mice typically is studied by the oral administration of a nonabsorbable marker such as carmine red and subsequent monitoring for the first appearance of the marker in stool.106, 107 Obviously, this test is an assay of the leading edge of the content within the digestive tract that may simplify or misrepresent the overall motility of the gastrointestinal tract. Terminal experiments, in which the orally administered tracers are assayed in numerous segments of the gastrointestinal tract to assess overall transit as measured by the geometric center,57, 58 improve the limitations of leading edge experiments such as carmine red, but re-introduce the requirement for terminal, rather than repeatable, tests of whole-gut transit.
A recent study described scintigraphy using steel beads and barium in mice to measure intestinal transit in a nonterminal manner.108 This is an intriguing development that may lead to better noninvasive preclinical studies; however, the relatively poor accuracy of clinical scintigraphy for intestinal transit, as presented earlier, may limit the translational potential of the method.
WMC for use in whole-gut transit was validated using dogs109 and has been used in several studies in dogs,110, 111, 112 pigs,113 rabbits,114 and horses.115 To our knowledge, validated technologies have not been developed for use in small animals.
Evaluation of Colon Transit
Radiopaque Markers
Radiopaque markers can be used to evaluate total and segmental colonic transit times. Several different methods to measure colonic transit with radiopaque markers have been described.116, 117, 118, 119, 120 The minimum number of markers to be ingested daily should be at least 10–12 for reporting colonic transit time in days or hours.121
Localization of retained markers in the rectosigmoid area suggests functional outlet obstruction, whereas diffuse distribution of the retained markers is suggestive of slow-transit constipation. However, this is not diagnostic because delayed rectosigmoid transit resulting from pelvic floor dyssynergia may inhibit proximal colonic transit and result in widespread distribution of markers.
Segmental transit times are measured in the right colon to the right of the vertebral spinous processes and above a line from the fifth lumbar vertebra to the pelvic outlet. The left colon is the area to the left of the vertebral spinous processes and the line above the fifth lumbar vertebra and the left anterior superior iliac crest. The rectosigmoid is the area under the line from the pelvic brim on the right to the superior iliac crest on the left. The advantages of the radiopaque marker method are the well-established normal values and standardization of methods, it also readily is available and is reasonably inexpensive. Sadik et al120 have quantitated rapid colonic transit using radiopaque markers. Radiopaque markers are the reference standard for colon transit evaluation in clinical practice.122
Colonic Scintigraphy
Measurement of colonic transit by scintigraphy is safe, noninvasive, correlates with radiopaque markers, and provides information on ascending colon (AC) emptying and overall colon transit.123 In the most widely published method, subjects ingest a pH-sensitive methacrylate-coated capsule containing indium-111–labeled activated charcoal particles after fasting overnight, the coated capsule dissolves in the neutral pH in the terminal ileum, releasing the radioisotope into the lumen.91 The alternative method follows the transit of radiolabeled liquid in a whole-gut transit test for a solid–liquid meal including colonic transit.124 Repeated anterior and posterior abdominal scans of 2 minutes’ duration acquired with a gamma camera at 4, 6, 8, 24, and 48 hours after ingestion to appraise colonic transit125 were summarized as a geometric center (weighted average) of radioactivity based on 5125 or 7 regions.124 The times of greatest interest for overall colonic transit are at 24, 48, and 72 hours. The delayed-release capsule facilitates the measurement of AC emptying as the time for emptying half of the radioactivity from the AC, which is calculated by linear interpolation of values on the AC emptying curve.126 Thus, by delivering radiolabeled charcoal particles to the ileocolonic region upon capsule dissolution, the particles empty from the ileum into the colon by bolus movements.127
Normal values, performance characteristics, coefficients of variation, application in patients with diarrhea or constipation, differences in transit profile between slow colonic transit constipation and defecation disorders, and relationship between colonic transit summaries at 24 and 48 hours and bowel function symptoms have been documented extensively.126, 128, 129, 130
The intraindividual coefficients of variation were 31% at 24 hours and 27% at 48 hours over a period of less than 3 weeks, and 38% at 24 hours and 30% at 48 hours over a median interval of 2 years.126 The degree of variation is similar across different mean values of colonic transit, and the vast majority of individuals have replicate values within 1 geometric center unit of measurement. This variation reflects the physiological, natural variation in stool frequency and consistency.
Whole colonic scintigraphic transit and AC emptying have been reported to be abnormal in several diseases of colonic motility, including idiopathic constipation, functional diarrhea, carcinoid diarrhea,131 and different subtypes of irritable bowel syndrome132 and bile acid diarrhea. It therefore is plausible that colonic transit measurement may serve as a biological marker of colonic function in disease and as a surrogate end point in the evaluation of drug therapy.133 For example, scintigraphic colonic transit correctly has predicted clinical efficacy with medications targeting different mechanisms, including prokinetics such as 5-HT4 agonists, bisacodyl, and neurotrophin-3; medications retarding transit such as 5-hydroxytryptophan3 antagonists and cholecystokinin-1 antagonist; and secretagogues such as linaclotide and lubiprostone. Equally important, colonic transit measurement correctly has predicted a lack of efficacy of medications to alter bowel dysfunction in irritable bowel syndrome in clinical trials when there were no significant effects of the drug on colonic transit, such as pexacerafont (corticotropin-releasing factor-1 antagonist) and solabegron (β3 adrenergic agonist).
Use of Wireless pH and Motility Capsule to Measure Colonic Transit
The passage of the WMC into the cecum is determined by the sudden decrease of pH by more than 1 U, which lasts for at least 5 minutes; colonic transit time is the time from entry into the cecum to the time the WMC passes out of the colon with the sudden decrease in temperature and loss of pressure recordings. Colonic transit time in 78 constipated and 87 healthy subjects showed a median value of 21.7 hours (IQR, 15.5–37.3 h; 95th percentile, 59 h) in healthy subjects and 46.7 hours (IQR, 24.0–91.9 h) in constipated patients.134 The correlation of the WMC to the percentage of radiopaque markers retained on day 5 was significant (r = 0.69) in constipated patients studied simultaneously with both methods.134
In a large multicenter study of 158 patients with constipation, there was overall agreement of 87% for classifying subjects as having slow or normal colonic transit.105 The WMC also has been used to characterize pressure activity in colons of healthy controls and in constipated patients.52
The advantages of the WMC assessment of colonic transit are performance in the patients’ usual surroundings, appraisal of whole gut and regional transit, and no radiation exposure. Sometimes, discerning the 1-U decrease in pH (entry into the cecum) may be difficult. The method is more expensive than other methods (radiopaque markers, scintigraphy) to measure colonic or whole-gut transit.
Tests to Evaluate Gastrointestinal, Colonic, and Anorectal Contractility
Antropyloroduodenal Manometry
The distal stomach, pylorus, and duodenum, with their relatively small diameters and ability to generate high-amplitude pressure activity, are suitable for manometric recordings (Figure 2). Antroduodenal manometry is available mainly at a few tertiary referral centers; the test is invasive, typically requiring tube placement with the aid of upper gastrointestinal endoscopy, time consuming, and requires skilled technical support. An alternative, a wireless motility capsule, detects the frequency and amplitude of phasic contractions during the process of capsule emptying from the stomach and its passage through the small intestine (and colon, see later).
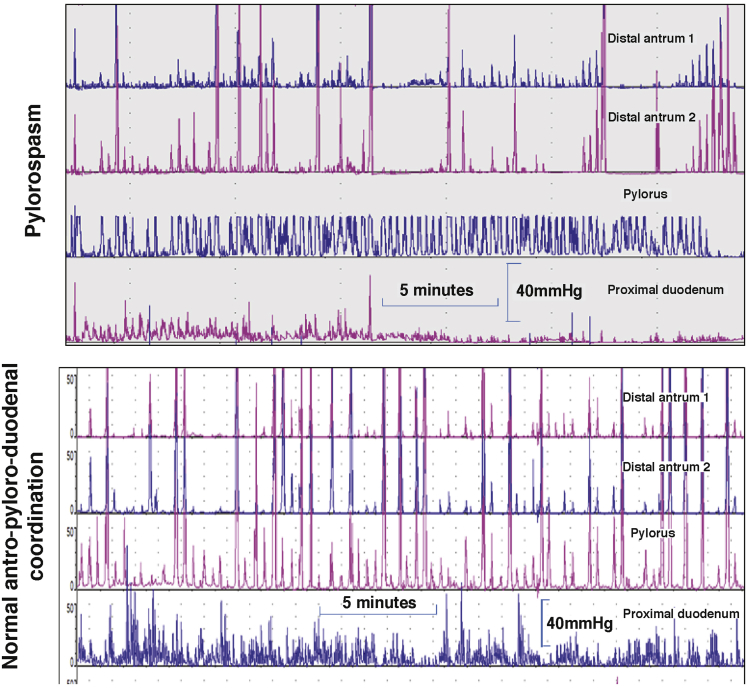
Figure 2.
Antroduodenal motility tracings in the postprandial period with sensors 1-cm apart. Note in the upper example the consistent phasic and tonic contractions at the pylorus with intermittent loss of distal antral contractions 1 and 2 cm proximal to the pylorus. In contrast, note the consistent antropyloric coordination in the normal example in the lower tracings.
Reproduced with permission from Camilleri.185
The main indications for antroduodenal manometry are as follows: evaluation of the cause of documented gastric or small-bowel transit (neuropathy or myopathy) and clarification of whether there is a generalized or localized dysmotility in patients with suspected colonic inertia. The method typically uses water-perfused manometric catheters or solid-state sensors mounted on motility catheters. The tube is positioned with the aid of fluoroscopy. The intragastric sensors should be 1 cm apart or less, to ensure optimal measurements of distal antral contractile activity proximal to the pylorus, which can be identified manometrically by a combination of distal antral and duodenal peaks and the presence of a high-pressure zone (tone). A solid-liquid meal is ingested during the test, which predominantly appraises the amplitude of contractions and the physiological responses in the fasting and postprandial periods. Thus, normal gastroduodenal motility consists of at least 1 MMC per 24 hours, conversion to the fed pattern with ingestion of a meal without return of the MMC for at least 2 hours after a meal of more than 400 kcal, and consistent distal postprandial antral (>40 mm Hg, with an average frequency of 1 contraction/min during the first postprandial hour) and small intestinal contractions (>20 mm Hg).135
Myopathic disorders (eg, scleroderma, amyloidosis, hollow visceral myopathy) are characterized by low-amplitude contractions (consistently <20 mm Hg in the small bowel and <40 mm Hg in the distal antrum).136 In neuropathic disorders, there is increased frequency (eg, 3 during 3 hours) of fasting MMCs in the duodenum while awake, postprandial antral hypomotility (average frequency of contractions in distal antrum <1/min during the first postprandial hour), and a return of phase III MMC-like activity within 2 hours of the ingestion of a meal of more than 400 kcal.137
Correlations of findings on manometry with histopathology are poor, but they are based on a few detailed reports138 of cases of pseudo-obstruction. Manometry has to be interpreted with caution because abnormal motor patterns do not necessarily imply causation of the patient’s symptoms. Stress related to the intubation and procedure may delay gastric emptying, impair antral contractility, suppress MMC cycling, and induce intestinal irregularity.
The greatest use of gastroduodenal manometry in research is in drug development, that is, in showing prokinetic effects of novel medications in addition to effects on gastric emptying, such as with the 5-HT4–receptor agonist, cisapride,93 and the ghrelin-receptor pentapeptide agonist, relamorelin.139
Colonic Phasic Contractility and Tone
Tone is measured by the barostat, phasic contractions can be measured by manometry or wireless pressure capsule. Stationary laboratory-based studies to assess motility usually are conducted for 6 hours, during which colonic compliance, fasting, and 2-hour postprandial recordings of contractions and tone are conducted. Ambulatory studies usually are conducted over 24 hours and involve measurement of phasic contractions.140 There is evidence that an increased number of solid-state sensors on the colonic tube or a fiberoptic manometry catheter, pioneered by thorough and systematic research conducted predominantly by Australian groups,141 results in high-resolution measurements of antegrade and retrograde contractions, as well as definition of the locus of origination of high-amplitude propagated contractions.
In clinical practice, the main indications for colonic manometry (usually with barostat assessment of compliance and tone) are as follows: severe constipation with slow colonic transit and no evidence of an evacuation disorder in patients who are unresponsive to medical therapy,142 and confirmation of chronic megacolon or megarectum (Figure 3) when viscus diameters exceed 10 and 15 cm, respectively.143 The most useful measurements in research and clinical practice are compliance142, 143 and high-amplitude propagated contractions,144 and the responses to intravenous neostigmine.145 The reproducibility and performance characteristics of compliance and tone measurements have been documented in the literature.146
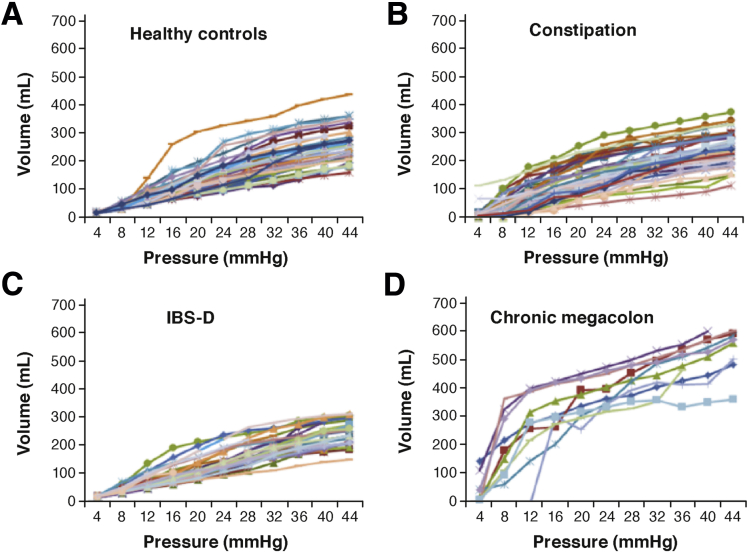
Figure 3.
Colonic compliance in (A) healthy, (B) functional constipation/constipation-predominant irritable bowel syndrome, (C) diarrhea-predominant irritable bowel syndrome (IBS-D) groups; and (D) patients with chronic megacolon. Note the markedly increased volume of the intracolonic balloon (10-cm long) in patients with megacolon compared with controls.
Reprinted with permission from O’Dwyer et al.143
Colonic motility and tone or compliance have been used to show the effects of biological agents (eg, bile acids),147 pharmacologic agents (eg, cholinergic modulation as with neostigmine),148 adrenergic agents,149 cannabinoid-receptor agonist,150 ghrelin-receptor agonist,151 and 5-HT4–receptor agonist.152
Colonic measurement of contractions has been used in several preclinical models recorded by surgically implanted strain-gauge transducers. For example, Briejer et al153 showed induction of giant migrating contractions by prucalopride in the dog, and Sarna et al154 examined several neurotransmitter substances that modulate contractile activity of the rat colon. High-resolution manometry has advanced rapidly and may become a preclinical model of choice.155
Similarly, measurement of colonic tone, compliance and reflexes, and responses to meals and pharmacologic agents have been measured in the canine colon, into which a barostat device was inserted, typically through a cecal cannula.156, 157, 158 More recently, barostat studies of the colon have been performed in the horse159 and pig.160
Anorectal Manometry
Anorectal manometry is essential for the evaluation of patients with constipation (to exclude evacuation disorders) or fecal incontinence.155 Two technologies that currently dominate in practice and research161, 162, 163 are as follows. First, high-resolution anorectal manometry (HRAM) with flexible catheters, typically with 8–12 longitudinal sensors spaced approximately 0.6- to 1-cm apart, and the most proximal 1 or 2 sensors within a balloon attached to the uppermost part of the catheter for rectal distension/sensory testing, and the most distal sensor, left outside of the anal canal, recording atmospheric pressure. Other configurations use 12–36 circumferential sensors or 4 radially arranged sensors at each level, from which pressures can be averaged to provide a mean pressure. Second, 3-dimensional high-definition anorectal pressure manometry/topography (3-D HDAM), which uses a rigid probe (100-mm length and 10.75-mm diameter) housing 256 pressure sensors arranged in 16 rows spaced 4-mm apart, each containing 16 circumferentially oriented sensors 2.1-mm apart). The area of measurement is 6.4-cm long and provides detailed anal morphology by linear interpolation through dedicated software to provide 2-dimensional or 3-D cylindric topography of the anal canal, which can be viewed from all sides.
Normal values that are influenced by age, sex, and body mass index have been published. In general, there is reasonable concordance between studies of average values reported for anal resting tone, although resting tone and squeeze pressures generally are higher when measured with HRAM/3-D HDAM techniques, possibly owing to reduced fidelity of traditional water-perfused systems. Further studies are required to validate the utility of HRAM/3-D HDAM techniques for evaluating and diagnosing dyssynergia in patients with defecatory disorders and fecal incontinence.155, 164
Anorectal manometry studies are applied less often in research studies other than for the assessment of different methods for normalizing functions in defecatory disorders or fecal incontinence.165
Colonic Motility and Transit in Preclinical Studies
Bead expulsion assays in mice have become the preclinical model of choice to understand effects on colonic motility.166, 167 The assay involves the intrarectal insertion of a 2- to 3-cm glass bead within the colon in awake mice and measurement of the time it takes for the bead to leave the anus. There is tremendous variability in the assay both between and within individual animals. This variability likely is owing to stress-induced defecation in mice in which 5–6 fecal pellets, the entire content of the mouse colon, are expelled at once.
Colonic motor activity in mice also has been assessed using water-based168 and solid-state manometers.169 Although these measures of pressure improve the ability to assess colonic motility beyond the limits of bead expulsion assays, they remain isolated measures of contractility. The development of dual-sensor manometric probes170 to assess propulsive and retropulsive contractions in mouse colon provides hope that more advanced technologies for mouse colonic motility are forthcoming.
Scintigraphy and manometry have been used to study colonic motility in rats.170, 171, 172, 173, 174 In addition, the defecation reflex has been well characterized in rats, making this animal model suitable to replace dogs for preclinical testing.175 Fluoroscopic analysis of colonic motility has been studied extensively in the pig.176 Although pigs typically are not used in drug discovery, the pig has been developed as a preclinical model of electrical stimulation for evacuation dysfunction and manometry to assess contractility.177 Colonic motility in the rabbit also has been characterized radiographically,178 and recent advances in high-resolution colonic manometry are being validated using rabbit colon,179 which may make rabbits a preclinical animal of choice in the near future.
New MRI Parameters of Gastrointestinal Function
MRI applications have been proposed to measure several gastrointestinal functions: gastric volumes and emptying; small-bowel water content; colonic volumes; bowel gas volumes; esophageal, gastric, small-bowel, and colonic motility; anorectal and pelvic floor motion and anatomic integrity; and whole-gut transit measured using MRI capsule markers filled with watery gel pellets or water labeled with an MRI contrast agent. These methods have been reviewed in detail elsewhere.180 It is interesting to note that software-quantified bowel motility using cine MRI has potential as a future tool to investigate enteric dysmotility, using a validated motility assessment technique based on motion-capture MRI.101 Another application of MRI to measure colonic motility involves challenge with a laxative preparation (polyethylene glycol 3350 electrolyte solution) and use of MRI marker pills.181
Conclusions
There have been numerous recent advances in measurement of gastrointestinal motility in both human and preclinical models. Imaging technologies especially have advanced rapidly, and it is encouraging to recognize that digestive disease researchers appear to be keeping pace with these advances. It is instructive to understand that, just as the invention of the Roentgen tube in 1895 was adapted rapidly by Cannon182, 183 to make the first seminal observations of modern gastrointestinal motility and formed the basis for the modern scintigraphic tests presented here,184 modern neurogastroenterologists rapidly are adapting other medical imaging technologies and high-resolution or fiberoptic measurements of intraluminal pressures into novel clinical tests. It also is important to recognize the value of reverse translating new clinical tests for use in preclinical studies. Nowhere is this idea better shown than the reverse translation of gastric emptying tests for use in mice. It is our sincere hope that the juxtaposition of state-of-the-art motility tests for both human beings and animals will inform researchers of the great potential of this bedside-to-bench-to-bedside approach for the future of neurogastroenterology.
Acknowledgment
The authors wish to thank Mrs Cindy Stanislav for secretarial assistance.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This work was supported by National Institutes of Health grants R56-DK67071 (M.C.), R01-DK92179 (M.C.), R01-DK106011 (D.R.L.), and P01-DK68055 (M.C. and D.R.L.).
References
- 1.Savarino E., Zentilin P., Savarino V. Functional testing: pharyngeal pH monitoring and high-resolution manometry. Ann N Y Acad Sci. 2013;1300:226–235. doi: 10.1111/nyas.12255. [DOI] [PubMed] [Google Scholar]
- 2.Carlson D.A., Pandolfino J.E. High-resolution manometry and esophageal pressure topography: filling the gaps of convention manometry. Gastroenterol Clin North Am. 2013;42:1–15. doi: 10.1016/j.gtc.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zerbib F., Roman S. Current therapeutic options for esophageal motor disorders as defined by the Chicago Classification. J Clin Gastroenterol. 2015;49:451–460. doi: 10.1097/MCG.0000000000000317. [DOI] [PubMed] [Google Scholar]
- 4.Hunt R.H., Camilleri M., Crowe S.E. The stomach in health and disease. Gut. 2015;64:1650–1668. doi: 10.1136/gutjnl-2014-307595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azpiroz F., Malagelada J.R. Physiological variations in canine gastric tone measured by an electronic barostat. Am J Physiol Gastrointest Liver Physiol. 1985;248:G229–G237. doi: 10.1152/ajpgi.1985.248.2.G229. [DOI] [PubMed] [Google Scholar]
- 6.Tutuian R., Vos R., Karamanolis G. An audit of technical pitfalls of gastric barostat testing in dyspepsia. Neurogastroenterol Motil. 2008;20:113–118. doi: 10.1111/j.1365-2982.2007.01005.x. [DOI] [PubMed] [Google Scholar]
- 7.Coulie B., Tack J., Sifrim D. Role of nitric oxide in fasting gastric fundus tone and in 5-HT1 receptor-mediated relaxation of gastric fundus. Am J Physiol. 1999;276:G373–G377. doi: 10.1152/ajpgi.1999.276.2.G373. [DOI] [PubMed] [Google Scholar]
- 8.Zhao J., Liao D., Gregersen H. Tension and stress in the rat and rabbit stomach are location- and direction-dependent. Neurogastroenterol Motil. 2005;17:388–398. doi: 10.1111/j.1365-2982.2004.00635.x. [DOI] [PubMed] [Google Scholar]
- 9.Tournadre J.P., Allaouchiche B., Malbert C.H. Metabolic acidosis and respiratory acidosis impair gastro-pyloric motility in anesthetized pigs. Anesth Analg. 2000;90:74–79. doi: 10.1097/00000539-200001000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Lorenzo-Figueras M., Jones G., Merritt A.M. Effects of various diets on gastric tone in the proximal portion of the stomach of horses. Am J Vet Res. 2002;63:1275–1278. doi: 10.2460/ajvr.2002.63.1275. [DOI] [PubMed] [Google Scholar]
- 11.Rouzade M.L., Fioramonti J., Bueno L. Decrease in gastric sensitivity to distension by 5-HT1A receptor agonists in rats. Dig Dis Sci. 1998;43:2048–2054. doi: 10.1023/a:1018859214758. [DOI] [PubMed] [Google Scholar]
- 12.Monroe M.J., Hornby P.J., Partosoedarso E.R. Central vagal stimulation evokes gastric volume changes in mice: a novel technique using a miniaturized barostat. Neurogastroenterol Motil. 2004;16:5–11. doi: 10.1046/j.1365-2982.2003.00464.x. [DOI] [PubMed] [Google Scholar]
- 13.Chial H.J., Camilleri C., Delgado-Aros S. A nutrient drink test to assess maximum tolerated volume and postprandial symptoms: effects of gender, body mass index and age in health. Neurogastroenterol Motil. 2002;14:249–253. doi: 10.1046/j.1365-2982.2002.00326.x. [DOI] [PubMed] [Google Scholar]
- 14.Tack J., Caenepeel P., Piessevaux H. Assessment of meal induced gastric accommodation by a satiety drinking test in health and in severe functional dyspepsia. Gut. 2003;52:1271–1277. doi: 10.1136/gut.52.9.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouras E.P., Delgado-Aros S., Camilleri M. SPECT imaging of the stomach: comparison with barostat and effects of sex, age, body mass index, and fundoplication. Gut. 2002;51:781–786. doi: 10.1136/gut.51.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Schepper H.U., Cremonini F., Chitkara D. Assessment of gastric accommodation: overview and evaluation of current methods. Neurogastroenterol Motil. 2004;16:275–285. doi: 10.1111/j.1365-2982.2004.00497.x. [DOI] [PubMed] [Google Scholar]
- 17.Delgado-Aros S., Vella A., Camilleri M. Comparison of gastric volumes in response to isocaloric liquid and mixed meals in humans. Neurogastroenterol Motil. 2004;16:567–573. doi: 10.1111/j.1365-2982.2004.00533.x. [DOI] [PubMed] [Google Scholar]
- 18.Delgado-Aros S., Kim D.Y., Burton D.D. Effect of GLP-1 on gastric volume, emptying, maximum volume ingested and postprandial symptoms in humans. Am J Physiol Gastrointest Liver Physiol. 2002;282:G424–G431. doi: 10.1152/ajpgi.2002.282.3.G424. [DOI] [PubMed] [Google Scholar]
- 19.Liau S.S., Camilleri M., Kim D.Y. Pharmacological modulation of human gastric volumes demonstrated noninvasively using SPECT imaging. Neurogastroenterol Motil. 2001;13:533–542. doi: 10.1046/j.1365-2982.2001.00287.x. [DOI] [PubMed] [Google Scholar]
- 20.Delgado-Aros S., Vella A., Camilleri M. Effects of glucagon-like peptide-1 and feeding on gastric volumes in diabetes mellitus with cardio-vagal dysfunction. Neurogastroenterol Motil. 2003;15:435–444. doi: 10.1046/j.1365-2982.2003.00422.x. [DOI] [PubMed] [Google Scholar]
- 21.Bredenoord A.J., Chial H.J., Camilleri M. Gastric accommodation and emptying in evaluation of patients with upper gastrointestinal symptoms. Clin Gastroenterol Hepatol. 2003;1:264–272. [PubMed] [Google Scholar]
- 22.Breen M., Camilleri M., Burton D. Performance characteristics of the measurement of gastric volume using single photon emission computed tomography. Neurogastroenterol Motil. 2011;23:308–315. doi: 10.1111/j.1365-2982.2010.01660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Schepper H., Camilleri M., Cremonini F. Comparison of gastric volumes in response to isocaloric liquid and mixed meals in humans. Neurogastroenterol Motil. 2004;16:567–573. doi: 10.1111/j.1365-2982.2004.00533.x. [DOI] [PubMed] [Google Scholar]
- 24.Simonian H.P., Maurer A.H., Knight L.C. Simultaneous assessment of gastric accommodation and emptying: studies with liquid and solid meals. J Nucl Med. 2004;45:1155–1160. [PubMed] [Google Scholar]
- 25.Burton D.D., Kim H.J., Camilleri M. Relationship of gastric emptying and volume changes after a solid meal in humans. Am J Physiol Gastrointest Liver Physiol. 2005;289:G261–G266. doi: 10.1152/ajpgi.00052.2005. [DOI] [PubMed] [Google Scholar]
- 26.Padmanabhan P., Grosse J., Asad A.B. Gastrointestinal transit measurements in mice with 99mTc-DTPA-labeled activated charcoal using NanoSPECT-CT. EJNMMI Res. 2013;3:60. doi: 10.1186/2191-219X-3-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dadachova E., Carrasco N. The Na/I symporter (NIS): imaging and therapeutic applications. Semin Nucl Med. 2004;34:23–31. doi: 10.1053/j.semnuclmed.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Portulano C., Paroder-Belenitsky M., Carrasco N. The Na+/I- symporter (NIS): mechanism and medical impact. Endocr Rev. 2014;35:106–149. doi: 10.1210/er.2012-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suksanpaisan L., Pham L., McIvor S. Oral contrast enhances the resolution of in-life NIS reporter gene imaging. Cancer Gene Ther. 2013;20:638–641. doi: 10.1038/cgt.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilja O.H., Hausken T., Odegaard S. Monitoring postprandial size of the proximal stomach by ultrasonography. J Ultrasound Med. 1995;14:81–89. doi: 10.7863/jum.1995.14.2.81. [DOI] [PubMed] [Google Scholar]
- 31.Liao D., Gregersen H., Hausken T. Analysis of surface geometry of the human stomach using real-time 3D ultrasonography in vivo. Neurogastroenterol Motil. 2004;16:315–324. doi: 10.1111/j.1365-2982.2004.00522.x. [DOI] [PubMed] [Google Scholar]
- 32.Gilja O.H., Hausken T., Odegaard S. Ultrasonography and three-dimensional methods of the upper gastrointestinal tract. Eur J Gastroenterol Hepatol. 2005;17:277–282. doi: 10.1097/00042737-200503000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Manini M.L., Burton D.D., Meixner D.D. Feasibility and application of 3-dimensional ultrasound for measurement of gastric volumes in healthy adults and adolescents. J Pediatr Gastroenterol Nutr. 2009;48:1–7. doi: 10.1097/mpg.0b013e318189694f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Zwart I.M., Mearadji B., Lamb H.J. Gastric motility: comparison of assessment with real-time MR imaging or barostat measurement initial experience. Radiology. 2002;224:592–597. doi: 10.1148/radiol.2242011412. [DOI] [PubMed] [Google Scholar]
- 35.Fidler J., Bharucha A.E., Camilleri M. Application of magnetic resonance imaging to measure fasting and postprandial volumes in humans. Neurogastroenterol Motil. 2009;21:42–51. doi: 10.1111/j.1365-2982.2008.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janssen P., Verschueren S., Ly H.G. Intragastric pressure during food intake: a physiological and minimally invasive method to assess gastric accommodation. Neurogastroenterol Motil. 2011;23:316–322. doi: 10.1111/j.1365-2982.2011.01676.x. [DOI] [PubMed] [Google Scholar]
- 37.Papathanasopoulos A., Rotondo A., Janssen P. Effect of acute peppermint oil administration on gastric sensorimotor function and nutrient tolerance in health. Neurogastroenterol Motil. 2013;25:e263–e271. doi: 10.1111/nmo.12102. [DOI] [PubMed] [Google Scholar]
- 38.Rotondo A., Janssen P., Mulè F. Effect of the GLP-1 analog liraglutide on satiation and gastric sensorimotor function during nutrient-drink ingestion. Int J Obes (Lond) 2013;37:693–698. doi: 10.1038/ijo.2012.101. [DOI] [PubMed] [Google Scholar]
- 39.Camilleri M., Hasler W., Parkman H.P. Measurement of gastrointestinal motility in the GI laboratory. Gastroenterology. 1998;115:747–762. doi: 10.1016/s0016-5085(98)70155-6. [DOI] [PubMed] [Google Scholar]
- 40.Camilleri M., Zinsmeister A.R., Greydanus M.P. Towards a less costly but accurate test of gastric emptying and small bowel transit. Dig Dis Sci. 1991;36:609–615. doi: 10.1007/BF01297027. [DOI] [PubMed] [Google Scholar]
- 41.Tougas G., Eaker E.Y., Abell T.L. Assessment of gastric emptying using a low-fat meal: establishment of international control values. Am J Gastroenterol. 2000;95:1456–1462. doi: 10.1111/j.1572-0241.2000.02076.x. [DOI] [PubMed] [Google Scholar]
- 42.Abell T.L., Camilleri M., Donohoe K. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol. 2008;103:753–763. doi: 10.1111/j.1572-0241.2007.01636.x. [DOI] [PubMed] [Google Scholar]
- 43.Camilleri M., Shin A. Editorial: novel and validated approaches for gastric emptying scintigraphy in patients with suspected gastroparesis. Dig Dis Sci. 2013;58:1813–1815. doi: 10.1007/s10620-013-2715-9. [DOI] [PubMed] [Google Scholar]
- 44.Camilleri M., Iturrino J., Bharucha A.E. Performance characteristics of scintigraphic measurement of gastric emptying of solids in healthy participants. Neurogastroenterol Motil. 2012;24 doi: 10.1111/j.1365-2982.2012.01972.x. 1076–e562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi M.G., Camilleri M., Burton D.D. [13C]octanoic acid breath test for gastric emptying of solids: accuracy, reproducibility, and comparison with scintigraphy. Gastroenterology. 1997;112:1155–1162. doi: 10.1016/s0016-5085(97)70126-4. [DOI] [PubMed] [Google Scholar]
- 46.Tinker J., Kocak N., Jones T. Supersensitivity and gastric emptying after vagotomy. Gut. 1970;11:502–505. doi: 10.1136/gut.11.6.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuo B., Viazis N., Bahadur S. Noninvasive simultaneous measurement of intra-liminal pH and pressure: assessment of gastric emptying and upper GI manometry in healthy subjects. Neurogastroenterol Motil. 2004;16:666. [Google Scholar]
- 48.Cassilly D., Kantor S., Knight L.C. Gastric emptying of a non-digestible solid: assessment with simultaneous SmartPill pH and pressure capsule, antroduodenal manometry, gastric emptying scintigraphy. Neurogastroenterol Motil. 2008;20:311–319. doi: 10.1111/j.1365-2982.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- 49.Kuo B., McCallum R.W., Koch K.L. Comparison of gastric emptying of a non-digestible capsule to a radio-labeled meal in healthy and gastroparetic subjects. Aliment Pharmacol Ther. 2008;27:186–196. doi: 10.1111/j.1365-2036.2007.03564.x. [DOI] [PubMed] [Google Scholar]
- 50.Kloetzer L., Chey W.D., McCallum R.W. Motility of the antroduodenum in healthy and gastroparetics characterized by wireless motility capsule. Neurogastroenterol Motil. 2010;22:527–533. doi: 10.1111/j.1365-2982.2010.01468.x. [DOI] [PubMed] [Google Scholar]
- 51.Brun R., Michalek W., Surjanhata B.C. Comparative analysis of phase III migrating motor complexes in stomach and small bowel using wireless motility capsule and antroduodenal manometry. Neurogastroenterol Motil. 2012;24 doi: 10.1111/j.1365-2982.2011.01862.x. 332–e165. [DOI] [PubMed] [Google Scholar]
- 52.Hasler W.L., Saad R.J., Rao S.S. Heightened colon motor activity measured by a wireless capsule in patients with constipation: relation to colon transit and IBS. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1107–G1114. doi: 10.1152/ajpgi.00136.2009. [DOI] [PubMed] [Google Scholar]
- 53.Rozov-Ung I., Mreyoud A., Moore J. Detection of drug effects on gastric emptying and contractility using a wireless motility capsule. BMC Gastroenterol. 2014;14:2. doi: 10.1186/1471-230X-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Viramontes B.E., Kim D.Y., Camilleri M. Validation of a stable isotope gastric emptying test for normal accelerated or delayed gastric emptying. Neurogastroenterol Motil. 2001;13:567–574. doi: 10.1046/j.1365-2982.2001.00288.x. [DOI] [PubMed] [Google Scholar]
- 55.FDA approves breath test to aid in diagnosis of delayed gastric emptying. April 5, 2015. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm441370.htm. Accessed: March 9, 2016.
- 56.Szarka L.A., Camilleri M., Vella A. A stable isotope breath test with a standard meal for abnormal gastric emptying of solids in the clinic and in research. Clin Gastroenterol Hepatol. 2008;6:635–643. doi: 10.1016/j.cgh.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller M.S., Galligan J.J., Burks T.F. Accurate measurement of intestinal transit in the rat. J Pharmacol Methods. 1981;6:211–217. doi: 10.1016/0160-5402(81)90110-8. [DOI] [PubMed] [Google Scholar]
- 58.Moore B.A., Otterbein L.E., Türler A. Inhaled carbon monoxide suppresses the development of postoperative ileus in the murine small intestine. Gastroenterology. 2003;124:377–391. doi: 10.1053/gast.2003.50060. [DOI] [PubMed] [Google Scholar]
- 59.Bennink R.J., De Jonge W.J., Symonds E.L. Validation of gastric-emptying scintigraphy of solids and liquids in mice using dedicated animal pinhole scintigraphy. J Nucl Med. 2003;44:1099–1104. [PubMed] [Google Scholar]
- 60.Whited K.L., Hornof W.J., Garcia T. A non-invasive method for measurement of gastric emptying in mice: effects of altering fat content and CCK A receptor blockade. Neurogastroenterol Motil. 2004;16:421–427. doi: 10.1111/j.1365-2982.2004.00529.x. [DOI] [PubMed] [Google Scholar]
- 61.Symonds E.L., Butler R.N., Omari T.I. Assessment of gastric emptying in the mouse using the [13C]-octanoic acid breath test. Clin Exp Pharmacol Physiol. 2000;27:671–675. doi: 10.1046/j.1440-1681.2000.03318.x. [DOI] [PubMed] [Google Scholar]
- 62.Choi K.M., Zhu J., Stoltz G.J. Determination of gastric emptying in nonobese diabetic mice. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1039–G1045. doi: 10.1152/ajpgi.00317.2007. [DOI] [PubMed] [Google Scholar]
- 63.Sutton D.G., Bahr A., Preston T. Validation of the [13C]octanoic acid breath test for measurement of equine gastric emptying rate of solids using radioscintigraphy. Equine Vet J. 2003;35:27–33. doi: 10.2746/042516403775467423. [DOI] [PubMed] [Google Scholar]
- 64.Wyse C.A., Murphy D.M., Preston T. Assessment of the rate of solid-phase gastric emptying in ponies by means of the [13C]octanoic acid breath test: a preliminary study. Equine Vet J. 2001;33:197–203. doi: 10.1111/j.2042-3306.2001.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 65.McLeay L.M., Carruthers V.R., Neil P.G. Use of a breath test to determine the fate of swallowed fluids in cattle. Am J Vet Res. 1997;58:1314–1319. [PubMed] [Google Scholar]
- 66.McLellan J., Wyse C.A., Dickie A. Comparison of the carbon 13-labeled octanoic acid breath test and ultrasonography for assessment of gastric emptying of a semisolid meal in dogs. Am J Vet Res. 2004;65:1557–1562. doi: 10.2460/ajvr.2004.65.1557. [DOI] [PubMed] [Google Scholar]
- 67.Tsukamoto A., Ohno K., Maeda S. Effect of mosapride on prednisolone-induced gastric mucosal injury and gastric-emptying disorder in dog. J Vet Med Sci. 2012;74:1103–1108. doi: 10.1292/jvms.12-0066. [DOI] [PubMed] [Google Scholar]
- 68.Schoonjans R., Van Vlem B., Van Heddeghem N. The 13C-octanoic acid breath test: validation of a new noninvasive method of measuring gastric emptying in rats. Neurogastroenterol Motil. 2002;14:287–293. doi: 10.1046/j.1365-2982.2002.00334.x. [DOI] [PubMed] [Google Scholar]
- 69.Whited K.L., Lu D., Tso P. Apolipoprotein A-IV is involved in detection of lipid in the rat intestine. J Physiol. 2005;569:949–958. doi: 10.1113/jphysiol.2005.097634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Whited K.L., Thao D., Lloyd K.C. Targeted disruption of the murine CCK1 receptor gene reduces intestinal lipid-induced feedback inhibition of gastric function. Am J Physiol Gastrointest Liver Physiol. 2006;291:G156–G162. doi: 10.1152/ajpgi.00569.2005. [DOI] [PubMed] [Google Scholar]
- 71.The F.O., Boeckxstaens G.E., Snoek S.A. Activation of the cholinergic anti-inflammatory pathway ameliorates postoperative ileus in mice. Gastroenterology. 2007;133:1219–1228. doi: 10.1053/j.gastro.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 72.The F.O., de Jonge W.J., Bennink R.J. The ICAM-1 antisense oligonucleotide ISIS-3082 prevents the development of postoperative ileus in mice. Br J Pharmacol. 2005;146:252–258. doi: 10.1038/sj.bjp.0706303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morscher S., Driessen W.H., Claussen J. Semi-quantitative Multispectral Optoacoustic Tomography (MSOT) for volumetric PK imaging of gastric emptying. Photoacoustics. 2014;2:103–110. doi: 10.1016/j.pacs.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsukamoto A., Ohno K., Tsukagoshi T. Real-time ultrasonographic evaluation of canine gastric motility in the postprandial state. J Vet Med Sci. 2011;73:1133–1138. doi: 10.1292/jvms.11-0044. [DOI] [PubMed] [Google Scholar]
- 75.Yu D., Cheeseman F., Vanner S. Combined oro-caecal scintigraphy and lactulose hydrogen breath testing demonstrate that breath testing detects oro-caecal transit, not small intestinal bacterial overgrowth in patients with IBS. Gut. 2011;60:334–340. doi: 10.1136/gut.2009.205476. [DOI] [PubMed] [Google Scholar]
- 76.Sciarretta G., Furno A., Mazzoni M. Lactulose hydrogen breath test in orocecal transit assessment. Critical evaluation by means of scintigraphic method. Dig Dis Sci. 1994;39:1505–1510. doi: 10.1007/BF02088056. [DOI] [PubMed] [Google Scholar]
- 77.van Nieuwenhoven M.A., Kovacs E.M., Brummer R.J. The effect of different dosages of guar gum on gastric emptying and small intestinal transit of a consumed semisolid meal. J Am Coll Nutr. 2001;20:87–91. doi: 10.1080/07315724.2001.10719019. [DOI] [PubMed] [Google Scholar]
- 78.Ternent C.A., Thorson A.G., Blatchford G.J. Mouth to pouch transit after restorative proctocolectomy: hydrogen breath analysis correlates with scintigraphy. Am J Gastroenterol. 2001;96:1460–1463. doi: 10.1111/j.1572-0241.2001.03799.x. [DOI] [PubMed] [Google Scholar]
- 79.Staniforth D.H. Effect of drugs on oro-caecal transit time assessed by the lactulose/breath hydrogen method. Eur J Clin Pharmacol. 1987;33:55–58. doi: 10.1007/BF00610380. [DOI] [PubMed] [Google Scholar]
- 80.Yuan C.S., Foss J.F., O’Connor M. Gut motility and transit changes in patients receiving long-term methadone maintenance. J Clin Pharmacol. 1998;38:931–935. doi: 10.1002/j.1552-4604.1998.tb04389.x. [DOI] [PubMed] [Google Scholar]
- 81.Yuan C.S., Foss J.F., Osinski J. The safety and efficacy of oral methylnaltrexone in preventing morphine-induced delay in oral-cecal transit time. Clin Pharmacol Ther. 1997;61:467–475. doi: 10.1016/S0009-9236(97)90197-1. [DOI] [PubMed] [Google Scholar]
- 82.Gorard D.A., Libby G.W., Farthing M.J. Effect of a tricyclic antidepressant on small intestinal motility in health and diarrhea-predominant irritable bowel syndrome. Dig Dis Sci. 1995;40:86–95. doi: 10.1007/BF02063948. [DOI] [PubMed] [Google Scholar]
- 83.Morali G.A., Braverman D.Z., Lissi J. Effect of clonidine on gallbladder contraction and small bowel transit time in insulin-treated diabetics. Am J Gastroenterol. 1991;86:995–999. [PubMed] [Google Scholar]
- 84.Geypens B., Bennink R., Peeters M. Validation of the lactose-[13C]ureide breath test for determination of orocecal transit time by scintigraphy. J Nucl Med. 1999;40:1451–1455. [PubMed] [Google Scholar]
- 85.Coremans G., Vos R., Margaritis V. Small doses of the unabsorbable substance polyethylene glycol 3350 accelerate oro-caecal transit, but slow gastric emptying in healthy subjects. Dig Liver Dis. 2005;37:97–101. doi: 10.1016/j.dld.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 86.Priebe M.G., Wachters-Hagedoorn R.E., Landman K. Influence of a subsequent meal on the oro-cecal transit time of a solid test meal. Eur J Clin Invest. 2006;36:123–126. doi: 10.1111/j.1365-2362.2006.01601.x. [DOI] [PubMed] [Google Scholar]
- 87.Cloetens L., De Preter V., Swennen K. Dose-response effect of arabinoxylooligosaccharides on gastrointestinal motility and on colonic bacterial metabolism in healthy volunteers. J Am Coll Nutr. 2008;27:512–518. doi: 10.1080/07315724.2008.10719733. [DOI] [PubMed] [Google Scholar]
- 88.Lin H.C., Prather C., Fisher R.S. Measurement of gastrointestinal transit. Dig Dis Sci. 2005;50:989–1004. doi: 10.1007/s10620-005-2694-6. [DOI] [PubMed] [Google Scholar]
- 89.Maurer A.H., Krevsky B. Whole-gut transit scintigraphy in the evaluation of small-bowel and colonic transit disorders. Semin Nucl Med. 1995;25:326–338. doi: 10.1016/s0001-2998(95)80006-9. [DOI] [PubMed] [Google Scholar]
- 90.Charles F., Camilleri M., Phillips S.F. Scintigraphy of the whole gut: clinical evaluation of transit disorders. Mayo Clin Proc. 1995;70:113–118. doi: 10.4065/70.2.113. [DOI] [PubMed] [Google Scholar]
- 91.Camilleri M., Zinsmeister A.R. Towards a relatively inexpensive, noninvasive, accurate test for colonic motility disorders. Gastroenterology. 1992;103:36–42. doi: 10.1016/0016-5085(92)91092-i. [DOI] [PubMed] [Google Scholar]
- 92.Cremonini F., Mullan B.P., Camilleri M. Performance characteristics of scintigraphic transit measurements for studies of experimental therapies. Aliment Pharmacol Ther. 2002;16:1781–1790. doi: 10.1046/j.1365-2036.2002.01344.x. [DOI] [PubMed] [Google Scholar]
- 93.Camilleri M., Malagelada J.R., Abell T.L. Effect of six weeks of treatment with cisapride in gastroparesis and intestinal pseudoobstruction. Gastroenterology. 1989;96:704–712. [PubMed] [Google Scholar]
- 94.Camilleri M., Brown M.L., Malagelada J.R. Impaired transit of chyme in chronic intestinal pseudoobstruction. Correction by cisapride. Gastroenterology. 1986;91:619–626. doi: 10.1016/0016-5085(86)90631-1. [DOI] [PubMed] [Google Scholar]
- 95.Prather C.M., Camilleri M., Zinsmeister A.R. Tegaserod accelerates orocecal transit in patients with constipation-predominant irritable bowel syndrome. Gastroenterology. 2000;118:463–468. doi: 10.1016/s0016-5085(00)70251-4. [DOI] [PubMed] [Google Scholar]
- 96.Bouras E.P., Camilleri M., Burton D.D. Prucalopride accelerates gastrointestinal and colonic transit in patients with constipation without a rectal evacuation disorder. Gastroenterology. 2001;120:354–360. doi: 10.1053/gast.2001.21166. [DOI] [PubMed] [Google Scholar]
- 97.Shin A., Acosta A., Camilleri M. A randomized trial of 5-hydroxytryptamine4-receptor agonist, YKP10811, on colonic transit and bowel function in functional constipation. Clin Gastroenterol Hepatol. 2015;13:701–708. doi: 10.1016/j.cgh.2014.08.012. e1. [DOI] [PubMed] [Google Scholar]
- 98.Li Y., Hauenstein K. New imaging techniques in the diagnosis of inflammatory bowel diseases. Viszeralmedizin. 2015;31:227–234. doi: 10.1159/000435864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yacoub J.H., Obara P., Oto A. Evolving role of MRI in Crohn's disease. J Magn Reson Imaging. 2013;37:1277–1289. doi: 10.1002/jmri.24081. [DOI] [PubMed] [Google Scholar]
- 100.Hahnemann M.L., Nensa F., Kinner S. Quantitative assessment of small bowel motility in patients with Crohn's disease using dynamic MRI. Neurogastroenterol Motil. 2015;27:841–848. doi: 10.1111/nmo.12558. [DOI] [PubMed] [Google Scholar]
- 101.Menys A., Butt S., Emmanuel A. Comparative quantitative assessment of global small bowel motility using magnetic resonance imaging in chronic intestinal pseudo-obstruction and healthy controls. Neurogastroenterol Motil. 2016;28:376–383. doi: 10.1111/nmo.12735. [DOI] [PubMed] [Google Scholar]
- 102.Sarr M.G., Kelly K.A. Patterns of movement of liquids and solids through canine jejunum. Am J Physiol. 1980;239:G497–G503. doi: 10.1152/ajpgi.1980.239.6.G497. [DOI] [PubMed] [Google Scholar]
- 103.Zarate N., Mohammed S.D., O’Shaughnessy E. Accurate localization of a fall in pH within the ileocecal region: validation using a dual-scintigraphic technique. Am J Physiol. 2010;299:G1276–G1286. doi: 10.1152/ajpgi.00127.2010. [DOI] [PubMed] [Google Scholar]
- 104.Sarosiek I., Selover K.H., Katz L.A. The assessment of regional gut transit times in healthy controls and patients with gastroparesis using wireless motility technology. Aliment Pharmacol Ther. 2010;31:313–322. doi: 10.1111/j.1365-2036.2009.04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Camilleri M., Thorne N.K., Ringel Y. Wireless pH-motility capsule for colonic transit: prospective comparison with radiopaque markers in chronic constipation. Neurogastroenterol Motil. 2010;22:874–882. doi: 10.1111/j.1365-2982.2010.01517.x. e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nagakura Y., Naitoh Y., Kamato T. Compounds possessing 5-HT3 receptor antagonistic activity inhibit intestinal propulsion in mice. Eur J Pharmacol. 1996;311:67–72. doi: 10.1016/0014-2999(96)00403-7. [DOI] [PubMed] [Google Scholar]
- 107.Asuzu D.T., Hayashi Y., Izbeki F. Generalized neuromuscular hypoplasia, reduced smooth muscle myosin and altered gut motility in the klotho model of premature aging. Neurogastroenterol Motil. 2011;23:e309–e323. doi: 10.1111/j.1365-2982.2011.01730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reed D.E., Pigrau M., Lu J. Bead study: a novel method to measure gastrointestinal transit in mice. Neurogastroenterol Motil. 2014;26:1663–1668. doi: 10.1111/nmo.12442. [DOI] [PubMed] [Google Scholar]
- 109.Appleyard M., Fireman Z., Glukhovsky A. A randomized trial comparing wireless capsule endoscopy with push enteroscopy for the detection of small-bowel lesions. Gastroenterology. 2000;119:1431–1438. doi: 10.1053/gast.2000.20844. [DOI] [PubMed] [Google Scholar]
- 110.Boillat C.S., Gaschen F.P., Hosgood G.L. Assessment of the relationship between body weight and gastrointestinal transit times measured by use of a wireless motility capsule system in dogs. Am J Vet Res. 2010;71:898–902. doi: 10.2460/ajvr.71.8.898. [DOI] [PubMed] [Google Scholar]
- 111.Boillat C.S., Gaschen F.P., Gaschen L. Variability associated with repeated measurements of gastrointestinal tract motility in dogs obtained by use of a wireless motility capsule system and scintigraphy. Am J Vet Res. 2010;71:903–908. doi: 10.2460/ajvr.71.8.903. [DOI] [PubMed] [Google Scholar]
- 112.Boscan P., Cochran S., Monnet E. Effect of prolonged general anesthesia with sevoflurane and laparoscopic surgery on gastric and small bowel propulsive motility and pH in dogs. Vet Anaesth Analg. 2014;41:73–81. doi: 10.1111/vaa.12093. [DOI] [PubMed] [Google Scholar]
- 113.Kvetina J., Kunes M., Bures J. The use of wireless capsule enteroscopy in a preclinical study: a novel diagnostic tool for indomethacin-induced gastrointestinal injury in experimental pigs. Neuro Endocrinol Lett. 2008;29:763–769. [PubMed] [Google Scholar]
- 114.Shi Q1, Wang J., Chen D. In vitro and in vivo characterization of wireless and passive micro system enabling gastrointestinal pressure monitoring. Biomed Microdevices. 2014;16:859–868. doi: 10.1007/s10544-014-9890-0. [DOI] [PubMed] [Google Scholar]
- 115.Stokes A.M., Lavie N.L., Keowen M.L. Evaluation of a wireless ambulatory capsule (SmartPill®) to measure gastrointestinal tract pH, luminal pressure and temperature, and transit time in ponies. Equine Vet J. 2012;44:482–486. doi: 10.1111/j.2042-3306.2011.00533.x. [DOI] [PubMed] [Google Scholar]
- 116.Hinton J.M., Lennard-Jones J.E., Young A.C. A new method for studying gut transit times using radioopaque markers. Gut. 1969;10:842–847. doi: 10.1136/gut.10.10.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Arhan P., Devroede G., Jehannin B. Segmental colonic transit time. Dis Colon Rectum. 1981;24:625–629. doi: 10.1007/BF02605761. [DOI] [PubMed] [Google Scholar]
- 118.Metcalf A.M., Phillips S.F., Zinsmeister A.R. Simplified assessment of segmental colonic transit. Gastroenterology. 1987;92:40–47. doi: 10.1016/0016-5085(87)90837-7. [DOI] [PubMed] [Google Scholar]
- 119.Evans R.C., Kamm M.A., Hinton J.M. The normal range and a simple diagram for recording whole gut transit time. Int J Colorectal Dis. 1992;7:15–17. doi: 10.1007/BF01647654. [DOI] [PubMed] [Google Scholar]
- 120.Sadik R., Stotzer P.O., Simrén M. Gastrointestinal transit abnormalities are frequently detected in patients with unexplained GI symptoms at a tertiary centre. Neurogastroenterol Motil. 2008;20:197–205. doi: 10.1111/j.1365-2982.2007.01025.x. [DOI] [PubMed] [Google Scholar]
- 121.Abrahamsson H., Antov S. Accuracy in assessment of colonic transit time with particles: how many markers should be used? Neurogastroenterol Motil. 2010;22:1164–1169. doi: 10.1111/j.1365-2982.2010.01543.x. [DOI] [PubMed] [Google Scholar]
- 122.Rao S.S. Constipation: evaluation and treatment of colonic and anorectal motility disorders. Gastroenterol Clin North Am. 2007;36:687–711. doi: 10.1016/j.gtc.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 123.Proano M., Camilleri M., Phillips S.F. Transit of solids through the human colon: regional quantification in the unprepared bowel. Am J Physiol. 1990;258:G856–G862. doi: 10.1152/ajpgi.1990.258.6.G856. [DOI] [PubMed] [Google Scholar]
- 124.Bonapace E.S., Maurer A.H., Davidoff S. Whole gut transit scintigraphy in the clinical evaluation of patients with upper and lower gastrointestinal symptoms. Am J Gastroenterol. 2000;95:2838–2847. doi: 10.1111/j.1572-0241.2000.03195.x. [DOI] [PubMed] [Google Scholar]
- 125.Deiteren A., Camilleri M., Burton D. Effect of meal ingestion on ileocolonic and colonic transit in health and irritable bowel syndrome. Dig Dis Sci. 2010;55:384–391. doi: 10.1007/s10620-009-1041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Deiteren A., Camilleri M., Bharucha A.E. Performance characteristics of scintigraphic colon transit measurement in health and irritable bowel syndrome and relationship to bowel functions. Neurogastroenterol Motil. 2010;22:415–423. doi: 10.1111/j.1365-2982.2009.01441.x. e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Spiller R.C., Brown M.L., Phillips S.F. Emptying of the terminal ileum in intact humans. Influence of meal residue and ileal motility. Gastroenterology. 1987;92:724–729. doi: 10.1016/0016-5085(87)90024-2. [DOI] [PubMed] [Google Scholar]
- 128.Nullens S., Nelsen T., Camilleri M. Regional colon transit in patients with dyssynergic defecation or slow transit in patients with constipation. Gut. 2012;61:1132–1139. doi: 10.1136/gutjnl-2011-301181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zinsmeister A.R., Burton D., Camilleri M. Pharmacodynamic and clinical endpoints for functional colonic disorders: statistical considerations. Dig Dis Sci. 2013;58:509–518. doi: 10.1007/s10620-012-2369-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kolar G.J., Camilleri M., Burton D. Prevalence of colonic motor or evacuation disorders in patients presenting with chronic nausea and vomiting evaluated by a single gastroenterologist in a tertiary referral practice. Neurogastroenterol Motil. 2014;26:131–138. doi: 10.1111/nmo.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.von der Ohe M.R., Camilleri M., Kvols L.K. Motor dysfunction of the small bowel and colon in patients with the carcinoid syndrome and diarrhea. N Engl J Med. 1993;1329:1073–1078. doi: 10.1056/NEJM199310073291503. [DOI] [PubMed] [Google Scholar]
- 132.Camilleri M., McKinzie S., Busciglio I. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:772–781. doi: 10.1016/j.cgh.2008.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Camilleri M. Review article: biomarkers and personalized therapy in lower functional gastrointestinal disorders. Aliment Pharmacol Ther. 2015;42:818–828. doi: 10.1111/apt.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rao S.S., Kuo B., McCallum R.W. Investigation of colonic and whole-gut transit with wireless motility capsule and radiopaque markers in constipation. Clin Gastroenterol Hepatol. 2009;7:537–544. doi: 10.1016/j.cgh.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 135.Weston S., Thumshirn M., Wiste J. Clinical and upper gastrointestinal motility features in systemic sclerosis and related disorders. Am J Gastroenterol. 1998;93:1085–1089. doi: 10.1111/j.1572-0241.1998.00334.x. [DOI] [PubMed] [Google Scholar]
- 136.Thumshirn M., Bruninga K., Camilleri M. Simplifying the evaluation of postprandial antral motor function in patients with suspected gastroparesis. Am J Gastroenterol. 1997;92:1496–1500. [PubMed] [Google Scholar]
- 137.von Schönfeld J., Evans D.F., Renzing K. Human small bowel motor activity in response to liquid meals of different caloric value and different chemical composition. Dig Dis Sci. 1998;43:265–269. doi: 10.1023/a:1018885717947. [DOI] [PubMed] [Google Scholar]
- 138.Lindberg G., Törnblom H., Iwarzon M. Full-thickness biopsy findings in chronic intestinal pseudo-obstruction and enteric dysmotility. Gut. 2009;58:1084–1090. doi: 10.1136/gut.2008.148296. [DOI] [PubMed] [Google Scholar]
- 139.Nelson A.D., Camilleri M., Acosta A. A single-center, placebo-controlled, double-blind study to evaluate the effects of relamorelin on satiation, gastric volume, gastric accommodation and distal gastric function in healthy volunteers (abstr) Gastroenterology. 2016 In press. [Google Scholar]
- 140.Camilleri M., Bharucha A.E., di Lorenzo C. American Neurogastroenterology and Motility Society consensus statement on intraluminal measurement of gastrointestinal and colonic motility in clinical practice. Neurogastroenterol Motil. 2008;20:1269–1282. doi: 10.1111/j.1365-2982.2008.01230.x. [DOI] [PubMed] [Google Scholar]
- 141.Dinning P.G., Carrington E.V., Scott S.M. Colonic and anorectal motility testing in the high-resolution era. Curr Opin Gastroenterol. 2016;32:44–48. doi: 10.1097/MOG.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 142.Ravi K., Bharucha A.E., Camilleri M. Phenotypic variation of colonic motor functions in chronic constipation. Gastroenterology. 2010;138:89–97. doi: 10.1053/j.gastro.2009.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hurley O’Dwyer R., Acosta A., Camilleri M. Clinical features and colonic motor disturbances in chronic megacolon in adults. Dig Dis Sci. 2015;60:2398–2407. doi: 10.1007/s10620-015-3645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bharucha A.E. High amplitude propagated contractions. Neurogastroenterol Motil. 2012;24:977–982. doi: 10.1111/nmo.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mouchli M.A., Camilleri M., Lee T. Evaluating the safety and the effects on colonic compliance of neostigmine during motility testing in patients with chronic constipation. Neurogastroenterol Motil. 2016 doi: 10.1111/nmo.12786. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Odunsi S.T., Camilleri M., Bharucha A.E. Reproducibility and performance characteristics of colonic compliance, tone, and sensory tests in healthy humans. Dig Dis Sci. 2010;55:709–715. doi: 10.1007/s10620-009-0772-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bampton P.A., Dinning P.G., Kennedy M.L. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am J Physiol Gastrointest Liver Physiol. 2002;282:G443–G449. doi: 10.1152/ajpgi.00194.2001. [DOI] [PubMed] [Google Scholar]
- 148.Law N.M., Bharucha A.E., Undale A.S. Cholinergic stimulation enhances colonic motor activity, transit, and sensation in humans. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1228–G1237. doi: 10.1152/ajpgi.2001.281.5.G1228. [DOI] [PubMed] [Google Scholar]
- 149.Bharucha A.E., Camilleri M., Zinsmeister A.R. Adrenergic modulation of human colonic motor and sensory function. Am J Physiol. 1997;273:G997–G1006. doi: 10.1152/ajpgi.1997.273.5.G997. [DOI] [PubMed] [Google Scholar]
- 150.Esfandyari T., Camilleri M., Busciglio I. Effects of a cannabinoid receptor agonist on colonic motor and sensory functions in humans: a randomized, placebo-controlled study. Am J Physiol Gastrointest Liver Physiol. 2007;293:G137–G145. doi: 10.1152/ajpgi.00565.2006. [DOI] [PubMed] [Google Scholar]
- 151.Acosta A., Camilleri M., Busciglio I. Short-term effects of relamorelin on descending colon motility in chronic constipation: a randomized, controlled trial. Dig Dis Sci. 2016;61:852–860. doi: 10.1007/s10620-015-3876-5. [DOI] [PubMed] [Google Scholar]
- 152.Miner P.B., Camilleri M., Burton D. Prucalopride induces high-amplitude propagating contractions in the colon of patients with chronic constipation: a randomized study. Neurogastroenterol Motil. 2016 doi: 10.1111/nmo.12832. In press. [DOI] [PubMed] [Google Scholar]
- 153.Briejer M.R., Prins N.H., Schuurkes J.A. Effects of the enterokinetic prucalopride (R093877) on colonic motility in fasted dogs. Neurogastroenterol Motil. 2001;13:465–472. doi: 10.1046/j.1365-2982.2001.00280.x. [DOI] [PubMed] [Google Scholar]
- 154.Li M., Johnson C.P., Adams M.B. Cholinergic and nitrergic regulation of in vivo giant migrating contractions in rat colon. Am J Physiol Gastrointest Liver Physiol. 2002;283:G544–G552. doi: 10.1152/ajpgi.00114.2001. [DOI] [PubMed] [Google Scholar]
- 155.Dinning P.G., Carrington E.V., Scott S.M. The use of colonic and anorectal high-resolution manometry and its place in clinical work and in research. Neurogastroenterol Motil. 2015;27:1693–1708. doi: 10.1111/nmo.12632. [DOI] [PubMed] [Google Scholar]
- 156.Neri M., Phillips S.F., Fich A. Measurement of tone in canine colon. Am J Physiol. 1991;260:G505–G511. doi: 10.1152/ajpgi.1991.260.3.G505. [DOI] [PubMed] [Google Scholar]
- 157.Basilisco G., Phillips S.F., Cullen J.J. Tonic responses of canine proximal colon: effects of eating, nutrients, and simulated diarrhea. Am J Physiol. 1995;268:G95–G101. doi: 10.1152/ajpgi.1995.268.1.G95. [DOI] [PubMed] [Google Scholar]
- 158.Chen J.H., Sallam H.S., Lin L. Colorectal and rectocolonic reflexes in canines: involvement of tone, compliance, and anal sphincter relaxation. Am J Physiol Regul Integr Comp Physiol. 2010;299:R953–R959. doi: 10.1152/ajpregu.00439.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sanchez L.C., Merritt A.M. Colorectal distention in the horse: visceral sensitivity, rectal compliance and effect of i.v. xylazine or intrarectal lidocaine. Equine Vet J. 2005;37:70–74. doi: 10.2746/0425164054406955. [DOI] [PubMed] [Google Scholar]
- 160.Kreis M.E., Kasparek M.S., Starlinger M.J. Evaluation of the barostat for recordings of gastrointestinal motility. Digestion. 2002;66:213–221. doi: 10.1159/000068361. [DOI] [PubMed] [Google Scholar]
- 161.Jones M.P., Post J., Crowell M.D. High-resolution manometry in the evaluation of anorectal disorders: a simultaneous comparison with water-perfused manometry. Am J Gastroenterol. 2007;102:850–855. doi: 10.1111/j.1572-0241.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 162.Cheeney G., Remes-Troche J.M., Attaluri A. Investigation of anal motor characteristics of the sensorimotor response (SMR) using 3-D ano-rectal pressure topography. Am J Physiol Gastrointest Liver Physiol. 2011;300:G236–G240. doi: 10.1152/ajpgi.00348.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Raizada V., Bhargava V., Karsten A. Functional morphology of anal sphincter complex unveiled by high definition anal manometery and three dimensional ultrasound imaging. Neurogastroenterol Motil. 2011;23:1013–1019. doi: 10.1111/j.1365-2982.2011.01782.x. e1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Grossi U., Carrington E.V., Bharucha A.E. Diagnostic accuracy study of anorectal manometry for diagnosis of dyssynergic defecation. Gut. 2016;65:447–455. doi: 10.1136/gutjnl-2014-308835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rao S.S., Benninga M.A., Bharucha A.E. ANMS-ESNM position paper and consensus guidelines on biofeedback therapy for anorectal disorders. Neurogastroenterol Motil. 2015;27:594–609. doi: 10.1111/nmo.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Koslo R.J., Burks T.F., Porreca F. Centrally administered bombesin affects gastrointestinal transit and colonic bead expulsion through supraspinal mechanisms. J Pharmacol Exp Ther. 1986;238:62–67. [PubMed] [Google Scholar]
- 167.Raffa R.B., Mathiasen J.R., Jacoby H.I. Colonic bead expulsion time in normal and mu-opioid receptor deficient (CXBK) mice following central (ICV) administration of mu- and delta-opioid agonists. Life Sci. 1987;41:2229–2234. doi: 10.1016/0024-3205(87)90520-0. [DOI] [PubMed] [Google Scholar]
- 168.Jones O.M., Brading A.F., Mortensen N.J. Role of nitric oxide in anorectal function of normal and neuronal nitric oxide synthase knockout mice: a novel approach to anorectal disease. Dis Colon Rectum. 2003;46:963–970. doi: 10.1007/s10350-004-6694-y. [DOI] [PubMed] [Google Scholar]
- 169.Gourcerol G., Wang L., Adelson D.W. Cholinergic giant migrating contractions in conscious mouse colon assessed by using a novel noninvasive solid-state manometry method: modulation by stressors. Am J Physiol Gastrointest Liver Physiol. 2009;296:G992–G1002. doi: 10.1152/ajpgi.90436.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mulak A., Larauche M., Taché Y. Psychological stress induces visceral analgesic or hyperalgesic response in rodents: a role of preconditions. Front Gastrointest Res. 2012;30:106–114. doi: 10.1159/000338417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Spiessens C., Ceuterick L., Ponette E. Combined manometric and radiological study of the changes in colonic motility induced by sennosides in rats. Pharmacology. 1988;36(Suppl 1):66–72. doi: 10.1159/000138423. [DOI] [PubMed] [Google Scholar]
- 172.Croci T., Basilisco G., Bassani A. Manometric patterns of rat colonic motor activity and defecation. Effect of selective 5HT1A agonist 8-OH-DPAT. Dig Dis Sci. 1994;39:1968–1973. doi: 10.1007/BF02088133. [DOI] [PubMed] [Google Scholar]
- 173.Ravnefjord A., Brusberg M., Larsson H. Effects of pregabalin on visceral pain responses and colonic compliance in rats. Br J Pharmacol. 2008;155:407–416. doi: 10.1038/bjp.2008.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Suckow S.K., Anderson E.M., Caudle R.M. Lesioning of TRPV1 expressing primary afferent neurons prevents PAR-2 induced motility, but not mechanical hypersensitivity in the rat colon. Neurogastroenterol Motil. 2012;24:e125–e135. doi: 10.1111/j.1365-2982.2011.01848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nagano M., Ishimizu Y., Saitoh S. The defecation reflex in rats: fundamental properties and the reflex center. Auton Neurosci. 2004;111:48–56. doi: 10.1016/j.autneu.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 176.Hipper K., Ehrlein H.J. Motility of the large intestine and flow of digesta in pigs. Res Vet Sci. 2001;71:93–100. doi: 10.1053/rvsc.2001.0486. [DOI] [PubMed] [Google Scholar]
- 177.Sevcencu C., Rijkhoff N.J., Gregersen H. Electrical stimulation to induce propulsive contractions in the porcine descending colon. Artif Organs. 2005;29:246–249. doi: 10.1111/j.1525-1594.2005.29045.x. [DOI] [PubMed] [Google Scholar]
- 178.Ehrlein H.J., Reich H., Schwinger M. Physiological significance of the contractions of the rabbit proximal colon. Q J Exp Physiol. 1982;67:407–417. doi: 10.1113/expphysiol.1982.sp002656. [DOI] [PubMed] [Google Scholar]
- 179.Arkwright J.W., Underhill I.D., Dodds K.N. A composite fibre optic catheter for monitoring peristaltic transit of an intra-luminal bead. J Biophotonics. 2016;9:305–310. doi: 10.1002/jbio.201500187. [DOI] [PubMed] [Google Scholar]
- 180.Alyami J., Spiller R.C., Marciani L. Magnetic resonance imaging to evaluate gastrointestinal function. Neurogastroenterol Motil. 2015;27:1687–1692. doi: 10.1111/nmo.12726. [DOI] [PubMed] [Google Scholar]
- 181.Lam C., Chaddock G., Marciani L. Colonic response to laxative ingestion as assessed by MRI differs in constipated irritable bowel syndrome compared to functional constipation. Neurogastroenterol Motil. 2016 doi: 10.1111/nmo.12784. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cannon W.B., Mosher A. The movements of the food in the esophagus. Am J Physiol. 1898;1:435–444. [Google Scholar]
- 183.Cannon W.B. The movements of the intestines studied by means of the Röntgen rays. Am J Physiol. 1902;6:251–277. [PMC free article] [PubMed] [Google Scholar]
- 184.Szurszewski J.H. A 100-year perspective on gastrointestinal motility. Am J Physiol Gastrointest Liver Physiol. 1998;274:G447–G453. doi: 10.1152/ajpgi.1998.274.3.G447. [DOI] [PubMed] [Google Scholar]
- 185.Camilleri M. Review: novel diet, drugs and gastric interventions for gastroparesis. Clin Gastroenterol Hepatol. 2016 doi: 10.1016/j.cgh.2015.12.033. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]