Abstract
The widely used model for evolutionary relationships is a bifurcating tree with all taxa/observations placed at the leaves. This is not appropriate if the taxa have been densely sampled across evolutionary time and may be in a direct ancestral relationship, or if there is not enough information to fully resolve all the branching points in the evolutionary tree. In this article, we present a fast distance-based agglomeration method called family-joining (FJ) for constructing so-called generally labeled trees in which taxa may be placed at internal vertices and the tree may contain polytomies. FJ constructs such trees on the basis of pairwise distances and a distance threshold. We tested three methods for threshold selection, FJ-AIC, FJ-BIC, and FJ-CV, which minimize Akaike information criterion, Bayesian information criterion, and cross-validation error, respectively. When compared with related methods on simulated data, FJ-BIC was among the best at reconstructing the correct tree across a wide range of simulation scenarios. FJ-BIC was applied to HIV sequences sampled from individuals involved in a known transmission chain. The FJ-BIC tree was found to be compatible with almost all transmission events. On average, internal branches in the FJ-BIC tree have higher bootstrap support than branches in the leaf-labeled bifurcating tree constructed using RAxML. 36% and 25% of the internal branches in the FJ-BIC tree and RAxML tree, respectively, have bootstrap support greater than 70%. To the best of our knowledge the method presented here is the first attempt at modeling evolutionary relationships using generally labeled trees.
Keywords: generally labeled trees, densely sampled taxa, distance-based phylogenies, latent tree graphical models.
Introduction
Phylogenetic trees are models of evolutionary relationships. The general approach in phylogenetics is to represent evolutionary relationships using bifurcating trees with sampled taxa (represented by so-called labeled vertices) placed at the leaves. Neighbor-joining (NJ) is a popular method for constructing such trees and uses distances between each pair of taxa. Such trees have the maximum number of unsampled ancestors (represented by so-called latent vertices), each ancestor corresponding to a vertex comprising a branching point in the tree. This approach does not allow the labeled vertices to share an ancestor–descendant relationship, and thus may not be appropriate for data sets that have been densely sampled with respect to evolutionary time, for example, genomic sequences of pathogens that have been sampled from individuals who are part of the same transmission chain.
To account for ancestor–descendant relationships (Jombart et al. 2011) model evolutionary relationships using a directed acyclic graph in which each edge is directed from a parent to its child. This graph does not contain any latent vertices and is not necessarily connected. In case the graph is disconnected, it is an incomplete representation of the evolutionary relationships among all the labeled vertices.
In related work Gavryushkina et al. (2014) provide a method for constructing so-called sampled ancestor (SA) trees in which labeled vertices come to be placed at internal vertices by contracting terminal branches. The authors do this in a Bayesian inference framework where trees are generated under a model that does not allow labeled vertices to have degree greater than two and, in addition, does not allow latent vertices to have degree greater than three.
Two distance-based algorithms, recursive grouping (RG) and Chow–Liu recursive grouping (CLRG), have been developed by Choi et al. (2011) for constructing trees which may contain latent vertices with degree greater than two and labeled vertices with degree greater than 0 (so-called generally labeled trees). The authors additionally developed NJc, a method for constructing generally labeled trees by initially constructing a tree using NJ and subsequently contracting all branches that are incident to a latent vertex and are smaller than a preselected threshold. The performance of RG, CLRG, and NJc was compared on simulated data where only the tree topology was varied. In that study, no method clearly outperformed the others.
We developed a distance-based agglomeration method called family-joining (FJ). FJ iteratively identifies, on the basis of a distance threshold, vertices that are in a parent–child or sibling relationship, and introduces latent vertices if required. After inferring all the edges, the branch lengths are estimated using ordinary least-squares (OLS) regression.
RG, CLRG, and FJ require the setting of a threshold that determines the model complexity (number of branches) of the output tree. We tested three approaches to threshold selection which minimized Bayesian information criterion (BIC), Akaike information criterion (AIC), and cross-validation (CV) error, respectively.
We compared the performance of FJ-BIC, FJ-AIC, FJ-CV with NJc-BIC, RG-BIC, CLRG-BIC, and SA across diverse simulation scenarios. We applied FJ-BIC to an HIV-1 transmission chain data set (Vrancken et al. 2014) and checked if the known transmission events were compatible with the FJ-BIC tree. Additionally in the analysis of HIV-1 sequences, we compared the bootstrap support of branches in the FJ-BIC tree and the maximum likelihood tree constructed using RAxML (Stamatakis 2006).
New Approaches
An Overview of FJ
The FJ method consists of a distance-based agglomeration algorithm for constructing generally labeled trees, and an efficient algorithm for computing OLS branch lengths. Trees are inferred using the following agglomeration procedure. We initialize a vertex set with all labeled vertices. At each iteration we select from the vertex set, the vertex pair that optimizes the NJ objective, as defined by Saitou and Nei (1987), see eq. (1) in Materials and Methods. We classify the selected vertex pair as being either parent–child or siblings on the basis of a threshold ϵ, see eq. (2) in Materials and Methods. If they are found to be siblings we check if there is another vertex that is the parent of both the siblings. If no such vertex is found, a latent vertex is introduced as the parent of both the siblings. The distance matrix is augmented by adding distances from the newly introduced latent vertex to each of the other vertices, obtained using the formula described in Studier and Keppler (1988), see eq. (5) in Materials and Methods. Rows and columns of the distance matrix corresponding to the children are removed, and the procedure is iterated until a connected graph is obtained. Subsequently, we estimate branch lengths using OLS regression. For efficient calculation of OLS branch lengths we extended the algorithm by Bryant (1997), which was designed for leaf-labeled trees, to generally labeled trees. OLS branch lengths may be negative, which has no biological interpretation. To account for this, after estimating the branch lengths, all branches that are shorter than ϵ and are incident to a latent vertex are contracted. Overall, the procedure is similar to construct the NJ tree followed by contracting short branches.
We demonstrate FJ by applying it to a tree-additive distance matrix. A distance matrix is tree-additive if there exists a tree, in which the distance between each pair of labeled vertices is equal to the corresponding sum of lengths of the branches that lie along the unique path between the two vertices.
An Example Using Tree-Additive Distances
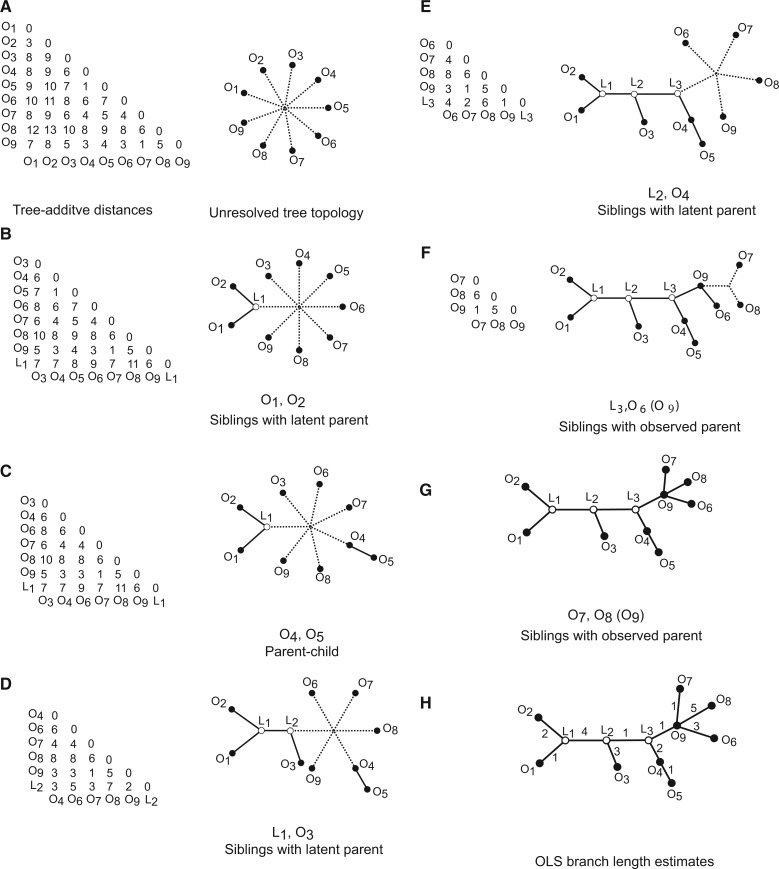
We simulated a generally labeled tree and computed corresponding tree-additive distances. We applied FJ to the resulting tree-additive distance matrix and describe the major steps below. See figure 1 for an illustration. The first iteration identified O1 and O2 as neighbors that share a sibling relationship. No parent was found for these siblings and a latent vertex L1 was introduced. Distances between L1 and vertices O3 through O9 were calculated and the rows and columns corresponding to O1 and O2 were removed from the distance matrix. Edges were added between L1 and O1, and between L1 and O2. The second iteration found O4 and O5 as neighbors that share a parent–child relationship with O4 being the parent. An edge was added between O4 and O5, and O5 was removed from the distance matrix. The following two iterations identified neighbors that are siblings with no parent thus introducing two latent vertices L2 and L3. The sibling pairs found in the third and fourth iteration are and respectively. The fifth iteration identified L3 and O6 as siblings, both of which are the children of O9. Similarly, the next iteration found O9 to be the parent of both O7 and O8. The final step involved estimating branch lengths using OLS. The estimated branch lengths are identical to the corresponding branch lengths in the simulated tree.
Fig. 1.
(A) The tree-additive distances used in this example. Labeled vertices are represented by solid circles and latent vertices by white circles with black border. (B–G) The agglomeration steps of FJ which identifies the correct tree topology. The edges that are inferred in each agglomeration step are shown as solid lines. The dotted lines connect the labeled and latent vertices that will be used in the next iteration. (H) The correct branch lengths estimated using OLS.
Results and Discussion
Simulated Data
Simulated data sets were constructed by varying either the tree type, proportion of labeled internal vertices, type of contracted edge, number of labeled vertices, sequence length or branch length. Each of these parameters is described in detail below. An overview of the parameter settings is provided in table 1.
Table 1.
Simulated Data Sets Were Constructed by Varying Either the Tree Type, Proportion of Labeled Internal Vertices, Type of Contracted Edge, Number of Labeled Vertices, and Sequence Length or Branch Length.
| Tree Type | Balanced | Random* | Unbalanced | ||
|---|---|---|---|---|---|
| Fraction of latent vertices | 0.5 | 0.37 | 0.25* | 0.12 | 0 |
| Contracted edge | leaf/latent | labeled/latent | any/latent* | latent/latent | |
| Average branch length | 0.001 | 0.004 | 0.016* | 0.064 | 0.256 |
| Number of labeled vertices | 20 | 40 | 80 | 160* | 320 |
| Sequence length | 250 | 500 | 1,000* | 2,000 | 4,000 |
Note.—All settings that were considered for each parameter are shown below. The default setting for each parameter is indicated with *.
Three types of binary trees were generated: balanced, unbalanced, and random. Unbalanced or ladder-like trees have the largest diameter among all the trees with the same number of vertices. The diameter of a tree is the number of edges that lie on the path in the tree with the maximum number of edges. We chose this tree type because it has been shown that the accuracy of the neighbor identification step (1), which forms a part of FJ, is inversely related to tree diameter (St. John et al. 2003). A balanced tree is complementary to an unbalanced tree and has the smallest diameter possible.
The fraction of latent vertices ranges from zero to where n is the number of labeled vertices. We simulated trees by varying the fraction of latent vertices over this range in four equal steps.
Trees with the desired proportion of labeled vertices were constructed by contracting edges of a binary tree. Depending on the type of simulation experiment, the following edges were contracted: leaf/latent, labeled/latent, latent/latent, and any/latent.
For each setting of tree type, fraction of latent vertices, and edge type, we randomly generated corresponding types of binary trees and contracted randomly selected edges of the appropriate type, until the desired fraction of latent vertices was reached. Once the topology was generated, branches were assigned lengths by uniformly sampling numbers between 1 and 100, and scaling them such that the expected branch length was equal to a preselected branch length average. Branch length averages took values of 0.001, 0.004, 0.016, 0.064, and 0.256 subs/site. A vertex was randomly selected as the root and sequences were evolved along the branches according to a GTR + Γ model of substitution (Lanave et al. 1984). The parameters of the GTR model were set using estimates from a real data set (Waddell and Steel 1997). The parameters shape and scale of the Γ model were set to 1 which resulted in a moderate variation of substitution rate across sites. Seq-Gen was used for simulating sequence evolution (Rambaut and Grassly 1997). Sequence lengths took values of 250, 500, 1,000, 2,000, and 4,000 nt. The number of labeled vertices (taxa) took values of 20, 40, 80, 160, and 320.
Simulation scenarios were defined by varying each parameter over its range while keeping the remaining parameters fixed at their default setting. The default settings for each parameter are described below. Note that this procedure would result in 22 different parameter combinations. We simulated the corresponding 22 scenarios.
For the categorical parameters tree type and contracted edge type, the respective default settings were random and any/latent. These settings were selected as the defaults as they do not restrict the generation of generally labeled trees.
For the continuous parameter, fraction of vertices that are latent, which has a bounded range the midpoint was considered as the default value.
For the following continuous parameters with no upper bound: number of labeled vertices, sequence length, and average branch length, we selected the appropriate range and default settings such that the trend in performance over each parameter range would be apparent.
The default setting for the number of labeled vertices was 160, for the sequence length it was 1,000 nt, for the average branch length was 0.016 subs/site.
For each setting of parameter values, 100 trees and corresponding sequences were simulated. For distance-based methods we computed pairwise distances using ML distance estimates under a GTR + Γ model, computed using RAxMLv8.2.8 (Stamatakis 2014). For SA which constructs rooted trees we provided sampling times for each labeled vertex. This was done by randomly selected a vertex as the root and defining the sampling time for each labeled vertex as the path length from the root. Note that this method of defining sampling times is equivalent to assuming a strict molecular clock with a clock rate of 1.0. When substitution rates (subs./site/time) follow a strict molecular clock, the distance from the root to each labeled vertex is proportional to the time elapsed since divergence from the root. SA recovers the correct clock rate of 1.0 under the strict molecular clock model in all scenarios except two where the average branch length is very small (0.001 and 0.004; see supplementary fig. 3, Supplementary Material online).
Performance Metrics
Precision and recall were used to quantify the accuracy of the various methods at reconstructing the simulated trees. These metrics are defined below.
where S and are the set of splits corresponding to the simulated tree T and the reconstructed tree , respectively. Please note that S contains the split of every branch in T, including the terminal branches. Precision and recall range from zero to one. Precision is equal to one only if all the splits in the reconstructed tree are present in the simulated tree. Similarly, recall is equal to one only if all the splits in the simulated tree are present in the reconstructed tree. Please note that we do not report Robinson–Foulds distance, which is popularly used for quantifying reconstruction accuracy, since it would be biased against methods that do not allow polytomies. Each of the reconstruction methods that we tested can achieve the highest and the lowest possible value of recall. Among the reconstruction methods that were compared, only SA cannot achieve a precision of one if the simulated tree contains polytomies. We feel that both precision and recall are important measures of reconstruction accuracy.
Results of Comparative Study on Simulated Data
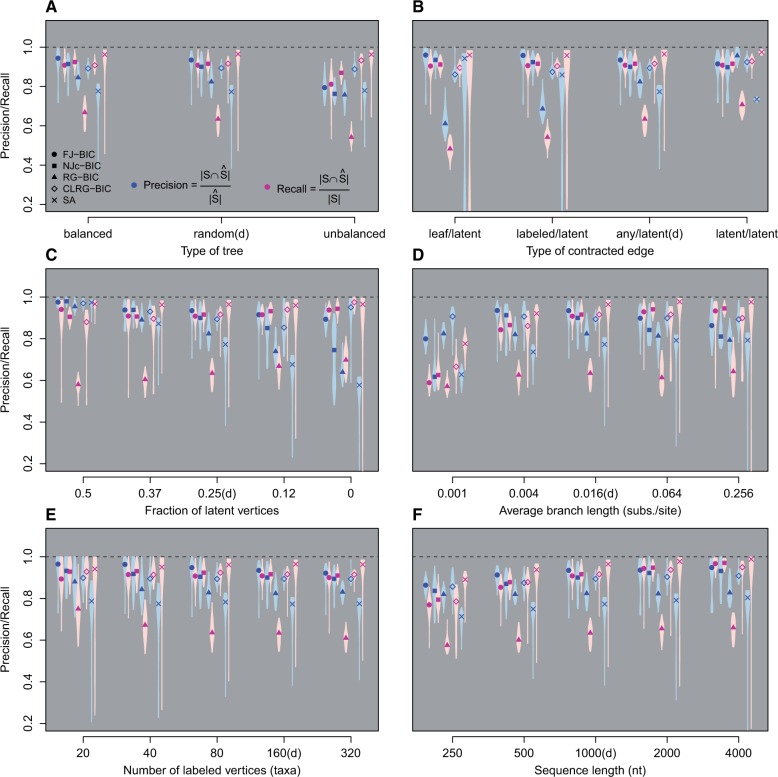
We present the results of applying FJ-BIC, NJc-BIC, RG-BIC, CLRG-BIC, and SA to all simulated data sets. For methods which have the suffix BIC, we performed threshold selection by minimizing BIC. For FJ, we also tested FJ-AIC and FJ-CV which optimized AIC, and CV error, respectively. As FJ-AIC and FJ-CV never performed higher than FJ-BIC in any simulation scenario we do not show the results in the main paper. These results are shown in supplementary figure 4, Supplementary Material online. A change in precision or recall is considered to be statistically significant if the corresponding Welch’s t-test has a P value that is smaller than 0.01. A method is said to have significantly high precision or recall if no other method has significantly higher precision or recall, respectively.
Tree Type
Both FJ-BIC and NJc-BIC have significantly higher precision and recall on balanced trees than on unbalanced trees. This behavior is expected, since the accuracy of the step of FJ, in which neighbors are identified, is inversely related to tree diameter (St. John et al. 2003). Even on unbalanced trees, which have large diameters, FJ-BIC and NJc-BIC have moderately large (median) precision/recall values of 0.79/0.81 and 0.76/0.87 respectively (see fig. 2A). Similarly RG-BIC performs low on unbalanced trees than on balanced trees, which is in agreement with previous work (Choi et al. 2011). RG iteratively partitions the entire vertex set into families. Balanced trees and unbalanced trees have families, and two families, of size two, respectively. This suggests that RG has a higher error rate for unbalanced trees than for balanced trees. In contrast, CLRG-BIC performs significantly higher on unbalanced trees than on balanced trees with median precision/recall values of 0.89/0.93 and 0.89/0.91, respectively. CLRG constructs the MST and then iteratively applies RG to the neighborhood of each internal vertex. The higher performance of CLRG-BIC on unbalanced trees is most likely due to the MST being topologically close to the unbalanced tree. SA has a median precision and recall of 0.77 and 0.96, respectively, across all tree types. The comparatively lower precision of SA is due to this methods constructing trees in which a labeled vertex can only have up to one descendant and all other internal vertices have degree three. Subsequently this results in trees with excess branches if the true tree contains polytomies.
Fig. 2.
A comparison of the reconstruction accuracy of all methods in six simulation categories. One parameter (x-axis) was varied in each category. The default parameter settings are denoted as parameterValue (d) on each x-axis. For each parameter setting, 100 data sets were created. Precision is shown in blue and recall is shown in pink.
Type of Contracted Edge
FJ-BIC has a significantly higher precision than other methods for all types of contracted edges, except latent/latent. SA has a high median recall of 0.96 for all types of contracted edges. However the recall values of SA are not significantly higher than those of FJ-BIC if the contracted edge is leaf/latent. This is due to a large variance in the performance of SA, quantified with an inter-quantile range of 0.26 (see fig. 2B). SA has high median precision of 0.94 if the contracted edge is leaf/latent. Contracting leaf/latent edges results in trees in which a labeled vertex can have up to one descendant and all other internal vertices have degree three. The high performance of SA in this category is because these are the same type of trees which SA samples when optimizing tree topology. SA has lower performance when any other edge type is contracted. RG-BIC and CLRG-BIC have significantly higher precision and recall if latent/latent edges are contracted, when compared with precision and recall for other edge types.
Fraction of Vertices that Are Latent
For leaf-labeled trees which have a maximal fraction (0.5) of latent vertices, all methods have a median precision higher than 0.95 (see fig. 2C). In this simulation scenario, with a median recall of 0.97, SA has significantly higher recall than other methods, even though FJ-BIC also has a high median recall of 0.94. In general, precision reduces and recall rises with a decrease in the fraction of latent vertices. FJ-BIC has a median precision and recall that is greater than 0.89 across all settings of fraction of latent vertices. CLRG-BIC has a significantly higher precision and recall than other methods when all vertices are labeled. This is because the CLRG algorithm involves the construction of a MST which should be topologically similar to the completely labeled tree.
Average Branch Length (Substitution Rate)
All methods perform badly on trees with short average branch lengths of 0.001 subs/site with median recall smaller than 0.8 each (see fig. 2D). This is because a significant portion of the simulated sequences are identical. Thus, in FJ-BIC, NJc-BIC, RG-BIC, and CLRG-BIC there is a preference for choosing parent–child relationship over siblings. CLRG-BIC has significantly higher precision than other methods if branch lengths are either very small or very large. FJ-BIC has high precision if the average branch length is between 0.004 and 0.064. In trees with larger branch lengths there is a high chance that sequences undergo multiple substitutions at the same site. This effect has been termed genetic saturation and results in an underestimation of the true evolutionary distance. Additionally, estimates of large distances are associated with large variance (Hoyle and Higgs 2003) which results in the selection of wrong neighbors in the NJ step. CLRG-BIC has higher performance for trees with large branch lengths because the input to CLRG-BIC is the MST and the construction of the MST is probably robust to noise in distance estimates. The performance of SA is not greatly affected by long branches.
Number of Labeled Vertices (Taxa)
The performance of all the methods is expected to worsen with increasing number of labeled vertices. RG shows significant change in precision/recall with corresponding median values changing from 0.88/0.75 (5 labeled vertices) to 0.83/0.61 (80 labeled vertices) (see fig. 2E). The change in precision and recall shown by SA is not significant. FJ-BIC and CLRG-BIC show a significant drop in precision but not in recall. Even for trees with 320 taxa, FJ-BIC has high median precision and recall of 0.92 and 0.9 respectively. NJc-BIC shows significant change in both precision and recall with median precision/recall changing from 0.93/0.93 to 0.89/0.91.
Sequence Length
The performance of all methods improves with increase in sequence length. For all settings of sequence length, FJ-BIC is among the methods with significantly high precision (see fig. 2F). FJ-BIC is among the methods with significantly high recall for sequences of length 1,000 to 4,000 nt. For all settings of sequence length, SA is among the methods with significantly high recall.
Summary of Performance
For the simulations performed at the default parameter settings, the methods listed in order of decreasing median precision are FJ-BIC (0.93), NJc-BIC (0.9), CLRG-BIC (0.89), RG-BIC (0.82), and SA (0.77), and the methods listed in order of decreasing median recall are SA (0.96), NJc-BIC (0.92), CLRG-BIC (0.92), FJ-BIC (0.91), and RG-BIC (0.63). In 15 out of the 22 simulated scenarios FJ-BIC is among the methods with significantly high precision (see Table 2). In 17/22 simulated scenarios SA is among the methods with significantly high recall. In 13/22 simulated scenarios NJc-BIC is among the methods with significantly high recall. FJ-BIC has a median recall that is greater than 0.9 in 16/22 simulated scenarios. The remaining scenarios are 1) trees with 20 taxa (recall of 0.89), 2) trees in which branches are very short (0.001 and 0.004 subs/site; recall of 0.6 and 0.84 respectively), 3) unbalanced trees (0.81), and 4) trees constructed using short sequences (250 and 500 nt; recall of 0.77 and 0.85 respectively).
Table 2.
Methods with the Significantly High Precision and Recall Are Shown Below.
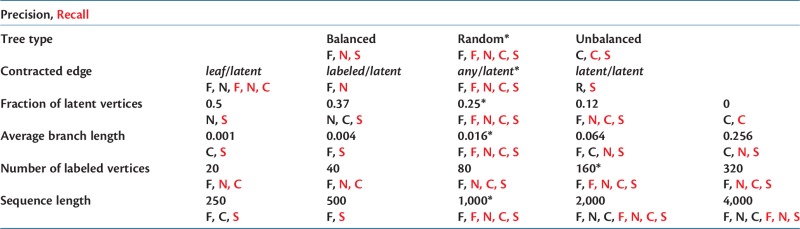 |
Note.—All methods that are not significantly worse than the best method are also shown. F, N, R, C, and S stand for FJ-BIC, NJc-BIC, RG-BIC, CLRG-BIC, and SA, respectively. Black and red indicate methods with the highest precision and recall, respectively. The default setting for each simulation parameter is indicated with *.
Comparison of Time-Complexities and Run Times
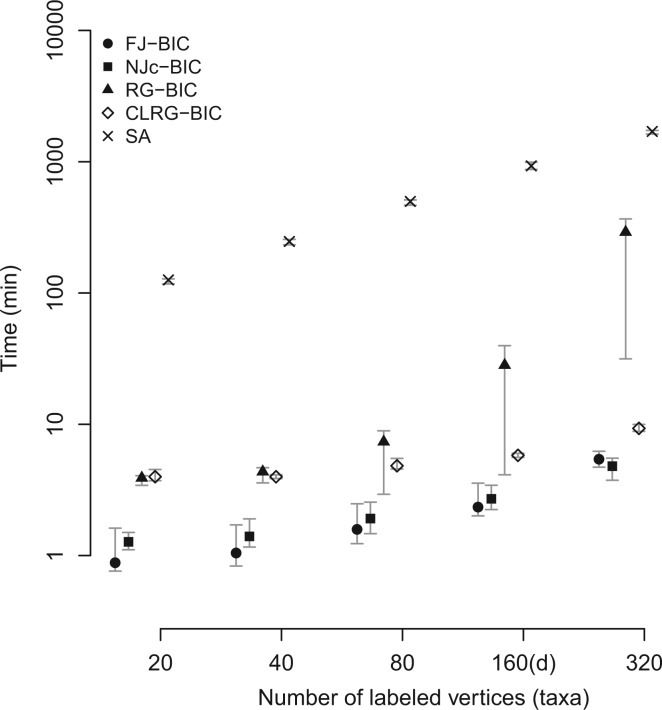
Clustering methods are deterministic procedures for which we report worst-case run times. Both FJ and NJ run in time . RG runs in time which makes it infeasible to run on large datasets. CLRG runs in where ni is the number of internal vertices of the MST and is the largest vertex degree in the MST. Model selection with BIC or AIC requires the repeated optimization of the likelihood function with respect to parameters of the substitution model. Computing the likelihood with Felsenstein’s dynamic programming algorithm (Felsenstein 1981) takes time where L is the sequence length and A is the size of the alphabet. A is 4 for genetic sequences and 20 for protein sequences. We used RAxML for computing and optimizing likelihoods; RAxML is highly optimized for this task. SA performs Bayesian inference by MCMC sampling, a stochastic procedure whose runtime depends on how easily the MCMC chain moves through the space of trees and model parameters. The observed run times (see fig. 3) suggest that FJ-BIC and NJc-BIC are the fastest methods for trees containing up to 320 taxa, with both the methods having a median run time of 5.4 and 4.8 min respectively. CLRG-BIC took around 9.3 min to reconstruct trees containing 320 taxa and showed the slowest growth in run time. RG showed the largest growth in run time taking 4.8 h for reconstructing trees with 320 taxa. SA was run with MCMC chain-length set to 108 states. SA took around 2 h to construct trees containing 20 taxa and 30 h for constructing trees containing 320 taxa.
Fig. 3.
A comparison of run times of all methods in the scenario where the number of labeled vertices was varied. Run times are shown on a log-scale.
HIV-1 Transmission Chain Data
We applied FJ-BIC to a dataset of HIV-1 subtype C env gene sequences that were sampled from 11 hosts who are part of a partially known transmission chain (Vrancken et al. 2014). We selected this dataset because it contains sequences from viruses that are evolutionarily closely related. We discarded 31 sequences which had gaps and analyzed the remaining 181 sequences of length 1,376 nt. The hosts are labeled . Sequences from multiple time points are available for . The sampling times for all sequences are known. All the pairs of hosts who were involved in a transmission event are known and have been inferred by interviewing the hosts. The direction of transmission is known for all transmission events except for the transmission between A and B.
Additionally we compared the bootstrap support of branches in the FJ-BIC tree with the branches in the maximum likelihood tree constructed using RAxMLv8.2. (Stamatakis 2006). We first identified the most appropriate model of substitution using JModelTest2 (Darriba et al. 2012). A BIONJ tree (Gascuel 1997) was constructed with Jukes–Cantor distances and AIC was computed for the following models of substitution: JC, F81, K80, HKY, TrNef, TrN, TPM1, TPM1uf, SYM, and GTR. Variants of all substitution models which included a parameter for invariant sites (I) and/or a Gamma model (Γ) for intersite rate variation were also tested. GTR + Γ+I was the best model, that is, the one with the smallest AIC score. We constructed a tree with RAXML using the original sequence alignment and the GTRCATI model of substitution, which we refer to as the RAxML tree. The CAT model approximates the Gamma model an enables fast computation (Stamatakis 2006).
We inferred a generally labeled tree using FJ-BIC. Pairwise distances were computed using RAxML which included the following steps (Stamatakis et al. 2005). First a maximum parsimony tree was constructed using stepwise addition and the parameters of the substitution model GTR + Γ were optimized. The optimized substitution model was used to compute maximum likelihood distances for all sequence pairs. For computing the likelihood of FJ trees at various values of the distance threshold we used RAxML as follows. FJ trees were converted to leaf-labeled trees by replacing each interior labeled vertex with a latent vertex and adding an edge of length zero between the newly added latent vertex and the former interior labeled vertex. This conversion was necessary since RAxML can only handle leaf-labeled trees. We then maximized the likelihood of the converted FJ tree by fixing the tree topology and branch lengths and optimizing the parameters of the substitution model GTR + Γ. The maximized log-likelihood was used for computing BIC.
The FJ-BIC tree was rooted assuming a strict molecular clock model. We define the optimal position of the root as that position which minimizes the RSS of regressing distances from the root to each labeled vertex against sampling times. We placed the root at the midpoint of each branch and computed the RSS for each branch. We then picked the branch that had the smallest RSS and searched along the branch for the position of the root with the smallest RSS. Subsequently this position was chosen as the root of the FJ-BIC tree.
Compatibility of the FJ-BIC Tree with Known Transmission Events
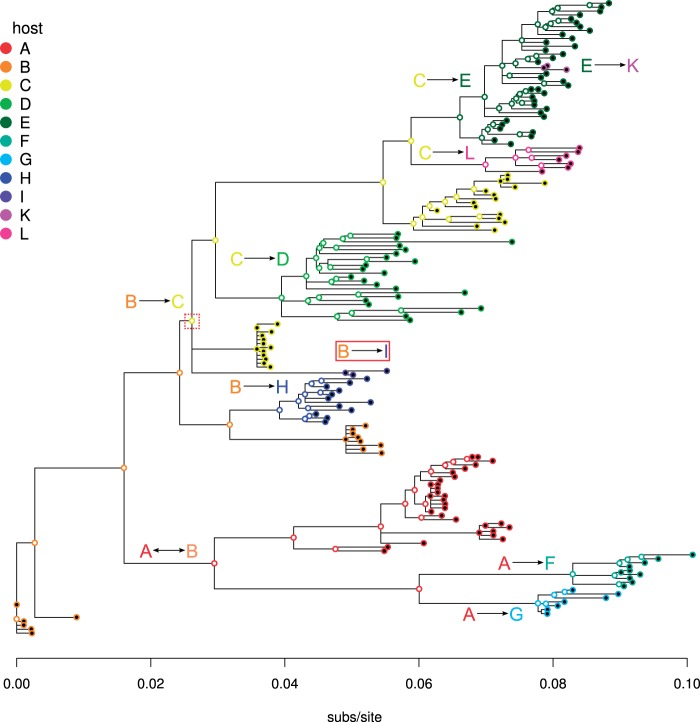
In order to check if the known transmission events are compatible with a rooted tree we needed to label all latent vertices with a host. Latent vertices were visually labeled with hosts using standard maximum parsimony. The labeling that we applied resulted in the minimum possible total cost of 10 (see fig. 4). Given a rooted tree with all vertices labeled with a host, we define a transmission event () to be compatible with the tree if there is a directed edge from a vertex labeled X to a vertex labeled Y. Nine out of ten transmission events are compatible with the FJ-BIC tree. The direction of transmission between A and B is not known. The FJ-BIC tree suggests that A was infected by B. The branch of the FJ-BIC tree that suggests this transmission event has been labeled with the known transmission event . Eight out the remaining nine transmission events are compatible with the FJ-BIC tree and branches indicative of these transmission events are labeled in fig. 4. The transmission event is not compatible with the FJ-BIC tree (red solid box in fig. 4) which may be due to insufficient sampling; only three sequences were available from host I. It is possible that the polytomy present inside the red dotted box in fig. 4 may be resolved if more sequences from I were available, in such a way that the resulting tree would be compatible with the transmission .
Fig. 4.
The FJ-BIC tree of 181 HIV-1 env gene sequences sampled from hosts involved in a known transmission chain. Each vertex is represented by a circle whose inner color is black if the vertex is labeled and white if the vertex is latent. The outer color of each circle indicates the host of the corresponding vertex. Branches reflecting transmission events have been labeled. Nine out of ten transmission events are compatible with the FJ-BIC tree. The red box highlights the transmission event which is not compatible with the FJ tree.
Branch Support in the FJ-BIC Tree and the RAxML Tree
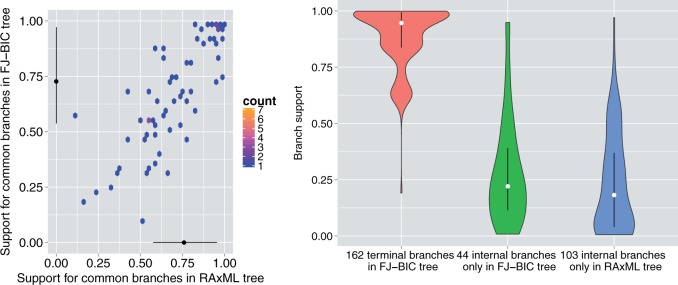
The bootstrap support of a branch is defined as the number of bootstrap replicate trees that contain this branch. The bootstrap support of branches in the FJ-BIC tree and the RAxML tree were computed using 1,000 replicates. Since each labeled vertex is a leaf in all bootstrap RAxML trees, all terminal branches of the RAxML tree trivially have a support of one. The support of a terminal branch in the FJ-BIC tree is not necessarily one.
75 internal branches were common to both the FJ-BIC tree and the RAxML tree. The median (IQR) supports for the common branches were 0.73 (0.43) and 0.76 (0.38) in the FJ-BIC and the RAxML tree respectively. Supports for the common branches in both trees were strongly correlated (Pearson’s , see fig. 5). There are 44 and 103 internal branches that are present only in the FJ-BIC tree and the RAxML tree respectively with lower median (IQR) branch supports of 0.22 (0.28) and 0.18 (0.33) respectively (see fig. 5). The 124 terminal branches in the FJ-BIC tree have a median (IQR) branch support of 0.95 (0.16).
Fig. 5.
Left: Comparing the support of common branches in the FJ-BIC tree and the RAxML tree. Right: Supports for branches that are only present in either the FJ-BIC tree or the RAxML tree.
On average an internal branch in the FJ-BIC tree has a higher support than an internal branch in the RAxML tree. 36% of FJ-BIC branches and 25% of RAxML branches have supports greater than 0.7.
Summary and Outlook
In this paper, we present a fast distance-based agglomeration method called FJ for constructing generally labeled trees. A key feature of the algorithm presented here is its low worst case time complexity, , where n is the number of taxa making it feasible for analyzing large data sets. For precomputed distances between 320 taxa, FJ-BIC took around 5.4 min (±0.76) to estimate a tree. At each agglomeration step FJ only adds branches (both internal and terminal) if there is sufficient data to support this move. The algorithm treats short branches as unreliable and identifies an optimal threshold by minimizing test error. We tested two methods, FJ-BIC and FJ-CV error, which minimize BIC and CV error, respectively. When compared with related methods FJ-BIC was best at reconstructing the correct tree across a wide range of simulation settings. FJ-BIC was applied to HIV sequences sampled from individuals involved in a known transmission chain. The FJ-BIC tree was compatible with ten out eleven transmission events. On average, internal branches in the FJ-BIC tree were found to have higher statistical support than internal branches in the tree constructed using RAxML. A method for reconstructing phylogenetic trees with high precision circumvents the need for a time-consuming bootstrap analysis. To the best of our knowledge the method presented here is the first attempt at modeling evolutionary relationships using generally labeled trees.
Materials and Methods
Terminology
A phylogenetic tree is an edge weighted undirected tree consisting of two types of vertices, labeled vertices (representing observed sequences) and latent vertices (representing unobserved sequences). Sequence information is present only at labeled vertices. Where appropriate, we refer to edges as branches and edge weights as branch lengths. A branch length quantifies the amount of expected change between the sequences corresponding to the respective incident vertices. Branch lengths are usually in units of substitutions per site. Labeled vertices and latent vertices have a minimum degree of one and three respectively. For a tree consisting of n labeled vertices the number of latent vertices lies between zero and . For trees with a maximal number of latent vertices, all labeled vertices are leaves (degree one) and all latent vertices have degree three. Trees are leaf-labeled if all labeled vertices are leaves, else they are generally labeled.
A distance matrix d is tree-additive in a tree T if the distance between each pair of labeled vertices equals the corresponding path length (sum of branch lengths along the unique path between the two vertices) in T. Each branch partitions the set of all labeled vertices into two disjoint sets which are referred to as the split of the branch. The two sets of labeled vertices that are present in a split are referred to as the sides of the split. A split is compatible with a tree if there is any branch in the tree such that removing the branch bipartitions the set of labeled vertices as defined by the split. S(T) denotes the set of splits that are defined by a branch in T.
A pair of vertices are siblings if both of them are leaves and are adjacent to the same vertex. A vertex pair is in a parent–child relationship if they are adjacent and one of them is a leaf. Thus we call siblings what in the context of the NJ algorithm is called neighbors.
A rooted tree is a tree with directed edges. In such trees there is one latent vertex (the root) which has indegree zero and outdegree greater than zero. All edges in a rooted tree are directed away from the root.
Edges incident to leaves are referred to as terminal edges while edges incident to internal vertices are referred to as internal edges.
FJ Algorithm
Our objective is, given a tree-additive distance matrix d, to infer the respective tree To. To may be generally labeled and may contain latent vertices with degree greater than three. We assume that all branch lengths in T0 are strictly greater than zero. We provide a method which correctly infers To using entries in d.
Let be the set of all trees that satisfy the following criteria: 1) their set of leaves includes all the labeled vertices in To, 2) they have the maximum number of latent vertices, and 3) d is the tree-additive distance matrix in every tree in . All splits in are compatible with every tree in . If this were not true for some tree in then there would be two branches, bo in To and in , such that labeled vertices {i, j} and {k, l} lie on different sides of b0 and {i, k} and {j, l} lie on different sides of . Applying Buneman’s 4-point condition (Buneman 1971) would result in the following contradictory inequalities:
The inequality is strict for b0 as all branch lengths in T0 are greater than zero.
Thus any tree in can be constructed as follows. If there is a labeled vertex in T0 with degree greater than one replace this vertex with a latent vertex and add a branch of length zero between the labeled vertex and the newly added latent vertex. If there is a latent vertex with degree greater than three () disconnect two randomly selected vertices adjacent to and connect them to a new latent vertex with a branch of length zero. Lengths of branches between the newly added latent vertex and each adjacent vertex are the same as the length of the original branch between and that vertex. Both of these augmentation operations are performed until all latent vertices have degree three and there are no labeled internal vertices.
Applying the NJ algorithm using distances in d yields a tree with the maximum number of latent vertices such that d is tree-additive in . Thus belongs to and consequently neighbors in are either parent–child or siblings in To.
NJ is an agglomerative clustering method that identifies, at each step, the pair of vertices to cluster by minimizing the following objective value (Saitou and Nei 1987; Studier and Keppler 1988).
| (1) |
where n is the number of vertices that are yet to be clustered.
Neighbors i and j can be classified as parent–child or siblings based on the following quantity.
It can be easily shown that:
These criteria are shown to be true in the following statements. If i is the parent of j then the path from j to any vertex , will visit i. Thus , which gives and . If i and j are siblings then where u is the vertex adjacent to both i and j. Similarly , which gives . It follows that .
When distances are estimated from sequences we use a threshold ϵ for classifying the relationship as parent–child or sibling. Specifically i is the parent of j if . The unordered vertex pair {i, j} are said to be in a parent–child relationship if
| (2) |
The criterion for selecting the appropriate ϵ is discussed in detail later. When d is tree-additive any sufficiently small ϵ can be used for correctly classifying the vertices.
The FJ algorithm consists of two main parts: GetTreeTopology which infers the tree topology, and GetBranchLengths which estimates the branch lengths. We describe these two steps in detail below.
Inferring Tree Topology
An overview of GetTreeTopology is provided in Algorithm 1. GetTreeTopology initializes a so-called active vertex set with the set of all labeled vertices. It then performs agglomerative clustering where the following actions are performed at each step.
The pair {i, j} of vertices in that minimizes (1) is identified. i and j are then classified as parent–child or siblings using (2). If i is the parent of j, or vice-versa, an edge is added between them and all distances from the child are removed from d. If i and j are found to be siblings then we search for another vertex k in that minimizes the following quantity.
| (3) |
If then k is the parent of both i and j. Corresponding edges are added and all distances from i and j are removed from d. i and j are removed from . Note that there are alternate criteria for checking if there is a vertex k that is the parent of both i and j. One such criterion is to compute
| (4) |
and consider k to be the parent of both i and j if . In the simulation study we found that reconstruction accuracy was higher when we used the quantity in eqn. (3) as opposed to eqn. (4) (see supplementary fig. 4, Supplementary Material online). This is probably because the quantity in eqn. (3) is more robust to noise in the estimates of large distances. If k is not the parent of both i and j, a latent vertex l is introduced as the parent of both i and j. Corresponding edges are added and distances from l to any vertex m in other than i and j are calculated using the following estimate by Studier and Keppler (1988).
| (5) |
i and j are removed from and all distances from i and j are removed from d. Distances from u are added to d and u is added to .

The agglomeration step described above is repeated until the number of vertices in is less than four. After each iteration reduces by either one or two vertices. If has reached the size three, we check using (3) if there are vertices i, j, and k in such that k is the parent of both i and j. If we find such vertices, corresponding edges are added. Otherwise a latent vertex u is introduced and edges are added between u and the three remaining vertices. If has reached size two, an edge is added between the two remaining vertices.
GetTreeTopology returns the list of edges of the estimated tree . has the same topology as the true tree if distances are additive in the true tree.
Upper Bound on the Time Complexity of GetTreeTopology
At first glance it appears that the neighbor identification step requires time. This can be reduced to with the observation that the NJ objective can be reformulated as follows (Studier and Keppler 1988):
| (6) |
From eq. (6) it is evident that initializing each row sum Ri with the original distances takes O(n) time. Updating each Ri after each agglomeration step is done by subtracting distances from children and, if applicable, adding distances to the newly introduced latent vertices. Thus the process of updating each Ri takes O(1) time. Additionally, storing all the Ri in memory requires O(n) space which incurs very little memory overhead compared with the space required to store all the pairwise distances. If all distances and row sums are stored in memory then identifying the neighbors takes time. Note that Δij can also be reformulated for faster computation as follows.
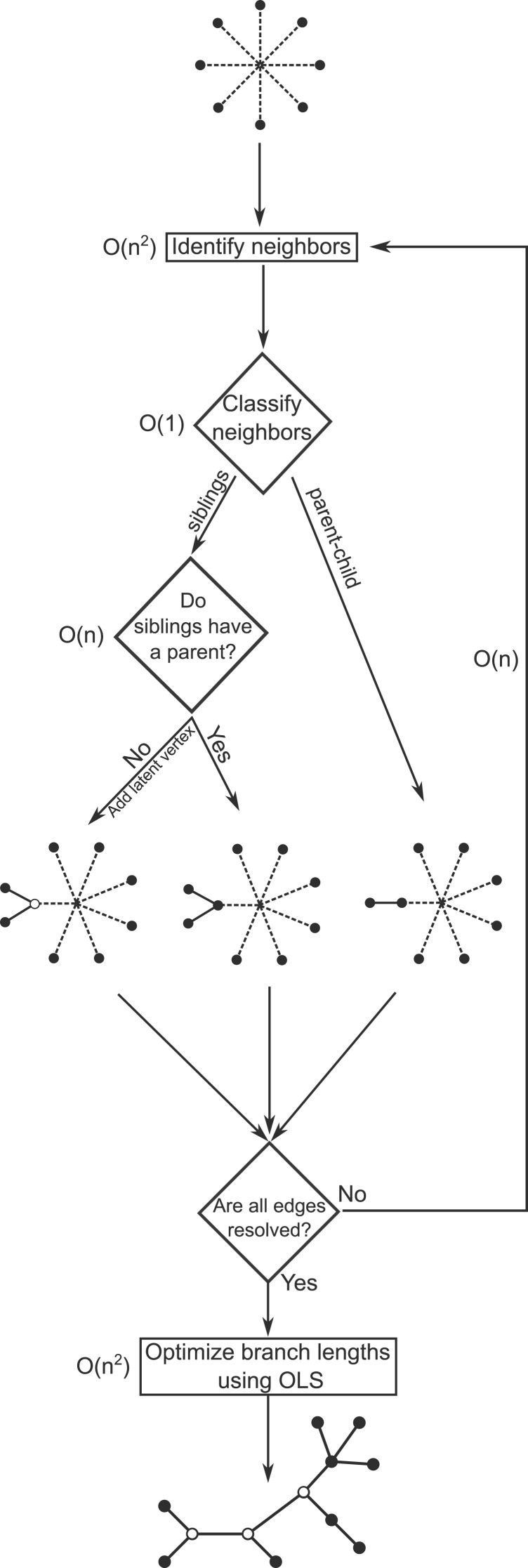
Thus, once the neighbors {i, j} have been identified, it takes O(1) time to compute both Δij and Δji. It takes O(n) time to find the vertex k which minimizes — —. The overall time-complexity of GetTreeTopology is . The time-complexities associated with the main steps of GetTreeTopology are shown in figure 6.
Fig. 6.
An illustration of the FJ algorithm. The main steps have been labeled with their time complexity.
Efficient Estimation of Branch Lengths
Branch lengths b of are estimated by ordinary least squares. This is done by solving where d is a column vector containing all those entries of d that are above or alternatively all those entries of d that are below the diagonal. A is the branch incidence matrix of and is constructed as follows. If the mth entry of the d is dij, then
| (7) |
A has the dimension where is the number of branches in the tree, n is the number of labeled vertices, and b is the vector of branch lengths that we wish to estimate.
The ordinary least squares (OLS) estimate of branch lengths is given by
| (8) |
For the estimation of OLS branch lengths we do not make the assumption that distances are tree-additive. For leaf-labeled trees there is a fast algorithm for computing the OLS branch lengths (Bryant 1997). Any algorithm that estimates OLS branch lengths by performing the matrix operations that are defined in eqn. (8) needs to use all entries of the distance vector, and thus must run in time (Bryant and Waddell 1998). Thus the algorithm by Bryant (1997) is time-optimal. We show that this algorithm extends to generally labeled trees. The main steps involved in this computation are computing first and then , each in time. We describe both of these steps below.
Computing
The ith entry of , is the sum of all distances between labeled vertices a and b that lie on either side of edge ei. δi is the ith column of A. For efficient computation of , edges are visited in order of increasing distance from leaves, keeping track of which edges have already been visited.
We first compute for every terminal edge ei which is defined as follows.
| (9) |
Next we compute for every internal edge ei which are visited in the order of increasing distance from leaves. Consider the internal vertex α with only one incident edge ei such that has not been calculated. Let the edges incident to ei be
Let Ci be the side of the split of the edge ei that does not contain α. Similarly is the side of the split of that does not contain α.
Depending on whether α is labeled or not labeled, is computed as follows:
Case 1: Vertex α is not labeled (Bryant 1997).
| (10) |
Case 2: Vertex α is labeled.
| (11) |
Computing each element of involves the summation of entries of the distance vector. Since each element of the distance vector is summed over just once, is computed in time.
Computing
There is a closed-form solution for the OLS branch length b0 of any edge e0 which is formulated in terms of the splits, and the elements of , that are defined by e0 and the edges adjacent to e0. A description of the branch length formula is given later.
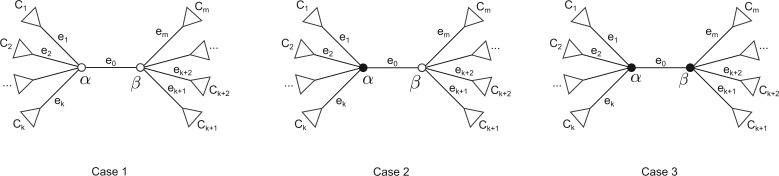
When computing branch lengths, edges can be visited in any order. We derive the branch length formula for an internal edge.
Consider the internal edge e0 shown in figure 7 with adjacent edges . e0 is incident to . The respective sizes of the parts of the split defined by e0 are and
Fig. 7.
The three cases for the internal edge e0. Case 1: Both α and β are not labeled. Case 2: Only α is labeled. Case 3: Both α and β are labeled. The triangles represent subtrees.
For each edge ei define where Ai and Bi are the parts of the split defined by edge ei. Here pxy denotes the length of the path from x to y when branch lengths are determined by OLS. It turns out that .
For each edge ei let Ci be the side of the split that does not contain α and β. ni is the cardinality of Ci. Define
For the case where both α and β are not labeled it can be shown that (Bryant 1997)
where N is the m × m diagonal matrix with on the diagonal, I is the identity matrix, , U is the m × m matrix of ones, is the vector with in positions 1 to k followed by in positions k + 1 to m, and .
Similarly for the internal edge e0
Letting and substituting gives the following branch length estimate.
| (12) |
For cases where only α and both α and β are labeled, respectively, the derivation of the above mentioned equations is similar to that described in Bryant (1997) and is provided in the supplementary material, Supplementary Material online.
The formula, eqn. (12), for branch length is valid only when exists. Bryant (1997) showed that X is invertible as long as there is at most one zero on the diagonal of the matrix . The ith diagonal element is zero if which occurs if there is an edge where both parts of the split have equal size. Even in generally labeled trees there can be at most one such edge.
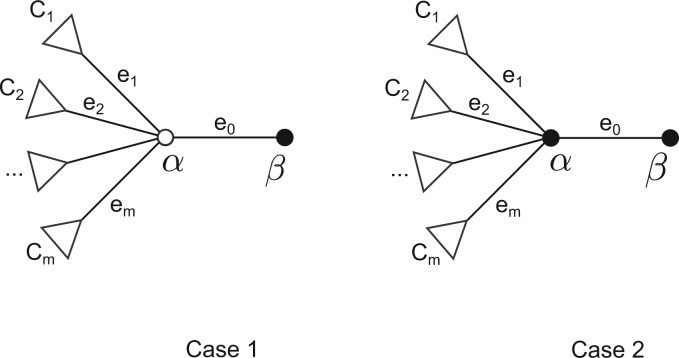
There are two cases to consider for external branches, one if α is not labeled and the other if α is labeled see figure 8. In both cases the derivation of the branch length formula is similar to what has been described earlier and is omitted.
Fig. 8.
The two cases for the terminal edge e0. α is not labeled in case 1 and is labeled in case 2. The triangles represent subtrees.
The branch length formulae turn out to be identical in all cases. The reader is referred to the supplementary material, Supplementary Material online for the proof.
For a more detailed description of the algorithm for computing OLS branch lengths, the reader is referred to Bryant (1997).
Once has been computed, all branch lengths can be calculated in O(n) time. Since there are O(n) edges the time complexity of computing OLS branch lengths is )
The overall time complexity of FJ is . This can be reduced further if heuristics are used at the neighbor identification step, eqn. (1).
OLS branch lengths may be negative which has no biological interpretation. After estimating the branch lengths all branches that are shorter than ϵ and are incident to a latent vertex are contracted. If there is a branch between two labeled vertices that has a negative length, its length is set to . is smaller than the smallest nonzero distance estimate computed in any of the simulation scenarios.
Model Selection
Values of ϵ are inversely related to the number of latent vertices and thus inversely related to model complexity.
We performed model selection using three estimates for test error, CV error, AIC, and BIC. In all cases, model selection is performed by identifying the value of ϵ that minimizes the estimate for test error. Please refer to the supplementary material, Supplementary Material online for a description on how CV error is computed.
AIC and BIC are Taylor series approximations of the Kullback–Leibler distance between the generative model which one wishes to recover and the model that is obtained by maximum likelihood estimation. These are formulated as,
Under the likelihood framework, phylogenetic trees are probabilistic graphical models which are completely described by tree topology and branch lengths. n denotes sample size and is given by sequence length. The number m of parameters equals the number of branches in the tree.
We use FJ branch lengths as approximations of the maximum likelihood branch lengths. Likelihood is computed using Felsenstein’s pruning algorithm which is a dynamic programming algorithm that enables efficient calculation of the likelihood (Felsenstein 1981).
The calculation of CV error is described on page 6 of the supplementary material, Supplementary Material online.
Related Methods Considered in the Comparative Validation
Sampled Ancestors
We used the SAs package (Gavryushkina et al. 2014) of BEASTv2.3.0 (Drummond et al. 2012) for the comparative validation of the FJ algorithm. The following models were considered: the GTR model for substitution, the four-category Γ model for rate heterogeneity across sites, the strict molecular clock model and the fossilized birth death model for generating trees. Uniform priors were set for all model parameters. For all datasets, 108 states were visited using Markov chain Monte Carlo (MCMC) and every 105th state was sampled. The first 5% of the sampled states were discarded as burn-in and the effective sample size (ESS) was computed for all model parameters using the R package CODA (Plummer et al. 2006). ESS were found to be greater than 200 for all parameters across all the MCMC chains indicating that the chains were sufficiently long. The trees that are produced by BEASTv2.3.0 are rooted and contain the maximum number of latent vertices. The sampled trees were postprocessed by unrooting them and contracting all terminal edges of length zero. We reported the average precision and recall of the postprocessed sampled trees from the true tree.
Recursive Grouping and Chow–Liu Recursive Grouping
For assessing the performance of RG and CLRG we used the Matlab implementation that was provided by the authors. Both of these methods are distance-based. RG initially sets the active vertex set to the set of all labeled vertices. At each iteration is partitioned into so-called families using k-means clustering. For each family containing more than one vertex, a relationship test similar to the one used in FJ is performed. If there is a vertex that is the parent of all other vertices in the family then edges are added from the parent to each child. If no such parent is found then a latent vertex is introduced as the parent to all vertices of the family and corresponding edges are added. is reduced by removing all the children. This procedure is iterated until a connected graph is obtained.
CLRG starts by constructing a minimum spanning tree over all the labeled vertices. For each internal vertex vi, the vertex set Vi comprising of vi and its neighbors is constructed and RG is applied to distances between vertices in Vi, producing the tree Ti. The subgraph in the minimum spanning tree that is induced by Vi is replaced by Ti.
Both RG and CLRG require the setting of two thresholds, ϵ and τ. The first threshold, ϵ is used for performing the relationship test. RG and CLRG additionally contract branches that are smaller than this threshold. The second threshold, τ is used to filter out large distances and only distances below this threshold are used when performing the relationship test. We optimized ϵ using BIC and set τ to a reasonably high value of 0.5.
We modified the implementation provided by the authors, in order to correctly evaluate distances of value zero. Such distance estimates were encountered, predominantly, when the average branch length was the shortest and when a large fraction of internal vertices were labeled. The modification is that all distances of value zero were changed to .
NJ with Edge Contraction
We implemented NJc in Python. NJc involves two steps. The first step is the construction of a tree using NJ. Subsequently all branches that are incident to a latent vertex and are smaller than a preselected threshold ϵ are contracted. We optimized ϵ using BIC.
Availability of Code
A program that constructs generally labeled trees using FJ-BIC is provided at https://bioinf.mpi-inf.mpg.de/publications/prabhavk/familyJoining.
Supplementary Material
Supplementary figures S1–S4 are available at Molecular Biology and Evolution online (http://www.mbe.oxfordjournals.org/).
Acknowledgments
This work was partially supported by the German Center for Infection Research, Grant No. DZIF 80008023 to P.K. This work has been performed in the context of the EuResist Network GEIE, and the project MASTER-HIV/HEP which is funded by the German Health Ministry.
References
- Bryant D. 1997. Building trees, hunting for trees, and comparing trees–theory and method in phylogenetic analysis [Ph.D. thesis]. [Christchurch, New Zealand: ]: University of Canterbury. [Google Scholar]
- Bryant D, Waddell P. 1998. Rapid evaluation of least-squares and minimum-evolution criteria on phylogenetic trees. Mol Biol Evol. 15(10):1346–1359. [Google Scholar]
- Buneman P. 1971. The recovery of trees from measures of dissimilarity In: Kendall DG, Tautu P, editors. Mathematics in the archaeological and historical sciences. Edinburgh, UK: Edinburgh University Press, p. 387–395. [Google Scholar]
- Choi MJ, Tan VYF, Anandkumar A, Willsky AS. 2011. Learning latent tree graphical models. J Mach Learn Res. 12:1771–1812. [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9(8):772–772.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond AJ, Suchard MA, Xie D, Rambaut A. 2012. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol. 29(8):1969–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. 1981. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol. 17:368–376. [DOI] [PubMed] [Google Scholar]
- Gascuel O. 1997. BIONJ: an improved version of the NJ algorithm based on a simple model of sequence data. Mol Biol Evol. 14(7):685–695. [DOI] [PubMed] [Google Scholar]
- Gavryushkina A, Welch D, Stadler T, Drummond AJ. 2014. Bayesian inference of sampled ancestor trees for epidemiology and fossil calibration. PLoS Comput Biol. 10(12):e1003919.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle DC, Higgs PG. 2003. Factors affecting the errors in the estimation of evolutionary distances between sequences. Mol Biol Evol. 20(1):1–9. [DOI] [PubMed] [Google Scholar]
- Jombart T, Eggo RM, Dodd PJ, Balloux F. 2011. Reconstructing disease outbreaks from genetic data: a graph approach. Heredity 106(2):383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanave C, Preparata G, Saccone C, Serio G. 1984. A new method for calculating evolutionary substitution rates. J Mol Evol. 20(1):86–93. [DOI] [PubMed] [Google Scholar]
- Plummer M, Best N, Cowles K, Vines K. 2006. CODA: convergence diagnosis and output analysis for MCMC. R News 6(1):7–11. [Google Scholar]
- Rambaut A, Grassly N. 1997. Seq-Gen: an application for the Monte Carlo simulation of DNA sequence evolution along phylogenetic trees. Comput Appl Biosci. 13(3):235–238. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 4(4):406–425. [DOI] [PubMed] [Google Scholar]
- St. John K, Warnow T, Moret BME, Vawter L. 2003. Performance study of phylogenetic methods: (Unweighted) quartet methods and neighbor-joining. J Algorithms 48:173–193. [Google Scholar]
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22(21):2688–2690. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30(9):1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A, Ludwig T, Meier H. 2005. RAxML-III: a fast program for maximum likelihood-based inference of large phylogenetic trees. Bioinformatics 21(4):456–463. [DOI] [PubMed] [Google Scholar]
- Studier JA, Keppler KJ. 1988. A note on the neighbor-joining algorithm of Saitou and Nei. Mol Biol Evol. 5(6):729–731. [DOI] [PubMed] [Google Scholar]
- Vrancken B, Rambaut A, Suchard MA, Drummond A, Baele G, Derdelinckx I, Van Wijngaerden E, Vandamme AM, Van Laethem K, Lemey P. 2014. The genealogical population dynamics of HIV-1 in a large transmission chain: bridging within and among host evolutionary rates. PLoS Comput Biol. 10(4):e1003505.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell PJ, Steel MA. 1997. General time-reversible distances with unequal rates across sites: mixing gamma and inverse Gaussian distributions with invariant sites. Mol Phylogenet Evol. 8(3):398–414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.