Abstract
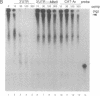
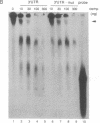
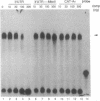
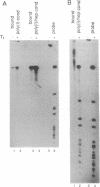
Adenosine+uridine (AU)-rich sequences in the 3' untranslated region (3'UTR) of the mRNA of many cytokines and oncogenes play an important role in mediating RNA degradation. Among the cytokines containing such AU-rich sequences in their 3'UTR is the hematopoietic growth factor granulocyte/macrophage colony-stimulating factor (GM-CSF). GM-CSF gene expression in T cells is regulated by modulation of mRNA half-life. Transfection studies using murine EL-4 thymoma cells have demonstrated that degradation depends on the presence of specific elements in the 3'UTR, including the AU-rich sequences. A number of AU-binding factors have recently been discovered, suggesting that specific regulation may occur through specific protein-mRNA interaction(s). We present evidence from gel-shift analyses and label-transfer experiments that murine cells contain proteins that bind specifically to AU-rich sequences. Three major proteins of 33, 39.5, and 42 kDa are detected. Phorbol ester treatment of cells does not alter the abundance or apparent binding affinity of the proteins. The 33-kDa protein is present in the cytoplasm of murine and human cells, whereas the 39.5- and 42-kDa proteins are present in murine extracts only. Constitutively expressed AU-binding proteins of the type that we describe may function by directing mRNA degradation in the absence of a stimulus to the contrary.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aghib D. F., Bishop J. M., Ottolenghi S., Guerrasio A., Serra A., Saglio G. A 3' truncation of MYC caused by chromosomal translocation in a human T-cell leukemia increases mRNA stability. Oncogene. 1990 May;5(5):707–711. [PubMed] [Google Scholar]
- Bernstein P., Ross J. Poly(A), poly(A) binding protein and the regulation of mRNA stability. Trends Biochem Sci. 1989 Sep;14(9):373–377. doi: 10.1016/0968-0004(89)90011-x. [DOI] [PubMed] [Google Scholar]
- Bickel M., Cohen R. B., Pluznik D. H. Post-transcriptional regulation of granulocyte-macrophage colony-stimulating factor synthesis in murine T cells. J Immunol. 1990 Aug 1;145(3):840–845. [PubMed] [Google Scholar]
- Bohjanen P. R., Petryniak B., June C. H., Thompson C. B., Lindsten T. An inducible cytoplasmic factor (AU-B) binds selectively to AUUUA multimers in the 3' untranslated region of lymphokine mRNA. Mol Cell Biol. 1991 Jun;11(6):3288–3295. doi: 10.1128/mcb.11.6.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawerman G. mRNA decay: finding the right targets. Cell. 1989 Apr 7;57(1):9–10. doi: 10.1016/0092-8674(89)90166-9. [DOI] [PubMed] [Google Scholar]
- Brewer G. An A + U-rich element RNA-binding factor regulates c-myc mRNA stability in vitro. Mol Cell Biol. 1991 May;11(5):2460–2466. doi: 10.1128/mcb.11.5.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Yen T. J. Multiple determinants of eukaryotic mRNA stability. New Biol. 1989 Nov;1(2):121–126. [PubMed] [Google Scholar]
- Dignam J. D. Preparation of extracts from higher eukaryotes. Methods Enzymol. 1990;182:194–203. doi: 10.1016/0076-6879(90)82017-v. [DOI] [PubMed] [Google Scholar]
- Farrar J. J., Fuller-Farrar J., Simon P. L., Hilfiker M. L., Stadler B. M., Farrar W. L. Thymoma production of T cell growth factor (Interleukin 2). J Immunol. 1980 Dec;125(6):2555–2558. [PubMed] [Google Scholar]
- Gillis P., Malter J. S. The adenosine-uridine binding factor recognizes the AU-rich elements of cytokine, lymphokine, and oncogene mRNAs. J Biol Chem. 1991 Feb 15;266(5):3172–3177. [PubMed] [Google Scholar]
- Inoue A., Yanagisawa M., Takuwa Y., Mitsui Y., Kobayashi M., Masaki T. The human preproendothelin-1 gene. Complete nucleotide sequence and regulation of expression. J Biol Chem. 1989 Sep 5;264(25):14954–14959. [PubMed] [Google Scholar]
- Iwai Y., Bickel M., Pluznik D. H., Cohen R. B. Identification of sequences within the murine granulocyte-macrophage colony-stimulating factor mRNA 3'-untranslated region that mediate mRNA stabilization induced by mitogen treatment of EL-4 thymoma cells. J Biol Chem. 1991 Sep 25;266(27):17959–17965. [PubMed] [Google Scholar]
- Jacob W. F., Silverman T. A., Cohen R. B., Safer B. Identification and characterization of a novel transcription factor participating in the expression of eukaryotic initiation factor 2 alpha. J Biol Chem. 1989 Dec 5;264(34):20372–20384. [PubMed] [Google Scholar]
- Jochum C., Voth R., Rossol S., Meyer zum Büschenfelde K. H., Hess G., Will H., Schröder H. C., Steffen R., Müller W. E. Immunosuppressive function of hepatitis B antigens in vitro: role of endoribonuclease V as one potential trans inactivator for cytokines in macrophages and human hepatoma cells. J Virol. 1990 May;64(5):1956–1963. doi: 10.1128/jvi.64.5.1956-1963.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. R., Cole M. D. Rapid cytoplasmic turnover of c-myc mRNA: requirement of the 3' untranslated sequences. Mol Cell Biol. 1987 Dec;7(12):4513–4521. doi: 10.1128/mcb.7.12.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malter J. S., Hong Y. A redox switch and phosphorylation are involved in the post-translational up-regulation of the adenosine-uridine binding factor by phorbol ester and ionophore. J Biol Chem. 1991 Feb 15;266(5):3167–3171. [PubMed] [Google Scholar]
- Malter J. S. Identification of an AUUUA-specific messenger RNA binding protein. Science. 1989 Nov 3;246(4930):664–666. doi: 10.1126/science.2814487. [DOI] [PubMed] [Google Scholar]
- Noshiro M., Nishimoto M., Okuda K. Rat liver cholesterol 7 alpha-hydroxylase. Pretranslational regulation for circadian rhythm. J Biol Chem. 1990 Jun 15;265(17):10036–10041. [PubMed] [Google Scholar]
- Raymond V., Atwater J. A., Verma I. M. Removal of an mRNA destabilizing element correlates with the increased oncogenicity of proto-oncogene fos. Oncogene Res. 1989;5(1):1–12. [PubMed] [Google Scholar]
- Ross H. J., Sato N., Ueyama Y., Koeffler H. P. Cytokine messenger RNA stability is enhanced in tumor cells. Blood. 1991 Apr 15;77(8):1787–1795. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Tobler A., Miller C. W., Norman A. W., Koeffler H. P. 1,25-Dihydroxyvitamin D3 modulates the expression of a lymphokine (granulocyte-macrophage colony-stimulating factor) posttranscriptionally. J Clin Invest. 1988 Jun;81(6):1819–1823. doi: 10.1172/JCI113525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakalopoulou E., Schaack J., Shenk T. A 32-kilodalton protein binds to AU-rich domains in the 3' untranslated regions of rapidly degraded mRNAs. Mol Cell Biol. 1991 Jun;11(6):3355–3364. doi: 10.1128/mcb.11.6.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodnar-Filipowicz A., Moroni C. Regulation of interleukin 3 mRNA expression in mast cells occurs at the posttranscriptional level and is mediated by calcium ions. Proc Natl Acad Sci U S A. 1990 Jan;87(2):777–781. doi: 10.1073/pnas.87.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]