Abstract
Recent discoveries have led to the identification of a novel group of immune cells, the innate lymphoid cells (ILCs). The members of this group are divided into three subpopulations: ILC1s, ILC2s, and ILC3s. ILC2s produce Th2 cytokines, IL-4, IL-5, and IL-13, upon activation by epithelial cell-derived cytokines, lipid mediators (cysteinyl leukotrienes and prostaglandin D2), and TNF family member TL1A and promote structural and immune cell responses in the airways after antigen exposure. In addition, ILC2 function is also influenced by inducible T cell costimulator (ICOS)/ ICOS-ligand (ICOS-L) interactions via direct contact between immune cells. The most common airway antigens are allergens and viruses which are highly linked to the induction of airway diseases with underlying type 2 inflammation including asthma and allergic rhinitis. Based on recent findings linking ILC2s and airway Th2 responses, there is intensive investigation into the role of ILC2s in human disease with the hope of a better understanding of the pathophysiology and the discovery of novel potential therapeutic targets. This review summarizes the recent advances made in elucidating ILC2 involvement in human Th2 airway disease.
Keywords: Group 2 innate lymphoid cells, ILC2, Allergy, Asthma, Airway
Introduction
Th2 inflammatory responses in the airway are characterized by the induction of Th2 cytokines including IL-4, IL-5, and IL-13 that lead to IgE production, increased mucus production, and tissue eosinophilia that play important roles in asthma, allergic rhinitis, and nasal polyposis [1, 2]. Conventionally, the increased Th2 cytokine production present in these diseases was largely attributed to CD4+ Th2 cells. However, a report in 2001 showed that a non-T/non-B accessory cell in mice produced IL-5 and IL-13 in response to IL-25 [3], and in 2010, three independent studies later identified these “accessory cells” as group 2 innate lymphoid cells (ILC2s, originally termed natural helper cells, nuocytes, and innate type 2 helper cells) [4–7].
ILC2s develop from common lymphoid progenitors but lack the surface expression of the common lineage markers associated with T cells (CD3, CD4, and αβ/γδ TCR), B cells (CD19 and CD20), and other leukocytes including CD11c, CD14, CD16, CD56, and FcεR1. ILC2s are part of a larger group of lineage-negative lymphoid cells that include ILC1s and ILC3s and are distinctly different in their role in immunity. ILC1s and ILC3s produce Th1 (IFNγ) and Th17 (IL-17, IL-22) cytokines, respectively. In contrast, ILC2s produce Th2 cytokines regulated by GATA-3, the master Th2 cytokine transcription factor, which is highly expressed in CD4+ Th2 cells and has been shown to be critical to the development and function of ILC2s [8–10, 11••]. ILC2s are especially potent producers of IL-5 that mediates eosinophil activation and survival and IL-13 that regulates multiple aspects of airway inflammation, mucus metaplasia, smooth muscle remodeling, and airway hyperresponsiveness (AHR) [12•]. ILC2s can also produce IL-4 after stimulation with specific mediators including cysteinyl leukotrienes (CysLTs) and prostaglandin D2 (PGD2) [13, 14]. Most of the early investigations of ILC2s have been performed in mice, and the precise role ILC2s play in human disease has not yet been elucidated in part due to the lack of ILC2-specific markers. Studies of ILCs utilize negative selection with lineage antibodies (to exclude T, B, NK, NKT cells as well as others) followed by positive selection with CD45 (hematopoietic marker) and one or more of IL-2Rα (CD25), CD44, IL-7Rα (CD127), ST2 (IL-33R), and CRTH2 (receptor for PGD2) [15].
ILC2 populations are found in several tissues, including the respiratory and gastrointestinal tracts as well as the skin, and there are increasing numbers of studies demonstrating strong correlations between levels and activation of ILC2s in asthma, chronic rhinosinusitis (CRS), atopic dermatitis, and eosinophilic esophagitis [16–18, 19••, 20••, 21]. Importantly, ILC2s are not directly activated in an antigen-specific manner supporting a possible role in propagation of inflammatory disease independent of specific allergen exposure. Thus, triggers including viruses that often exacerbate asthma and CRS may also activate ILC2s. The involvement of ILC2s in human diseases outside of the airway, including atopic dermatitis, eosinophilic esophagitis, and obesity, is reviewed elsewhere [22, 23]. Additionally, this review will largely focus on the involvement of ILC2s in human airway diseases with brief mention of mouse model studies where insight from human studies was unavailable, also reviewed elsewhere [8, 15].
Sources of ILC2 Activation
ILC2 activation results in the production and secretion of Th2 cytokines that play pivotal roles in Th2-induced airway diseases. Initial studies of ILC2s demonstrated robust production of IL-5 and IL-13 [5, 6]. IL-5 is essential in the recruitment, activation, and survival of eosinophils [24], and IL-13 promotes mucus production, AHR, and fibrosis [25]. Our work and others have demonstrated that ILC2s also produce IL-4 which has a critical role in promoting CD4+ Th2 cell differentiation and IgE class switching [26]. ILC2s also secrete IL-9, which promotes mast cell accumulation and mucus production [27]. Interestingly, ILC2s also secrete the epidermal growth factor receptor (EGFR) ligand amphiregulin (AREG) that may promote tissue repair in the lung after viral infections [28]. Human studies with asthmatics have shown the beneficial effects of blocking IL-4, IL-5, and IL-13, supporting the importance of Th2 cytokines in asthma [29]. Thus, ILC2s may regulate innate type 2 responses as well as critically link innate and adaptive responses in allergic airway diseases.
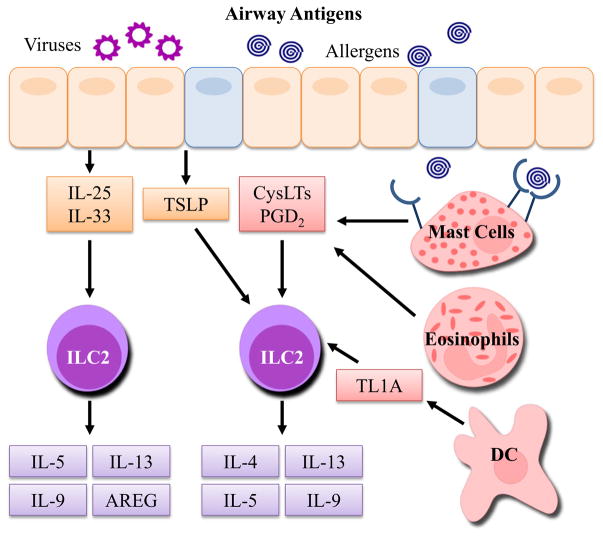
The activation of ILC2s is a downstream response to allergens and other antigens including viruses and therefore requires activation signals from other cell types. Interestingly, ILC2s have also recently been shown to interact directly (through cell contact) or indirectly (through mediator release) with several other cell types including T cells, basophils, mast cells, eosinophils, and epithelial cells [17]. This crosstalk between airway ILC2s and other cell types supports a significant role of ILC2s in orchestrating type 2 immune responses, as depicted in Fig. 1. The initial mediators found to initiate ILC2 activation were epithelial cell-derived cytokines: IL-25, IL-33, and thymic stromal lymphopoietin (TSLP). More recently, studies have shown that ILC2s are activated by lipid mediators (CysLTs and PGD2) and TNF-like ligand 1A (TL1A), which have all been associated with Th2-driven diseases [30–33]. Homeostatic survival and proliferation of ILC2s are regulated by IL-2 and IL-7 which signal through the common γ chain receptor [23, 34]. Importantly, other mediators reduce ILC2 activation including PGI2 and lipoxin A4 (LXA4) [35, 36]. The primary modulators of ILC2 function are described in more detail below.
Fig. 1.
Sources of ILC2 activation and downstream mediators. Upon exposure to environmental antigens including viruses and allergens, airway epithelial cells rapidly release cytokines IL-25, IL-33, and TSLP that directly activate ILC2s. Additionally, PGD2 and CysLTs from activated mast cells and eosinophils, as well as TL1A produced by DCs, can potently activate ILC2s. Allergens induce CysLT and PGD2 production from mast cells through binding of surface IgE. Activation of ILC2s results in the secretion of Th2 cytokines IL-5, IL-9, and IL-13. AREG is largely made by ILC2s after stimulation with IL-33, and IL-4 is produced by ILC2s after stimulation with CysLTs, PGD2, and TSLP. These mediators can then influence other airway cells to induce adaptive Th2 cell differentiation, eosinophil recruitment and activation, AHR, mucus production, and tissue repair and remodeling. CysLT cysteinyl leukotrienes, PGD2 prostaglandin D2, AREG amphiregulin
IL-33
IL-33 potently induces early type 2 responses in mice and is elevated in asthmatic airways compared to nonasthmatics [37]. The cellular sources of airway IL-33 in humans include bronchial epithelial cells and smooth muscle cells [38, 39]. In addition, genomewide association studies in asthma show that IL-33 and its receptor, ST2, are linked with susceptibility to asthma [40]. IL-33 has also been linked to chronic obstructive pulmonary disease (COPD), CRS, and allergic rhinitis [41–43]. Further, recent studies detected increased airway IL-33 levels in asthmatics that correlated with elevated ILC2s [21, 44]. IL-33 is a unique cytokine as it is bound to nuclear chromatin in a biologically active immature form prior to being released as a danger signal during cellular stress including exposure to the fungal allergen Alternaria [45]. One report showed that the secretion of IL-33 in human bronchial airway epithelial cells exposed to Alternaria is dependent on ATP release and calcium influx [46]. After secretion, IL-33 binds to a heterodimeric receptor made up of ST2 and IL-1R accessory protein (IL-1RAcP) [47]. Importantly, IL-33 has broad effects on multiple cell types including ILC2s, mast cells, eosinophils, and Th2 lymphocytes [22, 47, 48]. In 2011, Mjosberg et al. demonstrated that human peripheral blood and fetal gut CRTH2-expressing ILC2s responded to IL-33 to produce IL-13 and ILC2s were detected in the fetal and adult lung, suggesting that human airway ILC2s might also respond to IL-33 [49]. In support of these findings, previous mouse model studies have shown that IL-33 activates ILC2s to induce type 2 lung inflammation [10, 50–52]. Thus, IL-33 is a dominant trigger of ILC2 activation in humans.
IL-25
IL-25 (IL-17E) is a member of the IL-17 family but has a very distinct role in inflammatory responses compared with other IL-17 family members [3]. IL-25 is expressed in a variety of cell types including lung epithelial cells, eosinophils, mast cells, and Th2 cells [53]. Notably, IL-25 expression is increased in asthmatic airways after allergen challenge and may promote angiogenesis in asthma [54, 55]. Further, rhinovirus-induced airway inflammatory responses associated with asthma exacerbations are diminished by the blockade of IL-25 receptor in mice [56]. Together, these studies suggest a potential role for IL-25 in asthmatic airway responses.
Prior to the characterization of ILC2s, early work showed that a non-T/non-B cell type was activated by IL-25 [3]. Similar to IL-33, IL-25 promotes IL-5 and IL-13 production by ILC2s, though one study showed that IL-33 is more potent and faster acting than IL-25 in inducing ILC2 activation and airway inflammation in mice [49, 57]. Interestingly, another study demonstrated that IL-25 induces the expansion of a novel progenitor non-ILC2 subset of cells, termed MPPtype2 that can differentiate into basophils, mast cells, and macrophages in mice [58]. Thus, IL-25 may have a multitude of roles in type 2 inflammation apart from ILC2 activation.
TSLP
TSLP is primarily expressed by epithelial cells and is induced in response to toll-like receptor (TLR) agonists, infections, and allergens [59]. TSLP has effects on both innate and adaptive immunity during type 2 inflammatory responses. Harada et al. investigated the association between TSLP single nucleotide polymorphisms (SNPs) and allergic diseases and found several SNPs associated with asthma and pulmonary function [60]. TSLP expression is elevated in the airways of asthmatics, correlates with disease severity, and is also increased in atopic dermatitis, allergic rhinitis, and nasal polyposis [61, 62]. TSLP was initially shown to activate dendritic cells to induce CD4+ Th2 cell polarization through OX40/OX40L interactions [63]. The TSLP receptor is a heterodimer of TSLPR and IL-7Rα, which is expressed in human ILC2s [11••]. Importantly, TSLP induces IL-4, IL-5, and IL-13 secretion from human blood and nasal polyp ILC2s, as well as enhanced ILC2 GATA-3 expression [11••]. This study is in agreement with other studies performed in murine models, demonstrating TSLP activation of ILC2s [64, 65]. Finally, a recent allergen challenge study in asthmatics showed that anti-TSLP therapy reduced early- and late-phase responses [66]. Thus, there is strong support for TSLP as a key mediator in the pathogenesis of asthma and allergic inflammation.
Lipid Mediators
Eicosanoid lipid mediators contribute to many aspects of inflammation and include the prostaglandins and leukotrienes that are rapidly produced by activated mast cells, macrophages, dendritic cells, and eosinophils [32, 67]. PGD2 binds to CRTH2, which is expressed on Th2 cells, eosinophils, and ILC2s, and is produced in large quantities in response to IgE-induced mast cell activation [67]. Further, PGD2 is increased in patients with allergic rhinitis and severe asthma and is a potent bronchoconstrictor in both healthy and asthmatic subjects [33, 68–71]. Recently, PGD2 and CysLTs have been shown to promote ILC2 activation, whereas PGI2 and LXA4 reduce ILC2 activity [14, 35, 36, 72]. In human peripheral blood ILC2s, PGD2 has been shown to increase IL-13 secretion above IL-2, IL-25, and IL-33 stimulation [36]. In addition, our group showed enhanced PGD2-induced blood ILC2 chemotaxis from allergic compared with nonallergic individuals [72]. A subsequent report confirmed these findings and demonstrated that PGD2 induces migration of both human skin and blood ILC2s, as well as promotes ILC2 IL-4, IL-5, and IL-13 production, which were mimicked by stimulating ILC2s with supernatants of activated mast cells [13].
Similar to PGD2, CysLT levels are also found to be elevated in asthma, allergic rhinitis, aspirin-exacerbated respiratory disease, and CRS and can induce airway mucus secretion and bronchoconstriction [32, 73, 74]. Administration of leukotrienes (LT) LTC4, LTD4, and LTE4 alone has been shown to induce bronchoconstriction in both asthmatics and nonasthmatics [75]. LTD4 binds with high affinity to the type 1 CysLT receptor (CysLT1R), which is also antagonized by montelukast. Our group has shown that mouse ILC2s express CysLT1R and that stimulation of ILC2s with LTD4 rapidly induces the secretion of IL-4, IL-5, and IL-13 in a CysLT1R-dependent manner in vitro and in vivo [14]. A subsequent study demonstrated that human ILC2s respond to CysLTs suggesting that mouse ILC2 studies are translational to humans [13]. Thus, CysLTs and PGD2 that are present in human type 2 inflammatory airway diseases can potently activate ILC2s to produce Th2 cytokines.
While some lipid mediators promote ILC2 activation and Th2 inflammation, two lipid mediators have been shown to inhibit ILC2 function. A recent report by Zhou et al. demonstrated that a PGI2 analog inhibited human ILC2 expression of IL-5 and IL-13 after stimulation with IL-2 and IL-33 [35]. In addition, further findings in a mouse model showed that PGI2 inhibited ILC2 IL-5 and IL-13 secretion and reduced ILC2 proliferation. This study suggests a potential mechanism by which PGI2 may attenuate allergic airway inflammation through the inhibition of ILC2 activation. Another study previously demonstrated that LXA4, a proresolution and anti-inflammatory factor, inhibited IL-13 production from human ILC2s that were stimulated with PGD2, IL-25, and IL-33 [36]. LXA4 is thought to be decreased in severe asthma, and this study provides a possible mechanism by which reduced levels of LXA4 in asthmatics may result in increased ILC2 activity and ultimately unregulated asthmatic inflammation [76].
TL1A
The TNF family member TL1A (TNFSF15) is the ligand for DR3, a TNF receptor superfamily member (TNFRSF25) [77]. DR3 is commonly found on T cells and NKT cells but has recently been detected on ILC2s [31, 78]. Previous reports have suggested that TL1A/DR3 interactions contribute to type 2 inflammation in animal models [79]. Two recent reports have since demonstrated that TL1A promotes ILC2 responses [31, 78]. Treatment of human ILC2s with TL1A alone increased IL-5 and IL-13 production, and TL1A also enhanced IL-25 or IL-33 ILC2 activation [31]. Another report showed that TL1A costimulation induced ILC2 IL-13 secretion and that DR3 was required for ILC2 expansion after allergen challenge, but not after helminth infection in mice [78]. Together, these studies suggest that TL1A may promote ILC2 activation in specific conditions.
ICOS/ICOS-L Interactions
Direct cellular interactions between ICOS and ICOS-ligand have very recently been shown to regulate ILC2 function [80•, 81, 82]. ICOS is part of the immunoglobulin superfamily and was thought to be expressed solely on activated or memory Tcells, whereas ICOS-L was thought to be expressed by B cells and antigen-presenting cells [83]. ICOS/ICOS-L interactions were previously known to have a role in the regulation of Th2 cytokine production, AHR, IgE production, and B cell differentiation in mouse models [84]. A recent study by Maazi et al. demonstrated that human peripheral blood ILC2s express both the ICOS receptor as well as ICOS-L and their expression was increased by in vitro culture with IL-2 and IL-7 but not IL-33 [80•]. Blockade of ICOS/ICOS-L interactions on human ILC2s in culture diminished both IL-5 and IL-13 secretion after stimulation with IL-2, IL-7, and IL-33. Further, ICOS promoted ILC2 survival signaling and initiated ILC2-induced inflammation and AHR in Alternaria- or IL-33-challenged mice. Additional studies performed by other groups have supported ICOS/ICOS-L interactions as an important pathway of mouse ILC2 proliferation, homeostasis, and activation [81, 82]. Thus, the direct contact between ICOS and ICOS-ligand regulates ILC2 function and represents a therapeutic target of ILC2 activation and lung inflammation.
ILC2 Responses Induced by Allergens and Viruses
ILC2 activation can be triggered by allergens including house dust mite (HDM), Alternaria alternata, and papain, as well as viruses including influenza, rhinovirus (RV), and respiratory syncytial virus (RSV). Most investigations into human ILC2 activation in the airways have been correlative. In order to investigate the effects of allergens and viruses on human airways, studies have utilized mouse models of lung inflammation induced by airway challenges with allergens and viruses. In-depth evaluation of these individual studies is reviewed elsewhere [15, 34], though increasing evidence from human studies suggests that similar pathways may occur in human airway disease.
A. alternata is a fungal allergen that is associated with severe and sometimes fatal/near-fatal asthma exacerbations in humans after exposure [85]. One of the more novel aspects about this fungal allergen is that it specifically promotes the rapid release of IL-33, which in turn rapidly activates downstream ILC2 responses [46, 86]. A recent study in children with severe asthma with fungal sensitization reported increased IL-33 in both the bronchoalveolar lavage (BAL) fluid and endobronchial biopsy specimen compared to children without fungal sensitization [44]. Our group and others have shown that even a single dose of Alternaria in unsensitized mice can induce rapid IL-33 release, ILC2 activation, and IL-33-dependent airway eosinophilia [10, 46, 51, 86]. Further, we have shown that Alternaria administered to mice during an adaptive response to a different allergen (rye grass) leads to enhanced eosinophilia, AHR, and ILC2 activation [87]. Importantly, the ILC2-driven response to Alternaria occurs independent of adaptive immunity as mice lacking B and T cells are able to mount the same response as wild-type mice. The triggering of the IL-33-ILC2 axis by Alternaria suggests an IgE-independent mechanism of induction of AHR and type 2 inflammation.
HDM sensitization and exposure is thought to contribute to the development and symptoms of asthma [88]. Though adaptive Th2 responses to HDM are well characterized, recent evidence suggests that relevant innate airway responses may drive ILC2 activation. HDM activates TLRs on the surface of epithelial cells and induces epithelial cell-derived cytokine secretion as well as activates mast cells in an IgE-independent manner [89, 90]. Recently, one study found that HDM induces ILC2 expansion in the lung and BAL of mice and showed that ILC2s are important Th2 cytokine producers comparable to CD4+ cells during repetitive challenges [12•]. In another report, mice that lack ILC2s but have CD4 cells had reduced levels of Th2 cytokines and IgE after HDM challenges, thus highlighting the importance of ILC2s in bridging innate to adaptive Th2 responses [91].
Papain is an inhaled protease allergen known to induce occupational asthma [92]. Papain contains cysteine protease activity that activates epithelial IL-33 production, and several studies have investigated the effects of papain on ILC2 activation in mice [93–98]. Similar to Alternaria, papain induces eosinophilic lung inflammation in the absence of T and B cells, and depletion of ILC2s abrogates this response suggesting that ILC2s have a dominant role in papain-induced lung inflammation [94, 95]. Though studies in humans exposed to papain are limited, the animal models suggest a potential role for ILC2s in papain-induced occupational asthma.
Respiratory viruses including RV, influenza virus, and RSV have been implicated in asthma development and exacerbation [99, 100]. Mouse studies have shown that IL-33 is released in response to influenza infection leading to ILC2 activation that promoted AHR or induced tissue repair [50, 101–104]. Additionally, increased IL-25 secretion and ILC2 activation have been reported after RV infection in neonatal mice [105]. Another study found that RSV infection in mice induced ILC2 IL-13 production in an IL-33-ST2-dependent manner [106]. Importantly, there is one human study that investigated IL-33 production and ILC2 activation after in vivo rhinovirus challenges performed in asthmatics and controls [107••]. The authors found that IL-5, IL-13, and IL-33 levels were elevated in the BAL and nasal fluid of asthmatics after RV infection compared to baseline and nonasthmatics. Importantly, the in vitro addition of supernatants from rhinovirus-infected epithelium added to purified ILC2s led to IL-33-dependent Th2 cytokine production. Thus, rhinovirus may induce asthma exacerbations in part through an IL-33/ILC2 pathway.
ILC2s in Human Airway Disease
Animal model studies support the notion that ILC2s may play an important role in type 2 airway diseases. Recently, ILC2s have been detected in samples from patients with asthma, allergic rhinitis, and chronic rhinosinusitis with nasal polyposis (Table 1), suggesting that they may contribute to human disease. Combined with animal data, further human ILC2 studies may lead to advances in understanding disease pathogenesis as well as human ILC2 biology.
Table 1.
Relevant human studies of ILC2s in airway disease
| Disease | Outcome | Reference |
|---|---|---|
| Allergic rhinitis | Allergen challenge increased peripheral blood ILC2 levels in allergic individuals. | [108••] |
| ILC2 levels in peripheral blood increased during grass pollen season in individuals with seasonal allergic rhinitis. | [109] | |
| Asthma | ILC2s are present in the peripheral blood of asthmatics and healthy controls, however, not in significantly different levels. | [36] |
| ILC2s were elevated in the peripheral blood of atopic asthmatics, and circulating ILC2s were found responsive to IL-33 and secrete IL-5 and IL-13. | [110] | |
| IL-33, ILC2, and IL-13-producing ILC2 levels were increased in the BAL fluid of asthmatics. | [111] | |
| IL-5- and IL-13-producing ILC2s were elevated in both the blood and sputum of severe asthmatics compared to mild asthmatics. | [19••] | |
| ILC2 levels were higher in the sputum collected from severe asthmatic children compared to children with recurrent lower respiratory tract infections. | [20••] | |
| Chronic rhinosinusitis | ILC2s are enriched in nasal polyps of CRS patients. | [49] |
| Increased ILC2s in inflamed sinonasal mucosa in patients with CRSwNP compared to patients with CRSsNP and increased IL-33 induced IL-13 secretion. | [112] | |
| ILC2s enriched in eosinophilic nasal polyp endotype and were inversely related to systemic steroid use. | [113] | |
| CRSwNP and allergic CRS positively correlated with ILC2, IL-5, and IL-13 levels. | [114] | |
| CRS patients with coexisting asthma or allergy had increased ILC2 levels. ILC2 levels positively correlated with nasal symptom score and blood eosinophil counts. | [115] |
Asthma
Asthma is a chronic inflammatory disorder, resulting in shortness of breath, wheezing, excessive mucus production, and airway remodeling. Evidence of Th2 inflammation has been detected in the airways of a large subset of asthmatics [116]. Though the adaptive CD4+ Th2 paradigm may explain IgE and CD4+ cell-specific response to allergens, other triggers including viral infections and tobacco smoke have been shown to worsen asthma symptoms suggesting that innate immune pathways contribute. Asthmatics have elevated airway levels of IL-33, TSLP, IL-25, PGD2, and CysLTs which have been shown to activate ILC2s [39, 54, 61, 68, 117]. Two studies set out to determine whether ILC2 levels differed in the peripheral blood of asthmatics. Barnig et al. found no differences in peripheral blood ILC2 levels between severe asthmatics, mild asthmatics, and healthy individuals [36]. However, Bartemes et al. detected higher ILC2 levels in the blood of allergic asthmatics compared to patients with allergic rhinitis and healthy individuals [110]. Further, they found that IL-5 production from peripheral blood ILC2s of allergic asthmatics was more responsive to IL-33 and IL-2 treatment compared with healthy individuals and patients with allergic rhinitis. The differences in findings may stem from diverse patient populations and whether or not they were allergic.
Importantly, several groups have recently examined the presence of ILC2s in the airways of asthmatics. Christianson et al. found elevated ILC2s, and specifically IL-13-producing ILC2s, in the BAL fluid of asthmatics compared to controls, which also suggests heightened activation of ILC2s in asthmatics [111]. In addition, two recent studies found elevated ILC2 levels in the sputum and blood of adult severe asthmatics compared to mild asthmatics, as well as in the BAL, induced sputum, and blood of children with severe asthma compared to children without [19••, 20••]. Notably, the adult asthmatics were treated with systemic corticosteroids (CS) and had activated airway ILC2s despite CS use. These reports implicate ILC2s in the pathogenesis of severe asthma for which there are limited treatment options, and targeting ILC2s or their regulatory cytokines represents a therapeutic strategy.
Allergic Rhinitis
Allergic rhinitis is an IgE-mediated response to inhaled allergens that involves many inflammatory cells from the innate and adaptive immune systems including T and B cells, mast cells, basophils, eosinophils, and dendritic cells. Cross-linking of IgE on mast cells induces a plethora of mediator release including histamine, CysLTs, and prostaglandins. CysLTs and PGD2 are therefore available to rapidly activate ILC2s, thus supporting a role for an adaptive IgE response in stimulating innate Th2 cytokine-producing cells. Interestingly, a recent study investigated the ability of human IgE-activated mast cells to generate active IL-33 and found that activation of mast cells results in the generation of proteases able to cleave full-length IL-33 into more biologically active forms [118]. In addition, the report showed that the cleaved forms of IL-33 were able to directly activate mouse lung ILC2s with greater potency than full-length IL-33, as demonstrated by increased IL-5 and IL-13 secretion and further providing a direct connection between IgE responses and ILC2 activation. This is in agreement with another mouse model study highlighting the mast cell/IL-33/ST2 axis in IgE-dependent inflammation [119].
There are currently only a few human studies investigating ILC2s in allergic rhinitis in humans [108••, 109]. Our group has demonstrated a significant increase in the percent of peripheral blood ILC2s 4 h postnasal cat allergen challenge of cat-allergic individuals compared with diluent challenge in the same individuals at a separate visit [108••]. One possible mechanism of the increase in peripheral blood ILC2s during nasal allergen exposure may be that ILC2s are recruited from the bone marrow into circulation. Another group investigated the association between seasonal allergic rhinitis (SAR) and peripheral blood ILC2 levels measured in and out of the grass pollen season from nonatopic, SAR, and SAR individuals who received subcutaneous immunotherapy (SCIT) [109]. Consistent with our findings, the group demonstrated that ILC2 levels were significantly increased in SAR individuals during grass pollen season and this increase was not present in nonatopic and SAR with SCIT individuals. A key question remains in both reports regarding the origin and destination of the peripheral blood ILC2s during allergen exposure.
Chronic Rhinosinusitis with Nasal Polyposis
CRS is defined by inflammation of the epithelial lining of the nose and sinuses for over 12 weeks and is often associated with the presence of nasal polyps [120]. Comorbidities including asthma and COPD are associated with CRS [121]. Several groups have recently shown an enrichment of ILC2s in sinus and nasal polyp tissue (CRSwNP), suggesting that ILC2s may contribute to the pathophysiology of CRSwNP [49, 113–115]. Mjosberg et al. first reported the presence of ILC2s in nasal polyps that respond to IL-33 and TSLP (expressed by nasal polyp epithelium) and induce polyp ILC2 IL-13 production [11••, 49]. In addition, the authors showed that ILC2 GATA-3 is enhanced by TSLP and required for ILC2 IL-13 production. Shaw et al. demonstrated elevated ST2+ ILC2s, as well as an increase in ST2 expression in the inflamed sinus mucosa, and an increase in IL-33 production from sinonasal epithelial cells from CRSwNP patients compared to CRS patients without nasal polyps (CRSsNP) and healthy controls [112]. They further showed that ILC2s responded to IL-2 and IL-33 stimulation and produced IL-13, thus suggesting a possible mechanism by which sinonasal epithelial cells secrete IL-33 and activate ILC2s to produce IL-13 in CRSwNP. Our group has shown that ILC2s were significantly elevated in eosinophilic compared with noneosinophilic nasal polyp endotypes thus further implicating ILC2s in human eosinophilic disease [113]. Additionally, we found that patients treated with systemic CS had reduced NP ILC2s and, using a mouse model, showed that mouse ILC2s are susceptible to CS treatment. However, another study has suggested that TSLP induces ILC2 CS resistance, and perhaps the context dictates whether ILC2s are sensitive or resistant to CS [122].
Other Lung Diseases
Aside from asthma, some studies have investigated the role of ILC2s in other lung diseases including pulmonary fibrosis and primary spontaneous pneumothorax (PSP). Hams et al. demonstrated increased IL-25 and ILC2 levels in the lung tissue and BAL of subjects with idiopathic pulmonary fibrosis [123]. In addition, they utilized a mouse model of pulmonary fibrosis and found that IL-25-driven ILC2 secretion of IL-13 was sufficient to cause collagen deposition in the lungs of challenged mice. Another report demonstrated an increase in ILC2 levels in the pleural fluid of patients with PSP and that either IL-33 alone or pleural fluid from PSP patients could induce ILC2 IL-5 production resulting in eosinophilic pleural effusion [124]. These studies highlight ILC2 involvement in a wider array of diseases and may identify novel pathways induced by ILC2 responses in human disease.
Summary
ILC2s are newly discovered innate immune cell members that have been shown to have an important role in airway Th2 immunity. ILC2s are activated by several key inflammatory mediators including epithelial cell-derived cytokines and lipid mediators. These mediators are released from epithelial cells as well as other structural cells and immune cells. Activation of ILC2s results in a rapid and robust release of IL-4, IL-5, and IL-13 known to participate in airway eosinophilia, mucus production, AHR, and tissue remodeling. The activation of ILC2s provides a mechanism by which the innate immune response to allergens and viruses can facilitate Th2 inflammation independent of adaptive immunity. Current ILC2 investigations are challenging due to the rarity of ILC2s in tissues and the lack of specific positive cell surface markers as well as consistent methods of identification and isolation. Despite these limitations, there are a growing number of successful human studies demonstrating ILC2s in Th2 airway diseases, and mouse models strongly support the contribution of ILC2s in these diseases. However, studies beyond the scope of this review also suggest a protective role for ILC2s in tissue repair and metabolism. Therefore, although ILC2s are becoming promising candidates for targeting Th2 inflammatory diseases, more work is needed to characterize the role of this novel population of cells in health and human disease.
Acknowledgments
This study was supported by NIH R01 AI114585 to T.A.D.; AI 107779, AI 38425, and AI 70535 to D.H.B.; and T32 AI007469 to M.R.K.
Footnotes
Compliance with Ethical Standards
Conflict of Interest Drs. Broide and Doherty report grants from NIH and grants and honoraria from Janssen. Dr. Karta has no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2008;8:218–30. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest. 2008;118:3546–56. doi: 10.1172/JCI36130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–95. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 4.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–70. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–94. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–4. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 7.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells—a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–9. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 8.Walker JA, McKenzie AN. Development and function of group 2 innate lymphoid cells. Curr Opin Immunol. 2013;25:148–55. doi: 10.1016/j.coi.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diefenbach A, Colonna M, Koyasu S. Development, differentiation, and diversity of innate lymphoid cells. Immunity. 2014;41:354–65. doi: 10.1016/j.immuni.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doherty TA, Khorram N, Chang JE, Kim HK, Rosenthal P, Croft M, et al. STAT6 regulates natural helper cell proliferation during lung inflammation initiated by Alternaria. Am J Physiol Lung Cell Mol Physiol. 2012;303:L577–588. doi: 10.1152/ajplung.00174.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Mjosberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–59. doi: 10.1016/j.immuni.2012.08.015. Study demonstrating the critical role of transcription factor GATA-3 as an important regulator of human peripheral blood and nasal polyp ILC2 function. [DOI] [PubMed] [Google Scholar]
- 12•.Klein Wolterink RG, Kleinjan A, van Nimwegen M, Bergen I, de Bruijn M, Levani Y, et al. Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur J Immunol. 2012;42:1106–16. doi: 10.1002/eji.201142018. Study performed multiple mouse models of asthma to show that ILC2s are major IL-5 and IL-13 producers in the lung compared with other lymphocytes. [DOI] [PubMed] [Google Scholar]
- 13.Xue L, Salimi M, Panse I, Mjosberg JM, McKenzie AN, Spits H, et al. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J Allergy Clin Immunol. 2014;133:1184–94. doi: 10.1016/j.jaci.2013.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol. 2013;132:205–13. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doherty TA. At the bench: understanding group 2 innate lymphoid cells in disease. J Leukoc Biol. 2015;97:455–67. doi: 10.1189/jlb.5BT0814-374R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halim TY. Group 2 innate lymphoid cells in disease. Int Immunol. 2015 doi: 10.1093/intimm/dxv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lund S, Walford HH, Doherty TA. Type 2 innate lymphoid cells in allergic disease. Curr Immunol Rev. 2013;9:214–21. doi: 10.2174/1573395510666140304235916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doherty TA, Baum R, Newbury RO, Yang T, Dohil R, Aquino M, et al. Group 2 innate lymphocytes (ILC2) are enriched in active eosinophilic esophagitis. J Allergy Clin Immunol. 2015;136:792–794.e3. doi: 10.1016/j.jaci.2015.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Smith SG, Chen R, Kjarsgaard M, Huang C, Oliveria JP, O’Byrne PM, et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.05.037. The first study demonstrating increased Th2 cytokine producing ILC2s in the airways of patients with severe asthma on systemic corticosteroids. [DOI] [PubMed] [Google Scholar]
- 20••.Nagakumar P, Denney L, Fleming L, Bush A, Lloyd CM, Saglani S, et al. Type 2 innate lymphoid cells in induced sputum from children with severe asthma. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.06.038. First report demonstrating ILC2 levels are increased in the sputum of children with severe asthma and providing validation in human airways for ILC2 association with asthma. [DOI] [PubMed] [Google Scholar]
- 21.Christianson CA, Goplen NP, Zafar I, Irvin C, Good JT, Jr, Rollins DR, et al. Persistence of asthma requires multiple feedback circuits involving type 2 innate lymphoid cells and IL-33. J Allergy Clin Immunol. 2015;136(1):59–68.e14. doi: 10.1016/j.jaci.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mjosberg J, Eidsmo L. Update on innate lymphoid cells in atopic and non-atopic inflammation in the airways and skin. Clin Exp Allergy. 2014;44:1033–43. doi: 10.1111/cea.12353. [DOI] [PubMed] [Google Scholar]
- 23.McKenzie AN, Spits H, Eberl G. Innate lymphoid cells in inflammation and immunity. Immunity. 2014;41:366–74. doi: 10.1016/j.immuni.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Takatsu K, Nakajima H. IL-5 and eosinophilia. Curr Opin Immunol. 2008;20:288–94. doi: 10.1016/j.coi.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Rael EL, Lockey RF. Interleukin-13 signaling and its role in asthma. World Allergy Organ J. 2011;4:54–64. doi: 10.1097/WOX.0b013e31821188e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gour N, Wills-Karp M. IL-4 and IL-13 signaling in allergic airway disease. Cytokine. 2015;75:68–78. doi: 10.1016/j.cyto.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilhelm C, Turner JE, Van Snick J, Stockinger B. The many lives of IL-9: a question of survival? Nat Immunol. 2012;13:637–41. doi: 10.1038/ni.2303. [DOI] [PubMed] [Google Scholar]
- 28.Zaiss DM, Gause WC, Osborne LC, Artis D. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity. 2015;42:216–26. doi: 10.1016/j.immuni.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walford HH, Doherty TA. Diagnosis and management of eosinophilic asthma: a US perspective. J Asthma Allergy. 2014;7:53–65. doi: 10.2147/JAA.S39119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Islam SA, Luster AD. T cell homing to epithelial barriers in allergic disease. Nat Med. 2012;18:705–15. doi: 10.1038/nm.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu X, Pappu R, Ramirez-Carrozzi V, Ota N, Caplazi P, Zhang J, et al. TNF superfamily member TL1A elicits type 2 innate lymphoid cells at mucosal barriers. Mucosal Immunol. 2014;7:730–40. doi: 10.1038/mi.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyce JA. Mast cells and eicosanoid mediators: a system of reciprocal paracrine and autocrine regulation. Immunol Rev. 2007;217:168–85. doi: 10.1111/j.1600-065X.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- 33.Fajt ML, Gelhaus SL, Freeman B, Uvalle CE, Trudeau JB, Holguin F, et al. Prostaglandin D(2) pathway upregulation: relation to asthma severity, control, and TH2 inflammation. J Allergy Clin Immunol. 2013;131:1504–12. doi: 10.1016/j.jaci.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klein Wolterink RG, Hendriks RW. Type 2 innate lymphocytes in allergic airway inflammation. Curr Allergy Asthma Rep. 2013;13:271–80. doi: 10.1007/s11882-013-0346-z. [DOI] [PubMed] [Google Scholar]
- 35.Zhou W, Toki S, Zhang J, Goleniewska K, Newcomb DC, Cephus JY, et al. PGI signaling inhibits group 2 innate lymphoid cell responses. Am J Respir Crit Care Med. 2015 doi: 10.1164/rccm.201410-1793OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barnig C, Cernadas M, Dutile S, Liu X, Perrella MA, Kazani S, et al. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med. 2013;5:174ra126. doi: 10.1126/scitranslmed.3004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller AM. Role of IL-33 in inflammation and disease. J Inflamm (Lond) 2011;8:22. doi: 10.1186/1476-9255-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prefontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J, et al. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol. 2010;125:752–4. doi: 10.1016/j.jaci.2009.12.935. [DOI] [PubMed] [Google Scholar]
- 39.Prefontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, et al. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol. 2009;183:5094–103. doi: 10.4049/jimmunol.0802387. [DOI] [PubMed] [Google Scholar]
- 40.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–21. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xia J, Zhao J, Shang J, Li M, Zeng Z, Zhao J, et al. Increased IL-33 expression in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2015;308:L619–627. doi: 10.1152/ajplung.00305.2014. [DOI] [PubMed] [Google Scholar]
- 42.Lam M, Hull L, Imrie A, Snidvongs K, Chin D, Pratt E, et al. Interleukin-25 and interleukin-33 as mediators of eosinophilic inflammation in chronic rhinosinusitis. Am J Rhinol Allergy. 2015;29:175–81. doi: 10.2500/ajra.2015.29.4176. [DOI] [PubMed] [Google Scholar]
- 43.Lloyd CM, Saglani S. Epithelial cytokines and pulmonary allergic inflammation. Curr Opin Immunol. 2015;34:52–8. doi: 10.1016/j.coi.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Castanhinha S, Sherburn R, Walker S, Gupta A, Bossley CJ, Buckley J, et al. Pediatric severe asthma with fungal sensitization is mediated by steroid-resistant IL-33. J Allergy Clin Immunol. 2015;136:312–322.e317. doi: 10.1016/j.jaci.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cayrol C, Girard JP. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol. 2014;31:31–7. doi: 10.1016/j.coi.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186:4375–87. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lloyd CM. IL-33 family members and asthma—bridging innate and adaptive immune responses. Curr Opin Immunol. 2010;22:800–6. doi: 10.1016/j.coi.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lamkanfi M, Dixit VM. IL-33 raises alarm. Immunity. 2009;31:5–7. doi: 10.1016/j.immuni.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 49.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–62. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 50.Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–8. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage−CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188:1503–13. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kondo Y, Yoshimoto T, Yasuda K, Futatsugi-Yumikura S, Morimoto M, Hayashi N, et al. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol. 2008;20:791–800. doi: 10.1093/intimm/dxn037. [DOI] [PubMed] [Google Scholar]
- 53.Sharma J, Balakrishnan L, Datta KK, Sahasrabuddhe NA, Khan AA, Sahu A, et al. A knowledgebase resource for interleukin-17 family mediated signaling. J Cell Commun Signal. 2015;9(3):291–3. doi: 10.1007/s12079-015-0297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Corrigan CJ, Wang W, Meng Q, Fang C, Eid G, Caballero MR, et al. Allergen-induced expression of IL-25 and IL-25 receptor in atopic asthmatic airways and late-phase cutaneous responses. J Allergy Clin Immunol. 2011;128:116–24. doi: 10.1016/j.jaci.2011.03.043. [DOI] [PubMed] [Google Scholar]
- 55.Corrigan CJ, Wang W, Meng Q, Fang C, Wu H, Reay V, et al. T-helper cell type 2 (Th2) memory T cell-potentiating cytokine IL-25 has the potential to promote angiogenesis in asthma. Proc Natl Acad Sci U S A. 2011;108:1579–84. doi: 10.1073/pnas.1014241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beale J, Jayaraman A, Jackson DJ, Macintyre JD, Edwards MR, Walton RP, et al. Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci Transl Med. 2014;6:256ra134. doi: 10.1126/scitranslmed.3009124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barlow JL, Peel S, Fox J, Panova V, Hardman CS, Camelo A, et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J Allergy Clin Immunol. 2013;132:933–41. doi: 10.1016/j.jaci.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 58.Saenz SA, Siracusa MC, Monticelli LA, Ziegler CG, Kim BS, Brestoff JR, et al. IL-25 simultaneously elicits distinct populations of innate lymphoid cells and multipotent progenitor type 2 (MPPtype2) cells. J Exp Med. 2013;210:1823–37. doi: 10.1084/jem.20122332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y, Zhou B. Functions of thymic stromal lymphopoietin in immunity and disease. Immunol Res. 2012;52:211–23. doi: 10.1007/s12026-012-8264-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harada M, Hirota T, Jodo AI, Hitomi Y, Sakashita M, Tsunoda T, et al. Thymic stromal lymphopoietin gene promoter polymorphisms are associated with susceptibility to bronchial asthma. Am J Respir Cell Mol Biol. 2011;44:787–93. doi: 10.1165/rcmb.2009-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ying S, O’Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–90. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 62.Kimura S, Pawankar R, Mori S, Nonaka M, Masuno S, Yagi T, et al. Increased expression and role of thymic stromal lymphopoietin in nasal polyposis. Allergy Asthma Immunol Res. 2011;3:186–93. doi: 10.4168/aair.2011.3.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med. 2006;203:269–73. doi: 10.1084/jem.20051745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5:170ra116. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mohapatra A, Van Dyken SJ, Schneider C, Nussbaum JC, Liang HE, Locksley RM, et al. Group 2 innate lymphoid cells utilize the IRF4-IL-9 module to coordinate epithelial cell maintenance of lung homeostasis. Mucosal Immunol. 2015 doi: 10.1038/mi.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gauvreau GM, O’Byrne PM, Boulet LP, Wang Y, Cockcroft D, Bigler J, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014;370:2102–10. doi: 10.1056/NEJMoa1402895. [DOI] [PubMed] [Google Scholar]
- 67.Claar D, Hartert TV, Peebles RS., Jr The role of prostaglandins in allergic lung inflammation and asthma. Expert Rev Respir Med. 2015;9:55–72. doi: 10.1586/17476348.2015.992783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu MC, Bleecker ER, Lichtenstein LM, Kagey-Sobotka A, Niv Y, McLemore TL, et al. Evidence for elevated levels of histamine, prostaglandin D2, and other bronchoconstricting prostaglandins in the airways of subjects with mild asthma. Am Rev Respir Dis. 1990;142:126–32. doi: 10.1164/ajrccm/142.1.126. [DOI] [PubMed] [Google Scholar]
- 69.Johnston SL, Freezer NJ, Ritter W, O’Toole S, Howarth PH. Prostaglandin D2-induced bronchoconstriction is mediated only in part by the thromboxane prostanoid receptor. Eur Respir J. 1995;8:411–5. doi: 10.1183/09031936.95.08030411. [DOI] [PubMed] [Google Scholar]
- 70.Wenzel SE, Westcott JY, Smith HR, Larsen GL. Spectrum of prostanoid release after bronchoalveolar allergen challenge in atopic asthmatics and in control groups. An alteration in the ratio of bronchoconstrictive to bronchoprotective mediators. Am Rev Respir Dis. 1989;139:450–7. doi: 10.1164/ajrccm/139.2.450. [DOI] [PubMed] [Google Scholar]
- 71.Sampson SE, Sampson AP, Costello JF. Effect of inhaled prostaglandin D2 in normal and atopic subjects, and of pretreatment with leukotriene D4. Thorax. 1997;52:513–8. doi: 10.1136/thx.52.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chang JE, Doherty TA, Baum R, Broide D. Prostaglandin D2 regulates human type 2 innate lymphoid cell chemotaxis. J Allergy Clin Immunol. 2014;133:899–901. e893. doi: 10.1016/j.jaci.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors: cellular distribution and function in immune and inflammatory responses. J Immunol. 2004;173:1503–10. doi: 10.4049/jimmunol.173.3.1503. [DOI] [PubMed] [Google Scholar]
- 74.Laidlaw TM, Boyce JA. Pathogenesis of aspirin-exacerbated respiratory disease and reactions. Immunol Allergy Clin North Am. 2013;33:195–210. doi: 10.1016/j.iac.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors; emerging concepts. Allergy Asthma Immunol Res. 2014;6:288–95. doi: 10.4168/aair.2014.6.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Planaguma A, Kazani S, Marigowda G, Haworth O, Mariani TJ, Israel E, et al. Airway lipoxin A4 generation and lipoxin A4 receptor expression are decreased in severe asthma. Am J Respir Crit Care Med. 2008;178:574–82. doi: 10.1164/rccm.200801-061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meylan F, Richard AC, Siegel RM. TL1A and DR3, a TNF family ligand-receptor pair that promotes lymphocyte costimulation, mucosal hyperplasia, and autoimmune inflammation. Immunol Rev. 2011;244:188–96. doi: 10.1111/j.1600-065X.2011.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meylan F, Hawley ET, Barron L, Barlow JL, Penumetcha P, Pelletier M, et al. The TNF-family cytokine TL1A promotes allergic immunopathology through group 2 innate lymphoid cells. Mucosal Immunol. 2014;7:958–68. doi: 10.1038/mi.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Richard AC, Tan C, Hawley ET, Gomez-Rodriguez J, Goswami R, Yang XP, et al. The TNF-family ligand TL1A and its receptor DR3 promote T cell-mediated allergic immunopathology by enhancing differentiation and pathogenicity of IL-9-producing T cells. J Immunol. 2015;194:3567–82. doi: 10.4049/jimmunol.1401220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80•.Maazi H, Patel N, Sankaranarayanan I, Suzuki Y, Rigas D, Soroosh P, et al. ICOS:ICOS-ligand interaction is required for type 2 innate lymphoid cell function, homeostasis, and induction of airway hyperreactivity. Immunity. 2015;42:538–51. doi: 10.1016/j.immuni.2015.02.007. This study identified a critical role of ICOS/ ICOS-L interactions in ILC2 responses in mouse lung inflammation and showed that ILC2 function is regulated through direct contact with other cells via ICOS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paclik D, Stehle C, Lahmann A, Hutloff A, Romagnani C. ICOS regulates the pool of group 2 innate lymphoid cells under homeostatic and inflammatory conditions in mice. Eur J Immunol. 2015;45:2766–72. doi: 10.1002/eji.201545635. [DOI] [PubMed] [Google Scholar]
- 82.Kamachi F, Isshiki T, Harada N, Akiba H, Miyake S. ICOS promotes group 2 innate lymphoid cell activation in lungs. Biochem Biophys Res Commun. 2015;463:739–45. doi: 10.1016/j.bbrc.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 83.Kallinich T, Beier KC, Gelfand EW, Kroczek RA, Hamelmann E. Co-stimulatory molecules as potential targets for therapeutic intervention in allergic airway disease. Clin Exp Allergy. 2005;35:1521–34. doi: 10.1111/j.1365-2222.2005.02369.x. [DOI] [PubMed] [Google Scholar]
- 84.Lombardi V, Singh AK, Akbari O. The role of costimulatory molecules in allergic disease and asthma. Int Arch Allergy Immunol. 2010;151:179–89. doi: 10.1159/000242355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bush RK, Prochnau JJ. Alternaria-induced asthma. J Allergy Clin Immunol. 2004;113:227–34. doi: 10.1016/j.jaci.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 86.Doherty TA, Khorram N, Sugimoto K, Sheppard D, Rosenthal P, Cho JY, et al. Alternaria induces STAT6-dependent acute airway eosinophilia and epithelial FIZZ1 expression that promotes airway fibrosis and epithelial thickness. J Immunol. 2012;188:2622–9. doi: 10.4049/jimmunol.1101632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim HK, Lund S, Baum R, Rosenthal P, Khorram N, Doherty TA. Innate type 2 response to Alternaria extract enhances ryegrass-induced lung inflammation. Int Arch Allergy Immunol. 2014;163:92–105. doi: 10.1159/000356341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Calderon MA, Linneberg A, Kleine-Tebbe J, De Blay F, de Rojas Hernandez Fernandez D, Virchow JC, et al. Respiratory allergy caused by house dust mites: what do we really know? J Allergy Clin Immunol. 2015;136:38–48. doi: 10.1016/j.jaci.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 89.Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457:585–8. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–6. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gold MJ, Antignano F, Halim TY, Hirota JA, Blanchet MR, Zaph C, et al. Group 2 innate lymphoid cells facilitate sensitization to local, but not systemic, TH2-inducing allergen exposures. J Allergy Clin Immunol. 2014;133:1142–8. doi: 10.1016/j.jaci.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 92.Baur X, Bakehe P. Allergens causing occupational asthma: an evidence-based evaluation of the literature. Int Arch Occup Environ Health. 2014;87:339–63. doi: 10.1007/s00420-013-0866-9. [DOI] [PubMed] [Google Scholar]
- 93.Motomura Y, Morita H, Moro K, Nakae S, Artis D, Endo TA, et al. Basophil-derived interleukin-4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity. 2014;40:758–71. doi: 10.1016/j.immuni.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 94.Oboki K, Ohno T, Kajiwara N, Arae K, Morita H, Ishii A, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci U S A. 2010;107:18581–6. doi: 10.1073/pnas.1003059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–63. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 96.Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–35. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wilhelm C, Hirota K, Stieglitz B, Van Snick J, Tolaini M, Lahl K, et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol. 2011;12:1071–7. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morita H, Arae K, Unno H, Miyauchi K, Toyama S, Nambu A, et al. An interleukin-33-mast cell-interleukin-2 axis suppresses papain-induced allergic inflammation by promoting regulatory T cell numbers. Immunity. 2015;43:175–86. doi: 10.1016/j.immuni.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gern JE. Viral respiratory infection and the link to asthma. Pediatr Infect Dis J. 2008;27:S97–103. doi: 10.1097/INF.0b013e318168b718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Saglani S. Viral infections and the development of asthma in children. Ther Adv Infect Dis. 2013;1:139–50. doi: 10.1177/2049936113497202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Le Goffic R, Arshad MI, Rauch M, L’Helgoualc’h A, Delmas B, Piquet-Pellorce C, et al. Infection with influenza virus induces IL-33 in murine lungs. Am J Respir Cell Mol Biol. 2011;45:1125–32. doi: 10.1165/rcmb.2010-0516OC. [DOI] [PubMed] [Google Scholar]
- 102.Gorski SA, Hahn YS, Braciale TJ. Group 2 innate lymphoid cell production of IL-5 is regulated by NKT cells during influenza virus infection. PLoS Pathog. 2013;9:e1003615. doi: 10.1371/journal.ppat.1003615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shim DH, Park YA, Kim MJ, Hong JY, Baek JY, Kim KW, et al. Pandemic influenza virus, pH1N1, induces asthmatic symptoms via activation of innate lymphoid cells. Pediatr Allergy Immunol. 2015 doi: 10.1111/pai.12462. [DOI] [PubMed] [Google Scholar]
- 104.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–54. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hong JY, Bentley JK, Chung Y, Lei J, Steenrod JM, Chen Q, et al. Neonatal rhinovirus induces mucous metaplasia and airways hyperresponsiveness through IL-25 and type 2 innate lymphoid cells. J Allergy Clin Immunol. 2014;134:429–39. doi: 10.1016/j.jaci.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu J, Wu J, Qi F, Zeng S, Xu L, Hu H, et al. Natural helper cells contribute to pulmonary eosinophilia by producing IL-13 via IL-33/ST2 pathway in a murine model of respiratory syncytial virus infection. Int Immunopharmacol. 2015;28:337–43. doi: 10.1016/j.intimp.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 107••.Jackson DJ, Makrinioti H, Rana BM, Shamji BW, Trujillo-Torralbo MB, Footitt J, et al. IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am J Respir Crit Care Med. 2014;190:1373–82. doi: 10.1164/rccm.201406-1039OC. First human study connecting the IL-33/ILC2 axis to rhinovirus-induced asthma exacerbations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108••.Doherty TA, Scott D, Walford HH, Khorram N, Lund S, Baum R, et al. Allergen challenge in allergic rhinitis rapidly induces increased peripheral blood type 2 innate lymphoid cells that express CD84. J Allergy Clin Immunol. 2014;133:1203–5. doi: 10.1016/j.jaci.2013.12.1086. First report to demonstrate a direct correlation between allergen exposure and increased circulating ILC2s in allergic individuals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lao-Araya M, Steveling E, Scadding GW, Durham SR, Shamji MH. Seasonal increases in peripheral innate lymphoid type 2 cells are inhibited by subcutaneous grass pollen immunotherapy. J Allergy Clin Immunol. 2014;134:1193–1195. e1194. doi: 10.1016/j.jaci.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 110.Bartemes KR, Kephart GM, Fox SJ, Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol. 2014;134:671–678.e4. doi: 10.1016/j.jaci.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Christianson CA, Goplen NP, Zafar I, Irvin C, Good JT, Jr, Rollins DR, et al. Persistence of asthma requires multiple feedback circuits involving type 2 innate lymphoid cells and IL-33. J Allergy Clin Immunol. 2015;136:59–68.e14. doi: 10.1016/j.jaci.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shaw JL, Fakhri S, Citardi MJ, Porter PC, Corry DB, Kheradmand F, et al. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2013;188:432–9. doi: 10.1164/rccm.201212-2227OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Walford HH, Lund SJ, Baum RE, White AA, Bergeron CM, Husseman J, et al. Increased ILC2s in the eosinophilic nasal polyp endotype are associated with corticosteroid responsiveness. Clin Immunol. 2014;155:126–35. doi: 10.1016/j.clim.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miljkovic D, Bassiouni A, Cooksley C, Ou J, Hauben E, Wormald PJ, et al. Association between group 2 innate lymphoid cells enrichment, nasal polyps and allergy in chronic rhinosinusitis. Allergy. 2014;69:1154–61. doi: 10.1111/all.12440. [DOI] [PubMed] [Google Scholar]
- 115.Ho J, Bailey M, Zaunders J, Mrad N, Sacks R, Sewell W, et al. Group 2 innate lymphoid cells (ILC2s) are increased in chronic rhinosinusitis with nasal polyps or eosinophilia. Clin Exp Allergy. 2015;45:394–403. doi: 10.1111/cea.12462. [DOI] [PubMed] [Google Scholar]
- 116.Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16:45–56. doi: 10.1038/ni.3049. [DOI] [PubMed] [Google Scholar]
- 117.Wenzel SE, Larsen GL, Johnston K, Voelkel NF, Westcott JY. Elevated levels of leukotriene C4 in bronchoalveolar lavage fluid from atopic asthmatics after endobronchial allergen challenge. Am Rev Respir Dis. 1990;142:112–9. doi: 10.1164/ajrccm/142.1.112. [DOI] [PubMed] [Google Scholar]
- 118.Lefrancais E, Duval A, Mirey E, Roga S, Espinosa E, Cayrol C, et al. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc Natl Acad Sci U S A. 2014;111:15502–7. doi: 10.1073/pnas.1410700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hsu CL, Neilsen CV, Bryce PJ. IL-33 is produced by mast cells and regulates IgE-dependent inflammation. PLoS ONE. 2010;5:e11944. doi: 10.1371/journal.pone.0011944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Akdis CA, Bachert C, Cingi C, Dykewicz MS, Hellings PW, Naclerio RM, et al. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2013;131:1479–90. doi: 10.1016/j.jaci.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hirsch AG, Yan X, Sundaresan A, Tan BK, Schleimer RP, Kern RC, et al. Five-year risk of incident disease following a diagnosis of chronic rhinosinusitis. Allergy. 2015;70:1613–21. doi: 10.1111/all.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kabata H, Moro K, Fukunaga K, Suzuki Y, Miyata J, Masaki K, et al. Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat Commun. 2013;4:2675. doi: 10.1038/ncomms3675. [DOI] [PubMed] [Google Scholar]
- 123.Hams E, Armstrong ME, Barlow JL, Saunders SP, Schwartz C, Cooke G, et al. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc Natl Acad Sci U S A. 2014;111:367–72. doi: 10.1073/pnas.1315854111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kwon BI, Hong S, Shin K, Choi EH, Hwang JJ, Lee SH. Innate type 2 immunity is associated with eosinophilic pleural effusion in primary spontaneous pneumothorax. Am J Respir Crit Care Med. 2013;188:577–85. doi: 10.1164/rccm.201302-0295OC. [DOI] [PubMed] [Google Scholar]