Abstract
Red blood cell (RBC) transfusion is a common and lifesaving therapy for anemic neonates and infants, particularly among those born prematurely or undergoing surgery. However, evidence-based indications for when to administer RBCs and adverse effects of RBC transfusion on important outcomes including necrotizing enterocolitis, survival and long-term neurodevelopmental impairment remain uncertain. In addition, blood-banking practices for preterm and term neonates and infants have been largely developed using studies from older children and adults. Use of and refinements in emerging technologies and advances in biomarker discovery and neonatal-specific RBC transfusion databases may allow clinicians to better define and tailor RBC transfusion needs and practices to individual neonates. Decreasing the need for RBC transfusion and developing neonatal-specific approaches in the preparation of donor RBCs has potential for reducing resource utilization and cost, improving outcomes, and assuring blood safety. Finally, large donor-recipient linked cohort studies can provide data to better understand the balance of the risks and benefits of RBC transfusion in neonates. These studies may also guide the translation of new research into best practices that can rapidly be integrated into routine care. This review highlights key opportunities in transfusion medicine and neonatology for improving the preparation and transfusion of RBCs into neonates and infants. We focus on timely, currently addressable knowledge gaps that can increase the safety and efficacy of preterm and term neonatal and infant RBC transfusion practices.
Keywords: infant, newborn, prematurity, blood bank, hemoglobin, anemia
INTRODUCTION
Red blood cell (RBC) transfusion is a common and lifesaving therapy for anemic neonates and infants, particularly those born prematurely [1] or undergoing surgery [2]. The goal of this review is to highlight our view of the current state of neonatal RBC transfusion practices and review opportunities to address critical research knowledge gaps in these practices. We have focused on the following thematic areas in neonatal RBC transfusion: hemovigilance, blood supply management, patient blood management, and translation of evidence into practice. Many of the critical research gaps we highlight in neonates also apply to infants. Using the PICO format, we describe a neonatal (and infant) population (P), intervention (I), comparison (C) and outcome (O),[3] to frame critical and timely research questions in the context of the goal of improving clinical outcomes among patients needing RBC transfusion. We have emphasized what we view as the most important, feasible, and relevant priority research opportunities for the next 5–10 years. Where relevant to these research questions and opportunities, we identify and discuss current ongoing studies (Table 1) and identify areas where additional research is needed.
Table 1.
Ongoing Registered Neonatal RBC Transfusion Studies.
| Study Title | Target Sample size |
Study Goal | Registry identifier (status)* |
|---|---|---|---|
| Combining Restrictive Guidelines and a NIRS Score to Decrease RBC Transfusions |
82 | To determine the number of RBC transfusions received by preterm infants cared for using restrictive Hb transfusion guidelines in combination with splanchnic to cerebral oxygenation ratio assessment compared to liberal guidelines alone based on Hb level below defined values. |
clinicaltrials.gov NCT02535208 (recruiting) |
| Withholding Feeds During Red Blood Cell Transfusion and TRAGI |
154 (actual) |
To investigate the effect of withholding feeds during transfusion, on the development of TRAGI |
clinicaltrials.gov NCT02132819 (active, not recruiting) |
| Transfusion of Prematures (TOP) Trial |
1824 | To determine whether higher Hb thresholds for transfusing ELBW infants resulting in higher Hb levels lead to improvement in the primary outcome of survival and rates of neurodevelopmental impairment at 22–26 months of age. |
clinicaltrials.gov NCT01702805 (recruiting) |
| Timing of Umbilical Cord Occlusion in Premature Babies: Delayed vs Early |
150 | To test the hypothesis that delayed cord clamping in the premature newborn <33 week gestation significantly decreases the need for RBC transfusions and intraventricular hemorrhage compared with early cord clamping. |
clinicaltrials.gov NCT02187874 (not yet recruiting) |
| Umbilical Cord Blood Use For Admission Blood Tests of VLBW Preterm Neonates |
225 | To compare the use of cord blood vs. infant blood with the primary outcome of comparing both the absolute Hb concentration and the percent change in Hb concentration from baseline around 24 hours of life. |
clinicaltrials.gov NCT02103296 (recruiting) |
| Effects of Placental Transfusion on Early Brain Development in Infants Born at Term |
100 | To measure the effects of cord clamping time on the structure and function of the developing brain at three and four years of age in healthy term infants. |
clinicaltrials.gov NCT02619006 (recruiting) |
| Effects of Transfusion Thresholds on Neurocognitive Outcome of ELBW Infants (ETTNO) Trial |
920 | To compare restrictive vs. liberal RBC transfusion guidelines on long-term neuro-developmental outcome in ELBW infants. |
clinicaltrials.gov NCT01393496 (active, not recruiting) |
| Preterm Erythropoietin Neuroprotection (PENUT) Trial |
940 | To determine whether EPO treatment of extremely low gestational age newborns decreases the combined outcome of death or NDI at 24–26 months corrected age. |
clinicaltrials.gov NCT01378273 (recruiting) |
| Use of EPO to reduce top-up transfusions in neonates with RBC alloimmunization |
64 | To determine if treatment with EPO reduces the need for top-up transfusions in neonates with hemolytic disease of the newborn due to RBC alloimmunization treated with intrauterine transfusion |
EU Clinical Trials Register 2010- 023955-27 (ongoing) |
Abbreviations: NIRS, near infrared spectroscopy; RBC, red blood cell; Hb, hemoglobin; TRAGI, transfusion related acute gut injury; ELBW, extremely low birth weight; VLBW, very low birth weight; EPO, recombinant human erythropoietin; EU, European Union.
Status as of 02/28/2016 from a search of trial registries in the US (www.clinicaltrials.gov), Europe (www.clinicaltrialsregister.eu) and Australia/New Zealand (www.anzctr.org.au).
BIG DATA: NEED FOR RBC DONOR-RECIPIENT LINKED COHORT STUDIES IN TRANSFUSED NEONATES
Question
Among anemic neonates of all birth weights (including those born preterm), can knowledge of RBC transfusion practices informed by large, longitudinal RBC donor-recipient linked cohort studies, compared to current RBC transfusion recipient-only data, lead to improved (i.e., safer, more effective, and less expensive) RBC transfusion utilization and outcomes?
Rationale/current knowledge
Limited studies have evaluated the relationship between utilization of donor RBC products and recipient outcomes in neonates—a group that ranks among the most highly transfused patients [4]. With a lifetime ahead for neonates, improving RBC transfusion-related health outcomes, such as safe and effective transfusions themselves, short- and long-term functional health outcomes, and long-term cognitive functioning, is of vital importance. Despite this opportunity, there is currently no large, national neonatal RBC transfusion dataset with sufficient detail to adequately evaluate relationships between RBC transfusion practices and outcomes. Such a dataset would allow for the evaluation of uncommon adverse effects of RBC transfusion and provide greater ability to evaluate the risks and benefits of RBC transfusion in various subgroups of patients and settings. Studies using these datasets would not supplant but rather inform and guide the development of randomized trials which are better able to evaluate cause and effect. Additionally, a comprehensive longitudinal cohort database could facilitate evaluation of the effectiveness of blood products in clinical practice and provide an assessment of strategies to reduce inappropriate transfusion practices. Fortunately, there are potential opportunities that can serve to address this deficiency. Opportunities include expanding data collection on RBC transfusion in current neonatal-focused networks [5] with the potential for temporally linking RBC transfusion exposures with neonatal outcomes. More comprehensive reporting of the types and quantity of RBCs products utilized would afford opportunities to examine RBC exposure characteristics in relationship to important safety and efficacy outcomes and better understand practice variation.
Knowledge gaps
Ongoing studies
No databases, registries, or networks currently exist that include neonatal RBC transfusion-relevant data with donor linkage in sufficient detail.
Areas where new research is needed
Through its funded REDS programs [6], the National Heart, Lung, and Blood Institute (NHLBI) has developed longitudinal databases that have the advantage of capturing contextual information relative to donor characteristics, timing of transfusions and methods of delivery. These studies have informed critically important facets of RBC transfusion treatment in adults [7]—but not in neonates (or infants and children). Thus, a similar approach in neonates and children is needed to better inform the extent and safety of, and approaches to RBC transfusion. In addition, understanding variation in donor characteristics is necessary to fully assess the effect of “vein-to-vein” variability in RBC donor characteristics on recipient outcomes [8]. Small, single center, narrowly focused studies have not, and cannot, provide sufficient data to guide neonatal RBC transfusion. Presently, the utilization and expansion of existing pediatric databases to gather these important, basic RBC transfusion data, in combination with institutional-level data regarding patient blood management, is potentially feasible and urgently needed. Longitudinal data from such databases would—for the first time—provide an opportunity to evaluate a range of outcomes and their relationship to RBC transfusion exposures, including important covariates, important in assessing associations with minimal bias [9]. Furthermore, when to transfuse, what to transfuse, how much to transfuse are all complicated by the patient condition, physician practice, and available resources. Ideally such transfusion databases should build on existing neonatal databases with important outcomes already included with support from existing organizations (e.g., Vermont Oxford Network, American Academic of Pediatrics Section on Neonatal-Perinatal Medicine Task Force for Neonatal Perinatal Therapeutic Development (NeoPeritD), Pediatrix Clinical Data Warehouse, or the NICHD Neonatal Research Network). While large databases can evaluate variations and uptake in practice, associations between exposures and outcomes, and potential risk factors, it is acknowledged that they are limited in determining the efficacy and effectiveness of approaches to RBC transfusion. Randomized trials embedded within big data platforms or existing networks offer an exciting opportunity to address the priority research opportunities listed below. As transfusion-specific databases and cohorts emerge, their presence will provide the basis for establishing rigorous randomized, pragmatic clinical trials that can subsequently lay the groundwork for a more solid, informed foundation for evaluating the safety and effectiveness of RBC products and for developing future, highly relevant, evidenced-based treatments [10].
Priority research opportunities
Establishment of neonatal (and pediatric) REDS-like collaborative to perform detailed epidemiological studies, along with nested prospective observational and small clinical trials, focused on priority areas (e.g., transfusion practices, effect of donor characteristics on recipients).
Longitudinal data evaluating post-RBC transfusion outcomes such as neurodevelopment, growth and quality of life, with examination of the relationship of these outcomes to RBC transfusion and other early-life exposures.
Rigorous randomized and pragmatic clinical trials informed by large databases, focused on the most important and feasible areas of investigation in which there is substantial variation in practice or observational evidence to suggest benefit or harm.
HEMOVIGILANCE AND RBC TRANSFUSION-RELEVANT OUTCOMES
RBC transfusion, survival and neurodevelopmental outcomes
Question
Among preterm and term neonates and infants, does liberal RBC transfusion (i.e., higher hemoglobin thresholds), compared to conservative RBC transfusion (lower hemoglobin thresholds) result in better survival and neurodevelopment outcomes?
Rationale/current knowledge
Among preterm neonates, RBC transfusion is a widespread and common treatment for anemia of prematurity, with ≥90% of extremely low birth weight (<1000g at birth) infants receiving at least one RBC transfusion during hospitalization [11]. There are conflicting data on neurologic outcomes from two prior randomized trials comparing the effect of liberal vs. conservative RBC transfusion approaches [12, 13]. In addition, these studies were underpowered to assess the effect of the two strategies on clinically significant differences in mortality. In the 18–21 month follow-up of infants in the Prematures in Need of Transfusion (PINT) trial, a conservative transfusion approach was associated with a non-significant increase in cognitive delay (odds ratio 1.74; 95% CI 0.98–3.11), which in a post-hoc analysis using a lower cognitive threshold was statistically significant [14]. By contrast, a 12-year follow-up of 44 out of 100 subjects enrolled in the Iowa Trial demonstrated lower brain volumes among liberally transfused infants compared to those conservatively transfused [15]. The study also suggested heterogeneity by sex, with more severe MRI abnormalities in girls compared to boys. Among term infants, neonates undergoing cardiac surgery [16] and those receiving Extracorporeal Membrane Oxygenation (ECMO) [17] are both highly transfused populations with limited data from randomized trials on the risks and benefits of various approaches to RBC transfusion to guide informed clinical care.
Knowledge gaps
Ongoing studies
Two large, ongoing randomized trials, the Transfusion of Prematures (TOP) and Effects of Transfusion Thresholds on Neurocognitive Outcome of ELBW Infants (ETTNO) trials [18] (Table 1), will compare neurodevelopment outcomes among infants receiving liberal vs. conservative transfusion. The primary outcome of the TOP trial will be death or neurodevelopmental impairment assessed at 22–26 months corrected age and has a projected sample size of 1824 ELBW infants. The primary outcome of the ETTNO trial is death or major developmental impairment at 24 months corrected age with a projected sample size of 920 ELBW infants. The estimated completion of follow-up for the primary outcome is August 2018 for TOP and April 2017 for ETTNO. The large sample size of both trials will ensure sufficient power to detect clinically important differences in death or neurodevelopmental impairment.
Areas where new research is needed
Additional study is needed to understand the biological mechanisms underlying the effect of RBC transfusion and anemia on neurodevelopment. In addition, future studies should evaluate gender-specific differences based on findings of heterogeneity in treatment effects on neurodevelopmental outcomes by gender in trials of RBC transfusion practices [15] and delayed cord clamping [19]. Advanced technologies such as cerebral near-infrared spectroscopy (NIRS) and amplitude integrated electroencephalogram (aEEG) may allow for an improved understanding of cerebral oxygenation and its relation to short-and long-term neurologic outcome across different transfusion thresholds. Early studies involving NIRS in this area appear promising [20, 21]. In addition, aEEG may potentially predict long-term outcome in preterm infants [22] and be able to detect responses to RBC transfusion [23], although additional studies are needed. There is also a gap in how to apply these newer technologies in routine practice in guiding RBC transfusion in individual neonates, particularly if cerebral oxygenation is shown to be associated with long-term neurocognitive outcomes. For term infants, large studies among highly transfused populations such as infants with congenital heart disease or infants requiring ECMO are necessary to compare the effect of restrictive vs. liberal transfusion on survival, and, if feasible, long-term neurocognitive outcome.
Priority research opportunities
Refinement of RBC transfusion strategies and thresholds to more precisely incorporate individual patients’ baseline factors and physiological state by including variables, such as gender and cerebral oxygenation measured by NIRS, to individualize RBC transfusion to improve survival without neurodevelopmental impairment.
Studies of liberal versus conservation transfusion strategies in critically ill late-preterm and term neonates, including those with congenital heart disease or needing ECMO in which survival and neurologic outcomes, using either neuroimaging or functional assessments, are evaluated.
Clinical and pre-clinical mechanistic studies to understand the relationship between anemia, RBC transfusion strategies, cerebral oxygenation and neurodevelopmental outcomes.
RBC transfusion and necrotizing enterocolitis
Question
Among neonates and infants born prematurely and those with congenital heart disease, does RBC transfusion, compared to no RBC transfusion, result in a higher risk of necrotizing enterocolitis?
Rationale/current knowledge
Necrotizing enterocolitis (NEC) is a leading cause of death and long-term disability in preterm infants [24, 25] and also affects term infants with congenital heart disease [26]. Among preterm infants, a systematic review and meta-analysis of observational case-control or cohort studies reported an association between RBC transfusion and NEC [27]. Additionally, some studies have suggested an association of anemia with NEC [28–30]. By contrast, results from randomized trials have not shown a higher risk of NEC with more liberal RBC transfusion compared to more conservative RBC transfusion [31], although these studies may have been underpowered to detect clinically significant differences in NEC. These studies have largely relied on hemoglobin thresholds, along with respiratory support and postnatal age, as determinants of RBC transfusion without incorporating measures of oxygen delivery. Mesenteric NIRS, a tool to measure regional oxygen saturation of the gut, offers the ability to assess oxygen delivery in real-time and follow trends that may provide insight into both the need for RBC transfusion and an individual’s risk for developing NEC [32].
Knowledge gaps
Ongoing studies
As discussed above, two large, ongoing randomized trials, the TOP and ETTNO trials (Table 1) are currently studying whether tolerance of more anemia with conservative RBC transfusion, compared to liberal RBC transfusion, results in a higher risk of death or neurodevelopmental impairment. Both of these trials will include NEC as secondary outcomes. In addition, the TOP trial will include a secondary study that will evaluate the use of NIRS as a potential tool in assessing responses to RBC transfusion.
Areas where new research is needed
Additional studies are needed to identify new biomarkers that can inform clinicians of insufficient intestinal oxygen delivery before NEC occurs. Combining existing technologies such as NIRS, with novel biomarkers such as Intestinal Fatty Acid-Binding Protein has the potential to better tailor transfusion practices among infants at high risk of NEC [33, 34]. In addition, new “omic” biomarkers [35] and heart rate assessment [36] could potentially be integrated to provide more individualized, patient-centered RBC transfusion practices, using designs similar to those employed in adults [37]. Continuous non-invasive hemoglobin monitoring may also help identify whether acute decreases in hemoglobin are an early diagnostic marker of NEC, rather than causal risk factor. However, recent adult studies highlight the need for new research before non-invasive hemoglobin assessment can replace current invasive laboratory testing [38].
Priority research opportunities
Studies of NEC evaluating the effects of RBC transfusion on gut oxygenation in preterm infants, including the potential interaction with the severity of anemia and feeding practices around the time of RBC transfusion.
Preclinical and clinical mechanistic studies to understand the effects of anemia and RBC transfusion on gut inflammation, permeability and injury that provide biologic insight into the pathophysiology of NEC and the potential role of RBC transfusion.
BLOOD SUPPLY MANAGEMENT
Donor RBC preparation and variability in blood banking practices
Question
Among transfused neonates, can standardization of blood banking practices informed by the best available evidence, compared to current blood bank practice variability, improve clinical outcomes?
Rationale/current Knowledge
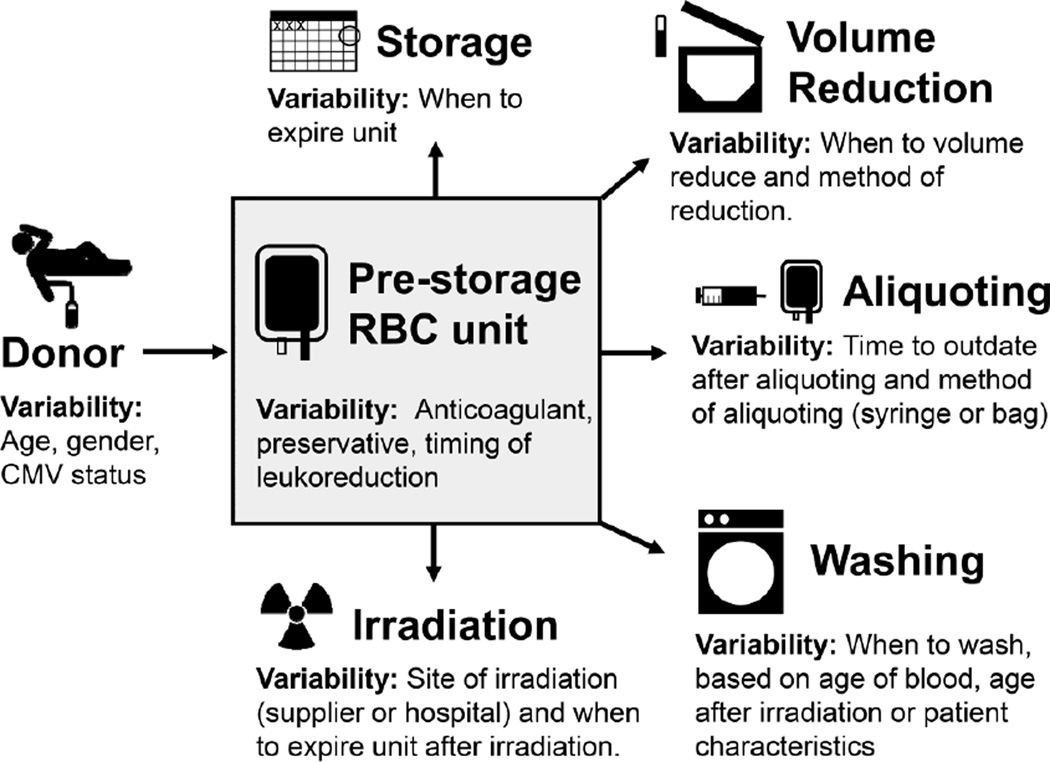
Patients less than 4 months of age not only have small blood volumes but also underdeveloped organ systems requiring special processing of blood products, which vary by center (Figure 1, Table 2). Small-volume blood product aliquots are transfused to neonates in order to limit donor exposure, prevent circulatory overload, and potentially decrease donor-related risks [39]. There are several well-accepted technical approaches for creating blood product aliquots [40]. Yet there is no national standard with regard to the optimal type of RBC anticoagulant-preservative solution to transfuse, when to wash or irradiate RBC units, what age of RBC units to use, and when to outdate aliquots. Controversy has existed within the transfusion community with regard to the ideal age of RBC units to transfuse. The Age of Red Blood Cells in Premature Infants (ARIPI) trial randomized low birth weight infants to receive either RBCs <7 days old (mean 5.1 days, n=188) vs. standard practice of transfusing single units of RBCs (mean 14.6 days, n=189) [41]. The ARIPI trial found no difference in the primary endpoint (composite of major neonatal morbidity, including NEC and intraventricular hemorrhage) between infants in either arm, suggesting in this study population the age of RBCs does not impact common morbidities of premature neonates. Yet the ARIPI trial’s study design may not reflect transfusion practices of many U.S. centers [42]. More recently, two large trials that did not include neonates compared clinical outcomes after fresh vs. stored RBC transfusions (≤10 days vs. ≥ 21 days in one trial and < 8 days vs. standard issue (mean 22 days) in the other trial) [43, 44]. These trials found no serious adverse outcomes from transfusion of stored blood. The cumulative data from these trials suggest that transfusion of older RBC units, compared to younger RBC units, does not lead to harm. Regardless, the majority of institutions have standard operating procedures limiting the age of blood transfused to neonates [45, 46]. In addition, multiple other aspects of RBC preparation such as irradiation, using washed vs. unwashed RBCs [47], and the comparative effectiveness of strategies to prevent transfusion-transmission of infections [48] have not been as rigorously studied with sufficient power to adequately evaluate their safety.
Figure 1. Variability in the processing of donor RBCs.
Some examples of sources of variability in blood banking practices for neonatal RBC transfusion are highlighted. Abbreviations: RBC, red blood cell; CMV, cytomegalovirus.
Table 2.
Variation in Neonatal RBC Product Modification Survey Results.
| Survey population |
RBC Anticoagulant or Preservative Products* |
RBC Washing Procedures |
Irradiation | Dedication of Donor Units |
|---|---|---|---|---|
| 47 blood banks at academic medical centers part of the University Health Consortium in the US [45]. |
6% - CPD or CP2D only 15% - CPDA-1 allowed 60% - at least one type of AS‡ 45% - all three forms of AS‡ |
82% - No policy 18% - Policy addressing risk of hyperkalemia 9% - Policy specifying number of days after irradiation or storage |
Not asked | n/a |
| 29 NICUs participating in the Transfusion of Prematures (TOP) Trial in the US [46]. |
38% - CPD/CPDA only 21% - AS‡ only 41% - Combination |
34% - Policy for large volume transfusions 17% - Policy specifying number of days after irradiation or storage |
93% irradiate (66% performed on site, 34% by off-site donor center) |
77% - aliquot from unit until expiration date 21% - Do not dedicate unit§ |
Abbreviations: CPD, citrate phosphate dextrose; CP2D, citrate phosphate double dextrose, CPDA-1, citrate phosphate dextrose adenine; AS, additive solution.
Blood Banks often maintain a varied inventory of RBC products.
AS-1, AS-3, AS-5 units.
These sites will switch to another unit when the RBC unit ages to a certain point (age range to switch ranges from 5 to 28 days of RBC age).
Knowledge gaps
Though regulatory and accrediting agencies provide guidance and standards for many transfusion practices, neonatal blood banking techniques and policies remain predominately institutionally-based with substantial variability among hospitals (Table 2). A 2010 survey of 28 academic medical centers transfusing neonatal patients showed inherent blood banking practice variability with regard to the type of RBC anticoagulant-preservative solution transfused [45]. More recently, the blood banks participating in the Transfusion of Prematures (TOP) trial were surveyed on their blood banking practices for extremely low birth weight (ELBW) infants [46]. Similar to the 2010 survey [45], this survey of 29 institutions also showed blood bank practice variability in target RBC ages for neonatal transfusion, type of RBC anticoagulant-preservative solution transfused, and timing of irradiation of products.
Priority research opportunities
Comprehensive assessment of neonatal blood banking practices nationally including community and academic centers accomplished through surveys or prospective multicenter observational studies.
Comparative effectiveness studies evaluating the effect of specific neonatal blood banking practices, such as irradiation, cytomegalovirus infection prevention, and specific anticoagulant solutions, on clinically important outcomes.
Assessing effects of donor RBC processing/storage on RBC survival
Question
Among neonatal patients, does biotin labeling of red blood cells (BioRBCs), compared to traditional radiolabeling, help identify blood banking practices to increase RBC transfusion survival in this vulnerable population?
Rationale/current knowledge
Neonatal and pediatric blood banking practices have been based on adult experience with substantial variability within and between regions of the US and internationally. For example, RBC products in the US are manufactured in accordance with FDA-mandated thresholds so that >70% of RBC products will have RBC in vivo 24-hr recovery >75% [49–51]. The FDA accepted methodology for RBC survival (RCS) studies employs radiolabeling of RBCs by at least two laboratories from at least 20 healthy adults [51]. This threshold in adults sets the standard for products transfused not only to ill adults but also children, infants, and neonates. Institutions have various practices regarding preparation of neonatal aliquots, volume reduction of products, type of RBC unit to use (e.g., additive solution or CPDA), outdating of RBC aliquots, and age of blood for neonatal transfusions (Figure 1, Table 2). Institutional blood bank standard operating procedures are traditionally based on local preference and experience and utilize RBC products manufactured according to adult RCS standards.
Knowledge gaps
Neonates are among the most heavily transfused groups[52, 53]. Their immature organ functions require modification for optimal blood banking practices (e.g., aliquoting, washing, volume reduction and irradiation), and yet there are few direct studies upon which to base optimized practices. Biotin labelling of populations of red cells (BioRBCs) has been used to determine RBC lifespan and time-dependent changes in RBC populations [54]. Because BioRBCs can track several different cell populations at the same time, they have also been used to simultaneously determine the survival characteristics of both autologous neonatal and allogeneic adult donor RBCs in very low birth weight infants, with resulting observations substantially at odds with current assumptions about autologous and allogeneic RCS in neonates [55, 56].
Priority research opportunities
BioRBCs studies in neonates to elucidate mechanisms of and factors influencing RBC survival.
Utilization of BioRBCs to evaluate different RBC unit manufacturing and blood banking practices in an effort to identify optimal blood supply management.
PATIENT BLOOD MANAGEMENT
Decreasing the need for RBC transfusion
Question
Among critically ill neonates requiring intensive laboratory testing, can non-pharmacological interventions, compared to no intervention, reduce RBC transfusion?
Rationale/current knowledge
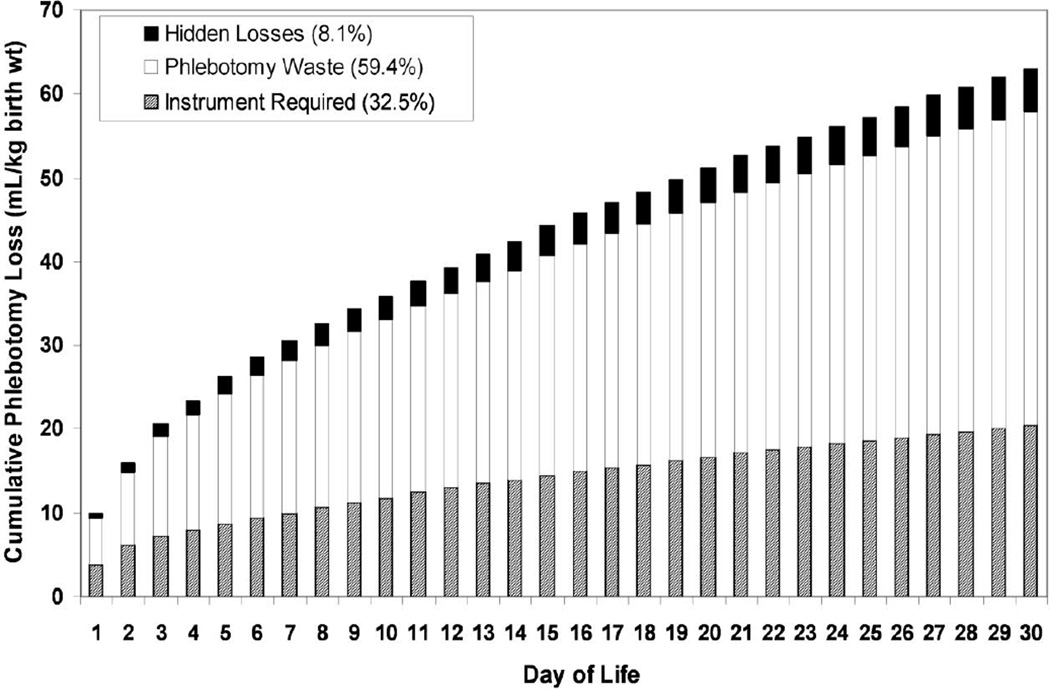
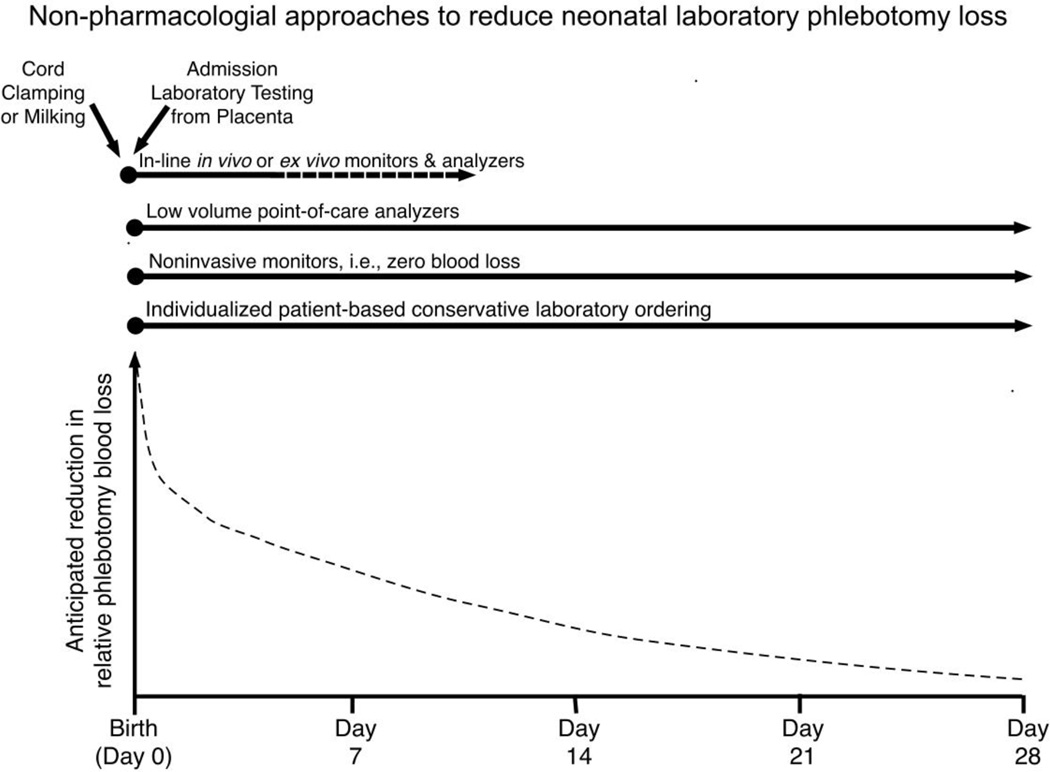
The development of anemia after birth in surviving very premature, critically ill newborn infants is certain [57]. Iatrogenic phlebotomy loss—the result of intensive clinical monitoring in critically ill newborns—can result in the loss of an entire ~80 mL/kg blood volume during the neonatal period and is the primary cause of neonatal anemia and driver of RBC transfusion (Figure 2) [58]. Because neonatal anemia is deemed most severe during the first month of life, the large majority of RBC transfusions are administered during this period. While it was initially hoped that erythropoiesis stimulating agents (ESAs), such as erythropoietin and darbepoetin, would be effective in the treatment and prevention of neonatal anemia and in reducing the need for RBC transfusion, heavy, iatrogenic phlebotomy blood loss was too large to be overcome by ESAs alone [59, 60]. As a result, subsequent efforts have focused on non-pharmacological blood conservation strategies that have been applied immediately at birth or during the neonatal period—and beyond (Figure 3) [61]. These include: 1) increasing the hemoglobin endowment and blood volume at birth from placental transfusion (e.g. delayed cord clamping or cord milking) [62]; and 2) removing less blood for lab testing [58]. While these approaches are feasible for most NICUs and have been demonstrated to be effective, they can be improved upon by basic and clinical research focused on: 1) implementation studies of delayed cord clamping and milking that address barriers and latent risk across various health delivery systems; 2) defining necessary and unnecessary laboratory testing and frequency for the population of infants cared for in the NICU; 3) continued innovation and improvement in micro- and nano-instrumentation; and 4) education of caregivers on optimal application of non-pharmacological approaches to reducing laboratory phlebotomy blood loss. Moreover, when several approaches are combined, an even greater cumulative effect can be achieved in reducing neonatal RBC transfusions [63].
Figure 2. “Bleeding into the laboratory:” Cumulative phlebotomy loss as a result of laboratory blood testing of neonates.
Mean data are shown for 26 ventilated VLBW infants of which 33% of laboratory blood loss was required by laboratory instrumentation for analysis, 59% of blood sampled was discarded as waste, and 8% represents hidden blood loss (e.g. blood left in syringes or on gauze pads, bandages). Reprinted with permission from Transfusion 53:1353–1360, 2013.
Figure 3. Non-pharmacological approaches to reduce laboratory phlebotomy blood loss during the neonatal period.
On the top half are six effective approaches to reduce phlebotomy loss, which all begin at birth and extend to various time periods when they are effective. The bottom half illustrates the anticipated relative cumulative reduction in laboratory blood loss if all six interventional approaches are concurrently applied.
Knowledge gaps
Ongoing studies
See “Umbilical Cord Blood Use for Admission Blood Tests of Very Low Birth Weight Preterm Neonates” in Table 1.
Areas where new research is needed
Although effective in premature infants [64], questions remain regarding delayed cord clamping and cord milking at delivery: 1) what is the optimal time—or physiologic status—to delay umbilical cord clamping to maximize benefits?; 2) are the benefits and risks similar at all gestational ages?; 3) does the benefit of immediate resuscitation outweigh the risk of no placental transfusion?; 4) what are the practical obstacles for implementing delayed cord clamping and cord milking into practice and can they be overcome?; 5) what is the method for optimal cord milking?; 6) does delivery mode make delayed cord clamping or milking more effective?; and 7) does cord milking confer the same—or greater—benefits with the equivalent low risk as delayed clamping? [65] Obtaining admission laboratory tests from umbilical cord blood is another strategy that has proven effective—even following delayed cord clamping or milking—in small, single center studies that needs to be tested on a larger scale. Studies are needed to optimize the methods, including timing, of cord blood admission laboratory testing. The need for accurate, minimally and non-invasive laboratory testing with point-of-care and in-line devices on ever smaller micro-and nano-sample volumes continues as an urgent—and increasingly feasible—need. Once laboratory phlebotomy losses are decreased to 10–20% of current levels, the efficacy of ESAs may well achieve initial expectations in the prevention and treatment of neonatal anemia. Finally, a constellation of non-pharmacological approaches applied together with ESAs has considerable potential in significantly decreasing neonatal RBC transfusions.
Priority research opportunities
Innovations and improvements in micro- and nanotechnology to safely and effectively reduce phlebotomy blood loss by use of improved bench top instruments and point-of-care analyzers and monitors.
Implementation and optimization studies of delayed cord clamping and/or milking at delivery to improve the success and efficacy of these therapies and potentially enhance immune function by increasing stem cell retention.
Comparative effectiveness studies of different laboratory testing approaches (e.g. invasive, non-invasive, point-of-care) and testing frequency where applicable (e.g. pCO2, pO2, glucose), to reduce blood loss and improve outcome.
Effective education and comprehensive quality improvement approaches (e.g. toolkits), that consistently motivate, inform and guide caregivers to implement multiple proven strategies to reduce laboratory phlebotomy loss.
Translating evidence into routine practice
Question
Among pediatric transfusion medicine specialists and neonatologists, does clinical decision making based on easily accessible, consistent and high-level of evidence of neonatal RBC transfusion practice, compared to individual experience-based opinions, lead to more rapid dissemination and application of new knowledge to improve RBC transfusion safety and efficacy?
Rationale/current knowledge
The National Institutes of Health (NIH) and other funders of research spend millions of dollars on studies in which results fail to reach the practitioners caring for neonates. Practitioners could potentially benefit their patients by incorporating and applying study results in a timelier manner to more effectively and safely treat anemic neonates with RBC transfusions. There is an urgent need to improve provider awareness and adoption of new, more effective and safer RBC transfusion and blood banking practices [66]. Important results found in multicenter randomized clinical trials and in well-performed clinical studies are available for being thoughtfully assimilated in pragmatic, diagnostic and therapeutic approaches that can be disseminated more effectively, rapidly, and widely.
Knowledge gaps
Ongoing studies
There are no randomized or pragmatic educational trials specific to neonatal RBC transfusion.
Areas where new research is needed
The effectiveness—or lack thereof—of educational tools to translate new evidence in transfusion medicine into safe and effective widespread practice in a timely fashion will require the development of key knowledge translational strategies. Many studies on neonatal RBC transfusion practices have been thoughtfully and pragmatically incorporated into meta-analyses, systematic reviews, consensus statements and recommendations of expert panels. However, meaningful collaboration of both transfusion medicine specialists and neonatologists could improve both the assessment and assimilation of current evidence into practice guidelines. Potential opportunities include collaboration of the American Academy of Pediatrics (AAP) Committee on the Fetus and Newborn and the AABB’s Pediatric Transfusion Medicine Subsection. While electronic and other means for dissemination of this information exist, how to most effectively and rapidly implement this in a cost-effective manner that will positively impact health outcomes remains to be explored to correct the current slow, uncertain, and cumbersome means of disseminating this information. Potential opportunities include improved integration of clinical decision support and other informatics approaches into routine workflows, with joint development by transfusion medicine and neonatal clinicians and sharing of modules among hospitals. These could include outcomes comparisons of hospital transfusion audit results as impacted by different educational programs, audit feedback, and hands on training. There is also a compelling need to develop solid, evidence based opportunities for the rapid dissemination of innovative educational research to ensure that research findings are being thoughtfully and effectively applied in clinical practice [10].
Priority research opportunities
Pragmatic RCTs and other rigorously controlled studies to improve neonatal RBC transfusion—provided the necessary databases and accompanying infrastructure exist—that include approaches applying lessons from business marketing and/or collaborations outside of academia with companies engaged in promoting and dissemination of research beneficial in patient care (e.g. www.KindeaLabs.com).
Requiring grant applications focused on neonatal RBC transfusion to include specific plans for post-publication dissemination of results directed at key practitioner groups while employing novel, effective methodologies that can be compared (e.g., smartphones, smartphone apps, social media, etc.
Development of guideline statements by joint societies (e.g. American Academy of Pediatrics and AABB) for neonatal RBC transfusion practices that include well-accepted methods to appraise evidence understood by clinicians such as GRADE [67].
Evaluation of approaches that provide practitioners with ongoing incentives to appraise and incorporate the highest-level and most recent evidence regarding neonatal RBC transfusion practices into routine practice, such as maintenance of certification, and/or receipt of CME credits.
SUMMARY
In the next 5–10 years, results from ongoing trials (Table 1) will address important knowledge gaps in neonatal RBC transfusion practices. These studies will provide important data on the risks and benefits of RBC transfusion on neurocognitive outcomes and NEC. Additional large donor RBC-recipient linked cohort studies are needed to inform RBC transfusion practices, guide new studies, and provide more precise estimates of the risks and benefits of transfusion. New biomarkers and pragmatic studies applying currently available technologies such as NIRS may allow for RBC transfusions that are better tailored to individual patients. Advances in technologies and implementation of currently available evidence have the potential to reduce resource utilization and cost, improve outcomes and assure blood safety. Nonetheless, new approaches are needed to improve implementation of the best available data into neonatal care and blood banking practice through collaborative efforts between transfusion medicine and neonatal care providers. Although RBC transfusion can be lifesaving, precision in the preparation, application and use of this therapy has the greatest potential for leading to more effective and safer patient outcomes at lower cost.
Acknowledgments
We appreciate the input and critical review of portions of this work by Mary A.M. Rogers, Ph.D., M.S. (University of Michigan), Donald M. Mock, M.D., Ph.D. (University of Arkansas for Medical Sciences), Ronald G. Strauss, M.D., (University of Iowa), Patrick D. Carroll M.D., M.P.H. (Intermountain Healthcare), Edward Bell, M.D. (University of Iowa), Cassandra Josephson, M.D. (Emory University), and Jeanne Hendrickson, M.D. (Yale University).
SOURCES OF SUPPORT
This publication was supported in part by US Public Health Service National Institutes of Health Grants P01 HL046925 (J.A.W), KL2 TR000455 (R.M.P), UL1 TR000454 (R.M.P), K23 HL128942 (R.M.P) and by the Thrasher Research Fund 0285-3 (J.A.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
Ravi M. Patel and Erin K. Meyer have no potential conflicts of interest for this publication. John A. Widness serves on the scientific advisory board for HemoGenix Corporation.
REFERENCES
- 1.Keir AK, Yang J, Harrison A, Pelausa E, Shah PS, Canadian Neonatal N. Temporal changes in blood product usage in preterm neonates born at less than 30 weeks' gestation in Canada. Transfusion. 2015;55:1340–1346. doi: 10.1111/trf.12998. [DOI] [PubMed] [Google Scholar]
- 2.Wesley MC, Yuki K, Daaboul DG, Dinardo JA. Blood utilization in neonates and infants undergoing cardiac surgery requiring cardiopulmonary bypass. World J Pediatr Congenit Heart Surg. 2011;2:382–392. doi: 10.1177/2150135111403779. [DOI] [PubMed] [Google Scholar]
- 3.Straus SE, Richardson WS, Glasziou P, Haynes RB. Evidence-based medicine: how to practice and teach it. 4th. Edinburgh: Churchill Livingstone; 2004. [Google Scholar]
- 4.Josephson CD, Mondoro TH, Ambruso DR, Sanchez R, Sloan SR, Luban NL, Widness JA. One size will never fit all: the future of research in pediatric transfusion medicine. Pediatr Res. 2014;76:425–431. doi: 10.1038/pr.2014.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Profit J, Soll RF. Neonatal networks: clinical research and quality improvement. Semin Fetal Neonatal Med. 2015;20:410–415. doi: 10.1016/j.siny.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Kleinman S, Busch MP, Murphy EL, Shan H, Ness P, Glynn SA L. National Heart, E. Blood Institute Recipient, S. Donor Evaluation. The National Heart, Lung, and Blood Institute Recipient Epidemiology and Donor Evaluation Study (REDS-III): a research program striving to improve blood donor and transfusion recipient outcomes. Transfusion. 2014;54:942–955. doi: 10.1111/trf.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma G, Parwani AV, Raval JS, Triulzi DJ, Benjamin RJ, Pantanowitz L. Contemporary issues in transfusion medicine informatics. J Pathol Inform. 2011;2:3. doi: 10.4103/2153-3539.74961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chasse M, McIntyre L, English SW, Tinmouth A, Knoll G, Wolfe D, Wilson K, Shehata N, Forster A, van Walraven C, Fergusson DA. Effect of Blood Donor Characteristics on Transfusion Outcomes: A Systematic Review and Meta-Analysis. Transfus Med Rev. 2016 doi: 10.1016/j.tmrv.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Pencina MJ, Peterson ED. Moving From Clinical Trials to Precision Medicine: The Role for Predictive Modeling. JAMA. 2016;315:1713–1714. doi: 10.1001/jama.2016.4839. [DOI] [PubMed] [Google Scholar]
- 10.Christensen RD, Carroll PD, Josephson CD. Evidence-based advances in transfusion practice in neonatal intensive care units. Neonatology. 2014;106:245–253. doi: 10.1159/000365135. [DOI] [PubMed] [Google Scholar]
- 11.Maier RF, Sonntag J, Walka MM, Liu G, Metze BC, Obladen M. Changing practices of red blood cell transfusions in infants with birth weights less than 1000 g. J Pediatr. 2000;136:220–224. doi: 10.1016/s0022-3476(00)70105-3. [DOI] [PubMed] [Google Scholar]
- 12.Kirpalani H, Whyte RK, Andersen C, Asztalos EV, Heddle N, Blajchman MA, Peliowski A, Rios A, LaCorte M, Connelly R, Barrington K, Roberts RS. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149:301–307. doi: 10.1016/j.jpeds.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Bell EF, Strauss RG, Widness JA, Mahoney LT, Mock DM, Seward VJ, Cress GA, Johnson KJ, Kromer IJ, Zimmerman MB. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 2005;115:1685–1691. doi: 10.1542/peds.2004-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whyte RK, Kirpalani H, Asztalos EV, Andersen C, Blajchman M, Heddle N, LaCorte M, Robertson CM, Clarke MC, Vincer MJ, Doyle LW, Roberts RS, Group PS. Neurodevelopmental outcome of extremely low birth weight infants randomly assigned to restrictive or liberal hemoglobin thresholds for blood transfusion. Pediatrics. 2009;123:207–213. doi: 10.1542/peds.2008-0338. [DOI] [PubMed] [Google Scholar]
- 15.Nopoulos PC, Conrad AL, Bell EF, Strauss RG, Widness JA, Magnotta VA, Zimmerman MB, Georgieff MK, Lindgren SD, Richman LC. Long-term outcome of brain structure in premature infants: effects of liberal vs restricted red blood cell transfusions. Arch Pediatr Adolesc Med. 2011;165:443–450. doi: 10.1001/archpediatrics.2010.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkinson KL, Brunskill SJ, Doree C, Trivella M, Gill R, Murphy MF. Red cell transfusion management for patients undergoing cardiac surgery for congenital heart disease. Cochrane Database Syst Rev. 2014;2:CD009752. doi: 10.1002/14651858.CD009752.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson HT, Oyetunji TA, Thomas A, Oyetunji AO, Hamrick M, Nadler EP, Wong E, Qureshi FG. The impact of leukoreduced red blood cell transfusion on mortality of neonates undergoing extracorporeal membrane oxygenation. J Surg Res. 2014;192:6–11. doi: 10.1016/j.jss.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Investigators E. The 'Effects of Transfusion Thresholds on Neurocognitive Outcome of Extremely Low Birth-Weight Infants (ETTNO)' Study: Background, Aims, and Study Protocol. Neonatology. 2012;101:301–305. doi: 10.1159/000335030. [DOI] [PubMed] [Google Scholar]
- 19.Andersson O, Lindquist B, Lindgren M, Stjernqvist K, Domellof M, Hellstrom-Westas L. Effect of Delayed Cord Clamping on Neurodevelopment at 4 Years of Age: A Randomized Clinical Trial. JAMA Pediatr. 2015;169:631–638. doi: 10.1001/jamapediatrics.2015.0358. [DOI] [PubMed] [Google Scholar]
- 20.Plomgaard AM, van Oeveren W, Petersen TH, Alderliesten T, Austin T, van Bel F, Benders M, Claris O, Dempsey E, Franz A, Fumagalli M, Gluud C, Hagmann C, Hyttel-Sorensen S, Lemmers P, Pellicer A, Pichler G, Winkel P, Greisen G. The SafeBoosC II randomized trial: treatment guided by near-infrared spectroscopy reduces cerebral hypoxia without changing early biomarkers of brain injury. Pediatr Res. 2015 doi: 10.1038/pr.2015.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyttel-Sorensen S, Pellicer A, Alderliesten T, Austin T, van Bel F, Benders M, Claris O, Dempsey E, Franz AR, Fumagalli M, Gluud C, Grevstad B, Hagmann C, Lemmers P, van Oeveren W, Pichler G, Plomgaard AM, Riera J, Sanchez L, Winkel P, Wolf M, Greisen G. Cerebral near infrared spectroscopy oximetry in extremely preterm infants: phase II randomised clinical trial. BMJ. 2015;350:g7635. doi: 10.1136/bmj.g7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wikstrom S, Pupp IH, Rosen I, Norman E, Fellman V, Ley D, Hellstrom-Westas L. Early single-channel aEEG/EEG predicts outcome in very preterm infants. Acta Paediatr. 2012;101:719–726. doi: 10.1111/j.1651-2227.2012.02677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greisen G, Pryds O, Rosen I, Lou H. Poor reversibility of EEG abnormality in hypotensive, preterm neonates. Acta Paediatr Scand. 1988;77:785–790. doi: 10.1111/j.1651-2227.1988.tb10756.x. [DOI] [PubMed] [Google Scholar]
- 24.Patel RM, Kandefer S, Walsh MC, Bell EF, Carlo WA, Laptook AR, Sanchez PJ, Shankaran S, Van Meurs KP, Ball MB, Hale EC, Newman NS, Das A, Higgins RD, Stoll BJ H. Eunice Kennedy Shriver National Institute of Child, N. Human Development Neonatal Research. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med. 2015;372:331–340. doi: 10.1056/NEJMoa1403489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacob J, Kamitsuka M, Clark RH, Kelleher AS, Spitzer AR. Etiologies of NICU deaths. Pediatrics. 2015;135:e59–e65. doi: 10.1542/peds.2014-2967. [DOI] [PubMed] [Google Scholar]
- 26.Baxi AC, Josephson CD, Iannucci GJ, Mahle WT. Necrotizing enterocolitis in infants with congenital heart disease: the role of red blood cell transfusions. Pediatr Cardiol. 2014;35:1024–1029. doi: 10.1007/s00246-014-0891-9. [DOI] [PubMed] [Google Scholar]
- 27.Mohamed A, Shah PS. Transfusion associated necrotizing enterocolitis: a meta-analysis of observational data. Pediatrics. 2012;129:529–540. doi: 10.1542/peds.2011-2872. [DOI] [PubMed] [Google Scholar]
- 28.Derienzo C, Smith PB, Tanaka D, Bandarenko N, Campbell ML, Herman A, Goldberg RN, Cotten CM. Feeding practices and other risk factors for developing transfusion-associated necrotizing enterocolitis. Early Hum Dev. 2014;90:237–240. doi: 10.1016/j.earlhumdev.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh R, Shah BL, Frantz ID., 3rd Necrotizing enterocolitis and the role of anemia of prematurity. Semin Perinatol. 2012;36:277–282. doi: 10.1053/j.semperi.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Patel RM, Knezevic A, Shenvi N, et al. Association of red blood cell transfusion, anemia, and necrotizing enterocolitis in very low-birth-weight infants. JAMA. 2016;315:889–897. doi: 10.1001/jama.2016.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whyte R, Kirpalani H. Low versus high haemoglobin concentration threshold for blood transfusion for preventing morbidity and mortality in very low birth weight infants. Cochrane Database Syst Rev. 2011;11:CD000512. doi: 10.1002/14651858.CD000512.pub2. [DOI] [PubMed] [Google Scholar]
- 32.Marin T, Moore J, Kosmetatos N, Roback JD, Weiss P, Higgins M, McCauley L, Strickland OL, Josephson CD. Red blood cell transfusion-related necrotizing enterocolitis in very-low-birthweight infants: a near-infrared spectroscopy investigation. Transfusion. 2013;53:2650–2658. doi: 10.1111/trf.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zamora IJ, Stoll B, Ethun CG, Sheikh F, Yu L, Burrin DG, Brandt ML, Olutoye OO. Low Abdominal NIRS Values and Elevated Plasma Intestinal Fatty Acid-Binding Protein in a Premature Piglet Model of Necrotizing Enterocolitis. PLoS One. 2015;10:e0125437. doi: 10.1371/journal.pone.0125437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gregory KE, Winston AB, Yamamoto HS, Dawood HY, Fashemi T, Fichorova RN, Van Marter LJ. Urinary intestinal fatty acid binding protein predicts necrotizing enterocolitis. J Pediatr. 2014;164:1486–1488. doi: 10.1016/j.jpeds.2014.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ng PC, Ma TP, Lam HS. The use of laboratory biomarkers for surveillance, diagnosis and prediction of clinical outcomes in neonatal sepsis and necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed. 2015;100:F448–F452. doi: 10.1136/archdischild-2014-307656. [DOI] [PubMed] [Google Scholar]
- 36.Stone ML, Tatum PM, Weitkamp JH, Mukherjee AB, Attridge J, McGahren ED, Rodgers BM, Lake DE, Moorman JR, Fairchild KD. Abnormal heart rate characteristics before clinical diagnosis of necrotizing enterocolitis. J Perinatol. 2013;33:847–850. doi: 10.1038/jp.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellis L, Murphy GJ, Culliford L, Dreyer L, Clayton G, Downes R, Nicholson E, Stoica S, Reeves BC, Rogers CA. The Effect of Patient-Specific Cerebral Oxygenation Monitoring on Postoperative Cognitive Function: A Multicenter Randomized Controlled Trial. JMIR Res Protoc. 2015;4:e137. doi: 10.2196/resprot.4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Broderick AJ, Desmond F, Leen G, Shorten G. Clinical evaluation of a novel technology for non-invasive and continuous measurement of plasma haemoglobin concentration. Anaesthesia. 2015;70:1165–1170. doi: 10.1111/anae.13146. [DOI] [PubMed] [Google Scholar]
- 39.Fung MK, Grossman BJ, Hillyer CD, Westhoff CM. Technical Manual. 18th. Bethesda: AABB; 2013. [Google Scholar]
- 40.Roseff SD. Pediatric blood collection and transfusion technology. In: Herman JK, Manno CS, editors. Pediatric transfusion therapy. Bethesda: AABB Press; 2002. pp. 217–247. [Google Scholar]
- 41.Fergusson DA, Hebert P, Hogan DL, LeBel L, Rouvinez-Bouali N, Smyth JA, Sankaran K, Tinmouth A, Blajchman MA, Kovacs L, Lachance C, Lee S, Walker CR, Hutton B, Ducharme R, Balchin K, Ramsay T, Ford JC, Kakadekar A, Ramesh K, Shapiro S. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA. 2012;308:1443–1451. doi: 10.1001/2012.jama.11953. [DOI] [PubMed] [Google Scholar]
- 42.Patel RM, Josephson CD. Storage age of red blood cells for transfusion of premature infants. JAMA. 2013;309:544–545. doi: 10.1001/jama.2012.177439. [DOI] [PubMed] [Google Scholar]
- 43.Lacroix J, Hebert PC, Fergusson DA, Tinmouth A, Cook DJ, Marshall JC, Clayton L, McIntyre L, Callum J, Turgeon AF, Blajchman MA, Walsh TS, Stanworth SJ, Campbell H, Capellier G, Tiberghien P, Bardiaux L, van de Watering L, van der Meer NJ, Sabri E, Vo D A. Investigators, G. Canadian Critical Care Trials. Age of transfused blood in critically ill adults. N Engl J Med. 2015;372:1410–1418. doi: 10.1056/NEJMoa1500704. [DOI] [PubMed] [Google Scholar]
- 44.Steiner ME, Ness PM, Assmann SF, Triulzi DJ, Sloan SR, Delaney M, Granger S, Bennett-Guerrero E, Blajchman MA, Scavo V, Carson JL, Levy JH, Whitman G, D'Andrea P, Pulkrabek S, Ortel TL, Bornikova L, Raife T, Puca KE, Kaufman RM, Nuttall GA, Young PP, Youssef S, Engelman R, Greilich PE, Miles R, Josephson CD, Bracey A, Cooke R, McCullough J, Hunsaker R, Uhl L, McFarland JG, Park Y, Cushing MM, Klodell CT, Karanam R, Roberts PR, Dyke C, Hod EA, Stowell CP. Effects of red-cell storage duration on patients undergoing cardiac surgery. N Engl J Med. 2015;372:1419–1429. doi: 10.1056/NEJMoa1414219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fung MK, Roseff SD, Vermoch KL. Blood component preferences of transfusion services supporting infant transfusions: a University HealthSystem Consortium benchmarking study. Transfusion. 2010;50:1921–1925. doi: 10.1111/j.1537-2995.2010.02668.x. [DOI] [PubMed] [Google Scholar]
- 46.Josephson CD, Friedman D, Pizzini DS, Higgins HNRD, Haresh MTHK, Bell EF, Tan S, Crawford MM, Luban NL. Pediatric Academic Society. San Diego, CA: 2015. Variability in Preparation, Storage, and Processing of Red Blood Cell Products for Extremely Low Birth Weight Infants: A Blood Bank Survey for the Transfusion of Prematures (TOP) Trial. [Google Scholar]
- 47.Keir AK, Wilkinson D, Andersen C, Stark MJ. Washed versus unwashed red blood cells for transfusion for the prevention of morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2016;1:CD011484. doi: 10.1002/14651858.CD011484.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Josephson CD, Caliendo AM, Easley KA, Knezevic A, Shenvi N, Hinkes MT, Patel RM, Hillyer CD, Roback JD. Blood transfusion and breast milk transmission of cytomegalovirus in very low-birth-weight infants: a prospective cohort study. JAMA Pediatr. 2014;168:1054–1062. doi: 10.1001/jamapediatrics.2014.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ross JF, Finch CA, Peacock WC, Sammons ME. The in Vitro Preservation and Post-Transfusion Survival of Stored Blood. J Clin Invest. 1947;26:687–703. doi: 10.1172/JCI101853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grindon AJ. Blood collection. In: McClatchey KD, editor. Clinical Laboratory Medicine. Williams and Wilkins; 2001. pp. 1526–1528. [Google Scholar]
- 51.Food and Drug Administration Blood Products Advisory Committee 80th Meeting Transcript. [Accessed 16.02.21];Dating of Irradiated Red Blood Cells. 2004 http://www.fda.gov/ohrms/dockets/ac/04/transcripts/2004-4057t1.htm.
- 52.Levy GJ, Strauss RG, Hume H, Schloz L, Albanese MA, Blazina J, Werner A, Sotelo-Avila C, Barrasso C, Blanchette V, et al. National survey of neonatal transfusion practices: I. Red blood cell therapy. Pediatrics. 1993;91:523–529. [PubMed] [Google Scholar]
- 53.Hume H, Bard H. Small volume red blood cell transfusions for neonatal patients. Transfus Med Rev. 1995;9:187–199. doi: 10.1016/s0887-7963(05)80109-9. [DOI] [PubMed] [Google Scholar]
- 54.Mock DM, Widness JA, Veng-Pedersen P, Strauss RG, Cancelas JA, Cohen RM, Lindsell CJ, Franco RS. Measurement of posttransfusion red cell survival with the biotin label. Transfus Med Rev. 2014;28:114–125. doi: 10.1016/j.tmrv.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Widness JA, Nalbant D, Matthews NI, Strauss RG, Schmidt RL, Cress GA, Zimmerman MB, Mock DM. Tracking donor RBC survival in premature infants: agreement of multiple populations of biotin-labeled RBCs with Kidd antigen-mismatched RBCs. Pediatr Res. 2013;74:689–697. doi: 10.1038/pr.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Widness JA, Kuruvilla DJ, Mock DM, Matthews NI, Nalbant D, Cress GA, Schmidt RL, Strauss RG, Zimmerman MB, Veng-Pedersen P. Autologous Infant and Allogeneic Adult Red Cells Demonstrate Similar Concurrent Post-Transfusion Survival in Very Low Birth Weight Neonates. J Pediatr. 2015;167:1001–1006. doi: 10.1016/j.jpeds.2015.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Widness JA. Pathophysiology of Anemia During the Neonatal Period, Including Anemia of Prematurity. Neoreviews. 2008;9:e520. doi: 10.1542/neo.9-11-e520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosebraugh MR, Widness JA, Nalbant D, Veng-Pedersen P. A mathematical modeling approach to quantify the role of phlebotomy losses and need for transfusions in neonatal anemia. Transfusion. 2013;53:1353–1360. doi: 10.1111/j.1537-2995.2012.03908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aher SM, Ohlsson A. Late erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2014;4:CD004868. doi: 10.1002/14651858.CD004868.pub4. [DOI] [PubMed] [Google Scholar]
- 60.Ohlsson A, Aher SM. Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2014;4:CD004863. doi: 10.1002/14651858.CD004863.pub4. [DOI] [PubMed] [Google Scholar]
- 61.Carroll PD, Widness JA. Nonpharmacological, blood conservation techniques for preventing neonatal anemia--effective and promising strategies for reducing transfusion. Semin Perinatol. 2012;36:232–243. doi: 10.1053/j.semperi.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rabe H, Diaz-Rossello JL, Duley L, Dowswell T. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst Rev. 2012;8:CD003248. doi: 10.1002/14651858.CD003248.pub3. [DOI] [PubMed] [Google Scholar]
- 63.Rabe H, Alvarez JR, Lawn C, Seddon P, Amess PN. A management guideline to reduce the frequency of blood transfusion in very-low-birth-weight infants. Am J Perinatol. 2009;26:179–183. doi: 10.1055/s-0028-1103024. [DOI] [PubMed] [Google Scholar]
- 64.Rabe H, Reynolds G, Diaz-Rossello J. Early versus delayed umbilical cord clamping in preterm infants. Cochrane Database Syst Rev. 2004;4:CD003248. doi: 10.1002/14651858.CD003248.pub2. [DOI] [PubMed] [Google Scholar]
- 65.Posencheg M, Kirpalani H. Placental transfusion at birth: do we have all of the answers? JAMA Pediatr. 2015;169:9–11. doi: 10.1001/jamapediatrics.2014.2347. [DOI] [PubMed] [Google Scholar]
- 66.Demaret P, Tucci M, Ducruet T, Trottier H, Lacroix J. Red blood cell transfusion in critically ill children (CME) Transfusion. 2014;54:365–375. doi: 10.1111/trf.12261. quiz 364. [DOI] [PubMed] [Google Scholar]
- 67.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ, Group GW. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]