Abstract
Among the various hypotheses put forward to explain the modulatory influence of helminth infection on allergic effector responses in humans, the IL-10 induced suppression of Th2-associated responses has been the leading candidate. To explore this helminth/allergy interaction more fully, parasite- and allergen--specific T cell responses CD4+ T cell responses in 12 subjects with filarial infections and coincident allergic sensitization (Fil+A+) were compared these to the responses to 3 appropriate control groups [Fil−A− (n=13), Fil−A+(n=12), Fil+A−(n=11)]. The most important findings revealed that Fil+A+ had marked (p<0001 for all cytokines) increases in parasite antigen-driven Th2 (IL-4, IL-5, IL-13), Th9 (IL-9) and the regulatory (IL-10) cytokines when compared with Fil+A−. Moreover, using multiparameter flow cytometry, filarial parasite antigen induced a marked increase in not only the frequency of CD4+ T cells producing IL-4, IL-5, IL-2 and TNF-α in Fil+A+ when compared to Fil+A− patients but also in the frequencies of polyfunctional Th2-like (CD4+IL-4+IL-5+ and CD4+IL-2+IL-4+IL-5+TNF-α+) cells. The Th2-associated responses seen in the Fil+A+ group was correlated with serum IgE levels (p<0.01, r=0.5165 for IL-4; p<0.001, r=0.5544 for IL-5; and, p<0.001, r=0.4901 for IL-13), levels of circulating eosinophils (p<0.0116, r=0.5656) and their degranulation/activation products [major basic protein (p<0.001, r=0.7353) and, eosinophil derived neurotoxin (p<0.01, r=0.7059)]. CD4+ responses to allergen were not different (to a large extent) among the groups. Taken together, our data suggest that allergic sensitization coincident with filarial infection drives parasite antigen-specific T cell hyperresponsiveness, characterized largely by an augmented Th2-dominated immune response.
Keywords: filarial infection, atopy, immunoregulation, hygiene hypothesis, T cells, helminth infection, cytokine production
Introduction
Currently, more than three billion people worldwide are infected with helminth parasites and/or suffer from allergic diseases including bronchial asthma, allergic rhinitis, food allergy and eczema (1, 2). A common feature of both atopic disorders and helminth infections is their association with type 2 immune responses (3). Indeed, the canonical human immune response to filarial parasites is of the T helper 2 (Th2) type, characterized by the production of IL-4, IL-5, IL-9, IL-10 and IL-13, induction of IgG4 and IgE antibodies, as well as tissue and peripheral blood eosinophilia (4). This Th2-associated immune response has been implicated in mediating protection to helminth infection but has also been shown to play an important role in the pathogenesis of allergic diseases (5).
The role of helminth infection in modulating allergic reactivity [a concept grouped within the “hygiene hypothesis” (6)] is based on initial studies from Gabon (7) and Ecuador (8) that demonstrated that children infected with intestinal helminths or Schistosoma haematobium have a lower risk of allergic sensitization (based on skin testing to aeroallergen extracts) in comparison to children without helminth parasite infections from the same geographical regions. It has been postulated that this allergen-specific immune response modulation is mediated by parasite-specific IL-10 and possibly by increases in the frequency of regulatory T cells (9–11). Other studies, in contrast, have indicated that infection by gastrointestinal helminths is associated with an increased incidence of allergic reactions (12–14). The explanation that perhaps allow for a reconciling of these distinct set of findings may rely on the relative acuteness of the helminth infection (15, 16). Indeed, given that most helminth infections are longstanding (chronic infection is the rule), it is known that the effector T cell responses (including the Th2 responses) become muted [modified Th2 response (17)] allowing for a response that balances Th2-mediated inflammation and their regulatory controls. Further, it has been shown that this modulation of the antigen-specific T cell response in the context of chronic helminth infection has a propensity to induce bystander effects on responses to vaccinations, other infectious agents, and allergens (18, 19).
Thus, the present study examined the nature of the T cell response induced by parasite antigens and aeroallergens in the context of filarial-infected individuals with or without coincident allergic sensitization. Our data demonstrate that allergic sensitization coincident with filarial infection drives parasite antigen-specific T cell hyperresponsiveness, characterized largely by an augmented Th2-dominated immune response. These responses are associated with a marked elevation of IgE, eosinophilia, and eosinophil activation, the latter potentially responsible for the associated clinical signs (angioedema, urticaria) often seen in these infections.
Material and methods
Study population
From a previously described cohort of 308 subjects (16, 20) used to identify cross reactive epitopes among parasite antigens and aeroallergens, 49 individuals were included in the present study population based solely on the availability of sufficient numbers of cryopreserved peripheral blood mononuclear cells (PBMCs). The study population is described in Table 1 and consisted of four study groups based on the presence or absence of atopy and/or filarial infection: Group 1 Filaria+Allergy+ (Fil+A+; n=12), Group 2 Filaria+Allergy− (Fil+A−; n=11); Group 3 Filaria−Allergy+ (Fil−A+; n=13); Group 4 Filaria−Allergy− (Fil−A−; n=13).
Table 1.
Description of the study population
| Group | ID | Diagnosis | Phadiatop (kUA/L)* |
Der p- specific IgE (kUA/L) |
Total IgE (kUA/L) |
Eosinophil (cells/mm3) |
|---|---|---|---|---|---|---|
| Fil+A+ | 1 | Loa loa | 22.5 | 11.8 | 497.0 | 400 |
| Fil+A+ | 2 | W. bancrofti | 5.4 | 13.3 | 120.0 | 427 |
| Fil+A+ | 3 | O. volvulus | 48.0 | 16.1 | 755.0 | 3,080 |
| Fil+A+ | 4 | Loa loa | 43.4 | 38.5 | 1,244.0 | 7,520 |
| Fil+A+ | 5 | Loa loa | 5.3 | 4.8 | 9,296.0 | 1,408 |
| Fil+A+ | 6 | Loa loa | 26.4 | 2.3 | 11,794.0 | 10,414 |
| Fil+A+ | 7 | Loa loa | 30.8 | 45.7 | 1,269.0 | 7,283 |
| Fil+A+ | 8 | Loa loa | 4.1 | 3.2 | 6,206.0 | 1,404 |
| Fil+A+ | 9 | Loa loa | 1.4 | 0.6 | 606.0 | 4,218 |
| Fil+A+ | 10 | Loa loa | 35.7 | 2.1 | 1,774.0 | 3,267 |
| Fil+A+ | 11 | Loa loa | 1.4 | 0.7 | 1,506.0 | 1,709 |
| Fil+A+ | 12 | Loa loa | 3.5 | 3.3 | 4,969.0 | 1,334 |
| Fil+A− | 1 | Loa loa | 0.0 | - | 43.3 | 1,240 |
| Fil+A− | 2 | Loa loa | 0.0 | - | 115.0 | 560 |
| Fil+A− | 3 | Loa loa | 0.0 | - | 187.0 | 141 |
| Fil+A− | 4 | Loa loa | 0.0 | - | 33.9 | 4,104 |
| Fil+A− | 5 | Loa loa | 0.0 | - | 2,794.0 | 675 |
| Fil+A− | 6 | Loa loa | 0.0 | - | 106.0 | 1,650 |
| Fil+A− | 7 | O. volvulus | 0.0 | - | 172.0 | 252 |
| Fil+A− | 8 | W. bancrofti | 0.0 | - | 99.2 | 88 |
| Fil+A− | 9 | Loa loa | 0.0 | - | 787.0 | 1,580 |
| Fil+A− | 10 | W. bancrofti | 0.0 | - | 26.0 | 200 |
| Fil+A− | 11 | Loa loa | 0.0 | - | 28.0 | 960 |
| Fil−A+ | 1 | Negative | 21.9 | 3.5 | 457.0 | - |
| Fil−A+ | 2 | Negative | 5.6 | 2.6 | 107.0 | - |
| Fil−A+ | 3 | Negative | 11.9 | 8.7 | 43.8 | - |
| Fil−A+ | 4 | Negative | 11.1 | 0.8 | 35.2 | - |
| Fil−A+ | 5 | Negative | 24.1 | 5.7 | 74.3 | - |
| Fil−A+ | 6 | Negative | 13.7 | 1.2 | 283.0 | - |
| Fil−A+ | 7 | Negative | 7.7 | 6.8 | 10.7 | - |
| Fil−A+ | 8 | Negative | 26.6 | 8.1 | 60.1 | - |
| Fil−A+ | 9 | Negative | 5.6 | 2.6 | 18.4 | - |
| Fil−A+ | 10 | Negative | 2.7 | 2.0 | 63.2 | - |
| Fil−A+ | 11 | Negative | 14.5 | 2.1 | 121.8 | - |
| Fil−A+ | 12 | Negative | 56.0 | 0.4 | 399.3 | - |
| Fil−A+ | 13 | Negative | 39.8 | 9.4 | 191.1 | - |
| Fil−A− | 1 | Negative | 0.0 | - | 5.6 | - |
| Fil−A− | 2 | Negative | 0.0 | - | 8.4 | - |
| Fil−A− | 3 | Negative | 0.0 | - | 2.4 | - |
| Fil−A− | 4 | Negative | 0.0 | - | 9.7 | - |
| Fil−A− | 5 | Negative | 0.0 | - | 8.9 | - |
| Fil−A− | 6 | Negative | 0.0 | - | 24.3 | - |
| Fil−A− | 7 | Negative | 0.0 | - | 2.0 | - |
| Fil−A− | 8 | Negative | 0.0 | - | 143.0 | - |
| Fil−A− | 9 | Negative | 0.0 | - | 7.1 | - |
| Fil−A− | 10 | Negative | 0.0 | - | 81.1 | - |
| Fil−A− | 11 | Negative | 0.0 | - | 2.6 | - |
| Fil−A− | 12 | Negative | 0.0 | - | 3.1 | - |
| Fil−A− | 13 | Negative | 0.0 | - | 7.7 | - |
Allergens specific-IgE levels > 0.35 kUA/L were considered allergic/atopic.
All individuals (and all samples collected) were part of registered protocols approved by the Institutional Review Boards of the National Institute of Allergy and Infectious Diseases (NCT00001230 and NCT00001345) for the filarial-infected subjects and of the Department of Transfusion Medicine, Clinical Center, National Institutes of Health (IRB# 99-CC-0168) for the healthy donors. Written informed consent was obtained from all subjects.
In vitro culture
For the analysis of cytokine production by PBMCs after in vitro stimulation by parasite antigen and environmental allergens, aliquots of cell suspension in RPMI 1640 medium supplemented with 10% human sera, 1% L-glutamine (Sigma, USA), 1% antibiotic (Invitrogen, USA) and 1% non-essentials amino acids (Sigma, USA) were cultured in media alone, with the filarial parasite antigen BMA (10µg/mL), dust mite Dermatophagoides pteronyssinus extract allergen (AlkAbelló, Port Washington, NY, USA) (Der p EXT 15 AU) or with PMA/ionomycin (Sigma, USA) (0.5/0.05 pg/mL) for 72 hours in 5% CO2 at 37°C for the measurement of cytokine production. To identify the source of these cytokines by flow cytometry, PBMCs of all group’s patients were cultured and stimulated in the same conditions as mentioned above, but for 12 hours, with the addition of CD28/CD45d co-stimulatory molecules (BD, FastImmune™, BD Bioscience, USA) and 10µg/mL Brefeldin A/Monensin (Sigma, USA).
Luminex assay
The quantification of IL-2, IL-4, IL-5, IL-9, IL-10, IL-13, IL-17A, IL-22, TNF-α and IFN-γ levels was assessed by a Luminex multiplex assay (Milliplex®, Millipore, USA) in accordance with the manufacturer’s recommendations. The eosinophil granule proteins were measured using a previously described assay (21).
Mulitparameter flow cytometry
To identify the source of the Th1/Th2/Th9/Th17-associated and regulatory cytokines, cells from in vitro cultures were harvested and washed with FACS buffer (PBS, FBS 2% and 0.1% sodium azide) and then incubated with Fc blocking solution for 20 minutes. The cells were then stained for viability (Live/Dead® Fixable Blue, Molecular Probes, USA), washed with FACS buffer and then incubated with fluorochrome-conjugated anti-CD3, anti-CD4 and anti-CD8 for 30 min in the dark at RT. The cells were next washed twice with FACS buffer, then fixed and permeabilized using a Fix/Perm buffer kit (BioLegend, USA) for 20 min in the dark at 4°C. The cells were washed twice with perm buffer (BioLegend, USA) and re-suspended with the fluorochrome-conjugated anti-IL-2, anti-IL-4, anti-IL-5, anti-IL-10, anti-IL-17A, anti-TNF-α, anti-IFN-γ and anti-CD25 pool (Supplemental Table 1) for 30 min in the dark at 4°C. Finally, the cells were washed twice with perm buffer and then acquired using the BD LSR Fortessa™ flow cytometer (BD Biosciences, USA) and FACSDiva™ software (BD Biosciences, USA) for acquisition. All analyses were performed using FlowJov10.0.8 (Flow Jo LLC, USA). For the flow cytometry multiparameter analyses, PESTLE/SPICE v5.0 (NIAID, USA) software was used. In addition, the frequency of CD4+ T cells producing single and multiple cytokines were analyzed by the FlowJo software based on the gating strategy shown in Supplemental Figure 1.
Data analysis
All statistical analyses were performed using Prism 5.0 for Windows (GraphPad Software Inc., USA). Unless stated otherwise, geometric means (GM) and upper 95% CI of geometric mean (between parentheses) were used as measures of central tendency. The D’Agostino and Pearce normality test was used to determine whether data sets were normally distributed. Kruskal-Wallis followed by Dunn’s multiple comparison tests were used to evaluate the statistical differences in the cytokine production by the different groups, as well as, the frequency of CD4+ T cells producing cytokines. Correlations were performed using the Spearman rank correlation. Net production of cytokines (in pg/ml) and net frequency of CD4+ T cell producing cytokines (in %) were calculated by subtracting the baseline level from the level following stimulation. The differences were considered statistically significant when the corrected p values< 0.05.
Results
Study population
The clinical diagnoses, IgE (total and allergen-specific) levels and peripheral eosinophil counts for the study population can be found in Table 1. Peripheral blood eosinophil counts were greater in the Fil+A+ patients compared to those filarial-infected patients without allergy (Fil+A−). [GM=2269 (4449) cells/uL vs. 590 (1312) cells/uL; p=0.0151]. Interestingly, the Fil+A+ patients also demonstrated a significant increase in the levels of IgE (GM =1644 (3865) kUA/L) when compared to Fil+A− patients (GM=122 (323.1) kUA/L p<0.05], Fil−A+ individuals [GM= 84.0 (168.1) kUA/L p<0.05] and Fil−A− healthy donors [GM 9.0 (19.74) kUA/L; p<0.001].
Environmental allergy induces a hyperresponsiveness in the concomitant immune response to filarial infection mediated by parasite antigens
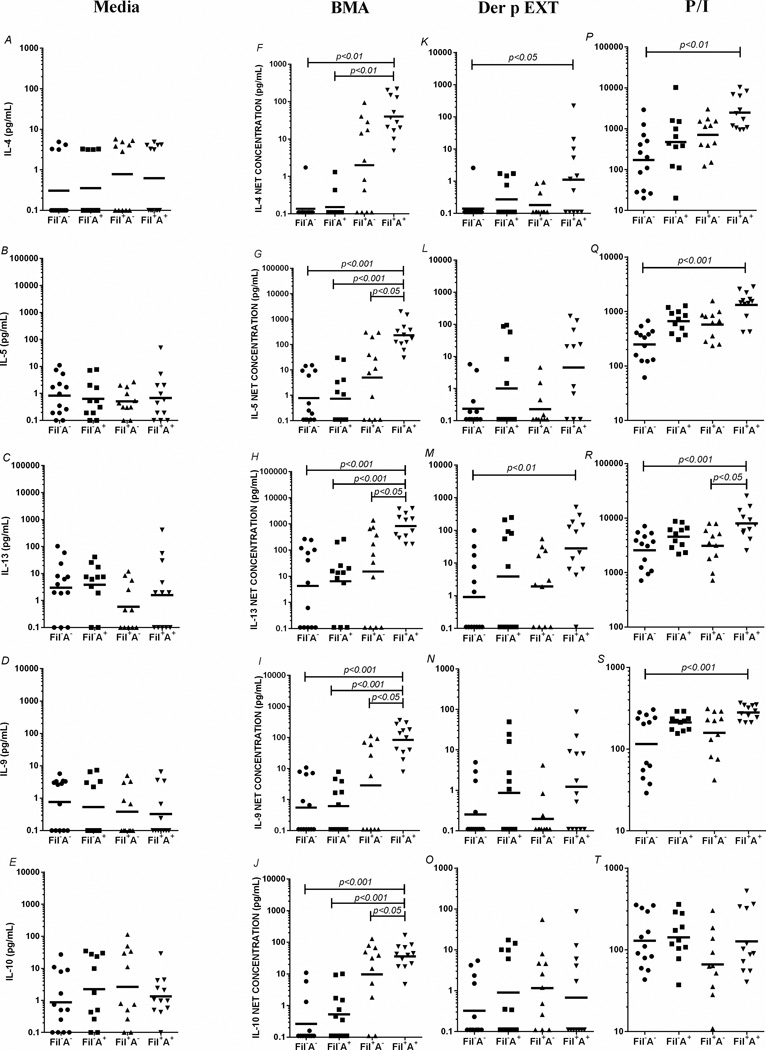
To elucidate the influence of atopy on the parasite- and allergen-induced immune response, we first assessed cytokine production. As can be seen in Figure 1, when spontaneous production of the various Th2-associated cytokines was measured (in the absence of a stimulus), there were no differences among the 4 groups in any of the cytokines measured (Fig. 1 A–E). In marked contrast, when stimulated with the filarial parasite antigen BMA, those filarial-infected individuals with concomitant atopy (Fil+A+) had marked and significant increases in the levels of IL-5 [GM=232.1 (504.3) pg/mL vs 4.9 (38.34) pg/mL, p<0.05], IL-13 [GM=826.9 (1732) pg/mL vs 14.9 (175.6) pg/mL, p<0.05], IL-9 [GM= 83.1 (178.8) pg/mL vs 2.8 (24.69) pg/mL, p<0.05] and IL-10 [GM=36.1 (65.45) vs 9.6 (52.82) pg/mL, p<0.05] when compared to Fil+A−. These differences were even more pronounced when the IL-4 [GM=0.1 (0.24) pg/mL, p<0.01 and GM=0.1 (0.21) pg/mL, p<0.01], IL-5 [GM=0.7 (2.98) pg/mL, p<0.001 and GM=0.7 (2.89) pg/mL, p<0.001], IL-13 [GM=6.3 (35.66) pg/mL, p<0.001 and GM=4.2 (32.76) pg/mL, p<0.001], IL-9 [GM=0.6 (1.99) pg/mL, p<0.001 and GM=0.5 (1.82) pg/mL, p<0.001], and IL-10 [GM=0.5 (1.56) pg/mL, p<0.001 and GM= 0.2 (0.72) pg/mL, p<0.001] response were compared to the other 2 groups (Fil−A+ and Fil−A−, respectively Fig. 1 F–J). No relevant differences were observed among the groups in the cytokine production after Der p allergen stimulation (Fig. 1 K–O). Cytokine responses following PMA/ionomycin (P/I) stimulation showed that Fil+A+ had a marked increase in the levels of IL-4 (p<0.01), IL-5 (p<0.001) and IL-9 (p<0.001) when compared to Fil−A− (Fig. 1 P–Q,S). When levels of IL-13 were evaluated, the Fil+A+ had levels of IL-13 that were strikingly more pronounced than those seen in comparison to Fil+A− (p<0.05) and Fil−A− (p<0.001) (Fig. 1R). No differences were seen among the groups for P/I driven IL-10. The analysis of spontaneous production and BMA antigen, Der p allergen and P/I-driven Type-1 and Type-17-associated cytokines showed no differences in the production of IL-2, TNF- α, IFN-γ, IL-17 and IL-22 production among the 4 groups (Supplemental Figure 2).
Figure 1. Th2-associated cytokines driven by parasite antigens, allergens and mitogen in the filarial-infected individuals with or without coincident allergic sensitization.
In vitro production of IL-4 (A,F,K,P), IL-5 (B,G,L,Q), IL-13 (C,H,M,R), IL-9 (D,I,N,S) and IL-10 (E,J,O,T) at baseline (A–E) and after stimulation by filaria antigen (F–J), Der p Ext (K–O) and P/I (P–T) in the 4 groups (FIL−A−, Fil−A+, Fil+A−, Fil+A+). Each dot represents a single individual and the horizontal bars are the geometric mean (GM). Net production of cytokines (in pg/ml) calculated by subtracting the baseline level from the level following stimulation.
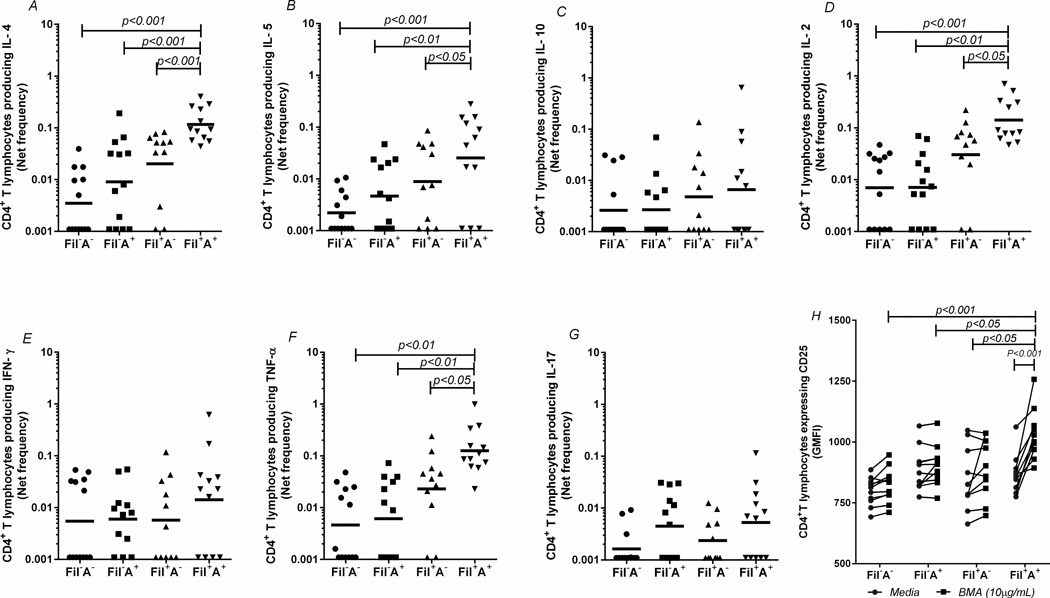
When the frequencies of cytokine-producing CD4+ T cells were assessed ex vivo and in response to antigenic and mitogenic stimuli, a unique pattern of immune response was seen in those filarial-infected individuals with concomitant atopy (Fil+A+). In the ex vivo analyses, there were no significant differences among the 4 groups in the frequency of T cells producing a given cytokine in the absence of a stimulus (Supplemental Figure 3). However, when stimulated with the filarial parasite antigen BMA (Figure 2), Fil+A+ patients demonstrated a marked increase in the frequency of CD4+ T cells producing IL-4 (p<0.001; Fig. 2A), IL-5 (p<0.05; Fig. 2B), IL-2 (p<0.05; Fig. 2D) and TNF-α (P<0.05; Fig. 2F) when compared to the Fil+A− group. There was an even more marked disparity in the responses when the Fil+A+ group was compared to the other 2 groups (Fil−A−, Fil−A+) (p<0.01 for each comparison). Moreover, when the expression of CD25 was measured on the CD4+ T cells of the 4 groups, (to assess antigen-specific T cell activation), it was clear that BMA induced a marked increase in the per cell surface expression of CD25 in the Fil+A+ group, both in comparison with the baseline of the same group (p<0.001) and when compared to the BMA stimulation in the other 3 groups (Fil+A− (p<0.05, Fil−A+(p<0.05), Fil−A− (p<0.001) (Fig. 2H). No differences were observed after Der p allergen stimulation (Supplemental Figure 3) or after P/I stimulation, confirming the relative antigen-specificity of the hyper-reactive CD4+ T cell response in the Fil+A+ group (Supplemental Figure 3).
Figure 2. Antigen-specific CD4+ T-cells hyperresponsiveness in the filarial-infected individuals with or without coincident allergic sensitization.
Frequency of CD4+ T lymphocytes of the 4 groups, producing IL-4 (A), IL-5 (B), IL-10 (C), IL-2 (D), IFN-γ (E), TNF-α (F), and IL-17A (G), and expressing surface CD25 (H) after stimulation with filarial parasite antigen (BMA 10µg/mL). Each dot represents a single individual and the horizontal bars are GMs.
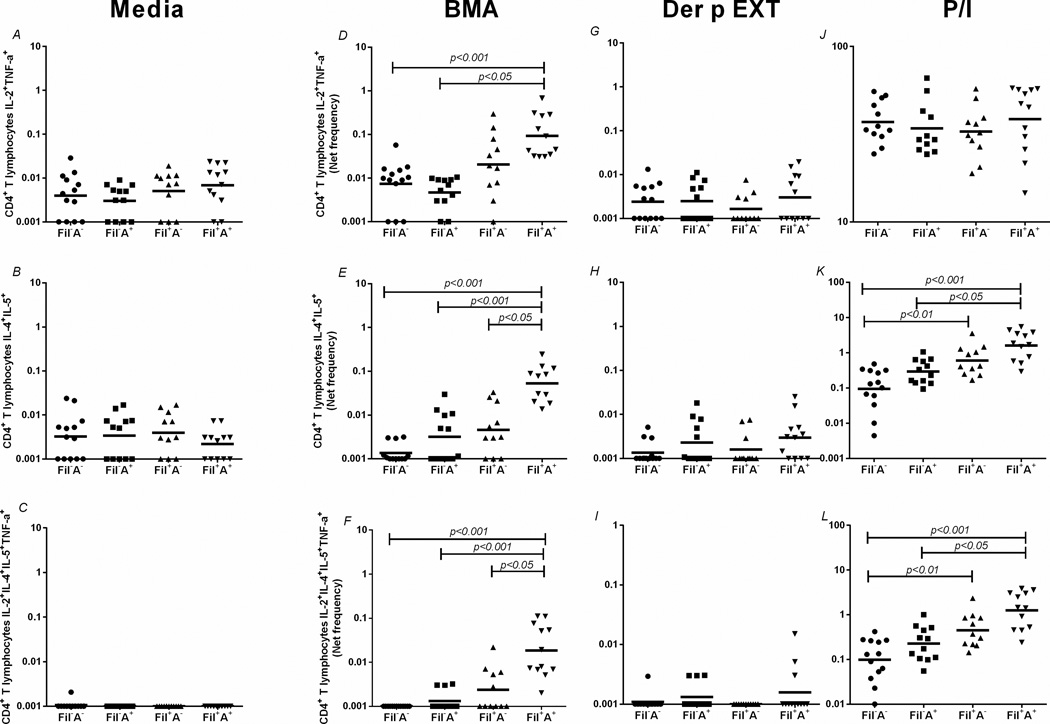
Having demonstrated a marked increase in CD4+ T cells producing Th1- or Th-2-associated cytokines in Fil+A+ individuals, we performed multiparameter analysis (Fig. 3) on the CD4 response in all groups. As can be seen in Fig. 3A–C there were no differences among the groups in the spontaneous/homeostatic frequencies of CD4+IL-2+TNF-α+ cells, CD4+IL-4+IL-5+ cells and, CD4+IL-2+IL-4+IL-5+TNF-α cells. However in response to the filarial antigen BMA, those in the Fil+A+ group, had a marked increase in the frequency of CD4+IL-4+IL-5+T cells [GM=0.053 (0.01)%], when compared to Fil+A− [GM=0.004 (0.01)%, p<0.05], Fil−A+ [GM=0.003 (0.007)%, p<0.001] and Fil−A− [GM=0.001 (0.001)%, p<0.001], Fig. 3E. Interestingly, the Fil+A+ group also had a marked increase in the frequency of the relatively infrequent polyfunctional CD4+ subset CD4+IL-2+IL-4+IL-5+TNF-α+ when compared to Fil+A− patients [GM=0.018 (0.043)% vs. 0.002 (0.004)%, p<0.05], Fig. 3F. There were no differences among the groups when multiparameter flow cytometric analyses were examined in response to dust mite extract.
Figure 3. Flow cytometry multiparameter analysis highlighting the increased frequency of polyfunctional CD4+ T cell subsets.
Frequency of CD4+ T lymphocytes producing multiple Th1 (A,D,G,J), Th2 (B,E,H,K) and mixed Th1/Th2 (C,F,I,L) at baseline (A,B,C) and after stimulation with the filarial antigen (BMA) (D,E,F), Der p EXT (G,H,I) and P/I (J,K,L). Each dot represents a single individual and the horizontal bars are GMs.
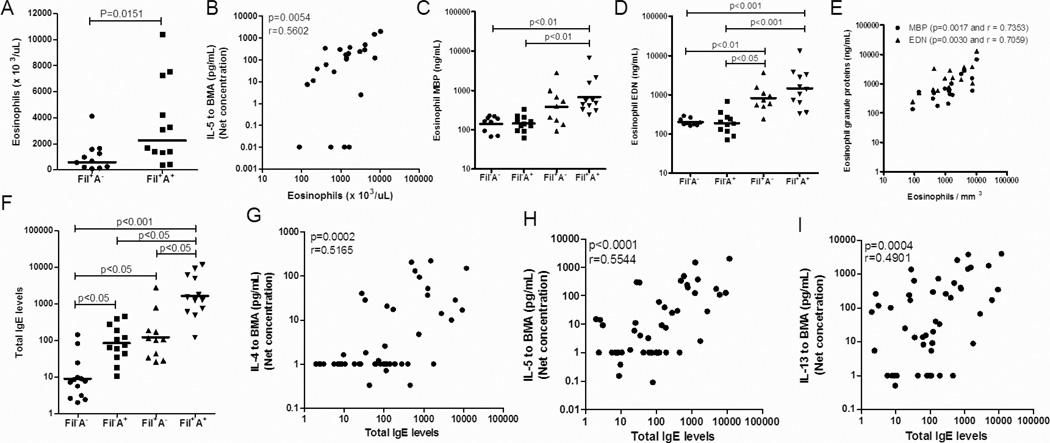
The polarized Th2 response –mediated by filarial parasite antigen may be responsible for sustaining the elevated levels of blood eosinophils and IgE in the Fil+A+ patients
To explore the relationship between the parasite-induced T cell responses and the regulation of eosinophilia, eosinophil activation and IgE, we examined the interrelationships among these various parameters (Figure 4). As can be seen in Figure 4A, the Fil+A+ group had a distinctly increased number of circulating peripheral eosinophils compared to Fil+A− patients (p<0.05) and those cells were strongly and positively correlated with the IL-5 levels driven by BMA antigen (p=0.005 and r=0.560) (Fig. 4B). Although increases in the number of peripheral blood eosinophils are often seen in helminth infections (and some allergic disorders as well), it is the activation of these eosinophils that often drive the eosinophil-mediated pathology, activation that is reflected in the serum levels of eosinophil-specific granule proteins (MBP, EDN, ECP, EPO). As can be seen in Fig. 4C and 4D, there was an increase in the levels of major basic protein-MBP (p<0.01 and p<0.01; Fig. 4C) and eosinophil-derived neurotoxin –EDN (p<0001 and p<0.001; Fig. 4D) in the Fil+A+ patients in comparison to Fil−A+ and Fil−A− groups, respectively. There was also a clear relationship between the levels of these granule proteins and the number of circulating eosinophils (p=0.0017 and r=0.7353; p=0.0030 and r=0.7059; Fig. 4E). Not unlike what was seen for eosinophil levels, Fil+A+ individuals demonstrated significantly increased levels of IgE when compared to Fil+A− (p<0.05), Fil−A+ (p<0.05) and Fil−A− (p<0.001) groups. Moreover, the IgE was shown to be strongly correlation with the levels of IL-4 (p=0.0002 and r=0.5165; Fig. 4G), IL-5 (p<0.001 and r=0.5544; Fig. 4H) and IL-13 (p<0004 and r=0.4901; Fig. 4I)] driven by BMA.
Figure 4. Eosinophilia and IgE regulation in the 4 groups.
Eosinophils counts (A) and their association with BMA-specific IL-5 (B); eosinophil granule proteins (C,D) and their correlation with eosinophils (E); IgE levels (F) and its association with BMA-specific TH2 cytokines (G,H,I). Each dot represents a single individual and the horizontal bars are GMs.
Discussion
Put into the context of the “hygiene hypothesis” it is felt that the lack of exposure by children early in their development to microbes/parasites (as seen in most high and middle-income countries) may explain the increased incidence of allergic diseases (6, 22) seen in these same countries, providing a causal link between helminth infection and protection from allergy. However, a wide range of studies in humans and model systems have provided substantive but conflicting evidence of the relationship between helminths and allergy, with different studies providing evidence of a positive association (23, 24), a negative association (25) or no association at all (26). This lack of consensus likely reflects real differences among the studies, if the modulatory effects of helminth infections on allergic reactivity differ either because of species differences among particular helminths or because of differences in the timing of parasite infection in relation to immune maturation or sensitization (27).
While initial exposures to helminths may be associated with enhanced allergic inflammatory responses to the parasite, in long-term infections and with repeated infections, the host inflammatory response becomes more tightly controlled (28, 29). Chronic helminth infections induce potent immunoregulatory pathways (30–32), such as immunosuppressive cytokines [e.g. IL-10 or TGF-β (33, 34)] or regulatory T cell populations (35, 36) that may facilitate parasite survival. This regulation, however, may not just affect responses to parasite antigens but also to bystander antigens and aeroallergens. Such helminth-associated regulatory effects may contribute to the decreased prevalence of allergic diseases (29, 37) reported from the rural tropics.
The present study was designed to understand the mechanisms underlying the parasite-driven CD4+ T cell response and the effect of coincident allergic sensitization on this process. The most important findings revealed that filarial-infected patients with coincident atopy had marked increases in parasite antigen-driven Th2 (IL-4, IL-5, IL-13), Th9 (IL-9) cytokines and associated with adaptive Tregs (IL-10) when compared with filarial-infected but non-allergic patients. Moreover, filarial parasite antigen induced a marked increase in the frequency of CD4+ T cells producing IL-4, IL-5, IL-2 and TNF-α in Fil+A+ when compared to Fil+A− patients, as well as, a dominant-Th2 cell expansion, characterized by a marked increase in the frequencies of polyfunctional (CD4+IL-4+IL-5+) and (CD4+IL-2+IL-4+IL-5+TNF-α+) cells in the Fil+A+ group when compared to Fil+A− patients. Furthermore, we found that this increase in Th2-associated cytokine production was reflected in IgE serum levels, increases in circulating eosinophils and measurements of eosinophil activation. These increases were also strongly positively correlated with the ability to produce IL-4, IL-5 and IL-13 in vitro.
The current explanation related to the association between helminth infection and reduced allergic reactivity --based on studies in both mice and humans-- is that parasite-driven production of IL-10 (along with moderate increases in the frequency of regulatory T cells (Tregs) are responsible for the modulation of allergic reactivity (9–11). The ability of the Tregs to suppress immune-mediated pathology (38) was suggested in studies in mice infected with the intestinal nematode Heligmosomoides polygyrus, in which this parasite induced an expansion of natural Tregs (39–42) that prevented allergen-induced pathology (43, 44).
However, the present study suggests that on a background of allergic sensitization immune responses to parasite antigens may actually be accentuated in patients with relatively acute filarial-infections. This accentuated immune responsiveness may either lead to 1) control of the parasite and/or resistance to re-infection, or 2) alteration of the helminth infection –associated pathology.
The Th2-dominant immune response as observed in the Fil+A+ in this study, has been the object of discussion for many years in the context of both allergy and helminth infections. IL-4, the major cytokine that induces IgE isotype switching, has been implicated as being essential for the clearance/control of microfilariae in a murine infection model (45). Moreover, it has been postulated that helminth-induced alternatively activated macrophages that are driven by IL-4 and/or IL-13 have been shown to play an important role in the control of Th1-type inflammation, wound healing, and worm expulsion (46, 47).
IgE levels have also been associated with immunity to some helminth infections (48, 49). Indeed, Fitzsimmons and collaborators (50) reviewed a number of studies in human schistosomiasis, in which levels of parasite-specific IgE were shown to be correlated with resistance to re-infection (48, 51–55). Moreover, parasite-specific IgE responses have also been associated with immunity in human infections with hookworms (56, 57), Trichuris (58), and Ascaris infection (59, 60). In addition, eosinophils and their granule proteins have been implicated the in host defense against helminth parasites (61) and in mediating resistance to helminth infections, either alone or in conjunction with antibody, complement, or innate cells (62–66).
In contrast, this marked increase in the Th2-associated IgE levels and eosinophilia driven by filarial antigens in Fil+A+ patients has also been implicated in the pathogenesis of some syndromes associated with filarial infections (67–70) including tropical pulmonary eosinophilia (TPE) (68, 71, 72) and the localized hyperreactive dermatitis (sowda) seen in Onchocerca volvulus-infected patients (73–75). Finally, given that IL-10-mediated modulation of Th1/Th2/Th17/Th9 responses (76) has been associated with clinically asymptomatic filarial infection, it is possible that allergic sensitization coincident with these filarial infections results in altering the balance of regulatory and effector responses that could in turn alter the longevity of the parasites in their human hosts.
Taken together, our data suggest that allergic sensitization coincident with filarial infection drives a hyper-reactive parasite antigen-specific T cell response characterized by an augmented Th2-associated immune response, eosinophilia and elevated serum levels of IgE that might limit parasite burden but, as a consequence, allow for immune-mediated, parasite-associated morbidity.
Supplementary Material
Acknowledgments
This work was funded entirely by the Division of Intramural Research (DIR) of the National Institute of Allergy and Infectious Diseases
PHGG was supported by a sandwich pre-doctoral degree fellowship from the “Ciências sem Fronteiras” program of CNPq, Brazil.
Abbreviations
- BMA
crude extract from Brugia malayi adult worms
- Der p
Dermatophagoides pteronyssinus
- Fil−A−
Filaria negative and Allergy negative
- Fil−A+
Filaria negative and Allergy positive
- Fil+A−
Filaria positive and Allergy negative
- Fil+A+
Filaria positive and Allergy positive
- GM
geometric mean
- PBMC
peripheral blood mononuclear cell
- P/I
PMA/ionomycin.
References
- 1.Feasey N, Wansbrough-Jones M, Mabey DC, Solomon AW. Neglected tropical diseases. Br Med Bull. 2010;93:179–200. doi: 10.1093/bmb/ldp046. [DOI] [PubMed] [Google Scholar]
- 2.Pawankar R, Canonica GW, Holgate ST, Lockey RF. Allergic diseases and asthma: a major global health concern. Curr Opin Allergy Clin Immunol. 2012;12:39–41. doi: 10.1097/ACI.0b013e32834ec13b. [DOI] [PubMed] [Google Scholar]
- 3.Pulendran B, Artis D. New paradigms in type 2 immunity. Science. 2012;337:431–435. doi: 10.1126/science.1221064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol. 2011;11:375–388. doi: 10.1038/nri2992. [DOI] [PubMed] [Google Scholar]
- 5.Cooper PJ, Chico ME, Rodrigues LC, Ordonez M, Strachan D, Griffin GE, Nutman TB. Reduced risk of atopy among school-age children infected with geohelminth parasites in a rural area of the tropics. J Allergy Clin Immunol. 2003;111:995–1000. doi: 10.1067/mai.2003.1348. [DOI] [PubMed] [Google Scholar]
- 6.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van den Biggelaar AH, Lopuhaa C, van Ree R, van der Zee JS, Jans J, Hoek A, Migombet B, Borrmann S, Luckner D, Kremsner PG, Yazdanbakhsh M. The prevalence of parasite infestation and house dust mite sensitization in Gabonese schoolchildren. Int Arch Allergy Immunol. 2001;126:231–238. doi: 10.1159/000049519. [DOI] [PubMed] [Google Scholar]
- 8.Cooper PJ, Chico ME, Bland M, Griffin GE, Nutman TB. Allergic symptoms, atopy, and geohelminth infections in a rural area of Ecuador. Am J Respir Crit Care Med. 2003;168:313–317. doi: 10.1164/rccm.200211-1320OC. [DOI] [PubMed] [Google Scholar]
- 9.Yazdanbakhsh M, Wahyuni S. The role of helminth infections in protection from atopic disorders. Curr Opin Allergy Clin Immunol. 2005;5:386–391. doi: 10.1097/01.all.0000182541.52971.eb. [DOI] [PubMed] [Google Scholar]
- 10.Mitre E, Chien D, Nutman TB. CD4(+) (and not CD25+) T cells are the predominant interleukin-10-producing cells in the circulation of filaria-infected patients. J Infect Dis. 2008;197:94–101. doi: 10.1086/524301. [DOI] [PubMed] [Google Scholar]
- 11.Metenou S, Dembele B, Konate S, Dolo H, Coulibaly SY, Coulibaly YI, Diallo AA, Soumaoro L, Coulibaly ME, Sanogo D, Doumbia SS, Traore SF, Mahanty S, Klion A, Nutman TB. At homeostasis filarial infections have expanded adaptive T regulatory but not classical Th2 cells. J Immunol. 2010;184:5375–5382. doi: 10.4049/jimmunol.0904067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dold S, Heinrich J, Wichmann HE, Wjst M. Ascaris-specific IgE and allergic sensitization in a cohort of school children in the former East Germany. J Allergy Clin Immunol. 1998;102:414–420. doi: 10.1016/s0091-6749(98)70129-0. [DOI] [PubMed] [Google Scholar]
- 13.Palmer LJ, Celedon JC, Weiss ST, Wang B, Fang Z, Xu X. Ascaris lumbricoides infection is associated with increased risk of childhood asthma and atopy in rural China. Am J Respir Crit Care Med. 2002;165:1489–1493. doi: 10.1164/rccm.2107020. [DOI] [PubMed] [Google Scholar]
- 14.Hunninghake GM, Soto-Quiros ME, Avila L, Ly NP, Liang C, Sylvia JS, Klanderman BJ, Silverman EK, Celedon JC. Sensitization to Ascaris lumbricoides and severity of childhood asthma in Costa Rica. J Allergy Clin Immunol. 2007;119:654–661. doi: 10.1016/j.jaci.2006.12.609. [DOI] [PubMed] [Google Scholar]
- 15.Steel C, Nutman TB. Altered T cell memory and effector cell development in chronic lymphatic filarial infection that is independent of persistent parasite antigen. PLoS One. 2011;6:e19197. doi: 10.1371/journal.pone.0019197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santiago HC, Bennuru S, Boyd A, Eberhard M, Nutman TB. Structural and immunologic cross-reactivity among filarial and mite tropomyosin: implications for the hygiene hypothesis. J Allergy Clin Immunol. 2011;127:479–486. doi: 10.1016/j.jaci.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733–744. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 18.Babu S, Nutman TB. Immunology of lymphatic filariasis. Parasite Immunol. 2014;36:338–346. doi: 10.1111/pim.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chatterjee S, Nutman TB. Helminth-induced immune regulation: implications for immune responses to tuberculosis. PLoS Pathog. 2015;11:e1004582. doi: 10.1371/journal.ppat.1004582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santiago HC, LeeVan E, Bennuru S, Ribeiro-Gomes F, Mueller E, Wilson M, Wynn T, Garboczi D, Urban J, Mitre E, Nutman TB. Molecular mimicry between cockroach and helminth glutathione S-transferases promotes cross-reactivity and cross-sensitization. J Allergy Clin Immunol. 2012;130:248–256. e249. doi: 10.1016/j.jaci.2012.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makiya MA, Herrick JA, Khoury P, Prussin CP, Nutman TB, Klion AD. Development of a suspension array assay in multiplex for the simultaneous measurement of serum levels of four eosinophil granule proteins. J Immunol Methods. 2014;411:11–22. doi: 10.1016/j.jim.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu AH, Leung DY. Renaissance of the hygiene hypothesis. J Allergy Clin Immunol. 2006;117:1063–1066. doi: 10.1016/j.jaci.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 23.Hagel I, Cabrera M, Hurtado MA, Sanchez P, Puccio F, Di Prisco MC, Palenque M. Infection by Ascaris lumbricoides and bronchial hyper reactivity: an outstanding association in Venezuelan school children from endemic areas. Acta Trop. 2007;103:231–241. doi: 10.1016/j.actatropica.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Wordemann M, Diaz RJ, Heredia LM, Collado Madurga AM, Ruiz Espinosa A, Prado RC, Millan IA, Escobedo A, Rojas Rivero L, Gryseels B, Gorbea MB, Polman K. Association of atopy, asthma, allergic rhinoconjunctivitis, atopic dermatitis and intestinal helminth infections in Cuban children. Trop Med Int Health. 2008;13:180–186. doi: 10.1111/j.1365-3156.2007.01988.x. [DOI] [PubMed] [Google Scholar]
- 25.van den Biggelaar AH, van Ree R, Rodrigues LC, Lell B, Deelder AM, Kremsner PG, Yazdanbakhsh M. Decreased atopy in children infected with Schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet. 2000;356:1723–1727. doi: 10.1016/S0140-6736(00)03206-2. [DOI] [PubMed] [Google Scholar]
- 26.van der Werff SD, Twisk JW, Wordemann M, Campos Ponce M, Diaz R, Junco Nunez FA, Rojas Rivero L, Bonet Gorbea M, Polman K. Deworming is not a risk factor for the development of atopic diseases: a longitudinal study in Cuban school children. Clin Exp Allergy. 2013;43:665–671. doi: 10.1111/cea.12129. [DOI] [PubMed] [Google Scholar]
- 27.Cooper PJ, Barreto ML, Rodrigues LC. Human allergy and geohelminth infections: a review of the literature and a proposed conceptual model to guide the investigation of possible causal associations. Br Med Bull. 2006;79–80:203–218. doi: 10.1093/bmb/ldl015. [DOI] [PubMed] [Google Scholar]
- 28.Fallon PG, Mangan NE. Suppression of TH2-type allergic reactions by helminth infection. Nat Rev Immunol. 2007;7:220–230. doi: 10.1038/nri2039. [DOI] [PubMed] [Google Scholar]
- 29.Cooper PJ. Interactions between helminth parasites and allergy. Curr Opin Allergy Clin Immunol. 2009;9:29–37. doi: 10.1097/ACI.0b013e32831f44a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teixeira-Carvalho A, Fujiwara RT, Stemmy EJ, Olive D, Damsker JM, Loukas A, Correa-Oliveira R, Constant SL, Bethony JM. Binding of excreted and/or secreted products of adult hookworms to human NK cells in Necator americanus-infected individuals from Brazil. Infect Immun. 2008;76:5810–5816. doi: 10.1128/IAI.00419-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujiwara RT, Cancado GG, Freitas PA, Santiago HC, Massara CL, Dos Santos Carvalho O, Correa-Oliveira R, Geiger SM, Bethony J. Necator americanus infection: a possible cause of altered dendritic cell differentiation and eosinophil profile in chronically infected individuals. PLoS Negl Trop Dis. 2009;3:e399. doi: 10.1371/journal.pntd.0000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gazzinelli-Guimaraes PH, Souza-Fagundes EM, Cancado GG, Martins VG, Dhom-Lemos LC, Ricci ND, Fiuza JA, Bueno LL, Miranda RR, Guatimosim S, Gazzinelli A, Correa-Oliveira R, Bartholomeu DC, Fujiwara RT. Cell apoptosis induced by hookworm antigens: a strategy of immunomodulation. Front Biosci (Elite Ed) 2013;5:662–675. doi: 10.2741/e647. [DOI] [PubMed] [Google Scholar]
- 33.King CL, Mahanty S, Kumaraswami V, Abrams JS, Regunathan J, Jayaraman K, Ottesen EA, Nutman TB. Cytokine control of parasite-specific anergy in human lymphatic filariasis. Preferential induction of a regulatory T helper type 2 lymphocyte subset. J Clin Invest. 1993;92:1667–1673. doi: 10.1172/JCI116752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geiger SM, Massara CL, Bethony J, Soboslay PT, Correa-Oliveira R. Cellular responses and cytokine production in post-treatment hookworm patients from an endemic area in Brazil. Clin Exp Immunol. 2004;136:334–340. doi: 10.1111/j.1365-2249.2004.02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ricci ND, Fiuza JA, Bueno LL, Cancado GG, Gazzinelli-Guimaraes PH, Martins VG, Matoso LF, de Miranda RR, Geiger SM, Correa-Oliveira R, Gazzinelli A, Bartholomeu DC, Fujiwara RT. Induction of CD4(+)CD25(+)FOXP3(+) regulatory T cells during human hookworm infection modulates antigen-mediated lymphocyte proliferation. PLoS Negl Trop Dis. 2011;5:e1383. doi: 10.1371/journal.pntd.0001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor MD, LeGoff L, Harris A, Malone E, Allen JE, Maizels RM. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J Immunol. 2005;174:4924–4933. doi: 10.4049/jimmunol.174.8.4924. [DOI] [PubMed] [Google Scholar]
- 37.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 38.Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. Helminth parasites--masters of regulation. Immunol Rev. 2004;201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 39.Finney CA, Taylor MD, Wilson MS, Maizels RM. Expansion and activation of CD4(+)CD25(+) regulatory T cells in Heligmosomoides polygyrus infection. Eur J Immunol. 2007;37:1874–1886. doi: 10.1002/eji.200636751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Setiawan T, Metwali A, Blum AM, Ince MN, Urban JF, Jr, Elliott DE, Weinstock JV. Heligmosomoides polygyrus promotes regulatory T-cell cytokine production in the murine normal distal intestine. Infect Immun. 2007;75:4655–4663. doi: 10.1128/IAI.00358-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rausch S, Huehn J, Kirchhoff D, Rzepecka J, Schnoeller C, Pillai S, Loddenkemper C, Scheffold A, Hamann A, Lucius R, Hartmann S. Functional analysis of effector and regulatory T cells in a parasitic nematode infection. Infect Immun. 2008;76:1908–1919. doi: 10.1128/IAI.01233-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hang L, Blum AM, Setiawan T, Urban JP, Jr, Stoyanoff KM, Weinstock JV. Heligmosomoides polygyrus bakeri infection activates colonic Foxp3+ T cells enhancing their capacity to prevent colitis. J Immunol. 2013;191:1927–1934. doi: 10.4049/jimmunol.1201457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson MS, Taylor MD, Balic A, Finney CA, Lamb JR, Maizels RM. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med. 2005;202:1199–1212. doi: 10.1084/jem.20042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaiss DM, van Loosdregt J, Gorlani A, Bekker CP, Grone A, Sibilia M, van Bergen en Henegouwen PM, Roovers RC, Coffer PJ, Sijts AJ. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity. 2013;38:275–284. doi: 10.1016/j.immuni.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Volkmann L, Saeftel M, Bain O, Fischer K, Fleischer B, Hoerauf A. Interleukin-4 is essential for the control of microfilariae in murine infection with the filaria Litomosoides sigmodontis. Infect Immun. 2001;69:2950–2956. doi: 10.1128/IAI.69.5.2950-2956.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kreider T, Anthony RM, Urban JF, Jr, Gause WC. Alternatively activated macrophages in helminth infections. Curr Opin Immunol. 2007;19:448–453. doi: 10.1016/j.coi.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Babu S, Kumaraswami V, Nutman TB. Alternatively activated and immunoregulatory monocytes in human filarial infections. J Infect Dis. 2009;199:1827–1837. doi: 10.1086/599090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rihet P, Demeure CE, Bourgois A, Prata A, Dessein AJ. Evidence for an association between human resistance to Schistosoma mansoni and high anti-larval IgE levels. Eur J Immunol. 1991;21:2679–2686. doi: 10.1002/eji.1830211106. [DOI] [PubMed] [Google Scholar]
- 49.Dunne DW, Butterworth AE, Fulford AJ, Kariuki HC, Langley JG, Ouma JH, Capron A, Pierce RJ, Sturrock RF. Immunity after treatment of human schistosomiasis: association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur J Immunol. 1992;22:1483–1494. doi: 10.1002/eji.1830220622. [DOI] [PubMed] [Google Scholar]
- 50.Fitzsimmons CM, Falcone FH, Dunne DW. Helminth Allergens, Parasite-Specific IgE, and Its Protective Role in Human Immunity. Front Immunol. 2014;5:61. doi: 10.3389/fimmu.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hagan P, Blumenthal UJ, Dunn D, Simpson AJ, Wilkins HA. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature. 1991;349:243–245. doi: 10.1038/349243a0. [DOI] [PubMed] [Google Scholar]
- 52.Dunne DW, Butterworth AE, Fulford AJ, Ouma JH, Sturrock RF. Human IgE responses to Schistosoma mansoni and resistance to reinfection. Mem Inst Oswaldo Cruz. 1992;87(Suppl 4):99–103. doi: 10.1590/s0074-02761992000800014. [DOI] [PubMed] [Google Scholar]
- 53.Dunne DW, Webster M, Smith P, Langley JG, Richardson BA, Fulford AJ, Butterworth AE, Sturrock RF, Kariuki HC, Ouma JH. The isolation of a 22 kDa band after SDS-PAGE of Schistosoma mansoni adult worms and its use to demonstrate that IgE responses against the antigen(s) it contains are associated with human resistance to reinfection. Parasite Immunol. 1997;19:79–89. doi: 10.1046/j.1365-3024.1997.d01-186.x. [DOI] [PubMed] [Google Scholar]
- 54.Jiz M, Friedman JF, Leenstra T, Jarilla B, Pablo A, Langdon G, Pond-Tor S, Wu HW, Manalo D, Olveda R, Acosta L, Kurtis JD. Immunoglobulin E (IgE) responses to paramyosin predict resistance to reinfection with Schistosoma japonicum and are attenuated by IgG4. Infect Immun. 2009;77:2051–2058. doi: 10.1128/IAI.00012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fitzsimmons CM, Jones FM, Pinot de Moira A, Protasio AV, Khalife J, Dickinson HA, Tukahebwa EM, Dunne DW. Progressive cross-reactivity in IgE responses: an explanation for the slow development of human immunity to schistosomiasis? Infect Immun. 2012;80:4264–4270. doi: 10.1128/IAI.00641-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pritchard DI, Quinnell RJ, Walsh EA. Immunity in humans to Necator americanus: IgE, parasite weight and fecundity. Parasite Immunol. 1995;17:71–75. doi: 10.1111/j.1365-3024.1995.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 57.Bethony J, Loukas A, Smout M, Brooker S, Mendez S, Plieskatt J, Goud G, Bottazzi ME, Zhan B, Wang Y, Williamson A, Lustigman S, Correa-Oliveira R, Xiao S, Hotez PJ. Antibodies against a secreted protein from hookworm larvae reduce the intensity of hookworm infection in humans and vaccinated laboratory animals. FASEB J. 2005;19:1743–1745. doi: 10.1096/fj.05-3936fje. [DOI] [PubMed] [Google Scholar]
- 58.Faulkner H, Turner J, Kamgno J, Pion SD, Boussinesq M, Bradley JE. Age- and infection intensity-dependent cytokine and antibody production in human trichuriasis: the importance of IgE. J Infect Dis. 2002;185:665–672. doi: 10.1086/339005. [DOI] [PubMed] [Google Scholar]
- 59.McSharry C, Xia Y, Holland CV, Kennedy MW. Natural immunity to Ascaris lumbricoides associated with immunoglobulin E antibody to ABA-1 allergen and inflammation indicators in children. Infect Immun. 1999;67:484–489. doi: 10.1128/iai.67.2.484-489.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turner JD, Faulkner H, Kamgno J, Kennedy MW, Behnke J, Boussinesq M, Bradley JE. Allergen-specific IgE and IgG4 are markers of resistance and susceptibility in a human intestinal nematode infection. Microbes Infect. 2005;7:990–996. doi: 10.1016/j.micinf.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 61.Specht S, Saeftel M, Arndt M, Endl E, Dubben B, Lee NA, Lee JJ, Hoerauf A. Lack of eosinophil peroxidase or major basic protein impairs defense against murine filarial infection. Infect Immun. 2006;74:5236–5243. doi: 10.1128/IAI.00329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kazura JW, Aikawa M. Host defense mechanisms against Trichinella spiralis infection in the mouse: eosinophil-mediated destruction of newborn larvae in vitro. J Immunol. 1980;124:355–361. [PubMed] [Google Scholar]
- 63.Lee TD. Helminthotoxic responses of intestinal eosinophils to Trichinella spiralis newborn larvae. Infect Immun. 1991;59:4405–4411. doi: 10.1128/iai.59.12.4405-4411.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rainbird MA, Macmillan D, Meeusen EN. Eosinophil-mediated killing of Haemonchus contortus larvae: effect of eosinophil activation and role of antibody, complement and interleukin-5. Parasite Immunol. 1998;20:93–103. doi: 10.1046/j.1365-3024.1998.00132.x. [DOI] [PubMed] [Google Scholar]
- 65.Venturiello SM, Giambartolomei GH, Costantino SN. Immune cytotoxic activity of human eosinophils against Trichinella spiralis newborn larvae. Parasite Immunol. 1995;17:555–559. doi: 10.1111/j.1365-3024.1995.tb00998.x. [DOI] [PubMed] [Google Scholar]
- 66.Galioto AM, Hess JA, Nolan TJ, Schad GA, Lee JJ, Abraham D. Role of eosinophils and neutrophils in innate and adaptive protective immunity to larval strongyloides stercoralis in mice. Infect Immun. 2006;74:5730–5738. doi: 10.1128/IAI.01958-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ottesen EA, Nutman TB. Tropical pulmonary eosinophilia. Annu Rev Med. 1992;43:417–424. doi: 10.1146/annurev.me.43.020192.002221. [DOI] [PubMed] [Google Scholar]
- 68.O'Bryan L, Pinkston P, Kumaraswami V, Vijayan V, Yenokida G, Rosenberg HF, Crystal R, Ottesen EA, Nutman TB. Localized eosinophil degranulation mediates disease in tropical pulmonary eosinophilia. Infect Immun. 2003;71:1337–1342. doi: 10.1128/IAI.71.3.1337-1342.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nutman TB. Evaluation and differential diagnosis of marked, persistent eosinophilia. Immunol Allergy Clin North Am. 2007;27:529–549. doi: 10.1016/j.iac.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lobos E, Nutman TB, Hothersall JS, Moncada S. Elevated immunoglobulin E against recombinant Brugia malayi gamma-glutamyl transpeptidase in patients with bancroftian filariasis: association with tropical pulmonary eosinophilia or putative immunity. Infect Immun. 2003;71:747–753. doi: 10.1128/IAI.71.2.747-753.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nutman TB, Vijayan VK, Pinkston P, Kumaraswami V, Steel C, Crystal RG, Ottesen EA. Tropical pulmonary eosinophilia: analysis of antifilarial antibody localized to the lung. J Infect Dis. 1989;160:1042–1050. doi: 10.1093/infdis/160.6.1042. [DOI] [PubMed] [Google Scholar]
- 72.Marshall BG, Wilkinson RJ, Davidson RN. Pathogenesis of tropical pulmonary eosinophilia: parasitic alveolitis and parallels with asthma. Respir Med. 1998;92:1–3. doi: 10.1016/s0954-6111(98)90022-1. [DOI] [PubMed] [Google Scholar]
- 73.Rubio de Kromer MT, Medina-De la Garza CE, Brattig NW. Differences in eosinophil and neutrophil chemotactic responses in sowda and generalized form of onchocerciasis. Acta Trop. 1995;60:21–33. doi: 10.1016/0001-706x(95)00099-z. [DOI] [PubMed] [Google Scholar]
- 74.Hoerauf A, Kruse S, Brattig NW, Heinzmann A, Mueller-Myhsok B, Deichmann KA. The variant Arg110Gln of human IL-13 is associated with an immunologically hyper-reactive form of onchocerciasis (sowda) Microbes Infect. 2002;4:37–42. doi: 10.1016/s1286-4579(01)01507-6. [DOI] [PubMed] [Google Scholar]
- 75.Katawa G, Layland LE, Debrah AY, von Horn C, Batsa L, Kwarteng A, Arriens S, D WT, Specht S, Hoerauf A, Adjobimey T. Hyperreactive onchocerciasis is characterized by a combination of Th17-Th2 immune responses and reduced regulatory T cells. PLoS Negl Trop Dis. 2015;9:e3414. doi: 10.1371/journal.pntd.0003414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: implications for parasite persistence. J Immunol. 2006;176:3248–3256. doi: 10.4049/jimmunol.176.5.3248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.