Abstract
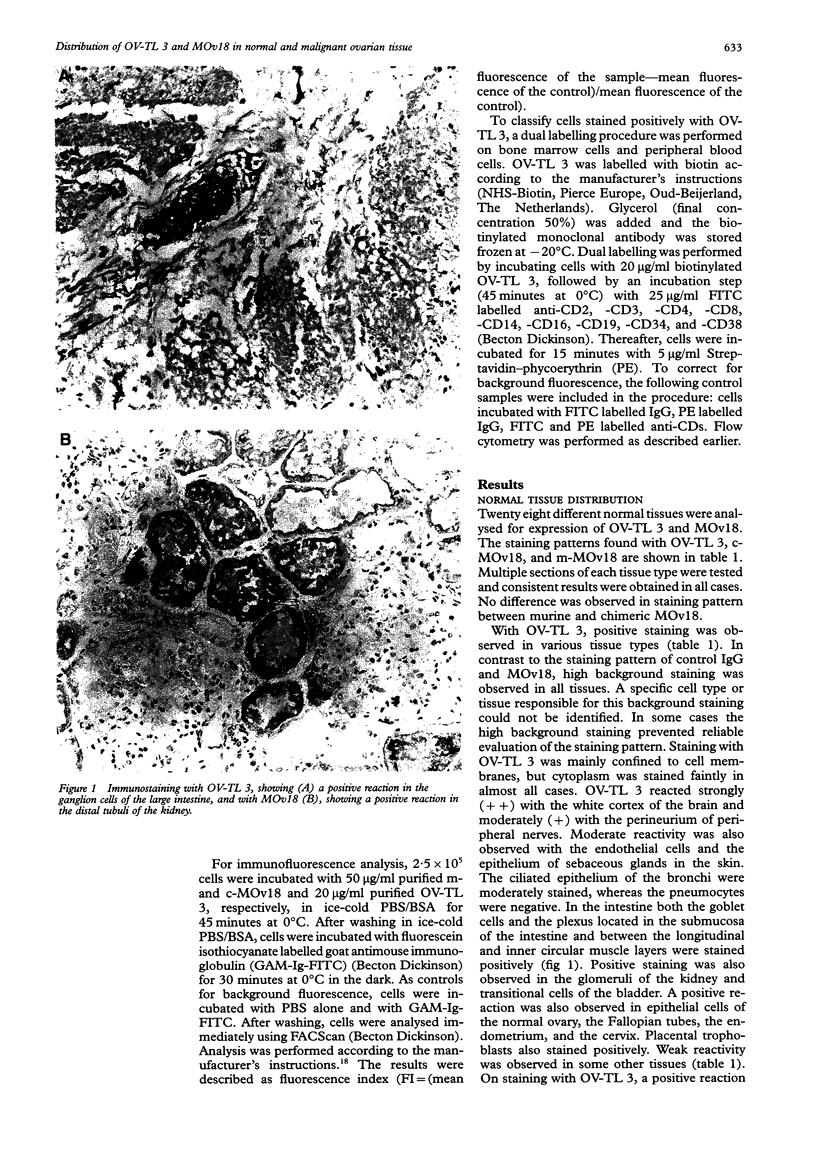
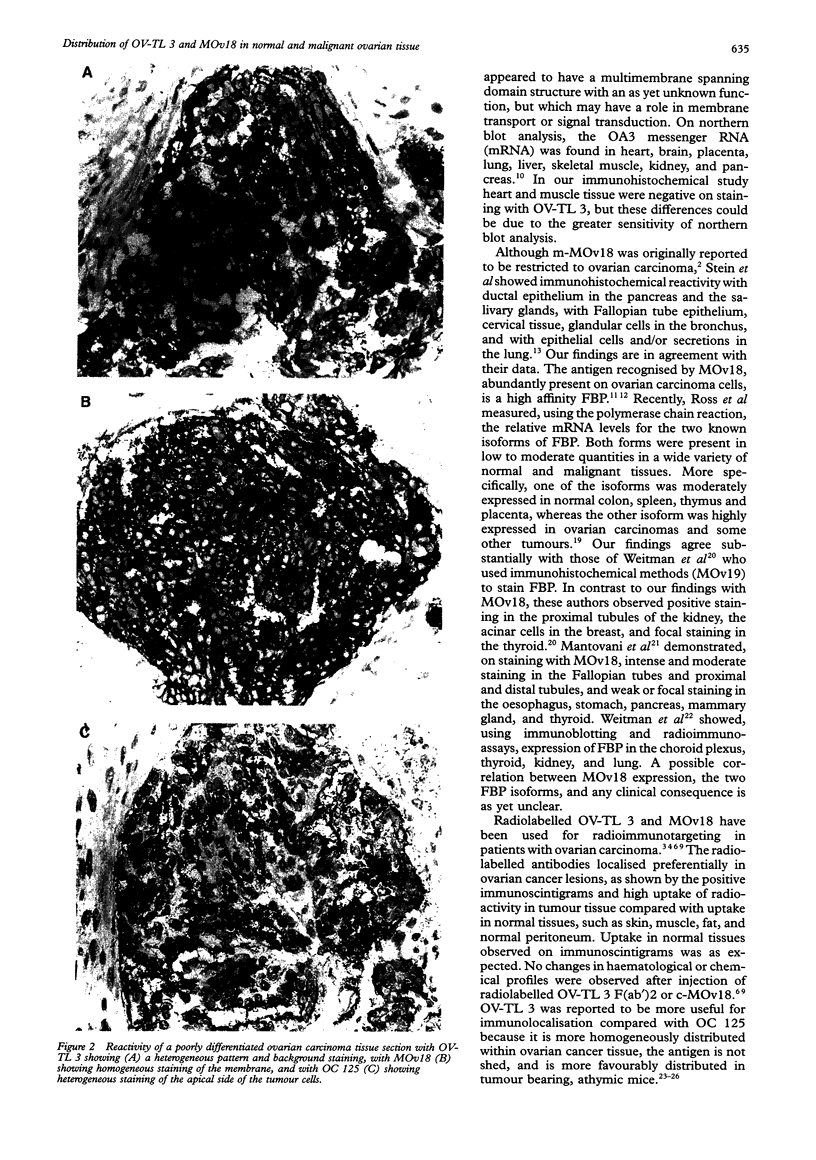
AIMS--To analyse the distribution of OV-TL 3 and MOv18 in normal ovarian tissue to determine which antibody is most suitable for (radio)immunotherapy of ovarian carcinoma. METHODS--The distribution of OV-TL 3 and MOv18 was determined using immunohistochemistry and flow cytometry. RESULTS--Epithelial and other cells in many tissues, and leucocytes in peripheral blood, bone marrow and spleen stained positively with OV-TL 3. The staining pattern of MOv18 in normal tissues was more restricted and was confined to epithelial cells in the lung, kidney, pancreas, salivary gland, ovary, Fallopian tubes, and cervix. Reactivity was also observed with pneumocytes in the lung, tubuli in the kidney, acinar cells in the salivary gland and pancreas, in the placenta, and with Kupffer cells in the liver. The staining pattern of chimeric MOv18 was identical with the murine form. OV-TL 3 and MOv18 reacted with 100% and 98% (45/46) of the 46 tested epithelial ovarian cancers, respectively. In ovarian carcinoma tissue homogeneous staining of epithelial cells was observed with OV-TL 3 and more heterogeneous staining with MOv18. In 12 and nine patients, respectively, a difference in staining intensity for OV-TL 3 and MOv18 was observed between various tumour samples from the same patient. CONCLUSION--MOv18 has greater therapeutic potential because of its restricted reactivity with normal tissues and especially, in contrast to OV-TL 3, its lack of reactivity with haematopoietic cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bast R. C., Jr, Klug T. L., St John E., Jenison E., Niloff J. M., Lazarus H., Berkowitz R. S., Leavitt T., Griffiths C. T., Parker L. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983 Oct 13;309(15):883–887. doi: 10.1056/NEJM198310133091503. [DOI] [PubMed] [Google Scholar]
- Berchuck A., Olt G. J., Soisson A. P., Kamel A., Soper J. T., Boyer C. M., Clarke-Pearson D. L., Leslie D. S., Bast R. C., Jr Heterogeneity of antigen expression in advanced epithelial ovarian cancer. Am J Obstet Gynecol. 1990 Apr;162(4):883–888. doi: 10.1016/0002-9378(90)91288-n. [DOI] [PubMed] [Google Scholar]
- Boerman O., Massuger L., Makkink K., Thomas C., Kenemans P., Poels L. Comparative in vitro binding characteristics and biodistribution in tumor-bearing athymic mice of anti-ovarian carcinoma monoclonal antibodies. Anticancer Res. 1990 Sep-Oct;10(5A):1289–1295. [PubMed] [Google Scholar]
- Buist M. R., Kenemans P., den Hollander W., Vermorken J. B., Molthoff C. J., Burger C. W., Helmerhorst T. J., Baak J. P., Roos J. C. Kinetics and tissue distribution of the radiolabeled chimeric monoclonal antibody MOv18 IgG and F(ab')2 fragments in ovarian carcinoma patients. Cancer Res. 1993 Nov 15;53(22):5413–5418. [PubMed] [Google Scholar]
- Buist M.R., Kenemans P., Vermorken J.B., Golding R.P., Burger C.W., Den Hollander W., Van Kamp G.J., Van Lingen A., Teule G.J.J., Baak J.P.A. Radioimmunotargeting in ovarian carcinoma patients with indium-111 labeled monoclonal antibody OV-TL 3 F(ab')2: pharmacokinetics, tissue distribution, and tumor imaging. Int J Gynecol Cancer. 1992 Jan;2(1):23–34. doi: 10.1046/j.1525-1438.1992.02010023.x. [DOI] [PubMed] [Google Scholar]
- Campbell I. G., Freemont P. S., Foulkes W., Trowsdale J. An ovarian tumor marker with homology to vaccinia virus contains an IgV-like region and multiple transmembrane domains. Cancer Res. 1992 Oct 1;52(19):5416–5420. [PubMed] [Google Scholar]
- Campbell I. G., Jones T. A., Foulkes W. D., Trowsdale J. Folate-binding protein is a marker for ovarian cancer. Cancer Res. 1991 Oct 1;51(19):5329–5338. [PubMed] [Google Scholar]
- Coney L. R., Mezzanzanica D., Sanborn D., Casalini P., Colnaghi M. I., Zurawski V. R., Jr Chimeric murine-human antibodies directed against folate binding receptor are efficient mediators of ovarian carcinoma cell killing. Cancer Res. 1994 May 1;54(9):2448–2455. [PubMed] [Google Scholar]
- Coney L. R., Tomassetti A., Carayannopoulos L., Frasca V., Kamen B. A., Colnaghi M. I., Zurawski V. R., Jr Cloning of a tumor-associated antigen: MOv18 and MOv19 antibodies recognize a folate-binding protein. Cancer Res. 1991 Nov 15;51(22):6125–6132. [PubMed] [Google Scholar]
- Haisma H. J., Battaile A., Stradtman E. W., Knapp R. C., Zurawski V. R., Jr Antibody-antigen complex formation following injection of OC125 monoclonal antibody in patients with ovarian cancer. Int J Cancer. 1987 Dec 15;40(6):758–762. doi: 10.1002/ijc.2910400608. [DOI] [PubMed] [Google Scholar]
- Henzen-Logmans S. C., Schipper N. W., Poels L. G., Stolk K., Kenemans P., Meyer C. J. Use of statistical evaluation of antigen profiles in differential diagnosis between colonic and ovarian adenocarcinomas. J Clin Pathol. 1988 Jun;41(6):644–649. doi: 10.1136/jcp.41.6.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenemans P. CA 125 and OA 3 as target antigens for immunodiagnosis and immunotherapy in ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 1990 Sep;36(3):221–228. doi: 10.1016/0028-2243(90)90201-b. [DOI] [PubMed] [Google Scholar]
- Kühnel R., Rao B. R., Poels L. G., Delemarre J. F., Kenemans P., Stolk J. G. Multiple parameter analyses of human ovarian cancer: morphology, immunohistochemistry, steroid hormone receptors and aromatase. Anticancer Res. 1988 Mar-Apr;8(2):281–286. [PubMed] [Google Scholar]
- Mantovani L. T., Miotti S., Ménard S., Canevari S., Raspagliesi F., Bottini C., Bottero F., Colnaghi M. I. Folate binding protein distribution in normal tissues and biological fluids from ovarian carcinoma patients as detected by the monoclonal antibodies MOv18 and MOv19. Eur J Cancer. 1994;30A(3):363–369. doi: 10.1016/0959-8049(94)90257-7. [DOI] [PubMed] [Google Scholar]
- Massuger L. F., Kenemans P., Claessens R. A., Verheijen R. H., Schijf C. P., Strijk S. P., Poels L. G., van Hoesel R. G., Corstens F. H. Immunoscintigraphy of ovarian cancer with indium-111-labeled OV-TL 3 F(ab')2 monoclonal antibody. J Nucl Med. 1990 Nov;31(11):1802–1810. [PubMed] [Google Scholar]
- Molthoff C. F., Buist M. R., Kenemans P., Pinedo H. M., Boven E. Experimental and clinical analysis of the characteristics of a chimeric monoclonal antibody, MOv18, reactive with an ovarian cancer-associated antigen. J Nucl Med. 1992 Nov;33(11):2000–2005. [PubMed] [Google Scholar]
- Molthoff C. F., Pinedo H. M., Schlüper H. M., Nijman H. W., Boven E. Comparison of the pharmacokinetics, biodistribution and dosimetry of monoclonal antibodies OC125, OV-TL 3, and 139H2 as IgG and F(ab')2 fragments in experimental ovarian cancer. Br J Cancer. 1992 May;65(5):677–683. doi: 10.1038/bjc.1992.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poels L. G., Peters D., van Megen Y., Vooijs G. P., Verheyen R. N., Willemen A., van Niekerk C. C., Jap P. H., Mungyer G., Kenemans P. Monoclonal antibody against human ovarian tumor-associated antigens. J Natl Cancer Inst. 1986 May;76(5):781–791. [PubMed] [Google Scholar]
- Riethmüller G., Schneider-Gädicke E., Schlimok G., Schmiegel W., Raab R., Höffken K., Gruber R., Pichlmaier H., Hirche H., Pichlmayr R. Randomised trial of monoclonal antibody for adjuvant therapy of resected Dukes' C colorectal carcinoma. German Cancer Aid 17-1A Study Group. Lancet. 1994 May 14;343(8907):1177–1183. doi: 10.1016/s0140-6736(94)92398-1. [DOI] [PubMed] [Google Scholar]
- Ross J. F., Chaudhuri P. K., Ratnam M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Physiologic and clinical implications. Cancer. 1994 May 1;73(9):2432–2443. doi: 10.1002/1097-0142(19940501)73:9<2432::aid-cncr2820730929>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Weitman S. D., Lark R. H., Coney L. R., Fort D. W., Frasca V., Zurawski V. R., Jr, Kamen B. A. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992 Jun 15;52(12):3396–3401. [PubMed] [Google Scholar]
- Weitman S. D., Weinberg A. G., Coney L. R., Zurawski V. R., Jennings D. S., Kamen B. A. Cellular localization of the folate receptor: potential role in drug toxicity and folate homeostasis. Cancer Res. 1992 Dec 1;52(23):6708–6711. [PubMed] [Google Scholar]
- van Dam P. A., Watson J. V., Lowe D. G., Cox H., Curling M., Shepherd J. H. Tissue preparation for simultaneous flow cytometric quantitation of tumour associated antigens and DNA in solid tumours. J Clin Pathol. 1990 Oct;43(10):833–839. doi: 10.1136/jcp.43.10.833. [DOI] [PMC free article] [PubMed] [Google Scholar]