Abstract
The melanocortin peptides derived from pro-opiomelanocortin (POMC) were originally understood in terms of the biological actions of α-melanocyte-stimulating hormone (α-MSH) on pigmentation and adrenocorticotrophic hormone on adrenocortical glucocorticoid production. However, the discovery of POMC mRNA and melanocortin peptides in the CNS generated activities directed at understanding the direct biological actions of melanocortins in the brain. Ultimately, discovery of unique melanocortin receptors expressed in the CNS, the melanocortin-3 (MC3R) and melanocortin-4 (MC4R) receptors, led to the development of pharmacological tools and genetic models leading to the demonstration that the central melanocortin system plays a critical role in the regulation of energy homeostasis. Indeed, mutations in MC4R are now known to be the most common cause of early onset syndromic obesity, accounting for 2–5% of all cases. This review discusses the history of these discoveries, as well as the latest work attempting to understand the molecular and cellular basis of regulation of feeding and energy homeostasis by the predominant melanocortin peptide in the CNS, α-MSH.
Keywords: α-MSH, POMC, melanocortin, MC4R, food intake
Cloning of the melanocortin receptors
The cloning of the melanocortin receptors (MCR) in 1992 significantly advanced our understanding of the physiological roles and sites of action of α-melanocyte-stimulating hormone (α-MSH) (Mountjoy et al. 1992). The first two receptors reported corresponded to the previously characterized melanocyte-stimulating hormone receptor (MSHR or MC1R) and adrenocorticotrophic hormone receptor (ACTHR or MC2R). Ultimately, five MCR were cloned, and referred to as MC1R–MC5R. The latter three had no known physiological roles at the time, and therefore were referred to as melanocortin-3 (MC3R), melanocortin-4 (MC4R), and melanocortin-5 (MC5R), respectively.
MCRs are members of the rhodopsin-like, class A branch of the seven transmembrane-spanning domain G protein coupled receptor (GPCR) superfamily. They couple to, and cause dissociation of the heterotrimeric G protein complex. The Gα subunit types activated by ligand-bound MCRs are Gαs, Gαq, and Gα11. MC3R–MC5R have relatively short N- and C-termini, and intracellular and extracellular loops, placing them among the shortest GPCRs.
All MCRs except for MC2R, bind melanocortin peptides containing the conserved heptapeptide core ‘MEHFRWG’, found in α-MSH, while the ACTHR further requires a peptide motif C-terminal to the 13 amino acids found in α-MSH (Gantz et al. 1993a). Each MCR mediates diverse physiological responses to α-MSH, or ACTH, in the case of MC2R. The MC1R is expressed in skin melanocytes and hair follicles and regulate pigmentation. α-MSH binds to this receptor stimulating the synthesis of eumelanin (brown-black pigments). Agouti (agouti signaling protein) binds to the receptor, competitively inhibiting the binding of α-MSH, to stimulate the synthesis of pheomelanin (yellow-red pigments). MC2R binds α-MSH with lower affinity than other members of the family, but instead, is activated specifically by ACTH and it is exclusively expressed in the adrenal cortex. Activation of this receptor regulates cell proliferation and production of glucocorticoids in the adrenal cortex.
The MC3R is expressed mainly in the brain, including high expression in the arcuate nucleus (ARC), ventromedial nucleus of the hypothalamus (VMH), ventral tegmental area (VTA), central linear nucleus of raphe, with moderate expression in anteroventral preoptic nucleus, lateral hypothalamic area, posterior hypothalamic area, medial habenular nucleus, and paraventricular nucleus of the hypothalamus (PVH) (Roselli-Rehfuss et al. 1993, Gantz et al. 1993a). Disruption of this gene in knockout mouse models generate a nonhyperphagic increase in adiposity that is associated with reduced lean body mass and increased feed efficiency (Butler et al. 2000, Chen et al. 2000). The MC4R is widely expressed throughout the CNS (Mountjoy et al. 1994) as well as peripheral nervous system (Gautron et al. 2010), and in intestinal L cells (Panaro et al. 2014). MC4R functions to regulate food intake and energy expenditure, and this role for the receptor has been shown to be evolutionarily conserved in vertebrates from fish to human. MC4R knockout mice as well as human mutants present early onset severe obesity associated with increased fat and lean mass (Huszar et al. 1997, Yeo et al. 1998). Additionally, MC4R regulates insulin secretion, lipid metabolism, bone mineral density, and body length. MC5R appears to be expressed primarily in exocrine glands. MC5R knockout mice are defective in secretion of multiple exocrine gland products and lack pheromone-induced aggression behaviors (Chen et al. 1997, Morgan & Cone 2006).
As the effects of α-MSH on food intake are the focus of this review, we will center our discussion on the physiology, pharmacology, and neuroanatomy of pro-opiomelanocortin (POMC) and agouti-related peptide (AgRP), and their cognate receptors in the CNS, MC4R, and MC3R.
Cloning the MC4R
Historically, the earliest physiological evidence of effects of melanocortin peptides originates before cloning of MC4R, with reports that intracerebroventricular (ICV) injection of ACTH and α-MSH inhibited the feeding drive induced by i.p. injection of a κ-opiate receptor agonist in rats (Poggioli et al. 1986, Vergoni et al. 1986). Stimulation of food intake by α-MSH had also been reported (Shimizu et al. 1989), and thus the characterization of receptors for α-MSH in the brain was ultimately needed to clarify these conflicting findings.
Following the cloning of the MC1R and MC2R, three orphan MCRs were soon cloned as well. Two independent laboratories in 1993 cloned and mapped the human MC4R using homology-based cloning (Gantz et al. 1993b, Mountjoy et al. 1994). This gene, identified on chromosome 18 (q21.3) in humans, consisted of one large exon with an open reading frame of 1 kb encoding a protein of 332 amino acids. Based on sequence alignment analysis, the closest identified receptor was MC3R, with 58% homology (Gantz et al. 1993a, Magenis et al. 1994). MC4R couples to Gαs protein to activate adenylyl cyclase, resulting in elevation of intracellular cAMP. There is also evidence that this receptor can raise intracellular calcium levels through recruitment of Gαq and inositol trisphosphate production in heterologous overexpression systems (Konda et al. 1994, Mountjoy et al. 2001, Kim et al. 2002).
Discovery of the role of α-MSH in feeding behavior and energy homeostasis
When expression of MC4R was mapped in the CNS by in situ hybridization, the distribution suggested a role in neuroendocrine and autonomic control (Mountjoy et al. 1994). However, the first breakthrough in understanding the MC4R physiological function came from discoveries made in MC1R physiology and pharmacology (Lu et al. 1994).
Agouti, a 132-amino acid protein that is produced in the hair follicle, was demonstrated to be a high-affinity ligand of MC1R, competitively blocking α-MSH binding and inhibiting cAMP production (Lu et al. 1994). This finding correlated with observations in vivo that agouti blocked eumelanin production. Strikingly, agouti was also found to be a high-affinity competitive antagonist of α-MSH action at MC4R, but not other MCRs (Lu et al. 1994).
As agouti gene mutations were found to result from gene rearrangements that produced ectopic expression of agouti (Yen et al. 1994), it was inferred that the inhibition of MCRs in the brain by agouti underlie the obesity and metabolic syndrome observed in the yellow (Ay) mouse (Lu et al. 1994). The development of the first MC4R antagonist (Li et al. 1996), and the creation of two different genetic mouse models would ultimately confirm this hypothesis (Fan et al. 1997).
In 1997, several studies were published providing direct evidence supporting this hypothesis and establishing a central role of MC4R signaling in regulation of energy homeostasis. ICV injection of melanotan II (MTII), a cyclic analog of α-MSH, was shown to suppress food intake in four different mouse models: fasted C57BL/6J, ob/ob, Ay, and mice injected with neuropeptide Y (NPY). Conversely, this inhibition was blocked by coinjection of SHU9119, a cyclic peptide antagonist of MC3R and MC4R. Furthermore, ICV injection of SHU9119 alone increased food intake in mice (Hruby et al. 1995, Fan et al. 1997). These findings supported the hypothesis that hypothalamic POMC expressing neurons releasing α-MSH tonically to inhibit feeding via activation of MC4R target neurons, and chronic blockade of this signaling pathway by agouti is ultimately responsible for the obesity phenotype observed in Ay yellow mice (Fan et al. 1997).
Animal models manipulating the melanocortin system have been pivotal to further advancing our understanding of the role of α-MSH in regulation of feeding and energy homeostasis (Table 1). In 1997, the first animal model testing the role of α-MSH signaling in the brain was created. By targeting the Mc4r gene in embryonic stem cells, MC4R knockout mice were made and used to test the hypothesis that deletion of this receptor would recapitulate the agouti obesity syndrome (Huszar et al. 1997). Mice lacking both alleles displayed early onset obesity, hyperphagia, increased linear growth, hyperinsulinemia, and hyperglycemia. Loss of a single allele resulted in intermediate phenotypes compared with wildtype and homozygous siblings, indicating involvement of a gene–dosage effect (Huszar et al. 1997). These findings support a model in which the primary mechanism by which agouti induces obesity is by chronic antagonism of MC4R, establishing this receptor as central regulator of energy balance.
Table 1.
Mouse models used to study α-MSH signaling in obesity.
| Yellow lethal (Ay) and related alleles (Aiy, Asy, Avy, Aiapy) | Spontaneous mutation resulting in agouti (Asip) ectopic expression, homozygous lethal; unrelated to agouti ectopic expression, moderate hyperphagia, hyperinsulinemia (2–5 × normal), late-onset hyperglycemia, lean body mass, ↓ energy expenditure, ↑ sensitivity to stressors, milder obesity syndrome than ob/ob or db/db, yellow fur color | Dickie (1962, 1969), Frigeri et al. (1983), Bultman et al. (1992), Michaud et al. (1993, 1994a,b),Miller et al. (1993), Klebig et al. (1995) |
| AgRP transgene | Expression of the AgRP under the control of α-actin promoter, same metabolic phenotype as Avy, same fur color as wildtype littermates | Graham et al. (1997) |
| AgRPDTR/DTR | Cross of mice harboring a loxP flanked diphtheria toxin receptor with animals with Cre recombinase under control of AgRP promoter Ablation of AgRP/NPY neurons of the arcuate nucleus of the hypothalamus, ↓↓ food intake, ↓ body weight, complete ablation results in starvation and death | Gropp et al. (2005), Luquet et al. (2005), Wu et al. (2008a,b) |
| POMC−/− | Hyperphagic, ↓ energy expenditure, adrenal insufficiency, develop obesity exacerbated with high-fat diet, lighter than littermates-colored dorsal and yellow ventral fur | Yaswen et al. (1999), Challis et al. (2004) |
| POMC, AgRP−/− | Hyperphagic, ↓ energy expenditure, α-MSH but not AgRP administration rescues wildtype phenotype, lighter than littermates-colored dorsal and yellow ventral fur similar to POMC−/− | Corander et al. (2011) |
| MC4R−/− | Hyperphagic, less obese than ob/ob or db/db, early onset obesity (5–7 weeks), ↓ energy expenditure, dosage effect with, +/− animals have intermediate phenotype, increased linear growth, same fur color as wildtype littermates | Huszar et al. (1997) |
| Targeted deletion of MC4R at the PVH | Vglut2-ires-Cre;Mc4rlox/lox, targeted deletion of MC4R in glutamatergic neurons: same phenotype as MC4R null mice including ↑ hyperphagia, ↑ body weight, and ↑ linear growth Sim1-Cre;Mc4rlox/lox: targeted deletion of MC4R in single-minded 1 positive (SIM1+) neurons: intermediate phenotype between MC4R−/− and Mc4rlox/lox control mice |
Balthasar et al. (2004), Shah et al. (2014) |
| MC3R−/− | Moderate obesity syndrome of late onset (26 weeks), ↑ body weight predominant in females, ↑ adiposity, ↑ energy efficiency, no change in lean mass, no change in linear growth, same fur color as wildtype littermates | Butler et al. (2000), Chen et al. (2000), Sutton et al. (2006) |
| MC3R, MC4R−/− | Augmented obesity syndrome compared with MC4R−/− or MC3R−/−; including ↓ energy expenditure, no change in food ingestion after MTII administration, same fur color as wildtype littermates | Chen et al. (2000) |
| Mahogany (Atrnmg) and related alleles (Atrnmg-3j, Atrnmg-L) | Autosomal recessive mutation reverting the phenotype of Ay including obesity albeit hyperphagia is present, Atrn encodes for attractin, a melanocortin receptor coreceptor.Atrnmg is a defective splice variant allele that results from a 5 kb retroviral insertion in introns 26 and 27 Fur color reflects eumelanin predominance with diminished pheomelanin, darkened ears, and tail (umbrous coat). Atmmg-3j; a null allele of Atrn, has darker fur |
Lane & Green (1960), Dinulescu et al. (1998), Gunn et al. (1999) |
| Mahogunin, ring finger 1 (Mgrn1md) | Mgrn1 encodes an E3 ubiquitin ligaseThe Mgrn1md allele displays a similar phenotype to Atrnmg and related alleles | Dinulescu et al. (1998), Phan et al. (2002) |
| Mrap2−/− | Melanocortin receptor accessory protein 2 (MRAP2) knockoutLate-onset ↑ hyperphagia, early onset ↑ body weight, ↑ white fat tissue depots ↓ lean mass, same fur color as wildtype littermates | Asai et al. (2013) |
Subsequently, two research groups conducted two independent cohort screenings of the MC4R gene in individuals with childhood obesity and nonobese controls. They identified frameshift mutations in the MC4R gene in children with early onset obesity (Vaisse et al. 1998, Yeo et al. 1998). The majority of MC4R-induced obese individuals were heterozygous comprising about 6% of obese children. As observed in knockout mice, human MC4R-induced phenotypes exhibited a gene–dosage effect, unusual for other GPCRs. This finding demonstrated the evolutionarily conserved role of MC4R in energy balance in humans, and generated great interest in α-MSH signaling in the CNS in general, and the MC4R as a target for the development of new antiobesity therapeutics.
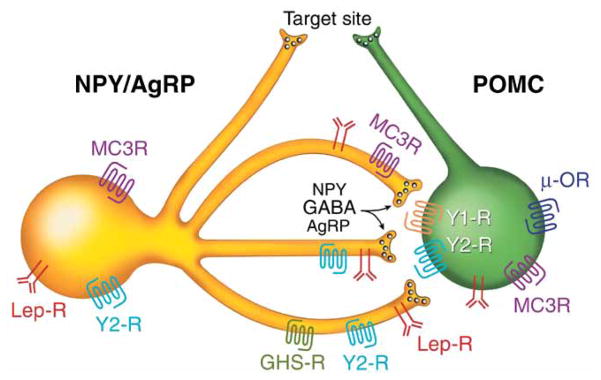
Another important event in 1997 was the discovery, characterization, and expression mapping of AgRP, an analog of agouti (Fong et al. 1997, Graham et al. 1997, Ollmann et al. 1997). AgRP mRNA is mainly expressed in the ARC of the hypothalamus and its levels are increased during fasting and in ob/ob mice, resulting in an increased drive to feed. AgRP binds with high affinity to MC3R and MC4R (Chai et al. 2003). Like agouti, it acts as competitive antagonist of α-MSH at these receptors, with low affinity for MC1R (Ollmann et al. 1997). A single ICV injection of AgRP increases food intake for up to a week, and coinjection of α-MSH does blunt the orexigenic effects of the former. Thus, AgRP functions as an inverse agonist of MC4R by decreasing cAMP levels produced by the constitutive activity of wildtype or mutant receptors (Haskell-Luevano et al. 2001, Nijenhuis et al. 2001, Chai et al. 2003). This finding substantiated the commonly held Yin–Yang view of feeding regulation, with POMC neurons and α-MSH inhibiting food intake and energy storage, and NPY/AgRP neurons stimulating it in part, through AgRP antagonism of MC4R signaling (Fig. 1).
Figure 1.
Yin–Yang model of control of feeding behavior and energy homeostasis. NPY/AgRP and POMC neurons within the arcuate nucleus form a coordinately regulated network due to dense NPY/ AgRP fibers that project to POMC cell bodies. Some of the receptors for a large number of hormones and neuropeptides known to regulate the network are indicated. These fibers project to many of the same nuclei, where dual release of α-MSH and AgRP were proposed to compete for MC4R binding, to coordinately regulate food intake and energy homeostasis. AgRP, agouti-related peptide; GABA, γ-aminobutyric acid; GHS, growth-hormone secretagogue receptor; Lep, leptin; MC3R, melanocortin 3 receptor; NPY, neuropeptide Y; μ-OR, μ-opiate receptor; R, receptor; GLP-1, glucagon-like peptide 1. Modified, with permission, from Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD & Low MJ (2001) Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411 480–484.
In agreement, transgenic mice ubiquitously overexpressing human AgRP exhibit obesity but not yellow fur, suggesting that AgRP, unlike agouti protein, is MC3R/MC4R specific, and unable to promote pheomelanin in hair follicle melanocytes (Graham et al. 1997, Ollmann et al. 1997). As MC4R knockout mice exhibit significant obesity, some expected that AgRP knockout mice might exhibit leanness. However, at first glance, these mice exhibit normal food intake, body composition, growth rates, and responses to starvation (Qian et al. 2002). Subsequent work demonstrated that homozygous AgRP knockout mice do exhibit a very modest reduction in body weight at 6 months of age and adiposity with increased metabolic rate, body temperature, and locomotor activity (Wortley et al. 2005). These mice also exhibit a blunted fast-induced refeeding response. It is now known that AgRP neurons are GABAergic, and of course express NPY as well, and all three agents regulate downstream MC4R neurons (Wu & Palmiter 2011). These findings underscore the importance of the AgRP neuronal system, as well as AgRP action on MC4R in the regulation of energy homeostasis.
Neuroanatomy of the central melanocortin system
POMC and AgRP neurons
To truly understand α-MSH action in the CNS, it is critical to recognize the neuroanatomical substrate underlying the central melanocortin system (Fig. 2). Neurons expressing MCR, and POMC and AgRP neurons collectively constitute the primary neural components of this system.
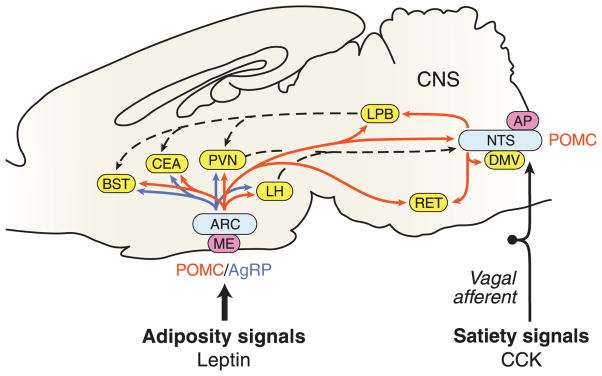
Figure 2.
A highly simplified schematic of the central melanocortin system. Receipt of long-term adipostatic signals and acute satiety signals by neurons in arcuate nucleus and brainstem, respectively. Light blue boxes indicate nuclei containing POMC neurons; yellow boxes indicate nuclei containing MC4R neurons that may serve to integrate adipostatic and satiety signals; and pink boxes show some circumventricular organs involved in energy homeostasis. Red arrows designate projections of POMC neurons; blue arrows show projections of agouti-related protein (AgRP neurons). AP, area postrema; ARC, arcuate nucleus; BST, bed nucleus of the stria terminalis; CCK, cholecystokinin; CEA, central nucleus of the amygdala; DMV, dorsal motor nucleus of the vagus; LH, lateral hypothalamic area; LPB, lateral parabrachial nucleus; ME, median eminence; NTS, nucleus tractus solitarius; PVH, paraventricular nucleus of the hypothalamus; RET, reticular formation. For simplicity, only a fraction of the >100 MC4R target sites are shown, and none of the MC3R target nuclei is indicated. Adapted, with permission, from Fan W, Boston BA, Kesterson RA, Hruby VJ & Cone RD (1997) Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385 165–168.
Within the CNS, AgRP neurons are restricted to the ARC of the hypothalamus (ARC). Most AgRP neurons (~90%) express another potently orexigenic peptide hormone, NPY. Unlike AgRP, NPY is one of the most abundant and widely expressed neuropeptides in the mammalian brain. Similarly, POMC neurons express another gene coding for an anorectic peptide called cocaine- and amphetamine-regulated transcript. NPY/AgRP and POMC neurons are chemically and anatomically distinct; however, approximately 25% of the NPY neurons are derived from the same lineage as POMC neurons during development (Padilla et al. 2010). In the rodent ARC, AgRP neurons are expressed homogenously throughout the rostrocaudal axis, while most POMC neurons are located in the anterior and medial ARC. In rat ARC, POMC neurons are more laterally distributed compared with the mouse (Cowley et al. 2001). Earlier studies quantifying POMC neurons by β-endorphin immunohistochemistry (Huo et al. 2006) or using mice expressing green fluorescent protein (GFP) under the POMC promoter (Cowley et al. 2001) have reported around 3000–3500 POMC neurons in rodent ARC. However, a recent report quantifying POMC neurons using an antibody specific to the POMC precursor, estimated around 9000 immunoreactive cells (Lemus et al. 2015). On the other hand, the number of NPY/AgRP neurons was estimated to be between 8000 and 10,000 (Betley et al. 2013, Lemus et al. 2015), analyzed using mice expressing GFP in an AgRP promoter-dependent manner.
Expression of POMC follows a dynamic pattern throughout gestation, and POMC-positive brain regions in the adult mice reveal that POMC expression in many regions in the developing embryo are transient. In adult rodents, POMC mRNA was detected by northern blot in hypothalamus, amygdala, and the cerebral cortex, but was not detectable in midbrain or cerebellar RNA preparations (Civelli et al. 1982). A 5′ truncated version of POMC mRNA lacking the signal sequence was also detected in amygdala, midbrain, and cortex as well as in several peripheral tissues. However, the role of this truncated version is unclear as it cannot produce active POMC-derived peptides (Clark et al. 1990). POMC was detected in the nucleus tractus solitarius (NTS) and lateral reticular formation of the rat brainstem initially by immunohistochemistry against ACTH (Joseph et al. 1983, Schwartzberg & Nakane 1983) and later by in situ hybridization (Bronstein et al. 1992). Most neurons identified as POMC positive by labeling strategies involving POMC-Cre-mediated recombination, which accounts for transient POMC expression that does not persist into adulthood, do not express POMC in the adult brain. In this context, mice expressing GFP under the POMC promoter yielded more satisfactory anatomical data as to ARC POMC neurons as there is almost 100% overlap of GFP and POMC expression in the ARC, but not in other brain regions (Pinto et al. 2004). More recent and comprehensive studies comparing POMC-Cre, POMC-GFP, and sensitive in situ hybridization techniques have concluded that POMC expression in the adult mouse brain is restricted to ARC and NTS (Padilla et al. 2012).
AgRP was identified by its homology to agouti and its expression is restricted to ARC and adrenal gland with very low expression in lung, kidney, testis, and ovaries (Ollmann et al. 1997, Shutter et al. 1997). AgRP and POMC neurons located in the ARC project to various intra- and extrahypothalamic brain regions, and many reciprocal connections are found. Within the hypothalamus, AgRP and POMC neurons send overlapping projections to many hypothalamic nuclei including PVH, lateral hypothalamus (LH), VMH, posterior hypothalamus, dorsomedial hypothalamus (DMH), and medial preoptic nucleus/area (Bagnol et al. 1999). AgRP neurons innervate extrahypothalamic sites such as the bed nucleus of the stria terminalis (BNST), and the lateral parabrachial nucleus (LPB), central nucleus of the amygdala (CEA), and periaqueductal gray (PAG) (Betley et al. 2013, Wang et al. 2015) outside the hypothalamus. ARC POMC neurons innervate many extrahypothalamic regions including BNST, lateral septum, nucleus accumbens, LPB, the periaqueductal gray, and the dorsal motor nucleus of the vagus (DMX). NTS POMC neurons innervate other neurons mostly within the brain stem (Wang et al. 2015). Collectively, POMC neurons appear to innervate multiple regions not receiving AgRP innervation, such as the DMX.
AgRP and POMC neurons receive inputs from other hypothalamic nuclei, predominantly from PVH, DMH, VMH, and LH (Wang et al. 2015). Extrahypothalamic brain regions innervating POMC and AgRP neurons include lateral septum and BNST. Neurons with cell bodies in the hippocampus, medial mammillary nucleus, and VTA appear to selectively innervate POMC neurons, but not AgRP neurons (Wang et al. 2015). NTS POMC neurons receive the majority of inputs from other neurons in the brainstem; however, neurons originating from paraventricular hypothalamic nucleus and amygdala (Wang et al. 2015) also innervate NTS POMC neurons.
The large degree of overlap in the regions innervated by both AgRP and POMC neurons also supports the dual regulation of central melanocortin signaling by α-MSH and AgRP (Fig. 1). Besides neuronal inputs, AgRP and POMC neurons are under direct regulation of hormonal and nutrient-related signals. Both neurons express leptin receptors (LepR), while only AgRP neurons express ghrelin receptors. A recent report suggested that the AgRP neurons that project within the hypothalamus do not express LepRb, which was not the case for POMC neurons (Betley et al. 2013). Around 30–40% of AgRP neurons respond to leptin with increased STAT3 phosphorylation (van de Wall et al. 2008). Virtually no POMC neurons in the NTS exhibit leptin-induced STAT3 phosphorylation or c-Fos expression induction (Huo et al. 2006). Nonetheless, about 60% of POMC neurons in the ARC are leptin responsive. In addition, fasting decreases POMC expression in both ARC and NTS, but only ARC POMC expression can be rescued by leptin (Huo et al. 2006, Perello et al. 2007). Deletion of LepRb from POMC or AgRP neurons results in obesity and hyperleptinemia (Balthasar et al. 2004, van de Wall et al. 2008) in both genders of mice, and the effect of deletion of LepRb from both neuronal populations is additive (van de Wall et al. 2008). Although these results should be evaluated taking into account the difficulties inherent to the developmental problems associated with the Cre lines used, they suggest that POMC and AgRP neurons mediate only part of leptin’s effects on energy homeostasis (Padilla et al. 2012).
Neuroanatomy of MC3R and MC4R neurons
Downstream of POMC and AgRP, MC3R/MC4R expressing target neurons serve as essential neural nodes for responding to integrated signals of energy status and relaying modulating signals throughout the CNS to maintain energy balance.
The MC3R was originally identified as the receptor more potently activated by the POMC-derived peptide γ-MSH (Roselli-Rehfuss et al. 1993), although the receptor also responds to α-MSH as well. In situ hybridization studies delineated the narrow distribution of MC3R within the adult CNS to approximately 30 mapped nuclei, where highest density is found in concentrated subregions of the hypothalamus including the VMH, ARC, anteroventral preoptic area, posterior hypothalamic area, and the medial preoptic area, the limbic system regions of nucleus accumbens (NuAcc) and VTA, and weak signals from a few brainstem nuclei (notably not the NTS) (Begriche et al. 2011, Mountjoy 2015). Double-labeled in situ hybridization validated before pharmacological studies by showing that a large component of ARC neurons expressing MC3R is also positive for AgRP or POMC mRNA (Bagnol et al. 1999). Deletion of the MC3R resulted in a mouse with increased adipose mass, decreased lean mass, and reduced bone density (Butler et al. 2000, Chen et al. 2000). Further physiological studies suggest MC3R regulates fast-induced refeeding (Renquist et al. 2012) and entrainment of anticipatory behavior to nutrient intake (Begriche et al. 2011). Recently conducted studies provided evidence for the functional expression of MC3R in the VTA and its role therein as a sexually dimorphic node for regulating the mesolimbic dopaminergic system and reward (Lippert et al. 2014). MC3R peripheral expression is detectable in the stomach, duodenum, kidneys, placenta, heart, monocytes, and macrophages; however, the function of the gene at these sites of action has not been well explored (Caruso et al. 2014). Nonetheless, it is apparent that one of the central MSH peptides likely mediates some effects on feeding and energy homeostasis via the MC3R.
The MC4R is more broadly distributed than MC3R, exhibiting expression in cellular nodes in every CNS region with a striking presence in the hypothalamus, NuAcc, and DMX. Mapping via in situ hybridization localized MC4R to over 100 distinct nuclei. Hypothalamic nodes of highest concentration include the suprachiasmatic preoptic nucleus, anteroventral periventricular nucleus, supraoptic nucleus, PVH, VMH, DMH, tuberomammillary nucleus, and the lateral hypothalamic area. Extensive brainstem labeling is found in the superior colliculus, DMX, substantia nigra, raphe, and reticular formation. Following these high expression zones, several regions of the amygdala and isocortex have moderate expression. MC4R may play a role in olfactory response due to its location in discrete cortical nodes. MC4R has also been identified in CA1 and CA2 regions of the hippocampus, throughout the BNST and striatum, and weakly in the thalamus (Mountjoy et al. 1992, 1994, Kishi et al. 2003, Cone 2005, Tao 2010, Cui et al. 2012, Siljee et al. 2013, Mountjoy 2015). Localization and distribution of MC4R were further confirmed by studies using a mouse model expressing GFP under control the MC4R promoter (Liu et al. 2003). Additional genetic and pharmacological modeling systems inducing or repressing MC4R gene function in specific neuronal populations have mapped some neuroanatomical functions of these cells bodies and begun to reveal how the MC4R serves to regulate energy homeostasis. For example, MC4R in the PVH is essential for regulating appetite, while MC4Rs expressed in cholinergic preganglionic parasympathetic neurons are necessary for regulating energy expenditure (Balthasar et al. 2005, Rossi et al. 2011). Outside of central neurons, MC4R mRNA expression has been detected in astrocytes, spinal cord, heart, lung, kidney, and testis (Caruso et al. 2014). One peripheral MC4R-mediated pathway was described after detection of high levels of MC4R expression in enteroendocrine L cells (Panaro et al. 2014). The data show the receptor mediates release of L cell products PYY and glucagon-like peptide 1 in either mouse or human, in response to exogenous administration of α-MSH, and of course these peptides have known secondary effects on feeding. The physiological role of MC4R at this site, and the origin of the ligand, likely an MSH peptide, remains unknown.
Pharmacological complexities of α-MSH signaling
The melanocortin signaling system regulates distinct physiological functions on a receptor-subtype specific basis, including energy homeostasis, coat color, hypothalamic–pituitary–adrenal axis, and exocrine gland function, as a result of the unique distributions of expression of each receptor (Vastermark & Schioth 2011, Cortes et al. 2014). In addition, an extended set of coreceptors, signaling partners, and alternate endogenous ligands enrich the tapestry of responses that result from α-MSH stimulation at each receptor (Fig. 3). This functional diversity played an important role in the discovery of many of the components of this system (Cone 2006).
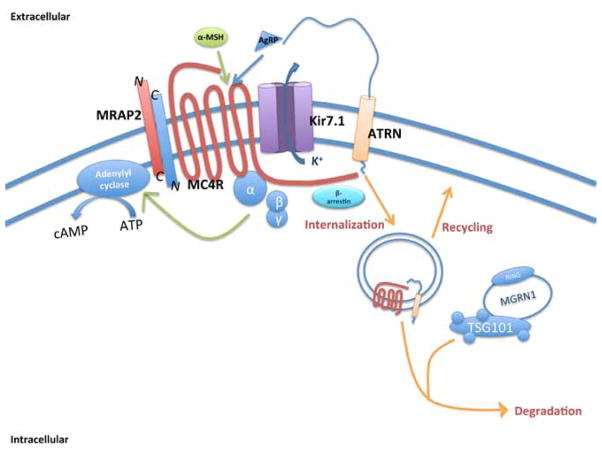
Figure 3.
The MC4R signaling complex. α-MSH and AgRP signaling at the MC4R involves a multiprotein signaling complex that appears to be highly regulated. In addition to interactions with well-characterized members of the G protein signaling cascade (α-, β, and γ subunits and β-arrestin), the MC4R appears to interact directly with the transmembrane protein MRAP2, which blocks constitutive activation of the receptor by the amino terminal domain, and enhances sensitivity to α-MSH action. The receptor also appears to interact with inward rectifier channels such as Kir7.1, with α-MSH and AgRP modulating channel activity in a receptor-dependent manner. Attractin and mahogunin are involved in receptor internalization, and attractin may also be involved in AgRP binding. For simplicity, syndecan and defensins are not indicated here.
An example of how variable signaling in the pigmentary system advanced research in the field of α-MSH signaling stemmed from studies on the determinants of fur coat coloration in mice and other mammals. As a natural outcome from the analysis of additional mouse coat color phenotypes (Silvers 1979, prepared a compilation of mouse fur color phenotypes also reviewed by Jackson et al. 1994), additional modifiers of MCR function were recognized. For example, mutations within the mahogany locus (Lane & Green 1960) rescued the agouti Ay dominant yellow fur phenotype and ameliorated the accompanying obesity syndrome (Dinulescu et al. 1998, Miller et al. 1997, He et al. 2001). Later analysis showed that the products of these genes regulate receptor membrane expression (Overton & Leibel 2011) as discussed below. An additional coat color-related allele was recognized in dogs (Kerns et al. 2003, 2004). Characterization of the product of this gene led to the identification of β-defensin 3, an additional endogenous ligand with agouti/AgRP-like activity on MC1R and MC4R (Candille et al. 2007). These and recently recognized coat color-unrelated partners for MCR that modulate agonist response or offer alternate signaling pathways are discussed in more detail in the following sections.
Mahogany, attractin-like protein, and mahoganoid
Attractin and mahogunin were originally identified as the suppressors of agouti action in mahogany and mahoganoid mice. Attractin is a type 1 transmembrane domain protein that is proposed to be necessary for the action of agouti at both the MC1R and MC4R. It is further suggested that attractin binds to the N-terminal domain of agouti and acts as coreceptor by assisting in the stability of the interaction between MC4R and the C-terminal domain of agouti (He et al. 2001). Attractin does not bind directly to MC4R or AgRP, and as such, it was thought to be unlikely that it was involved in the modulation of MC4R activity. However, loss-of-function mutations in attractin as well as mahoganoid (mahogunin ring finger-1) are able to rescue the obesity phenotype in Ay mice by blocking the agouti-dependent degradation of MC4R.
Mahoganoid is an E3 ubiquitin ligase that ubiquinates (Phan et al. 2002, He et al. 2003) tumor suppressor gene 101 (Tsg101), which is necessary for the trafficking of the ubiquitinated MC4R from the cell surface to the lysosome for degradation (Kim et al. 2007, Jiao et al. 2009). Attractin-like protein was found to be another binding partner of MC4R that is believed to play an important role in the receptor trafficking (Haqq et al. 2003). These modulators of MC4R activity impact the ability of α-MSH to regulate feeding and body weight.
Syndecans
An unrecognized fact of AgRP pharmacology is that most of the reported data are based on the use of the truncated 83–132 (human sequence) C-terminal peptide (Quillan et al. 1998, Nijenhuis et al. 2001). Studies with the full-length prohormone show lesser potency in quenching α-MSH-elicited cAMP responses (Creemers et al. 2006, Jackson et al. 2006). It is clear then, that any mechanism that acts on relative concentrations of truncated to full-length AgRP will have a discernible effect on α-MSH response and act as coreceptor as seen for the agouti–attractin duo. A provocative model suggests that the charged but amphipathic N-terminal domain of AgRP serves as ‘anchor’ to engage perisynaptic heparan sulfate proteoglycans.
One such heparan sulfate proteoglycan is the membrane-bound, predominantly brain-expressed syndecan-3 (Sarrazin et al. 2011). Syndecans are single-membrane spanning glycoproteins that associate three to five heparan sulfate polysaccharide chains (Bernfield et al. 1992). Also notable is that at the interface of the heparan sulfate-linked ecto- and the transmembrane domains, a juxtamembrane region that harbors protease cleavage sites is present. This region can potentially be targeted by metaloproteases and the ‘shedding’ of syndecan ectodomains by proteolytic cleavage adds an additional regulatory layer as a mechanism of clearance of bound ligands or paracrine ligand diffusion, as shed ligand-bound heparan sulfates retain activity in vivo (Elenius et al. 1992, Kim et al. 1994).
A link between the melanocortin system and heparan sulfate proteoglycans was first established by studying transgenic mice overexpressing syndecan-1, a predominantly peripherally expressed homolog, under the control of the CMV promoter (Reizes et al. 2001). These animals show late-onset obesity, which is additive to the phenotype of the yellow-lethal Ay mouse (Reizes et al. 2001). As the predominant form expressed in hypothalamus is syndecan-3, the latter is probably the form implicated in AgRP extracellular concentration regulation (Reizes et al. 2001). Further enforcing this hypothesis, fasting animals showed elevated membrane-bound syndecan-3, while refeeding induced proteolytic cleavage and syndecan-3 shedding (Reizes et al. 2001). Further evidence was obtained from the analysis of a syndecan-3 knockout mice, which was resistant to diet-induced obesity (Reizes et al. 2003, Strader et al. 2004, Zheng et al. 2010). Taken together, these results support a second-tier regulatory layer for AgRP signaling on MCR, where syndecan shedding might have a role.
Mechanistically, the model of syndecan–melanocortin interaction is contingent on the interaction of the AgRP N-terminus domain with the heparan sulfate moieties on syndecans (Reizes et al. 2003). For this to be possible, the full-length hormone, and not the truncated C-terminal domain forms, has to be the predominant species secreted. Creemers et al. (2006) raised the possibility that AgRP is intracellularly cleaved by proprotein convertases eliminating the anchoring N-terminal domain. If in fact, this is the case, the physiological relevance of syndecans in AgRP signaling would be diminished. Further work in this area will be necessary to establish the in vivo site of posttranslational cleavage of AgRP in or to settle this controversy.
Defensins
In opposition to mice where the AY allele that confers a yellow-fur phenotype is dominant, a third allele (AS) determining black coats was thought to be the dominant form in dogs (Kerns et al. 2003). Initially thought to be an agouti allele as opposed to an extension (i.e. MC1R-linked) allele, analysis of genome sequences in animals with red or black fur phenotypes showed that dominant black coats were conferred by a mutated allele of an unrelated gene that encodes for β-defensin (Kerns et al. 2004, 2007, Candille et al. 2007). Strikingly, transgenic expression of β-defensin 3 in the yellow-lethal background produced mice with black coats and lean phenotype (Candille et al. 2007). Moreover, β-defensin 3 blocked NDP-MSH binding to MC1R and with lesser affinity to MC4R (Candille et al. 2007). A later study showed that the human ortholog of β-defensin 3 is a weak partial agonist of MC1R transfected in HEK-293 cells (Beaumont et al. 2012) for cAMP accumulation and ERK1/2 phosphorylation, but a different group concluded that defensins might in fact have no intrinsic agonist action on its own (Swope et al. 2012).
Defensins are part of the innate immune system in mammals (Ganz 2003). The prospect of defensins serving as links between the immune and energy homeostasis systems is tantalizing. Cachexia is invariably linked to immunosuppression. As strongly charged molecules, defensins might interact with MCR solely through electrostatic interactions explaining their neutral antagonist effect (Nix et al. 2013, 2015). There is little doubt that these interactions play a role in determining melanocortin response on MC1R, as defensins seem to block agouti in live animals (Candille et al. 2007, Kerns et al. 2007). However, data are not as consistent for MC4R, and MC3R is yet to be tested as a potential defensin target. Electrostatic-based binding modalities open the possibility for additional undefined peptidic ligands to emerge as possible ‘modulators’ of AgRP and α-MSH signaling in energy balance (Nix et al. 2015).
MRAP1 and MRAP2
Melanocortin 2 receptor accessory proteins (MRAPs) are single-transmembrane proteins that form antiparallel homo- and heterodimers in the cell membrane. MRAP1 is essential for the trafficking, ligand binding, and signaling of MC2R in response to ACTH (Sebag & Hinkle 2007, Metherell et al. 2005). Null mutations in MRAP are linked to familial glucocorticoid deficiency type 2 (Metherell et al. 2005). MRAP2, a homolog to the former, is expressed in brain and adrenal tissue (Chan et al. 2009), interacts with all MCR subtypes, and alters the sensitivity of the MC4R to α-MSH (Sebag et al. 2013).
MRAP2 interacts with the MC4R in mammals as well as zebrafish and modulates the pharmacological response to α-MSH by suppressing the constitutive activity of the receptor and increasing sensitivity to α-MSH. In the zebrafish, MRAP2 exists in two isoforms, a and b. mrap2a stimulated growth in the larval zebrafish by suppressing the binding of α-MSH to MC4R, while mrap2b appears to control MC4R activity in the adult by increasing α-MSH sensitivity and suppressing receptor constitutive activity (Sebag et al. 2013). MRAP2 is the mammalian homolog of mrap2b, which is not expressed until hatching in the zebrafish. Upon binding, it causes an increase in MC4R expression as well as an increase in α-MSH binding and cAMP generation (Sebag et al. 2013). In the zebrafish, neither mrap2a nor mrap2b alters MC3R signaling (Sebag et al. 2013) suggesting that the role of MRAP2 in the regulation of feeding and body weight may be specific to its modulation of MC4R. However, other studies have shown that MRAP2 does alter mammalian MC3R signaling (Chan et al. 2009, Kay et al. 2013). The actual role of MRAP in the modulation of MC3R-regulated food intake and body weight, however, is still up for debate. Recently, MRAP2 has also been demonstrated to play a role in the function of the prokineticin-1 and -2 receptors, suggesting this protein may be a modulator of multiple GPCRs (Chaly et al. 2016), and exert effects on feeding and body weight through multifactorial effects.
To further validate the role of MRAP2 in the development of obesity, researchers created mice with whole-body and brain-specific deletion of MRAP2. It was found that at a young age, these MRAP2-deficient mice had a higher cumulative body weight than their wildtype littermates leading to the development of severe obesity (Asai et al. 2013). Similarly, in a study of genomes from 500 severely obese humans, one patient was found to have a deletion of one of the MRAP2 alleles and four were MRAP2 deficient, suggesting that, although rare, mutations in MRAP2 are potentially pathogenic contributors to the regulation of body weight in humans.
Kir7.1 and other ion channels
Based on the finding that AgRP couples MC4R to the pertussis toxin-sensitive Gi/o inhibitory protein in the hypothalamic GT1–7 cell line (Buch et al. 2009), it was suggested that MC4R might be coupling to G protein inwardly rectifying potassium channels (GIRKs). This result highlights the ability of MC4R to signal through different G proteins with opposing actions. One is the canonical (for MC4R) Gsα adenylyl cyclase stimulatory action activated by α-MSH vs Gi/o inhibitory protein-dependent pathways promoted by AgRP. The ability to signal through Gi/o led to the hypothesis that MC4R could potentially couple to GIRKs, known to be activated by Gβγ binding following release from Gi heterotrimers (Lei et al. 2000).
Physiologically relevant data on the mechanism of α-MSH and AgRP signaling through native MC4R derives from the use of electrophysiological slice preparations from mice in which PVH MC4R neurons have been transgenically labeled with GFP (Ghamari-Langroudi et al. 2010, 2011). In this preparation, α-MSH depolarizes and AgRP hyperpolarizes MC4R neurons when added to the bath. Notably, by using this experimental setup in the presence of G protein inhibitors such as GDPβS and gallein, the role of GIRKs in α-MSH induced depolarization in MC4R-positive PVH neurons was ruled out. Moreover, Gsα and cAMP signaling were also ruled out, when examined as a potential signaling pathway mediating α-MSH-induced depolarization (Ghamari-Langroudi et al. 2015). Current-voltage analyses demonstrated that the conductance involved in α-MSH-induced depolarization was a potassium inward rectifier channel (Kir), and a specific subunit, Kir7.1, was identified using a panel of specific Kir channel blockers.
Also of interest is that AgRP, the endogenous inverse agonist for cAMP responses from MC4R, hyperpolarizes PVH neurons (Ghamari-Langroudi et al. 2015). AgRP augmented membrane conductance, suggesting increased potassium channel opening and surface density, leading to hyperpolarization. Cell transfection studies reinforced the finding that AgRP couples the MC4R to Kir7.1 and regulate its opening. The molecular mechanism by which AgRP mediates this effect currently remains unknown and will require further studies to be definitively clarified. Other inward rectifiers such as Kir2.3 and Kir4.1 channels did not couple to MC4R in independent experiments using cultured cells. As Kir channels form homo- and heterotetramers, it remains to be determined whether MC4R modulates homotetramers of Kir7.1 or heterotetramers with other Kir channel subunits.
Kir7.1 modulation by MC4R also presented new pharmacological paradigms for the receptor, where some ligands have been found to favor the Gsα vs the Kir7.1 pathway. Ghamari-Langroudi et al. (2015) reported changes in potency for well-characterized tool compounds of MC4R, where the MSH analog MC4-NN2-0453, with an EC50 of 4.9 × 10−9 M for intracellular cAMP accumulation assays, was found to have an increased potency of 4.9 × 10−9 M in a thallium flux-based assay used to measure Kir7.1 coupling. Taken together, these findings reveal a novel MC4R signaling pathway, and the potential for the creation of biased ligands that favor the ion channel direct interaction/activation paradigm over classical G protein signaling, making this receptor one of only a handful of GPCRs that were shown to couple directly to ion channels (Zhang et al. 2014).
The microcircuitry of α-MSH and AgRP action
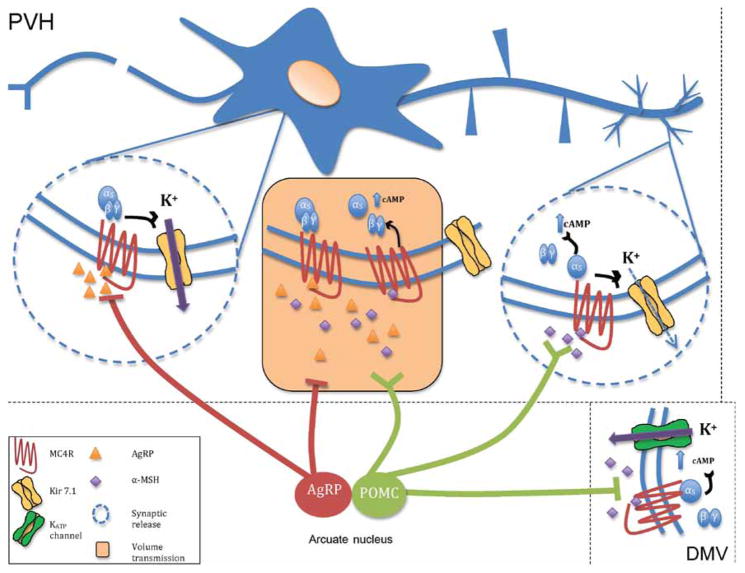
The discovery of G protein independent coupling of MC4R to Kir7.1 has suggested that AgRP is both a competitive antagonist of α-MSH action at the MC4R as well as a biased agonist that can act through MC4R to open Kir7.1 and hyperpolarize neurons (Fig. 4). New neuroanatomical data support both the original Yin–Yang model of α-MSH and AgRP competing for MC4R binding in areas of volume release from POMC and NPY/AgRP neurons (Fig. 1), as well as independent α-MSH and AgRP actions in different brain regions and on different subcellular domains of the target MC4R neurons. Confocal (Bouyer & Simerly 2013) and electron microscopy (Atasoy et al. 2008) of PVH neurons suggests that while there are areas of likely volume release of both peptide, PVH cell bodies receive mostly AgRP synaptic contacts, while small distal dendrites receive mostly POMC synaptic contacts. Thus, the microcircuitry is far more complex than originally suggested, in turn having profound impact on models of α-MSH and AgRP action in vivo (compare Figs 1 and 4).
Figure 4.
A new model of MC4R microcircuitry. The Yin–Yang model of α-MSH and AgRP action (Fig. 1) suggested competitive binding of these peptides to individual MC4R sites (orange box), and anatomical data suggest in regions where these peptides undergo volume release, that competition for binding to the MC4R may occur. New subcellular anatomical data suggest that in the PVH, AgRP synaptic contacts predominate at cell bodies, while POMC synaptic contacts predominate at distal dendrites. Along with the fact that AgRP immunoreactive fibers are only observed in a subset of MC4R expressing nuclei containing POMC-immunoreactive fibers, α-MSH may this often act independently of AgRP (right circle). At these sites, α-MSH may be expected to signal through both cAMP, and Kir7.1. The ability of AgRP to act independently of α-MSH as a potent hyperpolarizing agonist, via regulation of Kir7.1, suggests the likely existence of independent AgRP sites of action (left circle). Another MC4R signaling pathway, involving cAMP/PKA-dependent activation of KATP channels and α-MSH-induced hyperpolarization, has been demonstrated in MC4R neurons in the dorsal motor nucleus of the vagus in the brainstem (bottom right). Thus, α-MSH and AgRP utilize a diversity of signaling modalities to regulate feeding and energy homeostasis through the MC4R. Modified, with permission, from Ghamari-Langroudi M, Digby GJ, Sebag JA, Millhauser GL, Palomino R, Matthews R, Gillyard T, Panaro BL, Tough IR, Cox HM, et al. (2015) G-protein-independent coupling of MC4R to Kir7.1 in hypothalamic neurons. Nature 520 94–98.
Analysis of α-MSH action in the CNS using optogenetics and chemogenetics
Introduction to optogenetics and chemogenetics
The first circuit level genetic manipulation of the POMC and AgRP neurons involved the targeted expression of diphtheria toxin receptor (DTR) in each neuronal subpopulation, followed by administration of DT to ablate the entire neuronal population (Luquet et al. 2005). These studies demonstrated that AgRP neurons are essential for food intake, and that loss of POMC neurons in the ARC, but not NTS, results in hyperphagia, decreased energy expenditure, and obesity (Zhan et al. 2013). These findings undoubtedly stimulated interest in application of optogenetic and chemogenetic methods to the study of POMC and AgRP neurons. The development of optogenetics and designer receptors exclusively activated by designer drugs (DREADDs) has enabled circuit level manipulation of genetically defined neuronal populations involved in hunger and satiety. Optogenetics (Deisseroth 2011) employs microbial opsins to control neuronal firing properties. The principal opsin used in function studies of feeding circuits is humanized channel rhodopsin, a nonspecific cation channel that can depolarize cells when activated by 470 nm light (Boyden et al. 2005). By packaging hCh2R into a Cre-inducible adeno-associated viral vector, this construct can be selectively expressed in target populations via stereotactic injections (Atasoy et al. 2008, Sohal et al. 2009, Tsai et al. 2009). The connectivity and behavioral relevance of these neurons can then be investigated with ex vivo channel rhodopsin assisted circuit mapping (CRACM) and in vivo photostimulation (Adamantidis et al. 2007, Aravanis et al. 2007, Zhang et al. 2010). DREADDs (Sternson & Roth 2014) are a related strategy that enables remote control of neuronal activity without the need for specialized hardware (Armbruster et al. 2007, Alexander et al. 2009). The first-generation DREADDs are molecularly evolved human muscarinic receptors that have minimal endogenous activity and can be activated only by clozapine-N-oxide (CNO), an otherwise inert ligand (Armbruster et al. 2007). Importantly, DREADDs can be used to induce either Gq (hM3Gq) or Gi (hM4Gi) signaling pathways to depolarize or silence neurons (Sternson & Roth 2014), respectively. Together, these tools have been used to further understand how AgRP, POMC, and MC4R neurons regulate acute and chronic food intake.
Optogenetic and chemogenetic analysis of AgRP and POMC neurons
Initial studies found that 1 h hCh2R stimulation of ARC AgRP neurons induced 0.85 g of food intake in satiated mice (Aponte et al. 2011, Atasoy et al. 2012). The frequency of stimulation was proportional to the magnitude of food ingested indicating that this effect was presumably due to increased action potential frequency (Aponte et al. 2011). Interestingly, the acute phase of ARC AgRP-stimulated food intake was maintained on the Ay background and therefore independent of MC4R inhibition (Aponte et al. 2011). Similar experiments using DREADDs also found that hM3Gq activation of ARC AgRP neurons resulted in increased food intake and reduced oxygen consumption. hM4Gi-mediated inhibition reduced food intake indicating that ARC AgRP-regulated feeding is bidirectional (Krashes et al. 2011). Furthermore, chronic hM3Gq activation of ARC AgRP neurons was found to cause an obesity phenotype that could be reversed following cessation of CNO administration (Krashes et al. 2011). By combining ARC AgRP targeted DREADDs with existing KO mouse models, follow-up studies revealed that NPY or GABA was necessary for the acute effects on ARC AgRP neuron activation, while AgRP was sufficient for long-term effects (Krashes et al. 2013). More recent experiments have used hCh2R and DREADDs to further define how ARC AgRP neurons encode a negative balance signal for energy depletion (Betley et al. 2015) and how they evoke typified energy seeking behaviors even in the absence of food (Dietrich et al. 2015). Together, these studies establish a critical role of AgRP neurons in the regulation of feeding behavior and serve as a model by which to study the role of other genetically defined populations.
Optogenetics and DREADDs have also been used to study the role of POMC neurons in feeding behavior. Chronic but not acute optogenetic stimulation of ARC POMC neurons was found to reduce food intake and cause weight loss (Aponte et al. 2011, Zhan et al. 2013). Importantly, unlike ARC AgRP-dependent feeding, the satiating effect of ARC POMC stimulation was lost on the Ay background and therefore dependent on MC4R signaling (Aponte et al. 2011). This finding has been repeated with hM3Dq activation of ARC POMC neurons, whereby animals displayed a 50% reduction of food intake and a 6% drop in their total body mass (Zhan et al. 2013). Furthermore, chronic but not acute hM4Di-mediated inhibition of ARC POMC neurons was found to cause hyperphagia, but only after 24 h. Although this finding points toward a role for α-MSH signaling in regulating long-term energy balance, these studies are at odds with pharmacological data that have shown robust acute anorexic effects of MC4R agonists (Fan et al. 1997). Although it is possible that supraphysiological MSH dosing paradigms used during pharmacological studies might be responsible for some of this effect, NTS POMC neurons have also been implicated in acute feeding behavior. Indeed, acute hM3Dq stimulation of NTS POMC neurons has been found to cause acute anorexia while chronic activation of this population does not seem to affect food intake (Zhan et al. 2013). The mechanism that underlies the discordance between anatomical subsets of POMC neurons remains unknown; however, it is likely a result of their distinct projection fields (Wang et al. 2015). Alternatively, acute stimulation of ARC POMC has been shown to promote endocannabinoid evoked feeding, which may be responsible for the delayed response of ARC POMC neurons, but not NTS POMC neurons (Koch et al. 2015).
AgRP target sites that mediate acute feeding behavior
Optogenetics and DREADDs also enable the determination of the target sites that are necessary and sufficient and for evoked feeding behaviors. Despite evidence of a functional inhibitory ARC AgRP to ARC POMC projection, coactivation of these two populations did not blunt light evoked ARC AgRP feeding (Atasoy et al. 2012, Betley et al. 2013). However, site-specific photostimulation of GABAergic ARC AgRP efferents within the PVH was found to be sufficient for acute feeding behavior (Atasoy et al. 2012, Garfield et al. 2015). This effect was replicated with hM4Gi-mediated silencing of PVH SIM1 neurons indicating that inhibition of this population is sufficient for feeding behavior. Furthermore, coactivation of PVH SIM1 cell bodies and ARC AgRP efferents did not cause food intake. Initially, PVH OXT neurons (a subpopulation of PVH SIM1 neurons) were thought to be the mediators of ARC AgRP to PVH-induced food intake (Atasoy et al. 2012). However, both ex vivo and in vivo experiments have challenged this finding (Wu et al. 2012, Sutton et al. 2014, Garfield et al. 2015). Using CRACM, ARC AgRP fibers were found to evoke time locked inhibitory postsynaptic currents within 83% of PVH MC4R cells and 0% of PVH OXT expressing cells (Garfield et al. 2015). Importantly, MC4R neurons were found to be distinct from OXT neurons as evidenced by immunohistochemistry. Furthermore, costimulation of inhibitory ARC AgRP fibers and PVH MC4R were found to block evoked feeding behavior, while PVH OXT did not. Additionally, PVH MC4R neurons project to and activate the LPB, which evokes a satiety response (Garfield et al. 2015). In addition to the PVH, ARC AgRP are known to project to numerous other brain regions. Using a similar efferent projection stimulation strategy, ARC AgRP fiber activation in the BNST, LH, and PVT was sufficient for evoked feeding behavior (Betley et al. 2013). This contrasts with ARC AgRP fibers that project to ARC POMC neurons, PBN, CEA, and PAG, which do not evoke feeding. Interestingly, while the BNST and the LH both express MC4R, inhibition of MC4R neurons within these sites does not appear to be responsible for the increased food intake seen with ARC AgRP afferent stimulation (Garfield et al. 2015).
Summary
It has been a long road from the discovery 60 years ago of the POMC peptides, to the cloning of the POMC gene in 1978, identification of receptors for α-MSH in the brain in 1993, and demonstration of a role of central melanocortin signaling in the control of energy homeostasis in 1997. This year provides another landmark: in January 2016, the US Food and Drug Administration has just awarded orphan drug status to the first α-MSH-based therapeutic for obesity. The α-MSH analog RM-493 (Kievit et al. 2013, Chen et al. 2015), also known as setmelanotide, was awarded orphan drug status for POMC deficiency and Prader–Willi syndrome. This new innovation will surely generate additional interest in the field, both in terms of basic science aimed at understanding the molecular basis of regulation of energy homeostasis by α-MSH, as well as the development of therapeutics for obesity and cachexia modeled after the effects of α-MSH and AgRP at MCR in the CNS.
Acknowledgments
Funding
This work was supported by the National Institutes of Health RO1DK070332 to R D C. E J P A, T G, and M L are recipients of training grants: T32 DK07563, F31 DK107253, and F30 DK108476, respectively from NIH/NIDDK. M L is also supported by T32 GM07347 from NIH/NIGMS. S C is supported by the Vanderbilt International Scholar Program.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
References
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nature Neuroscience. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. Journal of Neural Engineering. 2007;4:S143–S156. doi: 10.1088/1741-2560/4/3/S02. [DOI] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. PNAS. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai M, Ramachandrappa S, Joachim M, Shen Y, Zhang R, Nuthalapati N, Ramanathan V, Strochlic DE, Ferket P, Linhart K, et al. Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science. 2013;341:275–278. doi: 10.1126/science.1233000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. Journal of Neuroscience. 2008;28:7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnol D, Lu XY, Kaelin CB, Day HE, Ollmann M, Gantz I, Akil H, Barsh GS, Watson SJ. Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain. Journal of Neuroscience. 1999;19:RC26. doi: 10.1523/JNEUROSCI.19-18-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Beaumont KA, Smit DJ, Liu YY, Chai E, Patel MP, Millhauser GL, Smith JJ, Alewood PF, Sturm RA. Melanocortin-1 receptor-mediated signalling pathways activated by NDP-MSH and HBD3 ligands. Pigment Cell & Melanoma Research. 2012;25:370–374. doi: 10.1111/j.1755-148X.2012.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begriche K, Levasseur PR, Zhang J, Rossi J, Skorupa D, Solt LA, Young B, Burris TP, Marks DL, Mynatt RL, et al. Genetic dissection of the functions of the melanocortin-3 receptor, a seven-transmembrane G-protein-coupled receptor, suggests roles for central and peripheral receptors in energy homeostasis. Journal of Biological Chemistry. 2011;286:40771–40781. doi: 10.1074/jbc.M111.278374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfield M, Kokenyesi R, Kato M, Hinkes MT, Spring J, Gallo RL, Lose EJ. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annual Review of Cell Biology. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- Betley JN, Cao ZF, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155:1337–1350. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley JN, Xu S, Cao ZF, Gong R, Magnus CJ, Yu Y, Sternson SM. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature. 2015;521:180–185. doi: 10.1038/nature14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyer K, Simerly RB. Neonatal leptin exposure specifies innervation of presympathetic hypothalamic neurons and improves the metabolic status of leptin-deficient mice. Journal of Neuroscience. 2013;33:840–851. doi: 10.1523/JNEUROSCI.3215-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nature Neuroscience. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Bronstein DM, Schafer MK, Watson SJ, Akil H. Evidence that beta-endorphin is synthesized in cells in the nucleus tractus solitarius: detection of POMC mRNA. Brain Research. 1992;587:269–275. doi: 10.1016/0006-8993(92)91007-2. [DOI] [PubMed] [Google Scholar]
- Buch TR, Heling D, Damm E, Gudermann T, Breit A. Pertussis toxin-sensitive signaling of melanocortin-4 receptors in hypothalamic GT1-7 cells defines agouti-related protein as a biased agonist. Journal of Biological Chemistry. 2009;284:26411–26420. doi: 10.1074/jbc.M109.039339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultman SJ, Michaud EJ, Woychik RP. Molecular characterization of the mouse agouti locus. Cell. 1992;71:1195–1204. doi: 10.1016/S0092-8674(05)80067-4. [DOI] [PubMed] [Google Scholar]
- Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- Candille SI, Kaelin CB, Cattanach BM, Yu B, Thompson DA, Nix MA, Kerns JA, Schmutz SM, Millhauser GL, Barsh GS. A-defensin mutation causes black coat color in domestic dogs. Science. 2007;318:1418–1423. doi: 10.1126/science.1147880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso V, Lagerstrom MC, Olszewski PK, Fredriksson R, Schioth HB. Synaptic changes induced by melanocortin signalling. Nature Reviews Neuroscience. 2014;15:98–110. doi: 10.1038/nrn3657. [DOI] [PubMed] [Google Scholar]
- Chai BX, Neubig RR, Millhauser GL, Thompson DA, Jackson PJ, Barsh GS, Dickinson CJ, Li JY, Lai YM, Gantz I. Inverse agonist activity of agouti and agouti-related protein. Peptides. 2003;24:603–609. doi: 10.1016/S0196-9781(03)00104-9. [DOI] [PubMed] [Google Scholar]
- Challis BG, Coll AP, Yeo GS, Pinnock SB, Dickson SL, Thresher RR, Dixon J, Zahn D, Rochford JJ, White A, et al. Mice lacking pro-opiomelanocortin are sensitive to high-fat feeding but respond normally to the acute anorectic effects of peptide-YY(3-36) PNAS. 2004;101:4695–4700. doi: 10.1073/pnas.0306931101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaly AL, Srisai D, Gardner EE, Sebag JA. The melanocortin receptor accessory protein 2 promotes food intake through inhibition of the prokineticin receptor-1. eLife. 2016;5:e12397. doi: 10.7554/eLife.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan LF, Webb TR, Chung TT, Meimaridou E, Cooray SN, Guasti L, Chapple JP, Egertova M, Elphick MR, Cheetham ME, et al. MRAP and MRAP2 are bidirectional regulators of the melanocortin receptor family. PNAS. 2009;106:6146–6151. doi: 10.1073/pnas.0809918106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Kelly MA, Opitz-Araya X, Thomas RE, Low MJ, Cone RD. Exocrine gland dysfunction in MC5-R-deficient mice: evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell. 1997;91:789–798. doi: 10.1016/S0092-8674(00)80467-5. [DOI] [PubMed] [Google Scholar]
- Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nature Genetics. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- Chen KY, Muniyappa R, Abel BS, Mullins KP, Staker P, Brychta RJ, Zhao X, Ring M, Psota TL, Cone RD, et al. RM-493, a melanocortin-4 receptor (MC4R) agonist, increases resting energy expenditure in obese individuals. Journal of Clinical Endocrinology & Metabolism. 2015;100:1639–1645. doi: 10.1210/jc.2014-4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civelli O, Birnberg N, Herbert E. Detection and quantitation of pro-opiomelanocortin mRNA in pituitary and brain tissue from different species. Journal of Biological Chemistry. 1982;257:6783–6787. [PubMed] [Google Scholar]
- Clark AJ, Lavender PM, Coates P, Johnson MR, Rees LH. In vitro and in vivo analysis of the processing and fate of the peptide products of the short proopiomelanocortin mRNA. Molecular Endocrinology. 1990;4:1737–1743. doi: 10.1210/mend-4-11-1737. [DOI] [PubMed] [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nature Neuroscience. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Cone RD. Studies on the physiological functions of the melanocortin system. Endocrine Reviews. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- Corander MP, Rimmington D, Challis BG, O’Rahilly S, Coll AP. Loss of agouti-related peptide does not significantly impact the phenotype of murine POMC deficiency. Endocrinology. 2011;152:1819–1828. doi: 10.1210/en.2010-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes R, Navarro S, Agulleiro MJ, Guillot R, Garcia-Herranz V, Sanchez E, Cerda-Reverter JM. Evolution of the melanocortin system. General and Comparative Endocrinology. 2014;209:3–10. doi: 10.1016/j.ygcen.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Creemers JW, Pritchard LE, Gyte A, Le Rouzic P, Meulemans S, Wardlaw SL, Zhu X, Steiner DF, Davies N, Armstrong D, et al. Agouti-related protein is posttranslationally cleaved by proprotein convertase 1 to generate agouti-related protein (AGRP)83-132: interaction between AGRP83-132 and melanocortin receptors cannot be influenced by syndecan-3. Endocrinology. 2006;147:1621–1631. doi: 10.1210/en.2005-1373. [DOI] [PubMed] [Google Scholar]
- Cui H, Sohn JW, Gautron L, Funahashi H, Williams KW, Elmquist JK, Lutter M. Neuroanatomy of melanocortin-4 receptor pathway in the lateral hypothalamic area. Journal of Comparative Neurology. 2012;520:4168–4183. doi: 10.1002/cne.23145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K. Optogenetics. Nature Methods. 2011;8:26–29. doi: 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie MM. A new viable yellow mutation in the house mouse. Journal of Heredity. 1962;53:84–86. doi: 10.1093/oxfordjournals.jhered.a107129. [DOI] [PubMed] [Google Scholar]
- Dickie MM. Mutations at the agouti locus in the mouse. Journal of Heredity. 1969;60:20–25. doi: 10.1093/oxfordjournals.jhered.a107920. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Zimmer MR, Bober J, Horvath TL. Hypothalamic Agrp neurons drive stereotypic behaviors beyond feeding. Cell. 2015;160:1222–1232. doi: 10.1016/j.cell.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinulescu DM, Fan W, Boston BA, McCall K, Lamoreux ML, Moore KJ, Montagno J, Cone RD. Mahogany (mg) stimulates feeding and increases basal metabolic rate independent of its suppression of agouti. PNAS. 1998;95:12707–12712. doi: 10.1073/pnas.95.21.12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenius K, Maatta A, Salmivirta M, Jalkanen M. Growth factors induce 3T3 cells to express bFGF-binding syndecan. Journal of Biological Chemistry. 1992;267:6435–6441. [PubMed] [Google Scholar]
- Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- Fong TM, Mao C, MacNeil T, Kalyani R, Smith T, Weinberg D, Tota MR, Van der Ploeg LH. ART (protein product of agouti-related transcript) as an antagonist of MC-3 and MC-4 receptors. Biochemical and Biophysical Research Communications. 1997;237:629–631. doi: 10.1006/bbrc.1997.7200. [DOI] [PubMed] [Google Scholar]
- Frigeri LG, Wolff GL, Robel G. Impairment of glucose tolerance in yellow (Avy/A) (BALB/c X VY) F-1 hybrid mice by hyperglycemic peptide(s) from human pituitary glands. Endocrinology. 1983;113:2097–2105. doi: 10.1210/endo-113-6-2097. [DOI] [PubMed] [Google Scholar]
- Gantz I, Konda Y, Tashiro T, Shimoto Y, Miwa H, Munzert G, Watson SJ, DelValle J, Yamada T. Molecular cloning of a novel melanocortin receptor. Journal of Biological Chemistry. 1993a;268:8246–8250. [PubMed] [Google Scholar]
- Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, DelValle J, Yamada T. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. Journal of Biological Chemistry. 1993b;268:15174–15179. [PubMed] [Google Scholar]
- Ganz T. Defensins: antimicrobial peptides of innate immunity. Nature Reviews Immunology. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- Garfield AS, Li C, Madara JC, Shah BP, Webber E, Steger JS, Campbell JN, Gavrilova O, Lee CE, Olson DP, et al. A neural basis for melanocortin-4 receptor-regulated appetite. Nature Neuroscience. 2015;18:863–871. doi: 10.1038/nn.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautron L, Lee C, Funahashi H, Friedman J, Lee S, Elmquist J. Melanocortin-4 receptor expression in a vago-vagal circuitry involved in postprandial functions. Journal of Comparative Neurology. 2010;518:6–24. doi: 10.1002/cne.22221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Vella KR, Srisai D, Sugrue ML, Hollenberg AN, Cone RD. Regulation of thyrotropin-releasing hormone-expressing neurons in paraventricular nucleus of the hypothalamus by signals of adiposity. Molecular Endocrinology. 2010;24:2366–2381. doi: 10.1210/me.2010-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Srisai D, Cone RD. Multinodal regulation of the arcuate/paraventricular nucleus circuit by leptin. PNAS. 2011;108:355–360. doi: 10.1073/pnas.1016785108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Digby GJ, Sebag JA, Millhauser GL, Palomino R, Matthews R, Gillyard T, Panaro BL, Tough IR, Cox HM, et al. G-protein-independent coupling of MC4R to Kir7.1 in hypothalamic neurons. Nature. 2015;520:94–98. doi: 10.1038/nature14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M, Shutter JR, Sarmiento U, Sarosi I, Stark KL. Overexpression of Agrt leads to obesity in transgenic mice. Nature Genetics. 1997;17:273–274. doi: 10.1038/ng1197-273. [DOI] [PubMed] [Google Scholar]
- Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nature Neuroscience. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- Gunn TM, Miller KA, He L, Hyman RW, Davis RW, Azarani A, Schlossman SF, Duke-Cohan JS, Barsh GS. The mouse mahogany locus encodes a transmembrane form of human attractin. Nature. 1999;398:152–156. doi: 10.1038/18217. [DOI] [PubMed] [Google Scholar]
- Haqq AM, Rene P, Kishi T, Khong K, Lee CE, Liu H, Friedman JM, Elmquist JK, Cone RD. Characterization of a novel binding partner of the melanocortin-4 receptor: attractin-like protein. Biochemical Journal. 2003;376:595–605. doi: 10.1042/bj20031241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell-Luevano C, Cone RD, Monck EK, Wan YP. Structure activity studies of the melanocortin-4 receptor by in vitro mutagenesis: identification of agouti-related protein (AGRP), melanocortin agonist and synthetic peptide antagonist interaction determinants. Biochemistry. 2001;40:6164–6179. doi: 10.1021/bi010025q. [DOI] [PubMed] [Google Scholar]
- He L, Gunn TM, Bouley DM, Lu XY, Watson SJ, Schlossman SF, Duke-Cohan JS, Barsh GS. A biochemical function for attractin in agouti-induced pigmentation and obesity. Nature Genetics. 2001;27:40–47. doi: 10.1038/83741. [DOI] [PubMed] [Google Scholar]
- He L, Lu XY, Jolly AF, Eldridge AG, Watson SJ, Jackson PK, Barsh GS, Gunn TM. Spongiform degeneration in mahoganoid mutant mice. Science. 2003;299:710–712. doi: 10.1126/science.1079694. [DOI] [PubMed] [Google Scholar]
- Hruby VJ, Lu D, Sharma SD, Castrucci AL, Kesterson RA, al-Obeidi FA, Hadley ME, Cone RD. Cyclic lactam alpha-melanotropin analogues of Ac-Nle4-cyclo[Asp5, D-Phe7, Lys10] alpha-melanocyte-stimulating hormone-(4-10)-NH2 with bulky aromatic amino acids at position 7 show high antagonist potency and selectivity at specific melanocortin receptors. Journal of Medicinal Chemistry. 1995;38:3454–3461. doi: 10.1021/jm00018a005. [DOI] [PubMed] [Google Scholar]
- Huo L, Grill HJ, Bjorbaek C. Divergent regulation of proopiomelanocortin neurons by leptin in the nucleus of the solitary tract and in the arcuate hypothalamic nucleus. Diabetes. 2006;55:567–573. doi: 10.2337/diabetes.55.03.06.db05-1143. [DOI] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/S0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Jackson IJ, Budd P, Horn JM, Johnson R, Raymond S, Steel K. Genetics and molecular biology of mouse pigmentation. Pigment Cell Research. 1994;7:73–80. doi: 10.1111/j.1600-0749.1994.tb00024.x. [DOI] [PubMed] [Google Scholar]
- Jackson PJ, Douglas NR, Chai B, Binkley J, Sidow A, Barsh GS, Millhauser GL. Structural and molecular evolutionary analysis of Agouti and Agouti-related proteins. Chemistry & Biology. 2006;13:1297–1305. doi: 10.1016/j.chembiol.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J, Sun K, Walker WP, Bagher P, Cota CD, Gunn TM. Abnormal regulation of TSG101 in mice with spongiform neurodegeneration. Biochimica et Biophysica Acta. 2009;1792:1027–1035. doi: 10.1016/j.bbadis.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph SA, Pilcher WH, Bennett-Clarke C. Immunocytochemical localization of ACTH perikarya in nucleus tractus solitarius: evidence for a second opiocortin neuronal system. Neuroscience Letters. 1983;38:221–225. doi: 10.1016/0304-3940(83)90372-5. [DOI] [PubMed] [Google Scholar]
- Kay EI, Botha R, Montgomery JM, Mountjoy KG. hMRAPa increases alphaMSH-induced hMC1R and hMC3R functional coupling and hMC4R constitutive activity. Journal of Molecular Endocrinology. 2013;50:203–215. doi: 10.1530/JME-12-0221. [DOI] [PubMed] [Google Scholar]
- Kerns JA, Olivier M, Lust G, Barsh GS. Exclusion of melanocortin-1 receptor (mc1r) and agouti as candidates for dominant black in dogs. Journal of Heredity. 2003;94:75–79. [PubMed] [Google Scholar]
- Kerns JA, Newton J, Berryere TG, Rubin EM, Cheng JF, Schmutz SM, Barsh GS. Characterization of the dog Agouti gene and a nonagoutimutation in German Shepherd Dogs. Mammalian Genome. 2004;15:798–808. doi: 10.1007/s00335-004-2377-1. [DOI] [PubMed] [Google Scholar]
- Kerns JA, Cargill EJ, Clark LA, Candille SI, Berryere TG, Olivier M, Lust G, Todhunter RJ, Schmutz SM, Murphy KE, et al. Linkage and segregation analysis of black and brindle coat color in domestic dogs. Genetics. 2007;176:1679–1689. doi: 10.1534/genetics.107.074237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kievit P, Halem H, Marks DL, Dong JZ, Glavas MM, Sinnayah P, Pranger L, Cowley MA, Grove KL, Culler MD. Chronic treatment with a melanocortin-4 receptor agonist causes weight loss, reduces insulin resistance, and improves cardiovascular function in diet-induced obese rhesus macaques. Diabetes. 2013;62:490–497. doi: 10.2337/db12-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CW, Goldberger OA, Gallo RL, Bernfield M. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Molecular Biology of the Cell. 1994;5:797–805. doi: 10.1091/mbc.5.7.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CS, Lee SH, Kim RY, Kim BJ, Li SZ, Lee IH, Lee EJ, Lim SK, Bae YS, Lee W, et al. Identification of domains directing specificity of coupling to G-proteins for the melanocortin MC3 and MC4 receptors. Journal of Biological Chemistry. 2002;277:31310–31317. doi: 10.1074/jbc.M112085200. [DOI] [PubMed] [Google Scholar]
- Kim BY, Olzmann JA, Barsh GS, Chin LS, Li L. Spongiform neurodegeneration-associated E3 ligase Mahogunin ubiquitylates TSG101 and regulates endosomal trafficking. Molecular Biology of the Cell. 2007;18:1129–1142. doi: 10.1091/mbc.E06-09-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. Journal of Comparative Neurology. 2003;457:213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- Klebig ML, Wilkinson JE, Geisler JG, Woychik RP. Ectopic expression of the agouti gene in transgenic mice causes obesity, features of type II diabetes, and yellow fur. PNAS. 1995;92:4728–4732. doi: 10.1073/pnas.92.11.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Varela L, Kim JG, Kim JD, Hernandez-Nuno F, Simonds SE, Castorena CM, Vianna CR, Elmquist JK, Morozov YM, et al. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature. 2015;519:45–50. doi: 10.1038/nature14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konda Y, Gantz I, DelValle J, Shimoto Y, Miwa H, Yamada T. Interaction of dual intracellular signaling pathways activated by the melanocortin-3 receptor. Journal of Biological Chemistry. 1994;269:13162–13166. [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. Journal of Clinical Investigation. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Koda S, Lowell BB. Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell Metabolism. 2013;18:588–595. doi: 10.1016/j.cmet.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane PW, Green MC. Mahogany, a recessive color mutation in linkage group V of the mouse. Journal of Heredity. 1960;51:228–230. [Google Scholar]
- Lei Q, Jones MB, Talley EM, Schrier AD, McIntire WE, Garrison JC, Bayliss DA. Activation and inhibition of G protein-coupled inwardly rectifying potassium (Kir3) channels by G protein beta gamma subunits. PNAS. 2000;97:9771–9776. doi: 10.1073/pnas.97.17.9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemus MB, Bayliss JA, Lockie SH, Santos VV, Reichenbach A, Stark R, Andrews ZB. A stereological analysis of NPY, POMC, Orexin, GFAP astrocyte, and Iba1 microglia cell number and volume in diet-induced obese male mice. Endocrinology. 2015;156:1701–1713. doi: 10.1210/en.2014-1961. [DOI] [PubMed] [Google Scholar]
- Li SJ, Varga K, Archer P, Hruby VJ, Sharma SD, Kesterson RA, Cone RD, Kunos G. Melanocortin antagonists define two distinct pathways of cardiovascular control by alpha- and gamma-melanocyte-stimulating hormones. Journal of Neuroscience. 1996;16:5182–5188. doi: 10.1523/JNEUROSCI.16-16-05182.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippert RN, Ellacott KL, Cone RD. Gender-specific roles for the melanocortin-3 receptor in the regulation of the mesolimbic dopamine system in mice. Endocrinology. 2014;155:1718–1727. doi: 10.1210/en.2013-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. Journal of Neuroscience. 2003;23:7143–7154. doi: 10.1523/JNEUROSCI.23-18-07143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Willard D, Patel IR, Kadwell S, Overton L, Kost T, Luther M, Chen W, Woychik RP, Wilkison WO, et al. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature. 1994;371:799–802. doi: 10.1038/371799a0. [DOI] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Magenis RE, Smith L, Nadeau JH, Johnson KR, Mountjoy KG, Cone RD. Mapping of the ACTH, MSH, and neural (MC3 and MC4) melanocortin receptors in the mouse and human. Mammalian Genome. 1994;5:503–508. doi: 10.1007/BF00369320. [DOI] [PubMed] [Google Scholar]
- Metherell LA, Chapple JP, Cooray S, David A, Becker C, Ruschendorf F, Naville D, Begeot M, Khoo B, Nurnberg P, et al. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nature Genetics. 2005;37:166–170. doi: 10.1038/ng1501. [DOI] [PubMed] [Google Scholar]
- Michaud EJ, Bultman SJ, Stubbs LJ, Woychik RP. The embryonic lethality of homozygous lethal yellow mice (Ay/Ay) is associated with the disruption of a novel RNA-binding protein. Genes & Development. 1993;7:1203–1213. doi: 10.1101/gad.7.7a.1203. [DOI] [PubMed] [Google Scholar]
- Michaud EJ, Bultman SJ, Klebig ML, van Vugt MJ, Stubbs LJ, Russell LB, Woychik RP. A molecular model for the genetic and phenotypic characteristics of the mouse lethal yellow (Ay) mutation. PNAS. 1994a;91:2562–2566. doi: 10.1073/pnas.91.7.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud EJ, van Vugt MJ, Bultman SJ, Sweet HO, Davisson MT, Woychik RP. Differential expression of a new dominant agouti allele (Aiapy) is correlated with methylation state and is influenced by parental lineage. Genes & Development. 1994b;8:1463–1472. doi: 10.1101/gad.8.12.1463. [DOI] [PubMed] [Google Scholar]
- Miller MW, Duhl DM, Vrieling H, Cordes SP, Ollmann MM, Winkes BM, Barsh GS. Cloning of the mouse agouti gene predicts a secreted protein ubiquitously expressed in mice carrying the lethal yellow mutation. Genes & Development. 1993;7:454–467. doi: 10.1101/gad.7.3.454. [DOI] [PubMed] [Google Scholar]
- Miller KA, Gunn TM, Carrasquillo MM, Lamoreux ML, Galbraith DB, Barsh GS. Genetic studies of the mouse mutations mahogany and mahoganoid. Genetics. 1997;146:1407–1415. doi: 10.1093/genetics/146.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C, Cone RD. Melanocortin-5 receptor deficiency in mice blocks a novel pathway influencing pheromone-induced aggression. Behavior Genetics. 2006;36:291–300. doi: 10.1007/s10519-005-9024-9. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG. Pro-Opiomelanocortin (POMC) neurones, POMC-derived peptides, melanocortin receptors and obesity: how understanding of this system has changed over the last decade. Journal of Neuroendocrinology. 2015;27:406–418. doi: 10.1111/jne.12285. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Molecular Endocrinology. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Kong PL, Taylor JA, Willard DH, Wilkison WO. Melanocortin receptor-mediated mobilization of intracellular free calcium in HEK293 cells. Physiological Genomics. 2001;5:11–19. doi: 10.1152/physiolgenomics.2001.5.1.11. [DOI] [PubMed] [Google Scholar]
- Nijenhuis WA, Oosterom J, Adan RA. AgRP(83-132) acts as an inverse agonist on the human-melanocortin-4 receptor. Molecular Endocrinology. 2001;15:164–171. doi: 10.1210/mend.15.1.0578. [DOI] [PubMed] [Google Scholar]
- Nix MA, Kaelin CB, Ta T, Weis A, Morton GJ, Barsh GS, Millhauser GL. Molecular and functional analysis of human beta-defensin 3 action at melanocortin receptors. Chemistry & Biology. 2013;20:784–795. doi: 10.1016/j.chembiol.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nix MA, Kaelin CB, Palomino R, Miller JL, Barsh GS, Millhauser GL. Electrostatic similarity analysis of human beta-defensin binding in the melanocortin system. Biophysical Journal. 2015;109:1946–1958. doi: 10.1016/j.bpj.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- Overton JD, Leibel RL. Mahoganoid and mahogany mutations rectify the obesity of the yellow mouse by effects on endosomal traffic of MC4R protein. Journal of Biological Chemistry. 2011;286:18914–18929. doi: 10.1074/jbc.M111.224592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla SL, Carmody JS, Zeltser LM. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nature Medicine. 2010;16:403–405. doi: 10.1038/nm.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla SL, Reef D, Zeltser LM. Defining POMC neurons using transgenic reagents: impact of transient Pomc expression in diverse immature neuronal populations. Endocrinology. 2012;153:1219–1231. doi: 10.1210/en.2011-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaro BL, Tough IR, Engelstoft MS, Matthews RT, Digby GJ, Moller CL, Svendsen B, Gribble F, Reimann F, Holst JJ, et al. The melanocortin-4 receptor is expressed in enteroendocrine L cells and regulates the release of peptide YY and glucagon-like peptide 1 in vivo. Cell Metabolism. 2014;20:1018–1029. doi: 10.1016/j.cmet.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perello M, Stuart RC, Nillni EA. Differential effects of fasting and leptin on proopiomelanocortin peptides in the arcuate nucleus and in the nucleus of the solitary tract. American Journal of Physiology – Endocrinology and Metabolism. 2007;292:E1348–E1357. doi: 10.1152/ajpendo.00466.2006. [DOI] [PubMed] [Google Scholar]
- Phan LK, Lin F, LeDuc CA, Chung WK, Leibel RL. The mouse mahoganoid coat color mutation disrupts a novel C3HC4 RING domain protein. Journal of Clinical Investigation. 2002;110:1449–1459. doi: 10.1172/JCI0216131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- Poggioli R, Vergoni AV, Bertolini A. ACTH-(1-24) and alpha-MSH antagonize feeding behavior stimulated by kappa opiate agonists. Peptides. 1986;7:843–848. doi: 10.1016/0196-9781(86)90104-X. [DOI] [PubMed] [Google Scholar]
- Qian S, Chen H, Weingarth D, Trumbauer ME, Novi DE, Guan X, Yu H, Shen Z, Feng Y, Frazier E, et al. Neither Agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Molecular and Cellular Biology. 2002;22:5027–5035. doi: 10.1128/MCB.22.14.5027-5035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillan JM, Sadee W, Wei ET, Jimenez C, Ji L, Chang JK. A synthetic human Agouti-related protein-(83-132)-NH2 fragment is a potent inhibitor of melanocortin receptor function. FEBS Letters. 1998;428:59–62. doi: 10.1016/S0014-5793(98)00487-6. [DOI] [PubMed] [Google Scholar]
- Reizes O, Lincecum J, Wang Z, Goldberger O, Huang L, Kaksonen M, Ahima R, Hinkes MT, Barsh GS, Rauvala H, et al. Transgenic expression of syndecan-1 uncovers a physiological control of feeding behavior by syndecan-3. Cell. 2001;106:105–116. doi: 10.1016/S0092-8674(01)00415-9. [DOI] [PubMed] [Google Scholar]
- Reizes O, Benoit SC, Strader AD, Clegg DJ, Akunuru S, Seeley RJ. Syndecan-3 modulates food intake by interacting with the melanocortin/AgRP pathway. Annals of the New York Academy of Sciences. 2003;994:66–73. doi: 10.1111/j.1749-6632.2003.tb03163.x. [DOI] [PubMed] [Google Scholar]
- Renquist BJ, Murphy JG, Larson EA, Olsen D, Klein RF, Ellacott KL, Cone RD. Melanocortin-3 receptor regulates the normal fasting response. PNAS. 2012;109:E1489–E1498. doi: 10.1073/pnas.1201994109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, Entwistle ML, Simerly RB, Cone RD. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. PNAS. 1993;90:8856–8860. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, Choi MJ, Lauzon D, Lowell BB, Elmquist JK. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metabolism. 2011;13:195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harbor Perspectives in Biology. 2011;3:a004952. doi: 10.1101/cshperspect.a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzberg DG, Nakane PK. ACTH-related peptide containing neurons within the medulla oblongata of the rat. Brain Research. 1983;276:351–356. doi: 10.1016/0006-8993(83)90746-1. [DOI] [PubMed] [Google Scholar]
- Sebag JA, Hinkle PM. Melanocortin-2 receptor accessory protein MRAP forms antiparallel homodimers. PNAS. 2007;104:20244–20249. doi: 10.1073/pnas.0708916105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebag JA, Zhang C, Hinkle PM, Bradshaw AM, Cone RD. Developmental control of the melanocortin-4 receptor by MRAP2 proteins in zebrafish. Science. 2013;341:278–281. doi: 10.1126/science.1232995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah BP, Vong L, Olson DP, Koda S, Krashes MJ, Ye C, Yang Z, Fuller PM, Elmquist JK, Lowell BB. MC4R-expressing glutamatergic neurons in the paraventricular hypothalamus regulate feeding and are synaptically connected to the parabrachial nucleus. PNAS. 2014;111:13193–13198. doi: 10.1073/pnas.1407843111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Shargill NS, Bray GA, Yen TT, Gesellchen PD. Effects of MSH on food intake, body weight and coat color of the yellow obese mouse. Life Sciences. 1989;45:543–552. doi: 10.1016/0024-3205(89)90105-7. [DOI] [PubMed] [Google Scholar]
- Shutter JR, Graham M, Kinsey AC, Scully S, Luthy R, Stark KL. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes & Development. 1997;11:593–602. doi: 10.1101/gad.11.5.593. [DOI] [PubMed] [Google Scholar]
- Siljee JE, Unmehopa UA, Kalsbeek A, Swaab DF, Fliers E, Alkemade A. Melanocortin 4 receptor distribution in the human hypothalamus. European Journal of Endocrinology. 2013;168:361–369. doi: 10.1530/EJE-12-0750. [DOI] [PubMed] [Google Scholar]
- Silvers WK. The Coat Colors of Mice: A Model for Mammalian Gene Action and Interaction. New York, NY, USA: Springer-Verlag; 1979. [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternson SM, Roth BL. Chemogenetic tools to interrogate brain functions. Annual Review of Neuroscience. 2014;37:387–407. doi: 10.1146/annurev-neuro-071013-014048. [DOI] [PubMed] [Google Scholar]
- Strader AD, Reizes O, Woods SC, Benoit SC, Seeley RJ. Mice lacking the syndecan-3 gene are resistant to diet-induced obesity. Journal of Clinical Investigation. 2004;114:1354–1360. doi: 10.1172/JCI20631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton GM, Trevaskis JL, Hulver MW, McMillan RP, Markward NJ, Babin MJ, Meyer EA, Butler AA. Diet-genotype interactions in the development of the obese, insulin-resistant phenotype of C57BL/6J mice lacking melanocortin-3 or -4 receptors. Endocrinology. 2006;147:2183–2196. doi: 10.1210/en.2005-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton AK, Pei H, Burnett KH, Myers MG, Jr, Rhodes CJ, Olson DP. Control of food intake and energy expenditure by Nos1 neurons of the paraventricular hypothalamus. Journal of Neuroscience. 2014;34:15306–15318. doi: 10.1523/JNEUROSCI.0226-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swope VB, Jameson JA, McFarland KL, Supp DM, Miller WE, McGraw DW, Patel MA, Nix MA, Millhauser GL, Babcock GF, et al. Defining MC1R regulation in human melanocytes by its agonist alpha-melanocortin and antagonists agouti signaling protein and beta-defensin 3. Journal of Investigative Dermatology. 2012;132:2255–2262. doi: 10.1038/jid.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocrine Reviews. 2010;31:506–543. doi: 10.1210/er.2009-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nature Genetics. 1998;20:113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, Jo YH, MacKenzie RG, Allison DB, Dun NJ, et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149:1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastermark A, Schioth HB. The early origin of melanocortin receptors, agouti-related peptide, agouti signalling peptide, and melanocortin receptor-accessory proteins, with emphasis on pufferfishes, elephant shark, lampreys, and amphioxus. European Journal of Pharmacology. 2011;660:61–69. doi: 10.1016/j.ejphar.2010.10.106. [DOI] [PubMed] [Google Scholar]
- Vergoni AV, Poggioli R, Bertolini A. Corticotropin inhibits food intake in rats. Neuropeptides. 1986;7:153–158. doi: 10.1016/0143-4179(86)90091-0. [DOI] [PubMed] [Google Scholar]
- Wang D, He X, Zhao Z, Feng Q, Lin R, Sun Y, Ding T, Xu F, Luo M, Zhan C. Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Frontiers in Neuroanatomy. 2015;9:40. doi: 10.3389/fnana.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortley KE, Anderson KD, Yasenchak J, Murphy A, Valenzuela D, Diano S, Yancopoulos GD, Wiegand SJ, Sleeman MW. Agouti-related protein-deficient mice display an age-related lean phenotype. Cell Metabolism. 2005;2:421–427. doi: 10.1016/j.cmet.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Wu Q, Palmiter RD. GABAergic signaling by AgRP neurons prevents anorexia via a melanocortin-independent mechanism. European Journal of Pharmacology. 2011;660:21–27. doi: 10.1016/j.ejphar.2010.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Howell MP, Cowley MA, Palmiter RD. Starvation after AgRP neuron ablation is independent of melanocortin signaling. PNAS. 2008a;105:2687–2692. doi: 10.1073/pnas.0712062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Howell MP, Palmiter RD. Ablation of neurons expressing agouti-related protein activates fos and gliosis in postsynaptic target regions. Journal of Neuroscience. 2008b;28:9218–9226. doi: 10.1523/JNEUROSCI.2449-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Xu Y, Zhu Y, Sutton AK, Zhao R, Lowell BB, Olson DP, Tong Q. An obligate role of oxytocin neurons in diet induced energy expenditure. PLoS ONE. 2012;7:e45167. doi: 10.1371/journal.pone.0045167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nature Medicine. 1999;5:1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- Yen TT, Gill AM, Frigeri LG, Barsh GS, Wolff GL. Obesity, diabetes, and neoplasia in yellow A(vy)/-mice – ectopic expression of the agouti gene. FASEB Journal. 1994;8:479–488. doi: 10.1096/fasebj.8.8.8181666. [DOI] [PubMed] [Google Scholar]
- Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O’Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nature Genetics. 1998;20:111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- Zhan C, Zhou J, Feng Q, Zhang JE, Lin S, Bao J, Wu P, Luo M. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. Journal of Neuroscience. 2013;33:3624–3632. doi: 10.1523/JNEUROSCI.2742-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Gradinaru V, Adamantidis AR, Durand R, Airan RD, de Lecea L, Deisseroth K. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nature Protocols. 2010;5:439–456. doi: 10.1038/nprot.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Li M, Lu R, Alioua A, Stefani E, Toro L. The angiotensin II type 1 receptor (AT1R) closely interacts with large conductance voltage- and Ca2+-activated K+ (BK) channels and inhibits their activity independent of G-protein activation. Journal of Biological Chemistry. 2014;289:25678–25689. doi: 10.1074/jbc.M114.595603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q, Zhu J, Shanabrough M, Borok E, Benoit SC, Horvath TL, Clegg DJ, Reizes O. Enhanced anorexigenic signaling in lean obesity resistant syndecan-3 null mice. Neuroscience. 2010;171:1032–1040. doi: 10.1016/j.neuroscience.2010.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]