Abstract
Rationale: Hospital readmission for chronic obstructive pulmonary disease (COPD) has attracted attention owing to the burden to patients and the health care system. There is a knowledge gap on approaches to reducing COPD readmissions.
Objectives: To determine the effect of comprehensive health coaching on the rate of COPD readmissions.
Methods: A total of 215 patients hospitalized for a COPD exacerbation were randomized at hospital discharge to receive either (1) motivational interviewing–based health coaching plus a written action plan for exacerbations (the use of antibiotics and oral steroids) and brief exercise advice or (2) usual care.
Measurements and Main Results: We evaluated the rate of COPD-related hospitalizations during 1 year of follow-up. The absolute risk reductions of COPD-related rehospitalization in the health coaching group were 7.5% (P = 0.01), 11.0% (P = 0.02), 11.6% (P = 0.03), 11.4% (P = 0.05), and 5.4% (P = 0.24) at 1, 3, 6, 9, and 12 months, respectively, compared with the control group. The odds ratios for COPD hospitalization in the intervention arm compared with the control arm were 0.09 (95% confidence interval [CI], 0.01–0.77) at 1 month postdischarge, 0.37 (95% CI, 0.15–0.91) at 3 months postdischarge, 0.43 (95% CI, 0.20–0.94) at 6 months postdischarge, and 0.60 (95% CI, 0.30–1.20) at 1 year postdischarge. The missing value rate for the primary outcome was 0.4% (one patient). Disease-specific quality of life improved significantly in the health coaching group compared with the control group at 6 and 12 months, based on the Chronic Respiratory Disease Questionnaire emotional score (emotion and mastery domains) and physical score (dyspnea and fatigue domains) (P < 0.05). There were no differences between groups in measured physical activity at any time point.
Conclusions: Health coaching may represent a feasible and possibly effective intervention designed to reduce COPD readmissions.
Clinical trial registered with www.clinicaltrials.gov (NCT01058486).
Keywords: chronic obstructive pulmonary disease, health coaching, hospitalizations, quality of life, communication skills
At a Glance Commentary
Scientific Knowledge on the Subject
Hospital readmission for chronic obstructive pulmonary disease (COPD) has attracted attention owing to the burden to patients and the health care system. There is a knowledge gap on approaches to reducing COPD readmissions.
What This Study Adds to the Field
To our knowledge, this study provides the first randomized trial evidence of a feasible, innovative, and possibly effective intervention designed to reduce short-term readmissions for patients with COPD and able to be translated to care.
Chronic obstructive pulmonary disease (COPD) is responsible for nearly 700,000 hospitalizations annually (1), and these hospitalizations, which account for a large proportion of the annual direct medical costs of COPD (2), are potentially preventable readmissions (3). Reducing COPD readmissions is currently an important health care goal driven by the Hospital Readmissions Reduction Program, which penalizes hospitals if admissions for COPD exacerbations occur at a higher than expected rate (4).
It is known that multiple factors contribute to readmissions, many of which are not specific to COPD, such as comorbidities and psychosocial issues (5–9). Effective prevention strategies need to be comprehensive and directed at more than COPD alone.
Health coaching by trained health care providers is an innovative form of health care delivery designed to comprehensively improve the quality of patient care. Health coaching programs that include individualized, patient-centered information with decision-making and self-management support as well as coordination of care have been shown to improve outcomes for patients with chronic conditions (10, 11). These goals are achieved by motivating patients and taking advantage of their willingness to change their lifestyle, as well as by supporting the patient’s home-based self-care (10). In the management of COPD, health coaches may provide patient support in several dimensions: adherence to medications, engagement in activities (including exercise), building collaborative plans on how to respond to exacerbations of COPD, and coordinating care between the patient and providers. One-to-one phone-based health coaching using motivational interviewing (MI) skills has been associated with behavior change (12, 13) and improved self-management abilities (14), self-efficacy (15), health status (16, 17), and medication adherence (16).
A knowledge gap exists with regard to preventing rehospitalizations in COPD. The scarcity of evidence on this topic leaves hospitals and health care systems with little guidance regarding approaches to reducing readmissions among patients with COPD (18). In this study, we aimed to test the effect of comprehensive health coaching, an innovative form of health care delivery for patients with COPD, on the rate of COPD rehospitalizations following hospital discharge.
Methods
Design Overview
We conducted a multisite, randomized trial, comparing a health coaching intervention with guideline-based usual care for patients after a hospitalization for a COPD exacerbation. The primary outcome of the study was the rate of COPD-related rehospitalization (19).
Setting and Patients
Patients admitted for a COPD exacerbation (primary inclusion criterion) were contacted in the hospital before their discharge and invited to participate. Additional inclusion criteria were age older than 40 years, current or past cigarette smoking history of more than 10 pack-years, ability to speak English, and access to a telephone. Patients were excluded if they had any medical conditions that would impair their ability to participate in the study or to provide informed consent or if they were receiving hospice care. There were no other exclusion criteria. The trial ran from September 2010 to August 2014 at two sites: the Mayo Clinic (Rochester, MN) and HealthPartners Regions Hospital (St. Paul, MN). The institutional review boards at both sites approved the study, and all patients gave their written informed consent to participate.
Randomization and Interventions
We randomly assigned subjects using an online, computer-generated, simple binomial randomization program to one of the two groups, stratified by center.
Intervention
The health coaching intervention was reported in detail in a previous publication (20). Each site had one dedicated coach or interventionist; the Mayo Clinic had a registered nurse and HealthPartners Regions Hospital had a respiratory therapist. Each met with the patient in the hospital and at least once in person after discharge, with subsequent sessions conducted by telephone. After the participants completed informed consent forms, they reviewed the study procedures with the health coach. The first visit was in person and lasted approximately 2 hours. Visit 1 included providing the patient with a written emergency plan (prednisone 40 mg orally and a $4 generic antibiotic [either ciprofloxacin or doxycycline], both for 5 days) to be activated in the event of an exacerbation. During this visit, the self-management concepts, goal setting, action planning, and the details of the telephone session to come were discussed. Each encounter embodied the spirit of MI: The patient is the expert; the coach is willing to listen deeply to understand the patient; and the participant is empowered to adopt the behavior he or she feels is important to pursue, even if not related to the pulmonary condition. The coaches asked about what the patient was already doing to be more comfortable living with chronic lung disease and to be and/or stay healthy in general, and they tried to explore the patient’s near-term hopes and goals to work on. Specific instructions on the use of the emergency plan are provided in the online supplement.
A copy of the book Living a Healthy Life with Chronic Conditions (21) was provided to introduce the concept of self-management. The patient was provided with a Stamina InMotion Elliptical Trainer (Stamina Products, Springfield, MO) to use daily while sitting (aiming for 20 min/d, a dose of exercise associated with improved outcomes in COPD [22]) and instructed on how to perform three simple upper extremity exercises (five repetitions) from the book. Instruction on slow pursed lip breathing was performed, followed by demonstration by the patient and discussion about how it felt. Participants were also invited to call the coach if they had concerns about worsening symptoms and self-initiating the emergency medications. The primary care provider of each patient in the intervention was engaged starting with the first visit, and this provider was kept updated by letter.
To evaluate fidelity to the intervention, one of the study investigators listened to 100 (5% of total) sessions to assess interventionists’ adherence to the intervention road map and MI principles (23, 24). A fidelity checklist was used to evaluate the general session content, self-management strategies, MI techniques, and global MI ratings using a range of 1 (low) to 5 (high) for MI spirit elements. Feedback was also provided to interventionists when indicated to strengthen their skills and fidelity to the intervention (23).
Control group: guideline-based usual care
Both the intervention arm and the usual care group received care in accordance with the Global Initiative for Chronic Obstructive Lung Disease and were also referred for conventional pulmonary rehabilitation (PR) (25).
Data and Safety Monitoring
The National Institutes of Health, which funded this study, required a data and safety monitoring board to monitor protocol adherence as well as patient accrual, outcomes, and complications.
Outcomes and Follow-up
The a priori primary outcome as funded was the rate of COPD hospitalization. The goal was to follow all patients for at least 12 months. Research staff blinded to study group allocation contacted patients every 3 months to determine whether they had developed symptoms of a COPD exacerbation or were hospitalized. When a hospitalization in the previous 3-month period was identified in the patient report, records were immediately requested to ascertain length of stay and discharge diagnosis. To ascertain that recall bias was not an issue in the study, the reported hospitalizations were compared with the electronic medical record or health plan claims data and found to be accurate. There were no differences in the rate of hospitalizations between HealthPartners Regions Hospital and the Mayo Clinic. Per protocol, three pulmonologists blinded to group allocation reviewed discharge summaries and other available information to determine the primary cause of all hospitalizations and classified them as COPD related (COPD exacerbation or pneumonia), cardiovascular, or other. Secondary outcomes included disease-specific quality of life (QoL) and measured physical activity at 6 and 12 months.
Baseline and Follow-up Assessments
At baseline, 6-month, and 1-year study visits, the disease-specific health-related QoL was measured with the Chronic Respiratory Disease Questionnaire (CRQ). The minimal clinically important difference accepted for this instrument is 0.5 points (26).
Physical activity was measured with a monitor validated in COPD (BodyMedia armband; BodyMedia, Pittsburgh, PA) (27, 28). Activity was recorded in terms of the average number of steps and minutes per day spent in daily physical activities of at least moderate intensity.
Statistical Analysis
The a priori primary endpoint as funded was the percentage of patients with a COPD-related hospitalization at 12 months. The analyses at 1, 3, and 6 months were considered secondary endpoints. These percentages were compared between the two treatment groups using χ2 tests with 5% type I error rates.
Patients with missing or unknown outcomes were excluded from this analysis. Logistic models were used to compare these rates between arms after adjusting for the confounding factors of age, modified Medical Research Council dyspnea scale (mMRC) score, and FEV1. The sensitivity of these models was evaluated using 1,000 bootstrapped samples. Intent-to-treat analyses were also run to account for the missing values (almost none for the primary outcome). Patients with missing values were considered to have died or to have had COPD hospitalization in these analyses. Because there were very few missing values and results from intent-to-treat analyses were similar to the original analyses, no imputations were done. Secondary analyses included the percentage of any hospitalization and percentage of deaths. Patients who died before an evaluation time point and had no hospitalizations were considered as missing with regard to whether they had a hospitalization before that evaluation time point.
To reach 80% power, an estimated sample size of 101 for each group was needed to detect a 20% difference (50% control to 30% intervention) between the group readmission rates. The test statistic used in the calculations was a two-sided z-test with continuity correction and unpooled variance. The significance level of the test was 0.05. Analyses were done using SAS version 9.4 software (SAS Institute Inc., Cary, NC).
Results
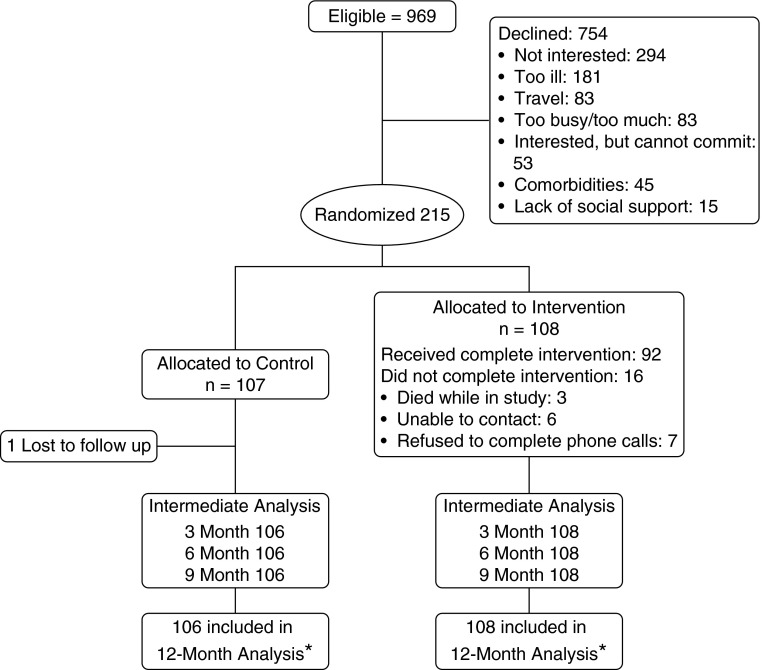
Recruitment details are shown in the Consolidated Standards of Reporting Trials (CONSORT) diagram in Figure 1. In our previous report (29), we compared the age, FEV1 percentage of predicted value, mMRC score, and age–dyspnea–obstruction index of the individuals who declined the intervention with these data for the subjects who were included in the study, and we found no differences, suggesting that the population recruited reflected the intended population without bias.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram of the study plan. *Death or chronic obstructive pulmonary disease–related hospitalization.
Baseline demographic, background information, comorbidities, and COPD outcomes were balanced between groups, as shown in Table 1. Groups were well balanced, as expected in a randomized study; there were no differences between groups in age, education, sex, insurance carrier, marital status, use of oxygen, exacerbation history, comorbidity burden, lung function, QoL, or measured physical activity. In the intervention group, 85% of the individuals received a complete intervention, defined as 15 (>70%) of 21 calls completed. Reasons for not completing the intervention were death during the study period (n = 3), unable to contact (n = 6), and refusal to complete the scheduled calls (n = 7).
Table 1.
Baseline Clinical and Demographic Characteristics
| Characteristic | Control (n = 107) | Intervention (n = 108) |
|---|---|---|
| Age, yr | 68.1 (9.2) | 67.9 (9.8) |
| Site | ||
| HealthPartners Regions Hospital | 52 (49%) | 52 (48%) |
| Mayo Clinic | 55 (51%) | 56 (52%) |
| Postsecondary education | 48 (48%) | 55 (54%) |
| Medicare coverage | 82 (82%) | 79 (77%) |
| Married | 48 (46%) | 54 (50%) |
| Male sex | 51 (48%) | 46 (43%) |
| Continuous supplemental oxygen | 32 (34%) | 40 (40%) |
| Supplemental oxygen with activity or sleep | 49 (51%) | 54 (53%) |
| Patient-reported exacerbation | ||
| Within the last 12 mo | 79 (90%) | 83 (86%) |
| Two or more exacerbations in past 12 mo | 61 (71%) | 61 (69%) |
| Hospitalization in past 12 mo | 60% | 57% |
| Charlson comorbidity index | 3.0 (1.5) | 3.7 (1.8) |
| FEV1, % predicted | 40.3 (17.2) | 40.5 (17.1) |
| TLC, % predicted | 115.2 (25.2) | 110.0 (24.4) |
| RV, % predicted | 173.7 (58.0) | 174.6 (72.8) |
| DlCO, % predicted | 38.7 (23.1) | 35.8 (19.3) |
| mMRC score | ||
| 0 | 3 (3%) | 2 (2%) |
| 1 | 18 (20%) | 11 (12%) |
| 2 | 9 (10%) | 12 (13%) |
| 3 | 51 (57%) | 62 (67%) |
| 4 | 9 (10%) | 6 (6%) |
| CRQ quality of life domains | ||
| Physical function (dyspnea and fatigue) | 4.1 (1.2) | 4.1 (1.1) |
| Emotional function (mastery and emotion) | 4.7 (1.4) | 4.6 (1.2) |
| Daily physical activity | ||
| Steps | 2,789.9 (3,000.7) | 2,448.0 (2,030.7) |
| Physical activity level | 1.3 (0.2) | 1.3 (0.2) |
| Sedentary (<2 METS), min | 1,220.5 (175.5) | 1,239.8 (125.0) |
| Light activity (2–4 METS), min | 130.0 (107.5) | 133.4 (115.5) |
| Moderate activity (4–6 METS), min | 14.0 (29.0) | 8.0 (12.6) |
| Vigorous activity (>6 METS), min | 0.7 (2.5) | 0.3 (0.9) |
| Resting metabolic rate, cal/24 h | 1,590.3 (379.4) | 1,529.8 (309.7) |
| Total energy expenditure, cal/24 h | 2,069.8 (495.5) | 2,016.7 (442.8) |
Definition of abbreviations: CRQ = Chronic Respiratory Disease Questionnaire; DlCO = diffusing capacity of the lung for carbon monoxide; METS = metabolic equivalents; mMRC = modified Medical Research Council dyspnea scale; RV = residual volume; TLC = total lung capacity.
Data are presented as mean (SD) or count (%).
The health coaching intervention had a significant effect on the main outcome (rates of COPD hospitalization at 1, 3, and 6 months post–hospital discharge), but this effect faded at 12 months (Table 2). The significance levels found at 1, 3, and 6 months for prevention of COPD readmission (Table 2) permitted calculation of the number needed to treat (NNT). On the basis of that analysis, 13 patients needed to be going through health coaching to prevent a hospitalization at 1 month, about 9 patients to prevent a hospitalization at 3 months, and 8 patients to prevent a hospitalization at 6 months.
Table 2.
Main Study Outcomes
| Characteristic | Control (%) (n = 107) | Intervention (%) (n = 108) | ARR (%) | NNT | P Value |
|---|---|---|---|---|---|
| Confirmed COPD-related hospitalization | |||||
| 1 mo AD | 9.4 | 1.9 | 7.5 | 13 | 0.0174 |
| 3 mo AD | 20.4 | 9.4 | 11.0 | 9 | 0.0280 |
| 6 mo AD | 27.7 | 15.4 | 11.6 | 8 | 0.0315 |
| 9 mo AD | 32.7 | 20.6 | 11.4 | 0.0514 | |
| 12 mo AD | 36.0 | 28.4 | 5.2 | 0.2496 | |
| All-cause hospitalization | |||||
| 1 mo AD | 11.3 | 4.6 | 6.7 | 0.213 | |
| 3 mo AD | 25.5 | 13.9 | 11.6 | 9 | 0.039 |
| 6 mo AD | 37.7 | 25.9 | 11.8 | 8 | 0.036 |
| 9 mo AD | 44.3 | 35.2 | 9.1 | 0.174 | |
| 12 mo AD | 50.0 | 40.7 | 9.3 | 0.172 |
Definition of abbreviations: AD = after hospital discharge; ARR = absolute risk reduction; COPD = chronic obstructive pulmonary disease; NNT = number needed to treat.
We also found that days in the hospital after discharge were fewer in the intervention group. For every 10 patients treated, there were 3 fewer hospital days at 1 month (mean length of stay in a COPD hospitalization at 1 month, 0.3 vs. 0.0; P = 0.015), 4 fewer hospital days at 3 months (mean length of stay in a COPD hospitalization at 3 months, 0.8 vs. 0.4; P = 0.027), and 8 fewer hospital days at 6 months (mean length of stay in a COPD hospitalization at 6 months, 1.5 vs. 0.7; P = 0.027).
Given the findings of significant differences in hospitalizations at intermediate time points 1, 3, and 6 months during the 1-year follow-up, we built logistic models (Table 3) to test the validity and robustness of the intervention on COPD hospitalization at those specific time points after adjusting for the most meaningful covariates in COPD that predict a COPD hospitalization: age, lung function (FEV1), level of breathlessness (mMRC score), and the number of hospitalizations in the previous year (2, 30, 31).
Table 3.
Logistic Models for Chronic Obstructive Pulmonary Disease Hospitalization at Each Time Point
| Time Point | Odds Ratio for COPD Hospitalization in Intervention Arm Compared with Control Arm | 95% Confidence Interval | P Value for a Difference Between the Intervention and Control Arms |
|---|---|---|---|
| 1 mo | 0.092 | 0.011–0.769 | 0.0276 |
| 3 mo | 0.371 | 0.150–0.916 | 0.0315 |
| 6 mo | 0.430 | 0.196–0.940 | 0.0344 |
| 12 mo | 0.600 | 0.300–1.203 | 0.1502 |
Definition of abbreviation: COPD = chronic obstructive pulmonary disease.
Models were adjusted for age, lung function (FEV1), level of breathlessness (modified Medical Research Council dyspnea scale), and the number of hospitalizations in the year before baseline.
We found a significant and sustained beneficial effect on disease-specific, health-related QoL as measured by CRQ physical function (dyspnea and fatigue domains) and CRQ emotional function (mastery and emotion domains) at 6 and 12 months after hospital discharge (Table 4). We also found that a higher percentage of patients in the intervention group had improved their QoL based on the minimal clinically important difference (Table 4). We did not find a difference in any physical activity outcome between the intervention and control arms at any time point. The effect of the intervention on the main outcomes was not different at the two study sites.
Table 4.
Secondary Outcomes
| Characteristic | Control | Intervention | NNT | P Value |
|---|---|---|---|---|
| CRQ emotional function | ||||
| Month 6 change from baseline, mean (SD) | 0.10 (1.0) | 0.50 (1.0) | 0.004 | |
| Month 12 change from baseline, mean (SD) | 0.15 (0.9) | 0.43 (1.0) | 0.058 | |
| Improved more than MCID at 6 mo, % | 14.1 | 29.9 | 6 | 0.018 |
| Improved more than MCID at 12 mo, % | 14.3 | 28.8 | 6 | 0.036 |
| CRQ physical function | ||||
| Month 6 change from baseline, mean (SD) | −0.01 (1.0) | 0.33 (0.9) | 0.036 | |
| Month 12 change from baseline, mean (SD) | −0.04 (1.0) | 0.27 (1.0) | 0.016 | |
| Improved more than MCID at 6 mo, % | 12.8 | 26.0 | 7 | 0.038 |
| Improved more than MCID at 12 mo, % | 11.4 | 19.2 | 0.199 | |
| Physical activity level change at Month 6 | 0.01 | 0.01 | NS | |
| Physical activity level change at Month 12 | 0.01 | −0.10 | NS | |
| Died in first 12 mo, n (%) | 12 (11.3%) | 10 (9.3%) | 0.620 | |
| Patient-reported prednisone/antibiotic use, n (%) | ||||
| Baseline to Month 3 | 40 (46.5%) | 51 (56.7%) | 0.178 | |
| Month 3 to Month 6 | 37 (44.0%) | 46 (51.1%) | 0.351 | |
| Month 6 to Month 9 | 35 (43.2%) | 49 (57.6%) | 0.063 | |
| Month 9 to Month 12 | 34 (43.0%) | 54 (65.9%) | 0.004 | |
| Any use in first 12 mo | 64 (68.8%) | 83 (87.4%) | 0.002 |
Definition of abbreviations: CRQ = Chronic Respiratory Disease Questionnaire; MCID = minimal clinically important difference, NNT = number needed to treat, NS = nonsignificant.
There was no difference between groups in the use of an antibiotic-prednisone combination (written action plan) at 3, 6, or 9 months after discharge (when the intervention was significant in decreasing hospitalizations). There was greater use of the written action plan only from Months 9 to 12 in the intervention group (when there was not an effect for the intervention on reducing hospitalizations). The mean number of confirmed exacerbations, defined as emergency department visits, nurse triage, or urgent care clinics, was greater in the control group than in the intervention group during the 12-month period of the study (mean [SD], 1.15 [1.5] vs. 0.8 [1.5]; P = 0.03).
Attendance at PR visits in the first 3 months after discharge (as part of the patient’s discharge plan, not research) was greater in the intervention group than in the control group (50% vs. 33%; P = 0.017). Attendance at PR visits anytime in the first 12 months after discharge tended to be higher in the intervention group (53% vs. 43%; P = 0.056).
The fidelity of the health coaching intervention to MI principles, as measured by the global ratings of the MI Treatment Integrity (24) tool, were as follows on a 1–5 scale and expressed as mean (SD): empathy 3.9 (0.5), collaboration 4 (0.6), autonomy/support 4 (0.3), direction 3.9 (0.5), and evocation 3.9 (0.5). A score greater than 3 was required to maintain minimal proficiency.
Discussion
In this study, we found that a comprehensive health coaching intervention that included MI-based intervention delivered via telephone, a written action plan for exacerbations, and an exercise prescription decreased COPD-related hospitalizations at 1, 3, and 6 months after hospital discharge, but not at 1 year after discharge. Our results may suggest a negative study result if only hospitalization at 12 months is considered. However, our study indicates a possibly effective intervention to decrease short-term (30 d) rehospitalizations (18), an outcome that is avidly pursued now in every health care system because there is no intervention that has been tested and has shown effectiveness in a randomized or nonrandomized study (4, 32).
Our results support health coaching as an innovative form of delivering care to patients with COPD after a hospital admission. We found health coaching to be a simple and trainable intervention with low cost and that was feasible (not requiring transportation, unlike PR) and had a high likelihood of replication, given the expansive availability of health coaching training programs. Health coaching is versatile; it can be incorporated into treatment of patients with other prevalent chronic diseases, such as diabetes and heart failure. NNT analysis indicates that 8–13 patients need to be treated to prevent one hospitalization at different time points. A higher NNT (less effective) has been reported for bronchodilators to prevent an exacerbation, not a hospitalization (33, 34).
Effect on Disease-Specific Quality of Life
We found a sustained improvement in health-related QoL in all domains tested, including physical function, representing dyspnea and fatigue (the two most common symptoms in COPD [35]) as well as emotional function. The latter may become a groundbreaking finding, as there is a knowledge gap regarding interventions to improve emotions in COPD, which are responsible for poor QoL and increased health care use (36). So far, PR has been shown to be the best intervention to improve emotions in COPD; however, PR can be completed by only 10% of patients with COPD after a hospitalization (37). We fully recognize that while we found a statistically significant difference in CRQ, not all patients improved to a clinically meaningful extent. However, our analysis of the percentage of patients who had clinically significant improvement showed that about six or seven patients needed to be treated (NNT = 6–7) (Table 4) for one patient to achieve a meaningful improvement in QoL at 6 months in both physical (dyspnea and fatigue domains) and emotional (emotion and mastery domains) summary CRQ scores. Also, the NNT is six for a meaningful improvement at 12 months in the emotion domain summary score. The improvement in QoL—and in particular the sustained improvement in emotion—is clearly of significance, given that we are in need of interventions that improve emotions in COPD that are independently responsible for high health care use. Also, the meaningfulness of our results is rooted in the simplicity and feasibility of health coaching as an intervention, in contrast to PR, which, while highly effective and able to produce the same outcomes, is available for only a small percentage of patients. The overwhelming adherence to health coaching speaks of its feasibility and acceptability. It is plausible that the effect size seen in CRQ is comparable and likely not inferior to the one seen in PR, an established and accepted intervention in COPD (38). The improvement in QoL found in our study is not a minor event, as there may be long-term effects beyond 1 year and patients whose health status improves have less likelihood of exacerbation, hospitalization, or dying during a 2-year follow-up (39, 40).
Plausible Explanations for the Lack of Effect at 12 Months
Two factors may contribute in the lack of effect at 12 months. First, at the time this study was launched, the rate of 12-month readmission was about 50% in our preliminary data (Table 1), consistent with previously published data (41). However, as the study evolved, we observed that the rate of readmission decreased, possibly related to increased use of macrolides and written action plans to prevent exacerbations, based on the positive results of two large studies (42, 43) and the implementation of readmission reduction programs at both institutions due to the upcoming readmission initiative (44). The second factor that may have resulted in lesser effects at 12 months is that the intensity of the intervention decreased from weekly calls to monthly calls at Month 3 after discharge and that the ripple effect of the lower intensity of calls and less patient support may have translated into less effectiveness.
The intervention did not increase measured physical activity by a validated monitor used for at least 4 days. This is not surprising, as there is a knowledge gap on how to improve physical activity in COPD. There is limited and inconsistent evidence on the effectiveness of interventions for improving physical activity (45, 46). Importantly, the intervention tested produced no harm, which is relevant, given the results of a recent study in which researchers tested a self-management program using a written action plan and stopped it due to increased mortality in the intervention arm (19).
Limitations
In this study, we found significant differences in the rates of COPD hospitalizations at 1, 3, and 6 months postdischarge, and the study was powered to find a difference in the rate of hospitalization at 12 months, which was not found. We believe that the differences at the intermediate time points are robust, as supported by the bootstrapped logistic models adjusted to the most meaningful factors that can predict a hospitalization. Also, the absolute risk reduction showed consistency over time (roughly 10% reduction of hospitalizations).
The only other randomized study done in the United States to test an intervention to decrease 1- and 3-month rehospitalizations proposed the same sample size (32), suggesting that our study may not have been underpowered for the intermediate outcomes. Our results represent the first available randomized evidence of a feasible and possibly effective intervention designed to reduce short-term readmissions of patients with COPD that could be translated to care, representing a true health care delivery innovation that can palliate the current “translational shortfall—that is, the delay in getting promising innovations in care delivery to the populations they can help” (47).
We tested a comprehensive health coaching intervention that included multiple components; we cannot know the exact contributory effect of each component. We did prescribe a simple exercise program that did not improve daily physical activity but could have had a rehabilitation-like effect. It is known that attending PR visits after discharge from a COPD hospitalization is a desirable but not widely feasible intervention for most communities, as published data indicate that only 10% of post–hospital discharge patients complete PR (37). However, there is conflicting evidence on the effect of posthospitalization PR (48, 49). There was higher attendance at PR visits in the intervention and control groups than in published standards (37), indicating that greater attention to patients, like in a clinical trial, may increase attendance. However, only individuals in the intervention group showed decreased hospitalization, suggesting that mindful attention (not any attention) such as that received in health coaching may matter because health coaching may motivate people to engage in behaviors (e.g., attending PR visits) that translate into improvement of “hard” outcomes (e.g., hospitalizations).
The use of antibiotics and prednisone in the intervention group was similar to that in the control group when the intervention showed effectiveness in decreasing rehospitalizations, downplaying the role of the written action plan for the use of antibiotics and steroids in explaining the differences in rehospitalization at 1, 3, and 6 months. In addition, there is clear evidence that the mere prescription of a written action plan for exacerbations is not sufficient to produce a difference in hospitalization in COPD (41).
On the basis of these latter points, we speculate that the health coaching component may be the most plausible factor associated with the differences found between groups in terms of hospitalizations, perhaps by the promotion of better patient activation and self-management abilities, which may improve coping and lead to less intensive medical care in the context of severe COPD and often the frailty phenotype (29).
Strengths
This study is seminal to informing knowledge about interventions that decrease short-term rehospitalizations among patients with COPD (18). We found only one other published study in the United States and/or Canada in which researchers examined rehospitalization for patients with COPD recruited while in the hospital, rather than stable patients (19, 50). We were able to recruit a patient population that is very inactive (physical activity level, <1.4) (28, 51) and most of the time reluctant to participate in research (29). Accruing participants for a study of this population is difficult: the National Institutes of Health COPD Clinical Research Network, a multicenter group of large and highly reputable centers, failed to accrue a sufficient number of participants in a study aimed at decreasing exacerbations that targeted the same population we did in this study (patients hospitalized with a COPD exacerbation) (52). We had minimal missing data, and 85% of the individuals in the intervention group received the intervention as intended.
Above all, we tested an intervention aimed primarily at promoting patient activation and mindful communication, two critical and perhaps underrated aspects of care that we firmly believe are at the heart of practicing the art of medicine (53). In concert with the MI spirit, we heavily emphasized aspects such as compassion (a true will to decrease suffering in the patient), empathy (an active effort to understand others’ internal perspectives and see the world through their eyes), deep listening, autonomy for self-care (honoring and respecting each person’s autonomy and irrevocable right to and capacity for self-direction), and the role of the health coach to facilitate activation of the person’s own motivation and resources for change (24).
Future Directions
The importance of the publication and dissemination of this work is to increase awareness of the possible effectiveness of this intervention to generate validating studies. Given that the intervention is simple, safe, of low cost, and feasible, further studies with randomized or hybrid designs that combine elements of clinical effectiveness and implementation research are needed to confirm, implement, and disseminate our results (54, 55).
Conclusions
This study may represent the first available randomized evidence of a feasible and possibly effective intervention to reduce short-term readmissions for patients with COPD that could be translated to care, representing a true health care delivery innovation.
Supplementary Material
Acknowledgments
Acknowledgment
The authors recognize the work of Kathryn Chaloner, Ph.D., University of Iowa Data and Safety Monitoring Board statistician, who died in October 2014. The authors also thank deeply Dennis Niewoehner, M.D., University of Minnesota, and Robert Wise, M.D., Johns Hopkins University, for their role as COPD experts on the data and safety monitoring board. Finally, the authors recognize the work of Marnie Wetzstein, R.N., and Donna Rasmussen, R.N., who worked in part of the study as health coaches, and Janae Kirsch, who helped with the final manuscript editing and submission.
Footnotes
Supported by NHLBI grant R01 HL09468 (R.B., principal investigator) from the National Institutes of Health.
Author Contributions: All authors had access to the data and take responsibility for the integrity of the data and the accuracy of the data analysis. R.B.: contributed to the study conception, hypothesis delineation, data analysis and interpretation, and manuscript writing and revision; P.J.N. and J.C.: contributed to data analysis and interpretation and revision of the manuscript; K.V. and C.M.: contributed to the design of the study, acquisition and interpretation of the data, and revision of the manuscript; and S.T., P.N., J.H., and K.L.: contributed to data acquisition and writing and revision of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201512-2503OC on March 8, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Almagro P, Barreiro B, Ochoa de Echaguen A, Quintana S, Rodríguez Carballeira M, Heredia JL, Garau J. Risk factors for hospital readmission in patients with chronic obstructive pulmonary disease. Respiration. 2006;73:311–317. doi: 10.1159/000088092. [DOI] [PubMed] [Google Scholar]
- 2.Roberts CM, Lowe D, Bucknall CE, Ryland I, Kelly Y, Pearson MG. Clinical audit indicators of outcome following admission to hospital with acute exacerbation of chronic obstructive pulmonary disease. Thorax. 2002;57:137–141. doi: 10.1136/thorax.57.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medicare Payment Advisory Commission (Medpac)Report to the Congress: promoting greater efficiency in Medicare. Washington, DC: Medpac; June 2007 [accessed 2016 Mar 16]. Available from: http://www.medpac.gov/documents/reports/Jun07_EntireReport.pdf
- 4.Elixhauser A, Au DH, Podulka J.Readmissions for chronic obstructive pulmonary disease, 2008. Statistical Brief 121. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville, MD: Agency for Health Care Policy and Research; September 2011 [accessed 2016 Mar 16]. Available from: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb121.pdf
- 5.Kasper EK, Gerstenblith G, Hefter G, Van Anden E, Brinker JA, Thiemann DR, Terrin M, Forman S, Gottlieb SH. A randomized trial of the efficacy of multidisciplinary care in heart failure outpatients at high risk of hospital readmission. J Am Coll Cardiol. 2002;39:471–480. doi: 10.1016/s0735-1097(01)01761-2. [DOI] [PubMed] [Google Scholar]
- 6.Jack BW, Chetty VK, Anthony D, Greenwald JL, Sanchez GM, Johnson AE, Forsythe SR, O’Donnell JK, Paasche-Orlow MK, Manasseh C, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150:178–187. doi: 10.7326/0003-4819-150-3-200902030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAlister FA, Lawson FM, Teo KK, Armstrong PW. A systematic review of randomized trials of disease management programs in heart failure. Am J Med. 2001;110:378–384. doi: 10.1016/s0002-9343(00)00743-9. [DOI] [PubMed] [Google Scholar]
- 8.Phillips CO, Wright SM, Kern DE, Singa RM, Shepperd S, Rubin HR. Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: a meta-analysis. JAMA. 2004;291:1358–1367. doi: 10.1001/jama.291.11.1358. [DOI] [PubMed] [Google Scholar]
- 9.Yu DS, Thompson DR, Lee DT. Disease management programmes for older people with heart failure: crucial characteristics which improve post-discharge outcomes. Eur Heart J. 2006;27:596–612. doi: 10.1093/eurheartj/ehi656. [DOI] [PubMed] [Google Scholar]
- 10.Kivelä K, Elo S, Kyngäs H, Kääriäinen M. The effects of health coaching on adult patients with chronic diseases: a systematic review. Patient Educ Couns. 2014;97:147–157. doi: 10.1016/j.pec.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 11.Dennis SM, Harris M, Lloyd J, Powell Davies G, Faruqi N, Zwar N. Do people with existing chronic conditions benefit from telephone coaching? A rapid review. Aust Health Rev. 2013;37:381–388. doi: 10.1071/AH13005. [DOI] [PubMed] [Google Scholar]
- 12.Eakin MN, Rand CS, Borrelli B, Bilderback A, Hovell M, Riekert KA. Effectiveness of motivational interviewing to reduce Head Start children’s secondhand smoke exposure: a randomized clinical trial. Am J Respir Crit Care Med. 2014;189:1530–1537. doi: 10.1164/rccm.201404-0618OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borrelli B, Riekert KA, Weinstein A, Rathier L. Brief motivational interviewing as a clinical strategy to promote asthma medication adherence. J Allergy Clin Immunol. 2007;120:1023–1030. doi: 10.1016/j.jaci.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Walters J, Cameron-Tucker H, Wills K, Schüz N, Scott J, Robinson A, Nelson M, Turner P, Wood-Baker R, Walters EH. Effects of telephone health mentoring in community-recruited chronic obstructive pulmonary disease on self-management capacity, quality of life and psychological morbidity: a randomised controlled trial. BMJ Open. 2013;3:e003097. doi: 10.1136/bmjopen-2013-003097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linden A, Butterworth SW, Prochaska JO. Motivational interviewing-based health coaching as a chronic care intervention. J Eval Clin Pract. 2010;16:166–174. doi: 10.1111/j.1365-2753.2009.01300.x. [DOI] [PubMed] [Google Scholar]
- 16.Wolever RQ, Dreusicke M, Fikkan J, Hawkins TV, Yeung S, Wakefield J, Duda L, Flowers P, Cook C, Skinner E. Integrative health coaching for patients with type 2 diabetes: a randomized clinical trial. Diabetes Educ. 2010;36:629–639. doi: 10.1177/0145721710371523. [DOI] [PubMed] [Google Scholar]
- 17.Thomas ML, Elliott JE, Rao SM, Fahey KF, Paul SM, Miaskowski C. A randomized, clinical trial of education or motivational-interviewing-based coaching compared to usual care to improve cancer pain management. Oncol Nurs Forum. 2012;39:39–49. doi: 10.1188/12.ONF.39-49. [DOI] [PubMed] [Google Scholar]
- 18.Prieto-Centurion V, Markos MA, Ramey NI, Gussin HA, Nyenhuis SM, Joo MJ, Prasad B, Bracken N, Didomenico R, Godwin PO, et al. Interventions to reduce rehospitalizations after chronic obstructive pulmonary disease exacerbations: a systematic review. Ann Am Thorac Soc. 2014;11:417–424. doi: 10.1513/AnnalsATS.201308-254OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan VS, Gaziano JM, Lew R, Bourbeau J, Adams SG, Leatherman S, Thwin SS, Huang GD, Robbins R, Sriram PS, et al. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized, controlled trial. Ann Intern Med. 2012;156:673–683. doi: 10.7326/0003-4819-156-10-201205150-00003. [DOI] [PubMed] [Google Scholar]
- 20.Benzo R, Vickers K, Ernst D, Tucker S, McEvoy C, Lorig K. Development and feasibility of a self-management intervention for chronic obstructive pulmonary disease delivered with motivational interviewing strategies. J Cardiopulm Rehabil Prev. 2013;33:113–123. doi: 10.1097/HCR.0b013e318284ec67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorig K, Holman H, Sobel D, Laurent D. Living a healthy life with chronic conditions: self-management of heart disease, arthritis, diabetes, asthma, bronchitis, emphysema and others. 3rd ed. Boulder, CO: Bull Publishing; 2006. [Google Scholar]
- 22.Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Antó JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006;61:772–778. doi: 10.1136/thx.2006.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hettema JE, Ernst D, Williams JR, Miller KJ. Parallel processes: using motivational interviewing as an implementation coaching strategy. J Behav Health Serv Res. 2014;41:324–336. doi: 10.1007/s11414-013-9381-8. [DOI] [PubMed] [Google Scholar]
- 24.Miller WR, Rose GS. Toward a theory of motivational interviewing. Am Psychol. 2009;64:527–537. doi: 10.1037/a0016830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 26.Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. Eur Respir J. 2002;19:398–404. doi: 10.1183/09031936.02.00063702. [DOI] [PubMed] [Google Scholar]
- 27.Patel SA, Benzo RP, Slivka WA, Sciurba FC. Activity monitoring and energy expenditure in COPD patients: a validation study. COPD. 2007;4:107–112. doi: 10.1080/15412550701246658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watz H, Pitta F, Rochester CL, Garcia-Aymerich J, ZuWallack R, Troosters T, Vaes AW, Puhan MA, Jehn M, Polkey MI, et al. An official European Respiratory Society statement on physical activity in COPD. Eur Respir J. 2014;44:1521–1537. doi: 10.1183/09031936.00046814. [DOI] [PubMed] [Google Scholar]
- 29.Benzo R, Wetzstein M, Neuenfeldt P, McEvoy C. Implementation of physical activity programs after COPD hospitalizations: lessons from a randomized study. Chron Respir Dis. 2015;12:5–10. doi: 10.1177/1479972314562208. [DOI] [PubMed] [Google Scholar]
- 30.Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, Miller B, Lomas DA, Agusti A, MacNee W, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Aymerich J, Serra Pons I, Mannino DM, Maas AK, Miller DP, Davis KJ. Lung function impairment, COPD hospitalisations and subsequent mortality. Thorax. 2011;66:585–590. doi: 10.1136/thx.2010.152876. [DOI] [PubMed] [Google Scholar]
- 32.Jennings JH, Thavarajah K, Mendez MP, Eichenhorn M, Kvale P, Yessayan L. Predischarge bundle for patients with acute exacerbations of COPD to reduce readmissions and ED visits: a randomized controlled trial. Chest. 2015;147:1227–1234. doi: 10.1378/chest.14-1123. [DOI] [PubMed] [Google Scholar]
- 33.Suissa S. Number needed to treat in COPD: exacerbations versus pneumonias. Thorax. 2013;68:540–543. doi: 10.1136/thoraxjnl-2012-202709. [DOI] [PubMed] [Google Scholar]
- 34.Gershon AS, Campitelli MA, Croxford R, Stanbrook MB, To T, Upshur R, Stephenson AL, Stukel TA. Combination long-acting β-agonists and inhaled corticosteroids compared with long-acting β-agonists alone in older adults with chronic obstructive pulmonary disease. JAMA. 2014;312:1114–1121. doi: 10.1001/jama.2014.11432. [DOI] [PubMed] [Google Scholar]
- 35.Meek PM, Lareau SC. Critical outcomes in pulmonary rehabilitation: assessment and evaluation of dyspnea and fatigue. J Rehabil Res Dev. 2003;40(5) Suppl 2:13–24. doi: 10.1682/jrrd.2003.10.0013. [DOI] [PubMed] [Google Scholar]
- 36.Panagioti M, Scott C, Blakemore A, Coventry PA. Overview of the prevalence, impact, and management of depression and anxiety in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:1289–1306. doi: 10.2147/COPD.S72073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones SE, Green SA, Clark AL, Dickson MJ, Nolan AM, Moloney C, Kon SS, Kamal F, Godden J, Howe C, et al. Pulmonary rehabilitation following hospitalisation for acute exacerbation of COPD: referrals, uptake and adherence. Thorax. 2014;69:181–182. doi: 10.1136/thoraxjnl-2013-204227. [DOI] [PubMed] [Google Scholar]
- 38.Gillespie P, O’Shea E, Casey D, Murphy K, Devane D, Cooney A, Mee L, Kirwan C, McCarthy B, Newell J PRINCE study team. The cost-effectiveness of a structured education pulmonary rehabilitation programme for chronic obstructive pulmonary disease in primary care: the PRINCE cluster randomised trial. BMJ Open. 2013;3:e003479. doi: 10.1136/bmjopen-2013-003479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilke S, Jones PW, Müllerova H, Vestbo J, Tal-Singer R, Franssen FM, Agusti A, Bakke P, Calverley PM, Coxson HO, et al. One-year change in health status and subsequent outcomes in COPD. Thorax. 2015;70:420–425. doi: 10.1136/thoraxjnl-2014-205697. [DOI] [PubMed] [Google Scholar]
- 40.Fan VS, Curtis JR, Tu SP, McDonell MB, Fihn SD Ambulatory Care Quality Improvement Project Investigators. Using quality of life to predict hospitalization and mortality in patients with obstructive lung diseases. Chest. 2002;122:429–436. doi: 10.1378/chest.122.2.429. [DOI] [PubMed] [Google Scholar]
- 41.Bucknall CE, Miller G, Lloyd SM, Cleland J, McCluskey S, Cotton M, Stevenson RD, Cotton P, McConnachie A. Glasgow Supported Self-management Trial (GSuST) for patients with moderate to severe COPD: randomised controlled trial. BMJ. 2012;344:e1060. doi: 10.1136/bmj.e1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JAD, Jr, Criner GJ, Curtis JL, Dransfield MT, Han MK, Lazarus SC, et al. COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689–698. doi: 10.1056/NEJMoa1104623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rice KL, Dewan N, Bloomfield HE, Grill J, Schult TM, Nelson DB, Kumari S, Thomas M, Geist LJ, Beaner C, et al. Disease management program for chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med. 2010;182:890–896. doi: 10.1164/rccm.200910-1579OC. [DOI] [PubMed] [Google Scholar]
- 44.Feemster LC, Au DH. Penalizing hospitals for chronic obstructive pulmonary disease readmissions. Am J Respir Crit Care Med. 2014;189:634–639. doi: 10.1164/rccm.201308-1541PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larson JL, Vos CM, Fernandez D. Interventions to increase physical activity in people with COPD: systematic review. Annu Rev Nurs Res. 2013;31:297–326. doi: 10.1891/0739-6686.31.297. [DOI] [PubMed] [Google Scholar]
- 46.Troosters T, van der Molen T, Polkey M, Rabinovich RA, Vogiatzis I, Weisman I, Kulich K. Improving physical activity in COPD: towards a new paradigm. Respir Res. 2013;14:115. doi: 10.1186/1465-9921-14-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berwick D, Bauchner H, Fontanarosa PB. Innovations in health care delivery: call for papers for a yearlong series. JAMA. 2015;314:675–676. [Google Scholar]
- 48.Greening NJ, Williams JE, Hussain SF, Harvey-Dunstan TC, Bankart MJ, Chaplin EJ, Vincent EE, Chimera R, Morgan MD, Singh SJ, et al. An early rehabilitation intervention to enhance recovery during hospital admission for an exacerbation of chronic respiratory disease: randomised controlled trial. BMJ. 2014;349:g4315. doi: 10.1136/bmj.g4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seymour JM, Moore L, Jolley CJ, Ward K, Creasey J, Steier JS, Yung B, Man WD, Hart N, Polkey MI, et al. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax. 2010;65:423–428. doi: 10.1136/thx.2009.124164. [DOI] [PubMed] [Google Scholar]
- 50.Bourbeau J, Julien M, Maltais F, Rouleau M, Beaupré A, Bégin R, Renzi P, Nault D, Borycki E, Schwartzman K, et al. Chronic Obstructive Pulmonary Disease axis of the Respiratory Network Fonds de la Recherche en Santé du Québec. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Intern Med. 2003;163:585–591. doi: 10.1001/archinte.163.5.585. [DOI] [PubMed] [Google Scholar]
- 51.Manini TM, Everhart JE, Patel KV, Schoeller DA, Colbert LH, Visser M, Tylavsky F, Bauer DC, Goodpaster BH, Harris TB. Daily activity energy expenditure and mortality among older adults. JAMA. 2006;296:171–179. doi: 10.1001/jama.296.2.171. [DOI] [PubMed] [Google Scholar]
- 52.Woodruff PG, Albert RK, Bailey WC, Casaburi R, Connett JE, Cooper JA, Jr, Criner GJ, Curtis JL, Dransfield MT, Han MK, et al. Copd Clinical Research Network. Randomized trial of zileuton for treatment of COPD exacerbations requiring hospitalization. COPD. 2011;8:21–29. doi: 10.3109/15412555.2010.540273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benzo RP. Mindfulness and motivational interviewing: two candidate methods for promoting self-management. Chron Respir Dis. 2013;10:175–182. doi: 10.1177/1479972313497372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50:217–226. doi: 10.1097/MLR.0b013e3182408812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berwick DM, Feeley D, Loehrer S. Change from the inside out: health care leaders taking the helm. JAMA. 2015;313:1707–1708. doi: 10.1001/jama.2015.2830. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.