Lung cancer remains the most common cancer worldwide and the leading cause of cancer death in the United States and in the world, with an estimated 224,390 cases and 158,080 deaths in the United States in 2016 (1). Lung cancer is caused by cigarette smoking in the vast majority of cases, and trends in smoking prevalence have led to declines in lung cancer deaths in the United States and in other high-income countries. Research of mechanisms of lung cancer tumorigenesis and response to therapy has been translated into new precision medicine strategies that have revolutionized lung cancer treatment. Concomitant advances in lung cancer clinical research have led to widespread implementation of lung cancer screening in the United States. In this review, we highlight advances in these areas that promise to continue to reduce lung cancer mortality.
Epidemiology
Similar to other high-income countries, rates of lung cancer incidence and deaths are declining in men and women in the United States, in concert with trends in smoking rates. Several of the observed differences in lung cancer incidence and mortality among different sex, racial, and ethnic groups that suggested disparities in lung cancer susceptibility, diagnosis, and treatment may in fact be predominantly attributable to smoking prevalence trends. DeSantis and colleagues showed that the disparity in lung cancer death rates between black and white men has decreased from 40% to 20% over the past 20 years, and it has been eliminated in adults younger than 40 years of age; this reduction also parallels declines in smoking prevalence, which have been more rapid in black individuals than in white individuals (2). Patel and colleagues showed that among women enrolled in the prospective Women’s Health Initiative cohort, Hispanic women had lower lung cancer incidence than non-Hispanic women, but there were no racial/ethnic differences in mortality (3).
Independent of smoking, there is a persistent difference in 5-year lung cancer survival after diagnosis stage for stage in black individuals compared with white individuals, with an overall 5-year survival rate of 14% in black individuals versus 18% in white individuals (2). Fewer lung cancers are detected at early stage, and studies indicate that treatments differ for early-stage disease, after accounting for socioeconomic confounders. These issues will be important to address directly as widespread lung cancer screening is more widely implemented in the United States in 2016. Tanner and colleagues have taken an important step by reporting on racial differences in outcomes within the National Lung Screening Trial that enrolled 2,361 black individuals in the 53,452-person study cohort (4). Consistent with prior reports, black individuals experienced higher all-cause mortality than white individuals. Importantly, among individuals who underwent screening with low-dose computed tomography (LDCT), the reduction in all-cause mortality was significantly greater in black individuals (hazard ratio, 0.61 vs. 0.86 in white individuals); however, there was no significant difference in lung cancer–specific mortality.
Approximately 15% of lung cancers occur in never-smokers, which suggests that exposure to carcinogens other than cigarette smoke at work or home can cause disease in susceptible individuals. Couraud and colleagues report on the epidemiological and molecular features of lung cancer in French never-smokers (5). They showed that occupational exposure to carcinogens such as asbestos and polycyclic aromatic hydrocarbons was significantly higher in men than in women, whereas domestic exposure to cooking oil and to passive smoking was higher in women (5). Seventy-three percent of the tumors had a targetable somatic mutation, which is between the 55% rate in American and the 80% rate in Asian never-smokers. The risks of biomass exposure were examined in a systematic review by Bruce and colleagues (6). The odds ratio for lung cancer risk with biomass for cooking and/or heating was 1.17 overall and 1.15 for cooking alone. Exposure-response risk was highest in women in developing countries, consistent with higher exposure compared with men and in developed countries. Interestingly, the genomic impact of smoky coal exposure on the airway epithelium of women in rural China was similar to that seen with tobacco smoke (7).
Lung Cancer Screening
The Final Coverage Decision issued by the United States Centers for Medicare and Medicaid Services recommended coverage for lung cancer screening services for high-risk individuals within screening programs that meet strict eligibility criteria and are committed to reporting data in a national registry (8). Key components of the coverage decision include: (1) high-risk individuals are defined as current or former smokers aged 55 to 77 years with at least 30 pack-years of exposure and with smoke exposure within 15 years; (2) for the initial low-dose CT scan, there must be a written order from a licensed provider after completion of a shared decision-making visit that uses decision aids such as the American Thoracic Society (ATS) Decision Aid for Lung Cancer Screening with Computerized Tomography (9); (3) scanning center eligibility is restricted to centers with radiologists experienced in reading chest CT studies with capability to provide LDCT scans at less than or equal to 3.0 mGy; (4) centers are required to collect and submit demographic and imaging data to a Center for Medicare and Medicaid Services–approved registry. As issued, the Coverage Decision is intended to provide a structured, standardized screening program that addresses key components of screening (10) that can balance the benefits and harms of screening outlined in the comprehensive review by Tanoue and colleagues (11). In a joint statement from the ATS and the American College of Chest Physicians, Wiener and colleagues outline the pragmatic considerations that are useful for centers that plan to implement lung cancer screening programs (12).
It remains unclear whether the benefits of lung cancer screening observed in the NLST (National Lung Screening Trial) (13) are generalizable to populations outside of the United States. Wille and colleagues reported on the Danish Lung Screening Trial that enrolled 4,105 subjects with a lower lung cancer risk profile than individuals enrolled in the NLST (14). After 5 years of follow up, no difference in lung cancer mortality was detected. A post hoc analysis suggested that individuals at highest risk had fewer deaths in the screening group. Similarly, Infante and colleagues reported long-term follow-up results of the DANTE (Detection and Screening of Early Lung Cancer with Novel Imaging Technology) trial that randomized 2,450 male Italian smokers aged 60 to 74 years with greater than 20 pack-years of cigarette smoke exposure to screening with LDCT versus control (15). There was no difference in lung cancer mortality. Neither study had sufficient statistical power to directly address the hypothesis that lung cancer screening with low-dose chest CT will reduce lung cancer mortality. Thus, the results of these small studies that enrolled a lower-risk population than NLST do not refute the conclusions drawn from the NLST study, but neither do they confirm that NLST’s conclusions are generalizable to European populations. Further data to address this issue are anticipated from the final results of the Dutch-Belgian NELSON trial that enrolled 15,000 participants (16). In the meantime, the European Society and of Radiology and the European Respiratory Society have issued a white paper on lung cancer screening that recommends that screening be restricted to comprehensive, quality-assured longitudinal programs within a clinical trial or in routine clinical practice at certified multidisciplinary medical centers (17). Specifically, the recommendations include the use of risk models to increase pretest probability, the use of standardized nodule reporting and data systems, and reduction of radiation exposure to 1 mSv or less.
As lung cancer screening clinical programs expand, intense research focus continues to be directed toward optimizing the benefits and minimizing the harms of screening. Sanchez-Salcedo and colleagues examined whether selection criteria for lung cancer screening could be improved by focusing on emphysema diagnosis (18). Using patients enrolled in two screening cohorts, they noted that inclusion of patients who met NLST criteria and had CT-detected emphysema would detect more than 88% of the incident cancers and would reduce the number of screened participants by 52%. These results were extended to develop and validate a risk assessment score that showed good performance in discriminating low-risk from high-risk cohorts (19). Similarly, Young and colleagues (20) evaluated prebronchodilator spirometry data acquired from 18,475 participants in the NLST cohort. They showed that in patients with flow limitation suggestive of the diagnosis of chronic obstructive pulmonary disease, lung cancer incidence was doubled. Together, these results suggest that consideration of chronic obstructive pulmonary disease, and emphysema in particular, merit further study of their utility in refining screening criteria, acknowledging the potential for an increased risk of complications and competing mortality in these patients.
Pulmonary Nodule Evaluation
Gould and colleagues reported on the epidemiology of pulmonary nodules, which is likely to change as lung cancer screening programs disseminate in the United States (21). Using natural language processing of data acquired by the Kaiser Permanente Southern California healthcare system data registry, they identified an increase in the annual rate of pulmonary nodule identification from 3.9 to 6.6 per 1,000 person-years between 2006 and 2012. The data were extrapolated to predict that more than 1.5 million adult Americans will have a pulmonary nodule identified each year. This study highlights the need and urgency for further research on pulmonary nodule characterization and evaluation, for which the ATS has proposed a research framework (22).
Nodule evaluation strategies address the challenge of distinguishing benign from malignant nodules (23) and, as importantly, of distinguishing indolent from aggressive lung carcinomas. Using data acquired from the NLST, Pinsky and colleagues retrospectively examined the performance of the Lung-Reporting and Data System (RADS) algorithm in identifying malignant nodules (24). The sensitivity and specificity of using Lung-RADS category 3 or higher as indicative of malignancy in this cohort were 84.9 and 87.2%, respectively, compared with 93.5 and 73.4% using the NLST criteria. Thus, Lung-RADS lowered the false-positive rate but at the cost of decreased sensitivity. We expect that analysis of data reported to the U.S. lung cancer screening program registry will provide further data on the clinical utility of Lung-RADS.
To distinguish clinically indolent from aggressive lung cancers, Maldonado and colleagues applied a Computer-aided Nodule Assessment and Risk Yield (CANARY) image analysis algorithm to 294 patients diagnosed with lung adenocarcinomas in the NLST (25). CANARY assigned each lesion one of three risk groups, and a multivariate Cox regression hazard model demonstrated significantly different hazard ratios for progression-free survival among the CANARY risk groups. These data and others suggest that observation may be an appropriate management strategy for screen-detected tumors with indolent properties, such as ground-glass nodules, which frequently represent adenocarcinoma in situ tumors. The safety of close monitoring approaches in these selected cases is supported by the experiences reported by the International Early Lung Cancer Action Project investigators (26) and by the NELSON investigators (27). Both studies showed that all of the monitored subsolid nodules had a lung cancer survival rate of 100%.
Diagnosis of Lung Cancer
As a complementary approach to LDCT, which is particularly effective in detecting peripheral adenocarcinomas, autofluorescent bronchoscopy shows promise for identifying premalignant squamous cell carcinoma lesions in the central airways. Given their variable natural history, it remains unclear which patients with premalignant airway lesions are likely to progress to invasive carcinoma requiring more aggressive monitoring and potential intervention. van Boerdonk and colleagues conducted one of the largest longitudinal studies of premalignant lesions to date, following 164 subjects for up to 12 years (median, 30 mo) with serial autofluorescent bronchoscopy and chest CT scans (28). During that follow-up period, 61 lung cancers were detected in 55 subjects (median time to event, 16.5 mo), with the majority of these cancers (∼60%) developing from separate (rather than the initial lesion) sites both in the airway or lung parenchyma. Subjects with high-grade dysplastic lesions were more likely to develop lung cancer, suggesting that the presence of these lesions may serve as biomarkers of cancer risk. Additional molecular studies are needed to better stratify cancer risk in this population and define the optimal management strategy for patients with premalignant airway lesions
Technological advances have produced instrumentation that facilitates bronchoscopy biopsy access to peripheral nodules. Oki and colleagues performed a prospective noninferiority study design to compare diagnostic yields using a 3.0-mm ultrathin bronchoscope with a 4.0-mm thin bronchoscope (29). Navigational bronchoscopy and endoscopic ultrasound were used in all procedures guided toward peripheral pulmonary nodules less than or equal to 30 mm. The diagnostic yield was 74% for the ultrathin bronchoscopy group and 59% for the thin bronchoscopy group, with a complication rate of 3 and 5%, respectively. Another option for diagnosis of these nodules is bronchoscopic transparenchymal nodule access. Herth and colleagues reported a feasibility study of this approach in 12 patients in whom a tunnel tract was created through an avascular path from the airway to the nodule using fused fluoroscopy guidance (30). Adequate biopsies were acquired from 10 patients, and no adverse events issues were reported other than an elevated postprocedure troponin level in one subject. Ost and colleagues reported results on diagnostic accuracy for peripheral lung lesions using the AQuIRE (American College of Chest Physicians Quality Improvement Registry, Evaluation, and Education) Registry (31). They noted lower-than-expected diagnostic accuracy of 57 and 39% for radial endobronchial ultrasound (EBUS) and electromagnetic navigation, respectively. They suggested that increased use of transbronchial needle aspiration (TBNA) may improve diagnostic yield for peripheral lesions. It will be important to continuously evaluate bronchoscopic diagnostic accuracy and safety as demand increases for nodule diagnosis in the screening era and as technological advances permit better access to peripheral nodules. Registries such as AQuIRE will be important resources to help guide the field.
A key issue is to ensure that diagnostic procedures acquire and process specimens in a manner that is suitable for complete diagnostic testing, which frequently requires molecular testing. Schneider and colleagues retrospectively examined lung cancer percutaneous CT-guided lung fine-needle aspirates and core needle biopsies between 2011 and 2013 (32). Fine-needle aspiration (FNA) specimens were sufficient for molecular testing in 46% of cases, compared with 67% of core needle biopsy cases. Importantly, there were significant interoperator differences in FNA yields. FNA has consistently been shown to be sufficient for diagnostic testing in the setting of EBUS-TBNA. Casadio and colleagues reported a molecular testing rate of 96.9% of samples obtained in EBUS-TBNA procedures from 306 consecutive patients (33). Taken together, these studies emphasize the importance of optimizing diagnostic specimen acquisition and processing procedures to ensure that all procedures provide sufficient material for pathological and molecular testing in this era of precision medicine.
Lung Cancer Pathology and Staging
The World Health Organization Classification of Tumours of the Lung, Pleura, Thymus, and Heart, fourth edition, has just been published (Table 1) (34). The most significant changes in this edition are: (1) adoption of the adenocarcinoma carcinoma classification proposed by the 2011 panel of the International Association for the Study of Lung Cancer/ATS/European Respiratory Society (35); (2) reclassifying squamous carcinoma into keratinizing, nonkeratinizing, and basaloid subtypes; (3) grouping neuroendocrine tumors (small cell, carcinoid, and large cell neuroendocrine carcinoma) together into one category; (4) restricting the diagnosis of large cell carcinoma only to resected tumors that lack any clear differentiation by morphology or immunohistochemistry; and (5) new classification for small biopsies and cytology specimens.
Table 1.
Major 2015 World Health Organization Classifications of Tumors of the Lung
| Adenocarcinoma | Preinvasive: AIS, MIA, and AAH |
| Lepidic predominant adenocarcinoma with acinar, papillary, micropapillary, or solid morphology | |
| Invasive adenocarcinoma with acinar, papillary, micropapillary, or mucinous morphology | |
| Squamous cell carcinoma | Keratinizing and nonkeratinizing |
| Basaloid | |
| Preinvasive—squamous cell CIS | |
| Neuroendocrine tumors | Small cell carcinoma |
| Large cell neuroendocrine carcinoma | |
| Carcinoid—typical and atypical | |
| Preinvasive—DIPNECH | |
| Large cell carcinoma | |
| Adenosquamous carcinoma |
Definition of abbreviations: AAH = atypical adenomatous hyperplasia; AIS = adenocarcinoma in situ; CIS = carcinoma in situ; DIPNECH = diffuse idiopathic neuroendocrine cell hyperplasia; MIA = minimally invasive adenocarcinoma.
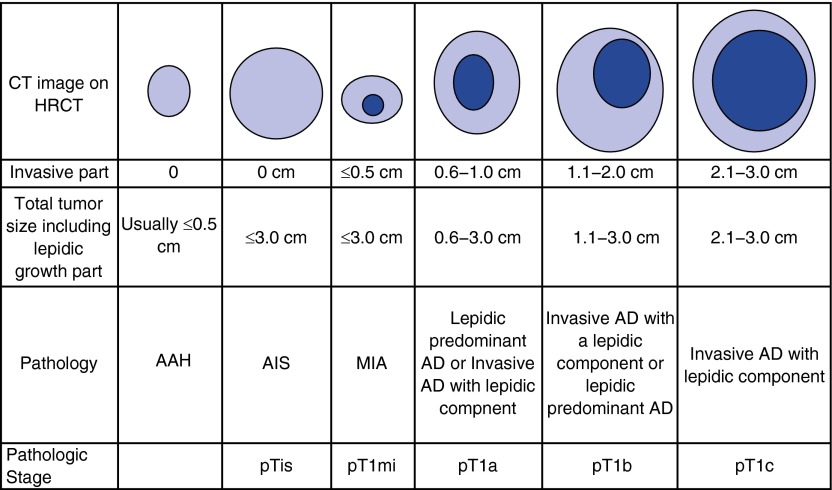
The clinical correlations of the histology and molecular classification schema proposed in the World Health Organization fascicle have helped to inform the eighth edition of the TNM Classification of Lung Cancer Staging. The most important changes are those applied to T categories for subsolid nodules and assessment of tumor size (36, 37). For lung nonmucinous adenocarcinoma, it is recommended that only the size of the invasive component is to be used for assessment of the T category (Figure 1). This is a significant difference from prior classifications that used the entire tumor size for T category assignment. New T categories of pTis and Tmi have been added for assignment of adenocarcinoma tumors with no invasive component and for minimally invasive tumors with an invasive size of less than or equal to 5 mm, respectively. These revisions should provide T staging that correlates with prognosis with more precision than prior versions and will permit a standardized approach to T staging that will facilitate prospective validation and the conduct of early-stage lung cancer research protocols. The eighth edition also addressed the issue of multiple lung tumors with a schema intended to clarify the classifications proposed in the seventh edition (38).
Figure 1.
T staging for subsolid nodules, American Joint Committee on Cancer eighth edition (36). Size is determined by measurement of the invasive component for lung nonmucinous adenocarcinoma. AAH = atypical adenomatous hyperplasia; AD = adenocarcinoma; AIS = adenocarcinoma in situ; CT = computed tomography; HRCT = high-resolution computed tomography; MIA = minimally invasive adenocarcinoma. Adapted by permission from Reference 82.
Molecular Biomarkers for Early Lung Cancer Detection
Given the growing challenges with lung cancer screening and diagnosis in the post-NLST era, there have been significant advances made toward development and validation of molecular markers that hold the potential to impact early lung cancer detection. In two prospective multicenter validation AEGIS (Airway Epithelial Gene Expression in the Diagnosis of Lung Cancer) trials (AEGIS-1 and AEGIS-2) of current and former smokers undergoing bronchoscopy for suspect lung cancer (n = 639), Silvestri and colleagues demonstrated that a gene-expression classifier (23 genes) measured in the cytologically normal bronchial epithelium from the mainstem bronchus can improve the diagnostic performance of bronchoscopy, with the combination of the genomic classifier plus bronchoscopy having a sensitivity of 97% for lung cancer detection (as compared with 75% sensitivity for bronchoscopy alone) (39). Among smokers with intermediate pretest risk of lung cancer (n = 101), where lung cancer prevalence was 41%, the genomic classifier in this “field of injury” had a sensitivity of 88%, specificity of 48%, and negative predictive value of 91%, potentially enabling physicians to pursue surveillance imaging instead of unnecessary invasive procedures (transthoracic needle aspiration and/or surgical lung biopsy) after a nondiagnostic bronchoscopy. To evaluate the potential clinical utility of this molecular biomarker, Vachani and colleagues leveraged the AEGIS trial data to retrospectively estimate that a significant proportion (∼50%) of invasive procedures among patients with benign disease could have been avoided after an inconclusive bronchoscopy with the use of this airway genomic classifier (40). Given that physicians were blinded to the results of the classifier in the AEGIS trials, this estimate is based on the assumption that physicians would pursue CT surveillance among all those with a negative genomic classifier.
A number of additional promising biomarkers are emerging in the both the screening and diagnostic space, although prospective clinical validation of these markers in the setting in which the test will be used (prediagnostic) remains key for translation to the bedside. Montani and colleagues (41) refined and validated a serum microRNA (miRNA) signature (13 miRNAs) as a screening biomarker among subjects (n = 1,115) enrolled in the COSMOS (Continuous Observation of Smoking Subjects) lung cancer screening trial. The miR-Test had a sensitivity and specificity of 77.8 and 74.8%, respectively. Importantly, 820 out of the 1,115 individuals were miR-Test negative (73.5%), including 810 out of the 1,067 individuals without lung cancer and 10 of the 48 individuals who developed lung cancer. Given the high negative predictive value, the test holds the potential to identify individuals who can safely avoid subsequent LDCT scans in the screening setting.
miRNA-based biomarkers as well as other blood-based molecular assays are also emerging as potential tools in the diagnostic setting. A panel of 24 circulating miRNAs was found to distinguish lung cancer cases from matched control subjects (area under the curve of 0.78) in cross-validation (42). Xing and colleagues (43) developed and validated a panel of three miRNA measured in sputum as diagnostic biomarkers in the solitary pulmonary nodule (SPN) setting. Using sputum collected from two cohorts of patients with benign and malignant SPNs, the sensitivity and specificity of the biomarkers in the two validation sets were 82 and 88%, and 80 and 86%, respectively. Tsay and colleagues characterized the mRNA and miRNA changes in peripheral airway brushings contralateral to the tumor (44), supporting the notion of an airway-wide “field of injury” that can be leveraged for lung cancer diagnosis. In two case-control retrospective studies, Fahrmann and colleagues showed the potential for metabolic markers in both the serum and plasma to detect lung adenocarcinoma (45). Finally, Vachani and colleagues (46) validated a plasma multiprotein classifier (11 proteins) in a retrospective case-control study of SPNs, demonstrating 70 to 92% sensitivity and 20 to 48% specificity. Importantly, the authors demonstrated that the classifier was independent of clinical risk factors for disease, enabling it to add to the diagnostic performance of a four-parameter clinical model.
Lung Cancer Genomics
There have been a number of key advances in our understanding of lung cancer genomics with direct therapeutic implications. In a landmark paper, Rizvi and colleagues (47) performed whole-exome sequencing on non–small cell lung cancers (NSCLCs) treated with pembrolizumab (antibody targeting PD-1) to demonstrate that the genomic landscape of lung cancer correlates with the clinical response to immunotherapy. Specifically, the authors demonstrated that higher nonsynonymous mutation burden in tumors was associated with progression-free survival in two independent cohorts. Importantly, higher neoantigen burden and DNA repair pathway mutations correlated with therapeutic efficacy.
Wilson and colleagues (48) characterized genomic mechanisms of resistance to anaplastic lymphoma receptor tyrosine kinase (ALK) inhibition in the subset of NSCLC where the echinoderm microtubule-associated protein like 4–ALK fusion protein is an oncogenic driver. Using systematic perturbations of gene expression, the authors identified neuregulin-1 (NRG1), the ligand that activates HER3, as the gene that most strongly induced resistance to ALK inhibition in a number of cell lines. They further identified members of the P2Y purinergic receptor family of G-protein–coupled receptors as mediating resistance through a protein kinase C–dependent mechanism. Finally, the authors demonstrated enrichment of these in vitro gene expression signatures associated with resistance with those found in crizotinib-resistant ALK-rearranged lung tumors.
George and colleagues (49) provided the first comprehensive genome atlas of somatic mutations in small cell lung cancer (SCLC). By sequencing the genomes of 110 SCLCs, the authors demonstrated that there was biallelic inactivation of TP53 and RB1 in nearly all tumors studied, suggesting that loss of the tumor suppressors TP53 and RB1 is required for SCLC. They also found kinase gene mutations in rare cases of SCLC, providing a possible therapeutic target in a small number of patients with this disease. Importantly, they found inactivating mutations in Notch family genes in 25% of cases and demonstrated that activation of Notch signaling led to a therapeutic response in an SCLC mouse model, providing a potential novel therapeutic target for this deadly form of lung cancer.
There have also been a number of key advances to characterizing the genomic landscape of lung cancer among underrepresented populations who suffer disproportionately from this disease. Araujo and colleagues (50) performed massively parallel sequencing of 81 NSCLC-related genes as well as studying ALK translocation by fluorescent in situ hybridization in 99 African American patients with NSCLC. They found that the frequency of driver mutations was not significantly different from that of white individuals. Importantly, there was no association between genetic ancestry and the presence of somatic mutations. By characterizing epidermal growth factor receptor (EGFR) and KRAS mutations in 5,738 patients with NSCLC (95% adenocarcinoma) from Latin America, Arrieta and colleagues (51) found that the frequency of EGFR and KRAS mutations was 26 and 14%, respectively, in this population. While confirming that the frequency of EGFR mutations in Latin America is intermediate between that observed in the Asian and Caucasian populations, the authors also found heterogeneity within Latin American countries, with highest rates in Peru (51%) and lowest in Argentina (14%). EGFR mutations were independently associated with female sex, nonsmoker status, ethnicity (mestizo/indigenous), and the absence of KRAS mutation.
NSCLC Early-Stage Management
Data continue to accumulate to suggest that sublobar surgical resection and stereotactic ablative body radiation (SABR) may be equivalent to traditional surgical lobectomy in terms of oncological outcomes in selected cases. Chang and colleagues pooled results from two incomplete randomized trials designed to compare SABR to surgical lobectomy with mediastinal lymph node dissection or sampling in 58 patients with stage T1 to 2a N0M0 operable NSCLC (52). Recurrence-free survival at 3 years was similar, 86% in the SABR group compared with 80% in the surgery group, with an overall survival (OS) rate at 3 years of 95% in the SABR group and 79% in the surgery group. The results are compelling but need to be interpreted in line with the study limitations that include small sample size, interinstitutional heterogeneity in evaluation procedures, and the low use of video-assisted thoracic surgery lobectomy in the surgical arm. Using the large Surveillance, Epidemiology, and End Results (SEER)-Medicare registry, Ezer and colleagues compared outcomes of SABR and sublobar resection in patients older than 65 years with stage I to II NSCLC (53). Survival of patients who underwent SABR was equivalent to patients treated with wedge surgical resection but was lower than in patients treated with lobectomy. The importance of patient selection, histology, and tumor biology is demonstrated by Veluswamy and colleagues, who used the SEER-Medicare database to compare outcomes in patients treated with lobectomy versus limited surgical resection (54). Propensity score–adjusted survival analysis showed that lobectomy outcomes were better than those for limited resection for invasive tumors and squamous histology.
Adjuvant Therapy
After a decade of herculean effort, results of the ECOG1505 study were presented at the World Lung meeting, unfortunately demonstrating no benefit for the addition of the anti–vascular endothelial growth factor antibody bevacizumab to doublet platinum-based chemotherapy in the management of resected stage IB to IIIA NSCLC (55). An important next phase for early-stage lung cancer management will be incorporation of personalized medicine strategies. The ALCHEMIST (Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trial) is a National Cancer Institute–sponsored national clinical trials network initiative to screen patients with operable lung adenocarcinoma to determine whether the tumors harbor EGFR or ALK alterations, who would then be randomized to targeted therapy after completion of standard adjuvant therapy (56).
NSCLC Locally Advanced Disease Management
A notable development in 2015 was the publication of results of the pivotal RTOG 0617 study demonstrating no advantage (in fact potentially showing harm) for higher radiation dose (74 vs. 60 Gy) in the management of locally advanced NSCLC (57). The 2 × 2 factorial design study also failed to demonstrate benefit for the anti-EGFR monoclonal antibody cetuximab along with concurrent low-dose weekly carboplatin/paclitaxel chemotherapy; however, excellent results for the conventional dose radiation arm (median OS, 28.7 mo) were notable.
NSCLC Advanced Disease Management
Squamous Cell Lung Cancer
The year 2015 turned out to be a breakthrough year after decades of few to no major advances in the management of advanced squamous cell lung cancer. The Checkmate-017 study showed an unprecedented survival advantage favoring the anti-programmed cell death protein 1 (PD1) antibody nivolumab over docetaxel chemotherapy (OS, 9.2 vs. 6.0 mo; hazard ratio [HR], 0.59) in the treatment of biomarker-unselected patients with advanced squamous cell lung cancer after failure of platinum-based doublet chemotherapy (58). The year 2015 also saw the approval of the anti-EGFR antibody necitumumab on the basis of results of the positive randomized SQUIRE (front-line cisplatin/gemcitabine chemotherapy with or without necitumumab in patients with stage IV squamous non–small-cell lung cancer) trial (59). The OS was 11.5 versus 9.9 months in favor of the necitumumab arm (HR, 0.84), leading to U.S. Food and Drug Administration (FDA) approval of the compound for the above indication (Table 2). In addition, the afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX Lung-8) study presented at the 2015 American Society of Clinical Oncology meeting demonstrated a modest but significant OS advantage of the irreversible pan-ErbB inhibitor afatinib versus erlotinib (median OS of 7.8 vs. 6.7 mo; HR, 0.81) in the second-line management of advanced squamous cell lung cancer, also culminating in FDA approval earlier this year (60).
Table 2.
Recent U.S. Food and Drug Administration Approvals of Nonchemotherapy Drugs for the Treatment of Lung Cancer
| Class | Drug | Year Approved | Biomarker | Indication |
|
|---|---|---|---|---|---|
| Tumor | Sequence | ||||
| Antiangiogenic agents | Avastin (bevacizumab) | 2006 | None | Advanced nonsquamous NSCLC | First line, in combination with carboplatin/paclitaxel chemotherapy |
| Cyramza (ramucirumab) | 2014 | None | Advanced NSCLC | After failure of platinum-based chemotherapy, in combination with docetaxel | |
| Targeted drugs: EGFR | Tarceva (erlotinib) | 2013 | Cobas EGFR mutation test | EGFR exon 19 deletion or L858R mutation–positive advanced NSCLC | First-line treatment |
| Gilotrif (afatinib) | 2013 | Therascreen EGFR RGQ PCR kit | EGFR exon 19 deletion or L858R mutant advanced NSCLC | First-line treatment | |
| Gilotrif (afatinib) | 2016 | None | Advanced squamous cell lung cancer | After failure of platinum-based chemotherapy | |
| Iressa (gefitinib) | 2015 | Therascreen EGFR RGQ PCR kit | EGFR exon 19 deletion or L858R mutant advanced NSCLC | First-line treatment | |
| Tagrisso (osimertinib) | 2015 | Cobas EGFR mutation test v2 | EGFR T790M–positive advanced NSCLC | ||
| Portrazza (necitumumab) | 2015 | None | Metastatic squamous cell lung cancer | First line, in combination with cisplatin/gemcitabine chemotherapy | |
| Targeted drugs: ALK | Xalkori (crizotinib) | 2011 | ALK FISH positive (Vysis ALK FISH) | ALK-positive locally advanced or advanced NSCLC | |
| Zykadia (ceritinib) | 2014 | ALK positive | Advanced ALK-positive NSCLC | Crizotinib-refractory or intolerant | |
| Alecensa (alectinib) | 2015 | ALK positive | Advanced ALK-positive NSCLC | Crizotinib-refractory or intolerant | |
| Targeted drugs: ROS-1 | Xalkori (crizotinib) | 2016 | ROS-1 positive | Advanced ROS-1 gene alteration–positive NSCLC | |
| Immunotherapeutic agents | Keytruda (pembrolizumab) | 2015 | PD-L1 IHC 22C3 pharmDx | Metastatic NSCLC | After failure of platinum-based chemotherapy |
| Opdivo (nivolumab) | 2015 | None mandated (PD-L1 IHC 28-8 pharmDx coapproved) | Metastatic NSCLC | After failure of platinum-based chemotherapy | |
Definition of abbreviations: ALK = anaplastic lymphoma kinase; EGFR = epidermal growth factor receptor; FISH = fluorescent in situ hybridization; IHC = immunohistochemistry; NSCLC = non–small cell lung cancer; PCR = polymerase chain reaction; PD-L1 = programmed death-ligand 1; RGQ = rotor-gene Q; ROS-1 = ROS proto-oncogene-1.
Nonsquamous NSCLC
EGFR
We have seen several significant advances in the last year in the management of EGFR-mutated lung adenocarcinomas. Although the upfront management of advanced EGFR-mutated lung adenocarcinoma is now well established to be an EGFR tyrosine kinase inhibitor (TKI), a recent randomized phase IIb study (LUX Lung-7) does suggest some benefit for the irreversible pan-Her inhibitor afatinib over gefitinib (61), as assessed by response rates and progression-free survival—at the cost of increased toxicity. Acquired resistance is a major shortcoming in the long-term benefit of EGFR-directed therapy. Whether EGFR-TKI continuation after progression is beneficial has been addressed in several studies. The randomized U.S. phase 2 Case2507 (62) and the IMPRESS (randomized phase 3 gefitinib plus chemotherapy vs. placebo plus chemotherapy in EGFR-mutation–positive NSCLC after progression on first-line gefitinib) studies (63), consistently show no significant benefit for continuing a first-generation EGFR TKI on progression. This is not surprising, given the very high level of resistance to these compounds by the most common resistance mutation, EGFR-T790M. The remaining question in this setting is whether EGFR TKI continuation might be helpful in the T790M-negative cases.
Excellent progress has been made in efforts to address EGFR-T790M–mediated resistance by the rapid and successful development of third-generation, T790M-targeting EGFR inhibitors. The two leading compounds, osimertinib (64) and rociletinib (65), demonstrate excellent activity (around 60% response rates). Accelerated FDA approval was secured for osimertinib in 2015 on the basis of robust data from the AZD9291 in pretreated T790M-positive advanced NSCLC (AURA-1 and AURA-2) studies, also demonstrating a very favorable side effect profile. Ongoing research efforts are now focusing on the use of these inhibitors in earlier line and stage settings as well as on combinations with other targeted and immunotherapies. Overall, outcomes for this subgroup of patients have drastically improved, with a recent article demonstrating a 15% 5-year survival rate, which is unprecedented in advanced NSCLC (66).
In clinical practice, a major hurdle in the use of third-generation inhibitors has been the challenge of obtaining sufficient tumor tissue for appropriate testing for EGFR T790M. The rapid and successful development of circulating tumor DNA assays that appear to have high specificity and sensitivity for the detection of EGFR T790M provide an excellent complement to tissue-based assays (67) for treatment of advanced adenocarcinoma and have entered daily clinical routine use. The Cobas EGFR Mutation Test v2 (Roche Molecular Systems) was just recently approved as a plasma-based companion diagnostic for erlotinib to detect EGFR gene mutations in NSCLC to guide initial management. This is the first “liquid biopsy test” approved by the FDA.
ALK
Good news continues to unfold in the management of patients with ALK-positive lung adenocarcinoma. After the recent approval of the second-generation agent ceritinib for patients with crizotinib-refractory disease, promising data demonstrating a high level of efficacy, including significant central nervous system activity thanks to its excellent central nervous system penetration, led to the recent approval by the FDA of alectinib (68, 69). This is now another highly potent second-generation inhibitor compound available for patients with crizotinib-resistant or refractory disease. We await results of the ALEX study, a randomized phase III study comparing alectinib with crizotinib in treatment-naive ALK-positive NSCLC participants.
MET proto-oncogene receptor tyrosine kinase
Despite being positioned for the past decade as a potentially actionable oncogene in lung cancer, a series of studies have failed to demonstrate clinical benefit of MET proto-oncogene receptor tyrosine kinase (MET) inhibition (e.g., with the MET TKI tivantinib and the anti-MET antibody, MetMab). A succession of recent pivotal manuscripts brings new clarity and hope to the field. Recurrent MET genetic abnormalities, most commonly leading to skipping of the entire sequence of exon 14, lead to a unique type of mutation generating a constitutively activated Met molecule deficient in Cbl-mediated degradation (70–72). Met exon skipping appears to occur at a frequency of around 3 to 4% in multiple NSCLC histotypes and appears more frequent in the highly aggressive and rare sarcomatoid variant that is characterized by mesenchymal differentiation and treatment resistance (72). On the basis of multiple case reports and case series, it seems that advanced NSCLCs harboring MET exon 14 skipping mutations are highly responsive to small molecule MET inhibitors, such as crizotinib and cabozantinib. Thus, analysis for Met exon 14 skipping mutations (best achieved through next-generation sequencing approaches) should be considered for inclusion in molecular testing algorithms for patients with advanced NSCLC.
Immunotherapy
The year 2015 was a exceptional year in lung cancer research that established immunotherapeutic agents targeting the PD1/programmed death-ligand 1 (PD-L1) axis as effective drugs in the second-line treatment of both squamous and nonsquamous NSCLC. This research culminated in the approval of nivolumab and pembrolizumab. It should be noted that these immunotherapy trials were able to demonstrate a significant difference in OS, whereas trials of tyrosine kinase inhibitors to date have failed to do so. Interestingly, progression-free survival appeared to be a highly unreliable surrogate endpoint for OS benefit in these immunotherapy studies, highlighting the unique nature/benefit of these drugs and suggesting reconsideration of classical clinical trial endpoints for immunotherapeutic studies.
The Checkmate-017 study demonstrated a dramatic survival benefit for nivolumab as compared with docetaxel for patients with advanced squamous cell lung cancer (58). The Checkmate-057 study in patients with advanced nonsquamous NSCLC after failure of front-line platinum-based chemotherapy showed similarly impressive results, with a median OS benefit of 12.2 versus 9.4 months (HR, 0.74) for nivolumab versus docetaxel (73). Correlative analysis of immunotherapy biomarker PD-L1 expression, as determined by the Pharm-DC28.8 assay, did not show predictive power for the assay in squamous cell cancer. Conversely, significant trends were noted in the nonsquamous study that demonstrated a higher magnitude of benefit for tumors with positive expression, leading to the FDA label recommending but not mandating PD-L1 testing for nivolumab.
The pivotal Keynote-010 study enrolled patients with PD-L1+ (>1 as determined by PharmDX 22C3 immunohistochemistry assay) advanced squamous and nonsquamous cell cancer and demonstrated a significant OS benefit for the anti-PD1 antibody pembrolizumab over docetaxel (74). This three-arm study compared two different doses of pembrolizumab (2 mg/kg and 10 mg/kg) versus docetaxel, and the OS for the entire study population was 10.4 months and 12.7 months for the lower- and higher-dose arms and 8.5 months for the control arm (HR, 0.71 and HR, 0.61 for lower/higher dose versus docetaxel, respectively). The results were even more striking when analyzing results for patients with high PD-L1 expression (>50%, prespecified endpoint), with OS of 14.7 months for the lower dose, 17.3 months for the higher dose pembrolizumab arm, and 8.2 months for the control group. Current FDA approval for pembrolizumab is based on results of the prior Keynote-001 study with a mandated companion PD-L1 biomarker (75) and restricts the indication to PD-L1+ tumors. Toxicity in all listed studies also favored immunotherapy; however, significant immune toxicities, such as pneumonitis and colitis, as well as endocrinopathies will require careful monitoring and management.
Further exciting data are also coming in from newer immunotherapeutic regimens, such as impressive data from the randomized phase 2 atezolizumab versus docetaxel for patients with previously treated NSCLC (POPLAR) study (76) of the anti–PD-L1 targeting agent adalimumab (in particular for biomarker-positive patients based on a unique PD-L1 assay using both tumor and immune cell expression). Promising early data of combination immunotherapy regimens, such as combinations of the anti–PD-L1 agent durvalumab and the anti–cytotoxic T-lymphocyte associated protein 4-targeting drug tremelimumab, show promising activity with reasonable tolerability, which is seemingly independent of PD-L1 expression (77).
Although immunotherapy has become the de facto second-line regimen for most patients with advanced NSCLC, several issues require further study, such as the proper use of biomarker selection, the safety of compounds in selected patient populations, the potentially lower activity in non–smoking-related EGFR/ALK-mutated tumors carrying lesser mutation burden, as well as the utility of combination regimens. In addition, a wide array of studies are ongoing or have been recently completed to assess the benefit of these compounds in addition to or instead of conventional doublet chemotherapy as well as in earlier-stage settings as adjuvant therapy or after concurrent chemoradiation for locally advanced disease. Indeed, the excitement continues to build, with several studies showing a significant tail suggestive of some long-term survivors with immunotherapy. The use of immunotherapy in the first-line setting is supported by the pivotal Keynote-024 study that compared doublet chemotherapy with pembrolizumab in PD-L1 high+ (50%+) patients. The trial was halted early due to significant superiority of the experimental arm, likely again completely transforming the treatment landscape in first-line management and calling for routine PD-L1 testing (78).
SCLC
Although we are still waiting for new drug approvals in the management of SCLC, at least some significant rays of hope have been noted in the last year, including early-phase studies clearly showing convincing signals of activity for immunotherapeutic agents for SCLC (79). In addition, exciting results have been published in both preclinical and early-phase clinical studies in high-grade pulmonary neuroendocrine cancers, including SCLC, for an innovative antibody-drug conjugate, rovalpituzumab, for tumors expressing the drug target delta-like 3 (Dll-3), which is preferentially expressed on cancer-initiating cells of high-grade pulmonary tumors (80). Although the efficacy of the approach is promising, significant toxicity remains a concern at this phase of development.
Mesothelioma
Practice-changing results have been published for the management of surgically unresectable malignant mesothelioma. The Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS) (81) demonstrated a significant survival benefit for the addition of the anti–vascular endothelial growth factor monoclonal antibody bevacizumab to doublet platinum/pemetrexed chemotherapy (median OS of 18.8 vs. 16.1 mo; HR, 0.77) in patients who were appropriate candidates for antiangiogenic therapy. In light of the significant survival benefit as well as quality-of-life gains, the platinum/pemetrexed/bevacizumab regimen is now positioned as the new standard of care for appropriate treatment candidates.
Supplementary Material
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Siegel RL, Sauer AG, Miller KD, Fedewa SA, Alcaraz KI, Jemal A. Cancer statistics for African Americans, 2016: progress and opportunities in reducing racial disparities. CA Cancer J Clin. 2016;66:290–308. doi: 10.3322/caac.21340. [DOI] [PubMed] [Google Scholar]
- 3.Patel MI, Wang A, Kapphahn K, Desai M, Chlebowski RT, Simon MS, Bird CE, Corbie-Smith G, Gomez SL, Adams-Campbell LL, et al. Racial and ethnic variations in lung cancer incidence and mortality: results from the Women’s Health Initiative. J Clin Oncol. 2016;34:360–368. doi: 10.1200/JCO.2015.63.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanner NT, Gebregziabher M, Hughes Halbert C, Payne E, Egede LE, Silvestri GA. Racial differences in outcomes within the National Lung Screening Trial: implications for widespread implementation. Am J Respir Crit Care Med. 2015;192:200–208. doi: 10.1164/rccm.201502-0259OC. [DOI] [PubMed] [Google Scholar]
- 5.Couraud S, Souquet PJ, Paris C, Dô P, Doubre H, Pichon E, Dixmier A, Monnet I, Etienne-Mastroianni B, Vincent M, et al. French Cooperative Intergroup IFCT. BioCAST/IFCT-1002: epidemiological and molecular features of lung cancer in never-smokers. Eur Respir J. 2015;45:1403–1414. doi: 10.1183/09031936.00097214. [DOI] [PubMed] [Google Scholar]
- 6.Bruce N, Dherani M, Liu R, Hosgood HD, III, Sapkota A, Smith KR, Straif K, Lan Q, Pope D. Does household use of biomass fuel cause lung cancer? A systematic review and evaluation of the evidence for the GBD 2010 study. Thorax. 2015;70:433–441. doi: 10.1136/thoraxjnl-2014-206625. [DOI] [PubMed] [Google Scholar]
- 7.Wang TW, Vermeulen RC, Hu W, Liu G, Xiao X, Alekseyev Y, Xu J, Reiss B, Steiling K, Downward GS, et al. Gene-expression profiling of buccal epithelium among non-smoking women exposed to household air pollution from smoky coal. Carcinogenesis. 2015;36:1494–1501. doi: 10.1093/carcin/bgv150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Medicare & Medicaid Services. 2015. Proposed decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N) [accessed 2016 April 29]. Available from: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274. [Google Scholar]
- 9.American Thoracic SocietyDecision aid: for lung cancer screening with computerized tomography (CT). 2015[accessed 2015 May 12]. Available from: http://www.thoracic.org/patients/patient-resources/resources/decision-aid-lcs.pdf
- 10.Mazzone P, Powell CA, Arenberg D, Bach P, Detterbeck F, Gould MK, Jaklitsch MT, Jett J, Naidich D, Vachani A, et al. Components necessary for high-quality lung cancer screening: American College of Chest Physicians and American Thoracic Society Policy Statement. Chest. 2015;147:295–303. doi: 10.1378/chest.14-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanoue LT, Tanner NT, Gould MK, Silvestri GA. Lung cancer screening. Am J Respir Crit Care Med. 2015;191:19–33. doi: 10.1164/rccm.201410-1777CI. [DOI] [PubMed] [Google Scholar]
- 12.Wiener RS, Gould MK, Arenberg DA, Au DH, Fennig K, Lamb CR, Mazzone PJ, Midthun DE, Napoli M, Ost DE, et al. ATS/ACCP Committee on Low-Dose CT Lung Cancer Screening in Clinical Practice. An official American Thoracic Society/American College of Chest Physicians policy statement: implementation of low-dose computed tomography lung cancer screening programs in clinical practice. Am J Respir Crit Care Med. 2015;192:881–891. doi: 10.1164/rccm.201508-1671ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wille MM, Dirksen A, Ashraf H, Saghir Z, Bach KS, Brodersen J, Clementsen PF, Hansen H, Larsen KR, Mortensen J, et al. Results of the randomized danish lung cancer screening trial with focus on high-risk profiling. Am J Respir Crit Care Med. 2016;193:542–551. doi: 10.1164/rccm.201505-1040OC. [DOI] [PubMed] [Google Scholar]
- 15.Infante M, Cavuto S, Lutman FR, Passera E, Chiarenza M, Chiesa G, Brambilla G, Angeli E, Aranzulla G, Chiti A, et al. DANTE Study Group. Long-term follow-up results of the DANTE trial, a randomized study of lung cancer screening with spiral computed tomography. Am J Respir Crit Care Med. 2015;191:1166–1175. doi: 10.1164/rccm.201408-1475OC. [DOI] [PubMed] [Google Scholar]
- 16.Horeweg N, van der Aalst CM, Thunnissen E, Nackaerts K, Weenink C, Groen HJ, Lammers JW, Aerts JG, Scholten ET, van Rosmalen J, et al. Characteristics of lung cancers detected by computer tomography screening in the randomized NELSON trial. Am J Respir Crit Care Med. 2013;187:848–854. doi: 10.1164/rccm.201209-1651OC. [DOI] [PubMed] [Google Scholar]
- 17.Kauczor HU, Bonomo L, Gaga M, Nackaerts K, Peled N, Prokop M, Remy-Jardin M, von Stackelberg O, Sculier JP European Society of Radiology (ESR); European Respiratory Society (ERS) ESR/ERS white paper on lung cancer screening. Eur Respir J. 2015;46:28–39. doi: 10.1183/09031936.00033015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez-Salcedo P, Wilson DO, de-Torres JP, Weissfeld JL, Berto J, Campo A, Alcaide AB, Pueyo J, Bastarrika G, Seijo LM, et al. Improving selection criteria for lung cancer screening: the potential role of emphysema. Am J Respir Crit Care Med. 2015;191:924–931. doi: 10.1164/rccm.201410-1848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de-Torres JP, Wilson DO, Sanchez-Salcedo P, Weissfeld JL, Berto J, Campo A, Alcaide AB, García-Granero M, Celli BR, Zulueta JJ. Lung cancer in patients with chronic obstructive pulmonary disease: development and validation of the COPD Lung Cancer Screening Score. Am J Respir Crit Care Med. 2015;191:285–291. doi: 10.1164/rccm.201407-1210OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young RP, Duan F, Chiles C, Hopkins RJ, Gamble GD, Greco EM, Gatsonis C, Aberle D. Airflow limitation and histology shift in the National Lung Screening Trial. The NLST-ACRIN Cohort Substudy. Am J Respir Crit Care Med. 2015;192:1060–1067. doi: 10.1164/rccm.201505-0894OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gould MK, Tang T, Liu IL, Lee J, Zheng C, Danforth KN, Kosco AE, Di Fiore JL, Suh DE. Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med. 2015;192:1208–1214. doi: 10.1164/rccm.201505-0990OC. [DOI] [PubMed] [Google Scholar]
- 22.Slatore CG, Horeweg N, Jett JR, Midthun DE, Powell CA, Wiener RS, Wisnivesky JP, Gould MK ATS Ad Hoc Committee on Setting a Research Framework for Pulmonary Nodule Evaluation. An Official American Thoracic Society Research Statement: a research framework for pulmonary nodule evaluation and management. Am J Respir Crit Care Med. 2015;192:500–514. doi: 10.1164/rccm.201506-1082ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callister ME, Baldwin DR, Akram AR, Barnard S, Cane P, Draffan J, Franks K, Gleeson F, Graham R, Malhotra P, et al. British Thoracic Society Pulmonary Nodule Guideline Development Group; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax. 2015;70:ii1–ii54. doi: 10.1136/thoraxjnl-2015-207168. [DOI] [PubMed] [Google Scholar]
- 24.Pinsky PF, Gierada DS, Black W, Munden R, Nath H, Aberle D, Kazerooni E. Performance of Lung-RADS in the National Lung Screening Trial: a retrospective assessment. Ann Intern Med. 2015;162:485–491. doi: 10.7326/M14-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maldonado F, Duan F, Raghunath SM, Rajagopalan S, Karwoski RA, Garg K, Greco E, Nath H, Robb RA, Bartholmai BJ, et al. Noninvasive computed tomography-based risk stratification of lung adenocarcinomas in the National Lung Screening Trial. Am J Respir Crit Care Med. 2015;192:737–744. doi: 10.1164/rccm.201503-0443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yankelevitz DF, Yip R, Smith JP, Liang M, Liu Y, Xu DM, Salvatore MM, Wolf AS, Flores RM, Henschke CI. International Early Lung Cancer Action Program Investigators Group. CT screening for lung cancer: nonsolid nodules in baseline and annual repeat rounds. Radiology. 2015;277:555–564. doi: 10.1148/radiol.2015142554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scholten ET, de Jong PA, de Hoop B, van Klaveren R, van Amelsvoort-van de Vorst S, Oudkerk M, Vliegenthart R, de Koning HJ, van der Aalst CM, Vernhout RM, et al. Towards a close computed tomography monitoring approach for screen detected subsolid pulmonary nodules? Eur Respir J. 2015;45:765–773. doi: 10.1183/09031936.00005914. [DOI] [PubMed] [Google Scholar]
- 28.van Boerdonk RA, Smesseim I, Heideman DA, Coupé VM, Tio D, Grünberg K, Thunnissen E, Snijders PJ, Postmus PE, Smit EF, et al. Close surveillance with long-term follow-up of subjects with preinvasive endobronchial lesions. Am J Respir Crit Care Med. 2015;192:1483–1489. doi: 10.1164/rccm.201504-0822OC. [DOI] [PubMed] [Google Scholar]
- 29.Oki M, Saka H, Ando M, Asano F, Kurimoto N, Morita K, Kitagawa C, Kogure Y, Miyazawa T. Ultrathin Bronchoscopy with multimodal devices for peripheral pulmonary lesions: a randomized trial. Am J Respir Crit Care Med. 2015;192:468–476. doi: 10.1164/rccm.201502-0205OC. [DOI] [PubMed] [Google Scholar]
- 30.Herth FJ, Eberhardt R, Sterman D, Silvestri GA, Hoffmann H, Shah PL. Bronchoscopic transparenchymal nodule access (BTPNA): first in human trial of a novel procedure for sampling solitary pulmonary nodules. Thorax. 2015;70:326–332. doi: 10.1136/thoraxjnl-2014-206211. [DOI] [PubMed] [Google Scholar]
- 31.Ost DE, Ernst A, Lei X, Kovitz KL, Benzaquen S, Diaz-Mendoza J, Greenhill S, Toth J, Feller-Kopman D, Puchalski J, et al. AQuIRE Bronchoscopy Registry. Diagnostic yield and complications of bronchoscopy for peripheral lung lesions. results of the AQuIRE registry. Am J Respir Crit Care Med. 2016;193:68–77. doi: 10.1164/rccm.201507-1332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider F, Smith MA, Lane MC, Pantanowitz L, Dacic S, Ohori NP. Adequacy of core needle biopsy specimens and fine-needle aspirates for molecular testing of lung adenocarcinomas. Am J Clin Pathol. 2015;143:193–200, quiz 306. doi: 10.1309/AJCPMY8UI7WSFSYY. [DOI] [PubMed] [Google Scholar]
- 33.Casadio C, Guarize J, Donghi S, Di Tonno C, Fumagalli C, Vacirca D, Dell’Orto P, De Marinis F, Spaggiari L, Viale G, et al. Molecular testing for targeted therapy in advanced non-small cell lung cancer: suitability of endobronchial ultrasound transbronchial needle aspiration. Am J Clin Pathol. 2015;144:629–634. doi: 10.1309/AJCPXGRAIMB4CTQ3. [DOI] [PubMed] [Google Scholar]
- 34.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO classification of tumours of the lung, pleura, thymus, and heart. Lyon, France: IARC Press; 2015. [DOI] [PubMed] [Google Scholar]
- 35.Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JH, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB, et al. WHO Panel. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 36.Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P, Mitchell A, Bolejack V International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Feinstein MB, DeSouza SA, Moreira AL, Stover DE, Heelan RT, Iyriboz TA, Taur Y, Travis WD. A comparison of the pathological, clinical and radiographical, features of cryptogenic organising pneumonia, acute fibrinous and organising pneumonia and granulomatous organising pneumonia. J Clin Pathol. 2015;68:441–447. doi: 10.1136/jclinpath-2014-202626. [DOI] [PubMed] [Google Scholar]
- 38.Detterbeck FC, Nicholson AG, Franklin WA, Marom EM, Travis WD, Girard N, Arenberg DA, Bolejack V, Donington JS, Mazzone PJ, et al. The IASLC Lung Cancer Staging Project: summary of proposals for revisions of the classification of lung cancers with multiple pulmonary sites of involvement in the forthcoming eighth edition of the TNM Classification. J Thorac Oncol. 2016;11:639–650. doi: 10.1016/j.jtho.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 39.Silvestri GA, Vachani A, Whitney D, Elashoff M, Porta Smith K, Ferguson JS, Parsons E, Mitra N, Brody J, Lenburg ME, et al. AEGIS Study Team. A bronchial genomic classifier for the diagnostic evaluation of lung cancer. N Engl J Med. 2015;373:243–251. doi: 10.1056/NEJMoa1504601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vachani A, Whitney DH, Parsons EC, Lenburg M, Ferguson JS, Silvestri GA, Spira A. Clinical utility of a bronchial genomic classifier in patients with suspected lung cancer. Chest. 2016;150:210–218. doi: 10.1016/j.chest.2016.02.636. [DOI] [PubMed] [Google Scholar]
- 41.Montani F, Marzi MJ, Dezi F, Dama E, Carletti RM, Bonizzi G, Bertolotti R, Bellomi M, Rampinelli C, Maisonneuve P, et al. miR-Test: a blood test for lung cancer early detection. J Natl Cancer Inst. 2015;107:djv063. doi: 10.1093/jnci/djv063. [DOI] [PubMed] [Google Scholar]
- 42.Wozniak MB, Scelo G, Muller DC, Mukeria A, Zaridze D, Brennan P. Circulating microRNAs as non-invasive biomarkers for early detection of non-small-cell lung cancer. Plos One. 2015;10:e0125026. doi: 10.1371/journal.pone.0125026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xing L, Su J, Guarnera MA, Zhang H, Cai L, Zhou R, Stass SA, Jiang F. Sputum microRNA biomarkers for identifying lung cancer in indeterminate solitary pulmonary nodules. Clin Cancer Res. 2015;21:484–489. doi: 10.1158/1078-0432.CCR-14-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsay JC, Li Z, Yie TA, Wu F, Segal L, Greenberg AK, Leibert E, Weiden MD, Pass H, Munger J, et al. Molecular characterization of the peripheral airway field of cancerization in lung adenocarcinoma. Plos One. 2015;10:e0118132. doi: 10.1371/journal.pone.0118132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fahrmann JF, Kim K, DeFelice BC, Taylor SL, Gandara DR, Yoneda KY, Cooke DT, Fiehn O, Kelly K, Miyamoto S. Investigation of metabolomic blood biomarkers for detection of adenocarcinoma lung cancer. Cancer Epidemiol Biomarkers Prev. 2015;24:1716–1723. doi: 10.1158/1055-9965.EPI-15-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vachani A, Pass HI, Rom WN, Midthun DE, Edell ES, Laviolette M, Li XJ, Fong PY, Hunsucker SW, Hayward C, et al. Validation of a multiprotein plasma classifier to identify benign lung nodules. J Thorac Oncol. 2015;10:629–637. doi: 10.1097/JTO.0000000000000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al. Cancer immunology: mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson FH, Johannessen CM, Piccioni F, Tamayo P, Kim JW, Van Allen EM, Corsello SM, Capelletti M, Calles A, Butaney M, et al. A functional landscape of resistance to ALK inhibition in lung cancer. Cancer Cell. 2015;27:397–408. doi: 10.1016/j.ccell.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.George J, Lim JS, Jang SJ, Cun Y, Ozretić L, Kong G, Leenders F, Lu X, Fernández-Cuesta L, Bosco G, et al. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015;524:47–53. doi: 10.1038/nature14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Araujo LH, Timmers C, Bell EH, Shilo K, Lammers PE, Zhao W, Natarajan TG, Miller CJ, Zhang J, Yilmaz AS, et al. Genomic characterization of non-small-cell lung cancer in African Americans by targeted massively parallel sequencing. J Clin Oncol. 2015;33:1966–1973. doi: 10.1200/JCO.2014.59.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arrieta O, Cardona AF, Martín C, Más-López L, Corrales-Rodríguez L, Bramuglia G, Castillo-Fernandez O, Meyerson M, Amieva-Rivera E, Campos-Parra AD, et al. Updated frequency of EGFR and KRAS mutations in nonsmall-cell lung cancer in Latin America: the Latin-American Consortium for the Investigation of Lung Cancer (CLICaP) J Thorac Oncol. 2015;10:838–843. doi: 10.1097/JTO.0000000000000481. [DOI] [PubMed] [Google Scholar]
- 52.Chang JY, Senan S, Paul MA, Mehran RJ, Louie AV, Balter P, Groen HJ, McRae SE, Widder J, Feng L, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16:630–637. doi: 10.1016/S1470-2045(15)70168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ezer N, Veluswamy RR, Mhango G, Rosenzweig KE, Powell CA, Wisnivesky JP. Outcomes after stereotactic body radiotherapy versus limited resection in older patients with early-stage lung cancer. J Thorac Oncol. 2015;10:1201–1206. doi: 10.1097/JTO.0000000000000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Veluswamy RR, Ezer N, Mhango G, Goodman E, Bonomi M, Neugut AI, Swanson S, Powell CA, Beasley MB, Wisnivesky JP. Limited resection versus lobectomy for older patients with early-stage lung cancer: impact of histology. J Clin Oncol. 2015;33:3447–3453. doi: 10.1200/JCO.2014.60.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wakelee H, Dahlberg S, Keller S, Tester W, Gandara D, Graziano S, Adjei A, Leighl N, Aisner S, Rothman J, et al. Randomized phase iii trial of adjuvant chemotherapy with or without bevacizumab in resected non-small cell lung cancer (NSCLC): results of E1505. Presented at the 16th World Conference on Lung Cancer. September 6–9, 2015, Denver, COAbstract 1608 [Google Scholar]
- 56.Govindan R, Mandrekar SJ, Gerber DE, Oxnard GR, Dahlberg SE, Chaft J, Malik S, Mooney M, Abrams JS, Janne PA, et al. ALCHEMIST trials: a golden opportunity to transform outcomes in early-stage non-small cell lung cancer. Clin Cancer Res. 2015;21:5439–5444. doi: 10.1158/1078-0432.CCR-15-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, Bogart J, Hu C, Forster K, Magliocco A, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thatcher N, Hirsch FR, Luft AV, Szczesna A, Ciuleanu TE, Dediu M, Ramlau R, Galiulin RK, Bálint B, Losonczy G, et al. SQUIRE Investigators. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16:763–774. doi: 10.1016/S1470-2045(15)00021-2. [DOI] [PubMed] [Google Scholar]
- 60.Soria JC, Felip E, Cobo M, Lu S, Syrigos K, Lee KH, Göker E, Georgoulias V, Li W, Isla D, et al. LUX-Lung 8 Investigators. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2015;16:897–907. doi: 10.1016/S1470-2045(15)00006-6. [DOI] [PubMed] [Google Scholar]
- 61.Park K, Tan E, Zhang L.Afatinib versus gefitinib as first-line treatment for patients with advanced non-small cell lung cancer harboring activating EGFR mutations: results of the global, randomized, open-label, Phase IIb trial LUX-Lung 7. Presented at the European Society for Medical Oncology Asia Congress. December 18–21, 2015, SingaporeLBA2
- 62.Halmos B, Pennell NA, Fu P, Saad S, Gadgeel S, Otterson GA, Mekhail T, Snell M, Kuebler JP, Sharma N, et al. Randomized phase II trial of erlotinib beyond progression in advanced erlotinib-responsive non-small cell lung cancer. Oncologist. 2015;20:1298–1303. doi: 10.1634/theoncologist.2015-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soria JC, Wu YL, Nakagawa K, Kim SW, Yang JJ, Ahn MJ, Wang J, Yang JC, Lu Y, Atagi S, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol. 2015;16:990–998. doi: 10.1016/S1470-2045(15)00121-7. [DOI] [PubMed] [Google Scholar]
- 64.Jänne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372:1689–1699. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 65.Sequist LV, Soria JC, Goldman JW, Wakelee HA, Gadgeel SM, Varga A, Papadimitrakopoulou V, Solomon BJ, Oxnard GR, Dziadziuszko R, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2015;372:1700–1709. doi: 10.1056/NEJMoa1413654. [DOI] [PubMed] [Google Scholar]
- 66.Lin JJ, Cardarella S, Lydon CA, Dahlberg SE, Jackman DM, Jänne PA, Johnson BE. Five-year survival in EGFR-mutant metastatic lung adenocarcinoma treated with EGFR-TKIs. J Thorac Oncol. 2016;11:556–565. doi: 10.1016/j.jtho.2015.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karlovich C, Goldman JW, Sun JM, Mann E, Sequist LV, Konopa K, Wen W, Angenendt P, Horn L, Spigel D, et al. Assessment of EGFR mutation status in matched plasma and tumor tissue of NSCLC patients from a phase i study of rociletinib (CO-1686) Clin Cancer Res. 2016;22:2386–2395. doi: 10.1158/1078-0432.CCR-15-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tam A, Churg A, Wright JL, Zhou S, Kirby M, Coxson HO, Lam S, Man SF, Sin DD. Sex differences in airway remodeling in a mouse model of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;193:825–834. doi: 10.1164/rccm.201503-0487OC. [DOI] [PubMed] [Google Scholar]
- 69.Shaw AT, Gandhi L, Gadgeel S, Riely GJ, Cetnar J, West H, Camidge DR, Socinski MA, Chiappori A, Mekhail T, et al. Study Investigators. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol. 2016;17:234–242. doi: 10.1016/S1470-2045(15)00488-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frampton GM, Ali SM, Rosenzweig M, Chmielecki J, Lu X, Bauer TM, Akimov M, Bufill JA, Lee C, Jentz D, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 2015;5:850–859. doi: 10.1158/2159-8290.CD-15-0285. [DOI] [PubMed] [Google Scholar]
- 71.Paik PK, Drilon A, Fan PD, Yu H, Rekhtman N, Ginsberg MS, Borsu L, Schultz N, Berger MF, Rudin CM, et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov. 2015;5:842–849. doi: 10.1158/2159-8290.CD-14-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kachuri L, Amos CI, McKay JD, Johansson M, Vineis P, Bueno-de-Mesquita HB, Boutron-Ruault MC, Johansson M, Quirós JR, Sieri S, et al. Fine mapping of chromosome 5p15.33 based on a targeted deep sequencing and high density genotyping identifies novel lung cancer susceptibility loci. Carcinogenesis. 2016;37:96–105. doi: 10.1093/carcin/bgv165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 75.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, et al. KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 76.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 77.Antonia S, Goldberg SB, Balmanoukian A, Chaft JE, Sanborn RE, Gupta A, Narwal R, Steele K, Gu Y, Karakunnel JJ, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016;17:299–308. doi: 10.1016/S1470-2045(15)00544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brahmer JR, Kim ES, Zhang J, Smith MM, Rangwala RA, O'Brien MER. KEYNOTE-024: phase III trial of pembrolizumab (MK-3475) vs platinum-based chemotherapy as first-line therapy for patients with metastatic non-small cell lung cancer (NSCLC) that expresses programmed cell death ligand 1 (PD-L1). J Clin Oncol. 2015;33:TPS8103.
- 79.Ott P, Fernandez M, Hiret S, Kim D, Moss R, Winser T, Yuan S, Cheng J, Piperdi B, Mehnert J. Pembrolizumab (MK-3475) in patients (pts) with extensive-stage small cell lung cancer (SCLC): preliminary safety and efficacy results from KEYNOTE-028. J Clin Oncol. 2015;33:S7502. [Google Scholar]
- 80.Saunders LR, Bankovich AJ, Anderson WC, Aujay MA, Bheddah S, Black K, Desai R, Escarpe PA, Hampl J, Laysang A, et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med. 2015;7:302ra136. doi: 10.1126/scitranslmed.aac9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zalcman G, Mazieres J, Margery J, Greillier L, Audigier-Valette C, Moro-Sibilot D, Molinier O, Corre R, Monnet I, Gounant V, et al. French Cooperative Thoracic Intergroup. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet. 2015;387:1405–1414. doi: 10.1016/S0140-6736(15)01238-6. [DOI] [PubMed] [Google Scholar]
- 82.Travis WD, Asamura H, Bankier AA, Beasley MB, Detterbeck F, Flieder DB, Goo JM, MacMahon H, Naidich D, Nicholson AG, et al. International Association for the Staging of Lung Cancer Staging and Prognostic Factors Committee and Advisory Board Members. The IASLC Lung Cancer Staging Project: proposals for coding T categories for subsolid nodules and assessment of tumor size in part-solid tumors in the forthcoming eighth edition of the TNM Classification of Lung Cancer J Thorac Oncol 2016111204–1223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.