Abstract
Somatic and germline mutations in the inward rectifying K+ channel (KCNJ5) are a common cause of primary aldosteronism (PA) in aldosterone producing adenoma and familial hyperaldosteronism type III, respectively. Dysregulation of adrenal cell calcium signaling represents one mechanism for mutated KCNJ5 stimulation of aldosterone synthase (CYP11B2) expression and aldosterone production. However, the mechanisms stimulating acute and chronic production of aldosterone by mutant KCNJ5 have not been fully characterized. Herein, we defined the effects of the T158A KCNJ5 mutation (KCNJ5T158A) on acute and chronic regulation of aldosterone production using an adrenal cell line with a doxycycline inducible KCNJ5T158A gene (HAC15-TRE-KCNJ5T158A). Doxycycline incubation caused a time-dependent increase in KCNJ5T158A and CYP11B2 mRNA and protein levels. Electrophysiological analyses confirm the loss of inward rectification and increased Na+ permeability in KCNJ5T158A-expressing cells. KCNJ5T158A expression also led to activation of CYP11B2 transcriptional regulators, NURR1 and ATF2. Acutely, KCNJ5T158A stimulated the expression of total and phosphorylated steroidogenic acute regulatory protein (StAR). KCNJ5T158A expression increased synthesis of aldosterone and the hybrid steroids 18-hydroxycortisol and 18-oxocortisol, measured with liquid chromatography-tandem mass spectrometry (LC-MS/MS). All of these stimulatory effects of KCNJ5T158A were inhibited by the L-type Ca2+ channel blocker, verapamil. Overall, KCNJ5T158A increases CYP11B2 expression and production of aldosterone, corticosterone and hybrid steroids by upregulating both acute and chronic regulatory events in aldosterone production, and verapamil blocks KCNJ5T158A-mediated pathways leading to aldosterone production.
Keywords: KCNJ5 mutations, primary aldosteronism (PA), adrenal, aldosterone
INTRODUCTION
In normal physiology, aldosterone production is mainly regulated by the renin-angiotensin-aldosterone system and plasma potassium levels. Primary aldosteronism (PA) is characterized by renin-independent aldosterone excess and represents the most common cause of secondary hypertension. The main causes of PA are aldosterone producing adenomas (APA) and bilateral adrenal hyperplasia. In 2011, Choi and colleagues reported mutations in the selectivity filter of the inward-rectifying K+ channel KCNJ5 in 35 % of APA (Choi, et al. 2011). In the past few years, several other mutations localized in or near the KCNJ5 selectivity filter have been described, causing a change in permeability from only K+ ions to allow influx of Na+ ions (Akerstrom, et al. 2012; Akerstrom, et al. 2015; Boulkroun, et al. 2013; Charmandari, et al. 2012; Choi et al. 2011; Dutta, et al. 2014; Hardege, et al. 2015; Kuppusamy, et al. 2014; Lenzini, et al. 2015; Monticone, et al. 2015; Monticone, et al. 2012; Monticone, et al. 2013; Mulatero, et al. 2012b; Murthy, et al. 2012; Murthy, et al. 2014; Scholl and Lifton 2013; Scholl, et al. 2012; Thiel, et al. 2015; Williams, et al. 2013; Zennaro and Jeunemaitre 2011). The Na+ influx triggers the activation of voltage-gated Ca2+ channels and downstream signaling pathways, which leads to elevated expression of aldosterone synthase (CYP11B2) and aldosterone production in basal and angiotensin II treated conditions (Hardege et al. 2015; Kuppusamy et al. 2014; Monticone et al. 2015; Monticone et al. 2012; Monticone et al. 2013; Mulatero et al. 2012b; Murthy et al. 2014; Oki, et al. 2012; Wang, et al. 2015; Williams et al. 2013; Williams, et al. 2014). The use of L-type Ca2+ channel blockers, such as nifedipine or verapamil, have been shown to block mutated KCNJ5-mediated aldosterone production in vitro (Kuppusamy et al. 2014; Monticone et al. 2012; Monticone et al. 2013; Oki et al. 2012; Tauber, et al. 2014). However, the details regarding the effects of KCNJ5T158A on signaling events downstream of intracellular Ca2+ have not been well defined.
Most in vitro studies of mutant KCNJ5 have used strategies with constitutive transgene expression in adrenocortical cells. These studies have provided useful findings regarding the effects of mutated KCNJ5 on membrane potential, CYP11B2 and NURR1 transcription, and aldosterone production. Other studies have used transient methods of expression (such as electroporation), which by itself can be toxic to cells, requiring longer recovery period for cells, in addition to potentially activating stress-mediated signaling. Therefore, this strategy makes it difficult to define the acute actions of mutant KCNJ5 on adrenal cell function.
To overcome the toxic effects of transfections, we developed a doxycycline-inducible system for KCNJ5 harboring the T158A mutation (KCNJ5T158A, encoding the mutant channel Kir3.4T158A) in order to define the effect of KCNJ5T158A on acute and chronic events leading to aldosterone production.
MATERIALS AND METHODS
Cell Culture
Human adrenocortical carcinoma (HAC15) cells were cultured as described earlier (Monticone et al. 2012; Monticone et al. 2013; Parmar, et al. 2008; Wang, et al. 2012). The pLenti-CMV-rtTA3-Hygro vector was used to produce a lentivirus to generate the HAC15-CMV-rtTA3 cell line expressing the reverse tetracycline-controlled transactivator 3 (rtTA3) (Campeau, et al. 2009). These cells were further transduced with a lentivirus expressing KCNJ5 cDNA harboring the T158A mutation (KCNJ5T158A) under the CMV promoter containing a Tet Operator element (TO), to generate the HAC15-TRE-KCNJ5T158A cells. Incubation with doxycycline prevents rtTA3 from physically binding to the TO, thus permitting expression of KCNJ5T158A. The HAC15-CMV-rtTA3-hygro and HAC15-TRE- KCNJ5T158A cells were grown in Dulbecco’s Modified Eagle’s/Ham’s F-12 medium (DMEM/F-12) containing 5% Cosmic calf serum (CCS, Hyclone, Logon UT), 1% ITS (Gibco) and antibiotics (penicillin-streptomycin and gentamicin). HAC15-TRE-KCNJ5T158A cells were further selected with 10 µg/mL puromycin. For experiments, cells were plated at a density of 50,000 cells/well in a 48-well dish (for studies involving gene expression analyses) or at 1 million cells/well in a 6-well dish (for nuclear protein isolation) for 48 h. After incubation in low-serum medium (DMEM/F-12 containing 0.1% CCS and antibiotics) for 24 h, KCNJ5T158A induction was initiated with 1 µg/mL doxycycline for the indicated times. For LC-MS/MS measurement of steroids, cells were incubated in doxycycline for 60 h. Inhibitor studies involved treatment of cells with 10 µM verapamil for 30 min prior to and during the course of doxycycline treatment.
For electrophysiological studies, HAC15-TRE- KCNJ5T158A cells treated with doxycycline for 60 h were plated on circular coverslips in 12-well plates at a density of 200,000 cells/well for 8–10 hours. During patching, the coverslips were rinsed in normal Tyrode’s solution prior to patching. Due to previous observations that endogenous Kir3.4 is non-functional in HAC15 cells (Kienitz, et al. 2015), cells were transduced with lentiviruses for constitutive expression of wild-type KCNJ5 (KCNJ5WT, encoding channel Kir3.4WT) and selected with for antibiotic resistance for blasticidin. These cells over-expressing KCNJ5WT were used for the purpose of measuring control currents.
Viral transduction
Cells were plated for 24 h and treated with appropriate amounts of virus in growth medium devoid of any antibiotics and containing 8 µg/mL polybrene. Cells were centrifuged at 1200 rpm for 2 h and incubated overnight to attain maximal transduction efficiency (Wu, et al. 2009). Recovery was performed with the addition of 2× growth medium devoid of any antibiotics/polybrene. After an additional 48 h, cells were sub-cultured and maintained in normal growth medium with 10 µg/mL puromycin.
RNA isolation and real-time quantitative PCR (RT-qPCR)
Gene expression analyses were performed using Taqman primer-probes (to make a total of 900 nM of each primer and 400 nM probe per reaction; Life Technologies, Carlsbad, CA) and Kapa Probe fast qPCR kit master mix ABI Prism (Kapa Biosystems, Boston MA). Negative controls consisted of nuclease-free water in place of cDNA. The PCR program, consisting of 40 amplification cycles, was performed following the manufacturer’s recommendations. Normalization of gene expression within each sample was performed by using the respective expression levels of PPIA (cyclophilin A) to calculate the ΔCt. Relative increase in gene expression was calculated using the 2−ΔΔCt method.
LC-MS/MS measurements of steroids
A mixture of 60 µL aliquot of medium, internal standards and 3% isopropyl alcohol was loaded on supported liquid extraction plates (Novum, Phenomenex) and subsequently eluted with 2 mL of methyl-t-butyl ether. The organic phase was concentrated under nitrogen. The dried extract was reconstituted with 60 µL of methanol/deionized water (1:1) and transferred to a 0.25-mL vial insert. Samples (10 µL) were injected via autosampler and resolved with an Agilent 1290 binary pump HPLC on a Kinetex 50×2.1 mm, 3µm particle size biphenyl column (Phenomenex) using gradient elution with 0.25 mmol/L ammonium fluoride and methanol. The column effluent was directed into the source of an Agilent 6490 triple quadrupole mass spectrometer using electrospray ionization in positive ionization mode and analyzed using multiple reaction monitoring mode. Quantitation was accomplished by comparing ion currents for the monitored ions with 13-point quadratic external calibration curves (r2 was minimum 0.995) and corrected for specimen dilution and recovery of internal standards using ChemStation and MassHunter software (Agilent). The retention times for steroids and respective internal standards are provided in Supplemental Table 2. Steroid levels were normalized to cellular protein and expressed as fold over basal.
Electrophysiology
Ion currents were recorded in whole-cell patch-clamp configuration using a MultiClamp 700B amplifier and Digidata 1440A digitizer (Molecular Devices, Sunnyvale, CA). Patch pipettes had resistances of 6–10 MΩ when filled with intracellular pipette solution and placed in extracellular solution. The external solution consisted of a simple HEPES-based Tyrode’s solution (140 mM NaCl, 5 mM KCl, 1.8 mM CaCl2 and 1.8 mM MgCl2). The internal pipette solution contained 95 mM K-gluconate, 15 mM KCl, 15 mM NaCl, 0.726 mM CaCl2, 3 mM EGTA and 2.38 mM MgCl2, 3 mM K2-ATP, 0.025 mM K2-GTP, 0.5 mM GTP-γ-S (pH 7.2 adjusted with KOH). In order to confirm Na+ permeability of mutated KCNJ5 channels, NaCl in the external bath solution was replaced by choline chloride. Barium chloride (1 mM) was used to inhibit K+ conductance. Data acquisition and analysis were performed using pCLAMP software (ver.10.3; Molecular Devices, Sunnyvale, CA). Current amplitudes were divided by cell capacitance (Cm) and expressed as current densities (pA/pF) to normalize for variable cell sizes.
RESULTS
Doxycycline causes time-dependent induction of KCNJ5T158A
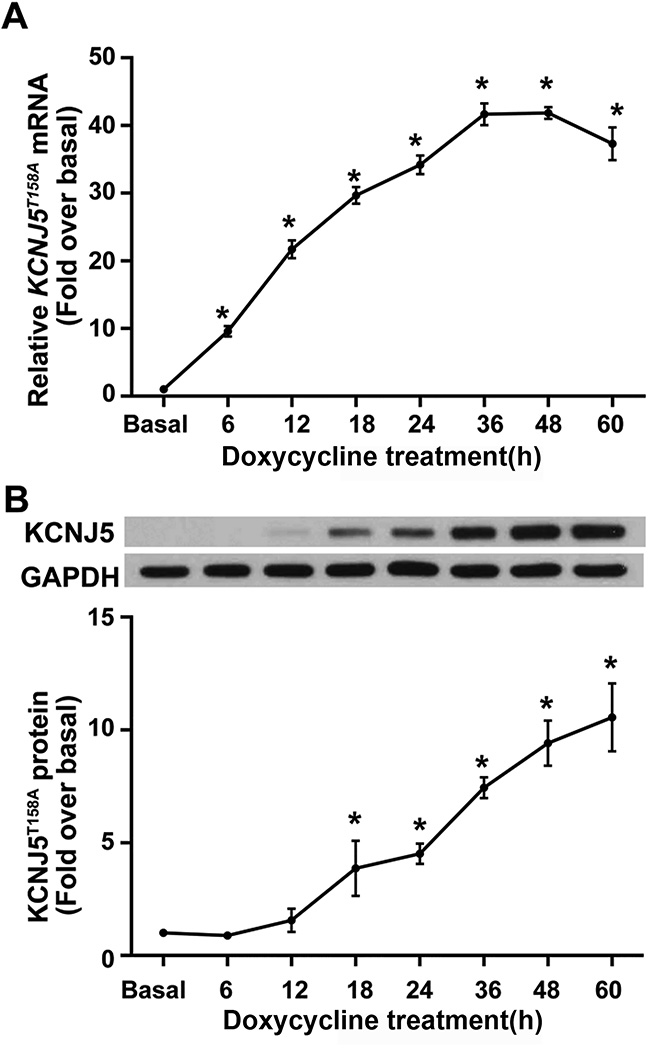
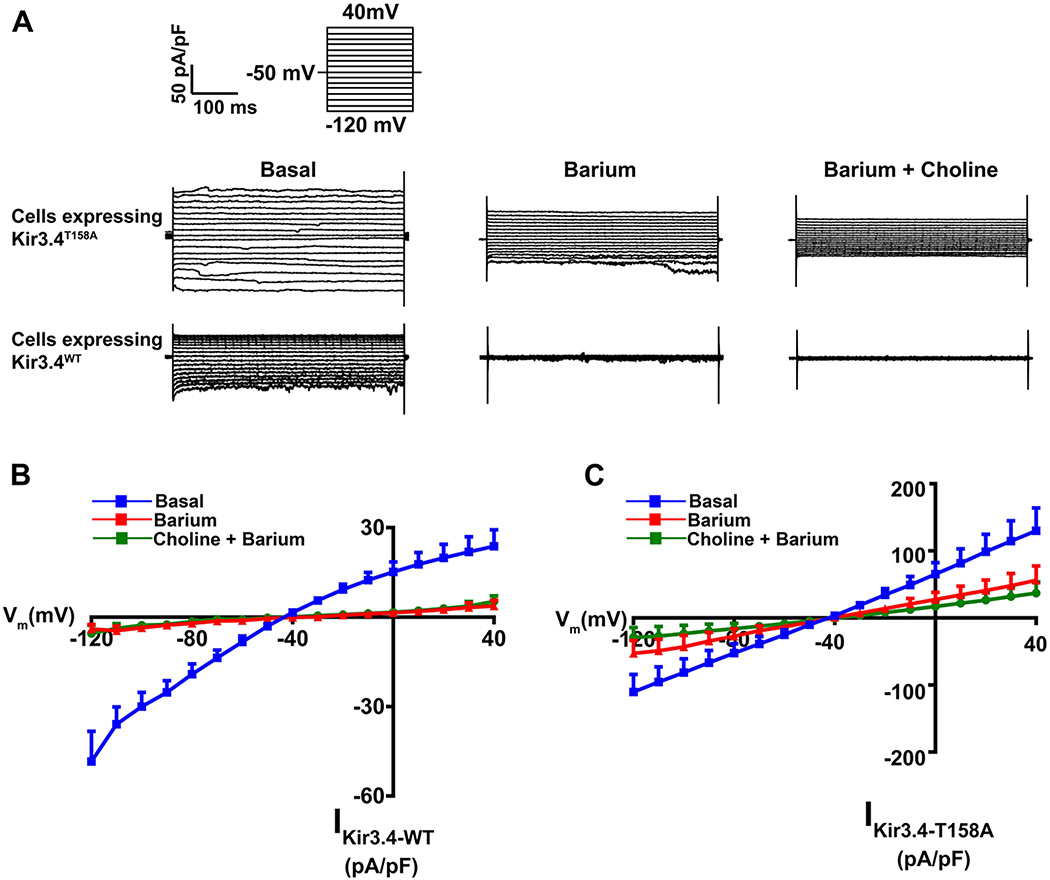
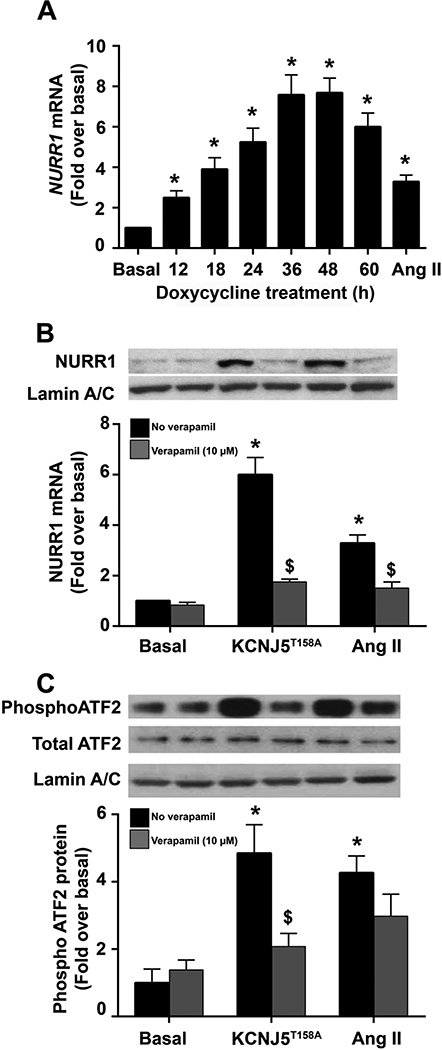
The HAC15-TRE-KCNJ5T158A cell model was generated with doxycycline-regulated KCNJ5T158A transgene expression using lentiviral transduction. HAC15-TRE-KCNJ5T158A cells were treated with 1 µg/mL doxycycline for the indicated times to induce KCNJ5T158A expression. KCNJ5T158A exhibited a time-dependent increase in mRNA (Figure 1, Panel A) and protein (Figure 1, Panel B). Transcript expression increased after 6 h of doxycycline incubation, peaked at 36 h (increasing by ~ 40-fold) and plateaued thereafter. Western analysis indicated an increase in KCNJ5T158A protein levels. Semi-quantitative densitometric analysis indicated that KCNJ5T158A protein increased approximately 5-fold to 10-fold between 24 h to 60 h. Electrophysiological analyses indicated that expression of Kir3.4T158A channel by doxycycline incubation also revealed increased conductance to Na+ in addition to K+, as indicated by sensitivity to both barium and choline. In contrast, in cells constitutively expressing the Kir3.4WT channel, channel conductance was sensitive to barium but not choline (Figure 2, Panels A–C). The Kir3.4T158A channel also showed a loss of the inward rectification property compared to the Kir3.4WT channel (Figure 2, Panels B and C).
Figure 1.
Figure 2.
KCNJ5T158A increased CYP11B2 expression and aldosterone production, which were reversed by blockade of calcium channels
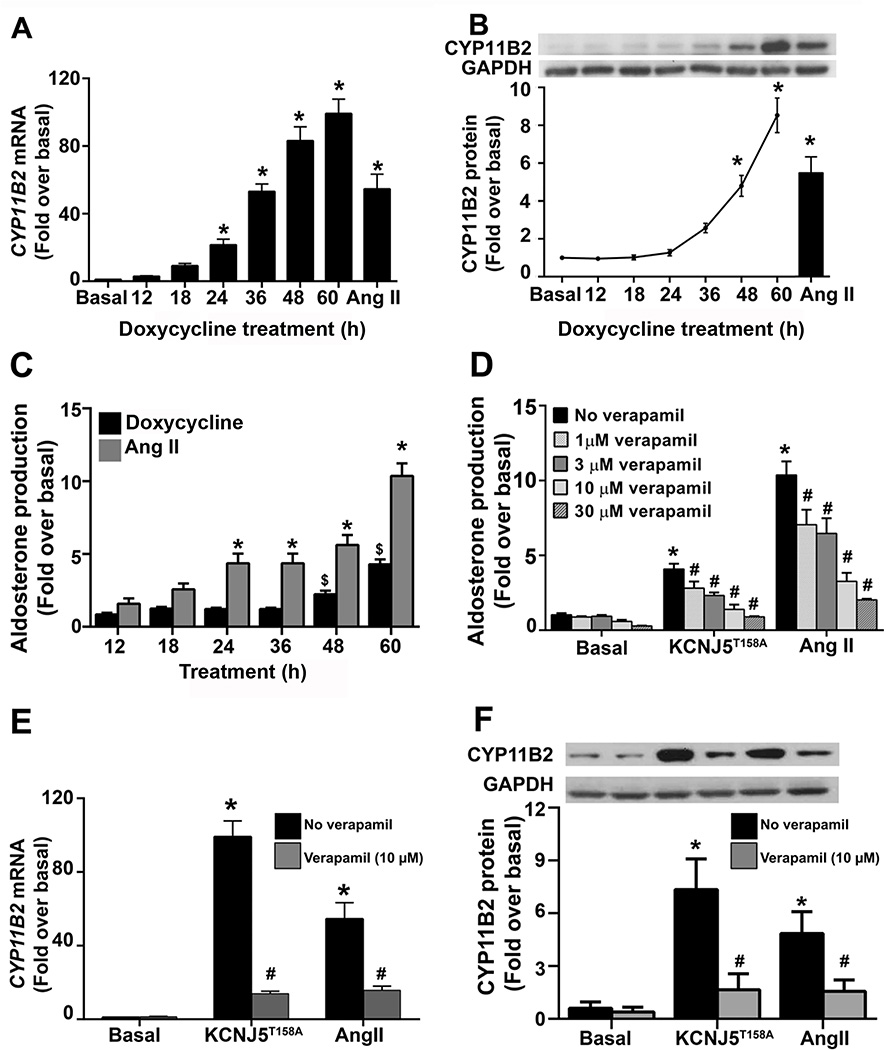
The temporal effects of doxycycline (1 µg/mL) on CYP11B2 expression and aldosterone production in HAC15-TRE-KCNJ5T158A cells were investigated. The data show that, along with increased KCNJ5T158A expression, there was a time-dependent stimulation in CYP11B2 mRNA and protein levels (Figure 3 Panels A and B). Specifically, transcript expression peaked at 36 h and plateaued thereafter (Figure 3 Panel A). CYP11B2 protein levels doubled every 12 h after 24 h doxycycline incubation, attaining a 5-fold increase over basal at 48 h and almost 10-fold over basal at 60 h. Treatment with the agonist angiotensin II (Ang II) served as a positive control and led to an increase in CYP11B2 mRNA (by ~60-fold over basal) and protein (by 8-fold) (Figure 3, Panels A and B). Aldosterone levels also exhibited a time-dependent increase in response to Ang II and KCNJ5T158A expression (Figure 3, Panel C). Ang II stimulatory effects were observed as early as 24 h, while KCNJ5T158A-induced aldosterone increase was detected at 48 h. At 60 h Ang II or doxycycline treatment, aldosterone levels were elevated (normalized to protein) by over 4-fold and 10-fold (compared to basal), respectively (Figure 2, Panel D). Finally, treatment with the L-type Ca2+ channel blocker verapamil (1, 3, 10 and 30 µM) blocked the stimulatory effects of Kir3.4T158A- and Ang II-induced aldosterone production in a concentration-dependent manner (Figure 3, Panel D). Although the highest inhibitory effect on aldosterone was observed using 30 µM verapamil, this dose also exhibited some cytotoxic effects. Therefore, the most efficient dose for verapamil was 10 µM, which inhibited Kir3.4T158A- and Ang II-stimulated aldosterone production by approximately 65% and 70%, respectively. Treatment with 10 µM verapamil also abrogated Kir3.4T158A- and Ang II-induced CYP11B2 mRNA and protein expression (by approximately 85% and 70%, respectively) (Figure 3, Panels E and F).
Figure 3.
Effect of KCNJ5T158A on adrenal steroidogenesis
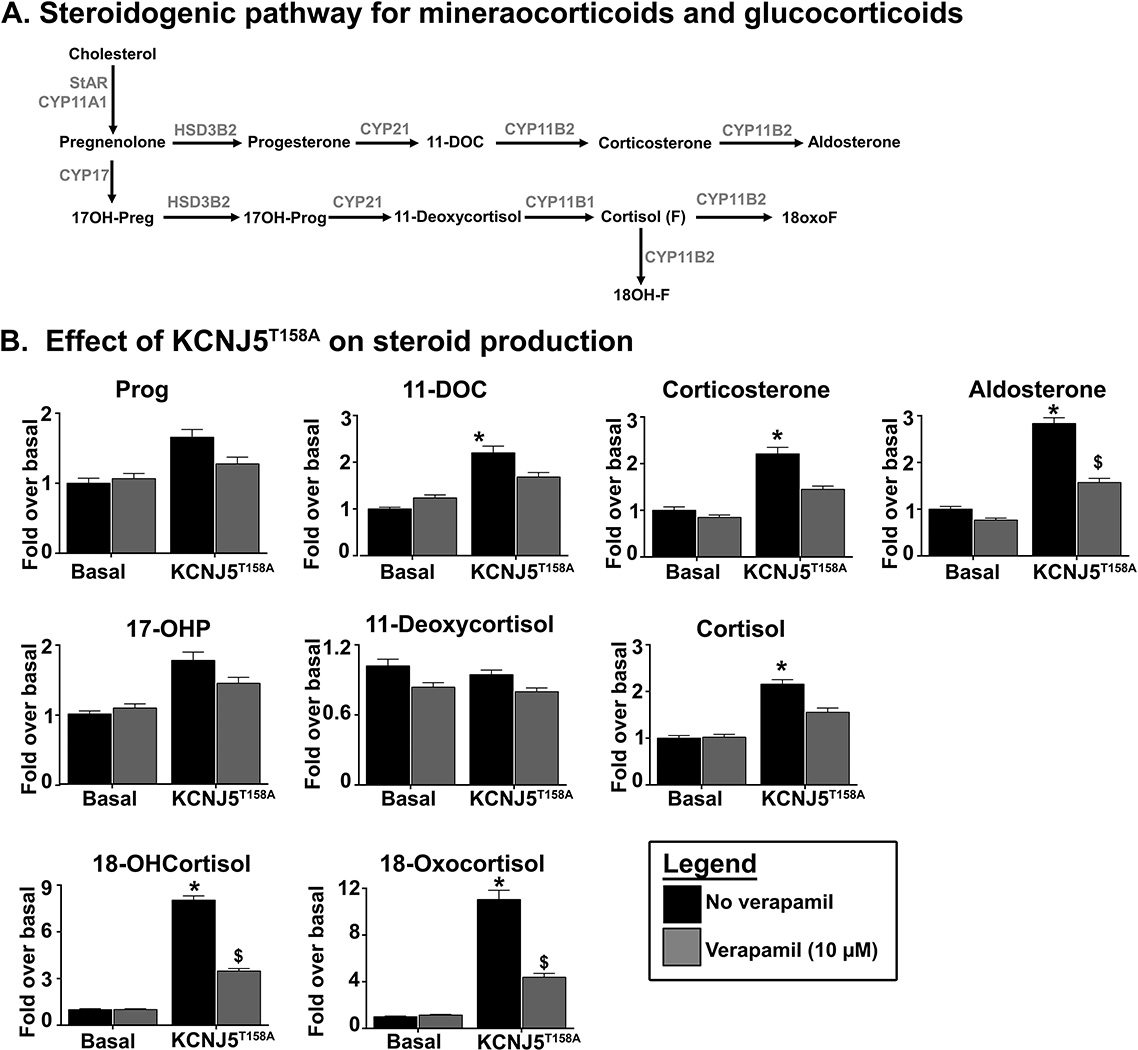
To better define the effects of KCNJ5T158A expression on adrenal steroidogenesis, cells were incubated in doxycycline for 60 h, and LC-MS/MS was performed to analyze the steroids in the pathway leading to aldosterone and cortisol production, as well as the hybrid steroids 18-hydroxycortisol (18OHF) and 18-oxocortisol (18oxoF). Figure 4, Panel A provides a schematic representation of the steroidogenic pathways for the synthesis of glucocorticoids and mineralocorticoids. KCNJ5T158A expression led to an increase in the production of aldosterone, as well as aldosterone precursors 11-deoxycorticosterone (DOC) and corticosterone, and cortisol (Figure 4, Panel B). No changes in progesterone, 17-hydroxyprogesterone and 11-deoxycortisol were observed. In addition, KCNJ5T158A increased the production of the hybrid steroids 18OHF and 18oxoF by over 6-fold.
Figure 4.
Effect of KCNJ5T158A on transcriptional events that regulate CYP11B2 expression
The transcription factors NURR1 and ATF2 are the main regulators of CYP11B2 promoter activation and are activated by intracellular calcium levels (Bassett, et al. 2004a; Bassett, et al. 2000; Hattangady, et al. 2012; Nogueira, et al. 2009). We, therefore, investigated the effect of KCNJ5T158A on the expression of NURR1 and the phosphorylation of ATF2. Analyses for NURR1 transcript levels indicate a time-dependent increase upon KCNJ5T158A induction, with NURR1 mRNA peaking at 8-fold over basal at 36 h and plateauing thereafter (Figure 5, Panel A). An increase in nuclear-localized NURR1 was also observed at 48 h of KCNJ5T158A activation by doxycycline treatment (Figure 5, Panel B). Stimulation of NURR1 mRNA and protein was inhibited by pre-treatment of cells with verapamil (10 µM) by approximately 70% (Figure 5, Panel B). Treatment with Ang II as a positive control similarly elevated NURR1 transcript and protein by 5-fold at 60 h of incubation. Verapamil pre-treatment inhibited the Ang II-mediated effects by approximately 50%. To define the effect of KCNJ5T158A on the activation of ATF2 (a member of the cAMP-response element-binding protein (CREB) family), nuclear protein from cells incubated with doxycycline or Ang II in the presence or absence of verapamil was assessed for phosphorylation. Both, Ang II and doxycycline treatment increased phosphorylation of ATF2 (normalized to total ATF2 levels) by ~ 5-fold and 4-fold, respectively (Figure 5, Panel C). These effects were reversible by calcium channel blockade with verapamil, which inhibited doxycycline and Ang II effects by 50% and 30%, respectively. Overall, these data indicate that KCNJ5T158A-mediated calcium dysregulation leads to the activation of transcriptional events needed for CYP11B2 transcription.
Figure 5.
Effect of KCNJ5T158A on acute regulatory events in aldosterone production
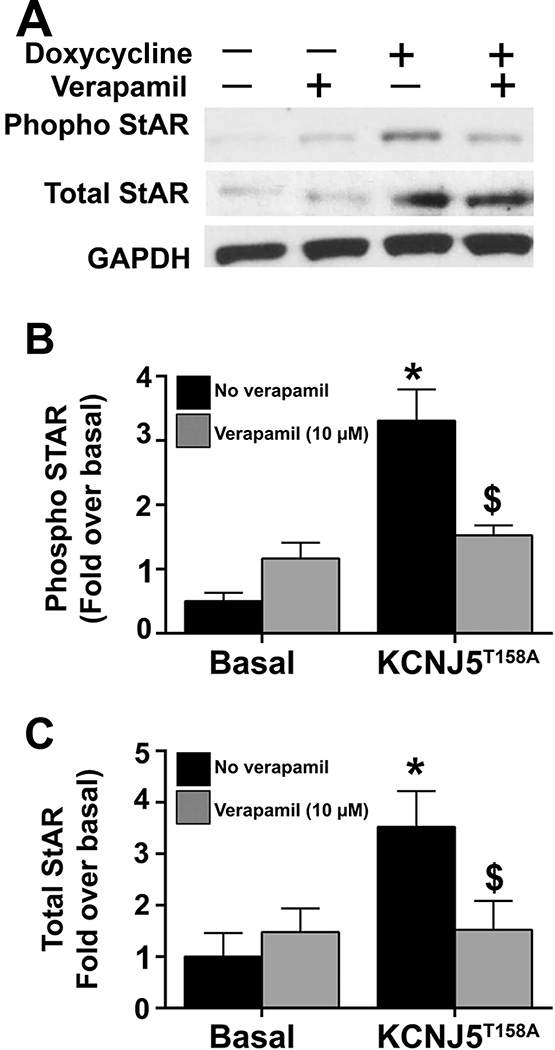
To further characterize the role of acute events in KCNJ5T158A-mediated events, we investigated the effects on steroidogenic acute regulatory protein (StAR), an established acute regulator of aldosterone synthesis. The regulation of STAR transcription as well as the post-translational modification of StAR, were determined. Figure 6, Panel A shows a representative western blot from three experiments examining phosphorylated StAR, total StAR and GAPDH (as a protein loading control). After 24 h of KCNJ5T158A transgene expression by doxycycline incubation, phospho-StAR was elevated by approximately 4-fold (p<0.05) after normalization to GAPDH (Figure 6, Panel B). A 3-fold (p<0.05) increase in total StAR was observed following KCNJ5T158A expression (Figure 6, Panel C). Pretreatment with verapamil (10 µM) inhibited KCNJ5T158A-mediated stimulatory effects on total and phospho-StAR. Initial time-response curves for total and phospho-StAR (data not shown) indicated that KCNJ5T158A increased phospho-StAR at 24 h. Increases in total StAR were observed starting 6 h post doxycycline treatment.
Figure 6.
DISCUSSION
Herein, we defined the acute and chronic events involved in KCNJ5T158A–induced aldosterone production. We developed a cell line with a doxycycline-inducible construct allowing for conditional expression of KCNJ5T158A, a mutation which occurs both as a germline mutation in familial hyperaldosteronism type III and as a somatic mutation in APA (Mulatero et al. 2012b; Oki et al. 2012). Transient or stable constitutive expression of mutated KCNJ5 causes increases in aldosterone production and expression of CYP11B2, through cell depolarization and resultant activation of calcium-dependent kinases (Monticone et al. 2012; Monticone et al. 2013; Oki et al. 2012; Williams et al. 2013). The specific advantage of the inducible expression of KCNJ5T158A is the ability to investigate the temporal events of molecular responses. Characterization of the cell line demonstrated time-dependent increases in KCNJ5T158A transgene mRNA and protein expression leading to increased CYP11B2 transcript and protein levels, as well as aldosterone production, while these effects were absent in control treated wild-type HAC15 cells (Supplemental Figure S1.). In summary, the cell line represents a model to study molecular pathophysiological changes following the initial event of KCNJ5 gene mutation.
The KCNJ5T158A mutation showed effects on the traditional steroids from both the mineralocorticoid and glucocorticoid pathways, as well as on the hybrid steroids, 18OHF and 18oxoF, which are frequently elevated in PA (Mulatero, et al. 2012a; Oki et al. 2012). The largest stimulatory effects were observed in 18OHF and 18oxoF, most likely mirroring increased CYP11B2 activity. Increases in aldosterone and its precursors, DOC and corticosterone were also observed. KCNJ5T158A expression resulted in a modest increase in cortisol production, in agreement with previous studies that demonstrate a modest increased in constitutive KCNJ5T158A-mediated CYP11B1 transcript levels (Oki et al. 2012). In addition, due to increased CYP11B2 expression, cortisol was further converted into hybrid steroids, 18OHF and 18oxoF by CYP11B2. Verapamil inhibited the KCNJ5T158A effects on aldosterone, 18OHF and 18oxoF synthesis, but not on corticosterone and DOC. Thus, stimulatory effects of KCNJ5T158A as well as the inhibitory effects of verapamil appear to be specific to CYP11B2.
From a molecular perspective, the physiological regulation of aldosterone production in response to Ang II or elevated serum potassium involves two key regulatory steps. The acute rate-limiting step includes the activation of StAR (occurring within seconds to minutes of agonist treatment). StAR regulates the transport of cholesterol from the outer to the inner mitochondrial membrane. The chronic limiting step involves CYP11B2 mediated conversion of DOC to aldosterone, which is mainly controlled on the transcriptional level. Ang II and K+ lead to the expression of NURR1, as well as the activation by phosphorylation of members of the CREB family, including ATF2 (Bassett, et al. 2004c; Bassett et al. 2004a; Hattangady et al. 2012; Nogueira et al. 2009; Xing, et al. 2011). Both of these steps are regulated by elevated intracellular calcium and the activation of calcium-calmodulin kinases (CaMKs) (Pezzi, et al. 1996; Pezzi, et al. 1997). These kinases activate the CREB family and NURR1 expression, which then translocate to the nucleus and bind the CYP11B2 promoter to initiate its transcription. In vitro studies have shown that mutated KCNJ5-mediated aldosterone excess in PA involves elevation of intracellular calcium. Additionally, our studies are the first to show increased nuclear protein levels of phosphorylated ATF2 after induction of KCNJ5T158A. These results are in agreement with recent findings in APA, which support the activation of calcium-calmodulin kinases I (CaMK I) and elevated nuclear localization of active, phosphorylated CREB (Sackmann, et al. 2011).
StAR regulates the movement of cholesterol from the outer to the inner mitochondrial membrane. StAR activity is regulated at the level of transcription and translation, as well as by post-translational modification. The transcriptional and translational regulation of StAR controls the level of expression of the 37 kDa StAR protein, while phosphorylation of StAR is crucial for its activity (Alberta, et al. 1989; Clark and Stocco 1997; Clark, et al. 1994; Estabrook and Rainey 1996; Krueger and Orme-Johnson 1983). Increased intracellular calcium levels, following Ang II treatment, cause increased StAR activation in the H295R cell line (Clark, et al. 1995; Nanba, et al. 2015; Olala, et al. 2014) as well as primary cultures of bovine adrenocortical cells (Betancourt-Calle, et al. 2001; Yamazaki, et al. 2006). In agreement with these studies, KCNJ5T158A expression increased expression and phosphorylation of StAR. Finally, maximum KCNJ5T158A-induced activation of StAR was observed at 24 h (earliest time point for StAR phosphorylation, data not shown), mirroring the fact that transcription of the transgene and translation of StAR protein following doxycycline treatment are necessary. Ang II treatment led to StAR activation as early as 30 min (data not shown).
We also evaluated the effects of constitutively expressed KCNJ5T158A in primary cultures of human adrenal fasciculata-reticularis (ZF/ZR) (Supplemental figure S2). While a dose-dependent increase in KCNJ5T158A mRNA was observed, no increase in CYP11B2 mRNA or aldosterone production was obtained. A positive control consisting of ZF/ZR cells treated with ACTH did yield increased CYP11B2 mRNA expression and aldosterone production. Importantly, the ZF/ZR cells were also unresponsive to Ang II or K+. These results, along with reports from other laboratories describing a ZF-like phenotype in APA harboring KCNJ5 mutations (Azizan, et al. 2012), may be the cause that mutations in KCNJ5 alone in ZF cells do not sufficiently lead to cause autonomous aldosterone production. The inherent challenge herein is the ability to obtain Ang II-responsive ZG cells, as normal adrenal glands generally lack a distinct CYP11B2-expressing ZG, but rather harbor a few, scattered CYP11B2-expressing cell clusters (Nishimoto, et al. 2010).
In summary, this study has defined the effects of KCNJ5T158A on 1) acute events (including StAR phosphorylation) and 2) chronic transcriptional events (including induction and activation of ATF2 and NURR1) 3) adrenal steroidogenesis in HAC15 cells and 4) confirmed the ability of verapamil to block KCNJ5T158A activation of aldosterone production.
Supplementary Material
Acknowledgments
FUNDING
This work was supported by National Institutes of Health Grants DK106618 (W.E.R and T.E.), DK103183 (R.J.A. and W.E.R.), and HL27255 (C.G-S.) and by grant AHA14SDG17990000 (T.E.) from the American Heart Association. The plasmid pLenti CMV rtTA3 Hygro (w785-1) (Plasmid #26730) and pLenti CMVTRE3G Puro DEST (w768-1) (Plasmid #27565) were created and deposited by Eric Campeau and obtained from www.Addgene.org.
Footnotes
DECLARATION OF INTEREST: We hereby declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
REFERENCES
- Akerstrom T, Crona J, Delgado Verdugo A, Starker LF, Cupisti K, Willenberg HS, Knoefel WT, Saeger W, Feller A, Ip J, et al. Comprehensive re-sequencing of adrenal aldosterone producing lesions reveal three somatic mutations near the KCNJ5 potassium channel selectivity filter. PLoS One. 2012;7:e41926. doi: 10.1371/journal.pone.0041926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerstrom T, Willenberg HS, Cupisti K, Ip J, Backman S, Moser A, Maharjan R, Robinson B, Iwen KA, Dralle H, et al. Novel somatic mutations and distinct molecular signature in aldosterone-producing adenomas. Endocr Relat Cancer. 2015;22:735–744. doi: 10.1530/ERC-15-0321. [DOI] [PubMed] [Google Scholar]
- Alberta JA, Epstein LF, Pon LA, Orme-Johnson NR. Mitochondrial localization of a phosphoprotein that rapidly accumulates in adrenal cortex cells exposed to adrenocorticotropic hormone or to cAMP. J Biol Chem. 1989;264:2368–2372. [PubMed] [Google Scholar]
- Azizan EA, Lam BY, Newhouse SJ, Zhou J, Kuc RE, Clarke J, Happerfield L, Marker A, Hoffman GJ, Brown MJ. Microarray, qPCR, and KCNJ5 sequencing of aldosterone-producing adenomas reveal differences in genotype and phenotype between zona glomerulosa- and zona fasciculata-like tumors. J Clin Endocrinol Metab. 2012;97:E819–E829. doi: 10.1210/jc.2011-2965. [DOI] [PubMed] [Google Scholar]
- Bassett MH, Suzuki T, Sasano H, De Vries CJ, Jimenez PT, Carr BR, Rainey WE. The orphan nuclear receptor NGFIB regulates transcription of 3beta-hydroxysteroid dehydrogenase. implications for the control of adrenal functional zonation. J Biol Chem. 2004c;279:37622–37630. doi: 10.1074/jbc.M405431200. [DOI] [PubMed] [Google Scholar]
- Bassett MH, Suzuki T, Sasano H, White PC, Rainey WE. The orphan nuclear receptors NURR1 and NGFIB regulate adrenal aldosterone production. Mol Endocrinol. 2004a;18:279–290. doi: 10.1210/me.2003-0005. [DOI] [PubMed] [Google Scholar]
- Bassett MH, Zhang Y, White PC, Rainey WE. Regulation of human CYP11B2 and CYP11B1: comparing the role of the common CRE/Ad1 element. Endocr Res. 2000;26:941–951. doi: 10.3109/07435800009048620. [DOI] [PubMed] [Google Scholar]
- Betancourt-Calle S, Calle RA, Isales CM, White S, Rasmussen H, Bollag WB. Differential effects of agonists of aldosterone secretion on steroidogenic acute regulatory phosphorylation. Mol Cell Endocrinol. 2001;173:87–94. doi: 10.1016/s0303-7207(00)00411-1. [DOI] [PubMed] [Google Scholar]
- Boulkroun S, Golib Dzib JF, Samson-Couterie B, Rosa FL, Rickard AJ, Meatchi T, Amar L, Benecke A, Zennaro MC. KCNJ5 mutations in aldosterone producing adenoma and relationship with adrenal cortex remodeling. Mol Cell Endocrinol. 2013;371:221–227. doi: 10.1016/j.mce.2013.01.018. [DOI] [PubMed] [Google Scholar]
- Campeau E, Ruhl VE, Rodier F, Smith CL, Rahmberg BL, Fuss JO, Campisi J, Yaswen P, Cooper PK, Kaufman PD. A versatile viral system for expression and depletion of proteins in mammalian cells. PLoS One. 2009;4:e6529. doi: 10.1371/journal.pone.0006529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmandari E, Sertedaki A, Kino T, Merakou C, Hoffman DA, Hatch MM, Hurt DE, Lin L, Xekouki P, Stratakis CA, et al. A novel point mutation in the KCNJ5 gene causing primary hyperaldosteronism and early-onset autosomal dominant hypertension. J Clin Endocrinol Metab. 2012;97:E1532–E1539. doi: 10.1210/jc.2012-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M, Scholl UI, Yue P, Bjorklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331:768–772. doi: 10.1126/science.1198785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ, Pezzi V, Stocco DM, Rainey WE. The steroidogenic acute regulatory protein is induced by angiotensin II and K+ in H295R adrenocortical cells. Mol Cell Endocrinol. 1995;115:215–219. doi: 10.1016/0303-7207(95)03683-0. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Stocco DM. Steroidogenic acute regulatory protein: the StAR still shines brightly. Mol Cell Endocrinol. 1997;134:1–8. doi: 10.1016/s0303-7207(97)00166-4. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR) J Biol Chem. 1994;269:28314–28322. [PubMed] [Google Scholar]
- Dutta RK, Welander J, Brauckhoff M, Walz M, Alesina P, Arnesen T, Soderkvist P, Gimm O. Complementary somatic mutations of KCNJ5, ATP1A1, and ATP2B3 in sporadic aldosterone producing adrenal adenomas. Endocr Relat Cancer. 2014;21:L1–L4. doi: 10.1530/ERC-13-0466. [DOI] [PubMed] [Google Scholar]
- Estabrook RW, Rainey WE. Twinkle, twinkle little StAR, how we wonder what you are. Proc Natl Acad Sci U S A. 1996;93:13552–13554. doi: 10.1073/pnas.93.24.13552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Sanchez CE, Foecking MF, Ferris MW, Chavarri MR, Uribe L, Gomez-Sanchez EP. The production of monoclonal antibodies against aldosterone. Steroids. 1987;49:581–587. doi: 10.1016/0039-128x(87)90097-3. [DOI] [PubMed] [Google Scholar]
- Hardege I, Xu S, Gordon RD, Thompson AJ, Figg N, Stowasser M, Murrell-Lagnado R, O'Shaughnessy KM. Novel Insertion Mutation in KCNJ5 Channel Produces Constitutive Aldosterone Release From H295R Cells. Mol Endocrinol. 2015;29:1522–1530. doi: 10.1210/me.2015-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattangady NG, Olala LO, Bollag WB, Rainey WE. Acute and chronic regulation of aldosterone production. Mol Cell Endocrinol. 2012;350:151–162. doi: 10.1016/j.mce.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienitz MC, Mergia E, Pott L. NCI-H295R cell line as in vitro model of hyperaldosteronism lacks functional KCNJ5 (GIRK4; Kir3.4) channels. Mol Cell Endocrinol. 2015;412:272–280. doi: 10.1016/j.mce.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Krueger RJ, Orme-Johnson NR. Acute adrenocorticotropic hormone stimulation of adrenal corticosteroidogenesis. Discovery of a rapidly induced protein. J Biol Chem. 1983;258:10159–10167. [PubMed] [Google Scholar]
- Kuppusamy M, Caroccia B, Stindl J, Bandulik S, Lenzini L, Gioco F, Fishman V, Zanotti G, Gomez-Sanchez C, Bader M, et al. A novel KCNJ5-insT149 somatic mutation close to, but outside, the selectivity filter causes resistant hypertension by loss of selectivity for potassium. J Clin Endocrinol Metab. 2014;99:E1765–E1773. doi: 10.1210/jc.2014-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzini L, Rossitto G, Maiolino G, Letizia C, Funder JW, Rossi GP. A Meta-Analysis of Somatic KCNJ5 K(+) Channel Mutations In 1636 Patients With an Aldosterone-Producing Adenoma. J Clin Endocrinol Metab. 2015;100:E1089–E1095. doi: 10.1210/jc.2015-2149. [DOI] [PubMed] [Google Scholar]
- Manolopoulou J, Bielohuby M, Caton SJ, Gomez-Sanchez CE, Renner-Mueller I, Wolf E, Lichtenauer UD, Beuschlein F, Hoeflich A, Bidlingmaier M. A highly sensitive immunofluorometric assay for the measurement of aldosterone in small sample volumes: validation in mouse serum. J Endocrinol. 2008;196:215–224. doi: 10.1677/JOE-07-0134. [DOI] [PubMed] [Google Scholar]
- Monticone S, Bandulik S, Stindl J, Zilbermint M, Dedov I, Mulatero P, Allgaeuer M, Lee CC, Stratakis CA, Williams TA, et al. A case of severe hyperaldosteronism caused by a de novo mutation affecting a critical salt bridge Kir3.4 residue. J Clin Endocrinol Metab. 2015;100:E114–E118. doi: 10.1210/jc.2014-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticone S, Hattangady NG, Nishimoto K, Mantero F, Rubin B, Cicala MV, Pezzani R, Auchus RJ, Ghayee HK, Shibata H, et al. Effect of KCNJ5 mutations on gene expression in aldosterone-producing adenomas and adrenocortical cells. J Clin Endocrinol Metab. 2012;97:E1567–E1572. doi: 10.1210/jc.2011-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticone S, Hattangady NG, Penton D, Isales CM, Edwards MA, Williams TA, Sterner C, Warth R, Mulatero P, Rainey WE. a Novel Y152C KCNJ5 mutation responsible for familial hyperaldosteronism type III. J Clin Endocrinol Metab. 2013;98:E1861–E1865. doi: 10.1210/jc.2013-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulatero P, di Cella SM, Monticone S, Schiavone D, Manzo M, Mengozzi G, Rabbia F, Terzolo M, Gomez-Sanchez EP, Gomez-Sanchez CE, et al. 18-hydroxycorticosterone, 18-hydroxycortisol, and 18-oxocortisol in the diagnosis of primary aldosteronism and its subtypes. J Clin Endocrinol Metab. 2012a;97:881–889. doi: 10.1210/jc.2011-2384. [DOI] [PubMed] [Google Scholar]
- Mulatero P, Tauber P, Zennaro MC, Monticone S, Lang K, Beuschlein F, Fischer E, Tizzani D, Pallauf A, Viola A, et al. KCNJ5 mutations in European families with nonglucocorticoid remediable familial hyperaldosteronism. Hypertension. 2012b;59:235–240. doi: 10.1161/HYPERTENSIONAHA.111.183996. [DOI] [PubMed] [Google Scholar]
- Murthy M, Azizan EA, Brown MJ, O'Shaughnessy KM. Characterization of a novel somatic KCNJ5 mutation delI157 in an aldosterone-producing adenoma. J Hypertens. 2012;30:1827–1833. doi: 10.1097/HJH.0b013e328356139f. [DOI] [PubMed] [Google Scholar]
- Murthy M, Xu S, Massimo G, Wolley M, Gordon RD, Stowasser M, O'Shaughnessy KM. Role for germline mutations and a rare coding single nucleotide polymorphism within the KCNJ5 potassium channel in a large cohort of sporadic cases of primary aldosteronism. Hypertension. 2014;63:783–789. doi: 10.1161/HYPERTENSIONAHA.113.02234. [DOI] [PubMed] [Google Scholar]
- Nanba K, Chen A, Nishimoto K, Rainey WE. Role of Ca(2+)/calmodulin-dependent protein kinase kinase in adrenal aldosterone production. Endocrinology. 2015;156:1750–1756. doi: 10.1210/en.2014-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, Mikami S, Shibata H, Itoh H, Mitani F, Yamazaki T, et al. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab. 2010;95:2296–2305. doi: 10.1210/jc.2009-2010. [DOI] [PubMed] [Google Scholar]
- Nogueira EF, Xing Y, Morris CA, Rainey WE. Role of angiotensin II-induced rapid response genes in the regulation of enzymes needed for aldosterone synthesis. J Mol Endocrinol. 2009;42:319–330. doi: 10.1677/JME-08-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oki K, Plonczynski MW, Luis Lam M, Gomez-Sanchez EP, Gomez-Sanchez CE. Potassium channel mutant KCNJ5 T158A expression in HAC-15 cells increases aldosterone synthesis. Endocrinology. 2012;153:1774–1782. doi: 10.1210/en.2011-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olala LO, Choudhary V, Johnson MH, Bollag WB. Angiotensin II-induced protein kinase D activates the ATF/CREB family of transcription factors and promotes StAR mRNA expression. Endocrinology. 2014;155:2524–2533. doi: 10.1210/en.2013-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar J, Key RE, Rainey WE. Development of an adrenocorticotropin-responsive human adrenocortical carcinoma cell line. J Clin Endocrinol Metab. 2008;93:4542–4546. doi: 10.1210/jc.2008-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzi V, Clark BJ, Ando S, Stocco DM, Rainey WE. Role of calmodulin-dependent protein kinase II in the acute stimulation of aldosterone production. J Steroid Biochem Mol Biol. 1996;58:417–424. doi: 10.1016/0960-0760(96)00052-0. [DOI] [PubMed] [Google Scholar]
- Pezzi V, Clyne CD, Ando S, Mathis JM, Rainey WE. Ca(2+)-regulated expression of aldosterone synthase is mediated by calmodulin and calmodulin-dependent protein kinases. Endocrinology. 1997;138:835–838. doi: 10.1210/endo.138.2.5032. [DOI] [PubMed] [Google Scholar]
- Sackmann S, Lichtenauer U, Shapiro I, Reincke M, Beuschlein F. Aldosterone producing adrenal adenomas are characterized by activation of calcium/calmodulin-dependent protein kinase (CaMK) dependent pathways. Horm Metab Res. 2011;43:106–111. doi: 10.1055/s-0030-1269899. [DOI] [PubMed] [Google Scholar]
- Scholl UI, Lifton RP. New insights into aldosterone-producing adenomas and hereditary aldosteronism: mutations in the K+ channel KCNJ5. Curr Opin Nephrol Hypertens. 2013;22:141–147. doi: 10.1097/MNH.0b013e32835cecf8. [DOI] [PubMed] [Google Scholar]
- Scholl UI, Nelson-Williams C, Yue P, Grekin R, Wyatt RJ, Dillon MJ, Couch R, Hammer LK, Harley FL, Farhi A, et al. Hypertension with or without adrenal hyperplasia due to different inherited mutations in the potassium channel KCNJ5. Proc Natl Acad Sci U S A. 2012;109:2533–2538. doi: 10.1073/pnas.1121407109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber P, Penton D, Stindl J, Humberg E, Tegtmeier I, Sterner C, Beuschlein F, Reincke M, Barhanin J, Bandulik S, et al. Pharmacology and pathophysiology of mutated KCNJ5 found in adrenal aldosterone-producing adenomas. Endocrinology. 2014;155:1353–1362. doi: 10.1210/en.2013-1944. [DOI] [PubMed] [Google Scholar]
- Thiel A, Reis AC, Haase M, Goh G, Schott M, Willenberg HS, Scholl UI. PRKACA mutations in cortisol-producing adenomas and adrenal hyperplasia: a single-center study of 60 cases. Eur J Endocrinol. 2015;172:677–685. doi: 10.1530/EJE-14-1113. [DOI] [PubMed] [Google Scholar]
- Wang B, Li X, Zhang X, Ma X, Chen L, Zhang Y, Lyu X, Tang Y, Huang Q, Gao Y, et al. Prevalence and characterization of somatic mutations in Chinese aldosterone-producing adenoma patients. Medicine (Baltimore) 2015;94:e708. doi: 10.1097/MD.0000000000000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Rowland JG, Parmar J, Nesterova M, Seki T, Rainey WE. Comparison of aldosterone production among human adrenocortical cell lines. Horm Metab Res. 2012;44:245–250. doi: 10.1055/s-0031-1298019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TA, Monticone S, Schack VR, Stindl J, Burrello J, Buffolo F, Annaratone L, Castellano I, Beuschlein F, Reincke M, et al. Somatic ATP1A1, ATP2B3, and KCNJ5 Mutations in Aldosterone-Producing Adenomas. Hypertension. 2013 doi: 10.1161/HYPERTENSIONAHA.113.01733. [DOI] [PubMed] [Google Scholar]
- Williams TA, Monticone S, Schack VR, Stindl J, Burrello J, Buffolo F, Annaratone L, Castellano I, Beuschlein F, Reincke M, et al. Somatic ATP1A1, ATP2B3, and KCNJ5 Mutations in Aldosterone-Producing Adenomas. Hypertension. 2014;63:188–195. doi: 10.1161/HYPERTENSIONAHA.113.01733. [DOI] [PubMed] [Google Scholar]
- Wu Y, Melton DW, Zhang Y, Hornsby PJ. Improved coinfection with amphotropic pseudotyped retroviral vectors. J Biomed Biotechnol. 2009;2009:901079. doi: 10.1155/2009/901079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Cohen A, Rothblat G, Sankaranarayanan S, Weibel G, Royer L, Francone OL, Rainey WE. Aldosterone production in human adrenocortical cells is stimulated by high-density lipoprotein 2 (HDL2) through increased expression of aldosterone synthase (CYP11B2) Endocrinology. 2011;152:751–763. doi: 10.1210/en.2010-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Kawasaki H, Takamasa A, Yoshitomi T, Kominami S. Ca2+ signal stimulates the expression of steroidogenic acute regulatory protein and steroidogenesis in bovine adrenal fasciculata-reticularis cells. Life Sci. 2006;78:2923–2930. doi: 10.1016/j.lfs.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Zennaro MC, Jeunemaitre X. Mutations in KCNJ5 gene cause hyperaldosteronism. Circ Res. 2011;108:1417–1418. doi: 10.1161/RES.0b013e318224a359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.