Abstract
This chapter describes our current understanding of the genetics of the Neurospora clock and summarizes the important findings in this area in the past decade. Neurospora is the most intensively studied clock system, and the reasons for this are listed. A discussion of the genetic interactions between clock mutants is included, highlighting the utility of dissecting complex mechanisms by genetic means. The molecular details of the Neurospora circadian clock mechanism are described, as well as the mutations that affect the key clock proteins, FRQ, WC-1, and WC-2, with an emphasis on the roles of protein phosphorylation. Studies on additional genes affecting clock properties are described and place these genes into two categories: those that affect the FRQ/WCC oscillator and those that do not. A discussion of temperature compensation and the mutants affecting this property is included. A section is devoted to the observations pertinent to the existence of other oscillators in this organism with respect to their properties, their effects, and their preliminary characterization. The output of the clock and the control of clock-controlled genes are discussed, emphasizing the phasing of these genes and the layers of control. In conclusion, the authors provide an outlook summarizing their suggestions for areas that would be fruitful for further exploration.
I. INTRODUCTION
A. The Neurospora clock system
Neurospora has been one of the primary organisms for circadian research for many years, and work on the Neurospora circadian system has often been reviewed (Brunner and Kaldi, 2008; Dunlap and Loros, 2006; Heintzen and Liu, 2007; Lakin-Thomas and Brody, 2004; Liu and Bell-Pedersen, 2006; Vitalini et al., 2010). One output of the circadian system, which is easily assayed under constant environmental conditions (usually constant darkness and constant temperature), is the daily rhythm of conidiation (asexual spore formation) on agar growth medium: the conidiation rhythm is seen as a pattern of areas of thick conidiating growth (bands) alternating with thinner areas of growth (interbands). These areas are formed as the endogenous clock directs the growth front to produce primarily apical extensions of the filamentous mycelium for approximately 11 h (forming an “interband”) followed by approximately 11 h of extension of the apical filaments plus the production of aerial hyphae at right angles to the surface of the medium on which the spores develop (forming a “band” region). This “fossil record” of the developmental state of the growth front can be seen on agar medium in petri plates (Fig. 3.1) or in long glass cylindrical tubes (“growth tubes” or “race tubes”). This conidiation rhythm has been the standard assay for the state of the circadian system in labs working with Neurospora. All known clock genes were identified and/or characterized by observing changes in the conidiation rhythm. Molecular rhythms can also be observed in the Neurospora system and are most often assayed in specialized cultures in which mycelial disks are submerged in liquid medium (Loros et al., 1989; Nakashima, 1981). Conidiation is suppressed to some extent by these culture conditions, but the underlying clock mechanisms continue functioning. Molecular rhythms can also be assayed on solid agar medium in conditions more physiologically similar to those the organism would encounter in nature (Gooch et al., 2008; Ramsdale and Lakin-Thomas, 2000; Schneider et al., 2009).
Figure 3.1.

The developmental rhythm of conidiation. (A) A 15-cm petri plate was inoculated at the lower edge with the bd; vvdP pan-2 strain of Neurospora and allowed to grow in constant light. Yellow bands of conidiation formed at approximately 11-h intervals. This strain is unusual in that it continues to form conidiation bands in constant light with a short period. (B) Two 30-cm race tubes were inoculated at the left with the csp-1, bd strain, exposed to light for 24 h and transferred to constant darkness for the remainder of growth. Growth was from left to right. The upper two images are photographs of the tops of the tubes; the lower two images are scans of the bottoms of the tubes. The black marks indicate the positions of the growth fronts of the colonies at 24-h intervals. The period of these cultures was approximately 21.5 h.
This review focuses on what genetics has told us about the mechanism of the underlying circadian oscillator that drives the observed rhythms, with an emphasis on findings within the past 10 years. We will also consider one aspect of the output of the oscillator: the downstream effects of the clock on the rhythmic control of the expression of “clock-controlled genes,” or ccgs. Circadian systems must also have input pathways to synchronize the clock with the external environment, but a consideration of input pathway genetics is beyond the scope of this review; the reader is directed to recent reviews (Chen et al., 2010; Liu, 2003; Price-Lloyd et al., 2005).
B. Neurospora as a model organism for circadian research
The circadian system of Neurospora is arguably the most intensively studied of all clocks and has many advantages as an experimental system. As a microorganism that is easily grown in culture, Neurospora has an advantage over other organisms in that large amounts of material can be synchronously harvested, making biochemical analysis easier. As a haploid organism, Neurospora has advantages for genetic analysis in the ease of isolation of new mutations and the expression of mutant phenotypes in the haploid without the complications of dominance relationships between alleles in a diploid. This has resulted in a huge collection of mutant strains, cataloged by (Perkins et al., 2001), now available online (http://www.fgsc.net/2000compendium/NewCompend.html), and continuously updated (http://bmbpcu36.leeds.ac.uk/~gen6ar/newgenelist/genes/gene_list.htm). The genome has been sequenced (Galagan et al., 2003), transformation methods are routine, and most molecular genetics methods are available for Neurospora including the use of an inducible promoter for dosage control (Campbell et al., 1994; Geever et al., 1989) and RNAi for gene silencing (Ziv and Yarden, 2010). Modern imaging techniques can be applied to Neurospora, including the use of luciferase (Gooch et al., 2008), GFP (Freitag et al., 2004), and mCherry (Castro-Longoria et al., 2010). Knockout mutants can be easily constructed (Colot et al., 2006), and knockouts are available for most identified genes from the Fungal Genetics Stock Center (McCluskey, 2003).
Neurospora has an 80-year history as a lab organism (Perkins and Davis, 2000), and the first report of circadian rhythmicity in Neurospora was published in 1959 (Pittendrigh et al., 1959). Classic circadian experiments describing the response of the Neurospora clock to pulses and steps of light and temperature were published in the 1960s and 1970s (Francis and Sargent, 1979; Sargent and Briggs, 1967). The primary output, the conidiation developmental pathway, has been extensively studied (Correa and Bell-Pedersen, 2002; Springer, 1993), and the characterization of ccgs at the molecular level is underway (see Section VI). The development of a transcription/translation feedback model to describe the mechanism of the Neurospora circadian oscillator (see Section III) has contributed to the development of similar models for other organisms and the finding of analogous genes in other clock systems (see Chapters 2, 4, 5, 6 and 7 in this volume). Neurospora was also one of the pioneer lab organisms for biochemical investigations (Davis, 2000), and there is a wealth of information about metabolic pathways, organelles such as mitochondria, signaling pathways, etc.; as the attention of circadian researchers shifts away from transcription/translation mechanisms and toward metabolic pathways (Bass and Takahashi, 2010; Harrisingh and Nitabach, 2008; Hastings et al., 2008), Neurospora will again prove its worth as a model organism.
II. EARLY GENETIC ANALYSIS
A. Identification of clock mutants
Neurospora was the second organism, after Drosophila (Konopka and Benzer, 1971), in which mutations affecting the circadian clock were isolated (Feldman and Hoyle, 1973). These were identified in brute force screenings for period changes as observed in the conidiation rhythm on solid agar medium. The first locus identified in these screens, frequency (frq), also turned out to be of extraordinary interest. Alleles with both shorter periods (frq1, frq2) and longer periods (frq3, frq7) were found at the same locus (see Table 3.1 for periods). These were found to be point mutations, introducing single amino acid changes (Aronson et al., 1994a), and were shown to be codominant in heterokaryons. Null alleles, either a truncated protein (frq9) (Loros and Feldman, 1986) or an engineered gene deletion (frq10) (Aronson et al., 1994a), were found to be “conditionally rhythmic” (see Section V) and recessive in heterokaryons. A great deal of subsequent work has been carried out on the biochemistry and genetics of the frq gene and is summarized below (Section III).
Table 3.1.
Interactions Involving cel and chol-1 Mutations in Neurospora
| wta | cela | wtb | chol-1b | |
|---|---|---|---|---|
| frq+ | 20 | 42 | 21 | 63 |
| frq1 | 14 | 34*,** | 16 | 55** |
| frq2 | 17 | 38*,** | 18 | 57 |
| frq3 | 23 | 41*,** | 23 | 70 |
| frq7 | 28 | 39*,** | 29 | 63* |
| prd-1 | 24 | 22*,** | ||
| olir | 19 | 19*,** |
Notes: Periods are rounded to the nearest whole hours. wt, wild type at other loci.
Significantly different from both additive and multiplicative models.
Significantly different from model of epistasis of cel or chol-1 over frq.
Grown on unsaturated fatty acid supplement; data from Lakin-Thomas and Brody (1985).
Grown on medium without choline supplementation; data from Lakin-Thomas (1998).
A number of other clock-affecting loci were identified in screens for period mutants, including five prd (period) loci (prd-1, prd-2, prd-3, prd-4, and prd-6) and chr (chrono). Additional period-affecting mutations were identified by screening existing libraries of mutants, including several amino acid auxotrophs, drug resistance mutants, and polymerase mutants. Comprehensive lists of mutations and their effects on period can be found in earlier reviews (Lakin-Thomas et al., 1990; Loros and Dunlap, 2001; Morgan et al., 2001). With the few exceptions noted in later sections, the molecular bases for the effects of most of these mutations on the period of the circadian rhythm have not been determined.
Two mutations affecting lipid synthesis are of particular interest: cel (fas) and chol-1. The cel (chain elongation) mutation impairs fatty acid elongation, and this strain requires exogenous saturated fatty acids for normal growth. When supplemented with unsaturated fatty acids, the period of the conidiation rhythm lengthens and temperature compensation is impaired. The chol-1 (choline) mutation impairs synthesis of the lipid phosphatidylcholine, and this strain requires choline for normal growth. On low-choline medium, the period of the rhythm lengthens in inverse proportion to the choline concentration in the medium, and temperature compensation is impaired. The cel and chol-1 strains will be discussed further in Sections II.B and V.
One large class of period-affecting mutants has a common target in mitochondrial functions and similar period-shortening effects. This class includes olir (oligomycin resistance), several cytochrome mutants, and both nuclear- and mitochondrially inherited genes (Lakin-Thomas et al., 1990). The short period of these mitochondrial mutants can be phenocopied by treatment of cultures with inhibitors of mitochondrial function such as chloramphenicol (Brody, 1992). A common effect of several of these mitochondrial mutations was found to be an increase in mitochondrial mass per cell volume (Brody, 1992), but the relationship between this effect and the circadian clock mechanism has not been uncovered.
In the absence of detailed molecular information about the roles of period-affecting mutations, there are several approaches to formulating hypotheses about their effects on the circadian clock. One type of hypothesis focuses on molecular mechanisms and changes in rates: short-period mutations may increase the rate of turnover of a negative-acting clock component, or increase the rate of synthesis of a positive-acting component; long-period mutations may have the opposite effects. Another possibility is a change in affinity of proteins in a regulatory complex: short-period mutations may increase the affinity of two proteins or of a protein for a promoter element, thus leading to precocious activation, and long-period mutations may decrease such affinities. A complementary approach is to consider the amplitude of the oscillator: an increase or decrease in amplitude may cause an increase or decrease in period. This change in amplitude may be brought about by any of the molecular mechanisms listed above. Evidence for changes in amplitude in clock mutants has been discussed in the framework of limit cycle theory (Lakin-Thomas et al., 1991; Shaw and Brody, 2000).
B. Interactions between clock mutants
With quantitative phenotypes such as circadian periods, it is possible to use genetics to ask questions about interactions between gene products in the absence of biochemical evidence. Constructing double- and triple-mutant strains combining several clock mutations in Neurospora has provided some insights into potential gene product interactions. The simplest interpretation of additive or multiplicative interactions (each mutation either adds/subtracts a fixed number of hours to the period or multiples the period by a fixed factor) is that each gene product acts independently on the final phenotype (the period). Epistatic, synergistic, or intermediate effects indicate the possibility of interactions between the gene products, and at least a common pathway for effects on the period (Lakin-Thomas and Brody, 1985; Morgan et al., 2001). It should be noted that most studies of genetic interactions have focused on period measurements and very little work has been reported on other clock properties such as phase resetting or temperature compensation.
The cel mutation described in Section II.A requires saturated fatty acids for normal growth, and when supplemented with unsaturated fatty acids, the period of the conidiation rhythm lengthens (Mattern et al., 1982). A series of double mutant strains was constructed between cel and the series of frq alleles (Lakin-Thomas and Brody, 1985), and the data are presented in Table 3.1. A systematic interaction between cel and the frq alleles was found, with greater divergence from the predicted additive or multiplicative periods found with the longer-period frq alleles (Lakin-Thomas and Brody, 1985). The prediction of complete epistasis of cel over frq (in which all cel frq double mutants would have the same period as cel frq+) also fails, but with the short-period frq alleles diverging the most from this prediction (Lakin-Thomas and Brody, 1985). In contrast, both the prd-1 and the olir mutations were found to be epistatic to cel, blocking the period-lengthening effects of unsaturated fatty acids (Lakin-Thomas and Brody, 1985) (Table 3.1). These data suggest that the gene products of frq, prd-1, and olir all interact with the unknown mechanism that produces long periods in cel.
The chol-1 mutant described above requires choline supplementation for normal growth and rhythmicity (Lakin-Thomas, 1996, 1998). Double mutants between chol-1 and the series of frq alleles were constructed, and the data are presented in Table 3.1. As with cel, the prediction of complete epistasis of chol-1 over frq (in which all chol-1 frq double mutants would have the same period as chol-1 frq+) also fails, again with the short-period frq alleles diverging the most from this prediction (Lakin-Thomas, 1998). The chol-1 frq1 strain is significantly different from chol-1 frq+ (Lakin-Thomas, 1998); an additive prediction fits this double mutant best. The chol-1 frq2 and chol-1 frq3 strains are not significantly different from additive, multiplicative, or epistatic predictions (Table 3.1). The chol-1 frq7 strain fits an epistatic prediction but not additive or multiplicative models (Table 3.1). These results indicate a complex series of interactions between the frq alleles and the period-lengthening effect of chol-1.
A large number of multiple mutant strains have been constructed using the series of prd mutants, frq alleles, and the chr mutant (Morgan and Feldman, 2001; Morgan et al., 2001), and the periods of some of these strains are presented in Table 3.2. These authors found significant synergistic interactions among a group of mutations including prd-2, prd-3, prd-6, and frq7, suggesting these gene products mutually interact (Table 3.2A and B). Synergistic effects on temperature compensation were also found in several double mutant combinations (Morgan and Feldman, 2001). Epistasis between prd-1 and prd-2 places them in the same pathway (Table 3.2B). It is interesting to note that not all alleles at the frq locus produced evidence of interactions; in the case of prd double mutants, only the double mutants carrying the frq7 allele, and in one case, frq3, differed significantly from the additive or multiplicative models (Table 3.2A). The chr mutant did not produce significant interactions in double mutants (Table 3.2A), suggesting that the chr gene product affects the period through a pathway independent of frq. Triple mutants with various combinations of mutations have also been constructed (Table 3.2C), and although the results are more difficult to interpret, the periods are roughly as would be predicted from the interactions revealed by the data in Table 3.2A and B.
Table 3.2.
Periods (in Hours) of Neurospora Double and Triple Clock Mutants from the Data of Morgan and Feldman (2001)
|
A. prd frq and chr frq double mutants
|
|||||
|---|---|---|---|---|---|
| frq+ | frq1 | frq2 | frq3 | frq7 | |
| prd+ | 22 | 17 | 19 | 24 | 29 |
| prd-1 | 26 | 19 | 23 | 28 | 35 |
| prd-2 | 26 | 19 | 22 | 28 | 38* |
| prd-3 | 25 | 19 | 23 | 30* | 41* |
| prd-4 | 18 | 14 | 16 | 20 | 24 |
| prd-6 | 18 | 15 | 15 | 20 | 21* |
| chr | 24 | 17 | 21 | 26 | 33 |
|
B. prd prd and chr prd double mutants
|
||||||
|---|---|---|---|---|---|---|
| chr+ | chr | prd-2 | prd-3 | prd-4 | prd-6 | |
| prd+ | 22 | 24 | ||||
| prd-1 | 26 | 28 | 26* | 30 | 21 | 24 |
| prd-2 | 26 | 29 | 33* | 21 | 18 | |
| prd-3 | 25 | 27 | 20 | 18* | ||
| prd-4 | 18 | 19 | 16 | |||
| prd-6 | 18 | 19 | ||||
| C. Triple mutants | |
|---|---|
| prd-1; chr; frq7 | 36 |
| prd-3; prd-1; frq7 | 49 |
| prd-3; prd-2; frq7 | 48 |
| prd-2 prd-6; frq7 | 21 |
| prd-3; prd-2 prd-6 | 18 |
| Note: Data from L. Morgan (personal communication). | |
Significantly different from both additive and multiplicative models.
III. THE GENETICS AND BIOCHEMISTRY OF THE FRQ/WCC FEEDBACK LOOP
A. Basic mechanism of the feedback loop
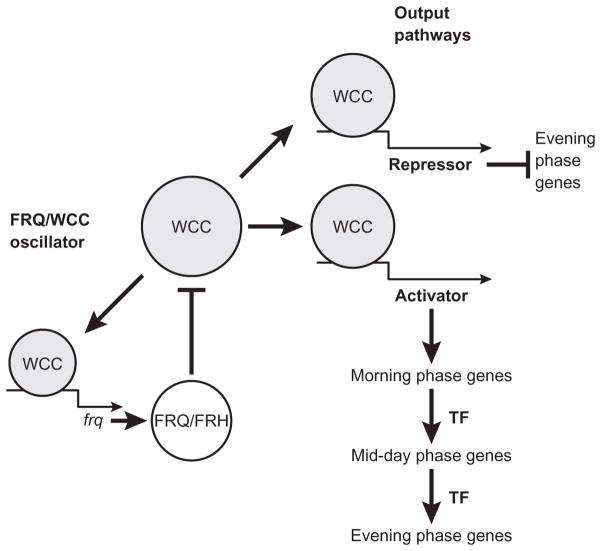
The phenotypes of frq mutations described above identified the frq gene as potentially a central component of the mechanism of the circadian clock in Neurospora, and cloning of the gene (McClung et al., 1989) allowed biochemical and molecular analysis of its function to begin. When negative feedback of frq transcription by its protein product FRQ was discovered (Aronson et al., 1994b), following the example of the per gene of Drosophila (Hardin et al., 1990), a transcription/translation negative feedback model was developed for the circadian clock mechanism of Neurospora with the FRQ protein and frq RNA as the core components (Aronson et al., 1994b). Additional clock components were later identified, the most important being the two white-collar proteins WC-1 and WC-2 (Crosthwaite et al., 1997). These were incorporated into a model that can be called the FRQ–white-collar complex (FRQ/WCC) model. In a simplified version of the current model for the Neurospora clock mechanism (for a detailed description of the model, see Vitalini et al., 2010), transcription of the frq gene is activated by a complex of the two white-collar proteins, WC-1 and WC-2, together called the white-collar complex (WCC; Froehlich et al., 2002, 2003). The level of frq mRNA increases when WCC is active, FRQ protein is translated, and FRQ protein inhibits the activity of WCC, thereby negatively regulating its own transcription and leading to a fall in frq mRNA levels (He et al., 2006; Schafmeier et al., 2006). FRQ protein is degraded (Liu et al., 2000), inhibition of transcription is relieved, and the cycle repeats as frq mRNA accumulates again. In a positive feedback loop, FRQ protein also acts to increase the levels of WCC, at posttranscriptional and/or posttranslational steps (Cheng et al., 2001b, 2003; Lee et al., 2000; Merrow et al., 2001; Schafmeier et al., 2006). It is the rhythmic activity of the WCC that drives the observed rhythms by regulating transcription of downstream genes (see Section VI). Interestingly, the levels of the wc transcripts do not cycle over the course of the day; however, WC-1 protein levels but not WC-2 protein do cycle (He et al., 2002; Lee et al., 2000; Merrow et al., 2001). Experiments in which wc-1 or wc-2 were overexpressed from an inducible promoter demonstrate that, while cycling levels of WC-1 protein are not required for overt rhythmicity, the positive feedback loops are important to stabilize and maintain proper amplitude of the rhythm (Cheng et al., 2001b).
B. Genetic analysis of FRQ and WCC
The earliest work on clock genes in Neurospora identified multiple alleles at the frq locus that altered the period, and subsequently, null alleles were found to be arrhythmic under standard growth conditions, or “conditionally rhythmic” (described in Section II.A). The two white-collar genes, wc-1 and wc-2, had been previously identified as essential for blue light photoresponses and were subsequently identified as clock components through the arrhythmic phenotypes of null mutants (Crosthwaite et al., 1997). Recent genetic studies have added to our understanding of the functions of these genes.
1. FRQ protein domains
No enzymatic activity has been attributed to the FRQ protein. Its function appears to be to help recruit enzymes involved in the negative feedback loop, including kinases, phosphatases, and a helicase. The full length FRQ protein is 989 amino acids long, with a coiled-coil domain near the N-terminus (Cheng et al., 2001a), and a nuclear localization signal located downstream of the coiled-coil domain. FRQ dimerizes through its coiled-coil domain, and dimerization is required for binding to the WCC. Two alternatively translated forms of FRQ, large FRQ (l-FRQ) and small FRQ (s-FRQ), that differ by 99 amino acids at the N-terminus are translated from two in frame AUGs (AUG1 and AUG3) (Garceau et al., 1997; Liu et al., 1997) within different transcripts that result from alternative splicing events (Colot et al., 2005; Diernfellner et al., 2005, 2007). Low levels of FRQ are present in the nucleus, and nuclear localization is required for FRQ’s role in the circadian oscillator (Luo et al., 1998). Despite the presence of the nuclear localization signal, most FRQ protein is cytoplasmic, suggesting that there is an active process to export FRQ from the nucleus. Using FRQ deletion mutants, the C-terminus of FRQ that includes the FRQ/FRQ-interacting RNA helicase (FRH) interaction domain was found to play an important role for cytoplasmic localization of FRQ (Cha et al., 2011). Other domains in FRQ include a casein kinase I (CK1) interaction domain located in the middle of the protein (Guo et al., 2010) and two PEST domains that function in FRQ turnover (Görl et al., 2001). The first PEST domain is just downstream of the CK1 interaction domain, and the second PEST domain is located at the carboxy-terminus of the protein. Between the two PEST domains is a binding site for FRH (Cheng et al., 2005; Guo et al., 2010). All FRQ protein interacts with FRH, and the FRQ/FRH complex is required for interaction of FRQ with the WCC and the inactivation of WCC in the negative feedback loop. The FRQ/FRH complex is also required for FRQ stability and for the positive activity of FRQ in promoting the accumulation of the WC proteins (Guo et al., 2010; Shi et al., 2010).
2. The WCC proteins
WC-1 and WC-2 are PAS domain-containing GATA-type zinc finger transcription factors, found primarily in the nucleus of cells (Ballario et al., 1996; Lee et al., 2000; Linden and Macino, 1997; Schwerdtfeger and Linden, 2000). The WC-1 protein has three PAS domains (A, B, and C), with the N-terminal PAS domain being a specialized LOV (light, oxygen-, voltage-sensing) domain that functions as a blue light sensory module (Briggs, 2007; Christie et al., 1999; Liu et al., 2003). The WC-2 protein has a single PAS domain. WC-1 and WC-2 bind to each other to form the WCC through the PASC domain of WC-1 and the PAS domain of WC-2 (Cheng et al., 2002, 2003; Talora et al., 1999).
3. wc-1 simple sequence repeats
Wild-type Neurospora isolates collected from a variety of locations and environments can provide information about natural genetic and phenotypic variation. A survey of circadian phenotypes among 143 accessions found a correlation between period of the conidiation rhythm and polymorphisms in simple sequence repeat (SSR) regions in the wc-1 gene (Michael et al., 2007). A correlation between period and latitude of collection was also found. These results suggest that the variation in wc-1 SSRs may be a target of selection for fitness by the local environment (Michael et al., 2007).
C. Role of protein phosphorylation in the FRQ/WCC feedback loop
Current work on the FRQ/WCC feedback loop focuses on filling in details of this model. It has become increasingly clear that both the mechanism of negative feedback, whereby FRQ protein inhibits the transcriptional activity of WCC, and the mechanism of positive feedback, whereby FRQ promotes accumulation of WCC, depend on posttranslational mechanisms, specifically the phosphorylation and dephosphorylation of the three proteins FRQ, WC-1, and WC-2. The activity and subcellular localization of these three major components of the FRQ/WCC are all modulated by phosphorylation. Kinase and phosphatase genes that have been identified as playing roles in regulating rhythmicity in Neurospora are listed in Table 3.3.
Table 3.3.
Recently Identified Clock-Associated Genes in Neurospora
| Gene name | Rhythm phenotypes of mutants | Function/Identity | References |
|---|---|---|---|
| camk-1 | Small effects on period, phase, and light-induced phase shifting of the conidiation rhythm | Calcium/calmodulin-dependent protein kinase | Yang et al. (2001) |
| chr (ckb-1) | Long period, low-amplitude rhythms of conidiation, altered temperature compensation, long-period rhythms of FRQ protein, frq, and ccg-1 RNA | Regulatory subunit of casein kinase 2 (CKII) | Mehra et al. (2009), Yang et al. (2003) |
| ck-1a | Long-period rhythms of conidiation, FRQ protein, and frq RNA | Casein kinase 1a | He et al. (2006) |
| csn-2 | Loss of FRQ protein rhythm, lengthened period of conidiation rhythm | Subunit of COP9 signalosome, involved in protein degradation | He et al. (2005a) |
|
csn-1
csn-4 csn-5 csn-6 csn-7 |
Irregular and long- period conidiation rhythms | Subunits of COP9 signalosome, involved in protein degradation | Wang et al. (2010) |
| csp-1 | Slightly shorter period of conidiation rhythm | Light-induced transcription factor, conidial separation | Schneider et al. (2009) |
| csp-2 | Slightly longer period of conidiation rhythm | Conidial separation | Brody et al. (2010) |
| csw-1 | Abnormal conidiation rhythm, loss of FRQ protein, and frq RNA rhythms | Chromatin remodeling enzyme | Belden et al. (2007b) |
| frh | Loss of conidiation rhythm and rhythms of FRQ protein, frq RNA, and ccg- 2 RNA | RNA helicase, component of exosome, regulates RNA processing | Cheng et al. (2005), Guo et al. (2009, 2010), Shi et al. (2010) |
| fwd1 | Loss of conidiation rhythm and rhythms of FRQ protein, frq RNA, and ccg-1 RNA | Component of SCF-type ubiquitin ligase complex, involved in protein degradation | He et al. (2003) |
| mcb | Loss of rhythms of conidiation, FRQ protein, and ccg-1 RNA | Regulatory subunit of protein kinase A | Huang et al. (2007) |
| pkac-1 | Loss of rhythms of conidiation, FRQ protein, and ccg-1 RNA | Catalytic subunit of protein kinase A | Huang et al. (2007) |
| ppp-1 | Short period and advanced phase of conidiation rhythm | Catalytic subunit of protein phosphatase 1 | Yang et al. (2004) |
| pp4 | Short period of conidiation and FRQ protein rhythms | Protein phosphatase 4 | Cha et al. (2008) |
| pp4 ppp-1 | Arrhythmic for conidiation and FRQ protein rhythms | Double mutant for protein phosphatases 1 and 4 | Cha et al. (2008) |
| prd-1 | Long period of conidiation rhythm in frq+, loss of fatty acid effect on FRQ-less rhythm in cel, long period of FRQ-less rhythm with geraniol, loss of FRQ-less rhythm in chol-1, severe effect on heat- entrainable FRQ-less rhythm | Unknown | Lakin-Thomas and Brody (1985), Li and Lakin-Thomas (2010), Lombardi et al. (2007) |
| prd-2 | Long period of conidiation rhythm in frq+, long period of FRQ- less rhythm with geraniol, loss of FRQ- less rhythm in chol-1, severe effect on heat- entrainable FRQ-less rhythm | Unknown | Li and Lakin-Thomas (2010), Lombardi et al. (2007) |
| prd-3 (cka) | Long period of conidiation rhythm, altered temperature compensation, loss of FRQ protein rhythm, and RNA rhythms of frq, ccg-1, and ccg-2 | Catalytic subunit of casein kinase 2 (CKII) | Mehra et al. (2009), Yang et al. (2002) |
| prd-4 | Short period of conidiation rhythm | Checkpoint kinase 2 (Chk2), links DNA damage to cell cycle arrest | Pregueiro et al. (2006) |
| prd-6 | Short period of FRQ- less rhythm with geraniol | Unknown | Lombardi et al. (2007) |
| rasbd (formerly bd) | Increased conidiation output, rhythm resistant to CO2 | ras-1, small G-protein involved in signaling pathways | Belden et al. (2007a) |
| rco-1 | Very long period | Transcription factor, regulates conidiation and photoadaptation | Brody et al. (2010), Olmedo et al. (2010a), Yamashiro et al. (1996) |
| rgb-1 | Low-amplitude, long- period rhythms of FRQ protein and ccg- 1 RNA | Regulatory subunit of protein phosphatase 2A | Yang et al. (2004) |
| rrp44 | Long period of FRQ protein and frq RNA molecular rhythms | Component of exosome, regulates RNA processing | Guo et al. (2009) |
| sod-1 | Phenocopies rasbd, slightly shorter period, double mutant with frq10 is rhythmic | Superoxide dismutase, removes reactive oxygen species | Belden et al. (2007a), Yoshida et al. (2008) |
| ult | Short-period conidiation rhythm, double mutant with frq10 or wc-2 is rhythmic, shortens the period of the FLO in the presence of geraniol | Unknown | Lombardi et al. (2007) |
| UV90 | Loss of FRQ-less rhythm in chol-1, severe effect on heat- entrainable FRQ-less rhythm, damping of amplitude of FRQ/ WCC oscillator | Unknown | Li et al. (2011) |
| vvd | Short-period conidiation rhythms in constant light in frq+ and frq10 | Photoreceptor involved in downregulation of light responses | Schneider et al. (2009) |
1. FRQ phosphorylation
The FRQ protein is phosphorylated at multiple sites, and a robust rhythm of phosphorylation can be seen on Western blots (Garceau et al., 1997). Newly synthesized FRQ protein is hypophosphorylated and undergoes progressive phosphorylation as it matures, until the hyperphosphorylated protein is degraded by the ubiquitin/proteasome pathway (He and Liu, 2005a). Recent work has identified numerous phosphorylation sites on the FRQ protein using MS techniques (Baker et al., 2009; Tang et al., 2009). At least 43 (Tang et al., 2009) or 75 (Baker et al., 2009) in vivo sites were identified, and the majority are progressively phosphorylated during maturation of the FRQ protein. Systematic mutagenesis of these sites did not identify individual residues essential for rhythmicity but did identify regions of the FRQ protein in which phosphorylation affects the period: mutations in the central region of FRQ lengthen the period, and mutations in the C-terminal region shorten the period; degradation rates of FRQ protein are correlated with period (Baker et al., 2009; Tang et al., 2009).
Several kinases have been identified as phosphorylating FRQ, including calcium/calmodulin-dependent kinase (CAMK-1) (Yang et al., 2001), casein kinase 1a (CK-1a) (Görl et al., 2001; He et al., 2006), casein kinase II (CKII) (Yang et al., 2002), and protein kinase A (PKA) (Huang et al., 2007). CK-1a and CKII were shown to phosphorylate the majority of sites identified by MS analysis (Tang et al., 2009). Knockout of the camk-1 gene produces slight effects on period, phase, and light-induced phase shifting of the conidiation rhythm (Yang et al., 2001). CK-1a physically associates with FRQ protein (Görl et al., 2001; He et al., 2006), and disruption of that association by mutation of the binding domain on FRQ results in hypophosphorylation of FRQ and arrhythmic conidiation (He et al., 2006). A knock-in mutation of ck-1a that disrupts the kinase domain also results in hypophosphorylation of FRQ and long-period rhythms of conidiation, FRQ protein levels and phosphorylation, and frq RNA levels (He et al., 2006). Disruption of cka, the gene coding for the catalytic subunit of CKII, abolishes rhythms of FRQ protein levels and phosphorylation and RNA levels of frq and two ccgs: ccg-1 and ccg-2 (Yang et al., 2002). Disruption of ckb1, the gene coding for the regulatory subunit CKB-1 of CKII, produces mostly arrhythmic conidiation patterns with a few long periods, and long-period rhythms of FRQ protein and frq and ccg-1 RNA (Yang et al., 2003). With either a knockout of the gene encoding the major PKA catalytic subunit pkac-1 or a mutation in the gene encoding the PKA regulatory subunit mcb, rhythms of conidiation, rhythms of FRQ levels and phosphorylation, and rhythms of ccg-1 RNA are abolished (Huang et al., 2007).
Three protein phosphatases, PP1, PP2A, and PP4, have been implicated in regulating the phosphorylation status of FRQ (Cha et al., 2008; Yang et al., 2004). The PP1 and PP2A proteins can dephosphorylate FRQ in vitro (Yang et al., 2004). A partially functional mutation of ppp-1, the gene that codes for the catalytic subunit of PP1, shortens the period and advances the phase of the conidiation rhythm (Yang et al., 2004). Disruption of rgb-1, the gene for a regulatory subunit of PP2A, produces low-amplitude, long-period rhythms of FRQ protein and ccg-1 RNA (Yang et al., 2004). Deletion of the pp4 gene shortens the period of the conidiation rhythm and the FRQ protein rhythm (Cha et al., 2008). FRQ protein is hyperphosphorylated and is at low levels in this mutant, and its degradation rate is faster than wild type (Cha et al., 2008). A double mutant strain carrying both the pp4 deletion and the partially functional ppp-1 mutation is arrhythmic for conidiation and for FRQ protein levels, and also displays hyperphosphorylated FRQ and a rate of FRQ degradation even faster than the pp4 deletion strain (Cha et al., 2008), indicating that both PP1 and PP4 normally dephosphorylate FRQ and stabilize it.
There may be several related functions for this phosphorylation of FRQ. Degradation of hyperphosphorylated FRQ protein is essential for the operation of the feedback loop to allow reactivation of WCC. A major role for FRQ phosphorylation is in determining the kinetics of FRQ degradation, and this, in turn, influences the period of the circadian oscillator (Baker et al., 2009; Liu et al., 2000; Ruoff et al., 2005; Tang et al., 2009).
A second role for FRQ phosphorylation may be modulating the interaction between FRQ and WCC. In the cka mutant that is defective in CKII, FRQ protein is hypophosphorylated and more FRQ was found to associate with WCC than in the wild type (Yang et al., 2002). In a time series analysis of proteins interacting with FRQ, WCC was found to associate with FRQ preferentially during the times in the circadian cycle when FRQ is hypophosphorylated (Baker et al., 2009). Lastly, mutation analysis of FRQ phosphorylation sites demonstrated that FRQ phosphorylation inhibits FRQ binding to the WCC and FRQ/CK-1a interactions (Cha et al., 2011).
Phosphorylation of FRQ may also modulate the rate of nucleocytoplasmic shuttling of FRQ (Diernfellner et al., 2009). Negative feedback requires hypophosphorylated nuclear FRQ (Schafmeier et al., 2006), and the slow accumulation of cytoplasmic FRQ as it becomes phosphorylated may contribute to the slow kinetics of the circadian cycle; this is supported by the observation that the mutant FRQ7 protein remains in the nucleus longer than the wild-type protein, and this correlates with the slower phosphorylation kinetics of FRQ7 (Diernfellner et al., 2009). However, recent data using FRQ kinase mutants and FRQ phosphorylation site mutations indicate that FRQ phosphorylation does not play a major role in FRQ localization in the cell (Cha et al., 2011).
2. WCC phosphorylation
Both the WC-1 and the WC-2 proteins are phosphorylated in vivo in the dark, and their phosphorylation increases in response to light exposure. This light-dependent phosphorylation may play a role in the function of WCC as the blue light receptor in Neurospora, but this function of WCC can be separated from its function in the FRQ/WCC feedback loop of the circadian system. We will be concerned in this review only with the clock-related function of WCC.
FRQ/FRH regulates the activity of WCC by regulating its phosphorylation status, inhibiting WCC activity as its phosphorylation increases (He et al., 2006; Schafmeier et al., 2005). Five phosphorylation sites on the WC-1 protein downstream of the DNA-binding zinc finger domain have been identified as light independent, and mutation of these sites produces short-period or arrhythmic conidiation (He et al., 2005b). Additional WC-1 phosphorylation sites have been identified, as well as one site on WC-2 (Sancar et al., 2009). The dark phosphorylation of both WC-1 and WC-2 depends on FRQ/FRH, and hypophosphorylated WCC is more transcriptionally active than the hyperphosphorylated forms (He et al., 2006; Schafmeier et al., 2005), supporting the conclusion that FRQ inactivates WCC in the negative feedback loop by promoting WCC phosphorylation.
Both CK-1a and CKII phosphorylate WC-1 and WC-2 in vivo in a FRQ-dependent manner (He et al., 2006). Mutations of FRQ that abolish the interaction between FRQ and CK-1a result in hypophosphorylation of WC proteins, as do both a kinase-defective ck-1a knock-in mutant and a cka mutant defective in CKII (He et al., 2006). The transcriptional activity of WCC is increased by mutations in either CK-1a or CKII (He et al., 2006). PKA also phosphorylates WC-1, but independently of FRQ (Huang et al., 2007). WC-1 protein is at low levels and is hypophosphorylated in the pkac-1 knockout, suggesting that PKA stabilizes WC-1 and primes it for phosphorylation by other kinases (Huang et al., 2007).
The phosphatases PP2A and PP4 also play roles in regulating WCC phosphorylation status. WCC is a substrate for PP2A, and the rgb-1 mutant, defective in a regulatory subunit of PP2A, increases phosphorylation of WC-1 and WC-2 (Schafmeier et al., 2005). Both WC-1 and WC-2 are also hyperphosphorylated when the pp4 gene is deleted, and the nuclear enrichment of WCC is lost (Cha et al., 2008).
3. Positive feedback and phosphorylation
The mechanism of positive feedback whereby FRQ promotes accumulation of WCC is not completely known. In one simple model (Schafmeier et al., 2008), positive feedback depends only on posttranslational regulation of WCC. Degradation of WCC may be triggered by DNA binding, so that FRQ can cause accumulation of WCC by inhibiting its binding to DNA through phosphorylation. In this way, the same mechanism (phosphorylation of WCC) that causes negative feedback (inhibition of DNA binding) may also cause positive feedback (accumulation of WCC). However, the system is more complex as FRQ-dependent transcriptional regulation of wc-2 has been demonstrated (Cheng et al., 2001b), and FRQ regulates WC-1 levels independent of WC-2 (Cheng et al., 2001b, 2002; Lee et al., 2000).
4. Nuclear localization and phosphorylation
As a transcription factor, WCC acts on its DNA targets in the nucleus, and therefore, the subcellular localizations and movements of WCC and FRQ are essential to the kinetics of the FRQ/WCC feedback loop. As indicated above, the phosphorylation and dephosphorylation cycle of FRQ is probably not a major player in this process. Instead, an unknown mechanism to actively export FRQ from the nucleus is thought to exist (Cha et al., 2011). Using the fluorescent protein mCherryNC fused to FRQ, the accumulation of FRQ in the nucleus can be visualized across a circadian cycle; a major peak of nuclear accumulation is found at CT 5 and a much smaller peak at CT 19, suggesting two phases of FRQ nuclear transport (Castro-Longoria et al., 2010). WCC is normally enriched in the nucleus, but this enrichment is lost when the pp4 gene, which codes for protein phosphatase 4, is deleted (Cha et al., 2008). There is conflicting evidence as to whether the rgb-1 mutation, which inactivates a subunit of PP2A, affects the nuclear localization of WCC (Cha et al., 2008; Schafmeier et al., 2008). The movement of WCC to the cytosol depends on the presence of a functional FRQ protein (Cha et al., 2008; Hong et al., 2008). FRAP analysis of GFP-tagged WC proteins showed rapid shuttling in and out of the nucleus on a time scale of minutes, modulated by FRQ-induced phosphorylation and PP2A phosphatase-mediated dephosphorylation (Schafmeier et al., 2008). These results suggest that FRQ promotes phosphorylation of WCC, which is counteracted by PP2A and PP4, and phosphorylated WCC preferentially accumulates in the cytosol. FRQ also physically interacts with WCC to promote clearance of the complex from the nucleus, and this may also contribute to negative feedback (Cha et al., 2008; Hong et al., 2008).
D. Identification of additional genes regulating the FRQ/WCC feedback loop
Included in this category are genes with clock-affecting phenotypes, either period effects or disruption of rhythmicity, whose functions in the FRQ/WCC feedback loop are known. See Table 3.3 for a summary of the genes described below.
1. frq antisense RNA
Two antisense transcripts from the frq locus have been identified, and their roles in regulating frq expression have been investigated (Kramer et al., 2003). Antisense frq RNA is rhythmically produced, but in antiphase to sense RNA, and its transcription is light induced. This light induction requires the WCC, which directly binds to the promoter of the antisense RNA following a short light pulse (Smith et al., 2010). When antisense frq expression is abolished, there is a delay in the expression of sense frq and a similar delay in the onset of the conidiation rhythm. The most dramatic effect is an increased response to phase resetting by light of the conidiation rhythm in the strains without antisense frq (Kramer et al., 2003). Antisense frq therefore seems to oppose the function of sense frq in the light response and may play a role in moderating the level of frq expression (Crosthwaite, 2004).
2. frh
A search for additional clock components that associate with FRQ using immunoprecipitation of FRQ protein revealed a protein that was given the name FRH. FRH forms a complex with the entire pool of FRQ protein and mediates the interaction of FRQ with WCC (Cheng et al., 2005; Guo et al., 2010). The frh gene is essential in Neurospora, but downregulation abolishes circadian conidiation rhythms as well as molecular rhythms in FRQ protein, frq RNA, and ccg-2 RNA, identifying FRH as an essential component of the FRQ/WCC feedback loop (Cheng et al., 2005). A genetic screen for mutations affecting negative feedback identified a mutant allele, frhR806H, that disrupts the interaction between FRQ/FRH and WCC and abolishes conidiation rhythmicity and molecular rhythms without affecting growth and viability (Shi et al., 2010). WC-1 phosphorylation and consequent stabilization appear to require the interaction of both FRQ and FRH with WCC: the frhR806H mutant disrupts those interactions, and both WC-1 and WC-2 are found at low levels in hypophosphorylated forms in this mutant (Shi et al., 2010).
FRQ/FRH associates with the exosome that regulates RNA processing and promotes decay of frq RNA (Guo et al., 2009). FRQ/FRH may therefore play two roles in the FRQ/WCC feedback loop: inhibiting WCC activity and promoting decay of frq RNA. Downregulating a component of the exosome, RRP44, increases the stability of frq RNA and lengthens the period of the molecular rhythms of FRQ protein and frq RNA, demonstrating the role of the exosome in regulating frq RNA decay (Guo et al., 2009). The exosome may also regulate rhythmicity of some ccgs by regulating their RNA stability: rrp44 RNA is rhythmic, and downregulating rrp44 affects the rhythmic expression of two ccgs (Guo et al., 2009). FRH has recently been shown to play a role in stability of FRQ protein as well (Guo et al., 2010): FRQ is degraded rapidly in the absence of FRQ–FRH interaction, by a pathway that is independent of FWD-1 (see Section III.D.3).
3. fwd1 and csn
The degradation of FRQ protein is an essential process in the functioning of the FRQ/WCC feedback loop. In frq mutants with different periods, the rate of degradation correlates with the period (Ruoff et al., 2005). Protein degradation in eukaryotes is often carried out by the ubiquitin–proteasome pathway, and this pathway has been shown to mediate FRQ degradation in the circadian feedback loop. FRQ protein is ubiquitylated, and the FWD1 protein was identified as the substrate-recruiting subunit of the SCF ubiquitin ligase complex responsible for FRQ degradation (He et al., 2003). Disruption of the fwd1 gene disrupts rhythms of FRQ protein, frq RNA, ccg-1 RNA, and conidiation rhythms (He et al., 2003). The COP9 signalosome (CSN) is a multisubunit complex that, among other functions, regulates the stability and activity of ubiquitin ligases. Disruption of the csn-2 gene that encodes a CSN subunit impairs degradation of FRQ, abolishes FRQ protein oscillations, and produces conidiation rhythms with very long periods (He et al., 2005a). A series of knockouts of other CSN subunits (csn-1, -2, -4, -5, -6, and -7) produces similar phentoypes of long-period and irregular conidiation rhythms (Wang et al., 2010). It should be noted that there is also evidence for another pathway for FRQ degradation independent of FWD-1, although the details of this pathway are not yet known (Guo et al., 2010).
4. csw-1
The WCC is the transcriptional activator that is responsible for activating frq expression, but little is known about events at the promoter. Chromatin remodeling and histone modifications are likely to be involved as they are in the activation of many genes. A survey of knockouts of putative chromatin-remodeling genes in Neurospora identified one gene, csw-1 (clockswitch), with a clock-associated phenotype (Belden et al., 2007b). The knockout produces abnormal, sporadic conidiation patterns and a loss of rhythmicity of frq RNA and FRQ protein expression. The CSW protein localizes to the frq promoter and affects chromatin structure and WCC association with the frq promoter (Belden et al., 2007b).
E. Temperature compensation
One of the basic properties of circadian oscillators is the property of temperature compensation: the period of the rhythm changes very little at different constant environmental temperatures. The wild-type Neurospora clock is well compensated in the physiological temperature range of 16–30 °C and poorly compensated between 30 and 36 °C (Gardner and Feldman, 1981; Gooch et al., 2008; Sargent et al., 1966). Not all period-affecting mutations in Neurospora have been assayed for their effects on temperature compensation; but of those that have, many have been found to have some effect on this property, either impairing compensation such that the period becomes longer at low temperature or in a few cases causing “overcompensation” in which the period becomes somewhat shorter at lower temperatures (Gardner and Feldman, 1981). Effects on temperature compensation can be slight, such as for prd-4 (Gardner and Feldman, 1981), or may be greater, as for cel (Mattern et al., 1982). Impaired temperature compensation may be uniform across a broad temperature range, as for frq7 (Gardner and Feldman, 1981), or may affect only a limited range of temperatures, as for chol-1 (Lakin-Thomas, 1998). The observation that many different gene products influence temperature compensation with a range of different effects may indicate that temperature compensation is a property of the system as a whole, rather than a special function of a particular temperature-independent regulatory mechanism. It also seems likely that some components of the circadian system may have evolved to play larger roles in maintaining temperature compensation.
The frq gene plays an important role in temperature compensation of the period, as shown by the impaired compensation in the null frq mutants frq9 (Loros and Feldman, 1986) and frq10 (Aronson et al., 1994a) and altered temperature compensation in period-affecting frq mutants (Gardner and Feldman, 1981). In frq mutants, FRQ protein stability is related to temperature compensation (Ruoff et al., 2005). Casein kinase 2 (CK2 or CKII, see Section III.C.1) has recently been identified as a regulator of temperature compensation through its phosphorylation of FRQ protein (Mehra et al., 2009). Two clock-affecting genes identified in early genetic screens as period-affecting mutants, chr (chrono) and prd-3 (period-3), also display unusual temperature compensation: chr extends temperature compensation beyond the wild-type range, and prd-3 is overcompensated (Gardner and Feldman, 1981). These two genes have now been identified as coding for subunits of CK2 (Mehra et al., 2009): chr (ckb-1) encodes subunit CKB-1 and prd-3 (cka) encodes subunit CK2α. The mutations were demonstrated to be hypomorphs that result in reduced FRQ phosphorylation and could be phenocopied by mutations in putative CK2 phosphorylation sites on FRQ. Manipulating the dosage of the CKB-1 protein alters temperature compensation and affects FRQ stability as predicted (Mehra et al., 2009). Importantly, not every kinase that phosphorylates FRQ affects temperature compensation. For example, manipulation of the levels of CK1a alters the period of the developmental rhythm, but the clock is still temperature compensated (Mehra et al., 2009). Together, these data suggest a specific role for CK2 in regulating the pace of the clock at different temperatures via temperature-dependent phosphorylation of FRQ altering FRQ stability.
IV. OTHER CLOCK-ASSOCIATED GENES
Included in this category are genes whose identity is known that affect rhythmicity but do not appear to directly affect the functioning of the FRQ/WCC feedback loop, and genes that affect the period of the conidiation rhythm but whose identity and function are as yet unknown. While the significance and underlying mechanisms for many of these observations are unknown, they provide fertile ground for future investigation. See Table 3.3 for a summary of the genes described below.
A. csp-1
This gene codes for a light-inducible transcription factor that affects the conidiation process by preventing conidial separation (Lambreghts et al., 2009; Smith et al., 2010). Strains carrying the csp-1 mutation show periods about 1 h shorter than wild type (Schneider et al., 2009).
B. csp-2
Mutations in csp-2 also prevent conidial separation, but unlike csp-1, this mutation lengthens the period by about 1.5 h (Brody et al., 2010). The csp-2 gene has been found by mapping and complementation to be allelic with ghh, the Neurospora homolog of the animal epidermal integrity gene Grainyhead; the period of the ghh mutant is about 3 h longer than wild type (A. Paré and B. McGinnis, personal communication).
C. prd-4
The prd-4 (period) gene was identified in the early screens for mutations affecting the period of the conidiation rhythm. The original prd-4 mutation shortens the period by about 3 h (Gardner and Feldman, 1981), but a null mutation (Pregueiro et al., 2006) displays a wild-type period and normal rhythmicity, indicating that prd-4 is not essential to the clock mechanism. Cloning of the gene (Pregueiro et al., 2006) provided an identity and function. Prd-4 is an ortholog of checkpoint kinase 2 (Chk2) that activates a cell cycle checkpoint in response to DNA damage in eukaryotes. DNA-damaging agents reset the clock in Neurospora, and this resetting requires PRD-4 (Pregueiro et al., 2006). PRD-4 promotes phosphorylation of FRQ protein in response to DNA damage, thereby resetting the clock. The prd-4 mutant is semidominant, suggesting a gain of function. Consistent with that, FRQ protein is phosphorylated earlier than normal during the circadian cycle in the prd-4 mutant, accounting for the decreased period (Pregueiro et al., 2006).
D. rasbd (formerly bd)
Almost all strains used in laboratories that study the circadian rhythm of conidiation in Neurospora carry the bd (band) mutation to make the rhythm easier to assay. The effect of the bd mutation was originally described as making the conidiation rhythm resistant to inhibition by high levels of CO2 that accumulate in closed culture vessels such as race tubes (Sargent and Kaltenborn, 1972). Cloning of the bd gene (Belden et al., 2007a) revealed that it is an allele of ras-1, the small G-protein involved in many signaling pathways in eukaryotes, and the mutation has been renamed rasbd. The addition of a source of reactive oxygen species (ROS) such as menadione to the growth medium can phenocopy the effects of rasbd, suggesting a signaling role for ROS in the conidiation output pathway (Belden et al., 2007a).
E. rco-1
This gene is a regulator of conidiation and photoadaptation (Olmedo et al., 2010a; Yamashiro et al., 1996). In the rasbd background, it lengthens the conidiation rhythm to 34–55 h, depending on the culture conditions (Brody et al., 2010).
F. Sod-1
This gene codes for superoxide dismutase, an enzyme that removes ROS. Mutation or deletion of this gene phenocopies the rasbd mutation in that a robust conidiation rhythm with a slightly shorter period can be seen under conditions where the wild type appears arrhythmic (Belden et al., 2007a; Yoshida et al., 2008). The addition of ROS to the growth medium also phenocopies rasbd (Belden et al., 2007a), while the removal of ROS reverses the banding phenotype of sod-1 (Yoshida et al., 2008), implicating ROS in the conidiation output pathway.
G. ult
This newly isolated mutation (ult, ultradian) shortens the conidiation period to about 12 h (see also Section V) and is the shortest clock mutant yet reported (Lombardi et al., 2007). It is dominant in heterokaryons and is not yet mapped and cloned. It produces a pattern of alternating thin and thick bands of conidiation (Lombardi et al., 2007). The addition of caffeine to the growth medium doubles the period of the ult mutant, suggesting a role for the cAMP pathway in the ult phenotype (Brody et al., 2010).
H. vvd
The vvd (vivid) gene has been previously identified as a component of the light input pathway in Neurospora. The gene is rapidly transcribed in response to light, and the protein functions to downregulate responses to light (Heintzen et al., 2001; Schwerdtfeger and Linden, 2003; Shrode et al., 2001). Wild-type cultures of Neurospora do not express the conidiation rhythm in constant light (LL), but vvd mutants express short-period (~11 h) conidiation rhythms in LL (Schneider et al., 2009). With increasing light intensity, temperature compensation is gradually lost and the period decreases to as little as 6–7 h. Upon transfer to the dark, the period gradually lengthens until a final steady-state period of 22 h is reached. The expression of rhythmicity depends on the culture: rhythms are seen in petri plates but not in race tubes (Schneider et al., 2009). A mutation in the csp-1 gene lengthens the period and reduces the sensitivity of the period to light effects. Cultures rhythmic in LL can be phase shifted by dark pulses. The level of FRQ protein is high and constant in vvd cultures when they are rhythmic in LL, indicating that a rhythm in FRQ is not essential for the conidiation rhythm; this result is consistent with the finding that the frq gene is not required for the vvd rhythm in LL (Schneider et al., 2009) (see Section V).
I. Quantitative trait loci
A method for identifying new clock-affecting genes is to exploit the genetic variation in natural populations. Free-running period and entrained phase were used to map quantitative trait loci (QTL) in three populations of progeny derived from crosses of isolates collected in different geographic locations (Kim et al., 2007). Thirty QTL were found that did not map near previously identified clock genes, and these regions are candidates for the locations of new clock-affecting genes (Kim et al., 2007).
J. Screening Neurospora knockout library for defects in circadian rhythms
Currently, almost all Neurospora’s predicted 10,000 open reading frames (ORFs) have been deleted, providing new tools for assaying the effects of specific gene knockouts on circadian rhythmicity. Screens are underway in several labs to assay alterations in the developmental rhythms in the knockout strain collection, as well as assaying rhythmic reporter genes for alterations in rhythmicity in the mutant strains. These approaches promise to provide a global view of the influence of the genome on circadian clock function.
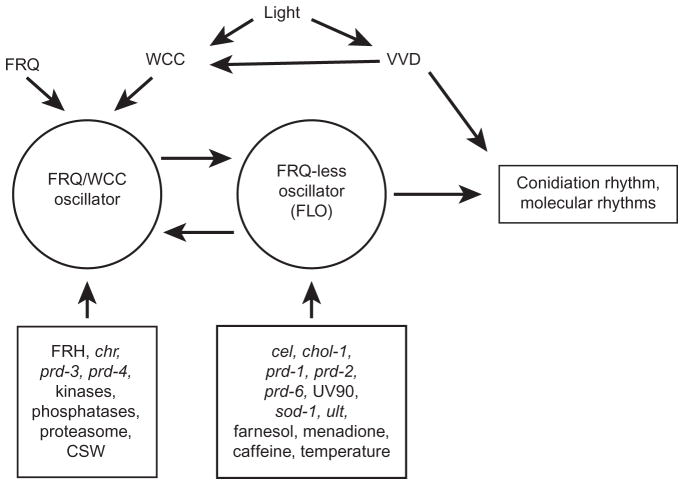
V. FRQ-LESS RHYTHMS
A. “Conditional rhythmicity” of frq and wc null mutants
Although the vast majority of research carried out on the Neurospora circadian system is focused on the FRQ/WCC feedback loop, it has been known for many years that rhythmic conidiation can be seen in the absence of a functional frq gene (Loros and Feldman, 1986). More examples of these FRQ-less rhythms have been reported over the years, and there is now a large collection of conditions and genetic backgrounds in which FRQ-less rhythmicity can be demonstrated. Although null mutants of frq, wc-1, or wc-2 are often described as “arrhythmic,” it might be more accurate to call them “conditionally rhythmic.” Rhythmicity can be induced in these null mutants by conditions such as introducing additional mutations, by adding chemicals to the growth medium, or by simply changing the geometry of the culture vessels.
Rhythmicity in the absence of FRQ/WCC function must be driven by an oscillator, and there has been much discussion over the years as to the relationship between the FRQ/WCC and the FRQ-less oscillator(s) (FLOs). The possibility that FRQ functions to transduce environmental information as part of an input pathway to another oscillator was discussed as early as 1997 (Lakin-Thomas et al., 1997). In 1998, Roenneberg and Merrow proposed the “zeitnehmer” model in which FRQ/WCC functions as a rhythmic input pathway to an oscillator (Roenneberg and Merrow, 1998). Evidence for multiple oscillators was discussed with reference to the restoration of rhythmicity to frq null strains by Lakin-Thomas and Brody in 2000 (Lakin-Thomas and Brody, 2000). Subsequently, the term FLOs was coined to describe the oscillator(s) driving FRQ-less rhythms (Iwasaki and Dunlap, 2000). While several labs have obtained a significant amount of data describing the FRQ-less oscillations, we still do not know the identity of the components of the FLOs. Until we do, we can only speculate on the role and organization of the FLOs in the circadian system.
B. FRQ-less conidiation rhythms and their properties
Most reports of FRQ-less rhythms have assayed rhythms of conidiation, and these are described below in approximate chronological order. In some cases, a mutation in the genetic background is required to observe the rhythm, and those genes that have been reported in the past 10 years are included in Table 3.3.
1. Long race tubes
FRQ-less conidiation rhythms were first reported in a frq9 null mutant (Loros and Feldman, 1986). Rhythms take several days to appear, so extra-long race tubes were used to gather more data, and not all cultures are rhythmic. The period is more variable than wild type and is affected by the nutritional composition of the medium. Temperature compensation of the period is defective. The rhythm can be damped out by constant light and entrained to cycles of light pulses. When the knockout frq10 became available, it was found to produce similar rhythms (Aronson et al., 1994a).
2. cel and chol-1
The two lipid-defective mutations cel and chol-1 described in Section II can reveal FRQ-less rhythms under appropriate conditions. When double mutants of cel and either frq10 or wc-2 are grown at low temperature or supplemented with unsaturated fatty acids, long-period conidiation rhythms are robustly produced (Lakin-Thomas and Brody, 2000). Double mutants of the choline-requiring mutant chol-1 with frq, wc-1, or wc-2 null mutants produce robust conidiation rhythms when choline supplementation is limited (Lakin-Thomas and Brody, 2000). At intermediate choline concentrations, the periods are in the circadian range and lengthen as choline is decreased. The period of the chol-1 frq10 double mutant is temperature compensated between 22 and 28 °C and lengthens below 22 °C. None of the double mutant cel or chol-1 strains are damped by constant light or entrain to light/dark cycles (Lakin-Thomas and Brody, 2000). Recent studies examining frq-driven luciferase rhythms in the chol-1 mutant strain revealed that under conditions in which the strain shows a long-period developmental rhythm, the frq:luc rhythms cycled with a 22-h period (Shi et al., 2007). Although it has been suggested that these data indicate independent functioning of two oscillators (Shi et al., 2007), the possibility of coupling between two oscillators in a frequency demultiplication relationship has not been fully explored.
3. Temperature entrainment
The circadian oscillator in Neurospora can be entrained to cycles of high and low temperature, and it was reported that such cycles could also entrain the conidiation rhythm in a frq null strain (Merrow et al., 1999; Roenneberg et al., 2005), suggesting that the same oscillator functions in both wild-type and frq null strains. This interpretation was challenged when a similar data set was analyzed with different methods and the authors concluded that the conidiation peaks represented “driven” or “masking” responses, not the output of an oscillator (Pregueiro et al., 2005). These experiments used symmetric temperature cycles, with equal periods of warm and cool temperature, which makes it difficult to separate masking effects from entrained peaks. An improved method using short pulses of high temperature demonstrated definitively that the conidiation peaks behave as if they are produced by an entrained oscillator (Lakin-Thomas, 2006a). This improved method uncovered both temperature-driven masking effects and temperature entrainment for both frq10 and a wc-1 null mutant (Lakin-Thomas, 2006a).
4. Farnesol and geraniol
The addition of the chemicals farnesol or geraniol to the growth medium produces reliable rhythms in frq, wc-1, and wc-2 null mutants with periods in the circadian range or somewhat longer (Granshaw et al., 2003). It takes several days before conidiation bands appear. Temperature compensation of the period is defective, and the carbon source in the growth medium affects the appearance of banding. Phase resetting by temperature pulses produces a strong type 0 phase response curve in both the frq10 and the frq+ strains grown on geraniol. However, frq10 did not entrain to light/dark cycles, unlike frq+ (Granshaw et al., 2003).
5. ult
This short-period mutant produces robust 12 h rhythms in frq10 double mutants. In a wc-2 null mutant background, ult produces rhythms with short periods of about 16.5 h (Lombardi et al., 2007).
6. sod-1
The frq10; sod-1 double mutant strain, displays a conidiation rhythm in constant darkness, although the triple-mutant frq10; bd; sod-1 is arrhythmic (Yoshida et al., 2008). Light/dark cycles induce a conidiation rhythm that behaves as if it is produced by masking effects of light and not by entrainment of an oscillator (Yoshida et al., 2008).
7. vvd
The short-period conidiation rhythms expressed in constant light (LL) by the vvd mutant are still expressed in the vvd; frq10 double mutant with no significant change in period, although the rhythms are not as robust as in vvd (Schneider et al., 2009). Introduction of the wc-1 knockout abolishes the LL rhythms in vvd; introducing wc-2 reduced the clarity of the vvd rhythms (Schneider et al., 2009). This suggests that wc-1 is required as the light receptor for the expression of rhythmicity in vvd; frq10 in LL.
8. Menadione
This chemical generates ROS and has been shown to phenocopy the rasbd mutation (see Section IV). When added to the growth medium, menadione induces conidiation rhythms in null mutants frq10, wc-1, and wc-2, in both DD and LL (Brody et al., 2010). The periods of frq10 and wc-1 are short (14–15 h), and wc-2 is long (25 h). In 24-h LD cycles, frq10 entrains and wc-1 produces two bands per cycle, while wc-2 fails to entrain. The addition of caffeine to the medium doubles the period of frq10. A mutation in the csp-1 gene shortens the period of the null frq9 mutant by about 1 h. The period of wc-2 is temperature compensated in the range 16–28 °C, and frq10 is compensated in the range 22–28 °C on menadione (Brody et al., 2010).
C. FRQ-less molecular rhythms and their properties
Several molecular species have been shown to be rhythmic in FRQ-less strains. Because these rhythms have been assayed under different growth conditions in different laboratories, it is not yet clear whether they are related to each other, and how they are related to FRQ-less conidiation rhythms.
1. DAG
The levels of the neutral lipid diacylglycerol (DAG) are rhythmic in wild-type Neurospora with a period that reflects the conidiation rhythm (Ramsdale and Lakin-Thomas, 2000). In a frq null strain grown on solid agar, the DAG rhythm has a period of about 12 h, similar to short-period conidiation rhythms seen in these cultures (Ramsdale and Lakin-Thomas, 2000).
2. Nitrate reductase
In ammonia-free medium, a rhythm in nitrate reductase activity can be measured in frq null and wc-1 null strains in both DD and LL (Christensen et al., 2004). This rhythm may be an indicator of rhythmic metabolism, or may be an autonomous oscillator constructed from the negative feedback of glutamine on nitrate reductase expression (Christensen et al., 2004).
3. ccg-16
The mRNA levels of a ccg, ccg-16, are rhythmic in DD in a frq knockout strain but are not seen in null mutants of either wc-1 or wc-2 (Correa et al., 2003). This FRQ-less rhythm is also seen in LL when FRQ protein levels are constantly elevated and is more responsive to temperature than to light cues for synchronization (de Paula et al., 2006). The period is temperature compensated between 22 and 27 °C (de Paula et al., 2006). A knockout of ccg-16 does not affect the conidiation rhythm in a frq+ background and does not affect the rhythm of WC-1 protein in a frq null background (see Section V.C.4), thereby defining ccg-16 as an output of the FLO and not a component of either the FRQ/WCC oscillator or the FLO (de Paula et al., 2006).
4. WC-1
A low-amplitude rhythm in WC-1 protein levels has been reported in frq+ strains (de Paula et al., 2006; Lee et al., 2000). This rhythm can also be seen in LL in wild-type strains, and in both DD and LL in a frq null strain, although the average levels are lower than in wild type (de Paula et al., 2006).
D. Mutations affecting FRQ-less rhythms
If additional mutations are introduced into frq or wc null strains and these double mutant strains are assayed under conditions in which FRQ-less rhythms can be seen, the effects of the additional mutations on the FLO(s) can be assayed. Several mutations have been found to affect FRQ-less rhythms, and they are summarized in Table 3.3.
1. frq
It may seem paradoxical to claim that frq mutations affect the period of FRQ-less rhythms. However, under some conditions, it appears that it is primarily the FLO that is controlling rhythmicity when there is a functional frq gene, and mutations in frq can affect the FLO, supporting the idea that the FLO functions downstream of the FRQ/WCC feedback loop. In the chol-1 strain, described above, long-period rhythms are seen when choline is limiting. Long-period rhythms are also seen in a chol-1 frq null double mutant, and these rhythms have characteristics similar to the rhythms in the frq+ background: the period is sensitive to the choline concentration and shows a similar pattern of temperature compensation (Lakin-Thomas and Brody, 2000). It is therefore reasonable to assume that the same oscillator is driving conidiation rhythms in both frq+ and frq null on limiting choline. As described in Section II.B and in Table 3.1, frq mutations can have significant effects on the long period in chol-1, indicating that the FLO can be affected by frq.
Additional evidence that mutations in the FRQ/WCC feedback loop can affect the FLO comes from the results with menadione in frq and wc null strains (Brody et al., 2010), as described in Section V.B.8. In the presence of menadione, the period of conidiation rhythms in frq10 and wc-1 is about 14–15 h, while the period in wc-2 is about 25 h, indicating that the alleles at these loci influence the period of the FRQ-less rhythm under these conditions.
2. ult
The periods of the FRQ-less rhythms produced by geraniol in frq10 and wc-2 mutants are shortened by the addition of the short-period ult mutation (Lombardi et al., 2007).
3. prds
The lipid-deficient cel strain produces long-period conidiation rhythms at low temperature and when supplemented with unsaturated fatty acids. These long-period rhythms continue when frq and wc null mutations are introduced (Lakin-Thomas and Brody, 2000), and therefore the long-period rhythms in cel frq+ strains may be said to be driven by the FLO. When the prd-1 mutation is introduced into cel, the period-lengthening effect of fatty acids is abolished, and therefore prd-1 appears to block the normal functioning of the FLO in cel (Lakin-Thomas and Brody, 1985).
The frq10 null mutant is rhythmic when supplemented with geraniol (described above), and this system was used to assay the effects of a series of prd mutations on the FLO (Lombardi et al., 2007). The prd-1 and prd-2 mutations, both long period in a frq+ background, also lengthen the period in the frq10 background. The short-period prd-6 mutation shortens the frq10 period (Lombardi et al., 2007). No change in period is seen with prd-3 or prd-4 in the frq10 background.
In a similar experiment, triple mutants were constructed between chol-1 frq10 and a series of prd mutations, and the effects of the prds on the long-period conidiation rhythm in chol-1 were assayed (Li and Lakin-Thomas, 2010). Both prd-1 and prd-2 severely disrupt rhythmicity in choline-depleted cultures, but prd-3 and prd-4 have only subtle effects on period and robustness of the conidiation rhythm. These strains were also grown on high choline (which repairs the chol-1 defect) to assay the effects of the prd mutations on the heat-entrainable oscillator in frq10. A similar pattern was seen: both prd-1 and prd-2 significantly affect the timing of the heat-entrainable peak, while prd-3 and prd-4 have little effect (Li and Lakin-Thomas, 2010).
Both prd-3 and prd-4 are known to encode subunits of protein kinases that phosphorylate FRQ protein (see Section III.C.1), and their lack of effect on FRQ-less rhythms is consistent with the assumption that they act primarily on the FRQ/WCC oscillator. The effects of prd-1 and prd-2 mutations on multiple FRQ-less rhythms may indicate that these genes act primarily on the FLO. The effects of both prd-1 and prd-2 on the period of the conidiation rhythm when FRQ/WCC is functional suggest that their effects on FLO can also affect the period of the intact system.
4. UV90
Using the chol-1 frq10 double mutant, a mutagenesis screen was carried out to look for genes that affect the FRQ-less rhythm on low choline (Li et al., 2011). A new mutation was found, named UV90, that abolishes the FRQ-less conidiation rhythm seen in low-choline cultures. UV90 also severely affects the heat-entrainable conidiation rhythm in choline-sufficient cultures. In a frq+ background, UV90 damps the amplitude of the conidiation rhythm and also damps the FRQ protein rhythm. The response of frq+ cultures to phase resetting by pulses of either light or high temperature is increased in the UV90 mutant background, consistent with a decrease in the amplitude of the circadian oscillator (Li et al., 2011). These results suggest that the UV90 gene product is an important component of the FLO and is also required to maintain the amplitude of the FRQ/WCC oscillator, indicating an influence of the FLO on FRQ/WCC.
E. Multiple independent FLOs, or an integrated system?
The existence of FRQ-less rhythms implies the existence of one or more oscillators (FLOs) that drive these rhythms in the absence of a functional FRQ/WCC oscillator. If we wish to fully understand the circadian system of Neurospora, one of the most fundamental and vigorously debated questions at the moment is: how many oscillators make up the complete system? There are several possible interpretations of the data. One view (Shi et al., 2007) describes FLOs as “metabolic oscillators” and proposes that there are different FLOs driving the observed rhythms for every reported condition; these multiple metabolic oscillators (of which there would need to be more than a dozen at the latest count) are not considered to be connected to the circadian system (Shi et al., 2007). Another view proposes multiple FLOs that, under normal circumstances, interact with one another and with the FRQ/WCC to produce a network of coupled oscillators, but that individual FLOs may act independently to drive a particular output (de Paula et al., 2007). If there are multiple oscillators, there is a formal possibility that they may function either “upstream” or “downstream” of FRQ/WCC; in either case, they would need to bypass FRQ/WCC to provide output to the conidiation pathway and biochemical rhythms when FRQ/ WCC is disabled. A third view proposes a single FLO, which may be the central rhythm generator for the circadian system, mutually coupled to the FRQ/WCC, which supplies stability, period control, and rhythmic input (Li and Lakin-Thomas, 2010; Roenneberg and Merrow, 1998).
The FRQ-less rhythms described above appear to have different properties, lending support to models in which every reported FRQ-less rhythm is driven by a different FLO. However, properties such as temperature compensation, response to alterations in growth media, and ability to entrain to light/ dark or temperature cycles have not been fully characterized for most of the reported FRQ-less rhythms. Claims of failure to entrain to environmental stimuli are particularly suspect unless a wide range of cycle lengths and stimulus strengths have been tested, as oscillators will fail to entrain to stimuli that are too weak or whose entraining periods are too far from the intrinsic period of the oscillator (Johnson et al., 2003). Because of the many different conditions and experimental paradigms, it is difficult to compare the properties of FRQ-less rhythms reported by different laboratories and impossible to say what they have in common.
Obviously, the best way to address the questions of how many FLOs there are and whether they function independently of the FRQ/WCC oscillator is to identify components of the FLOs. The analysis of genetic interactions in multiple mutant strains can suggest participation in common pathways, as described in Section II.B. The complex interactions between the series of frq alleles and the long periods in both cel and chol-1 (Lakin-Thomas, 1998; Lakin-Thomas and Brody, 1985) indicate that the FRQ gene product interacts with the FLO(s) that produce long periods in both cel and chol-1. The epistasis of prd-1 over cel (Lakin-Thomas and Brody, 1985) places prd-1 in the same pathway as the FLO in cel. The series of interactions among prd mutations and frq alleles (Morgan and Feldman, 2001; Morgan et al., 2001) place prd-1 and prd-2 in the same pathway, interacting with prd-6 and frq. All these interactions point to a system in which FRQ/WCC is not independent of the FLO(s) affected by cel, chol-1, prd-1, prd-2, and prd-6.
Additional genetic evidence comes from assaying the effects of mutations on more than one FRQ-less rhythm. Four different FRQ-less rhythms have been assayed in prd-1 and all four were found to be affected: in cel, in chol-1, with geraniol, and with heat pulses. Three FRQ-less rhythms have been assayed in prd-2 and all were affected: in chol-1, with geraniol, and with heat pulses. Two FRQ-less rhythms are affected by UV90: in chol-1 and with heat pulses. So far, the evidence indicates that these mutations all affect multiple FRQ-less rhythms. It may be that these gene products participate in several different FLOs, but the simplest explanation is that a single FLO is behind multiple observed rhythms.
It has recently been proposed (Brody et al., 2010) that the many conditions and mutations that allow expression of FRQ-less rhythms may all converge on a pathway that includes ROS and the activation of a RAS–cAMP–protein kinase signaling pathway leading to conidiation. Several of the mutations and treatments that affect the expression of FRQ-less rhythms can be linked to the production of or response to ROS, such as menadione, farnesol, and sod-1 (Brody et al., 2010). Menadione, by phenocopying rasbd, links ROS to the RAS signaling pathway (Belden et al., 2007a). The clock effects of genes involved in conidiation, such as rco-1 and csp-1, and the effects of caffeine may implicate a cAMP pathway leading to conidiation as a candidate for a component of the FLO (Brody et al., 2010). It is interesting to note that connections are now being reported between the circadian clockworks and the cAMP pathway, and metabolic regulation in general, in other organisms (Bass and Takahashi, 2010; Harrisingh and Nitabach, 2008; Hastings et al., 2008).
A model for the Neurospora circadian system is proposed in Fig. 3.2. In this model, the FRQ/WCC feedback loop is coupled to a single FLO. The mechanism of the FLO is unknown and may or may not include a transcription/translation feedback loop. Both oscillators are required in the intact system to maintain the period, temperature compensation, amplitude, and stability of the output. When the FRQ/WCC oscillator is defective in frq or wc null mutants, the FLO can drive the output at a low amplitude and without the full range of properties such as temperature compensation and period stability. The conditions and genotypes listed above that allow expression of FRQ-less rhythms may act on various parameters of the FLO to increase its amplitude. The differences in properties between different FRQ-less rhythms can be explained by effects of the various conditions and background genotypes on different parameters of the FLO. Versions of this model have been discussed previously (Lakin-Thomas, 2006b; Lakin-Thomas and Brody, 2004; Li and Lakin-Thomas, 2010).
Figure 3.2.
Model for the integrated circadian system of Neurospora. Circles represent oscillators; arrows represent causal influences. See text for details.
VI. CLOCK-CONTROLLED GENES
A. Output from the circadian oscillator: The hands of the clock
In Neurospora, circadian rhythms in several physiological properties have been documented (Bell-Pedersen et al., 1996a; Lakin-Thomas et al., 1990); however, it was the easily observable rhythm in the development of macroconidia that led to further investigation of the Neurospora clock mechanisms (Pittendrigh et al., 1959). Mutations that altered the pattern of rhythmic development led to the identification of FRQ/WCC oscillator components (Feldman et al., 1979). Thus, the study of circadian output pathways not only yields information on the cellular functions that are regulated by the clock and the mechanism of this regulation but also is instrumental in discovering oscillator components.
B. Identification and characterization of ccgs
Molecular investigation of circadian output pathways originated in Neurospora by using subtractive hybridization to isolate rhythmically expressed genes (Loros et al., 1989). Two genes were identified that expressed mRNA that accumulated with a circadian rhythm with a period that matched the genotype of the strain. In other words, in a wild-type strain, the period of the mRNA rhythm in DD at 25 °C was 22 h, whereas in the long-period frq7 mutant, the period of the mRNA rhythm was about 29 h. This landmark study provided evidence that the genes were bone fide ccgs and provided the criteria to establish a gene as a ccg. Subsequent screens for ccgs (Bell-Pedersen et al., 1996b; Zhu et al., 2001), including microarray studies, led to the identification of over 400 ccgs (Correa et al., 2003; Dong et al., 2008; Nowrousian et al., 2003). These data suggested that as much as 25% of the Neurospora transcriptome is under clock control, and that the dominant peak in rhythmic expression for most of the ccgs occurs just before dawn. However, ccgs were found to peak at all possible phases of the day (Correa et al., 2003).
C. The functions of the ccgs provide insights into the role of the clock
The predicted functions of the proteins encoded by the identified Neurospora ccgs have yielded key insights into processes that are clock regulated, many of which are conserved in higher eukaryotes, including development, metabolism, cell cycle, transport, and stress responses (Fig. 3.3). For example, genes encoding enzymes involved in carbon and nitrogen metabolism show circadian rhythms in mRNA accumulation, with peaks of expression occurring in the late night to early morning (Correa et al., 2003). In addition, the glycogen phosphorylase, mannitol-1-phosphate dehydrogenase, and a low-affinity glucose transporter genes peak in expression in the early night, suggesting that flux into the glycolytic pathway peaks at this time of day to prepare for increased energy requirements related to the consequent development of conidiospores. Several ccgs that are involved in stress responses, including genes encoding antioxidant enzymes that prevent damage due to ROS, are also under clock control, such as glutamate dehydrogenase, glutamine synthetase, oxidoreductase, and catalase. Genes encoding these enzymes peak in expression during the daytime, suggesting that the clock increases antioxidative defense mechanisms to cope with increases in free radicals that can result from light exposure.
Figure 3.3.
Representation of the phase of expression of functional classes of ccgs. (Above) The developmental rhythm in Neurospora grown on race tubes in a 12-h light: 12-h dark cycle. Growth is from left to right. The vertical black lines on the tube depict the dark to light transition every 24 h. (Below) Representative functional groups of ccgs with respect to the approximate phases in which they peak in expression.
In support for a role of the clock in providing a mechanism for anticipating stress responses, the highly conserved Neurospora osmosensing p-38 MAPK pathway (the OS pathway), essential for osmotic stress responses, functions as a circadian output pathway that regulates daily rhythms in expression of several downstream target genes of the pathway (de Paula et al., 2007; Vitalini et al., 2007). In this pathway, a circadian signal is relayed to the MAPK OS-2, resulting in rhythmic phosphorylation and activation of the MAPK. Phospho-OS-2, in turn, regulates downstream effector kinases, transcription factors, proteins involved in chromatin remodeling, and translation factors that then regulate rhythmic expression of downstream target genes of the pathway.
Two morning-specific ccgs known to be induced by osmotic stress under control of the OS-MAPK pathway include ccg-1 and ccg-9 (Vitalini et al., 2007; Yamashita et al., 2008) (D. Bell-Pedersen, unpublished data). Rhythms in ccg-1 (a gene encoding a protein of unknown function) are dependent on rhythmic activation of OS-2, and the ATF transcription factor that is activated in response to osmotic stress by phospho-OS-2 binds to the ccg-1 promoter (Yamashita et al., 2008). The ccg-9 gene encodes trehalose synthase, which catalyzes the synthesis of the disaccharide trehalose. Trehalose plays an important role in protecting cells from environmental stresses, including osmotic stress (Shinohara et al., 2002). A role in development for ccg-9 is also suggested by the finding that inactivation of ccg-9 results in altered conidiophore morphology and abolishes the normal circadian rhythm of conidial development. ccg-9-null strains have normal FRQ cycling, phosphorylation, and light induction, indicating that loss of the conidiation rhythm may be a defect in circadian output and is not due to changes in either the FRQ/WCC oscillator or light input into the clock (Shinohara et al., 2002). The phenotype of the ccg-9 deletion strain differs from os-2 null mutants (Vitalini et al., 2007), suggesting multiple levels of regulation of ccg-9.
Taken together, these data indicate that clock regulation of MAPK signaling pathways provides a mechanism to coordinately control major groups of genes such that they peak at the appropriate times of day to provide a growth and survival advantage to the organism likely by anticipating stresses (de Paula et al., 2007). Consistent with this idea, global gene profiling in Arabidopsis thaliana revealed that approximately 68% of the ccgs overlap with genes that are differently regulated in response to osmotic- and cold stresses (Kreps et al., 2002).
D. Signaling time-of-day information from the oscillator to the ccgs
A major goal has been to characterize, at the molecular and biochemical levels, the pathways by which the clock regulates phase-specific gene expression. One mechanism by which the output pathways are rhythmically controlled was believed to be through the rhythmic activity of the WCC transcription factor. Consistent with this idea, many ccgs contain a WCC binding site (LRE) in their promoters (Dong et al., 2008), and WCC was found to bind directly to the promoters of several ccgs, including vvd, al-3, fl, and sub-1 (Bell-Pedersen et al., 2005; Carattoli et al., 1994; Chen et al., 2009; Correa et al., 2003; He and Liu, 2005b; Olmedo et al., 2010b).
To further explore the idea that rhythmic WCC activity is the primary output signal from the FRQ/WCC, global approaches were used to identify the direct targets of the WCC. Using ChIP-sequencing (ChIP-seq) of WC-2 following a light pulse to activate the WCC, more than 400 regions of the chromosome were found to bind the WCC, with about 200 of these binding sites present in predicted promoters of at least one gene (Smith et al., 2010). A consensus WCC binding site (GATCGA) was derived from these studies, with two or more copies typically present in the binding site region. Consistent with previous studies on ccgs, the direct target genes of the WCC were enriched from genes with functions in the cell cycle, transcription, protein binding, and responses to the environment. As expected, most of the direct targets of the WCC were found to be light induced and/or clock regulated, peaking in the early morning. Interestingly, about 20% of the transcription factors known to be present in the Neurospora genome were found to be direct targets of the WCC. These 24 transcription factors include known activators and repressors of gene expression, that could, in theory, directly account for the differentially phased ccgs (Fig. 3.4). Further studies on one of the transcription factors, ADV-1, demonstrated that the adv-1 gene is both light induced and expressed with a circadian rhythm with peak transcript and protein levels in the early morning (Smith et al., 2010). Deletion of adv-1 had only a minor affect on the development of spores, but similar to the ccg-9 deletion strain, resulted in the complete loss of the circadian rhythm of spore development. FRQ protein rhythms were unaltered in the adv-1 deletion strain, suggesting that ADV-1 functions directly downstream of the FRQ/WCC oscillator to regulate the developmental rhythm.
Figure 3.4.
Model of differential phase regulation of ccgs. The WCC directly activates the expression of 24 transcription factors in the morning, including activators and repressors of transcription. The repressors would be active during the day, and therefore the targets of the repressors would be expressed in the night. In addition, the activators would turn on the first tier targets during the morning, resulting in morning-specific gene expression. One or more of the first tier targets may include transcription factors that activate or repress the second tier targets to regulate mid-day-specific ccgs, etc.
Taken together, the data suggest a “flat” hierarchical network in which the WCC activates the expression of a suite of second tier transcription factors, which, in turn, activate or repress their individual target genes. Some of these second tier targets may include additional transcription factors that activate or repress the next level. Conservatively, assuming that each of the 24 transcription factor regulates a set of about 20 downstream genes (and that WCC directly binds to regulate rhythms in about 180 additional morning-specific targets), this WCC-directed regulatory network can account for all of the known ccgs and their varied phases. Efforts are now underway to identify the direct targets of these 24 transcription factors using ChIP-seq to comprehensively map the WCC output network. In addition, it is clear that chromatin remodeling, posttranscriptional, translational, and posttranslational mechanisms provide additional combinatorial control of circadian rhythmicity, such as that observed for clock control of the MAPK signaling pathway, and these areas provide fertile ground for future work to decipher the mechanisms for clock control.
E. Output pathways can feed back to regulate oscillator activity
The modulating action of the clock-controlled component VVD on light input pathways provides a clear example of how output pathways can feed back to influence the circadian oscillator (Elvin et al., 2005; Heintzen et al., 2001). In addition to vvd, the ccg prd-4 was shown to influence input to the FRQ/ WCC oscillator in Neurospora (Gardner and Feldman, 1981; Pregueiro et al., 2006). PRD-4 is an ortholog of mammalian chk-2, which is involved in the response to DNA damage (see Section IV). Prd-4 mRNA accumulates rhythmically, peaking during the day. This regulation would allow cells to respond maximally to DNA damage caused by ionizing radiation during the day, resulting in the arrest of the cell cycle following PRD-4 phosphorylation in response to this damage. PRD-4 feeds back to the clock through an input pathway that signals DNA damage information to the FRQ/WCC oscillator via FRQ phosphorylation. This output to input loop involving prd-4 is predicted to provide a time-of-day-specific “gate” during which time DNA-damaging agents can affect the phase of the FRQ/WCC oscillator, which would, in turn, affect prd-4 expression.
In summary, while it is clear that different organisms use their clocks to regulate different biological processes, many of the output signaling pathways, such as the MAPK pathway and certain transcription factors, are conserved. Thus, a detailed understanding of the circadian output network in Neurospora will have a major impact on our understanding of circadian regulation in higher eukaryotes, including humans. In addition, these studies may provide key insights into the design of novel therapies for circadian disorders that result in changes in overt circadian rhythms and desynchrony.
VII. OUTLOOK
Great strides have been made in our understanding of the Neurospora “clock works” over the past 40 years, moving from biological phenomenology to the molecular and biochemical mechanism of the circadian clock system. We now have considerable detail regarding the nature of the core molecular feedback loops, their light input pathway, and their output. Further, the list of “clock-affecting” mutants continues to grow at a fast pace. More “effectors” are likely to continue to be discovered using the unusual resources that are available for Neurospora, such as the gene knockout collections. These genetic tools are likely to be supplemented by cytological and imaging techniques. Below is a partial list of some areas/techniques that are currently being studied, or are on the horizon.
Much remains to be discovered about the mechanism of the oscillator. More details will likely be uncovered regarding clock protein interactions. Perhaps these will be able to be described in biochemical terms, that is, km, ki, flux rates, etc. 3-D crystal structures of the components would be useful in this regard. The nature of the FLO components needs to be established, and how the FLO(s) relate to the FRQ/WCC oscillator. Studying the connection of circadian oscillators to the regulation of the biochemistry of the cell should prove worthwhile, particularly if the FLO(s) are not transcription/translation feedback loops. The role of redox sensing and low molecular weight molecules has been relatively neglected in the clock field. However, a start has been made to establish the light and clock metabolome (http://fungicyc.broadinstitute.org:1555/overviewsWeb/celOv.shtml).
More studies are likely on subcellular localization of clock components.Currently, little is known about the role of subcellular organelles, such as mitochondria, peroxisomes, Golgi, etc., in the clock, although mutations that affect the function of the organelles can alter circadian period.
The mechanism of temperature compensation will continue to be a fruitful area for studies in Neurospora. This will be aided by the already available mutants that affect temperature compensation and the identification of the mutated genes. A full description of temperature compensation will likely be aided by mathematical modeling.
Significant progress has been made in understanding the circadian output pathways using new sequencing technologies. Clearly, the complexities of these processes and their links to cellular biochemistry and metabolism will occupy many labs for quite a while.
Chromatin remodeling is an area that will continue to be of interest in understanding rhythmic gene expression. Neurospora is a good model system for these analyses as well, as it provides the opportunity to combine genetic, genomic (through ChIP-seq of histone and DNA modifying proteins), and biochemical techniques with imaging of histones using GFP, RFP, etc.
#x02022; (F) A systems biology approach to monitor rhythmicity in RNA, proteins, and the metabolome will be useful. This has been initiated for the transcriptome (Dong et al., 2008), but additional studies are needed to investigate the proteome and metabolome under different growth/lighting conditions and in specific genetic backgrounds.
Finally, there is a special feature of the Neurospora clock system that is fascinating to study and one that may be applicable to understanding synchrony in tissue-specific multioscillator model systems discussed in other chapters. This property is that Neurospora grows as a syncytium, with cytoplasm and nuclei streaming throughout the colony. It is intriguing how the growing front of the colony stays synchronized, and how stable the clock signals (phase information) are within a given area of the colony. Experiments involving fluorescent labeling of clock proteins and following their movement in a colony would provide one approach to study this unique feature, and genetics could be used to identify key molecules required for this synchrony.
The progress in understanding the details of the mechanism of the circadian clock, particularly with regard to the oscillator feedback loop, the roles of accessory proteins, temperature compensation, light input, and output, would not have been possible without the use of genetics. Neurospora is the undisputed leader in this arena. Importantly, many of these conceptual advances made in Neurospora have provided the foundation for our understanding of the clock mechanism in higher organisms. Undoubtedly, genetic tools, coupled with new technologies, will continue to provide the tools needed to further advance the field into uncharted areas of circadian biology.
References
- Aronson BD, Johnson KA, Dunlap JC. Circadian clock locus Frequency: Protein encoded by a single open reading frame defines period length and temperature compensation. Proc Natl Acad Sci USA. 1994a;91:7683–7687. doi: 10.1073/pnas.91.16.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson BD, Johnson KA, Loros JJ, Dunlap JC. Negative feedback defining a circadian clock: Autoregulation of the clock gene-frequency. Science. 1994b;263:1578–1584. doi: 10.1126/science.8128244. [DOI] [PubMed] [Google Scholar]
- Baker CL, Kettenbach AN, Loros JJ, Gerber SA, Dunlap JC. Quantitative proteomics reveals a dynamic interactome and phase-specific phosphorylation in the Neurospora circadian clock. Mol Cell. 2009;34:354–363. doi: 10.1016/j.molcel.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballario P, Vittorioso P, Magrelli A, Talora C, Cabibbo A, Macino G. White collar-1, a central regulator of blue light responses in Neurospora, is a zinc finger protein. EMBO J. 1996;15:1650–1657. [PMC free article] [PubMed] [Google Scholar]
- Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belden WJ, Larrondo LF, Froehlich AC, Shi M, Chen CH, Loros JJ, Dunlap JC. The band mutation in Neurospora crassa is a dominant allele of ras-1 implicating RAS signaling in circadian output. Genes Dev. 2007a;21:1494–1505. doi: 10.1101/gad.1551707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belden WJ, Loros JJ, Dunlap JC. Execution of the circadian negative feedback loop in Neurospora requires the ATP-dependent chromatin-remodeling enzyme CLOCK-SWITCH. Mol Cell. 2007b;25:587–600. doi: 10.1016/j.molcel.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Bell-Pedersen D, Garceau N, Loros JJ. Circadian rhythms in fungi. J Genet. 1996a;75:387–401. [Google Scholar]
- Bell-Pedersen D, Shinohara ML, Loros JJ, Dunlap JC. Circadian clock-controlled genes isolated from Neurospora crassa are late night- to early morning-specific. Proc Natl Acad Sci USA. 1996b;93:13096–13101. doi: 10.1073/pnas.93.23.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ. Circadian rhythms from multiple oscillators: Lessons from diverse organisms. Nat Rev Genet. 2005;6:544–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs WR. The LOV domain: A chromophore module servicing multiple photoreceptors. J Biomed Sci. 2007;14:499–504. doi: 10.1007/s11373-007-9162-6. [DOI] [PubMed] [Google Scholar]
- Brody S. Circadian rhythms in Neurospora crassa: The role of mitochondria. Chronobiol Int. 1992;9:222–230. doi: 10.3109/07420529209064531. [DOI] [PubMed] [Google Scholar]
- Brody S, Oelhafen K, Schneider K, Perrino S, Goetz A, Wang C, English C. Circadian rhythms in Neurospora crassa: Downstream effectors. Fungal Genet Biol. 2010;47:159–168. doi: 10.1016/j.fgb.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Brunner M, Kaldi K. Interlocked feedback loops of the circadian clock of Neurospora crassa. Mol Microbiol. 2008;68:255–262. doi: 10.1111/j.1365-2958.2008.06148.x. [DOI] [PubMed] [Google Scholar]
- Campbell JW, Enderlin CS, Selitrennikoff C. Vectors for expression and modification of cDNA sequences in Neurospora crassa. Fungal Genet News. 1994;41:20–21. [Google Scholar]
- Carattoli A, Cogoni C, Morelli G, Macino G. Molecular characterization of upstream regulatory sequences controlling the photoinduced expression of the albino-3 gene of Neurospora crassa. Mol Microbiol. 1994;13:787–795. doi: 10.1111/j.1365-2958.1994.tb00471.x. [DOI] [PubMed] [Google Scholar]
- Castro-Longoria E, Ferry M, Bartnicki-Garcia S, Hasty J, Brody S. Circadian rhythms in Neurospora crassa: Dynamics of the clock component frequency visualized using a fluorescent reporter. Fungal Genet Biol. 2010;47:332–341. doi: 10.1016/j.fgb.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J, Chang SS, Huang G, Cheng P, Liu Y. Control of WHITE COLLAR localization by phosphorylation is a critical step in the circadian negative feedback process. EMBO J. 2008;27:3246–3255. doi: 10.1038/emboj.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J, Yuan H, Liu Y. Regulation of the activity and cellular localization of the circadian clock protein FRQ. J Biol Chem. 2011;286:11469–11478. doi: 10.1074/jbc.M111.219782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Ringelberg CS, Gross RH, Dunlap JC, Loros JJ. Genome-wide analysis of light-inducible responses reveals hierarchical light signalling in Neurospora. EMBO J. 2009;28:1029–1042. doi: 10.1038/emboj.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Dunlap JC, Loros JJ. Neurospora illuminates fungal photoreception. Fungal Genet Biol. 2010;47:922–929. doi: 10.1016/j.fgb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, Yang YH, Heintzen C, Liu Y. Coiled-coil domain-mediated FRQ-FRQ interaction is essential for its circadian clock function in Neurospora. EMBO J. 2001a;20:101–108. doi: 10.1093/emboj/20.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, Yang YH, Liu Y. Interlocked feedback loops contribute to the robustness of the Neurospora circadian clock. Proc Natl Acad Sci USA. 2001b;98:7408–7413. doi: 10.1073/pnas.121170298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, Yang YH, Gardner KH, Liu Y. PAS domain-mediated WC-1/WC-2 interaction is essential for maintaining the steady-state level of WC-1 and the function of both proteins in circadian clock and light responses of Neurospora. Mol Cell Biol. 2002;22:517–524. doi: 10.1128/MCB.22.2.517-524.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, Yang YH, Wang LX, He QY, Liu Y. WHITE COLLAR-1, a multifunctional Neurospora protein involved in the circadian feedback loops, light sensing, and transcription repression of Wc-2. J Biol Chem. 2003;278:3801–3808. doi: 10.1074/jbc.M209592200. [DOI] [PubMed] [Google Scholar]
- Cheng P, He Q, He Q, Wang L, Liu Y. Regulation of the Neurospora circadian clock by an RNA helicase. Genes Dev. 2005;19:234–241. doi: 10.1101/gad.1266805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen MK, Falkeid G, Loros JJ, Dunlap JC, Lillo C, Ruoff P. A nitrate induced frq-less oscillator in Neurospora crassa. J Biol Rhythms. 2004;19:280–286. doi: 10.1177/0748730404265532. [DOI] [PubMed] [Google Scholar]
- Christie JM, Salomon M, Nozue K, Wada M, Briggs WR. LOV (light, oxygen, or voltage) domains of the blue-light photoreceptor phototropin (nph1): Binding sites for the chromophore flavin mononucleotide. Proc Natl Acad Sci USA. 1999;96:8779–8783. doi: 10.1073/pnas.96.15.8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colot HV, Loros JJ, Dunlap JC. Temperature-modulated alternative splicing and promoter use in the circadian clock gene frequency. Mol Biol Cell. 2005;16:5563–5571. doi: 10.1091/mbc.E05-08-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colot HV, Park G, Turner GE, Ringelberg C, Crew CM, Litvinkova L, Weiss RL, Borkovich KA, Dunlap JC. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc Natl Acad Sci USA. 2006;103:10352–10357. doi: 10.1073/pnas.0601456103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa A, Bell-Pedersen D. Distinct signaling pathways from the circadian clock participate in regulation of rhythmic conidiospore development in Neurospora crassa. Eukaryot Cell. 2002;1:273–280. doi: 10.1128/EC.1.2.273-280.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa A, Lewis AZ, Greene AV, March IJ, Gomer RH, Bell-Pedersen D. Multiple oscillators regulate circadian gene expression in Neurospora. Proc Natl Acad Sci USA. 2003;100:13597–13602. doi: 10.1073/pnas.2233734100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosthwaite SK. Circadian clocks and natural antisense RNA. FEBS Lett. 2004;567:49–54. doi: 10.1016/j.febslet.2004.04.073. [DOI] [PubMed] [Google Scholar]
- Crosthwaite SK, Dunlap JC, Loros JJ. Neurospora wc-1 and wc-2: Transcription, photoresponses, and the origins of circadian rhythmicity. Science. 1997;276:763–769. doi: 10.1126/science.276.5313.763. [DOI] [PubMed] [Google Scholar]
- Davis RH. Neurospora: Contributions of a Model Organism. Oxford University Press; Oxford: 2000. [Google Scholar]
- de Paula RM, Lewis ZA, Greene AV, Seo KS, Morgan LW, Vitalini MW, Bennett L, Gomer RH, Bell-Pedersen D. Two circadian timing circuits in Neurospora crassa cells share components and regulate distinct rhythmic processes. J Biol Rhythms. 2006;21:159–168. doi: 10.1177/0748730406288338. [DOI] [PubMed] [Google Scholar]
- de Paula RM, Vitalini MW, Gomer RH, Bell-Pedersen D. Complexity of the Neurospora crassa circadian clock system: Multiple loops and oscillators. Cold Spring Harb Symp Quant Biol. 2007;72:345–351. doi: 10.1101/sqb.2007.72.002. [DOI] [PubMed] [Google Scholar]
- Diernfellner ACR, Schafmeier T, Merrow M, Brunner M. Molecular mechanism of temperature sensing by the circadian clock of Neurospora crassa. Genes Dev. 2005;19:1968–1973. doi: 10.1101/gad.345905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diernfellner A, Colot HV, Dintsis O, Loros JJ, Dunlap JC, Brunner M. Long and short isoforms of Neurospora clock protein FRQ support temperature-compensated circadian rhythms. FEBS Lett. 2007;581:5759–5764. doi: 10.1016/j.febslet.2007.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diernfellner ACR, Querfurth C, Salazar C, Höfer T, Brunner M. Phosphoryla-tion modulates rapid nucleocytoplasmic shuttling and cytoplasmic accumulation of Neurospora clock protein FRQ on a circadian time scale. Genes Dev. 2009;23:2192–2200. doi: 10.1101/gad.538209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, Tang X, Yu Y, Nilsen R, Kim R, Griffith J, Arnold J, Schüttler HB. Systems biology of the clock in Neurospora crassa. PLoS One. 2008;3:e3105. doi: 10.1371/journal.pone.0003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap JC, Loros JJ. How fungi keep time: Circadian system in Neurospora and other fungi. Curr Opin Microbiol. 2006;9:579–587. doi: 10.1016/j.mib.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Elvin M, Loros JJ, Dunlap JC, Heintzen C. The PAS/LOV protein VIVID supports a rapidly dampened daytime oscillator that facilitates entrainment of the Neurospora circadian clock. Genes Dev. 2005;19:2593–2605. doi: 10.1101/gad.349305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JF, Hoyle MN. Isolation of circadian clock mutants of Neurospora crassa. Genetics. 1973;75:606–613. doi: 10.1093/genetics/75.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JF, Gardner GF, Denison R. Genetic analysis of the circadian clock of Neurospora. In: Suda M, Hayaishi O, Nakagawa H, editors. Biological Rhythms and Their Central Mechanism. Elsevier; Amsterdam: 1979. pp. 57–66. [Google Scholar]
- Francis CD, Sargent ML. Effects of temperature perturbations on circadian conidiation in Neurospora. Plant Physiol. 1979;64:1000–1004. doi: 10.1104/pp.64.6.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag M, Hickey PC, Raju NB, Selker EU, Read ND. GFP as a tool to analyze the organization, dynamics and function of nuclei and microtubules in Neurospora crassa. Fungal Genet Biol. 2004;41:897–910. doi: 10.1016/j.fgb.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Froehlich AC, Liu Y, Loros JJ, Dunlap JC. White collar-1, a circadian blue light photoreceptor, binding to the frequency promoter. Science. 2002;297:815–819. doi: 10.1126/science.1073681. [DOI] [PubMed] [Google Scholar]
- Froehlich AC, Loros JJ, Dunlap JC. Rhythmic binding of a WHITE COLLAR-containing complex to the frequency promoter is inhibited by FREQUENCY. Proc Natl Acad Sci USA. 2003;100:5914–5919. doi: 10.1073/pnas.1030057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galagan JE, Calvo SE, Borkovich KA, Selker EU, Read ND, Jaffe D, FitzHugh W, Ma LJ, Smirnov S, Purcell S, et al. The genome sequence of the filamentous fungus Neurospora crassa. Nature. 2003;422:859–868. doi: 10.1038/nature01554. [DOI] [PubMed] [Google Scholar]
- Garceau NY, Liu Y, Loros JJ, Dunlap JC. Alternative initiation of translation and time-specific phosphorylation yield multiple forms of the essential clock protein FREQUENCY. Cell. 1997;89:469–476. doi: 10.1016/s0092-8674(00)80227-5. [DOI] [PubMed] [Google Scholar]
- Gardner GF, Feldman JF. Temperature compensation of circadian period length in clock mutants of Neurospora crassa. Plant Physiol. 1981;68:1244–1248. doi: 10.1104/pp.68.6.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geever RF, Huiet L, Baum JA, Tyler BM, Patel VB, Rutledge BJ, Case ME, Giles NH. DNA sequence, organization and regulation of the qa gene cluster of Neurospora crassa. J Mol Biol. 1989;207:15–34. doi: 10.1016/0022-2836(89)90438-5. [DOI] [PubMed] [Google Scholar]
- Gooch VD, Mehra A, Larrondo LF, Fox J, Touroutoutoudis M, Loros JJ, Dunlap JC. Fully codon-optimized luciferase uncovers novel temperature characteristics of the Neurospora clock. Eukaryot Cell. 2008;7:28–37. doi: 10.1128/EC.00257-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görl M, Merrow M, Huttner B, Johnson J, Roenneberg T, Brunner M. A PEST-like element in FREQUENCY determines the length of the circadian period in Neurospora crassa. EMBO J. 2001;20:7074–7084. doi: 10.1093/emboj/20.24.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granshaw T, Tsukamoto M, Brody S. Circadian rhythms in Neurospora crassa: Farnesol or geraniol allow expression of rhythmicity in the otherwise arrhythmic strains frq10, wc-1, and wc-2. J Biol Rhythms. 2003;18:287–296. doi: 10.1177/0748730403255934. [DOI] [PubMed] [Google Scholar]
- Guo J, Cheng P, Yuan H, Liu Y. The exosome regulates circadian gene expression in a posttranscriptional negative feedback loop. Cell. 2009;138:1236–1246. doi: 10.1016/j.cell.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Cheng P, Liu Y. Functional significance of FRH in regulating the phosphorylation and stability of Neurospora circadian clock protein FRQ. J Biol Chem. 2010;285:11508–11515. doi: 10.1074/jbc.M109.071688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin PE, Hall JC, Rosbash M. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature. 1990;343:536–540. doi: 10.1038/343536a0. [DOI] [PubMed] [Google Scholar]
- Harrisingh MC, Nitabach MN. Circadian rhythms: Integrating circadian timekeeping with cellular physiology. Science. 2008;320:879–880. doi: 10.1126/science.1158619. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Maywood ES, O’Neill JS. Cellular circadian pacemaking and the role of cytosolic rhythms. Curr Biol. 2008;18:R805–R815. doi: 10.1016/j.cub.2008.07.021. [DOI] [PubMed] [Google Scholar]
- He Q, Liu Y. Degradation of the Neurospora circadian clock protein FREQUENCY through the ubiquitin-proteasome pathway. Biochem Soc Trans. 2005a;33:953–956. doi: 10.1042/BST20050953. [DOI] [PubMed] [Google Scholar]
- He Q, Liu Y. Molecular mechanism of light responses in Neurospora: From light-induced transcription to photoadaptation. Genes Dev. 2005b;19:2888–2899. doi: 10.1101/gad.1369605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He QY, Cheng P, Yang YH, Wang LX, Gardner KH, Liu Y. White collar-1, a DNA binding transcription factor and a light sensor. Science. 2002;297:840–843. doi: 10.1126/science.1072795. [DOI] [PubMed] [Google Scholar]
- He Q, Cheng P, Yang YH, He QY, Yu HT, Liu Y. FWD1-mediated degradation of FREQUENCY in Neurospora establishes a conserved mechanism for circadian clock regulation. EMBO J. 2003;22:4421–4430. doi: 10.1093/emboj/cdg425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Cheng P, He Q, Liu Y. The COP9 signalosome regulated the Neurospora circadian clock by controlling the stability of the SCFFWD-1 complex. Genes Dev. 2005a;19:1518–1531. doi: 10.1101/gad.1322205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Shu H, Cheng P, Chen S, Wang L, Liu Y. Light-independent phosphorylation of WHITE COLLAR-1 regulates its function in the Neurospora circadian negative feedback loop. J Biol Chem. 2005b;280:17526–17532. doi: 10.1074/jbc.M414010200. [DOI] [PubMed] [Google Scholar]
- He Q, Cha J, Lee HC, Yang Y, Liu Y. CKI and CKII mediate the FREQUENCY-dependent phosphorylation of the WHITE COLLAR complex to close the Neurospora circadian negative feedback loop. Genes Dev. 2006;20:2552–2565. doi: 10.1101/gad.1463506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzen C, Liu Y. The Neurospora crassa circadian clock. Adv Genet. 2007;58:25–66. doi: 10.1016/S0065-2660(06)58002-2. [DOI] [PubMed] [Google Scholar]
- Heintzen C, Loros JJ, Dunlap JC. The PAS protein VIVID defines a clock-associated feedback loop that represses light input, modulates gating, and regulates clock resetting. Cell. 2001;104:453–464. doi: 10.1016/s0092-8674(01)00232-x. [DOI] [PubMed] [Google Scholar]
- Hong CI, Ruoff P, Loros JJ, Dunlap JC. Closing the circadian negative feedback loop: FRQ-dependent clearance of WC-1 from the nucleus. Genes Dev. 2008;22:3196–3204. doi: 10.1101/gad.1706908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Chen S, Li S, Cha J, Long C, Li L, He Q, Liu Y. Protein kinase A and casein kinases mediate sequential phosphorylation events in the circadian negative feedback loop. Genes Dev. 2007;21:3283–3295. doi: 10.1101/gad.1610207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki H, Dunlap JC. Microbial circadian oscillatory systems in Neurospora and Synechococcus: Models for cellular clocks. Curr Opin Microbiol. 2000;3:189–196. doi: 10.1016/s1369-5274(00)00074-6. [DOI] [PubMed] [Google Scholar]
- Johnson CH, Elliot JA, Foster RG. Entrainment of circadian programs. Chronobiol Int. 2003;20:741–774. doi: 10.1081/cbi-120024211. [DOI] [PubMed] [Google Scholar]
- Kim TS, Logsdon BA, Park S, Mezey JG, Lee K. Quantitative trait loci for the circadian clock in Neurospora crassa. Genetics. 2007;177:2335–2347. doi: 10.1534/genetics.107.077958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci USA. 1971;68:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer C, Loros JJ, Dunlap JC, Crosthwaite SK. Role for antisense RNA in regulating circadian clock function in Neurospora crassa. Nature. 2003;421:948–952. doi: 10.1038/nature01427. [DOI] [PubMed] [Google Scholar]
- Kreps JA, Wu Y, Chang HS, Zhu T, Wang X, Harper JF. Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol. 2002;130:2129–2141. doi: 10.1104/pp.008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakin-Thomas PL. Effects of choline depletion on the circadian rhythm in Neurospora crassa. Biol Rhythm Res. 1996;27:12–30. doi: 10.1177/074873098129000101. [DOI] [PubMed] [Google Scholar]
- Lakin-Thomas PL. Choline depletion, frq mutations, and temperature compensation of the circadian rhythm in Neurospora crassa. J Biol Rhythms. 1998;13:268–277. doi: 10.1177/074873098129000101. [DOI] [PubMed] [Google Scholar]
- Lakin-Thomas PL. Circadian clock genes frequency and white collar-1 are not essential for entrainment to temperature cycles in Neurospora crassa. Proc Natl Acad Sci USA. 2006a;103:4469–4474. doi: 10.1073/pnas.0510404103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakin-Thomas PL. New models for circadian systems in microorganisms. FEMS Microbiol Lett. 2006b;259:1–6. doi: 10.1111/j.1574-6968.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- Lakin-Thomas PL, Brody S. Circadian rhythms in Neurospora crassa: Interactions between clock mutations. Genetics. 1985;109:49–66. doi: 10.1093/genetics/109.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakin-Thomas PL, Brody S. Circadian rhythms in Neurospora crassa: Lipid deficiencies restore robust rhythmicity to null frequency and white-collar mutants. Proc Natl Acad Sci USA. 2000;97:256–261. doi: 10.1073/pnas.97.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakin-Thomas PL, Brody S. Circadian rhythms in microorganisms: New complexities. Annu Rev Microbiol. 2004;58:489–519. doi: 10.1146/annurev.micro.58.030603.123744. [DOI] [PubMed] [Google Scholar]
- Lakin-Thomas PL, Cote GG, Brody S. Circadian rhythms in Neurospora crassa: Biochemistry and genetics. Crit Rev Microbiol. 1990;17:365–416. doi: 10.3109/10408419009114762. [DOI] [PubMed] [Google Scholar]
- Lakin-Thomas PL, Brody S, Cote GG. Amplitude model for the effects of mutations and temperature on period and phase resetting of the Neurospora circadian oscillator. J Biol Rhythms. 1991;6:281–297. doi: 10.1177/074873049100600401. [DOI] [PubMed] [Google Scholar]
- Lakin-Thomas PL, Brody S, Coté GG. Temperature compensation and membrane composition in Neurospora crassa. Chronobiol Int. 1997;14:445–454. doi: 10.3109/07420529709001467. [DOI] [PubMed] [Google Scholar]
- Lambreghts R, Shi M, Belden WJ, DeCaprio D, Park D, Henn MR, Galagan JE, Baştürkmen M, Birren BW, Sachs MS, et al. A high-density single nucleotide polymorphism map for Neurospora crassa. Genetics. 2009;181:767–781. doi: 10.1534/genetics.108.089292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Loros JJ, Dunlap JC. Interconnected feedback loops in the Neurospora circadian system. Science. 2000;289:107–110. doi: 10.1126/science.289.5476.107. [DOI] [PubMed] [Google Scholar]
- Li S, Lakin-Thomas PL. Effects of prd circadian clock mutations on FRQ-less rhythms in Neurospora. J Biol Rhythms. 2010;25:71–80. doi: 10.1177/0748730409360889. [DOI] [PubMed] [Google Scholar]
- Li S, Motavaze K, Kafes E, Suntharalingam S, Lakin-Thomas P. A new mutation affecting FRQ-less rhythms in the circadian system of Neurospora crassa. PLoS Genetics. 2011;7:e1002151. doi: 10.1371/journal.pgen.1002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden H, Macino G. White collar 2, a partner in blue-light signal transduction, controlling expression of light-regulated genes in Neurospora crassa. EMBO J. 1997;16:98–109. doi: 10.1093/emboj/16.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. Molecular mechanisms of entrainment in the Neurospora circadian clock. J Biol Rhythms. 2003;18:195–205. doi: 10.1177/0748730403018003002. [DOI] [PubMed] [Google Scholar]
- Liu Y, Bell-Pedersen D. Circadian rhythms in Neurospora crassa and other filamentous fungi. Eukaryot Cell. 2006;5:1184–1193. doi: 10.1128/EC.00133-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Garceau NY, Loros JJ, Dunlap JC. Thermally regulated translational control of FRQ mediates aspects of temperature responses in the Neurospora circadian clock. Cell. 1997;89:477–486. doi: 10.1016/s0092-8674(00)80228-7. [DOI] [PubMed] [Google Scholar]
- Liu Y, Loros J, Dunlap JC. Phosphorylation of the Neurospora clock protein FREQUENCY determines its degradation rate and strongly influences the period length of the circadian clock. Proc Natl Acad Sci USA. 2000;97:234–239. doi: 10.1073/pnas.97.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, He Q, Cheng P. Photoreception in Neurospora: A tale of two White Collar proteins. Cell Mol Life Sci. 2003;60:2131–2138. doi: 10.1007/s00018-003-3109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi L, Schneider K, Tsukamoto M, Brody S. Circadian rhythms in Neurospora crassa: Clock mutant effects in the absence of a frq-based oscillator. Genetics. 2007;175:1175–1183. doi: 10.1534/genetics.106.068270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loros JJ, Dunlap JC. Genetic and molecular analysis of circadian rhythms in Neurospora. Annu Rev Physiol. 2001;63:757–794. doi: 10.1146/annurev.physiol.63.1.757. [DOI] [PubMed] [Google Scholar]
- Loros JJ, Feldman JF. Loss of temperature compensation of circadian period length in the frq-9 mutant of Neurospora crassa. J Biol Rhythms. 1986;1:187–198. doi: 10.1177/074873048600100302. [DOI] [PubMed] [Google Scholar]
- Loros JJ, Denome SA, Dunlap JC. Molecular cloning of genes under control of the circadian clock in Neurospora. Science. 1989;243:385–388. doi: 10.1126/science.2563175. [DOI] [PubMed] [Google Scholar]
- Luo CH, Loros JJ, Dunlap JC. Nuclear localization is required for function of the essential clock protein FRQ. EMBO J. 1998;17:1228–1235. doi: 10.1093/emboj/17.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattern DL, Forman LR, Brody S. Circadian rhythms in Neurospora crassa: A mutation affecting temperature compensation. Proc Natl Acad Sci USA. 1982;79:825–829. doi: 10.1073/pnas.79.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CR, Fox BA, Dunlap JC. The Neurospora clock gene frequency shares a sequence element with the Drosophila clock gene period. Nature. 1989;339:558–562. doi: 10.1038/339558a0. [DOI] [PubMed] [Google Scholar]
- McCluskey K. The Fungal Genetics Stock Center: From molds to molecules. Adv Appl Microbiol. 2003;52:245–262. doi: 10.1016/s0065-2164(03)01010-4. [DOI] [PubMed] [Google Scholar]
- Mehra A, Shi M, Baker CL, Colot HV, Loros JJ, Dunlap JC. A role for casein kinase 2 in the mechanism underlying circadian temperature compensation. Cell. 2009;137:749–760. doi: 10.1016/j.cell.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrow M, Brunner M, Roenneberg T. Assignment of circadian function for the Neurospora clock gene frequency. Nature. 1999;399:584–586. doi: 10.1038/21190. [DOI] [PubMed] [Google Scholar]
- Merrow M, Franchi L, Dragovic Z, Gorl M, Johnson J, Brunner M, Macino G, Roenneberg T. Circadian regulation of the light input pathway in Neurospora crassa. EMBO J. 2001;20:307–315. doi: 10.1093/emboj/20.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael TP, Park S, Kim TS, Booth J, Byer A, Sun Q, Chory J, Lee K. Simple sequence repeats provide a substrate for phenotypic variation in the Neurospora crassa circadian clock. PLoS One. 2007;8:e795. doi: 10.1371/journal.pone.0000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan LW, Feldman JF. Epistatic and synergistic interactions between circadian clock mutations in Neurospora crassa. Genetics. 2001;159:537–543. doi: 10.1093/genetics/159.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan LW, Feldman JF, Bell-Pedersen D. Genetic interactions between clock mutations in Neurospora crassa: Can they help us to understand complexity? Philos Trans R Soc Lond B Biol Sci. 2001;356:1717–1724. doi: 10.1098/rstb.2001.0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima H. A liquid culture method for the biochemical analysis of the circadian clock of Neurospora crassa. Plant Cell Physiol. 1981;22:231–238. [Google Scholar]
- Nowrousian M, Duffield GE, Loros J, Dunlap J. The frequency gene is required for temperature-dependent regulation of many clock-controlled genes in Neurospora crassa. Genetics. 2003;164:922–933. doi: 10.1093/genetics/164.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmedo M, Navarro-Sampedro L, Ruger-Herreros C, Kim SR, Jeong BK, Lee BU, Corrochano LM. A role in the regulation of transcription by light for RCO-1 and RCM-1, the Neurospora homologs of the yeast Tup1-Ssn6 repressor. Fungal Genet Biol. 2010a;47:939–952. doi: 10.1016/j.fgb.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Olmedo M, Ruger-Herreros C, Corrochano LM. Regulation by blue light of the fluffy gene encoding a major regulator of conidiation in Neurospora crassa. Genetics. 2010b;184:651–658. doi: 10.1534/genetics.109.109975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DD, Davis RH. Neurospora at the millennium. Fungal Genet Biol. 2000;31:153–167. doi: 10.1006/fgbi.2000.1248. [DOI] [PubMed] [Google Scholar]
- Perkins DD, Radford A, Sachs MS. Chromosomal Loci. Academic Press; San Diego: 2001. The Neurospora Compendium. [Google Scholar]
- Pittendrigh CS, Bruce VG, Rosensweig NS, Rubin ML. A biological clock in Neurospora. Nature. 1959;184:169–170. [Google Scholar]
- Pregueiro AM, Price-Lloyd N, Bell-Pedersen D, Heintzen C, Loros J, Dunlap J. Assignment of an essential role for the Neurospora frequency gene in circadian entrainment to temperature cycles. Proc Natl Acad Sci USA. 2005;102:2210–2215. doi: 10.1073/pnas.0406506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pregueiro AM, Liu QY, Baker CL, Dunlap JC, Loros JJ. The Neurospora checkpoint kinase 2: A regulatory link between the circadian and cell cycles. Science. 2006;313:644–649. doi: 10.1126/science.1121716. [DOI] [PubMed] [Google Scholar]
- Price-Lloyd N, Elvin M, Heintzen C. Synchronizing the Neurospora crassa circadian clock with the rhythmic environment. Biochem Soc Trans. 2005;33:949–952. doi: 10.1042/BST20050949. [DOI] [PubMed] [Google Scholar]
- Ramsdale M, Lakin-Thomas PL. sn-1,2-diacylglycerol levels in the fungus Neurospora crassa display circadian rhythmicity. J Biol Chem. 2000;275:27541–27550. doi: 10.1074/jbc.M002911200. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Merrow M. Molecular circadian oscillators: An alternative hypothesis. J Biol Rhythms. 1998;13:167–179. doi: 10.1177/074873098129000011. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Dragovic Z, Merrow M. Demasking biological oscillators: Properties and principles of entrainment exemplified by the Neurospora circadian clock. Proc Natl Acad Sci USA. 2005;102:7742–7747. doi: 10.1073/pnas.0501884102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoff P, Loros JJ, Dunlap JC. The relationship between FRQ-protein stability and temperature compensation in the Neurospora circadian clock. Proc Natl Acad Sci USA. 2005;102:17681–17686. doi: 10.1073/pnas.0505137102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar G, Sancar C, Brunner M, Schafmeier T. Activity of the circadian transcription factor White Collar Complex is modulated by phosphorylation of SP-motifs. FEBS Lett. 2009;583:1833–1840. doi: 10.1016/j.febslet.2009.04.042. [DOI] [PubMed] [Google Scholar]
- Sargent ML, Briggs WR. The effects of light on a circadian rhythm of conidiation in Neurospora. Plant Physiol. 1967;42:1504–1510. doi: 10.1104/pp.42.11.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent ML, Kaltenborn SH. Effects of medium composition and carbon dioxide on circadian conidiation in Neurospora. Plant Physiol. 1972;50:171–175. doi: 10.1104/pp.50.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent ML, Briggs WR, Woodward DO. Circadian nature of a rhythm expressed by an invertaseless strain of Neurospora crassa. Plant Physiol. 1966;41:1343–1349. doi: 10.1104/pp.41.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafmeier T, Haase A, Káldi K, Scholz J, Fuchs M, Brunner M. Transcriptional feedback of Neurospora circadian clock gene by phosphorylation-dependent inactivation of its transcription factor. Cell. 2005;122:1–12. doi: 10.1016/j.cell.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Schafmeier T, Kaldi K, Diernfellner A, Mohr C, Brunner M. Phosphorylation-dependent maturation of Neurospora circadian clock protein from a nuclear repressor toward a cytoplasmic activator. Genes Dev. 2006;20:297–306. doi: 10.1101/gad.360906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafmeier T, Diernfellner A, Schäfer A, Dintsis O, Neiss A, Brunner M. Circadian activity and abundance rhythms of the Neurospora clock transcription factor WCC associated with rapid nucleo-cytoplasmic shuttling. Genes Dev. 2008;22:3397–3402. doi: 10.1101/gad.507408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K, Perrino S, Oelhafen K, Li S, Zatsepin A, Lakin-Thomas PL, Brody S. Rhythmic conidiation in constant light in Vivid mutants of Neurospora crassa. Genetics. 2009;181:917–931. doi: 10.1534/genetics.108.097808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwerdtfeger C, Linden H. Localization and light-dependent phosphorylation of white collar 1 and 2, the two central components of blue light signaling in Neurospora crassa. Eur J Biochem. 2000;267:414–421. doi: 10.1046/j.1432-1327.2000.01016.x. [DOI] [PubMed] [Google Scholar]
- Schwerdtfeger C, Linden H. VIVID is a flavoprotein and serves as a fungal blue light photoreceptor for photoadaptation. EMBO J. 2003;22:4846–4855. doi: 10.1093/emboj/cdg451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J, Brody S. Circadian rhythms in Neurospora: A new measurement, the reset zone. J Biol Rhythms. 2000;15:225–240. doi: 10.1177/074873040001500304. [DOI] [PubMed] [Google Scholar]
- Shi M, Larrondo LF, Loros JJ, Dunlap JC. A developmental cycle masks output from the circadian oscillator under conditions of choline deficiency in Neurospora. Proc Natl Acad Sci USA. 2007;104:20102–20107. doi: 10.1073/pnas.0706631104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Collett M, Loros JJ, Dunlap JC. FRQ-interacting RNA helicase mediates negative and positive feedback in the Neurospora circadian clock. Genetics. 2010;184:351–361. doi: 10.1534/genetics.109.111393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara ML, Correa A, Bell-Pedersen D, Dunlap JC, Loros JJ. Neurospora clock-controlled gene 9 (ccg-9) encodes trehalose synthase: Circadian regulation of stress responses and development. Eukaryot Cell. 2002;1:33–43. doi: 10.1128/EC.1.1.33-43.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrode LB, Lewis ZA, White LD, Bell-Pedersen D, Ebbole DJ. vvd is required for light adaptation of conidiation-specific genes of Neurospora crassa, but not circadian conidiation. Fungal Genet Biol. 2001;32:169–181. doi: 10.1006/fgbi.2001.1264. [DOI] [PubMed] [Google Scholar]
- Smith KM, Sancar G, Dekhang R, Sullivan CM, Li S, Tag AG, Sancar C, Bredeweg EL, Priest HD, McCormick RF, et al. Transcription factors in light and circadian clock signaling networks revealed by genomewide mapping of direct targets for Neurospora white collar complex. Eukaryot Cell. 2010;9:1549–1556. doi: 10.1128/EC.00154-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer ML. Genetic control of fungal differentiation: The three sporulation pathways of Neurospora crassa. Bioessays. 1993;15:365–374. doi: 10.1002/bies.950150602. [DOI] [PubMed] [Google Scholar]
- Talora C, Franchi L, Linden H, Ballario P, Macino G. Role of a white collar-1-white collar-2 complex in blue-light signal transduction. EMBO J. 1999;18:4961–4968. doi: 10.1093/emboj/18.18.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CT, Li S, Long C, Cha J, Huang G, Li L, Chen S, Liu Y. Setting the pace of the Neurospora circadian clock by multiple independent FRQ phosphorylation events. Proc Natl Acad Sci USA. 2009;106:10722–10727. doi: 10.1073/pnas.0904898106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitalini MW, De Paula RM, Goldsmith CS, Jones CA, Borkovich KA, Bell-Pedersen D. Circadian rhythmicity mediated by temporal regulation of the activity of p38 MAPK. Proc Natl Acad Sci USA. 2007;104:18223–18228. doi: 10.1073/pnas.0704900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitalini MW, Dunlap J, Heintzen C, Liu Y, Loros J, Bell-Pedersen D. Circadian rhythms. In: Borkovich KA, Ebbole DJ, editors. Cellular and Molecular Biology of Filamentous Fungi. ASM Press; Washington, DC: 2010. pp. 442–466. [Google Scholar]
- Wang J, Hu Q, Chen H, Zhou Z, Li W, Wang Y, Li S, He Q. Role of individual subunits of the Neurospora crassa CSN complex in regulation of deneddylation and stability of cullin proteins. PLoS Genet. 2010;6:1–15. doi: 10.1371/journal.pgen.1001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro CT, Ebbole DJ, Lee BUK, Brown RE, Bourland C, Madi L, Yanofsky C. Characterization of rco-1 of Neurospora crassa, a pleiotropic gene affecting growth and development that encodes a homolog of Tup1 of Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:6218–6228. doi: 10.1128/mcb.16.11.6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K, Shiozawa A, Watanabe S, Fukumori F, Kimura M, Fujimura M. ATF-1 transcription factor regulates the expression of ccg-1 and cat-1 genes in response to fludioxonil under OS-2 MAP kinase in Neurospora crassa. Fungal Genet Biol. 2008;45:1562–1569. doi: 10.1016/j.fgb.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Yang YH, Cheng P, Zhi G, Liu Y. Identification of a calcium/calmodulin-dependent protein kinase that phosphorylates the Neurospora circadian clock protein FREQUENCY. J Biol Chem. 2001;276:41064–41072. doi: 10.1074/jbc.M106905200. [DOI] [PubMed] [Google Scholar]
- Yang YH, Cheng P, Liu Y. Regulation of the Neurospora circadian clock by casein kinase II. Genes Dev. 2002;16:994–1006. doi: 10.1101/gad.965102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Cheng P, He Q, Wang L, Liu Y. Phosphorylation of FREQUENCY protein by casein kinase II is necessary for the function of the Neurospora circadian clock. Mol Cell Biol. 2003;23:6221–6228. doi: 10.1128/MCB.23.17.6221-6228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, He Q, Cheng P, Wrage P, Yarden O, Liu Y. Distinct roles for PP1 and PP2A in the Neurospora circadian clock. Genes Dev. 2004;18:255–260. doi: 10.1101/gad.1152604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Maeda T, Lee B, Hasunuma K. Conidiation rhythm and light entrainment in superoxide dismutase mutant in Neurospora crassa. Mol Genet Genomics. 2008;279:193–202. doi: 10.1007/s00438-007-0308-z. [DOI] [PubMed] [Google Scholar]
- Zhu H, Nowrousian M, Kupfer D, Colot HV, Berrocal-Tito G, Lai HS, Bell-Pedersen D, Roe BA, Loros JJ, Dunlap JC. Analysis of expressed sequence tags from two starvation, time- of-day-specific libraries of Neurospora crassa reveals novel clock-controlled genes. Genetics. 2001;157:1057–1065. doi: 10.1093/genetics/157.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv C, Yarden O. Gene silencing for functional analysis: Assessing RNAi as a tool for manipulation of gene expression. Methods Mol Biol. 2010;638:77–100. doi: 10.1007/978-1-60761-611-5_6. [DOI] [PubMed] [Google Scholar]