Abstract
Smith-Lemli-Opitz syndrome (SLOS) is an autosomal recessive, multiple malformation syndrome with neurocognitive impairment. SLOS arises from mutations in the 7-dehydrocholesterol reductase gene which results in impaired enzymatic conversion of 7-dehydrocholesterol to cholesterol. In the current work, we sought to measure proteins that were altered in the cerebrospinal fluid from SLOS patients compared to pediatric controls. Using a multi-analyte antibody-based assay, we found that 12 proteins are altered in SLOS patients. Validation studies were carried out and the findings from this study suggest alterations in extracellular matrix remodeling and further evidence of oxidative stress within the disease pathophysiology. The results of this study will be used to explore biological pathways altered in SLOS and identifies a set of cerebral spinal fluid proteins that can be evaluated as biomarkers in future therapeutic trials.
Introduction
Smith-Lemli-Opitz syndrome (SLOS) is an autosomal recessive multiple malformation syndrome with cognitive impairment caused by an inborn error in cholesterol synthesis. SLOS results from mutations in the 7-dehydrocholesterol reductase (DHCR7) gene, which encodes the enzyme that catalyzes the reduction of 7-dehydrocholesterol (7-DHC) to cholesterol (1-3). A recent report by Cross et al. (4) indicates the carrier frequency of pathogenic alleles in the general population to be on the order of 1% and predicts a disease incidence on the order of 1 in 40,000 which is consistent with prior clinical studies (5, 6). The SLOS phenotypic spectrum is very wide. Clinical signs and symptoms range from mild behavioral and learning deficits in combination with minor physical anomalies to a lethal disorder with multiple major congenital anomalies. Cutaneous syndactyly of the second and third toes is the most common reported malformation (7). Individuals with SLOS frequently have atypical facial appearance that includes ptosis, a small upturned nose, and micrognathia (8). Biochemically, SLOS is characterized by elevated serum levels of 7-DHC and the isomer, 8-dehydrocholesterol (8-DHC), as well as low to normal serum cholesterol levels (9).
The pathological mechanisms connecting the sterol abnormality to the phenotypic manifestations of SLOS have not been fully elucidated, likely in part due to the diverse biological functions of cholesterol. Cholesterol is an integral component of lipid rafts from which signal transduction pathways are initiated and is also a precursor of bile acids, oxysterols, and steroid hormones (9). Besides the impact of cholesterol deficiency in SLOS, elevated levels of 7-DHC and 7-DHC oxidation products appear to have toxic effects (10-13). A recent antioxidant study utilizing a mixture with Vitamin E as the active component normalized oxysterol levels in SLOS patient fibroblast cultures and neuronal cultures from mouse models (13, 14). Collectively the data indicates that a combination of decreased cholesterol levels, decreased sterol levels, and increased levels of 7-DHC and its oxidized derivatives collectively contribute to the pathology of SLOS.
Anecdotal reports suggest some therapeutic benefit of dietary cholesterol supplementation as reviewed (15), but a short-term, placebo-controlled trial was not able to demonstrate beneficial effects on behavior (16). Cholesterol does not cross the blood-brain barrier, thus limiting the potential of dietary cholesterol supplementation to treat the cognitive and behavioral abnormalities observed in SLOS patients. Although behavioral and cognitive problems arising due to the altered sterol biochemistry might be amenable to treatment, there are no effective therapies to modulate central nervous system sterol composition to date. Due to the limited number of subjects, challenges arise in conducting adequately powered clinical trials for rare disorders such as SLOS. The development of a series of disease-relevant biomarkers could provide insight into the pathology of SLOS and provide a tool to evaluate potential efficacy of experimental interventions. In this paper we utilize multi-analyte profiling of cerebral spinal fluid to identify a set of proteins that may be useful for monitoring disease status in clinical trials and further our understanding of SLOS pathology.
Materials and Methods
Clinical samples
SLOS subjects included in this study were enrolled in clinical studies (98-CH-0081 or 03-CH-0225) which were approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Institutional Review Board. Informed consent was obtained from parents or guardians. A diagnosis of SLOS was established by biochemical and molecular confirmation of the clinical diagnosis. Control cerebral spinal fluid (CSF) was obtained from gender- and age-matched patients who were undergoing CSF collection for another clinical indication. Adult control CSF samples were obtained from BioChemed Services (Winchester, VA). All CSF aliquots were stored at −80°C until use. Severity scores were assigned based on malformation analysis as previously reported (6). Two groups of SLOS subject samples were used including an initial group of 20 SLOS subject samples followed by a validation group of 15 SLOS patients. Samples from the same subjects, with different collection times were used as well as some samples unique to each group. The demographics, severity score, and sterol levels for the SLOS subject samples analyzed are listed in Supplemental Data Table 1.
Cerebral Spinal Fluid Analyte Measurements
CSF samples were analyzed by Rules-Based Medicine (RBM), Inc (Austin, TX) using the Human Discovery Multi-Analyte Profile (MAP) panel. A total of 20 SLOS patient samples and 30 pediatric control CSF samples were evaluated. Statistical analysis of analyte levels was performed as described previously (17). Briefly, measurements were log10 transformed to achieve normal distribution. For analytes with less than 40% of the measurements reported as below the limit of detection (LOD), the missing values were inputted as LOD/2 (18). An unpaired t-test (p<0.05) was used to compare patient and control CSF samples using GraphPad Prism (version 5). Correlation analysis was carried out with GraphPad Prism using the raw data and Spearman (nonparametric) analysis.
Western Blots
Western blot analysis was carried out using 15 μL of CSF per patient and prepared according to the manufacturer’s suggested protocol (Invitrogen, Carlsbad, CA). Proteins were separated on NuPAGE Novex 4-12% Bis-Tris gels. Following separation, proteins were transferred to nitrocellulose membranes using the iBlot Dry Transfer System according to the manufacturer’s instructions. Membranes were blocked overnight at 4°C using the WesternBreeze blocking reagents (Invitrogen #WB7050) and then incubated with Rabbit Anti-CTGF antibody (1:4000, Abcam #ab6992) overnight at 4°C. Membranes were washed with WesternBreeze wash buffer and incubated with HRP-linked Goat Anti-Rabbit secondary antibody (1:10000, Sigma-Aldrich, Saint Louis, MO, Item #A6154) for 1 hour at room temperature. Blots were developed using Clarity Western ECL Substrate (Bio-Rad, Hercules, CA, Item #170-5060). Band intensities were quantified (ChemiDoc Image Lab software v. 4.1, Bio-Rad, Hercules, CA) and normalized to the mean intensity of bands from the control patients.
Gelatin Zymography
For gelatin zymography, 25 μL of CSF or 15 μL of serum (diluted 1:15) was mixed with 2x Laemmli buffer (125 mM Tris-HCl pH 6.8), 20% (v/v) glycerol, 4% (w/v) SDS, 0.005% (w/v) bromophenol blue). Non-reduced, non-thermally denatured samples were separated on 10% precast polyacrylamide gels with gelatin (Bio-Rad, Hercules, CA Item: #161-1167) in 1x Tris/SDS/Glycine running buffer for ~2.5 hours at 120 V on ice. Gels were incubated in renaturing buffer (2.5% Triton X-100) at room temperature for 30 minutes, then the incubation was repeated. After rinsing five times with water, gels were incubated in renaturation buffer (Tris 50 mM, NaCl 200mM, CaCl2 5mM, pH 7.6) for 20 hours at 37°C. Gels were fixed for one hour in 30% methanol/7.5% acetic acid and incubated in Coomassie Blue R-250 staining solution (Bio-Rad, Hercules, CA, Item: #161-0436) for 15 minutes at room temperature. Gels were de-stained at room temperature in 30% methanol/7.5% acetic acid for 10 hours and imaged several times throughout the de-staining process with the ChemiDoc System (Bio-Rad, Hercules, CA). Band intensities representing the protease activity of the MMP isoforms were quantified as described above.
Enzyme-Linked Immunosorbent Assays (ELISAs)
Concentrations of MMP-2, TIMP-1, TIMP-2, and MCP-1 in CSF were measured using ELISA kits from R&D Systems according to the manufacturer’s instructions (Product #DMP2F0, #DTM100, #DTM200, #DCP00, respectively). S100B levels in CSF were measured using an ELISA kit from BioVendor R&D (Product #RD192090100R). Samples were run in triplicate for MMP-2, TIMP-1, TIMP-2 and diluted 1:2, 1:25, 1:50, respectively. Samples were run in duplicate for MCP-1 and diluted 1:5. Undiluted samples were run in duplicate for S100B. Both the pro- and mature forms of MMP-2 were detected, although active MMP-2 complexes were not detected.
Results
Multiplex immunoassay data analysis
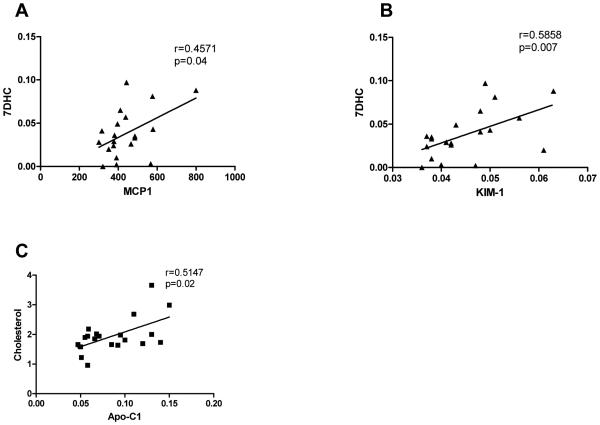
Our initial analysis of potential SLOS protein biomarkers in CSF included the quantitative measurements of 70 analytes in SLOS subjects and controls (Supplemental Data Table 2). From the 20 SLOS patient samples analyzed in the discovery portion of the study, the average age was 7.7 years and the gender breakdown was 13 males and 7 females. CSF DHC/total sterol ratio ranged from 0.3 to 13.6 percent. The analytes measured in this discovery assay included inflammatory markers, chemokines, cytokines, hormones, and growth factors. Of the 70 analytes that were measured, 14 were below the LOD for >40% of the samples and were excluded from further analysis. A list of these 14 analytes is provided (Supplemental Table 3). Of the 56 remaining analytes, 12 analytes demonstrated significant differences between the control and the SLOS patient samples (Table 1). These 12 candidate protein biomarkers were evaluated to determine if a correlation existed between protein concentration and disease severity, cholesterol, or 7-DHC concentrations in the CSF. Only three analytes had significant correlations; monocyte chemoattractant protein-1 vs. 7-DHC (p=0.04), kidney injury molecule-1 vs. 7-DHC (p=0.007) and apolipoprotein c-1 vs. cholesterol (p=0.02). These three correlation plots including Spearman’s correlation (r) values are provided in Figure 1.
Table 1.
List of altered proteins in SLOS patient CSF compared to control
| Analyte | Log10 concentration mean ±SD | P-value | |
|---|---|---|---|
| Control | SLOS | ||
| monocyte chemoattractant protein-1 (pg/ml) | 2.82±0.21 (n=30) | 2.63±0.11 (n=20) | 0.0001 |
| matrix metalloproteinase-2 (ng/ml) | 0.158±0.15 (n=30) | 0.602±0.435 (n=20) | 0.0002 |
| connective tissue growth factor (ng/ml) | 0.225±0.125 (n=24) | 0.350±0.11 (n=20) | 0.001 |
| calbindin (ng/ml) | 1.95±0.24 (n=25) | 2.17±0.17 (n=20) | 0.001 |
| macrophage inflammatory protein-1-β(pg/ml) | 1.20±0.35 (n=30) | 0.953±0.15 (n=19) | 0.001 |
| kidney injury molecule-1 (ng/ml) | −1.48±0.20 (n=27) | −1.35±0.07 (n=20) | 0.004 |
| serum glutamic oxaloacetic transaminase (ug/ml) | −0.361±0.15 (n=30) | −0.244±0.155 (n=20) | 0.009 |
| S100-β (ng/ml) | −0.152±0.320 (n=30) | 0.048±0.21 (n=20) | 0.02 |
| apolipoprotein C-1 (ng/ml) | −1.24±0.2B (n=30) | −1.09±0.17 (n=20) | 0.02 |
| superoxide dismutase-1 (ng/ml) | 1.81 ±0.28 (n=30) | 1.95±0.14 (n=20) | 0.03 |
| transferrin (mg/dl) | −0.0242±0.258 (n=30) | 0.0999±0.13 (n=20) | 0.03 |
| transthyretin (mg/dl) | 0.136±0.120 (n=27) | 0.073±O.OB4 (n=20) | 0.05 |
Figure 1.
Spearman’s correlation analysis of CSF candidate proteins and patient demographic information. Positive correlations were observed for CSF 7DHC vs. MCP-1 concentration (A), 7DHC vs. kidney injury molecule-1 (KIM-1) concentration (B) and Cholesterol vs. Apo-C1 (C).
Of the 12 analytes with differential CSF expression in SLOS, a subset of these proteins – monocyte chemoattractant protein-1 (MCP-1), matrix metalloproteinase-2 (MMP-2), connective tissue growth factor (CTGF), and S100B are commonly associated with extracellular matrix (ECM) turnover and brain injury (19-22). Elevated levels of MMP-2, CTGF, and S100B and decreased levels of MCP-1 were observed in SLOS patients relative to the control group. We thus elected to further explore these proteins in a validation set of CSF.
MCP-1
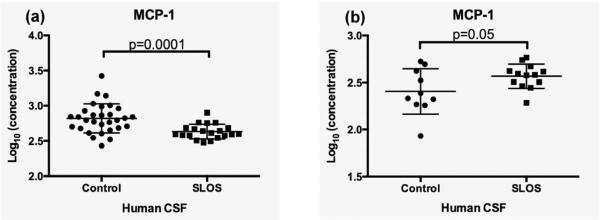
Monocyte chemoattractant protein-1 (MCP-1/CCL2) is a chemokine that is produced by multiple cell types following stimulation by cytokines, growth factors, or oxidative stress (21). MCP-1 is produced by glia as well as neurons, and functions to regulate the migration of monocytes, memory T cells, natural killer cells and microglia to areas of inflammation (20, 22). Semple et al. (23) observed elevated MCP-1 levels in patients suffering from traumatic brain injury and confirmed a functional role for MCP-1 in modulating brain damage. MCP-1/CCL2 deficient mice showed altered cerebral cytokine levels, reduction in macrophage/microglia accumulation, and astrogliosis that led to improved recovery rates following brain injury in these mice (23). We observed a decrease of MCP-1 in SLOS CSF compared to control CSF (Log10 transformed concentrations (pg/ml) for control: 2.82 ± 0.21, and SLOS: 2.6 3± 0.11; p=0.0001 (Table 1, Figure 2a). We sought to validate this observation by an independent ELISA using age- and gender-matched controls. Contrary to the initial results obtained by RBM we noted a trend toward elevated MCP-1 levels in SLOS patient CSF relative to control (Figure 2b). We performed a cell migration assay using human monocytic cells, THP1. Following exposure to media only, control CSF, or SLOS patient CSF, no difference in migratory cell number was observed between the groups (Supplemental Data Figure 1).
Figure 2.
Monocyte chemoattractant protein-1 levels in the CSF of SLOS patients. MCP-1 levels were measured (a) by a multiplex immunoassay and (b) by an independent ELISA. Results of the multiplex immunoassay showed a decreased amount of MCP-1 in SLOS CSF relative to control CSF. Measurements by ELISA in SLOS subjects (n=13) using age- and gender-matched controls (n=10) showed increased levels of MCP-1 in SLOS CSF relative to control CSF. Error bars represent +/− S.D. from the mean.
MMPs and TIMPs
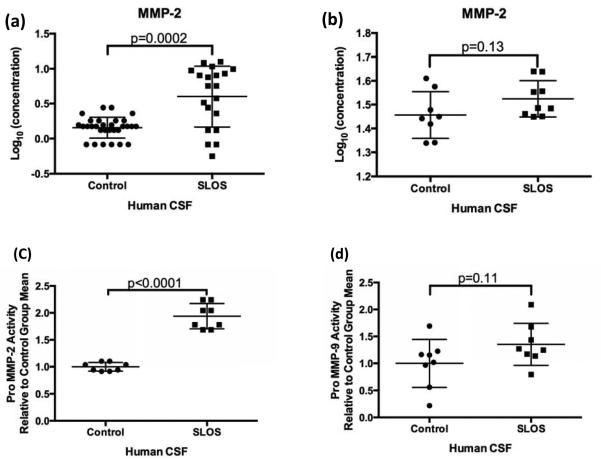
The family of matrix metalloproteinases (MMPs) consists of more than 20 zinc-dependent proteases that degrade components of the ECM and basement membranes. The family is divided into four subgroups: collagenases, gelatinases, stromelysins, and membrane-type MMPs (22). MMPs are secreted as inactive zymogens, which are then cleaved into active forms that regulate a variety of biological activities such as tissue remodeling, apoptosis, synaptic plasticity, angiogenesis, neurogenesis, and inflammation (22, 24). Gelatinase A (MMP-2) and gelatinase B (MMP-9) have been intensively studied in the brain due to their prominent role in injury and repair (22). Analysis of the multiplex immunoassay data showed that MMP-2 levels are significantly elevated in CSF from SLOS subjects when compared to controls (Log10 concentration (ng/ml): control 0.158 ± 0.15; SLOS: 0.602 ± 0.44, p=0.0002) (Table 1, Figure 3a). We observed a similar trend in MMP-2 levels using an independent ELISA, (Log10 concentration (ng/ml): control 1.46 ± 0.1; SLOS 1.52 ± 0.08 (Figure 3b).
Figure 3.
MMP-2 levels in CSF of SLOS patients. Assessment of MMP-2 levels by (a) a multiplex immunoassay and (b) an independent ELISA with age- and gender- matched controls (n=9) showed increased MMP-2 levels in the CSF of SLOS patients relative to control. Error bars represent +/− S.D. from the mean. Quantification of gelatinolytic activity (c and d) revealed a 2-fold increase in proMMP-2 activity and a trend in increased proMMP-9 activity in SLOS CSF compared to control CSF.
To further investigate the potential differences in the expression of MMP-2 in SLOS CSF, gelatin zymography was used to examine the relative amounts of pro- and active MMP-2. We also extended these studies to evaluate MMP-9, a closely associated family member, which was excluded from analysis of the MAP data due to the majority of the samples being below the LOD. For these studies, we used control and SLOS CSF. Strong protease bands at 92 kDa and 72 kDa indicated the presence of proMMP-9 and proMMP-2, respectively in all samples. Quantification of band intensities showed a significant 2-fold elevation of pro MMP-2 (72kDa) activity (Figure 3c, p<0.0001) and no significant difference was observed for proMMP-9 (92kDa) activity in SLOS CSF (p=0.11) (Figure 3d). Most of our samples showed no quantifiable active MMP-2 band, although faint bands at 63 kDa indicated the presence of some active MMP-2 in the SLOS subjects. Active MMP-9 bands at 92 kDa were also faintly visible in all patient samples. MMP-9 levels in CSF were undetectable by ELISA (data not shown). No changes in MMP-2 or MMP-9 gelatinolytic activity were observed in the serum of SLOS patients compared to control patients (Supplemental Data Figure 2).
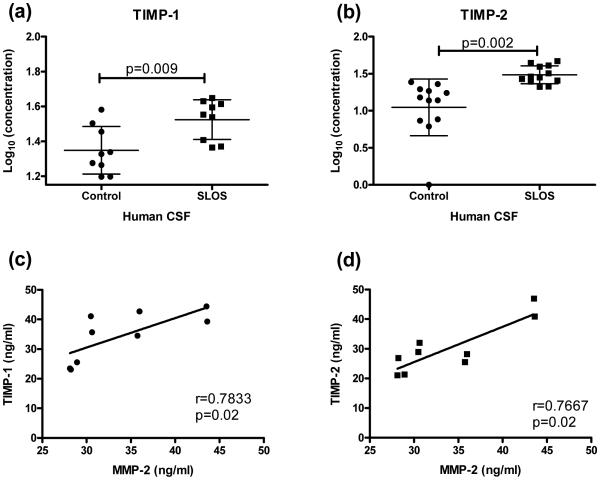
In addition to analyzing MMP-2 and MMP-9 levels, we examined if levels of their inhibitors, TIMP-2 and TIMP-1 respectively, were altered. TIMP-1 and TIMP-2 levels were measured in SLOS CSF and control CSF by ELISA (Figure 4). Both TIMP-1 (Log10 (ng/mL) of control: 1.35 ± 0.14, SLOS: 1.52± 0.11; p=0.01) and TIMP-2 (Log10 (ng/mL) of control: 1.05 ± 0.38, SLOS: 1.49± 0.12; p=0.002) concentrations were significantly elevated in SLOS patients (Figures 4a and 4b). Additionally, TIMP-1 (p=0.02) and TIMP-2 (p=0.02) levels had a positive correlation with MMP-2 levels (Figure 4c).
Figure 4.
TIMP-1 and TIMP-2 levels in CSF of SLOS patients. Concentrations of (a) TIMP-1 (n=9) and (b) TIMP-2 (n=12) were assessed by ELISA. Both were significantly elevated in SLOS CSF compared to age- and gender-matched controls. (c) TIMP-1 and TIMP-2 levels positively correlated with MMP-2 levels in CSF of SLOS patients. Error bars represent +/− S.D. from the mean.
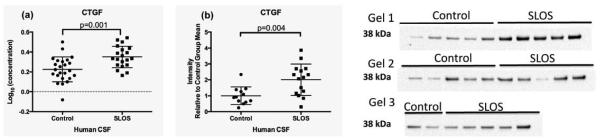
Connective Tissue Growth Factor (CTGF)
CTGF is a cysteine-rich 36-38 kDa protein secreted by multiple cell types (19). In the CNS, CTGF appears to be an important mediator of TGF-beta function in response to brain injury and inflammation (19). Our analysis of data from the multiplex immunoassays revealed a significant increase in CTGF levels in SLOS CSF compared to control CSF (control: Log10 0.2252 ng/ml ±0.03, SLOS: Log10 0.3502 ng/ml ±0.02; p=0.001) (Table 1, Figure 5a). We validated this finding in CSF by Western blot analysis (control n=12, SLOS n=15; p=0.004) (Figure 5b). While levels of CTGF positively correlate with MMP-2 levels in exfoliative glaucoma (25), we did not observe any correlation in our subjects (p=0.81) (Supplemental Data Figure 3).
Figure 5.
CTGF levels in CSF of SLOS patients. CTGF levels measured by (a) multiplex immunoassay revealed an increase in SLOS CSF relative to control CSF. This finding was validated by (b) Western blotting using age- and gender-matched controls, which revealed a 2-fold increase in CTGF levels in SLOS (n=15) compared to control (n=12).
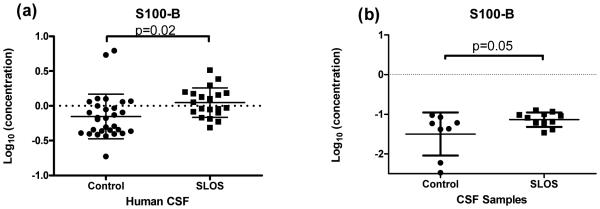
S100B
The S100B protein is a cytosolic, calcium binding protein with a variety of functions. In our study, we observed significantly elevated levels of S100B in the CSF of SLOS patients from the multiplex immunoassay data (Log10 concentration (ng/ml), SLOS: −0.152±0.32, control: 0.048±0.21; p=0.02) (Table 1, Figure 6a) and validated this observation by an independent ELISA with age- and gender-matched controls (Log10 concentrations (ng/ml): control 1.50±0.54 vs. SLOS: −1.87±0.18; p=0.05) (Figure 6b).
Figure 6.
S100B levels in CSF of SLOS patients. S100B concentrations in CSF measured by (a) multiplex immunoassay and (b) an independent ELISA using age- and gender-matched controls (n=10) showed a higher concentration of S100B in SLOS patients relative to control patients.
Discussion
Our study revealed that MCP-1 is altered in SLOS patients. Related to SLOS biochemistry defects, oxysterols which have strong pro-inflammatory effects, including a role in up-regulating NF-kappaB, are closely associated with the production of inflammatory cytokines such as TNF-alpha, IL-1beta, VCAM-1 and MCP-1 (26). Inflammation can lead to increased reactive oxygen species (ROS) production, which triggers further oxidation of cholesterol and oxysterols, thereby generating a detrimental self-propagating cycle (27). S100B is a member of the S100 family of Ca2+ binding proteins that are expressed in a cell-specific fashion (28). In the CNS, S100B is primarily expressed by astrocytes and appears to function in regeneration and repair through its ability to stimulate cell proliferation, migration, and inhibit apoptosis (28). However, over-expression of S100B may lead to neurotoxic effects (29). Similar to MCP-1, S100B which was found to be altered in SLOS, is also a marker of astrocyte activation (28), which can be linked to oxysterols. We observed an elevation of S100B in CSF from SLOS patients in our initial study; however validation of this finding was not statistically significant and might only indicate a minor elevation. Critical analysis of our initial data set did not reveal any outliers that may have skewed the dataset. However, the reduced number of unaffected “control” samples in our validation group may explain the loss of significance, as there appears to be greater variability in the levels of S100B present in the “normal population” (Figures 5a and 5b).
MMP-2, TIMP-1, and TIMP-2 levels are elevated in the CSF of SLOS patients. The elevation of MMP-2 is largely due to increased levels of the pro- form. ProMMP-9 also appears to be slightly increased. MMPs affect brain development through cleavage of proteins that function in synaptogenesis, synaptic plasticity, and long-term potentiation. Large CNS neurons are surrounded by proteoglycans such as brevican and the glycoprotein tenascin-R (TN-R). Cleavage of TN-R alters synaptic structure and result in learning and memory deficits (30). Other targets of MMP include activation of cytokines and growth factors, such as the pro-inflammatory cytokine TNF-alpha, the nerve growth factor, and brain derived neurotrophic factor which regulate neuronal survival, development, and synaptic plasticity (31). Correlation analysis suggests that elevated MMP-2 levels in SLOS patients and elevated TIMP levels such as TIMP-1 and TIMP-2 may inhibit MMP-2 and be responsible for regulating MMP proteolytic activity. It has been shown that over-expression of superoxide dismutase proteins in a mouse model results in increased MMPs upon cold injury induction suggesting a link between these two families of proteins (32). The family of tissue inhibitor of metalloproteinases (TIMPs) includes four proteins that inhibit MMP activity through the formation of non-covalent complexes (33). TIMPs are involved in a variety of biological activities including pro-apoptotic and anti-apoptotic processes, cellular interactions, ECM remodeling, brain injury and repair (22, 33). These proteins, together with CTGF and S100B that were found to be elevated as well, constitute a set of CSF protein biomarkers novel to SLOS. Proteins altered in the CSF will be useful in monitoring efficacy of potential therapies for SLOS and may also lead to a better understanding of the pathophysiology underlying SLOS.
The elevation of a subset of proteins in the CSF may be linked to increased oxysterol levels in SLOS patients, both in the CNS and systemically. Oxysterols are highly reactive biochemical compounds. Their oxygen groups allow them to cross lipophilic membranes, including the blood-brain barrier, and therefore impact brain pathophysiology in a significant manner (34). Studies have demonstrated that the accumulation of 7-DHC in SLOS may be particularly detrimental. The large lipid peroxidation rate constant for 7-DHC indicates that it is highly capable of triggering a free radical chain reaction that can lead to the production of ROS and the formation of additional oxysterols in tissues (11). In mouse peritoneal macrophages and human pro-monocytic cells, oxysterols have been shown to upregulate MMP-9 by inducing ROS production through the activation of Nox2, although TIMP-1 and TIMP-2 levels were unaffected (35). Furthermore, ROS were shown to independently upregulate pro-fibrotic and matrix remodeling proteins including CTGF and MMP-2 in mitral valves from humans with myxomatous mitral valve disease (36). Consistent with a hypothesis that oxidative stress is persistent in SLOS patients, our survey includes an increase of the protein superoxide dismutase-1 in SLOS patients. Correlations between oxidative stress markers and matrix metalloproteinase activity in coronary artery disease have also been reported (37). The increase in MMP activity observed in this study supports a role in oxidative stress. Additionally, the positive correlation of MMP activity and TIMP protein levels provide a means to evaluate these patients. Finally, TIMP proteins have been reported to be involved in modulating neurite outgrowth (38). Previous studies from our laboratory have reported on lengthened dendrites and axons in the SLOS mouse model (39) which is associated with the activation of Rho GTPases.
The enclosed report provides a collection of protein biomarkers related to SLOS, specifically focused on the CSF. While sample groups tend to be small when studying rare diseases, these findings are important in the development of therapeutic options and obtaining a better understanding the disease. While MCP-1, MMPs, TIMPs, CTGF, and S100B are distinct proteins, their altered levels in SLOS patient CSF relative to unaffected “control” CSF support the involvement of brain injury and repair in contributing to the pathophysiology of SLOS. Taken together, these data support defects in the extracellular matrix and remodeling of the brain in SLOS. Future studies will be geared towards extending this understanding of SLOS and providing optimal treatment options for SLOS patients.
Supplementary Material
Acknowledgements
This work has been supported by the intramural research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS. Financial support from the Smith-Lemli-Opitz/ RSH Foundation is also acknowledged. The authors would like to thank the patients and their families for their support and participation.
References
- 1.Waterham HR, Wijburg FA, Hennekam RCM, Vreken P, Poll-The BT, Dorland L, Duran M, Jira PE, Smeitink JAM, Wevers RA, Wanders RJA. Smith-Lemli-Opitz syndrome is caused by mutations in the 7-dehydrocholesterol reductase gene. Am J Hum Genet. 1998;63(2):329–38. doi: 10.1086/301982. doi: Doi 10.1086/301982. PubMed PMID: ISI:000075360800008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitzky BU, Witsch-Baumgartner M, Erdel M, Lee JN, Paik YK, Glossmann H, Utermann G, Moebius FF. Mutations in the Delta7-sterol reductase gene in patients with the Smith-Lemli-Opitz syndrome. Proc Natl Acad Sci U S A. 1998;95(14):8181–6. doi: 10.1073/pnas.95.14.8181. PubMed PMID: 9653161; PMCID: 20950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wassif CA, Maslen C, Kachilele-Linjewile S, Lin D, Linck LM, Connor WE, Steiner RD, Porter FD. Mutations in the human sterol delta7-reductase gene at 11q12-13 cause Smith-Lemli-Opitz syndrome. Am J Hum Genet. 1998;63(1):55–62. doi: 10.1086/301936. doi: 10.1086/301936. PubMed PMID: 9634533; PMCID: 1377256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cross JL, Iben J, Simpson CL, Thurm A, Swedo S, Tierney E, Bailey-Wilson JE, Biesecker LG, Porter FD, Wassif CA. Determination of the allelic frequency in Smith-Lemli-Opitz syndrome by analysis of massively parallel sequencing data sets. Clin Genet. 2014 doi: 10.1111/cge.12425. doi: 10.1111/cge.12425. PubMed PMID: 24813812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nowaczyk MM, Waye JS, Douketis JD. DHCR7 mutation carrier rates and prevalence of the RSH/Smith-Lemli-Opitz syndrome: Where are the patients? Am J Med Genet A. 2006;140a(19):2057–62. doi: 10.1002/ajmg.a.31413. doi: 10.1002/ajmg.a.31413. PubMed PMID: WOS:000241051200007. [DOI] [PubMed] [Google Scholar]
- 6.Lowry RB, Yong SL. Borderline Normal Intelligence in the Smith-Lemli-Opitz (Rsh) Syndrome. American journal of medical genetics. 1980;5(2):137–43. doi: 10.1002/ajmg.1320050205. doi: DOI 10.1002/ajmg.1320050205. PubMed PMID: WOS:A1980JL74900004. [DOI] [PubMed] [Google Scholar]
- 7.Kelley RI, Hennekam RC. The Smith-Lemli-Opitz syndrome. J Med Genet. 2000;37(5):321–35. doi: 10.1136/jmg.37.5.321. PubMed PMID: 10807690; PMCID: 1734573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nowaczyk MJ, Tan M, Hamid JS, Allanson JE. Smith-Lemli-Opitz syndrome: Objective assessment of facial phenotype. Am J Med Genet A. 2012;(5):158A, 1020–8. doi: 10.1002/ajmg.a.35285. doi: 10.1002/ajmg.a.35285. PubMed PMID: 22438180. [DOI] [PubMed] [Google Scholar]
- 9.Porter FD. Smith-Lemli-Opitz syndrome: pathogenesis, diagnosis and management. Eur J Hum Genet. 2008;16(5):535–41. doi: 10.1038/ejhg.2008.10. doi: 10.1038/ejhg.2008.10. PubMed PMID: 18285838. [DOI] [PubMed] [Google Scholar]
- 10.Gaoua W, Chevy F, Roux C, Wolf C. Oxidized derivatives of 7-dehydrocholesterol induce growth retardation in cultured rat embryos: a model for antenatal growth retardation in the Smith-Lemli-Opitz syndrome. J Lipid Res. 1999;40(3):456–63. PubMed PMID: 10064734. [PubMed] [Google Scholar]
- 11.Korade Z, Xu L, Shelton R, Porter NA. Biological activities of 7-dehydrocholesterol-derived oxysterols: implications for Smith-Lemli-Opitz syndrome. J Lipid Res. 2010;51(11):3259–69. doi: 10.1194/jlr.M009365. doi: 10.1194/jlr.M009365. PubMed PMID: 20702862; PMCID: 2952566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wassif CA, Yu JH, Cui JS, Porter FD, Javitt NB. 27-hydroxylation of 7-and 8-dehydrocholesterol in Smith-Lemli-Opitz syndrome: a novel metabolic pathway. Steroids. 2003;68(6):497–502. doi: 10.1016/s0039-128x(03)00090-4. doi: Doi 10.1016/S0039-128x(03)00090-4. PubMed PMID: ISI:000184846100002. [DOI] [PubMed] [Google Scholar]
- 13.Fliesler SJ. Antioxidants: The Missing Key to Improved Therapeutic Intervention in Smith-Lemli-Opitz Syndrome? Hereditary Genet. 2013;2(2):119. doi: 10.4172/2161-1041.1000119. doi: 10.4172/2161-1041.1000119. PubMed PMID: 24533230; PMCID: PMC3925008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korade Z, Xu L, Harrison FE, Ahsen R, Hart SE, Folkes OM, Mirnics K, Porter NA. Antioxidant supplementation ameliorates molecular deficits in Smith-Lemli-Opitz syndrome. Biological psychiatry. 2014;75(3):215–22. doi: 10.1016/j.biopsych.2013.06.013. doi: 10.1016/j.biopsych.2013.06.013. PubMed PMID: 23896203; PMCID: 3874268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Svoboda MD, Christie JM, Eroglu Y, Freeman KA, Steiner RD. Treatment of Smith-Lemli-Opitz Syndrome and Other Sterol Disorders. Am J Med Genet C. 2012;160c(4):285–94. doi: 10.1002/ajmg.c.31347. doi: 10.1002/ajmg.c.31347. PubMed PMID: WOS:000310072700005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tierney E, Conley SK, Goodwin H, Porter FD. Analysis of Short-Term Behavioral Effects of Dietary Cholesterol Supplementation in Smith-Lemli-Opitz Syndrome. Am J Med Genet A. 2010;152A(1):91–5. doi: 10.1002/ajmg.a.33148. doi: Doi 10.1002/Ajmg.A.33148. PubMed PMID: ISI:000273680500009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cologna SM, Cluzeau CV, Yanjanin NM, Blank PS, Dail MK, Siebel S, Toth CL, Wassif CA, Lieberman AP, Porter FD. Human and mouse neuroinflammation markers in Niemann-Pick disease, type C1. J Inherit Metab Dis. 2014;37(1):83–92. doi: 10.1007/s10545-013-9610-6. doi: 10.1007/s10545-013-9610-6. PubMed PMID: 23653225; PMCID: 3877698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganser GH, Hewett P. An accurate substitution method for analyzing censored data. J Occup Environ Hyg. 2010;7(4):233–44. doi: 10.1080/15459621003609713. doi: 10.1080/15459621003609713. PubMed PMID: 20169489. [DOI] [PubMed] [Google Scholar]
- 19.Friedrichsen S, Heuer H, Christ S, Winckler M, Brauer D, Bauer K, Raivich G. CTGF expression during mouse embryonic development. Cell Tissue Res. 2003;312(2):175–88. doi: 10.1007/s00441-003-0712-6. doi: 10.1007/s00441-003-0712-6. PubMed PMID: 12712324. [DOI] [PubMed] [Google Scholar]
- 20.Sorci G, Riuzzi F, Arcuri C, Tubaro C, Bianchi R, Giambanco I, Donato R. S100B protein in tissue development, repair and regeneration. World J Biol Chem. 2013;4(1):1–12. doi: 10.4331/wjbc.v4.i1.1. doi: 10.4331/wjbc.v4.i1.1. PubMed PMID: 23580916; PMCID: 3622753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29(6):313–26. doi: 10.1089/jir.2008.0027. doi: 10.1089/jir.2008.0027. PubMed PMID: 19441883; PMCID: 2755091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenberg GA. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. 2009;8(2):205–16. doi: 10.1016/S1474-4422(09)70016-X. doi: 10.1016/S1474-4422(09)70016-X. PubMed PMID: 19161911. [DOI] [PubMed] [Google Scholar]
- 23.Semple BD, Bye N, Rancan M, Ziebell JM, Morganti-Kossmann MC. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2-/- mice. J Cerebr Blood F Met. 2010;30(4):769–82. doi: 10.1038/jcbfm.2009.262. doi: 10.1038/jcbfm.2009.262. PubMed PMID: WOS:000276197200011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yong VW. Metalloproteinases: mediators of pathology and regeneration in the CNS. Nat Rev Neurosci. 2005;6(12):931–44. doi: 10.1038/nrn1807. doi: 10.1038/nrn1807. PubMed PMID: 16288297. [DOI] [PubMed] [Google Scholar]
- 25.Ghanem AA, Arafa LF, El-Baz A. Connective tissue growth factor and tissue inhibitor of matrix metalloproteinase-2 in patients with exfoliative glaucoma. Curr Eye Res. 2011;36(6):540–5. doi: 10.3109/02713683.2011.565541. Epub 2011/05/20. doi: 10.3109/02713683.2011.565541. PubMed PMID: 21591863. [DOI] [PubMed] [Google Scholar]
- 26.Vejux A, Lizard G. Cytotoxic effects of oxysterols associated with human diseases: Induction of cell death (apoptosis and/or oncosis), oxidative and inflammatory activities, and phospholipidosis. Mol Aspects Med. 2009;30(3):153–70. doi: 10.1016/j.mam.2009.02.006. doi: 10.1016/j.mam.2009.02.006. PubMed PMID: 19248805. [DOI] [PubMed] [Google Scholar]
- 27.Poli G, Biasi F, Leonarduzzi G. Oxysterols in the pathogenesis of major chronic diseases. Redox Biol. 2013;1(1):125–30. doi: 10.1016/j.redox.2012.12.001. doi: 10.1016/j.redox.2012.12.001. PubMed PMID: 24024145; PMCID: 3757713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, Weber DJ, Geczy CL. Functions of S100 proteins. Curr Mol Med. 2013;13(1):24–57. PubMed PMID: 22834835; PMCID: 3707951. [PMC free article] [PubMed] [Google Scholar]
- 29.Sedaghat F, Notopoulos A. S100 protein family and its application in clinical practice. Hippokratia. 2008;12(4):198–204. PubMed PMID: 19158963; PMCID: 2580040. [PMC free article] [PubMed] [Google Scholar]
- 30.Montag-Sallaz M, Montag D. Severe cognitive and motor coordination deficits in tenascin-R-deficient mice. Genes Brain Behav. 2003;2(1):20–31. doi: 10.1034/j.1601-183x.2003.00003.x. doi: DOI 10.1034/j.1601-183X.2003.00003.x. PubMed PMID: ISI:000180550300004. [DOI] [PubMed] [Google Scholar]
- 31.Ethell IM, Ethell DW. Matrix metalloproteinases in brain development and remodeling: synaptic functions and targets. Journal of neuroscience research. 2007;85(13):2813–23. doi: 10.1002/jnr.21273. doi: 10.1002/jnr.21273. PubMed PMID: 17387691. [DOI] [PubMed] [Google Scholar]
- 32.Morita-Fujimura Y, Fujimura M, Gasche Y, Copin JC, Chan PH. Overexpression of copper and zinc superoxide dismutase in transgenic mice prevents the induction and activation of matrix metalloproteinases after cold injury-induced brain trauma. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2000;20(1):130–8. doi: 10.1097/00004647-200001000-00017. Epub 2000/01/05. doi: 10.1097/00004647-200001000-00017. PubMed PMID: 10616801. [DOI] [PubMed] [Google Scholar]
- 33.Lorenzl S, Albers DS, LeWitt PA, Chirichigno JW, Hilgenberg SL, Cudkowicz ME, Beal MF. Tissue inhibitors of matrix metalloproteinases are elevated in cerebrospinal fluid of neurodegenerative diseases. J Neurol Sci. 2003;207(1-2):71–6. doi: 10.1016/s0022-510x(02)00398-2. PubMed PMID: 12614934. [DOI] [PubMed] [Google Scholar]
- 34.Bjorkhem I, Cedazo-Minguez A, Leoni V, Meaney S. Oxysterols and neurodegenerative diseases. Mol Aspects Med. 2009;30(3):171–9. doi: 10.1016/j.mam.2009.02.001. doi: 10.1016/j.mam.2009.02.001. PubMed PMID: 19248803. [DOI] [PubMed] [Google Scholar]
- 35.Gargiulo S, Sottero B, Gamba P, Chiarpotto E, Poli G, Leonarduzzi G. Plaque oxysterols induce unbalanced up-regulation of matrix metalloproteinase-9 in macrophagic cells through redox-sensitive signaling pathways: Implications regarding the vulnerability of atherosclerotic lesions. Free Radic Biol Med. 2011;51(4):844–55. doi: 10.1016/j.freeradbiomed.2011.05.030. doi: 10.1016/j.freeradbiomed.2011.05.030. PubMed PMID: 21664966. [DOI] [PubMed] [Google Scholar]
- 36.Hagler MA, Hadley TM, Zhang H, Mehra K, Roos CM, Schaff HV, Suri RM, Miller JD. TGF-beta signalling and reactive oxygen species drive fibrosis and matrix remodelling in myxomatous mitral valves. Cardiovasc Res. 2013;99(1):175–84. doi: 10.1093/cvr/cvt083. doi: 10.1093/cvr/cvt083. PubMed PMID: 23554457; PMCID: 3687751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kameda K, Matsunaga T, Abe N, Hanada H, Ishizaka H, Ono H, Saitoh M, Fukui K, Fukuda I, Osanai T, Okumura K. Correlation of oxidative stress with activity of matrix metalloproteinase in patients with coronary artery disease. Possible role for left ventricular remodelling. European heart journal. 2003;24(24):2180–5. doi: 10.1016/j.ehj.2003.09.022. Epub 2003/12/09. PubMed PMID: 14659769. [DOI] [PubMed] [Google Scholar]
- 38.Ould-yahoui A, Tremblay E, Sbai O, Ferhat L, Bernard A, Charrat E, Gueye Y, Lim NH, Brew K, Risso JJ, Dive V, Khrestchatisky M, Rivera S. A new role for TIMP-1 in modulating neurite outgrowth and morphology of cortical neurons. PloS one. 2009;4(12):e8289. doi: 10.1371/journal.pone.0008289. Epub 2009/12/17. doi: 10.1371/journal.pone.0008289. PubMed PMID: 20011518; PMCID: 2788270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang XS, Wassif CA, Backlund PS, Song L, Holtzclaw LA, Li Z, Yergey AL, Porter FD. Activation of Rho GTPases in Smith-Lemli-Opitz syndrome: pathophysiological and clinical implications. Human molecular genetics. 2010;19(7):1347–57. doi: 10.1093/hmg/ddq011. Epub 2010/01/14. doi: 10.1093/hmg/ddq011. PubMed PMID: 20067919; PMCID: 2838542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.