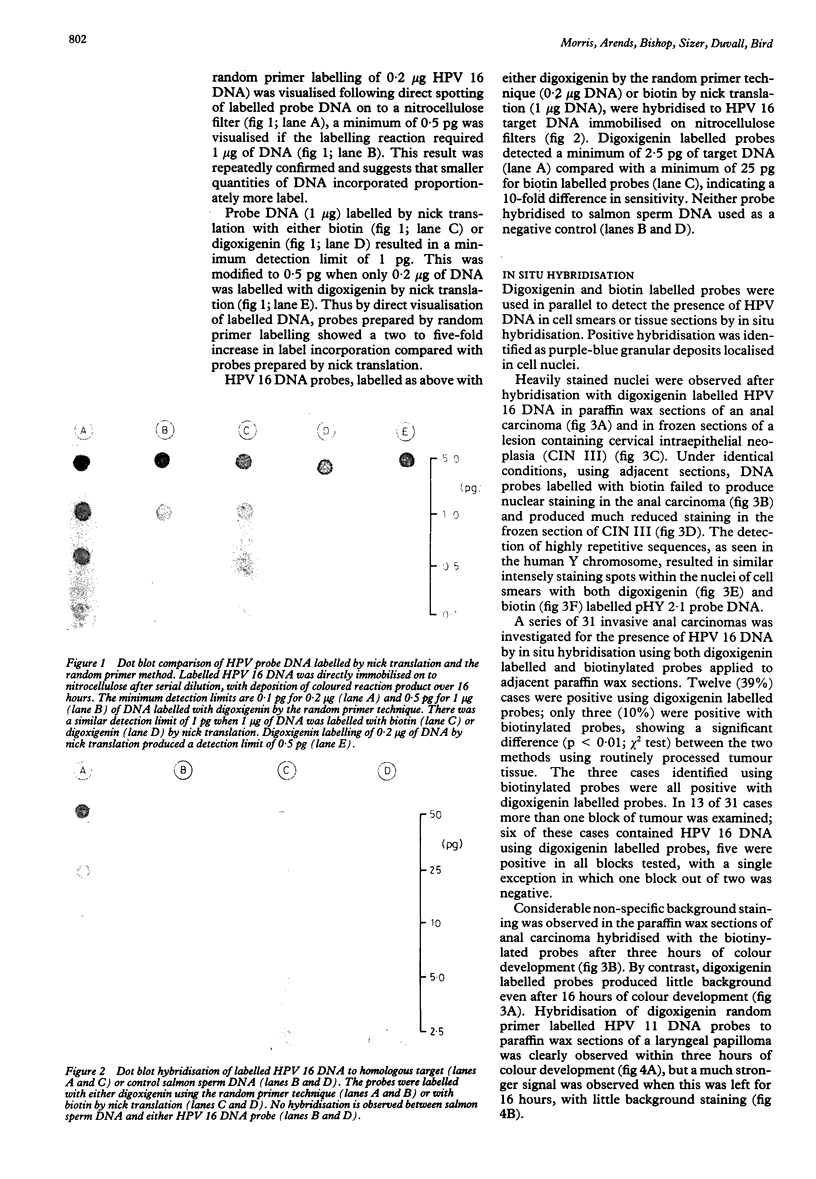
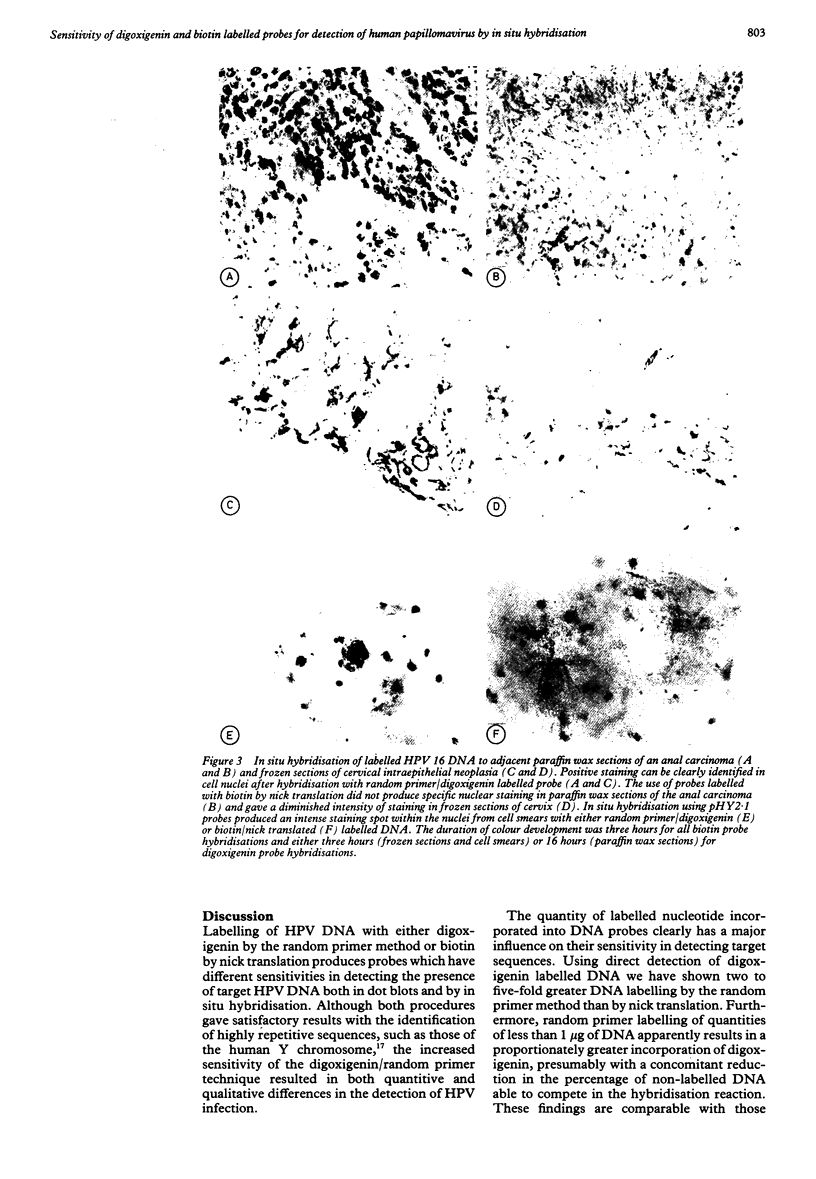
Abstract
The sensitivity of digoxigenin and biotin labelled DNA probes for the detection of human papillomavirus (HPV) by dot blotting and in situ hybridisation was compared in tissues from cervical, laryngeal, and anogenital neoplasia. Probes were either labelled with digoxigenin by the random primer technique and detected with anti-digoxigenin antibody, or labelled with biotin by nick translation and detected with streptavidin, both methods having a common final visualisation procedure using alkaline phosphatase. Digoxigenin labelled probes proved two to 10-fold more sensitive by quantitative dot blotting and four-fold more sensitive in detecting HPV 16 DNA in a series of 31 anal carcinomas, compared with biotinylated probes. The digoxigenin method also produced less non-specific background staining of tissue sections than biotin labelled probes. It is concluded that digoxigenin DNA labelling and detection provides a simple, reliable, and efficient alternative to the use of biotin or radioactive isotopes for the detection of HPV DNA by in situ hybridisation. Digoxigenin labelled probes also offer the possibility of double labelling in situ hybridisation procedures when used with biotin labelled probes to provide simultaneous identification of different DNA sequences.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arends M. J., Wyllie A. H., Bird C. C. Papillomaviruses and human cancer. Hum Pathol. 1990 Jul;21(7):686–698. doi: 10.1016/0046-8177(90)90027-3. [DOI] [PubMed] [Google Scholar]
- Banerjee D., Pettit S. Endogenous avidin-binding activity in human lymphoid tissue. J Clin Pathol. 1984 Feb;37(2):223–225. doi: 10.1136/jcp.37.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshart M., Gissmann L., Ikenberg H., Kleinheinz A., Scheurlen W., zur Hausen H. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 1984 May;3(5):1151–1157. doi: 10.1002/j.1460-2075.1984.tb01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigati D. J., Myerson D., Leary J. J., Spalholz B., Travis S. Z., Fong C. K., Hsiung G. D., Ward D. C. Detection of viral genomes in cultured cells and paraffin-embedded tissue sections using biotin-labeled hybridization probes. Virology. 1983 Apr 15;126(1):32–50. doi: 10.1016/0042-6822(83)90460-9. [DOI] [PubMed] [Google Scholar]
- Burns J., Graham A. K., Frank C., Fleming K. A., Evans M. F., McGee J. O. Detection of low copy human papilloma virus DNA and mRNA in routine paraffin sections of cervix by non-isotopic in situ hybridisation. J Clin Pathol. 1987 Aug;40(8):858–864. doi: 10.1136/jcp.40.8.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleridge J. C., Coleridge H. M. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol. 1984;99:1–110. doi: 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- Cooke H. J., Schmidtke J., Gosden J. R. Characterisation of a human Y chromosome repeated sequence and related sequences in higher primates. Chromosoma. 1982;87(5):491–502. doi: 10.1007/BF00333470. [DOI] [PubMed] [Google Scholar]
- Crum C. P., Nagai N., Levine R. U., Silverstein S. In situ hybridization analysis of HPV 16 DNA sequences in early cervical neoplasia. Am J Pathol. 1986 Apr;123(1):174–182. [PMC free article] [PubMed] [Google Scholar]
- Dürst M., Gissmann L., Ikenberg H., zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Ferenczy A., Braun L., Shah K. V. Human papillomavirus (HPV) in condylomatous lesions of cervix. Am J Surg Pathol. 1981 Oct;5(7):661–670. doi: 10.1097/00000478-198110000-00008. [DOI] [PubMed] [Google Scholar]
- Heiles H. B., Genersch E., Kessler C., Neumann R., Eggers H. J. In situ hybridization with digoxigenin-labeled DNA of human papillomaviruses (HPV 16/18) in HeLa and SiHa cells. Biotechniques. 1988 Nov-Dec;6(10):978–981. [PubMed] [Google Scholar]
- Lehn H., Villa L. L., Marziona F., Hilgarth M., Hillemans H. G., Sauer G. Physical state and biological activity of human papillomavirus genomes in precancerous lesions of the female genital tract. J Gen Virol. 1988 Jan;69(Pt 1):187–196. doi: 10.1099/0022-1317-69-1-187. [DOI] [PubMed] [Google Scholar]
- Lewis F. A., Griffiths S., Dunnicliff R., Wells M., Dudding N., Bird C. C. Sensitive in situ hybridisation technique using biotin-streptavidin-polyalkaline phosphatase complex. J Clin Pathol. 1987 Feb;40(2):163–166. doi: 10.1136/jcp.40.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitland N. J., Cox M. F., Lynas C., Prime S. S., Meanwell C. A., Scully C. Detection of human papillomavirus DNA in biopsies of human oral tissue. Br J Cancer. 1987 Sep;56(3):245–250. doi: 10.1038/bjc.1987.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrow R. S., Manias D. A., Clark B. A., Okagaki T., Twiggs L. B., Faras A. J. Detection of human papillomavirus DNA in invasive carcinomas of the cervix by in situ hybridization. Cancer Res. 1987 Jan 15;47(2):649–653. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Stanbridge C. M., Mather J., Curry A., Butler E. B. Demonstration of papilloma virus particles in cervical and vaginal scrape material: a report of 10 cases. J Clin Pathol. 1981 May;34(5):524–531. doi: 10.1136/jcp.34.5.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas-Cavallin M., Aït-Ahmed O. The random primer labeling technique applied to in situ hybridization on tissue sections. J Histochem Cytochem. 1988 Oct;36(10):1335–1340. doi: 10.1177/36.10.3138309. [DOI] [PubMed] [Google Scholar]
- Webb D. H., Rogers R. E., Fife K. H. A one-step method for detecting and typing human papillomavirus DNA in cervical scrape specimens from women with cervical dysplasia. J Infect Dis. 1987 Dec;156(6):912–919. doi: 10.1093/infdis/156.6.912. [DOI] [PubMed] [Google Scholar]
- Wood G. S., Warnke R. Suppression of endogenous avidin-binding activity in tissues and its relevance to biotin-avidin detection systems. J Histochem Cytochem. 1981 Oct;29(10):1196–1204. doi: 10.1177/29.10.7028859. [DOI] [PubMed] [Google Scholar]
- van Prooijen-Knegt A. C., Raap A. K., van der Burg M. J., Vrolijk J., van der Ploeg M. Spreading and staining of human metaphase chromosomes on aminoalkylsilane-treated glass slides. Histochem J. 1982 Mar;14(2):333–344. doi: 10.1007/BF01041225. [DOI] [PubMed] [Google Scholar]