Abstract
Mycobacterium tuberculosis (Mtb) is a facultative intracellular pathogen and the second largest contributor to global mortality caused by an infectious agent after HIV. In infected host cells, Mtb is faced with a harsh intracellular environment including hypoxia and the release of nitric oxide (NO) and carbon monoxide (CO) by immune cells. Hypoxia, NO and CO induce a state of in vitro dormancy where Mtb senses these gases via the DosS and DosT heme sensor kinase proteins, which in turn induce a set of ~47 genes, known as the Mtb Dos dormancy regulon. On the contrary, both iNOS and HO-1, which produce NO and CO, respectively, have been shown to be important against mycobacterial disease progression. In this review, we discuss the impact of O2, NO and CO on Mtb physiology and in host responses to Mtb infection as well as the potential role of another major endogenous gas, hydrogen sulfide (H2S), in Mtb pathogenesis.
Keywords: Mycobacterium tuberculosis, gasotransmitter, nitric oxide, carbon monoxide, hydrogen sulfide, heme oxygenase-1, reactive oxygen species, reactive nitrogen species, hypoxia
1. Introduction
1.1. Global TB burden
The World Health Organization (WHO) has estimated that one-third of the world’s population is infected with Mycobacterium tuberculosis (Mtb), the bacterium that causes tuberculosis disease (TB). In 2014 ~1.5 million people died from TB, making Mtb the second deadliest pathogen of the 21st century after human immunodeficiency virus (HIV) [1]. HIV/AIDS exacerbates the global TB burden, as is evidenced by that fact that of the 9.6 million people newly infected in 2014, ~1.1 million (12%) were HIV positive [1]. Bacille Calmette-Guerin (BCG), the only vaccine against TB, is effective only in children and does not provide consistent protection in vaccinated adults. TB can be treated with existing drugs; however, the lengthy treatment regimen of 6–9 months often leads to patient noncompliance, which can result in the development of drug-resistant TB. Globally, an estimated 5% of active TB cases are multi-drug resistant TB (MDR-TB), defined as resistance to isoniazid and rifampicin, the two most potent first-line anti-TB drugs. In 2013, an estimated 480,000 people worldwide developed MDR-TB, of which an estimated 210,000 died [2]. Together with HIV co-infection, the emergence of MDR-TB represents a growing challenge to global public health that threatens to undermine the gains achieved through the last 80 years of medical advances. Clearly, it is of great importance to better understand the molecular mechanisms of Mtb pathogenesis in order to develop more effective drugs and therapies against this global threat.
1.2 Scope
In this review, we aim to provide a new perspective on the role of gasotransmitters in TB, specifically nitric oxide (NO), carbon monoxide (CO) and the newly emerging gas, hydrogen sulfide (H2S). In addition, we will discuss the role of molecular oxygen (O2), including hypoxia, as the concentration of O2 plays a major role in TB disease progression. An emphasis is placed on the in vivo sources of these gases, and their roles in Mtb physiology and TB disease. This is followed by discussion of the mechanisms whereby Mtb senses and responds to these gases, including the Dos dormancy three-component system, the more recently described SenX-RegX two-component system, and iron-sulfur (Fe-S) cluster proteins. This review will not attempt in-depth descriptions of the chemical properties of these gases, but certain properties will be discussed where appropriate. For in-depth reviews on the chemical properties of these gases, we refer the reader to several excellent review articles [3–7]. This review will focus on the role of these host-derived intracellular gases in regulating Mtb disease progression with special focus on their specific roles in Mtb dormancy and in regulating host immune responses against TB.
1.3 Overview of Mtb physiology and disease dynamics
Mtb is a rod-shaped, acid-fast, obligate aerobe with a waxy lipid-rich cell wall and extremely slow growth rate (doubling time of ~18–20 hours in culture) [8]. Analysis of the Mtb genome has identified dedicated machinery for lipid biosynthesis, numerous virulence factors and sensor proteins that allow Mtb to respond to, and survive in, the hostile environment within host cells [9]. Mtb physiology is unique as studies have shown that Mtb can survive for up to 12 years in sealed tubes and remain fully virulent [10]. During infection Mtb is exposed to a wide range of host substrates including organic acids, virtually all amino acids, nucleic acid precursors, nucleotides, lipids and numerous carbohydrates [11]. Thus, it is clear that Mtb must adjust its metabolism in response to the availability of environmental nutrients during the different stages of infection.
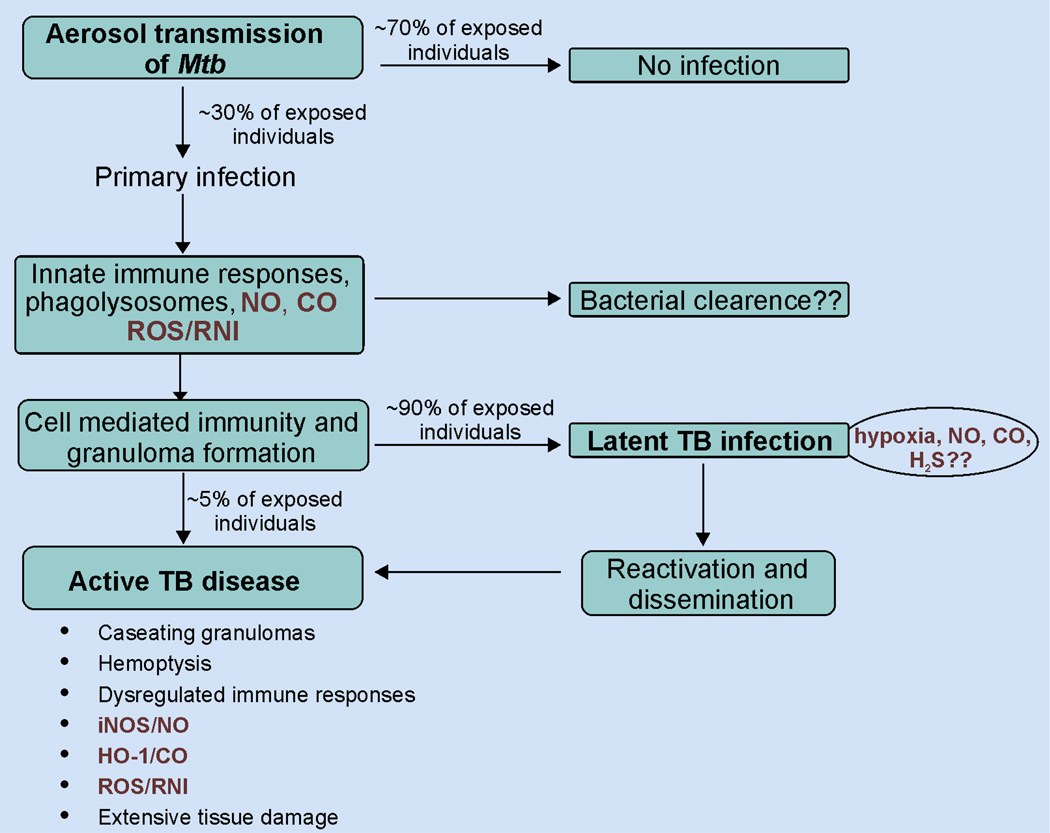
Mtb infection typically begins with the inhalation of Mtb-containing droplets aerosolized by an infected person [12]. The human lung has a total alveolar surface area of ~70 m2, and an adult inhales and exhales ~11,500 liters of air each day [13]. Since the only route of Mtb transmission is by inhalation, the lungs are the primary site of infection. The first step of infection is the uptake of bacilli by alveolar macrophages (AM) by phagocytosis and the formation of phagosomes. Within the AM, fusion of phagosomes with acidic lysosomes is critical for bactericidal activity [12]. However, Mtb uniquely blocks phagolysosome fusion and acidification, thereby eliciting the rapid recruitment of neutrophils, inflammatory monocytes, interstitial macrophages and T-cells in the lungs followed by formation of cellular aggregates called granulomas. Containment of Mtb within granulomas results in an asymptomatic latent TB infection (LTBI), which is clinically defined by a reactive TB skin test without clinical symptoms of disease or radiological findings [14]. Mtb granulomas have been shown to be hypoxic in guinea pig and non-human primate (NHP) models and in a specific mouse model of TB [15, 16] (Fig. 1).
Figure 1. Schematic overview of Mtb infection.
Mtb infection is initiated when an individual is exposed to aerosolized bacteria. Of those exposed, ~70% remain uninfected, whereas ~30% develop primary infection. The host immune response to the primary infection is initiated by innate immune responses of macrophages and neutrophils via phagocytosis, upregulation of iNOS and HO-1 and production of ROS/RNS. This is followed antigen presentation by dendritic cells, cell-mediated immunity and further infiltration of immune cells. These responses initially restrict bacterial replication and dissemination and lead to the formation of granulomas. In most cases, Mtb resides within the granuloma in a non-replicating dormant state leading to LTBI. Approximately 90% of individuals with LTBI remain asymptomatic while ~5% develop active TB disease, characterized by massive hemoptysis, dysregulated immune responses and extensive tissue damage.
The occurrence of LTBI highlights a unique aspect of Mtb physiology that has been extensively studied, but is not fully understood; that is, the ability of Mtb to persist for years or decades in the host in a genetically controlled state of low metabolic activity referred to as dormancy [17]. As an obligate aerobe, Mtb requires oxygen for growth, and therefore, it is rational to conclude that the low oxygen environment of the TB granuloma contributes to this metabolic shutdown of the bacillus. Studies in the 1950s showed that open (oxygen rich) lesions from resected lung tissue from human TB patients yielded actively growing Mtb that were predominantly drug resistant. In contrast, Mtb cells isolated from closed (oxygen poor) lesions showed delayed growth, but were drug sensitive despite being refractory to antimycobacterial drug therapy [18, 19]. This pointed to O2 as an important modulator of Mtb physiology and therapeutic intervention. Not surprisingly, a key objective in the TB field is to understand the mechanisms whereby Mtb persists in tissues for decades without replicating, to then abruptly resume growth and cause disease. Acquiring such knowledge will make a significant contribution towards new therapeutic interventions.
2. Role of oxygen in Mtb pathogenicity
2.1 Overview
Prior to the advent of TB drug therapy, patients were treated in sanatoria, where rest and fresh air were considered conducive to recovery [20]. The human host reaction to the bacillus was studied, and while many patients succumbed to the consumptive process, in other patients the bacilli were isolated and walled off by the body’s defense mechanisms, leading to LTBI. One of the theories postulated was that the lower oxygen levels in these “walled off” areas inhibited the growth of the obligate aerobic mycobacterium, and the concept of “resting the lung” while the infection took its course became popularized. Various techniques to artificially collapse a lung to render a relatively hypoxemic environment were then proposed, and “collapse therapy” marked the birth of thoracic surgery for the treatment of TB [21]. Methods of inducing “collapse therapy” included instilling air, liquid or spherical objects into the chest cavity to prevent the lung from completely expanding. By depriving the organism of oxygen, it was thought that the disease process would be curtailed. The advent of chemotherapy for TB marked the end of an era of collapse therapy for TB, and the attention of thoracic surgeons was then directed toward lung resection for TB (Fig. 2).
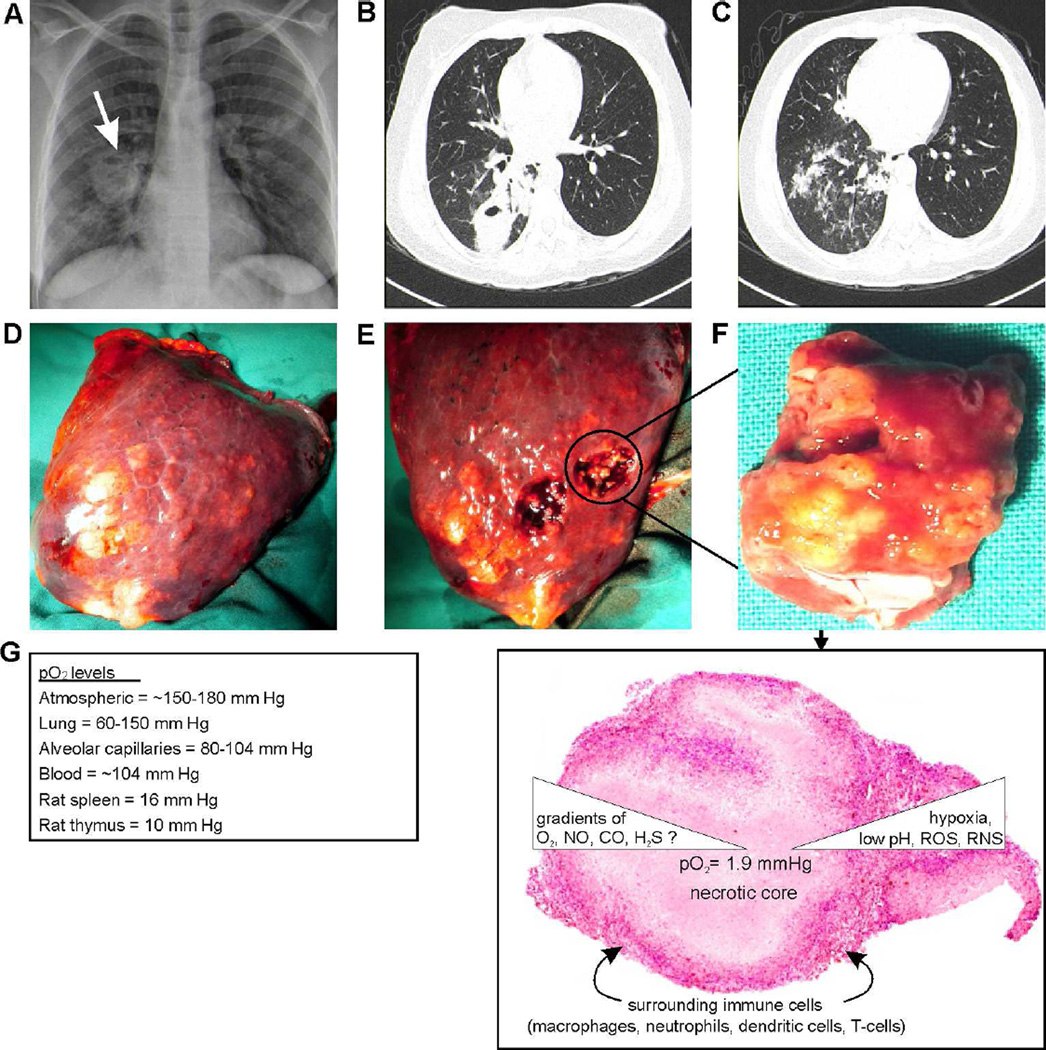
Figure 2. Typical chest radio graph, HRCT and granuloma of a TB patient.
(A) Plain chest radiograph of a 46 year old woman with active pulmonary tuberculosis, demonstrating a thick walled cavity in the right lung with associated parenchymal opacification. (B, C) High resolution computed tomography (HRCT) images illustrate the location of the cavity in the apical segment of the right lower lobe with the associated tree-in-bud appearance in the basal segments that are characteristic of active tuberculosis. (D, E, F) Right lower lobectomy specimen with macroscopic features of active pulmonary tuberculous nodules with central caseous necrosis that is evident after sectioning. (G) Representative tuberculous granuloma with central necrotic core surrounded by immune cells which create a gradient of O2, NO, CO and possibly H2S.
2.2 O2 gradients during Mtb infection
It was believed that patients with pulmonary TB improved if they lived at altitudes above 2,000 m due to the lower oxygen tension [22]. Using radioisotope techniques, West and Dollery [22] measured regional perfusion and ventilation and calculated oxygen tension in the upright human lung. They observed that the partial pressure of alveolar oxygen (PaO2) at sea level is approximately 132 mm Hg at the level of the first anterior interspace, decreasing to 89 mm Hg at the level of the fifth anterior [23]. These observations supported the popular belief that oxygen tension is a major determinant of the apical localization of pulmonary TB. However, an alternate explanation is that the effect of gravity on the erect human lung leads to a reduced pulmonary artery blood flow in the apical and subapical regions leading to higher O2 tension [24, 25]. This reduced blood flow would impede tissue clearance and favor accumulation of Mtb, making the apical lobes ideal for Mtb growth and proliferation [26]. Although it can be argued that conventional in vitro tissue culture experiments expose Mtb to hyperoxic conditions, recent in vitro studies have shown that the intracellular environment of Mtb-infected macrophages in vitro may be hypoxic (< 10 mm Hg) [27]. The above discussion clearly highlights O2 as a critical player in TB disease.
In the human TB lung, there is a gradient of oxygen tensions from the upper apices to the lower lobes. The flow rate of blood in the upper apex of the lung is reduced due to depressed pressure within the alveoli relative to pulmonary artery pressure in the upright position. Therefore, the apices have elevated oxygen tensions despite reduced ventilation due to restricted blood flow [28]. Several studies have shown that Mtb-infected lungs have various oxygen tensions, all of which are below the normal range seen in naïve subjects. This hypoxic environment within the Mtb-infected lung has important implications for drug distribution and bioavailability. The interplay between the host and Mtb requires in-depth analysis of various oxygen levels within the different compartments of human tuberculous lung, which could provide new insights into the underlying mechanisms of current anti-TB drugs activity. Despite recent progress, the physiology of Mtb-infected lung tissue is not yet fully resolved in terms of drug penetration and levels [29]. Animal models have been useful for shedding light on the dynamics of granuloma formation and TB disease progression. Granulomatous tissues in mouse models of TB have features that resemble those in humans despite some subtle differences [30]. Routine access to human tuberculous lung specimens for research purposes is unfortunately restricted by the paucity of these biopsies, and represents a major hindrance to TB research. This is important as there is no single animal model that accurately represents the full spectrum of human pulmonary TB. Another limitation of human TB lung studies is that the granuloma is dynamic in nature; hence real-time experimentation is not possible in the human host. Mice, guinea pigs, rabbits, and non-human primates have the ability to form well defined granulomas upon Mtb infection.
2.3 Reactive oxygen species (ROS) and Mtb physiology
As a diffusible diatomic gas, O2 plays important roles in host response to infection. As mentioned earlier, Mtb is exposed to low O2 levels or hypoxic conditions within the lungs. Further, ROS and reactive nitrogen species (RNS) generated by host immune cells within the lung create a harsh intracellular environment against Mtb. The oxidative burst created by NADPH oxidase (NOX) of host phagocytic cells via catalysis of a single electron reduction of O2 generates O2•− and further reduction of one more electron produces hydrogen peroxide (H2O2) by the activity of superoxide dismutase (SOD). Further, H2O2, in the presence of ferrous ions (Fe2+) undergoes the Fenton reaction to produce hydroxyl radical (OH•). Overall, hypoxia and these free radicals constitute a strong host innate defense that can damage bacterial cell wall components, DNA, and proteins that protects the host against a wide range of bacterial infections including Mtb [31–34]. Mice deficient in the cytosolic p47(phox) gene, which is required for O2•− production, are susceptible to Mtb infection [35]. However, Mtb has evolved complex mechanisms that provide protection from the damaging effects of ROS and hypoxia. Components of the Mtb cell wall, including lipoarabinomannan (LAM), mycolic acids and phenolic glycolic lipids (PGL-1), serve as effective ROS scavengers [36]. Mycothiol and recently discovered ergothioneine act as redox buffers and protect Mtb from endogenous ROS [37, 38]. Mtb also possesses several ROS-scavenging gene products, which contribute to ROS detoxification and enhance Mtb survival. Mtb katG (catalase peroxidase) decomposes H2O2 into water and oxygen thereby protecting cells from the damaging effects of H2O2 [39]. Mtb Mg/Fe and Cu/Zn superoxide dismutases (sodA and sodC, respectively) also protect the cells from oxidative bursts and Mtb strains lacking these genes are susceptible to ROS-mediated killing in both macrophages in vitro and in the murine model of TB [40–42]. Other genes including alkyl peroxide reductase genes (ahpC/D/E) and lipoamide dehydrogenase (lpd) also contribute to Mtb survival against host-generated ROS [43]. In response to hypoxic conditions generated in the lung, the Mtb three-component heme sensor/regulator system (DosS/R/T) senses and binds to host O2 thereby causing transcriptional adaptation that results in upregulation of Mtb-Dos dormancy regulon genes, which enables bacteria to persist in a non-replicative, low metabolic state [44] (see Section 6; Mtb gas sensors).
3. Nitric oxide
3.1 Overview
Since its initial characterization in the 1980s as a potent vasodilator followed by its recognition as ‘molecule of the year’ in 1992 [45], the gasotransmitter NO has been found to regulate a wide array of physiological functions such as neuromodulation, cellular signaling, maintenance of redox homeostasis and as an effector molecule during immune responses [46–49]. NO is produced endogenously by the nitric oxide synthase (NOS) enzyme family via NADPH- and O2-dependent oxidation of L-arginine to L-citrulline and NO [50]. Three distinct mammalian NOS isoforms have been identified based on the location of their expression: NOS enzymes expressed by endothelial cells (eNOS, also referred to as NOS3) and neurons (nNOS, also referred to as NOS1) are constitutively expressed and act distinctively in response to intracellular calcium levels in a calmodulin-dependent manner [51]. NO production for immunological functions is mediated by a calcium-independent inducible form, iNOS [51, 52]. iNOS is expressed predominantly in cells of myeloid lineage including macrophages and neutrophils and is generally induced by redox stress, exposure to microbial pathogens or bacterial endotoxins and pro-inflammatory cytokines such as IL-1, IFN-γ and TNFa (52). NO is a major RNS and reacts with atmospheric O2, superoxide anions (O2 •−), heme/non-heme iron and thiol groups (−SH) of proteins to regulate a wide array of cellular signaling responses. One such response includes formation of peroxynitrite (ONOO−) upon reaction with O2•−. NO and other RNS including ONOO− can modify bacterial DNA, proteins, and lipids in host cells as well as in invading pathogens. Further, NO also interacts with iron-sulfur clusters or heme groups of proteins. Nitrite and nitrate, which were thought to be end products of NO metabolism, can be recycled to produce NO. Bacteria in the gut have also been shown to form nitric oxide under varying physiological concentrations of O2 and L-arginine via acidification or reduction of nitrite [53–55].
3.2 Role of NO in host response to Mtb infection
Cells of the innate immune systems including macrophages and neutrophils are crucial for host defense against many bacterial infections including Mtb. One of their major anti-bacterial strategies includes upregulation of iNOS expression and production of NO [56–58]. Genetic knock-out studies with macrophages isolated from NADPH oxidase- or iNOS-deficient mice and in macrophages isolated from TB patients provided early evidence that ROS including O2•−, and RNS including NO are important for host defense against Mtb [59, 60]. This was further substantiated by MacMicking, et al., who demonstrated that iNOS-deficient mice are highly susceptible to Mtb infection and succumb to infection within ~45 days post infection [61]. Blocking iNOS function with inhibitors such as N6-(1-iminoethyl)-L-Lysine, aminoguanidine and NG-monomethyl-L-arginine resulted in rapid bacterial growth, increased disease pathology, high mortality and disease reactivation in murine TB models [62–64]. Similarly, inhibition of the iNOS-modulating cytokine TNFa also leads to Mtb reactivation in a murine model of latent TB [65]. A more recent study has shown that NO regulates TB immunopathology and tissue damage by regulating the levels of pro-inflammatory cytokine interleukin1β (IL1β) in both IFNγ-dependent and independent manners [66]. Compared to murine models, the protective role of NO in Mtb-infected humans is poorly understood. Nonetheless, immunohistochemical analysis of human TB lung tissue and AM revealed the presence of iNOS, NO, nitrotyrosine and increased exhaled NO in TB patients, suggesting a role for NO in human TB [67–69]. Further, AM from LTBI patients may kill Mtb in an iNOS-dependent manner [70, 71]. Similarly, Rich et al. reported that inhibition of intracellular Mtb growth correlated with elevated levels of NO and iNOS in AM following Mtb infection [72]. Kuo et, al. showed that increased NO generation by AM of TB patients plays an autoregulatory role in amplifying the synthesis of proinflammatory cytokines [73]. In the human promyelocytic cell line HL-60, vitamin D3 treatment resulted in significant increase in NO production and enhanced inhibition of Mtb growth [74]. NO is also thought to be important for the formation of granulomas thereby protecting the host from Mtb dissemination [75]. Interestingly, when peripheral blood monocytes obtained from patients with active TB where infected with Mtb in vitro, increases in both NO and TNF-α levels were observed. However, the same cells obtained from patients with MDR-TB produced little NO and TNF-α following Mtb infection, suggesting a role for NO in TB-associated immunosuppression and TB drug resistance [76]. Overall these studies clearly suggest that NO plays significant role in host response against Mtb.
3.3 Role of NO in Mtb physiology
As discussed above, iNOS/NO is an important component of the host defense strategy against Mtb infection; however, Mtb has developed a number of complex mechanisms to resist, subvert and counter the damaging effects of intracellular NO and other RNS. Darwin et al. have shown that the Mtb proteasome is crucial for bacterial resistance against host-generated oxidative and nitrosative stress, likely through the degradation of stress-damaged proteins [77]. Other studies showed that the noxR1 and noxR3 genes of Mtb confer protection against NO, RNS and ROS [78, 79] Further, Mtb ahpc was protective during RNS-induced necrosis, apoptosis and also protected the bacterium from DNA damage by detoxifying ONOO− [80]. Interestingly, transcriptomic analysis of iNOS-deficient mice revealed that Mtb faces significant oxidative and nitrosative stress within the phagosomal environment. However, under these conditions Mtb undergoes drastic transcriptional adaptation which enables to switch from aerobic to anaerobic respiration and entering dormancy [81]. Further, during hypoxia, nitrate is reduced by Mtb to nitrite which also assists in switching to anaerobic respiration and inducing dormancy [82]. Similarly, nitrate respiration was also important for Mtb survival under acidic and nitrosative stress environments [83]. Voskuil et al. showed that non-toxic concentrations of NO inhibit Mtb respiration and growth, and result in the induction of Mtb dormancy regulon genes [84]. Similar to O2, NO is also sensed by the Mtb three-component system DosS/R/T, which causes Mtb to enter dormancy in vitro [44, 85, 86]. Interestingly, Cunningham-Bussel et al. have shown that Mtb also produces significant levels of nitrite during infection of human macrophages, which assists its survival under hypoxic conditions by modulating bacterial respiration, ATP consumption and transcriptional modulation of stress associated genes [87]. Overall, these studies show that while NO is important for host defense, Mtb has evolved potent NO-mitigating machinery (Fig. 3).
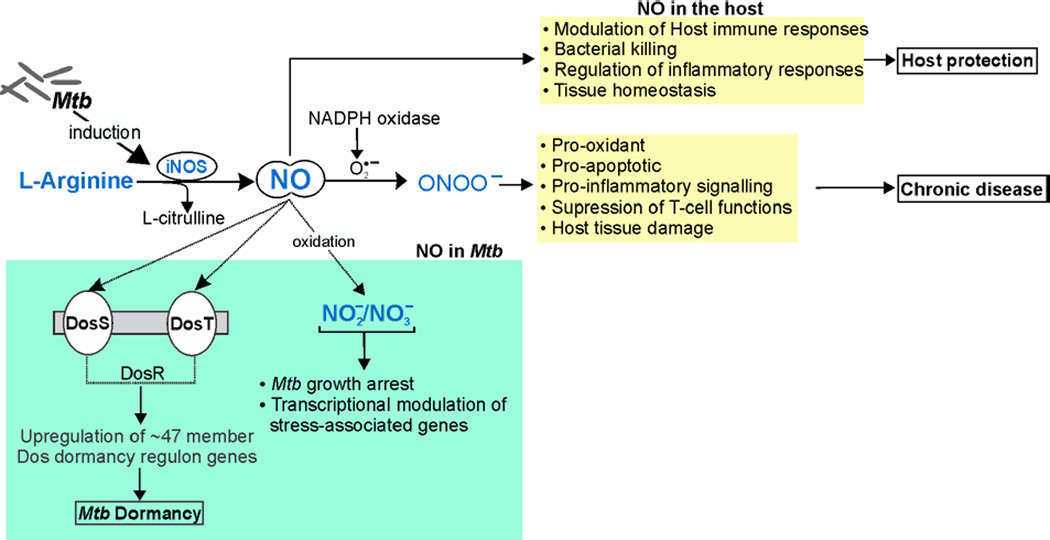
Figure 3. Schematic illustration of the role of NO in Mtb disease.
Mtb infection upregulates the expression of iNOS, which catalyzes the conversion of L-arginine into L-citrulline and NO. NO-mediated Mtb killing and modulation of host immune and inflammatory responses are crucial for host protection against Mtb infection. On the other hand, NO reacts with O2•− to produce ONOO− via NADPH oxidase. ONOO− is pro-inflammatory, pro-oxidant and a potent suppressor of T-cell responses and its overproduction leads to host tissue damage and increased disease pathology. NO also plays important roles in Mtb physiology where it is sensed by the Mtb heme sensor kinase proteins DosS and DosT, which activate the response regulator DosR thereby inducing the Mtb dormancy regulon. Also, NO can be converted to NO2− or NO3−, which arrest Mtb growth and lead to transcriptional adaptation of Mtb stress-associated genes.
4. Carbon monoxide (CO)
4.1 Overview
While initially regarded as a toxic environmental gas, CO is now known to be an enzymatic by-product of the heme oxygenase (HO) system and a key intracellular messenger that regulates numerous host physiological responses [5]. HO enzymes convert free heme, sourced primarily from hemoglobin released from erythrocytes, into CO, free iron (Fe2+) and biliverdin in equimolar ratios [88]. Two major HO enzymes have been characterized in mammals: the inducible form, HO-1, and a constitutive isoform, HO-2. While HO-2 is constitutively expressed almost exclusively in the brain, HO-1 is ubiquitously expressed at very low levels in almost all cell types, but its expression is highly induced by cellular redox stress, hypoxia, bacterial lipopolysaccharides and many pathogenic challenges including mycobacterial species [5, 89]. The HO system produces ~86% of endogenous CO; the remainder is contributed by lipid peroxidation, bacteria, photo-oxidation, and xenobiotic metabolism [5]. Verma et al. provided the first evidence that CO has a physiological role and, like NO, acts as a signaling molecule to regulate cGMP production [90]. Additional studies showed CO to be a key regulator of vascular and cardiac functions [91], inflammation [92, 93] and apoptosis [94, 95]. Later studies revealed cytoprotective roles for CO, which eventually lead to evaluation of CO as a therapeutic intervention in many pathophysiological conditions [96, 97].
4.2 Role of CO in bacterial pathogenesis
While the functions of CO in host responses to bacterial infections have been studied, demonstration of a direct role for CO in bacterial physiology has so far been limited to its bactericidal activity in E. coli, P. aeruginosa, and S. aureus, which results in death of the bacillus [98, 99]. Roles for CO in bacterial pathogenesis have been shown in models of bacterial disease, including sepsis and malaria [100]. Administration of a carbon monoxide releasing molecule (CO-RM) resulted in enhanced bacterial clearance and improved outcomes in a HO-1-deficient mouse model of severe sepsis [101]. CO has also been shown to enhance the rate of macrophage phagocytosis of E. coli through upregulation of TLR-4 expression via p38 MAPK signaling [102]. A similar study reported that macrophage-specific deletion of HO-1 increases macrophage death upon infection with E. coli and E. faecalis and that this effect was reversed by administration of exogenous CO [103]. In malarial disease caused by the parasite Plasmodium, Pamplona et al., have shown that pharmacological induction of HO-1 or CO prevents experimental cerebral malaria (ECM) in mice by inhibiting the release of free heme. This prevents disruption of the blood brain barrier, and regulates CD8 T-cell responses [104]. Similarly, Jeney et al. demonstrated that the HO/CO system, along with NO, provides disease tolerance during cerebral malaria via suppression of T-cell responses [105]. Taken together, these studies show that host-derived CO plays an important role in host defense and bacterial physiology.
4.3 Role of CO in Mtb physiology and pathogenicity
One anti-bacterial strategy of host phagocytes is the upregulation of HO-1 and the production of CO via HO-1 enzymatic activity. However, unlike other bacterial pathogens, Mtb tolerates physiological levels of CO due to its expression of a CO-resistance gene, cor [106]. The first major advancement in our understanding of a role for CO in Mtb physiology came from studies showing that like O2 and NO, CO can independently bind to Mtb heme sensor kinase proteins DosS and DosT as confirmed by the formation of heme-carbonyl species upon exposure to CO [44]. Further, exposure of Mtb cells to CO-RM-derived CO or CO gas results in increased expression of dosR, hspX and fdxA genes of the Mtb dormancy regulon [44]. In vitro infection studies using macrophages isolated from wild-type and HO-1−/− mice showed that physiological levels of host-generated CO induce a transcriptional profile of Mtb dormancy-inducing genes comparable to that of NO. This transcriptional regulation is specific to CO and not the other products of HO-1 enzymatic activity [107, 108]. These studies also demonstrated that while induction of the dormancy regulon genes (discussed later) by NO requires the activity of both heme sensor kinases DosS and DosT, the binding of CO to DosS alone is sufficient to induce the Mtb dormancy regulon. These data demonstrate a direct role for CO in initiation of Mtb dormancy, and that HO-1-derived CO and iNOS-derived NO may act in synchrony or independently to determine the outcome of infection.
We and others have shown showed that HO-1 levels are significantly increased in macrophages in vitro and in the lungs of mice following Mtb infection [107, 108]. In the infected lung, HO-1 is upregulated in immune cells that surround granulomas, suggesting that increased production of CO may be a host strategy to induce dormancy and restrict bacterial dissemination [108]. While these studies highlight the role of HO-1-derived CO in Mtb dormancy, the role of CO in active TB disease is still unclear. Nonetheless, during non-tuberculous infections (NTM) caused by Mycobacterium avium (M. avium), HO-1 confers host protection by suppressing the expression of monocyte chemoattractant protein-1 (MCP-1) and chemokine receptor 2 (CCR2) resulting in the formation of compact granulomas, thereby reducing bacterial dissemination. HO-1 also counteracts the cytotoxic effects of heme and inhibits apoptosis of infected macrophages [109, 110]. While based on a HO-1-deficient mouse model of M. avium infection, these studies did not demonstrate a direct role for CO, a technically challenging task. However, since CO is known to have anti-apoptotic, anti-inflammatory and cytoprotective functions in macrophages when challenged with bacterial lipopolysaccharide (LPS), it is reasonable to postulate that the cytoprotective activity of HO-1 during M. avium infection is attributable to CO [111]. Further, these studies point to HO-1 and CO as a potential targets for therapeutic interventions against M. avium infection.
HO-1 and HO-1-derived CO play an important role in host anti-inflammatory and anti-oxidative responses during various pathological conditions [92, 97]. Notably, active TB is characterized by dysregulated inflammatory responses such as heightened immune cell infiltration, increased levels of pro-inflammatory cytokines and hemorrhagic inflammation resulting in hemoptysis and extensive lung tissue damage [112–115]. Andrade et al. showed that HO-1 levels are significantly elevated in the serum of patients with active pulmonary TB compared to patients with LTBI, and suggested that elevated HO-1 levels could be a potential TB biomarker [116]. A later study by the same group showed that increases in HO-1 levels in TB patients corresponded with decreased expression of matrix metalloproteinase-1 (MMP-1), which drives immunopathology during TB [117, 118]. This inversely proportional expression of HO-1 and MMP-1 was specific to TB and was not observed in other lung diseases. The authors also demonstrated that CO specifically reduces the expression of MMP-1 via suppression of c-Jun/AP-1 activation and suggested a potential therapeutic role for CO in host protection against TB disease progression.
The dysregulated immune responses and increased influx of immune cells to the infection site during active TB results in substantial oxidative and nitrosative stresses that likely result in extensive tissue damage. In contrast, dormant Mtb within granuloma remain non-pathogenic and drug insensitive, leading to LTBI. These two disease phenotypes in TB are strikingly similar to malaria, wherein a clinically latent pre-erythrocytic stage is required to establish infection, and a subsequent erythrocytic stage is responsible for active malarial disease [119]. Since application of HO-1 and CO has been shown to protect mice from malarial disease pathology, a similar therapeutic strategy for Mtb infection may have merit.
Overall, while the role of CO in TB is not fully understood, it is clear that it plays a key role in both controlling mycobacterial dissemination and inducing dormancy in vitro. Further, as HO-1/CO are important for host defense against oxidative and inflammatory damage, their therapeutic application at the site of infection may play a significant role in minimizing exacerbated inflammation and oxidative damage during TB (Fig. 4).
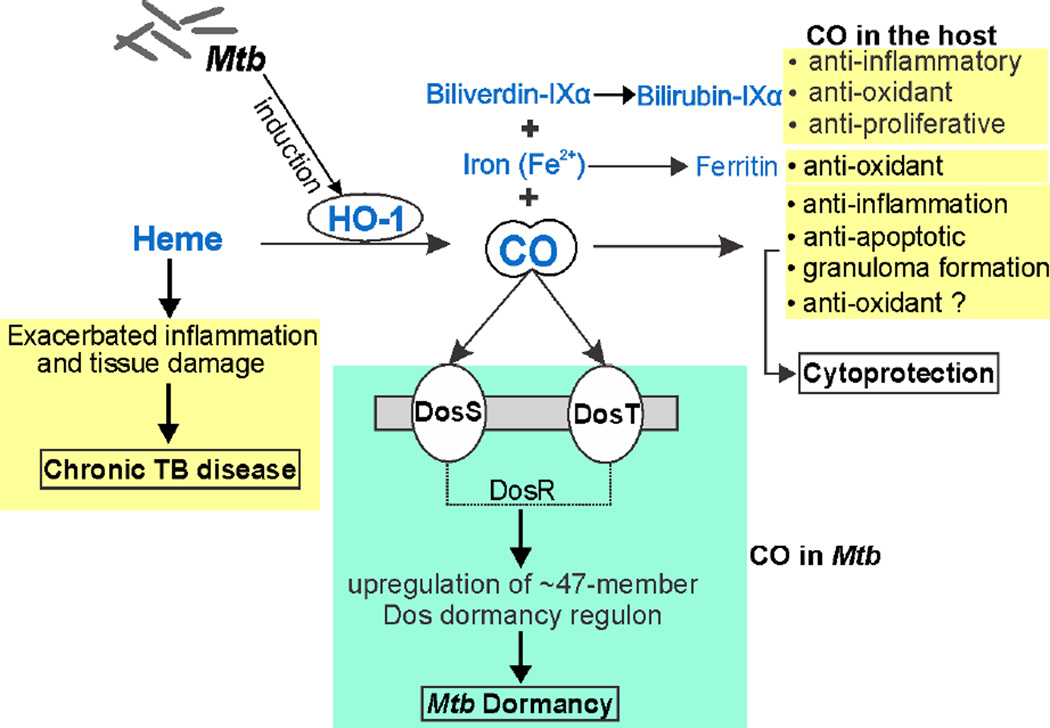
Figure 4. Schematic illustration of endogenous CO production and role in Mtb disease.
CO is produced by HO-1 via heme catabolism. Other products of this enzymatic reaction include free iron (Fe2+) and biliverdin, which is further converted into bilirubin. Iron further increases the expression of ferritin, an iron storage protein. CO, bilirubin and ferritin are potent antioxidant molecules. Further, CO and bilirubin also play important roles in regulating inflammation, immune cell proliferation and host cell apoptosis. Mtb infection induces HO-1 expression thereby increasing endogenous CO, which could protect against heme-mediated tissue damage via its anti-oxidative, anti-proliferative and anti-apoptotic properties. CO binds to Mtb heme sensor kinase proteins DosS and DosT, which like NO, induce the Mtb dormancy regulon.
5. Hydrogen Sulfide (H2S)
5.1 Overview
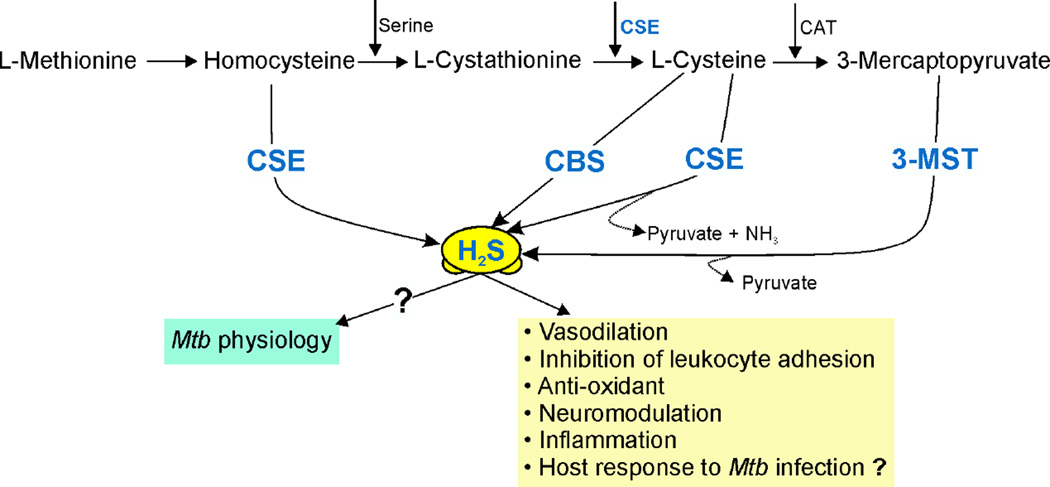
H2S has recently emerged as the fourth physiologically important gas that mediates multiple processes in mammalian systems including hypertension, atherosclerosis, heart failure, diabetes, inflammation, neurodegenerative diseases and asthma [120–126]. H2S can be produced in the human body by resident microbes [127] or by dedicated enzymatic machinery consisting of three independent enzymes, cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE) and 3-mercaptopyruvate sulfurtransferase β-MST) along with cysteine aminotransferase (CAT) [128] (Fig. 5). The overall chemical properties of sulfide (defined here as H2S + HS−) relevant to biological systems are briefly outlined below; further details can be found in recent reviews on the biochemical properties of sulfide [6, 129–131]. H2S is a weak acid, with a pKa of ca. 7 for the dissociation H2S ↔ H+ + HS−, the second pKa is high enough that the sulfide anion (S2−) almost certainly has no biological relevance. This means approximately 28% of sulfide is present as the protonated nonelectrolyte species (H2S, hydrogen sulfide) at pH 7.4. As an uncharged small lipophilic molecule, the fact that protonation/deprotonation is extremely rapid means that membranes are quite permeable and so sulfide produced in one compartment will rapidly distribute within the biological milieu. The volatility of H2S also demands relatively rapid exit to a headspace, requiring careful attention to experimental configuration. The chemical reactivity of sulfide is dominated by the fact that, like thiol, it is a strong nucleophile, much more so in the hydrosulfide anion form (HS-) than H2S. This means that HS- is the species that exerts the majority of (but not all) biochemical reactivity. The nucleophilicity of sulfide is reflected in its electronegativity, second only to oxygen (except for fluorine). Unlike oxygen, sulfur is able to utilize its 3d orbitals for an expanded valence shell thereby allowing multiple bonds and formal oxidation states ranging from −2 (e.g. H2S) to +6 (e.g. SO42−).
Figure 5. Schematic illustration of endogenous H2S production and functions.
H2S is produced endogenously by cystathionine-β-synthase (CBS), cystathionine-γ-lyase (CSE) and 3-mercaptopyruvate sulfurtransferase (3-MST). CBS and CSE use L-cysteine as the primary substrate with pyridoxal-5′-phosphate as a cofactor. H2S formation through 3-MST is downstream of cysteine aminotransferase (CAT), which catalyzes the reaction of L-cysteine with α-ketoglutarate leading to the formation of 3-mercaptopyruvate, the substrate used by 3-MST to form H2S and pyruvate. H2S is important for numerous physiological responses including vasodilation, neuromodulation, and inflammation and can also act as an anti-oxidant.
Being electrophiles, transition metal ions react with sulfide by both complete (oxidation-reduction) and partial (bonding) electron transfer. The product of oxidation-reduction is the reduced metal and elemental sulfur (So). Elemental sulfur exists as a polymer, the most common configuration being S8. In the case of bonding, the metal-sulfide bond is a special type of bond, called a coordinate bond. The toxicity of sulfide in humans is dominated by its reactions with metals, specifically, with mitochondrial cytochrome oxidase at the site of O2 binding (the binuclear CuB/heme a3) and with hemoglobin which, through the intermediacy of a high valency heme-oxygen complex, produces sulfhemoglobin, which contains a covalent heme modification. Sulfide anion is also a key component of a large class of iron proteins containing iron-sulfur centers, which serves both oxidation/reduction and signaling roles. Undoubtedly, the best studied biological system of metal-sulfide interaction is in a symbiotic mollusk/bacterial symbiosis, where cytoplasmic hemoglobin in the host delivers both O2 and sulfide to the bacterium for metabolism [132]. The properties of the mollusk hemoglobin are elegantly tuned to carry these two molecules, involving configuration of the heme pocket and proximal and distal protein residues.
Partially reduced oxygen species (O2•−, H2O2, and HO•) are also electrophiles and react with sulfide. The rates of reaction, however, are highly variable, and in the in vivo environment these reactions are not likely to be important under most conditions due to the low concentrations of both these species and also sulfide. Finally, sulfide also reacts with other electrophilic species, of particular interest being the newly discovered species 8-nitro-cyclic GMP [133]. There is evidence that this reaction plays a key role in the signaling properties of this molecule. Sulfide also reacts with oxidized sulfur-containing species, for example, disulfide RSSR’. The product is thiol and persulfide: HS− + RSSR’ → RSS− + R’S− (contrary to some statements in the literature, sulfide does not react with thiol; in general, nucleophiles do not react with other nucleophiles). Similar further reactions also produce polysulfur species R(S)nH, in great abundance. This is true for both small thiols (e.g. glutathione) and also protein cysteine thiol. Indeed, it may well be that these species are more important functionally than sulfide itself. In spite of the lack of relevant data, there are observations about the role of H2S in mammals and other prokaryotes [127, 134, 135].
5.2 A role for H2S in TB?
It is not known whether H2S impacts the outcome of Mtb infection and TB disease in humans. Regardless, the physicochemical properties of H2S, as well as its established role in modulating immunity point to a potentially important role for this gas in TB. Both CBS and CSE have been found critical for proper alveolarization and functioning of the lung [136]. Since the human lung is the primary organ of Mtb infection in which the bacillus can reside for decades, it is reasonable to posit that Mtb may have mechanisms for sensing and responding to H2S. Also, since CBS and CSE play essential roles in human health [136–138], it is plausible that H2S via its immunomodulatory function is necessary for protection against Mtb infection. Studies on the role of H2S on Mtb pathogenesis are eagerly awaited.
However, there are observations on the role of H2S in mammals and other prokaryotes that can be extrapolated to comment on the possible role that H2S may play in TB disease. For example, exposing mice to low amounts of H2S induces a reversible hibernation-like state characterized by lowering of body temperature, and reduced energy expenditure in the form of reduced ATP consumption and low metabolic rate (as evaluated by substantially decreased CO2 production and O2 consumption) [139]. Induction of this hypo-metabolic state by H2S, referred to as suspended animation, is an oxygen-dependent phenomenon [140]. It is believed that the inhibition of O2 utilization and induction of suspended animation in low O2 conditions could have a host-protective role [140]. There are parallels that can be drawn from this H2S-induced suspended animation state and the characteristic features associated with dormancy in Mtb. For example, during dormancy Mtb is exposed to severe environmental stresses (e.g., low pH, O2 and carbon source limitation) that induce a metabolic state characterized by decreased respiration, low ATP levels, reduced growth, low O2 consumption and reduced metabolic rates.
H2S primarily affects mitochondrial respiration by targeting complex IV of the respiratory chain, i.e., cytochrome c oxidase [141]. Based on historical studies using manometry [142] and in a more recent drug study [143], Mtb is known to exhibit immense respiratory flexibility, which is a key requirement for maintaining an intracellular pathogenic lifestyle. It possesses two proton-pumping complexes: NADH dehydrogenase I, which is dispensable for growth, and an aa3-type cytochrome c oxidase, which provides the means of O2 reduction [144]. More importantly, Mtb also possess an alternate terminal oxidase, cytochrome bd oxidase, a high-affinity alternate terminal oxidase that can partially compensate for the loss in activity of the bc1-aa3 complex [144]. Therefore, it will be of significant interest to determine whether H2S affects Mtb respiration and consequently in vivo pathogenesis.
Lastly, there is a growing appreciation for the complex interactions between different intracellular gases such as NO, CO and H2S as they can all interact with the host (and potentially Mtb) electron transport chain [145–150]. Furthermore, NO and CO can also regulate H2S levels by targeting the CBS heme prosthetic group [151–153].
6. Mtb gas sensors
6.1 The Mtb Dos dormancy regulon
The success of Mtb as a pathogen is based in part on its ability to enter into dormancy, a genetically and physiologically controlled non-replicative state characterized by metabolic quiescence. In this reversible state, Mtb can survive the harsh intracellular environment inside the host [154]. Of note, dormancy in Mtb is a physiological state of persistence and should not be confused with TB latency or LTBI, which are clinical descriptors indicating the absence of TB disease symptoms in infected individuals. The transition into dormancy occurs via marked changes in gene expression, including up-regulation of the widely studied 47-gene Dos regulon in response to CO, NO or hypoxia [155, 156]. In addition to the genes of the Dos regulon, other gene sets, such as the enduring hypoxia response (EHR) genes, may play a role in dormancy; however molecular mechanisms of their regulation are largely unknown [157, 158]. Transcriptional analysis under hypoxic conditions in vitro revealed that even though ribosomal and metabolism genes are downregulated under low-O2 conditions, the Dos regulon genes are significantly upregulated [159]. Similar to the response to hypoxia or low O2 levels, other host-generated stresses such as CO and NO also upregulate these dormancy regulon genes. While many genes of this regulon are still being characterized, they are predicted to be involved in modulating Mtb physiology. These include genes with chaperone functions (acr or Rv2031c), ferredoxin modulation (fdxA or Rv2007c), nitrate/nitrite transporter (nark2 or Rv 1737), ribonuclease reductase (nrdZ or Rv0570) and triglyceride synthase (tgs1 or Rv3130) [19, 160].
The genes of the dormancy regulon are upregulated when Mtb senses host-generated hypoxia, NO and CO via the heme-containing kinases DosS and DosT, which along with a single response regulator, DosR, comprise the DosR/S/T three-component system [44, 107, 161]. As discussed earlier, our studies and others have shown that the redox or ligation status of the heme iron in DosS and DosT modulates binding to O2, NO and CO either together or independently. This shows that these host-generated intracellular gases are central to Mtb disease progression [19, 44]. This also emphasizes the fact that the success of Mtb as a pathogen can be largely attributed to its unique ability to sense and respond to NO, CO or low O2. Several lines of evidence point to these diatomic gases as modulators of dormancy. Firstly, Mtb is an obligate aerobe that requires oxygen as a terminal electron acceptor. However, the uncanny ability of Mtb to survive for more than a decade in test tubes without oxygen (or at least extremely low concentrations of oxygen) points to a novel aspect of Mtb adaptation to low oxygen environments. Secondly, it has been conclusively demonstrated that iNOS, and therefore NO is crucial for protection of mice against Mtb [61]. Further studies in patients with active pulmonary TB confirm the importance of NO in Mtb pathogenesis [59, 60, 35–76, 162]. Unfortunately, despite several elegant in vitro studies [107, 108, 163] a clearly established role for either HO-1 or CO in Mtb pathogenesis has yet to be demonstrated and represents an unexplored area of investigation.
It should be noted that the lack of oxygen and the presence of sufficient levels of NO and/or CO inhibit Mtb respiration and ultimately retard growth. An important difference between NO and CO is that CO can only react with ferrous (Fe2+) iron whereas NO reacts with Fe2+ and Fe3+. This raises the obvious question as to how concentration gradients of oxygen, NO and CO, as well as the different affinities of DosS and DosT for these gases contribute to Mtb disease.
Most studies showing the effect of gases on Mtb have been performed in vitro or in mouse models of TB and their relevance to human TB is yet to be established. However, recent studies in our laboratory using tissue samples from freshly resected human tuberculous lungs have clearly shown that significant levels of ROS/RNS are indeed present in human TB lungs. Interestingly ROS/RNS levels were directly proportional to the extent of tissue damage, suggesting a gradual disease progression.
6.2 Mtb iron-sulfur (Fe-S) cluster proteins
Proteins that contain iron-sulfur cluster [Fe-S] prosthetic groups are found in virtually all organisms and provide a mechanism for cells to sense changes in redox and respond to intracellular gases. The Mtb WhiB family of proteins WhiB1 (Rv3219), WhiB2 (Rv3260c), WhiB3 (Rv3416), WhiB4 (Rv3681c), WhiB5 (Rv0022c), WhiB6 (Rv3862c) and WhiB7 (Rv3197A)] contain four cysteine residues arranged in a Cys–X-14–22-Cys-X2-Cys-X5-Cys motif, with the exception of WhiB5 [164]. Studies of homologs in Streptomyces spp suggested that these proteins are Fe-S clusters proteins [164]. Indeed, Mtb WhiB3 is a 4Fe-4S cluster protein that maintains intracellular redox balance and responds to oxygen and NO [165]. Recently, WhiB3 was shown to regulate the production of a redox buffer, ergothioneine, which is critical for Mtb virulence and drug susceptibility [38]. The WhiB proteins likely acts as redox sensors via their Fe-S clusters and are critical in maintaining redox homeostasis, a key determinant of Mtb virulence [19, 38]. The seven members of the Mtb WhiB family perform diverse functions including cell division (WhiB2) [166], fatty acid metabolism and pathogenesis (WhiB3) [165, 167, 168], oxidative stress (WhiB6) [169, 170] and antibiotic resistance (WhiB7) despite only moderate sequence homology among themselves [171, 172].
Since the discovery of a WhiB-like protein in Streptomyces spp in 1992, a long-standing question has been whether these proteins are DNA-binding proteins that regulate gene expression [173]. Nearly 20 years later, it was shown that Mtb WhiB3 binds DNA [168]. Important features of its DNA binding capacity, which was extrapolated to other WhiB members, was that oxidized apo-WhiB3 binds DNA much stronger than either holo-WhiB3 or reduced apo-WhiB3. Subsequently, it was shown that Mtb apo-WhiB1, apo-WhiB2 and apo-WHiBTM4 bind DNA strongly in contrast to the holo-forms, which bind DNA weakly or not at all [164]. These findings revealed that DNA binding and transcriptional activity are modified by environmental conditions such as hypoxia, free radicals such as NO and oxidative stressors that influence the redox state of the 4Fe-4S cluster (of the holo-form) or the oxidation states of the Cys residues (of the apo-form). Overall, it appears that Mtb has evolved a sophisticated family of proteins that function as a flexible redox switch in response to oxido-reductive stress that causes an imbalance in intracellular redox homeostasis.
Redox homeostasis in mycobacteria is distinct from other intracellular pathogens as they lack a conventional glutathione system and homologues of the prototype sensor proteins in other intracellular pathogens such as SoxR, ArcAB, FNR and OxyR (a pseudogene in Mtb) [172–174]. Instead, Mtb uses two distinct redox couples, mycothiol and ergothioneine, and harbors at least 50 proteins containing Fe–S clusters that facilitate an intercellular life cycle [37, 175]. Interestingly, Mtb has a higher incidence of Fe–S cluster proteins (~6.5 motifs/1000 ORFs) than other aerobic bacteria (2.8 motifs/1000 ORFs), underscoring the importance of Fe–S cluster proteins to pathogenic adaptations of Mtb [176, 177].
6.3 Other gas-sensing systems
Apart from DosS/R/T, Mtb has other heme sensor kinase and response regulator protein systems that are predicted to play roles in its metabolic adaptation, including the SenX3-RegX3 system and the PhoPR systems [178, 179]. Transcriptomic analysis of a senX3-regX3 knockout strain of Mtb showed downregulation of several DNA repair and protein synthesis genes as well as genes important for survival under hypoxic conditions such as cydB and gltA1 [180]. Further, this Mtb knockout strain fails to establish successful infection of macrophages in vitro and in guinea pig and murine models of TB [181–183]. More recently, SenX3 was shown to sense and bind O2, NO and CO. Binding of O2 to SenX3 is associated with growth stimulation during reactivation whereas binding of NO and CO to SenX3 inhibits growth via inhibition of kinase activity [184].
7. Future challenges and conclusions
The development of highly effective therapeutic interventions such as new antimycobacterial drugs and vaccines hinges on understanding the mechanisms by which Mtb can persist for decades in the human lung to ultimately cause disease. Given the ubiquitous nature of O2, NO, CO and H2S, it is not surprising that some of these endogenous gases play critical roles in a wide range of pathophysiological processes. The importance of these gases in human health is underlined by genetic deficiency and polymorphism studies. For example, the pathophysiological consequences of deficiencies in HO-1 (systemic inflammation, intravascular hemolysis, asplenia, vulnerability to stressful injury) [185, 186], CBS, CSE or 3-MST (hyper-homocysteinemia, mercaptolactate-cysteine disulfiduria, mental retardation and aggressive vascular disease, neuoroblastoma and hepatoblastoma) [187–189] and iNOS (susceptibility to TB, and a wide range of diseases) [190] have profound effects on human health. Therefore, dysregulation of the levels of these gases at the site of infection could lead to ineffective drug treatment or impair inflammatory responses to exacerbate disease.
A practical advantage in studying the role of NO, CO and H2S in Mtb infection is that mice with deficiencies in expression of iNOS, HO-1, CBS, CSE and 3-MST are available for experimentation. To confirm the clinical relevance of these gases, findings from these studies should be expanded to the analysis of human pulmonary TB tissue samples. Unfortunately, a major impediment to these studies is the paucity of freshly resected human TB lung tissue. Of particular interest would be to determine what cell populations in animal and human TB lung tissues express CBS, CSE, 3-MST, iNOS and HO-1, and their respective levels. It is important to note that in the case of iNOS and HO-1, which are expressed at the site of infection [67, 107, 108], gradients of NO, CO and O2 are likely to exist in hypoxic Mtb granulomas [19, 191]. This presents an interesting opportunity for studying crosstalk among the gases since CBS and iNOS contain heme moieties, which represent potential interaction sites for NO, CO and O2.
Another important albeit unexplored area of investigation is the role of these gases in antimycobacterial drug susceptibility in vivo. First considered for NO, it is tempting to speculate that gasotransmitters play a role in antimycobacterial drug efficacy [67]. On the other hand, since these gasotransmitters are ubiquitous, pharmacologic modulation of gene expression or the application of iNOS, HO-1, CBS, CSE or 3-MST end products has long been proposed as a therapeutic avenue based on model studies [3–7]. Understanding how Mtb directly interacts with these gases, their corresponding targets, how these targets discriminate between these gases, and the signaling responses induced by these gases will be of substantial value. NO, CO and O2 have been shown to target the heme irons of Mtb DosS and DosT. However, targets other than the WhiB family proteins and the SenX3-RegX3 two-component system also exist. Further, a detailed understanding of how these gasotransmitters target the Mtb electron transport chain, which contains numerous heme and Fe-S cluster prosthetic groups, should provide new information on energy metabolism, and how the bacillus is capable of surviving under harsh intracellular conditions including hypoxia and gradients of NO, CO and H2S. The Mtb electron transport chain is remarkably plastic and capable of rapidly re-routing electrons around specific inhibition by switching between terminal oxidases. Despite the many potential targets, very little is known about this ATP-generating pathway in Mtb. Many provocative questions can emerge from such studies. For example, do these gasotransmitters inhibits or stimulate respiration, and do they have a predominantly bactericidal or bacteriostatic effect on Mtb?
In conclusion, it is anticipated that as more is learned about how these gasotransmitters regulate host immunity and directly targets Mtb, many new avenues of research will open up, compelling researchers to reassess existing paradigms for Mtb persistence, and to seek new testable hypothesis.
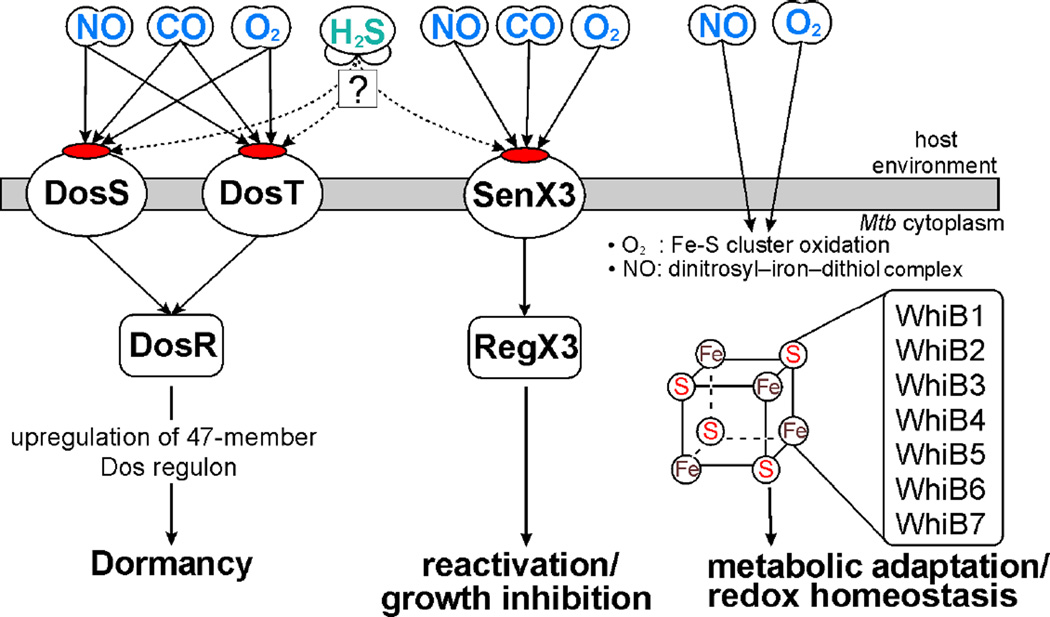
Figure 6. Schematic overview of gasotransmitters in Mtb physiology.
Mtb heme sensor kinase proteins DosS and DosT sense host-generated NO, CO and O2 and activate the response regulator DosR which induces the Mtb dormancy (Dos) regulon. SenX3 of the SenX3-RegX3 two-component system is a heme sensor kinase that also responds to host-generated O2, NO and CO. Binding of O2 to SenX3 stimulates Mtb growth during reactivation; however, binding of CO or NO inhibits growth. RegX3 is presumed to mediate these effects downstream of SenX3. Further, interaction of O2 or NO with members of the WhiB family of iron-sulfur cluster proteins regulates the intracellular redox homeostasis of Mtb as well as metabolic adaptation under conditions of host-generated stress.
Highlights.
One-third of the world’s population is infected with Mtb, the bacterium that causes TB.
Phagocytosis, ROS and RNS are important host responses to restrict initial Mtb infection.
iNOS and HO-1 enzymatic activity is important for host anti-mycobacterial responses.
Mtb heme sensor kinases bind to O2, NO and CO to modulate dormancy and reactivation.
Interaction of O2 and NO with Mtb 4Fe-4S cluster proteins regulates redox homeostasis and metabolism.
Acknowledgments
This work was supported by NIH grants AI058131 and AI111940 (to AJCS). AJCS is a Burroughs Wellcome Investigator in the Pathogenesis of Infectious Diseases. This work was also supported by the UAB Center for AIDS Research (CFAR) and UAB Center for Free Radical Biology (CFRB). The research was also co-funded by the South African Medical Research Council.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
REFERENCES
- 1.World Health Organization (WHO) Global tuberculosis report 2015. Available from: http://www.who.int/tb/publications/global_report/gtbr15_main_text.pdf.
- 2.Zumla A, George A, Sharma V, Herbert RH, Masham B, Oxley A, Oliver M. The WHO 2014 global tuberculosis report—further to go. Lancet Glob. Health. 2015;3(1):70361–70364. doi: 10.1016/S2214-109X(14)70361-4. [DOI] [PubMed] [Google Scholar]
- 3.Ricciardolo FL. Multiple roles of nitric oxide in the airways. Thorax. 2003;58(2):175–182. doi: 10.1136/thorax.58.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamattina L, García-Mata C, Graziano M, Pagnussat G. Nitric oxide: the versatility of an extensive signal molecule. Annu. Rev. Plant. Biol. 2003;54:109–136. doi: 10.1146/annurev.arplant.54.031902.134752. [DOI] [PubMed] [Google Scholar]
- 5.Wu L, Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol. Rev. 2005;57(4):585–630. doi: 10.1124/pr.57.4.3. [DOI] [PubMed] [Google Scholar]
- 6.Li Q, Lancaster JR., Jr Chemical foundations of hydrogen sulfide biology. Nitric Oxide. 2013;35:21–34. doi: 10.1016/j.niox.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimura H. The physiological role of hydrogen sulfide and beyond. Nitric Oxide. 2014;41:4–10. doi: 10.1016/j.niox.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Wayne LG, Sramek HA. Metronidazole is bactericidal to dormant cells of Mycobacterium tuberculosis. Antimicrob.. Agents. Chemother. 1994:2054–2058. doi: 10.1128/aac.38.9.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393(6685):537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 10.Corper HJ, Cohn ML. The viability and virulence of old cultures of tubercle bacilli. Annu. Rev. Tuberc. 1933;28:856–874. [Google Scholar]
- 11.Wheeler PR, Ratledge C. Metabolism of Mycobacterium tuberculosis. In: Bloom BR, editor. Tuberculosis: pathogenesis, protection, and control. ASM press; 1994. pp. 353–385. [Google Scholar]
- 12.Smith I. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin. Microbiol. Rev. 2003;16(3):463–496. doi: 10.1128/CMR.16.3.463-496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cumming BM, Lamprecht DA, Wells RM, Saini V, Mazorodze JH, Steyn AJ. The physiology and genetics of oxidative stress in mycobacteria. Microbiol. Spectr. 2014;2(3) doi: 10.1128/microbiolspec.MGM2-0019-2013. [DOI] [PubMed] [Google Scholar]
- 14.Salgame P, Geadas C, Collins L, Jones-López E, Ellner JJ. Latent tuberculosis infection--revisiting and revising concepts. Tuberculosis (Edinb) 2015;95(4):373–384. doi: 10.1016/j.tube.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Via LE, Lin LP, Ray SM, Carrillo J, Allen SS, et al. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect. Immun. 2008;76(6):2333–2340. doi: 10.1128/IAI.01515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harper J, Skerry C, Davis SL, Tasneen R, Weir M. Mouse model of necrotic tuberculosis granulomas develops hypoxic lesions. J. Infect. Dis. 2012;205(4):595–602. doi: 10.1093/infdis/jir786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wayne LG, Sohaskey CD. Nonreplicating persistence of Mycobacterium tuberculosis. Annu. Rev. Microbiol. 2001;55:139–163. doi: 10.1146/annurev.micro.55.1.139. [DOI] [PubMed] [Google Scholar]
- 18.Vandiviere HM, Loring WE, Melvin I, Willis S. The treated pulmonary lesion and its tubercle bacillus. II. The death and resurrection. Am. J. Med. Sci. 1956;232(1):30–37. doi: 10.1097/00000441-195607000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Kumar A, Farhana A, Guidry L, Saini V, Hondalus M, Steyn AJ. Redox homeostasis in mycobacteria: the key to tuberculosis control? Expert. Rev. Mol. Med. 2011;13:e39. doi: 10.1017/S1462399411002079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singer JJ. Collapse therapy in pulmonary tuberculosis. Cal. West. Med. 45(2):120–125. [PMC free article] [PubMed] [Google Scholar]
- 21.Pezella AT, Fang W. Surgical aspects of thoracic tuberculosis: a contemporary review -part 1. Curr. Probl. Surg. 2008;45:675–758. doi: 10.1067/j.cpsurg.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Murray JF. Tuberculosis high altitude Worth a try in extensively drug-resistant tuberculosis? Am. J. Respir. Crit. Care. Med. 2014;189(4):390–403. doi: 10.1164/rccm.201311-2043OE. [DOI] [PubMed] [Google Scholar]
- 23.West JB, Dollery CT. Distribution of blood flow and ventilation-perfusion ratio in the lung, measured with radioactive carbon dioxide. J. Appl. Physiol. 1960;15:405–410. doi: 10.1152/jappl.1960.15.3.405. [DOI] [PubMed] [Google Scholar]
- 24.Gurney JW. Cross-sectional physiology of the lung. Radiology. 1991;178(1):1–10. doi: 10.1148/radiology.178.1.1984285. [DOI] [PubMed] [Google Scholar]
- 25.Gurney JW, Schroeder BA. Upper lobe lung disease: physiologic correlates. Review. Radiology. 1988;167(2):359–366. doi: 10.1148/radiology.167.2.3282257. [DOI] [PubMed] [Google Scholar]
- 26.Goodwin RA, Des Prez RM. Apical localization of pulmonary tuberculosis, chronic pulmonary histoplasmosis, and progressive massive fibrosis of the lung. Chest. 1983;83(5):801–805. doi: 10.1378/chest.83.5.801. [DOI] [PubMed] [Google Scholar]
- 27.Cunningham-Bussel A, Nathan CF. Nitrite produced by Mycobacterium tuberculosis in human macrophages in physiologic oxygen impacts bacterial ATP consumption and gene expression. Proc. Natl. Acad. Sci. U.S.A. 2013;110(45):E4256–E4265. doi: 10.1073/pnas.1316894110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray JF. Bill Dock and the location of pulmonary tuberculosis: how bed rest might have helped consumption. Am. J. Respir. Crit. Care. Med. 2003;168(9):1029–1033. doi: 10.1164/rccm.200307-1016OE. [DOI] [PubMed] [Google Scholar]
- 29.Prideaux B, Via LE, Zimmerman MD, Eum S, Sarathy J. The association between sterilizing activity and drug distribution into tuberculosis lesions. Nat. Med. 2015;21(10):1223–1227. doi: 10.1038/nm.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guirado E, Schlesinger LS. Modeling the Mycobacterium tuberculosis granuloma – the critical battlefield in host immunity and disease. Front. Immunol. 2013;4:98. doi: 10.3389/fimmu.2013.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat. Rev. Immunol. 2009;9(9):609–617. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imlay JA. Pathways of oxidative damage. Annu. Rev. Microbiol. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 33.Spooner S, Yilmaz O. The role of reactive-oxygen-species in microbial persistence and inflammation. Int. J. Mol. Sci. 2011;12(1):334–352. doi: 10.3390/ijms12010334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabine E, Schnappinger D. Mycobacterial survival strategies in the phagosome: Defense against host stresses. Cell. Microbiol. 2009;11(8):1170–1178. doi: 10.1111/j.1462-5822.2009.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper AM, Segal BH, Frank AA, Holland SM, Orme IM. Transient loss of resistance to pulmonary tuberculosis in p47(phox−/−) mice. Infect. Immun. 2000;68(3):1231–1234. doi: 10.1128/iai.68.3.1231-1234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flynn JL, Chan J. Immunology of tuberculosis. Annu. Rev. Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 37.Buchmeier NA, Newton GL, Fahey RC. A mycothiol synthase mutant of Mycobacterium tuberculosis has an altered thiol-disulfide content and limited tolerance to stress. J. Bacteriol. 2006;188(17):6245–6252. doi: 10.1128/JB.00393-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saini V, Cumming BM, Guidry L, Lamprecht D, Adamson JH, et al. Ergothioneine maintains redox and bioenergetic homeostasis essential for drug susceptibility and virulence of Mycobacterium tuberculosis. Cell. Rep. 2016;14(3):572–585. doi: 10.1016/j.celrep.2015.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ng VH, Cox JS, Sousa AO, MacMicking JD, McKinney JD. Role of KatG catalase-peroxidase in mycobacterial pathogenesis: countering the phagocyte oxidative burst. Mol Microbiol. 2004;52(5):1291–1302. doi: 10.1111/j.1365-2958.2004.04078.x. [DOI] [PubMed] [Google Scholar]
- 40.Dussurget O, Stewart G, Neyrolles O, Pescher P, Young D, Marchal G. Role of Mycobacterium tuberculosis copper-zinc superoxide dismutase. Infect. Immun. 2001;69(1):529–533. doi: 10.1128/IAI.69.1.529-533.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edwards KM, Cynamon MH, Voladri RK, Hager CC, DeStefano MS. Iron-cofactored superoxide dismutase inhibits host responses to Mycobacterium tuberculosis. Am. J. Respir. Crit. Care. Med. 2001;164(12):2213–2219. doi: 10.1164/ajrccm.164.12.2106093. [DOI] [PubMed] [Google Scholar]
- 42.Piddington DL, Fang FC, Laessig T, Cooper AM, Orme IM, Buchmeier NA. Cu,Zn superoxide dismutase of Mycobacterium tuberculosis contributes to survival in activated macrophages that are generating an oxidative burst. Infect. Immun. 2001;69(8):4980–4987. doi: 10.1128/IAI.69.8.4980-4987.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bryk R, Lima CD, Erdjument-Bromage H, Tempst P, Nathan C. Metabolic enzymes of mycobacteria linked to antioxidant defense by a thioredoxin-like protein. Science. 2002;295(5557):1073–1077. doi: 10.1126/science.1067798. [DOI] [PubMed] [Google Scholar]
- 44.Kumar A, Toledo JC, Patel RP, Lancaster JR, Jr, Steyn AJ. Mycobacterium tuberculosis DosS is a redox sensor and DosT is a hypoxia sensor. Proc. Natl. Acad. Sci. U.S.A. 2007;104(28):11568–11573. doi: 10.1073/pnas.0705054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koshland DE., Jr The molecule of the year. Science. 1992;258(5090):1861. doi: 10.1126/science.1470903. [DOI] [PubMed] [Google Scholar]
- 46.Postorino A, Vetri T, Leggio L, Serio R, Bonvissuto F. Nitric oxide as neuromodulator of sympathetic transmission in rat vas deferens. J. Auton. Pharmacol. 1998;18(1):21–29. doi: 10.1046/j.1365-2680.1998.1810021.x. [DOI] [PubMed] [Google Scholar]
- 47.Blaise GA, Gauvin D, Gangal M, Authier S. Nitric oxide, cell signaling and cell death. Toxicology. 2005;208(2):177–192. doi: 10.1016/j.tox.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 48.Wink DA, Hines HB, Cheng RYS, Switzer CH, Flores-Santana W, et al. Nitric oxide and redox mechanisms in the immune response. J. Leukoc. Biol. 2011;89(6):873–891. doi: 10.1189/jlb.1010550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bogdan C. Nitric oxide and the immune response. Nat. Immunol. 2001;2(10):907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 50.Hill BG, Dranka BP, Bailey SM, Lancaster JR, Jr, Darley-Usmar VM. What part of NO don't you understand? Some answers to the cardinal questions in nitric oxide biology. J. Biol. Chem. 2010;285(26):19699–19704. doi: 10.1074/jbc.R110.101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Förstermann U, Closs EI, Pollock JS, Nakane M, Schwarz P, et al. Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension. 1994;23:1121–1131. doi: 10.1161/01.hyp.23.6.1121. [DOI] [PubMed] [Google Scholar]
- 52.Coleman JW. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001;1(8):1397–1406. doi: 10.1016/s1567-5769(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 53.Tiso M, Schechter AN. Nitrate reduction to nitrite, nitric oxide and ammonia by gut bacteria under physiological conditions. PLoS One. 2015;10(3):e0119712. doi: 10.1371/journal.pone.0119712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wheeler MA, Smith SD, García-Cardeña G, Nathan CF, Weiss RM, Sessa WC. Bacterial infection induces nitric oxide synthase in human neutrophils. J. Clin. Invest. 1997;99(1):110–116. doi: 10.1172/JCI119121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lundberg JO, Weitzberg E. Biology of nitrogen oxides in the gastrointestinal tract. Gut. 2013;62(4):616–629. doi: 10.1136/gutjnl-2011-301649. [DOI] [PubMed] [Google Scholar]
- 56.Adams LB, Dinauer MC, Morgenstern DE, Krahenbuhl JL. Comparison of the roles of reactive oxygen and nitrogen intermediates in the host response to Mycobacterium tuberculosis using transgenic mice. Tuber. Lung. Dis. 1997;78(5–6):237–246. doi: 10.1016/s0962-8479(97)90004-6. [DOI] [PubMed] [Google Scholar]
- 57.Sasaki S, Miura T, Nishikawa S, Yamada K, Hirasue M, Nakane A. Protective role of nitric oxide in Staphylococcus aureus infection in mice. Infect. Immun. 1998;66(3):1017–1022. doi: 10.1128/iai.66.3.1017-1022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chan J, Tanaka K, Carroll D, Flynn J, Bloom BR. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect. Immun. 1995;63(2):736–740. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang CH, Liu CY, Lin HC, Yu CT, Chung KF, Kuo HP. Increased exhaled nitric oxide in active pulmonary tuberculosis due to inducible NO synthase upregulation in alveolar macrophages. Eur. Respir. J. 1998;11(4):809–815. doi: 10.1183/09031936.98.11040809. [DOI] [PubMed] [Google Scholar]
- 60.Nicholson S, Bonecini-Almeida MG, Silva L, Jr, Nathan C, Xie QW, et al. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J. Exp. Med. 1996;183(5):2293–2302. doi: 10.1084/jem.183.5.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan C. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 1997;94(10):5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hernández-Pando R, Orozco-Esteves H, Maldonado HA, Aguilar-León D, Vilchis-Landeros MM. A combination of a transforming growth factor-β antagonist and an inhibitor of cyclooxygenase is an effective treatment for murine pulmonary tuberculosis. Clin. Exp. Immunol. 2006;144(2):264–272. doi: 10.1111/j.1365-2249.2006.03049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chan J, Tanaka K, Carroll D, Flynn J, Bloom BR. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect. Immun. 1995;63(2):736–740. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Botha T, Ryffel B. Reactivation of latent tuberculosis by an inhibitor of inducible nitric oxide synthase in an aerosol murine model. Immunology. 2002;107(3):350–357. doi: 10.1046/j.1365-2567.2002.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mohan VP, Scanga CA, Yu K, Scott HM, Tanaka KE, et al. Effects of tumor necrosis factor alpha on host immune response in chronic persistent tuberculosis: possible role for limiting pathology. Infect Immun. 2001;69(3):1847–1855. doi: 10.1128/IAI.69.3.1847-1855.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mishra BB, Rathinam VAK, Martens GW, Martinot AJ, Kornfeld H, et al. Nitric oxide controls tuberculosis immunopathology by inhibiting NLRP3 inflammasome-dependent IL-1β processing. Nat. Immunol. 2013;14(1):52–60. doi: 10.1038/ni.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. U.S.A. 2000;97(16):8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang CH, Liu CY, Lin HC, Yu CT, Chung KF, Kuo HP. Increased exhaled nitric oxide in active pulmonary tuberculosis due to inducible NO synthase upregulation in alveolar macrophages. Eur. Respir. J. 1998;11(4):809–815. doi: 10.1183/09031936.98.11040809. [DOI] [PubMed] [Google Scholar]
- 69.Nathan C. Inducible nitric oxide synthase in the tuberculous human lung. Am. J. Respir. Crit. Care. Med. 2002;166(2):130–131. doi: 10.1164/rccm.2205016. [DOI] [PubMed] [Google Scholar]
- 70.Choi HS, Rai PR, Chu HW, Cool C, Chan ED. Analysis of nitric oxide synthase and nitrotyrosine expression in human pulmonary tuberculosis. Am. J. Respir. Crit. Care. Med. 2002;166(2):178–186. doi: 10.1164/rccm.2201023. [DOI] [PubMed] [Google Scholar]
- 71.Jagannath C, Actor JK, Hunter RL., Jr Induction of nitric oxide in human monocytes and monocyte cell lines by Mycobacterium tuberculosis. Nitric Oxide. 1998;2(3):174–186. doi: 10.1006/niox.1998.9999. [DOI] [PubMed] [Google Scholar]
- 72.Rich EA, Torres M, Sada E, Finegan CK, Hamilton BD, Toossi Z. Mycobacterium tuberculosis (MTB)-stimulated production of nitric oxide by human alveolar macrophages and relationship of nitric oxide production to growth inhibition of Mtb. Tuber. Lung. Dis. 1997;78(5–6):247–255. doi: 10.1016/s0962-8479(97)90005-8. [DOI] [PubMed] [Google Scholar]
- 73.Kuo HP, Wang CH, Huang KS, Lin HC, Yu CT, et al. Nitric oxide modulates interleukin-1beta and tumor necrosis factor-alpha synthesis by alveolar macrophages in pulmonary tuberculosis. Am. J. Respir. Crit. Care. Med. 2000;161(1):192–199. doi: 10.1164/ajrccm.161.1.9902113. [DOI] [PubMed] [Google Scholar]
- 74.Rockett KA, Brookes R, Udalova I, Vidal V, Hill AV, Kwiatkowski D. 1,25-Dihydroxy vitamin D3 induces nitric oxide synthase and suppresses growth of Mycobacterium tuberculosis in a human macrophage-like cell line. Infect. Immun. 1998;66(11):5314–5321. doi: 10.1128/iai.66.11.5314-5321.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Facchetti F, Vermi W, Fiorentini S, Chilosi M, Caruso A, et al. Expression of inducible nitric oxide synthase in human granulomas and histiocytic reactions. Am. J. Pathol. 1999;154(1):145–152. doi: 10.1016/S0002-9440(10)65261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sharma S, Sharma M, Roy S, Kumar P, Bose M. Mycobacterium tuberculosis induces high production of nitric oxide in coordination with production of tumour necrosis factor-alpha in patients with fresh active tuberculosis but not in MDR tuberculosis. Immunol. Cell. Biol. 2004;82(4):377–382. doi: 10.1111/j.0818-9641.2004.01245.x. [DOI] [PubMed] [Google Scholar]
- 77.Darwin KH, Ehrt S, Gutierrez-Ramos JC, Weich N, Nathan C. The proteasome of Mycobacterium tuberculosis is required for resistance to nitric oxide. Science. 2003;302(5652):1963–1966. doi: 10.1126/science.1091176. [DOI] [PubMed] [Google Scholar]
- 78.Ehrt S, Shiloh MU, Ruan J, Choi M, et al. A novel antioxidant gene from Mycobacterium tuberculosis. J. Exp. Med. 1997;186(11):1885–1896. doi: 10.1084/jem.186.11.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ruan J, John G, Ehrt S, Riley L, Nathan C. noxR3, a Novel gene from Mycobacterium tuberculosis, protects Salmonella typhimurium from nitrosative and oxidative stress. Infect Immun. 1999;67(7):3276–3283. doi: 10.1128/iai.67.7.3276-3283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bryk R, Griffin P, Nathan C. Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature. 2000;407(6801):211–215. doi: 10.1038/35025109. [DOI] [PubMed] [Google Scholar]
- 81.Schnappinger D, Ehrt S, Voskuil MI, Liu Y, Mangan JA, et al. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages; Insights into the phagosomal environment. J. Exp. Med. 2003;198(5):693–704. doi: 10.1084/jem.20030846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tan MP, Sequeira P, Lin WW, Phong WY, Cliff P. Nitrate respiration protects hypoxic Mycobacterium tuberculosis against acid- and reactive nitrogen species stresses. PLoS One. 2010;5(10):e13356. doi: 10.1371/journal.pone.0013356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wayne LG, Hayes LG. Nitrate reduction as a marker for hypoxic shift down of Mycobacterium tuberculosis. Tuber. Lung. Dis. 1998;79(2):127–132. doi: 10.1054/tuld.1998.0015. [DOI] [PubMed] [Google Scholar]
- 84.Voskuil MI, Schnappinger D, Visconti KC, Harrell MI, Dolganov GM, et al. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J. Exp. Med. 2003;198(5):705–713. doi: 10.1084/jem.20030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Converse PJ, Karakousis PC, Klinkenberg LC, Kesavan AK, Ly LH, et al. Role of the dosR-dosS two-Component regulatory system in Mycobacterium tuberculosis virulence in three animal models. Infect. Immun. 2009;77(3):1230–1237. doi: 10.1128/IAI.01117-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mehra S, Foreman TW, Didier PJ, Ahsan MH, Hudock TA. The DosR regulon modulates adaptive immunity and is essential for Mycobacterium tuberculosis persistence. Am. J. Respir. Crit. Care. Med. 2015;191(10):1185–1196. doi: 10.1164/rccm.201408-1502OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cunningham-Bussel A, Zhang T, Nathana C. Nitrite produced by Mycobacterium tuberculosis in human macrophages in physiologic oxygen impacts bacterial ATP consumption and gene expression. Proc. Natl. Acad. Sci. U.S.A. 2013;110(45):E4256–E4265. doi: 10.1073/pnas.1316894110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kirkby KA, Adin CA. Products of heme oxygenase and their potential therapeutic applications. Am. J. Physiol. Renal. Physiol. 2006;290(3):563–571. doi: 10.1152/ajprenal.00220.2005. [DOI] [PubMed] [Google Scholar]
- 89.Agarwal A, Bolisetty S. Adaptive responses to tissue injury: Role of heme oxygenase-1. Trans. Am. Clin. Climatol. Assoc. 2013;124:111–122. [PMC free article] [PubMed] [Google Scholar]
- 90.Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science. 1993;259(5093):381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 91.Durante W, Johnson FK, Johnson RA. Role of carbon monoxide in cardiovascular function. J. Cell. Mol. Med. 2006;10(3):672–686. doi: 10.1111/j.1582-4934.2006.tb00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Otterbein LE, Bach FH, Alam J, Soares M, Tao LH, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000;6(4):422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 93.Sheikh SZ, Hegazi RA, Kobayashi T, Onyiah JC, Russo SM, et al. An anti-inflammatory role for carbon monoxide and heme oxygenase-1 in chronic Th2-mediated murine colitis. J. Immunol. 2011;186(9):5506–5513. doi: 10.4049/jimmunol.1002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH. Carbon monoxide generated by heme oxygenase-1 suppresses endothelial cell apoptosis. J. Exp. Med. 2000;192(7):1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Al-Owais MM, Scragg JL, Dallas ML, Boycott HE, Warburton P, et al. Carbon monoxide mediates the anti-apoptotic effects of heme oxygenase-1 in medulloblastoma DAOY cells via K+ channel inhibition. J. Biol. Chem. 2012;287(29):24754–24764. doi: 10.1074/jbc.M112.357012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takagi T, Uchiyama K, Naito Y. The therapeutic potential of carbon monoxide for inflammatory bowel disease. Digestion. 2015;91(1):13–18. doi: 10.1159/000368765. [DOI] [PubMed] [Google Scholar]
- 97.Motterlini R, Otterbein LE. The therapeutic potential of carbon monoxide. Nat. Rev. Drug Discov. 2010;9(9):728–743. doi: 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
- 98.Nobre LS, Seixas JD, Romão CC, Saraiva LM. Antimicrobial action of carbon monoxide-releasing compounds. Antimicrob. Agents Chemother. 2007;51(12):4303–4307. doi: 10.1128/AAC.00802-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Murray TS, Okegbe C, Gao Y, Kazmierczak BI, Motterlini R, et al. The carbon monoxide releasing molecule CORM-2 attenuates Pseudomonas aeruginosa biofilm formation. PLoS One. 2012;7(4):e35499. doi: 10.1371/journal.pone.0035499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chin BY, Otterbein LE. Carbon monoxide is a poison…to microbes! CO as a bactericidal molecule. Curr. Opin. Pharmacol. 2009;9(4):490–500. doi: 10.1016/j.coph.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 101.Chung SW, Liu X, Macias AA, Baron RM, Perrella MA. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J. Clin. Invest. 2008;118(1):239–247. doi: 10.1172/JCI32730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Otterbein LE, May A, Chin BY. Carbon monoxide increases macrophage bacterial clearance through Toll-like receptor (TLR) 4 expression. Cell. Mol. Biol. 2005;51(5):433–440. [PubMed] [Google Scholar]
- 103.Wegiel B, Larsen R, Gallo D, Chin BY, Harris C. Macrophages sense and kill bacteria through carbon monoxide-dependent inflammasome activation. J. Clin. Invest. 2014;124(11):4926–4940. doi: 10.1172/JCI72853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pamplona A, Ferreira A, Balla J, Jeney V, Balla G, et al. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat. Med. 2007;13(6):703–710. doi: 10.1038/nm1586. [DOI] [PubMed] [Google Scholar]
- 105.Jeney V, Ramos S, Bergman ML, Bechmann I, Tischer J, et al. Control of disease tolerance to malaria by nitric oxide and carbon monoxide. Cell. Rep. 2014;8(1):126–136. doi: 10.1016/j.celrep.2014.05.054. [DOI] [PubMed] [Google Scholar]
- 106.Zacharia VM, Manzanillo PS, Nair VR, Marciano DK, Kinch LN, et al. cor, a novel carbon monoxide resistance gene, is essential for Mycobacterium tuberculosis pathogenesis. MBio. 2013;4(6):e00721–e00713. doi: 10.1128/mBio.00721-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kumar A, Deshane JS, Crossman DK, Bolisetty S, Yan BS, et al. Heme oxygenase-1-derived carbon monoxide induces the Mycobacterium tuberculosis dormancy regulon. J. Biol. Chem. 2008;283(26):18032–18039. doi: 10.1074/jbc.M802274200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shiloh MU, Manzanillo P, Cox JS. Mycobacterium tuberculosis senses host-derived carbon monoxide during macrophage infection. Cell Host Microbe. 2008;3(5):323–330. doi: 10.1016/j.chom.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Regev D, Surolia R, Karki S, Zolak J, Montes-Worboys A, et al. Heme Oxygenase-1 promotes granuloma development and protects against dissemination of mycobacteria. Lab. Invest. 2012;92(11):1541–1552. doi: 10.1038/labinvest.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Silva-Gomes S, Appelberg R, Larsen R, Soares MP, Gomes MS. Heme catabolism by heme oxygenase-1 confers host resistance to mycobacterium Infection. Infect. Immun. 2013;81(7):2536–2545. doi: 10.1128/IAI.00251-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ryter SW, Choi AM. Carbon monoxide: present and future indications for a medical gas. Korean J. Intern. Med. 2013;28(2):123–140. doi: 10.3904/kjim.2013.28.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ciaramella A, Cavone A, Santucci MB, Amicosante M, Martino A, et al. Proinflammatory cytokines in the course of Mycobacterium tuberculosis-induced apoptosis in monocytes/macrophages. J. Infect. Dis. 2002;186(9):1277–1282. doi: 10.1086/344645. [DOI] [PubMed] [Google Scholar]
- 113.Ramakantan R, Bandekar VG, Gandhi MS, Aulakh BG, Deshmukh HL. Massive hemoptysis due to pulmonary tuberculosis: control with bronchial artery embolization. Radiology. 1996;200(3):691–694. doi: 10.1148/radiology.200.3.8756916. [DOI] [PubMed] [Google Scholar]
- 114.Dheda K, Booth H, Huggett JF, Johnson MA, Zumla A, Rook GA. Lung remodeling in pulmonary tuberculosis. J. Infect. Dis. 2005;192(7):1201–1209. doi: 10.1086/444545. [DOI] [PubMed] [Google Scholar]
- 115.Eruslanov EB, Majorov KB, Orlova MO, Mischenko VV, Kondratieva TK. Lung cell responses to M. tuberculosis in genetically susceptible and resistant mice following intratracheal challenge. Clin. Exp. Immunol. 2004;135(1):19–28. doi: 10.1111/j.1365-2249.2004.02328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Andrade BB, Kumar PN, Mayer-Barber KD, Barber DL, Sridhar R, et al. Plasma heme oxygenase-1 levels distinguish latent or successfully treated human tuberculosis from active disease. PLoS One. 2013;8(5):e62618. doi: 10.1371/journal.pone.0062618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Andrade BB, Kumar PN, Amaral EP, Riteau N, Mayer-Barber KD, et al. Heme oxygenase-1 regulation of matrix metalloproteinase-1 expression underlies distinct disease profiles in tuberculosis. J. Immunol. 2015;195(6):2763–2773. doi: 10.4049/jimmunol.1500942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Elkington P, Shiomi T, Breen R, Nuttall RK, Ugarte-Gil CA, et al. MMP-1 drives immunopathology in human tuberculosis and transgenic mice. J. Clin. Invest. 2011;121(5):1827–1833. doi: 10.1172/JCI45666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sinnis P, Ernst JD. CO-opting the host HO-1 pathway in tuberculosis and malaria. Cell Host Microbe. 2008;3(5):277–279. doi: 10.1016/j.chom.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 120.Meng G, Ma Y, Xie L, Ferro A, Ji Y. Emerging role of hydrogen sulfide in hypertension and related cardiovascular diseases. Br. J. Pharmacol. 2015;172(23):5501–5511. doi: 10.1111/bph.12900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mani S, Untereiner A, Wu L, Wang R. Hydrogen sulfide and the pathogenesis of atherosclerosis. Antioxid. Redox Signal. 2014;20(5):805–817. doi: 10.1089/ars.2013.5324. [DOI] [PubMed] [Google Scholar]
- 122.Polhemus DJ, Calvert JW, Butler J, Lefer DJ. The cardioprotective actions of hydrogen sulfide in acute myocardial infarction and heart failure. Scientifica (Cairo) 2014:768607. doi: 10.1155/2014/768607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Szabo C. Roles of hydrogen sulfide in the pathogenesis of diabetes mellitus and its complications. Antioxid. Redox. Signal. 2012;17(1):68–80. doi: 10.1089/ars.2011.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bhatia M. Role of hydrogen sulfide in the pathology of inflammation. Scientifica (Cairo) 2012:159680. doi: 10.6064/2012/159680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gong QH, Shi XR, Hong ZY, Pan LL, Liu XH, Zhu YZ. A new hope for neurodegeneration: possible role of hydrogen sulfide. J. Alzheimers. Dis. 2011:173–182. doi: 10.3233/JAD-2011-110128. [DOI] [PubMed] [Google Scholar]
- 126.Zhang G, Wang P, Yang G, Cao Q, Wang R. The inhibitory role of hydrogen sulfide in airway hyperresponsiveness and inflammation in a mouse model of asthma. Am. J. Pathol. 2013;182(4):1188–1195. doi: 10.1016/j.ajpath.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 127.Kamada N, Chen GY, Inohara N, Núñez G. Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol. 2013;14(7):685–690. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kimura H. Hydrogen sulfide: its production, release and functions. Amino Acids. 2011;41(1):113–121. doi: 10.1007/s00726-010-0510-x. [DOI] [PubMed] [Google Scholar]
- 129.Kolluru GK, Shen X, Bir SC, Kevil CG. Hydrogen sulfide chemical biology: Pathophysiological roles and detection. Nitric Oxide. 2013;35:5–20. doi: 10.1016/j.niox.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Basudhar D, Ridnour LA, Cheng R, Kesarwala AH, Heinecke J, Wink DA. Biological signaling by small inorganic molecules. Coord. Chem. Rev. 2016;306(Pt 2):708–723. doi: 10.1016/j.ccr.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nagy P. Mechanistic chemical perspective of hydrogen sulfide signaling. Methods Enzymol. 2015;554:3–29. doi: 10.1016/bs.mie.2014.11.036. [DOI] [PubMed] [Google Scholar]
- 132.Joyner JL, Peyer SM, Lee RW. Possible roles of sulfur-containing amino acids in a chemoautotrophic bacterium-mollusc symbiosis. Biol. Bull. 2003;205(3):331–338. doi: 10.2307/1543296. [DOI] [PubMed] [Google Scholar]
- 133.Nishida M, Sawa T, Kitajima N, Ono K, Inoue H, et al. Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nat. Chem. Biol. 2012;8(8):714–724. doi: 10.1038/nchembio.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol. Rev. 2012;92(2):791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 135.Lloyd D. Hydrogen sulfide: clandestine microbial messenger? Trends. Microbiol. 2006;14(10):456–462. doi: 10.1016/j.tim.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 136.Madurga A, Golec A, Pozarska A, Ishii I, Mižíková I. The H2S-generating enzymes cystathionine β-synthase and cystathionine γ-lyase play a role in vascular development during normal lung alveolarization. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2015;309(7):L710–L724. doi: 10.1152/ajplung.00134.2015. [DOI] [PubMed] [Google Scholar]
- 137.Miles EW, Kraus JP. Cystathionine beta-synthase: structure, function, regulation, and location of homocystinuria-causing mutations. J. Biol. Chem. 2004;279(29):29871–29874. doi: 10.1074/jbc.R400005200. [DOI] [PubMed] [Google Scholar]
- 138.Pan LL, Liu XH, Gong QH, Yang HB, Zhu YZ. Role of cystathionine γ-lyase/hydrogen sulfide pathway in cardiovascular disease: A novel therapeutic strategy? Antioxid. Redox. Signal. 2012;17(1):106–118. doi: 10.1089/ars.2011.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science. 2005;308(5721):518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- 140.Aslami H, Schultz MJ, Juffermans NP. Potential applications of hydrogen sulfide-induced suspended animation. Curr. Med. Chem. 2009;16(10):1295–1303. doi: 10.2174/092986709787846631. [DOI] [PubMed] [Google Scholar]
- 141.Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J. Bioenerg. Biomembr. 2008;40:533–539. doi: 10.1007/s10863-008-9166-6. [DOI] [PubMed] [Google Scholar]
- 142.Loebel RO, Richardson HB. The influence of adverse conditions upon the respiratory metabolism and growth of human tubercle bacilli. J. Bacteriol. 1933;26(2):167–200. doi: 10.1128/jb.26.2.167-200.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Arora K, Ochoa-Montaño B, Tsang PS, Blundell TL, Dawes SS, et al. Respiratory flexibility in response to inhibition of cytochrome C oxidase in Mycobacterium tuberculosis. Antimicrob. Agents. Chemother. 2014;58(11):6962–6965. doi: 10.1128/AAC.03486-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rao SP, Alonso S, Rand L, Dick T, Pethe K. The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc. Natl. Acad. Sci U.S.A. 2008;105(33):11945–11950. doi: 10.1073/pnas.0711697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Erusalimsky JD, Moncada S. Nitric oxide and mitochondrial signaling: from physiology to pathophysiology Arterioscler. Thromb. Vasc. Biol. 2007;27(12):2524–2531. doi: 10.1161/ATVBAHA.107.151167. [DOI] [PubMed] [Google Scholar]
- 146.Alonso JR, Cardellach F, López S, Casademont J, Miró O. Carbon monoxide specifically inhibits cytochrome c oxidase of human mitochondrial respiratory chain. Pharmacol. Toxicol. 2003;93(3):142–146. doi: 10.1034/j.1600-0773.2003.930306.x. [DOI] [PubMed] [Google Scholar]
- 147.Fu M, Zhang W, Wu L, Yang G, Li H, Wang R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc. Natl. Acad. Sci. U.S.A. 2012;109(8):2943–2948. doi: 10.1073/pnas.1115634109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kajimura M, Fukuda R, Bateman RM. Interactions of multiple gas-transducing systems: Hallmarks and uncertainties of CO, NO, and H2S gas biology. Antioxid. Redox. Signal. 2010;13(2):157–192. doi: 10.1089/ars.2009.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Everts B, Amiel E, van der Windt GJ, Freitas TC, Chott R, et al. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood. 2012;120(7):1422–1431. doi: 10.1182/blood-2012-03-419747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.D'Amico G, Lam F, Hagen T, Moncada S. Inhibition of cellular respiration by endogenously produced carbon monoxide. J. Cell. Sci. 2006;119(Pt 11):2291–2298. doi: 10.1242/jcs.02914. [DOI] [PubMed] [Google Scholar]
- 151.Hishiki T, Yamamoto T, Morikawa T, Kubo A, Kajimura M, Suematsu M. Carbon monoxide: impact on remethylation/transsulfuration metabolism and its pathophysiologic implications. J. Mol. Med (Berl) 2012;90(3):245–254. doi: 10.1007/s00109-012-0875-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Puranik M, Weeks CL, Lahaye D, Kabil O, Taoka S, et al. Dynamics of carbon monoxide binding to cystathionine beta-synthase. J. Biol. Chem. 2006;281(19):13433–13438. doi: 10.1074/jbc.M600246200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vicente JB, Colaço HG, Mendes MI, Sarti P, Leandro P, Giuffrè A. NO* binds human cystathionine β-synthase quickly and tightly. J. Biol. Chem. 2014;289(12):8579–8587. doi: 10.1074/jbc.M113.507533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gengenbacher M, Kaufmann SHE. Mycobacterium tuberculosis: Success through dormancy. FEMS Microbiol. Rev. 2012;36(3):514–532. doi: 10.1111/j.1574-6976.2012.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wayne LG. Dormancy of Mycobacterium tuberculosis and latency of disease. Eur. J. Clin. Microbiol. Infect. Dis. 1994;13(11):908–914. doi: 10.1007/BF02111491. [DOI] [PubMed] [Google Scholar]
- 156.Chao MC, Rubin EJ. Letting sleeping dos lie: does dormancy play a role in tuberculosis? Annu. Rev. Microbiol. 2010;64:293–311. doi: 10.1146/annurev.micro.112408.134043. [DOI] [PubMed] [Google Scholar]
- 157.Rustad TR, Harrell MI, Liao R, Sherman DR. The enduring hypoxic response of Mycobacterium tuberculosis. PLoS One. 2008;3:e1502. doi: 10.1371/journal.pone.0001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rustad TR, Sherrid AM, Minch KJ, Sherman DR. Hypoxia: a window into Mycobacterium tuberculosis latency. Cell Microbiol. 2009;11:1151–1159. doi: 10.1111/j.1462-5822.2009.01325.x. [DOI] [PubMed] [Google Scholar]
- 159.Voskuil MI, Visconti KC, Schoolnik GK. Mycobacterium tuberculosis gene expression during adaptation to stationary phase and low-oxygen dormancy. Tuberculosis (Edinb.) 2004;84:218–227. doi: 10.1016/j.tube.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 160.Yuan Y, Crane DD, Simpson RM, Zhu YQ, Hickey MJ, Sherman DR, Barry CE3rd. The 16-kDa alpha-crystallin (Acr) protein of Mycobacterium tuberculosis is required for growth in macrophages. Proc. Natl. Acad. Sci. U.S.A. 1998;95(16):9578–9583. doi: 10.1073/pnas.95.16.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sivaramakrishnan S, Montellano PRO. The DosS-DosT/DosR mycobacterial sensor system. Biosensors (Basel) 2013;3(3):259–282. doi: 10.3390/bios3030259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Long R, Jones R, Talbot J, Mayers I, Barrie J, Hoskinson M, Light B. Inhaled nitric oxide treatment of patients with pulmonary tuberculosis evidenced by positive sputum smears. Antimicrob. Agents. Chemother. 2005;49(3):1209–1212. doi: 10.1128/AAC.49.3.1209-1212.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hazleton EB. Carbon monoxide a predisposing cause of pulmonary tuberculosis. Br. Med. J. 1923;2(3281):945–946. [Google Scholar]
- 164.Saini V, Farhana A, Steyn AJ. Mycobacterium tuberculosis WhiB3: a novel iron-sulfur cluster protein that regulates redox homeostasis and virulence. Antioxid. Redox. Signal. 2012;16(7):687–697. doi: 10.1089/ars.2011.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Singh A, Guidry L, Narasimhulu KV, Mai D, Trombley J, et al. Mycobacterium tuberculosis WhiB3 responds to O2 and nitric oxide via its [4Fe-4S] cluster and is essential for nutrient starvation survival. Proc. Natl. Acad. Sci. U.S.A. 2007;104(28):11562–11567. doi: 10.1073/pnas.0700490104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Konar M, Alam MS, Arora C, Agrawal P. WhiB2/Rv3260c, a cell division-associated protein of Mycobacterium tuberculosis H37Rv, has properties of a chaperone. FEBS. J. 2012;279(15):2781–2792. doi: 10.1111/j.1742-4658.2012.08662.x. [DOI] [PubMed] [Google Scholar]
- 167.Singh A, Crossman DK, Mai D, Guidry L, Voskuil MI, et al. Mycobacterium tuberculosis WhiB3 maintains redox homeostasis by regulating virulence lipid anabolism to modulate macrophage response. PLoS Pathog. 2009;5(8):e1000545. doi: 10.1371/journal.ppat.1000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Steyn AJC, Collins DM, Hondalus MK, Jacobs WR, Jr, Kawakami RP, Bloom BR. Mycobacterium tuberculosis WhiB3 interacts with RpoV to affect host survival but is dispensable for in vivo growth. Proc. Natl. Acad. Sci. U.S.A. 2002;99(5):3147–3152. doi: 10.1073/pnas.052705399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Geiman DE, Raghunand TR, Agarwal N, Bishai WR. Differential gene expression in response to exposure to antimycobacterial agents and other stress conditions among seven Mycobacterium tuberculosis whiB-like genes. Antimicrob. Agents. Chemother. 2006;50(8):2836–2841. doi: 10.1128/AAC.00295-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Solans L, Aguiló N, Samper S, Pawlik A, Frigui W, et al. A specific polymorphism in Mycobacterium tuberculosis H37Rv causes differential ESAT-6 expression and identifies WhiB6 as a Novel ESX-1 component. Infect. Immun. 2014;82(8):3446–3456. doi: 10.1128/IAI.01824-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Morris RP, Nguyen L, Gatfield J, Visconti K, Nguyen K, et al. Ancestral antibiotic resistance in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 2005;102(34):12200–12205. doi: 10.1073/pnas.0505446102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Burian J, Ramón-García S, Sweet G, Gómez-Velasco A, Av-Gay Y, Thompson CJ. The mycobacterial transcriptional regulator whiB7 gene links redox homeostasis and intrinsic antibiotic resistance. J. Biol. Chem. 2012;287(1):299–310. doi: 10.1074/jbc.M111.302588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Davis NK, Chater KF. The Streptomyces coelicolor whiB gene encodes a small transcription factor-like protein dispensable for growth but essential for sporulation. Mol. Gen. Genet. 1992;232(3):351–358. doi: 10.1007/BF00266237. [DOI] [PubMed] [Google Scholar]
- 174.Deretic V, Philipp W, Dhandayuthapani S, Mudd MH, Curcic R. Mycobacterium tuberculosis is a natural mutant with an inactivated oxidative-stress regulatory gene: implications for sensitivity to isoniazid. Mol. Microbiol. 1995;17(5):889–900. doi: 10.1111/j.1365-2958.1995.mmi_17050889.x. [DOI] [PubMed] [Google Scholar]
- 175.Green J, Rolfe MD, Smith LJ. Transcriptional regulation of bacterial virulence gene expression by molecular oxygen and nitric oxide. Virulence. 2014;5(8):794–809. doi: 10.4161/viru.27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Saini V, Farhana A, Glasgow JN, Steyn AJC. Iron sulfur cluster proteins and microbial regulation: implications for understanding tuberculosis. Curr. Opin. Chem. Biol. 2012;16(0):45–53. doi: 10.1016/j.cbpa.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Major TA, Burd H, Whitman WB. Abundance of 4Fe-4S motifs in the genomes of methanogens and other prokaryotes. FEMS. Microbiol. Lett. 2004;239(1):117–123. doi: 10.1016/j.femsle.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 178.Zhou P, Long Q, Zhou Y, Wang H, Xie J. Mycobacterium tuberculosis two-component systems and implications in novel vaccines and drugs. Crit. Rev. Eukaryot. Gene. Expr. 2012;22(1):37–52. doi: 10.1615/critreveukargeneexpr.v22.i1.30. [DOI] [PubMed] [Google Scholar]
- 179.Parish T. Two-component regulatory systems of mycobacteria. Microbiol, Spectr. 2014;2(1) doi: 10.1128/microbiolspec.MGM2-0010-2013. MGM2-0010-2013. [DOI] [PubMed] [Google Scholar]
- 180.Roberts G, Vadrevu IS, Madiraju MV, Parish T. Control of CydB and GltA1 expression by the SenX3-RegX3 two component regulatory system of Mycobacterium tuberculosis. PLoS One. 2011;6(6):e21090. doi: 10.1371/journal.pone.0021090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Parish T, Smith DA, Roberts G, Betts J, Stoker NG. The senX3-regX3 two-component regulatory system of Mycobacterium tuberculosis is required for virulence. Microbiology. 2003;149(Pt 6):1423–1435. doi: 10.1099/mic.0.26245-0. [DOI] [PubMed] [Google Scholar]
- 182.Rifat D, Bishai WR, Karakousis PC. Phosphate depletion: a novel trigger for Mycobacterium tuberculosis persistence. J. Infect. Dis. 2009;200(7):1126–1135. doi: 10.1086/605700. [DOI] [PubMed] [Google Scholar]
- 183.Rickman L, Saldanha JW, Hunt DM, Hoar DN, Colston MJ, et al. A two-component signal transduction system with a PAS domain-containing sensor is required for virulence of Mycobacterium tuberculosis in mice. Biochem. Biophys. Res. Commun. 2004;314(1):259–267. doi: 10.1016/j.bbrc.2003.12.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Singh N, Kumar A. Virulence factor SenX3 is the oxygen-controlled replication switch of Mycobacterium tuberculosis. Antioxid. Redox. Signal. 2015;22(7):603–613. doi: 10.1089/ars.2014.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Radhakrishnan N, Yadav SP, Sachdeva A, Pruthi PK, Sawhney S. Human heme oxygenase-1 deficiency presenting with hemolysis, nephritis, and asplenia. J. Pediatr. Hematol. Oncol. 2011;33(1):74–78. doi: 10.1097/MPH.0b013e3181fd2aae. [DOI] [PubMed] [Google Scholar]
- 186.Kapturczak MH, Wasserfall C, Brusko T, Campbell-Thompson M, Ellis TM, et al. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am. J. Pathol. 2004;165(3):1045–1053. doi: 10.1016/S0002-9440(10)63365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang J, Hegele RA. Genomic basis of cystathioninuria (MIM 219500) revealed by multiple mutations in cystathionine gamma-lyase (CTH) Hum. Genet. 2003;112(4):404–408. doi: 10.1007/s00439-003-0906-8. [DOI] [PubMed] [Google Scholar]
- 188.Ichinohe A, Kanaumi T, Takashima S, Enokido Y, Nagai Y, Kimura H. Cystathionine beta-synthase is enriched in the brains of Down's patients. Biochem. Biophys. Res. Commun. 2005;338(3):1547–1550. doi: 10.1016/j.bbrc.2005.10.118. [DOI] [PubMed] [Google Scholar]
- 189.Billaut-Laden I, Rat E, Allorge D, Crunelle-Thibaut A, Cauffiez C, et al. Evidence for a functional genetic polymorphism of the human mercaptopyruvate sulfurtransferase (MPST), a cyanide detoxification enzyme. Toxicol. Lett. 2006;165(2):101–111. doi: 10.1016/j.toxlet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 190.Kröncke KD, Fehsel K, Kolb-Bachofen V. Inducible nitric oxide synthase in human diseases. Clin. Exp. Immunol. 1998;113(2):147–156. doi: 10.1046/j.1365-2249.1998.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Farhana A, Guidry L, Srivastava A, Singh A, Hondalus MK, Steyn AJ. Reductive stress in microbes: implications for understanding Mycobacterium tuberculosis disease and persistence. Adv. Microb. Physiol. 2010;57:43–117. doi: 10.1016/B978-0-12-381045-8.00002-3. [DOI] [PubMed] [Google Scholar]