Abstract
Electron microscopy (EM)-based techniques are mostly responsible for our current view of cell morphology at the subcellular level and continue to play an essential role in biological research. In cells from the immune system, such as eosinophils, EM has helped to understand how cells package and release mediators involved in immune responses. Ultrastructural investigations of human eosinophils enabled visualization of secretory processes in detail and identification of a robust, vesicular trafficking essential for the secretion of immune mediators via a non-classical secretory pathway associated with secretory (specific) granules. This vesicular system is mainly organized as large tubular-vesicular carriers (Eosinophil Sombrero Vesicles - EoSVs) actively formed in response to cell activation and provides a sophisticated structural mechanism for delivery of granule-stored mediators. In this review, we highlight the application of EM techniques to recognize pools of immune mediators at vesicular compartments and to understand the complex secretory pathway within human eosinophils involved in inflammatory and allergic responses.
Keywords: vesicular trafficking, cell secretion, eosinophils, immune mediators, transmission electron microscopy, immunogold electron microscopy, ultrastructure, leukocytes, immune response, inflammation
Graphical Abstract
Introduction
Cells from the immune system communicate by secreting mediators, which govern the course of immune responses. However, before being transferred from one group of cells to another, immune mediators need to be intracellularly mobilized, trafficked and secreted. Thus, the knowledge of secretory pathways and their related intracellular compartments are important to understand immune responses. In this sense, a central question is: how does a specific immune mediator traverse from intracellular sites to the cell surface in order to be released upon cell activation?
In leukocytes such as eosinophils, immune mediators are mostly transported en route to the plasma membrane in a ER-Golgi-independent manner, i. e., these messengers are stored as preformed pools within secretory (specific) granules, a major population present in the eosinophil cytoplasm (Fig. 1), from where they are mobilized and released in response to cell activation. Granules can fuse with the plasma membrane in order to secrete their contents, but the most frequent mechanism for the delivery of eosinophil mediators involve vesicular carriers, which recruit cargos directly from secretory granules, a secretory process termed piecemeal degranulation (reviewed in [1–3]). Thus, a granule-derived, vesicle-mediated secretion takes place within human eosinophils, enabling rapid release of specific mediators [3].
Figure 1.
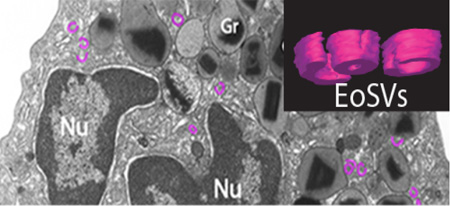
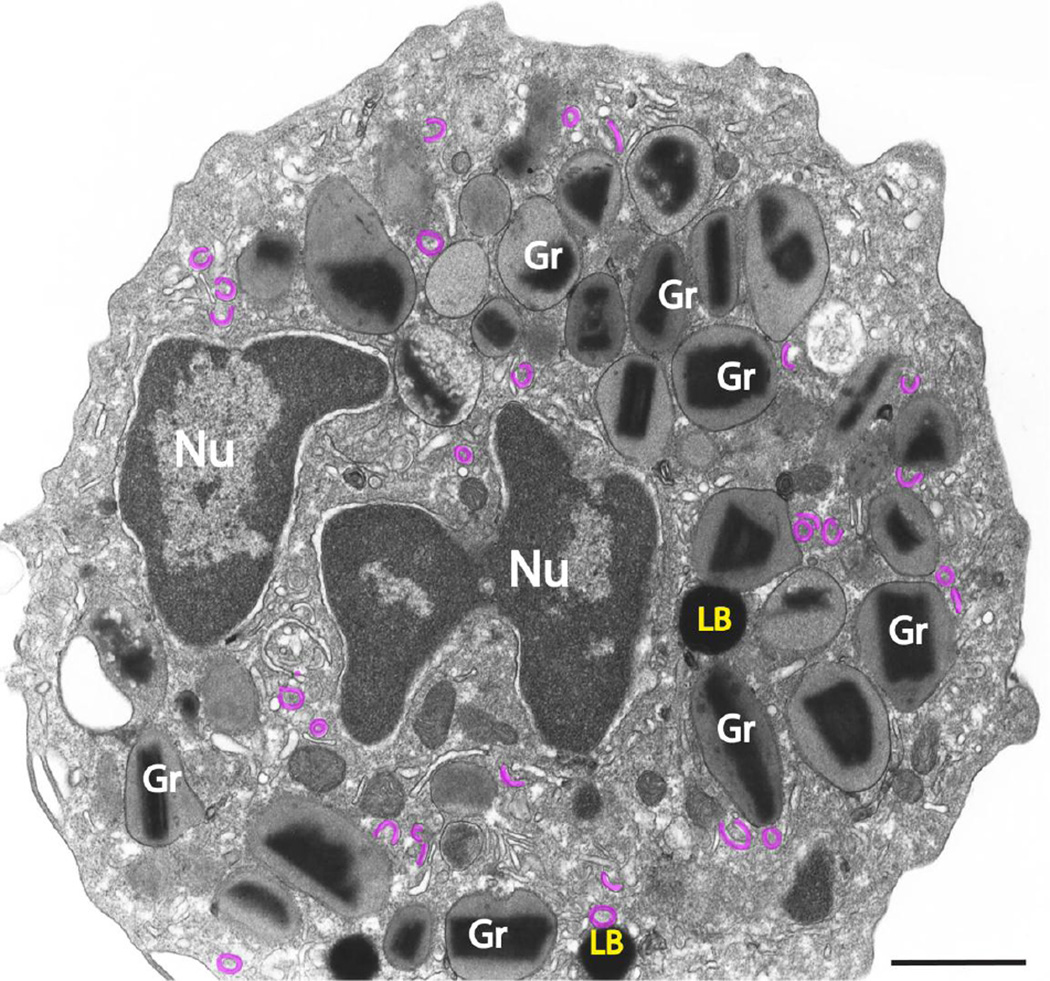
Conventional transmission electron microscopy (TEM) of a human eosinophil. The eosinophil cytoplasm contains a major population of secretory (specific) granules (Gr), lipid bodies (LBs), vesiculotubular structures termed Eosinophil Sombrero Vesicles (EoSVs, highlighted in pink) and an usually bilobed nucleus (Nu) with condensed, marginated chromatin. Note the unique morphology of specific granules (Gr) with an internal often electron-dense crystalline core surrounded by an electron-lucent matrix. Scale bar, 800 nm.
Our group has been using conventional transmission electron microscopy (TEM), immunonanogold EM and electron tomography to understand the cellular mechanisms involved in the release of immune mediators from human eosinophils activated by inflammatory stimuli [4–11]. Because TEM provides a comprehensive view of the interior of a cell at nanometer scale, application of this technique has enabled the identification of a consistent, granule-associated vesicular system represented by large vesiculotubular structures formed in response to eosinophil activation [5].
Combined with molecular detection methods (immunonanogold), TEM provides sufficient resolution to localize proteins to intracellular compartments [12]. The use of an improved approach for ultrastructural detection of proteins in leukocytes (pre-embedding immunonanogold EM) [13] led to the first ultrastructural identification of a vesicle-based transport of interleukin-4 (IL-4) [5] and major basic protein (MBP-1) [7] from eosinophil secretory granules. More recently, this technique located SNARES at secretory granules and vesicles [11] and identified an active intracellular CD63 trafficking connected to eosinophil granule-derived secretory pathways [14]. Here, we review the use of TEM to visualize and understand intracellular trafficking and secretion of immune mediators in eosinophil leukocytes during immune responses.
Eosinophil activation triggers formation of large transport carriers
It is well documented that large vesicular-tubular structures are fundamental to transfer secretory cargos within different cell types and secretory pathways [15–18]. These structures can show different sizes and shapes and complex plasticity [17].
In human eosinophils, vesiculotubular structures, considered as microgranules in the past, constitute one important morphological feature of these cells and are clearly identified in the cytoplasm by TEM [19] (Fig. 1 and 2). These structures have a typical and unique morphology, which differ from tubular vesicles found in other cells. We coined the term Eosinophil Sombrero Vesicles (EoSVs) for these eosinophil vesiculotubular carriers in 2005 when we identified them as morphologically distinct vesicles resembling a “sombrero hat” and involved in the trafficking of eosinophil products [5].
Figure 2.
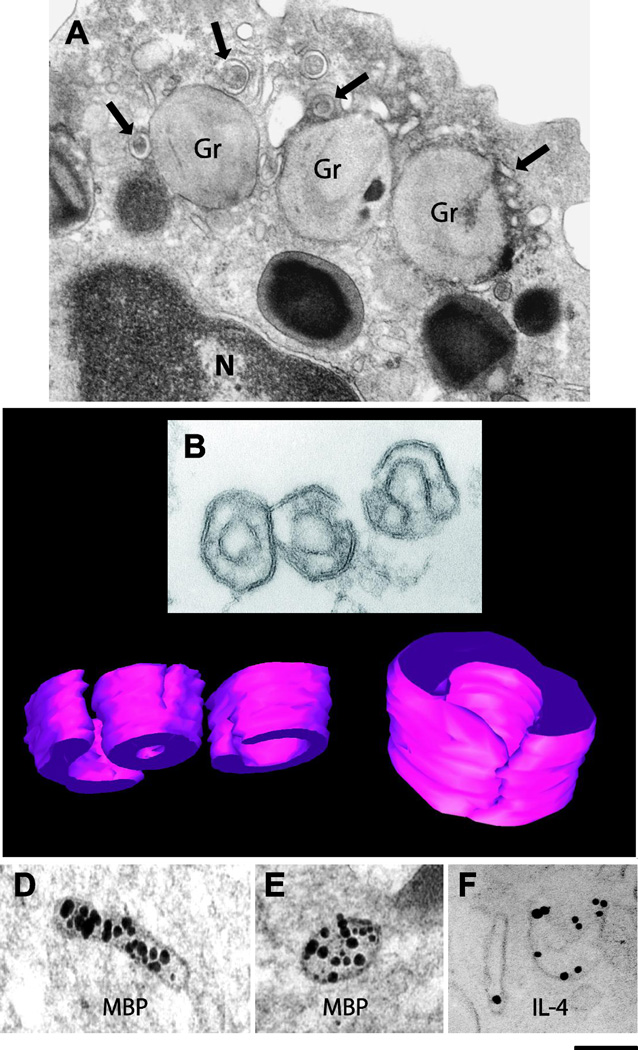
Vesicular trafficking of granule-derived products from human eosinophils. (A) Eosinophil sombrero vesicles - EoSVs - (arrows) are observed in the cytoplasm of a CCL11-stimulated eosinophil surrounding emptying, enlarged secretory granules (Gr). Intact granules (Gr) with typical morphology are also observed. (B) EoSVs visualized by conventional TEM after isolation by subcellular fractionation. (C) EoSV three-dimensional (3D) models obtained from electron tomographic analyses. (D–F) EoSVs labeled by immunonanogold EM for major basic protein (MBP) (D, E) and interleukin-4 (IL-4) (F). Note that EoSVs are seen as curved tubular and open structures surrounding a cytoplasmic center. While MBP is transported within the EoSVs lumen (D, E), IL-4 mobilization is associated with vesicle membrane (F). (A) was reprinted from [3]; (B) was reprinted from [5]; (D, E) from [6] and (F) from [8] with permission. Scale bar, 400 nm (A); 180 nm (B); 100 nm (C); 150 nm (D,E); 230 nm (F). N, nucleus.
As demonstrated by electron tomography, a technique that enables three-dimensional observation at high resolution [20, 21], EoSVs represent a dynamic and pleiomorphic vesicular system within human eosinophils with substantial membrane surfaces and remarkable ability to change their shape and to interact with secretory granules [17] (Fig. 2A). Three-dimensional reconstructions and models generated from serial sections revealed that individual EoSVs are curved tubular structures with cross-sectional diameters of approximately 150–300 nm, surrounding a cytoplasmic center – an intriguing architecture that confers the “sombrero hat” morphology. In addition to this typical aspect, large tubular vesicles with a “C” shaped appearance and elongated tubular profiles are frequently observed close to standard EoSVs in eosinophil thin sections [5] (Fig. 2A–C). Electron tomography showed that along the length of EoSVs, there were both continuous fully connected cylindrical and circumferential domains and incompletely connected and only partially circumferential curved domains (Fig. 2C). Thus, these two domains explain the different views of EoSVs in cross-sectional images of eosinophils [5] (Fig. 2A).
EoSVs are transport vesicles consistently formed in response to cell activation [17]. Stimulation with inflammatory stimuli such as CCL11 [5] and tumor necrosis factor alpha (TNF-α) [14] induces increased formation of EoSVs. Moreover, eosinophils naturally activated as observed in hypereosinophilic disorders also exhibit amplified numbers of EoSVs [7]. Ultrastructural analyses of blood eosinophils from patients with hypereosinophilic syndrome [7] or tissue eosinophils found in esophageal biopsy specimens from patients with eosinophilic esophagitis [22] revealed marked cytoplasmic vesiculation of EoSVs.
EoSVs are formed from secretory granules
As noted, EoSVs are typically found in mature human eosinophils [19]. Maturation of these cells is accompanied by increased numbers of EoSVs in parallel with the formation of secretory granules [23]. Conventional TEM has frequently identified EoSVs around and/or attached to secretory granules in the cytoplasm of human activated eosinophils. Ultrastructural studies showed that the increased genesis of EoSVs within these cells is associated with secretion. In eosinophils stimulated with inflammatory stimuli, not only did the total number of EoSVs increase but also did the number of EoSVs in contact with secretory granules undergoing release of their contents [5, 14]. Ultrastructural images obtained by conventional EM applied to human eosinophils have also indicated that EoSV were projecting from granule boundaries. Indeed, by tracking serial images with electron tomography and using ultrastructural immunodetection methods (discussed in next section), we demonstrated that EoSVs bud off from secretory granules, carrying selected mediators [5].
Budding of EoSVs from secretory granules would require substantial membranes from these organelles. In fact, it is documented that the secretory granules of human eosinophils encompass internal membranes organized as a tubular system in their matrix area and interconnected with the granule delimiting membrane, as revealed by electron tomography [4]. The presence of these internal membranes likely enables sequestration and relocation of specific granule products at budding EoSVs for rapid cargo delivery [4, 17]. Of note, pretreatment of human eosinophils with brefeldin-A, a potential inhibitor of vesicular transport, dramatically suppressed EoSVs [5] in addition to collapsing membranes within secretory granules, thus providing evidence for the EoSV origin from granules [4]. Another evidence for the close association of EoSVs with secretory granules was provided by TEM images of secretory granules isolated from human eosinophils by subcellular fractionation [7]. The granule containing-fractions clearly show a population of intact EoSVs structurally attached to secretory granules [7].
Interestingly, a recent work from our group indicated that EoSVs are able to translocate products not only from but also to granules in response to cell activation, that is, EoSVs have a remarkable ability to interact with granules acting as transient pools of granule-derived mediators [14]. Thus, these transport carriers seem to have a much more complex role within eosinophils, participating actively in the release of granule products as well as in other granule-associated events.
Visualizing pools of immune mediators at transport vesicles
Human eosinophils store in their secretory granules a plethora of molecules, mainly proteins such as cytokines and distinct cationic proteins (reviewed in [3, 24, 25]. Because vesicular traffic from secretory granules underlies secretion in eosinophils and other leukocytes, a challenge to understand this secretory pathway in more detail has been the identification of granule-derived products within transport vesicles.
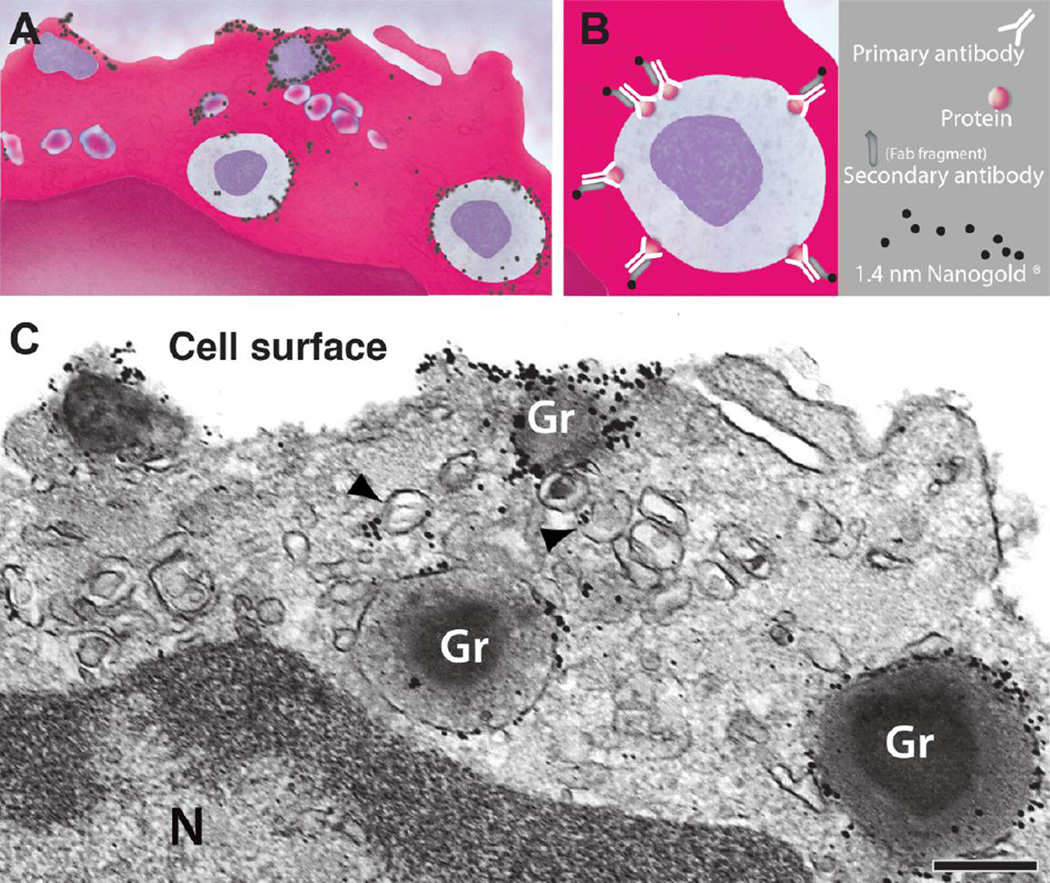
We have been using immunonanogold EM to localize typical granule-stored proteins in cytoplasmic vesicles. Molecular imaging of proteins in these subcellular compartments is achieved by using a protocol that combines several strategies for ultrastructure and antigen preservation in conjunction with robust blocking of nonspecific binding sites and improved antibody penetration [13]. Visualization is accomplished with electron-dense markers (very small gold particles with 1.4 nm diameter) covalently conjugated with Fab' fragments (Fig. 3), which are only one-third the size of a whole IgG molecule, thus facilitating antibody penetration. One important point of this technique is that antigen labeling is performed before all regular procedures for TEM – an approach referred as pre-embedding immunonanogold EM, which enables better antigen preservation [13].
Figure 3.
Immunonanogold electron microscopy (EM) technique. (A, B) The protein of interest is investigated by immunolabeling with a primary antibody against the target molecule followed by a secondary antibody (against primary antibody) conjugated with gold nanoparticles. In this protocol, we used affinity purified Fab fragments conjugated with 1.4 nm gold particles (Nanogold®). In (C), an electron micrograph shows subcellular sites of a human eosinophil leukocyte labeled for CD63. Cell surface microdomains and cytoplasmic secretory granules (Gr) and large vesicles (EoSVs, arrowheads) were labeled. Cells were isolated from the blood of healthy donors. N, nucleus. Reprinted from ref. [13], with permission. Scale bar, 1 µm.
Application of pre-embedding immunonanogold EM has enabled the identification of IL-4 in both small vesicles and EoSVs [5] (Fig. 2F). We also detected with the same approach the existence of specific receptors for IL-4 (IL-4R) at granule limiting membranes and EoSVs, revealing a unique mechanism contributing to the specificity of cytokine secretion in leukocytes [8]. This means that, depending on the stimulus, eosinophil leukocytes are able to select a specific granule-stored cytokine to be released. Further work revealed that human eosinophils are also able to transport MBP, the most abundant eosinophil granule cationic protein, at EoSVs [7]. EoSVs-stored pools of MBP were found in both stimulated and non-stimulated blood eosinophils [7] (Fig. 2D, E). Thus, EoSVs represent small storage/transient, extragranular sites for MBP. This may be important for the rapid release of small quantities of MBP in response to cell activation without immediate disarrangement of the intrincate crystalline cores within eosinophil specific granules [7]. Vesicular traffic of MBP also indicates that this cationic protein might act as an immunoregulatory mediator, underlying eosinophil functions as an immunoregulatory cell [7]. In fact, the role of eosinophils as modulators of the adaptive and innate immune responses has increasingly been discussed [26–28] and the identification of vesicle-mediated secretion of small packets of eosinophil immune mediators can help to understand this role.
Ultrastructural localization of tetraspanins and SNAREs at EoSVs
CD63, a member of the transmembrane-4 glycoprotein superfamily (tetraspanins) (reviewed in [29]), is known as a marker for eosinophil secretory granules [7, 30–32]. Ultrastructural immunodetection studies of human eosinophils localized CD63 on the limiting membranes of secretory granules (Fig. 3) [7].
A recent work using immunonanogold EM and EM quantitative analyses has identified an intracellular trafficking of CD63 associated with secretory granules within activated human eosinophils [14]. In response to eosinophil stimulation with inflammatory mediators (CCL11 and TNF-α), pools of CD63 traffic in the cytoplasm and accumulate within granules undergoing secretion likely acting as a “facilitator” molecule for the release of granulestored products [14]. TEM has unraveled how CD63 – a transmembrane protein – is transported in the cytoplasm. Ultrastructural molecular detection of this protein provided evidence that EoSVs act in the translocation of CD63 from/to intracellular compartments, particularly, secretory granules, in response to stimulation [14]. CD63-labeled EoSVs significantly increased in number upon cell activation and were seen in contact or fused with CD63-positive granules undergoing secretion [14]. Thus, it is now evident that EoSVs function as an important transport system within human eosinophils connected with the granule-derived secretory pathway.
The volume and complexity of vesicular traffic in eosinophils and other cells from the immune system require a selective machinery to ensure the accurate docking and fusion of carrier vesicles at their designated target membranes [33]. SNARE proteins (N-ethylmaleimide sensitive factor attachment protein receptors), coiled-coil forming proteins that are anchored to the membrane via a C-terminal anchor, likely mediate this fusion [33]. In a recent work, our group has identified, for the first time, the Qa-SNARE syntaxin17 (STX17) within human eosinophils [11]. This SNARE, which is involved in constitutive secretion in other cells [34], was visualized by immunonanogold EM in secretory granules and EoSVs from both unstimulated and stimulated eosinophils, suggesting a function in membrane trafficking from secretory granules to plasma membrane [11].
Vesiculotubular carriers in other cells from the immune system
As eosinophils, many cells from the immune system are specialized secretory cells. However, the complexity of the vesicular trafficking involved in the transport and secretion of immune mediators within/from these cells remains to be fully elucidated. Vesiculotubular structures were described in the cytoplasm of cells such as macrophages [16] and class II major histocompatibility complex (MHC)-positive cells [35]. In activated macrophages, vesiculotubular carriers budding from the Golgi complex were identified as transporters for the cytokines interleukin 6 (IL-6) alone or IL-6 together with TNF-α [16]. This means that large tubular carriers may have a broad distribution in cells from the immune system, being advantageous to accommodate and rapidly translocate immune mediators between membranes in different secretory pathways. Moreover, these carriers would provide, as compared to small round vesicles, a higher surface-to-volume ratio system for transport of membrane-bound proteins [5].
Concluding remarks and perspectives
A key function of human eosinophils is to secrete cytokines, chemokines and cationic proteins, trafficking and releasing these mediators for roles in inflammation and other immune responses. Immune mediators are stored as pre-formed products within secretory granules and then transported mainly by large vesiculotubular carriers (EoSVs) to the plasma membrane for release. EM has helped to understand this complex vesicular system within eosinophils. While conventional EM enabled unambiguous imaging of EoSVs at high resolution, immunonanogold EM identified EoSVs as transport carriers for cytokines, cationic proteins and tetraspanins. EoSVs are remarkably increased in number during eosinophil immune responses. Moreover, cytokine cognate receptors such as IL4-R and SNAREs were found in these structures, which likely have a much more complex function within human eosinophils.
Recent TEM data strongly indicated that EoSVs act in the translocation of products from/to granules and we can presume that they are also important for granule membrane replenishment, participating thus in a retrograde trafficking. The presence of intact, cell-free EoSVs in tissues detected by TEM after eosinophil lysis during human diseases raises the question if these carriers can function as transient stores of immune mediators outside of the cell. Continued study in this growing field using TEM, which has provided unique insights into cellular content and transport of immune mediators, combined with other approaches will certainly bring to light mechanisms more largely applicable to immune mediators secretion from other cells from the immune system.
Highlights.
Application of electron microscopy (EM) to understand the complex secretory pathway in human eosinophils
Conventional transmission EM, immunonanogold EM and electron tomography reveal a vesicular system associated with secretory granules
Large vesiculotubular structures are involved in the transport of granule-derived immune mediators.
Acknowledgments
Funding
This work was supported by National Institutes of Health (NIH grants, USA-R37AI020241, R01AI022571) and by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil-477475/2013-2; 469995/2014-9, 311083/2014-5), Brazilian Ministry of Health and Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG, Brazil-CBB-APQ-02239-14).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Melo RCN, Weller PF. Piecemeal degranulation in human eosinophils: a distinct secretion mechanism underlying inflammatory responses. Histol Histopathol. 2010;25:1341–1354. doi: 10.14670/hh-25.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melo RCN, Liu L, Xenakis JJ, Spencer LA. Eosinophil-derived cytokines in health and disease: unraveling novel mechanisms of selective secretion. Allergy. 2013;68:274–284. doi: 10.1111/all.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spencer LA, Bonjour K, Melo RCN, Weller PF. Eosinophil secretion of granule-derived cytokines. Front Immunol. 2014;5:496. doi: 10.3389/fimmu.2014.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melo RCN, Perez SA, Spencer LA, Dvorak AM, Weller PF. Intragranular vesiculotubular compartments are involved in piecemeal degranulation by activated human eosinophils. Traffic. 2005;6:866–879. doi: 10.1111/j.1600-0854.2005.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melo RCN, Spencer LA, Perez SA, Ghiran I, Dvorak AM, Weller PF. Human eosinophils secrete preformed, granule-stored interleukin-4 through distinct vesicular compartments. Traffic. 2005;6:1047–1057. doi: 10.1111/j.1600-0854.2005.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melo RCN, Dvorak AM, Weller PF. Electron tomography and immunonanogold electron microscopy for investigating intracellular trafficking and secretion in human eosinophils. J Cell Mol Med. 2008;12:1416–1419. doi: 10.1111/j.1582-4934.2008.00346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melo RCN, Spencer LA, Perez SA, Neves JS, Bafford SP, Morgan ES, Dvorak AM, Weller PF. Vesicle-mediated secretion of human eosinophil granule-derived major basic protein. Lab Invest. 2009;89:769–781. doi: 10.1038/labinvest.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spencer LA, Melo RCN, Perez SA, Bafford SP, Dvorak AM, Weller PF. Cytokine receptor-mediated trafficking of preformed IL-4 in eosinophils identifies an innate immune mechanism of cytokine secretion. Proc Natl Acad Sci U S A. 2006;103:3333–3338. doi: 10.1073/pnas.0508946103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melo RCN, Weller PF, Dvorak AM. Activated human eosinophils. Int Arch Allergy Immunol. 2005;138:347–349. doi: 10.1159/000089189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melo RCN, Dvorak AM, Weller PF. Contributions of electron microscopy to understand secretion of immune mediators by human eosinophils. Microsc Microanal. 2010;16:653–660. doi: 10.1017/S1431927610093864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmo LAS, Dias FF, Malta KK, Amaral KB, Shamri R, Weller PF, Melo RCN. Expression and subcellular localization of the Qa-SNARE syntaxin17 in human eosinophils. Exp Cell Res. 2015;337:129–135. doi: 10.1016/j.yexcr.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koster AJ, Klumperman J. Electron microscopy in cell biology: integrating structure and function. Nat Rev Mol Cell Biol Suppl. 2003:SS6–SS10. [PubMed] [Google Scholar]
- 13.Melo RCN, Morgan E, Monahan-Earley R, Dvorak AM, Weller PF. Pre-embedding immunogold labeling to optimize protein localization at subcellular compartments and membrane microdomains of leukocytes. Nature Protocols. 2014;9:2382–2394. doi: 10.1038/nprot.2014.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carmo LAS, Bonjour K, Ueki S, Neves JS, Liu L, Spencer LA, Dvorak AM, Weller PF, Melo RCN. CD63 is tightly associated with intracellular, secretory events chaperoning piecemeal degranulation and compound exocytosis in human eosinophils. J Leukoc Biol. 2016;100:391–401. doi: 10.1189/jlb.3A1015-480R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polishchuk RS, San Pietro E, Di Pentima A, Tete S, Bonifacino JS. Ultrastructure of long-range transport carriers moving from the trans Golgi network to peripheral endosomes. Traffic. 2006;7:1092–1103. doi: 10.1111/j.1600-0854.2006.00453.x. [DOI] [PubMed] [Google Scholar]
- 16.Manderson AP, Kay JG, Hammond LA, Brown DL, Stow JL. Subcompartments of the macrophage recycling endosome direct the differential secretion of IL-6 and TNF-alpha. J Cell Biol. 2007;178:57–69. doi: 10.1083/jcb.200612131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melo RCN, Spencer LA, Dvorak AM, Weller PF. Mechanisms of eosinophil secretion: large vesiculotubular carriers mediate transport and release of granule-derived cytokines and other proteins. J Leukoc Biol. 2008;83:229–236. doi: 10.1189/jlb.0707503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anitei M, Wassmer T, Stange C, Hoflack B. Bidirectional transport between the trans-Golgi network and the endosomal system. Molecular membrane biology. 2010;27:443–456. doi: 10.3109/09687688.2010.522601. [DOI] [PubMed] [Google Scholar]
- 19.Melo RCN, Dvorak AM, Weller PF. Eosinophil Ultrastructure. In: Lee J, Rosenberg H, editors. Eosinophils in health and disease. Vol. 1. New York: Elsevier; 2012. pp. 20–27. [Google Scholar]
- 20.McIntosh R, Nicastro D, Mastronarde D. New views of cells in 3D: an introduction to electron tomography. Trends Cell Biol. 2005;15:43–51. doi: 10.1016/j.tcb.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Frey TG, Perkins GA, Ellisman MH. Electron tomography of membrane-bound cellular organelles. Annu Rev Biophys Biomol Struct. 2006;35:199–224. doi: 10.1146/annurev.biophys.35.040405.102039. [DOI] [PubMed] [Google Scholar]
- 22.Saffari H, Hoffman LH, Peterson KA, Fang JC, Leiferman KM, Pease LF, 3rd, Gleich GJ. Electron microscopy elucidates eosinophil degranulation patterns in patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2014;133:1728–1734. e1721. doi: 10.1016/j.jaci.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Dvorak AM, Ishizaka T. Human eosinophils in vitro. An ultrastructural morphology primer. Histol Histopathol. 1994;9:339–374. [PubMed] [Google Scholar]
- 24.Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13:9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davoine F, Lacy P. Eosinophil cytokines, chemokines, and growth factors: emerging roles in immunity. Front Immunol. 2014;5:570. doi: 10.3389/fimmu.2014.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akuthota P, Wang HB, Spencer LA, Weller PF. Immunoregulatory roles of eosinophils: a new look at a familiar cell. Clin Exp Allergy. 2008;38:1254–1263. doi: 10.1111/j.1365-2222.2008.03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spencer LA, Weller PF. Eosinophils and Th2 immunity: contemporary insights. Immunol Cell Biol. 2010;88:250–256. doi: 10.1038/icb.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cadman ET, Lawrence RA. Granulocytes: effector cells or immunomodulators in the immune response to helminth infection? Parasite Immunol. 2010;32:1–19. doi: 10.1111/j.1365-3024.2009.01147.x. [DOI] [PubMed] [Google Scholar]
- 29.Pols MS, Klumperman J. Trafficking and function of the tetraspanin CD63. Exp Cell Res. 2009;315:1584–1592. doi: 10.1016/j.yexcr.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 30.Mahmudi-Azer S, Downey GP, Moqbel R. Translocation of the tetraspanin CD63 in association with human eosinophil mediator release. Blood. 2002;99:4039–4047. doi: 10.1182/blood.v99.11.4039. [DOI] [PubMed] [Google Scholar]
- 31.Neves JS, Perez SA, Spencer LA, Melo RCN, Reynolds L, Ghiran I, Mahmudi-Azer S, Odemuyiwa SO, Dvorak AM, Moqbel R, Weller PF. Eosinophil granules function extracellularly as receptormediated secretory organelles. Proc Natl Acad Sci U S A. 2008;105:18478–18483. doi: 10.1073/pnas.0804547105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JD, Willetts L, Ochkur S, Srivastava N, Hamburg R, Shayeganpour A, Seabra MC, Lee JJ, Moqbel R, Lacy P. An essential role for Rab27a GTPase in eosinophil exocytosis. J Leukoc Biol. 2013;94:1265–1274. doi: 10.1189/jlb.0812431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stow JL, Manderson AP, Murray RZ. SNAREing immunity: the role of SNAREs in the immune system. Nat Rev Immunol. 2006;6:919–929. doi: 10.1038/nri1980. [DOI] [PubMed] [Google Scholar]
- 34.Gordon DE, Bond LM, Sahlender DA, Peden AA. A targeted siRNA screen to identify SNAREs required for constitutive secretion in mammalian cells. Traffic. 2010;11:1191–1204. doi: 10.1111/j.1600-0854.2010.01087.x. [DOI] [PubMed] [Google Scholar]
- 35.Ohshima H, Maeda T, Takano Y. The distribution and ultrastructure of class II MHC-positive cells in human dental pulp. Cell Tissue Res. 1999;295:151–158. doi: 10.1007/s004410051221. [DOI] [PubMed] [Google Scholar]