Abstract
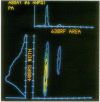
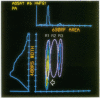
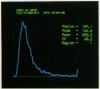
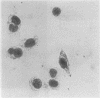
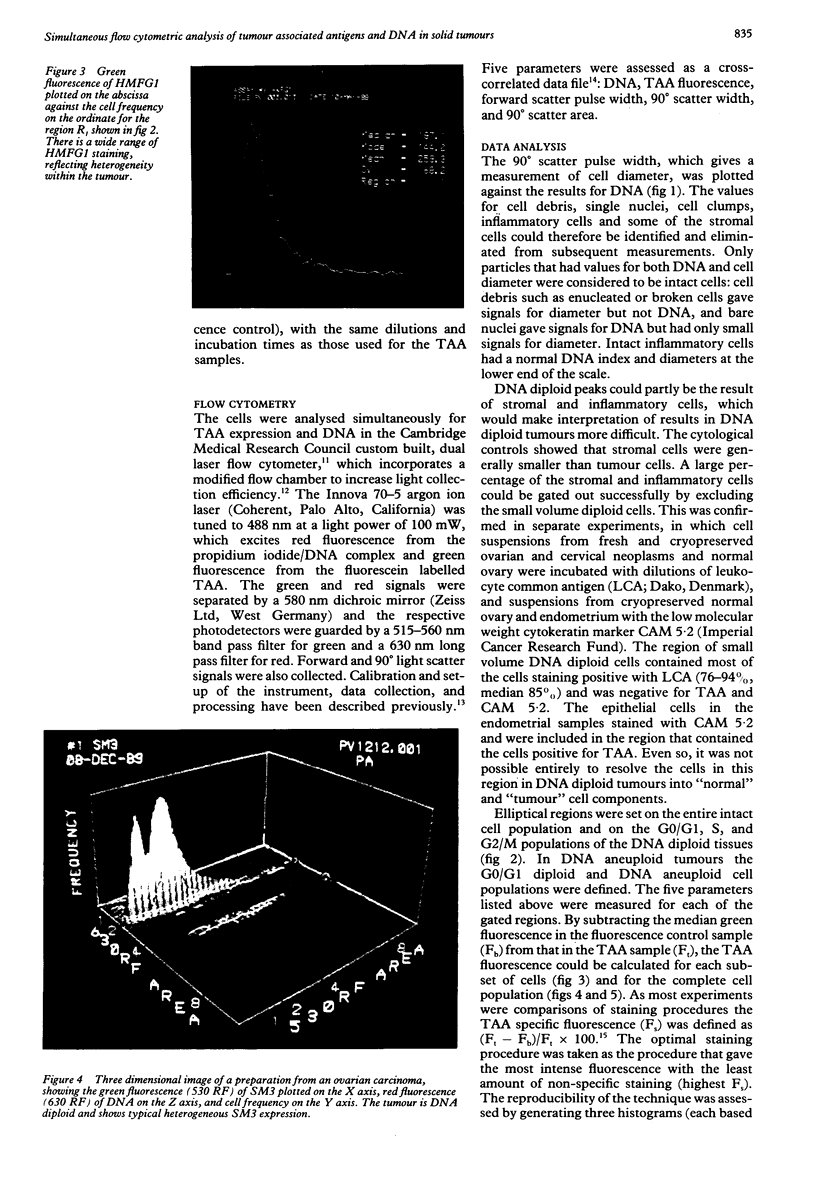
A multiparameter flow cytometric assay for the simultaneous study of tumour associated antigens (TAA) and DNA in fresh solid tumours was devised. Cell suspensions were prepared by disaggregating unfixed solid tumour samples mechanically over a stainless steel mesh. Indirect immunofluorescence was used to identify the TAA, and DNA was stained with propidium iodide. Cell morphology was well preserved, cell clumping was negligible, and high quality indirect immunofluorescence quality indirect immunofluorescence and DNA staining were obtained. The technique is simple, rapid, and reproducible. Multiparameter assays can be developed to study prognostic indicators such as membrane oncoproteins, receptors, and multidrug resistance in solid tumours. With a suitable panel of antibodies the technique might become an aid in the differential diagnosis and biochemical diagnosis of some solid tumours.
Full text
PDF

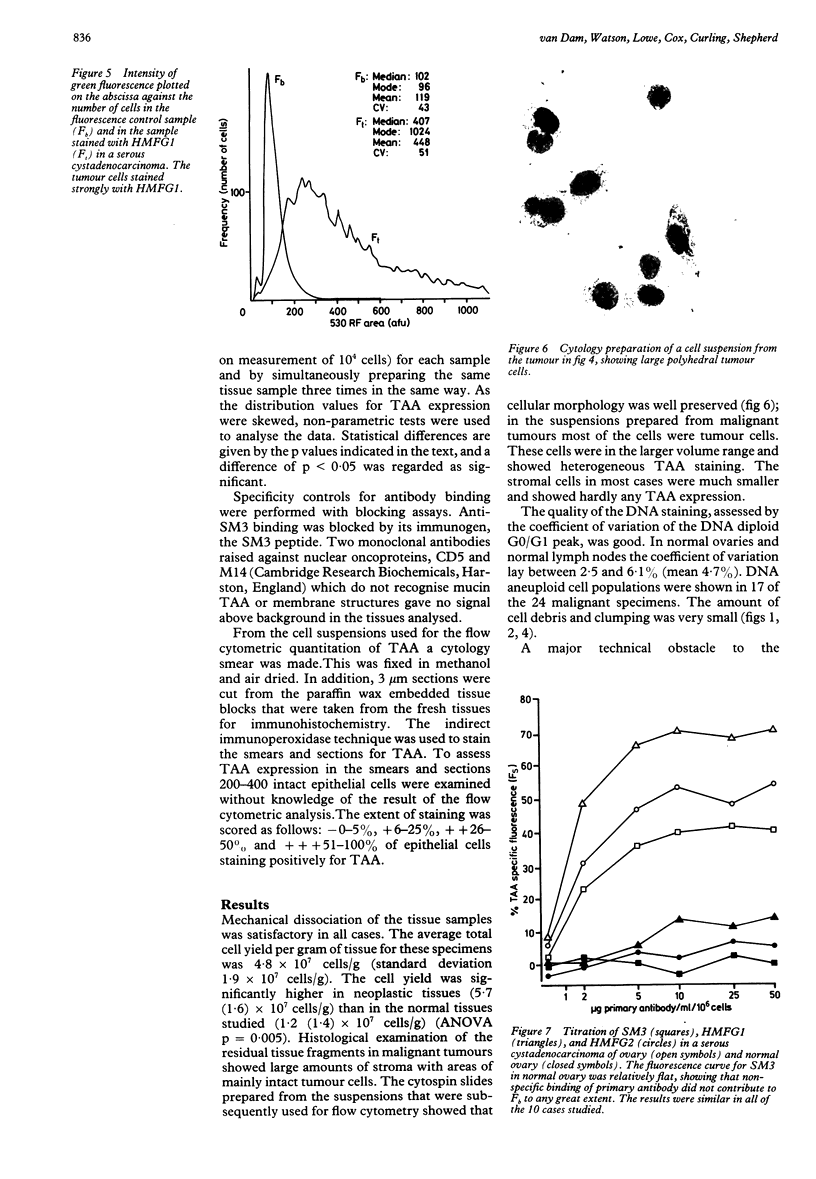
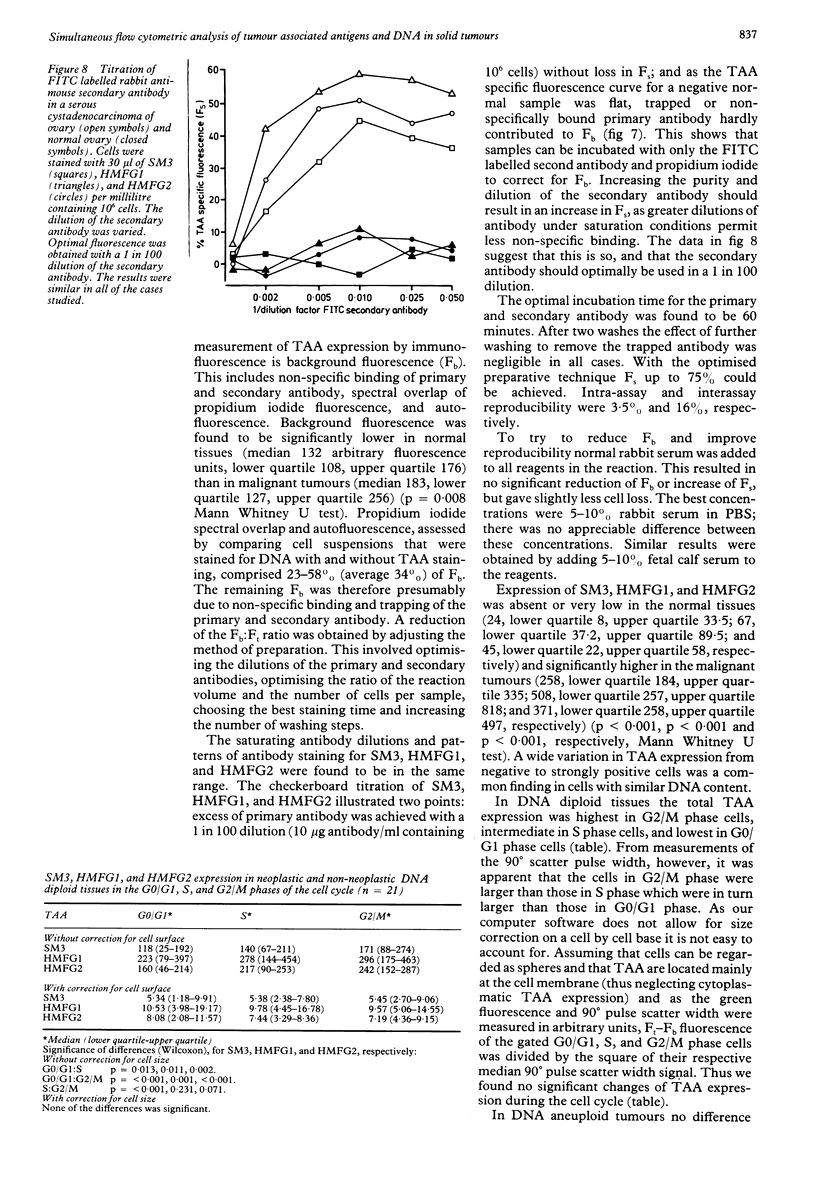
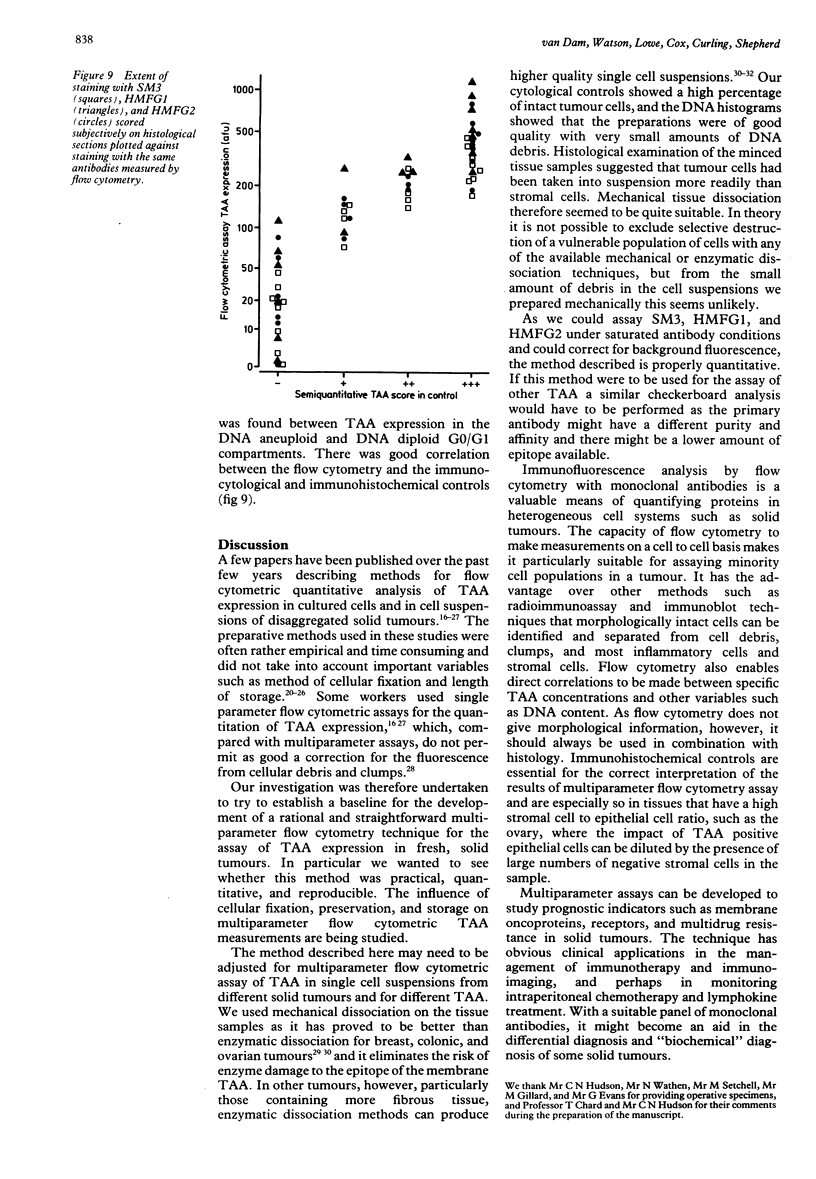

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer K. D., Clevenger C. V., Endow R. K., Murad T., Epstein A. L., Scarpelli D. G. Simultaneous nuclear antigen and DNA content quantitation using paraffin-embedded colonic tissue and multiparameter flow cytometry. Cancer Res. 1986 May;46(5):2428–2434. [PubMed] [Google Scholar]
- Chassevent A., Daver A., Bertrand G., Coic H., Geslin J., Bidabe M. C., George P., Larra F. Comparative flow DNA analysis of different cell suspensions in breast carcinoma. Cytometry. 1984 May;5(3):263–267. doi: 10.1002/cyto.990050308. [DOI] [PubMed] [Google Scholar]
- Clevenger C. V., Bauer K. D., Epstein A. L. A method for simultaneous nuclear immunofluorescence and DNA content quantitation using monoclonal antibodies and flow cytometry. Cytometry. 1985 May;6(3):208–214. doi: 10.1002/cyto.990060306. [DOI] [PubMed] [Google Scholar]
- Coon J. S., Landay A. L., Weinstein R. S. Advances in flow cytometry for diagnostic pathology. Lab Invest. 1987 Nov;57(5):453–479. [PubMed] [Google Scholar]
- Czerniak B., Darzynkiewicz Z., Staiano-Coico L., Herz F., Koss L. G. Expression of Ca antigen in relation to cell cycle in cultured human tumor cells. Cancer Res. 1984 Oct;44(10):4342–4346. [PubMed] [Google Scholar]
- Czerniak B., Koss L. G. Expression of Ca antigen on human urinary bladder tumors. Cancer. 1985 May 15;55(10):2380–2383. doi: 10.1002/1097-0142(19850515)55:10<2380::aid-cncr2820551013>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Ensley J. F., Maciorowski Z., Pietraszkiewicz H., Hassan M., Kish J., Al-Sarraf M., Jacobs J., Weaver A., Atkinson D., Crissman J. Solid tumor preparation for clinical application of flow cytometry. Cytometry. 1987 Sep;8(5):488–493. doi: 10.1002/cyto.990080509. [DOI] [PubMed] [Google Scholar]
- Fowler W. C., Jr, Maddock M. B., Moore D. H., Haskill S. Significance of multiparameter flow cytometric analysis of ovarian cancer. Am J Obstet Gynecol. 1988 Apr;158(4):838–845. doi: 10.1016/0002-9378(88)90081-6. [DOI] [PubMed] [Google Scholar]
- Girling A., Bartkova J., Burchell J., Gendler S., Gillett C., Taylor-Papadimitriou J. A core protein epitope of the polymorphic epithelial mucin detected by the monoclonal antibody SM-3 is selectively exposed in a range of primary carcinomas. Int J Cancer. 1989 Jun 15;43(6):1072–1076. doi: 10.1002/ijc.2910430620. [DOI] [PubMed] [Google Scholar]
- Inoue S., Ito M., Mizuno S., Isobe H., Miyamoto H., Kawakami Y., Ohnuma T. Simultaneous flow cytometric detection of nuclear DNA and tumor-associated antigens in lung cancers. Anal Quant Cytol Histol. 1988 Aug;10(4):243–250. [PubMed] [Google Scholar]
- Jacobberger J. W., Fogleman D., Lehman J. M. Analysis of intracellular antigens by flow cytometry. Cytometry. 1986 Jul;7(4):356–364. doi: 10.1002/cyto.990070410. [DOI] [PubMed] [Google Scholar]
- Lincoln S. T., Bauer K. D. Limitations in the measurement of c-myc oncoprotein and other nuclear antigens by flow cytometry. Cytometry. 1989 Jul;10(4):456–462. doi: 10.1002/cyto.990100414. [DOI] [PubMed] [Google Scholar]
- Marder P., Apelgren L. D., Bumol T. F. Comparative analysis of monoclonal antibody-drug conjugate binding by flow cytometry. J Immunol Methods. 1987 Feb 11;96(2):165–170. doi: 10.1016/0022-1759(87)90310-3. [DOI] [PubMed] [Google Scholar]
- Nozawa S., Sakayori M., Ohta K., Iizuka R., Mochizuki H., Soma M., Fujimoto J., Hata J., Iwamori M., Nagai Y. A monoclonal antibody (MSN-1) against a newly established uterine endometrial cancer cell line (SNG-II) and its application to immunohistochemistry and flow cytometry. Am J Obstet Gynecol. 1989 Oct;161(4):1079–1086. doi: 10.1016/0002-9378(89)90787-4. [DOI] [PubMed] [Google Scholar]
- Orntoft T. F., Petersen S. E., Wolf H. Dual-parameter flow cytometry of transitional cell carcinomas. Quantitation of DNA content and binding of carbohydrate ligands in cellular subpopulations. Cancer. 1988 Mar 1;61(5):963–970. doi: 10.1002/1097-0142(19880301)61:5<963::aid-cncr2820610518>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Parratto N. P., Kimura A. K. Isolation and visualization of Met-72-positive, metastatic variants present in B16 melanoma tumor masses. J Cell Biochem. 1988 Mar;36(3):311–322. doi: 10.1002/jcb.240360311. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Murakami T., Kawasaki S., Okita K., Takemoto T., Takahashi M. Change of alpha-fetoprotein content during cell cycle of human hepatoma cells in vitro: flow cytometric analysis. Tumour Biol. 1986;6(5):483–489. [PubMed] [Google Scholar]
- Sasaki K., Nagai M., Kato H., Torigoe T., Nagamine Y., Takahashi M. Flow cytometric analysis of tumor antigen TA-4 in cervical squamous cells. Gan. 1984 Aug;75(8):703–706. [PubMed] [Google Scholar]
- Sikora K., Chan S., Evan G., Gabra H., Markham N., Stewart J., Watson J. c-myc oncogene expression in colorectal cancer. Cancer. 1987 Apr 1;59(7):1289–1295. doi: 10.1002/1097-0142(19870401)59:7<1289::aid-cncr2820590710>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Slocum H. K., Pavelic Z. P., Rustum Y. M., Creaven P. J., Karakousis C., Takita H., Greco W. R. Characterization of cells obtained by mechanical and enzymatic means from human melanoma, sarcoma, and lung tumors. Cancer Res. 1981 Apr;41(4):1428–1434. [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Peterson J. A., Arklie J., Burchell J., Ceriani R. L., Bodmer W. F. Monoclonal antibodies to epithelium-specific components of the human milk fat globule membrane: production and reaction with cells in culture. Int J Cancer. 1981 Jul 15;28(1):17–21. doi: 10.1002/ijc.2910280104. [DOI] [PubMed] [Google Scholar]
- Valet G., Ormerod M. G., Warnecke H. H., Benker G., Ruhenstroth-Bauer G. Sensitive three-parameter flow-cytometric detection of abnormal cells in human cervical cancers: a pilot study. J Cancer Res Clin Oncol. 1981;102(2):177–184. doi: 10.1007/BF00410669. [DOI] [PubMed] [Google Scholar]
- Valet G., Rüssmann L., Wirsching R. Automated flow-cytometric identification of colo-rectal tumour cells by simultaneous DNA, CEA-antibody and cell volume measurements. J Clin Chem Clin Biochem. 1984 Dec;22(12):935–942. [PubMed] [Google Scholar]
- Watson J. V. A method for improving light collection by 600% from square cross section flow cytometry chambers. Br J Cancer. 1985 Mar;51(3):433–435. doi: 10.1038/bjc.1985.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. V. A method for improving light collection by 600% from square cross section flow cytometry chambers. Br J Cancer. 1985 Mar;51(3):433–435. doi: 10.1038/bjc.1985.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. V., Curling O. M., Munn C. F., Hudson C. N. Oncogene expression in ovarian cancer: a pilot study of c-myc oncoprotein in serous papillary ovarian cancer. Gynecol Oncol. 1987 Oct;28(2):137–150. doi: 10.1016/0090-8258(87)90207-1. [DOI] [PubMed] [Google Scholar]
- Watson J. V., Horsnell T. S., Smith P. J. Data compression: 8-dimensional flow cytometric data processing with 28K addressable computer memory. J Immunol Methods. 1988 Oct 26;113(2):205–214. doi: 10.1016/0022-1759(88)90333-x. [DOI] [PubMed] [Google Scholar]
- Watson J. V., Sikora K., Evan G. I. A simultaneous flow cytometric assay for c-myc oncoprotein and DNA in nuclei from paraffin embedded material. J Immunol Methods. 1985 Oct 24;83(1):179–192. doi: 10.1016/0022-1759(85)90071-7. [DOI] [PubMed] [Google Scholar]
- van Dam P. A., Shepherd J. H., Lowe D. G., Watson J. V. Flow cytometric quantitation of tumor-associated antigens in solid tumors. Am J Obstet Gynecol. 1990 Aug;163(2):698–699. doi: 10.1016/0002-9378(90)91265-e. [DOI] [PubMed] [Google Scholar]