Abstract
Background
Although a wealth of literature points to the importance of social factors on health, a detailed understanding of the complex interplay between social and biological systems is lacking. Social status is one aspect of social life that is made up of multiple structural (humans: income, education; animals: mating system, dominance rank) and relational components (perceived social status, dominance interactions). In a nonhuman primate model we use novel network techniques to decouple two components of social status, dominance rank (a commonly used measure of social status in animal models) and dominance certainty (the relative certainty vs. ambiguity of an individual’s status), allowing for a more complex examination of how social status impacts health.
Methods
Behavioral observations were conducted on three outdoor captive groups of rhesus macaques (N = 252 subjects). Subjects’ general physical health (diarrhea) was assessed twice weekly, and blood was drawn once to assess biomarkers of inflammation (interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP)).
Results
Dominance rank alone did not fully account for the complex way that social status exerted its effect on health. Instead, dominance certainty modified the impact of rank on biomarkers of inflammation. Specifically, high-ranked animals with more ambiguous status relationships had higher levels of inflammation than low-ranked animals, whereas little effect of rank was seen for animals with more certain status relationships. The impact of status on physical health was more straightforward: individuals with more ambiguous status relationships had more frequent diarrhea; there was marginal evidence that high-ranked animals had less frequent diarrhea.
Discussion
Social status has a complex and multi-faceted impact on individual health. Our work suggests an important role of uncertainty in one’s social status in status-health research. This work also suggests that in order to fully explore the mechanisms for how social life influences health, more complex metrics of social systems and their dynamics are needed.
Keywords: Dominance certainty, Inflammation, Social network analysis, Nonhuman primate, Status
Introduction
Social life influences mental and physical health (Thoits, 2011; Nunn et al., 2015). For example, a lack of satisfactory social relationships has been shown to be associated with poor health and high quality relationships can buffer individuals from stress (Hostinar, Sullivan & Gunnar, 2014; Hawkley & Capitanio, 2015). Treatment and prevention of illness thus requires a detailed understanding of the complex interplay between social and biological systems. Although past research has clearly shown that the social environment plays a critical role in shaping health, the effect of the complex and multi-scale dynamic nature of social relationships on health remains poorly understood (Thoits, 2011). For example, although the absence of social relationships has been associated with poor health outcomes, social life consists of more than just the presence or absence of social relationships. Qualities of these relationships, such as stability and role within the community, are also important factors (Sapolsky, 1992; Thoits, 2011). Therefore, approaches that empirically recognize the inherent complexity of social life are critical to improve our understanding of how social life influences health McCowan et al. (2016). In this paper, we use a nonhuman primate model to investigate an understudied aspect of social status and its effects on health outcomes. We use a measure of status certainty to test whether greater uncertainty in status relationships is coincident with greater levels of inflammation and poor health outcomes.
Status is one component of social life whose impact on health has been widely studied. While in humans there is a clear general pattern that individuals of low status (i.e. socioeconomic status) often have greater disease incidence and shorter lifespans (Adler et al., 1994; Marmot & Sapolsky, 2014; Chetty et al., 2016), across species the impact of status on health is less clear (Creel, 2001; Habig & Archie, 2015). For example, in species such as baboons, macaques, marmots, and meerkats, low status individuals are at greater risk of poor health outcomes, such as poor cardiovascular health, reduced immune function, and higher levels of glucocorticoids (GCs) (Sapolsky & Mott, 1987; Sapolsky & Share, 1994; Shively & Clarkson, 1994; Hackländer, Möstl & Arnold, 2003; Young et al., 2006; Archie, Altmann & Alberts, 2012). While GCs are not a health outcome, they are frequently used as a biomarker for increased risk for negative health outcomes because they are released in response to social stress and play an important role in regulating immune function (Sapolsky, Romero & Munck, 2000). In contrast, the potential costs of high status center on GCs and parasite loads. In social carnivores and many cooperative breeding species, high status individuals tend to have higher GCs than low status individuals (summarized in: Creel (2001) and Creel et al. (2013)). In addition, high status individuals across a variety of species have been shown to experience higher parasite loads than low status individuals (Habig & Archie, 2015). Compounding this confusion are species in which status effects differ by sex or study population (Schoech, Mumme & Moore, 1991; Creel, MarushaCreel & Monfort, 1996; Arnold & Dittami, 1997) as well as species in which no status effects on health have been found (Mays, Vleck & Dawson, 1991; Wingfield, Hegner & Lewis, 1991; Lynch, Ziegler & Strier, 2002). Recently, researchers have attempted to reconcile these differences to examine broad associations between social status and health across species. For example, a recent meta-analysis examining the impact of status on immune parameters and parasite load found little evidence for consistent effects of status on immune function but did find that high status males are at greater risk for parasitic infections (Habig & Archie, 2015).
The lack of cross species consensus on the impact of social status on health may be partly due to the fact that social status is more complex than a simple linear ranking of individuals based upon income, education level, or, in the case of nonhumans, dominance. The advancement of novel network techniques provides an opportunity to begin measuring the complexity of social life in new ways McCowan et al. (2016). Social networks have been shown to impact both mental and physical health outcomes, highlighting the utility of these methods in understanding population health (Pachucki et al., 2015; Perkins, Subramanian & Christakis, 2015). One understudied, and potentially critical, aspect of status is the relative stability and predictability associated with one’s status. In the human literature, unpredictability in access to resources (e.g. food, medical care, and housing) and job insecurity have been suggested to be features of low socioeconomic status that contribute to poorer health outcomes (Adler et al., 1994). In the animal literature, group-level instability of the hierarchy and dominance rank reversals have similarly been suggested to influence patterns of association between social status and health (e.g. Sapolsky, 1992; Muller & Wrangham, 2004; Sapolsky, 2005). Even within a stable social group, individual-level social relationships are dynamic and can vary in stability (Hinde, 1976). For example, while a change in rank may be stressful for both individuals involved, the negative impact on health is often greater for the animal that loses rank compared to the animal that gains rank (e.g. Sapolsky, 1992; Shively & Clarkson, 1994). This body of work highlights that the impact of status on health may depend not only on one’s position in the hierarchy, but also on the stability and predictability of one’s status relationships. Therefore, we propose that a measure of social status that quantifies such individual-level instability or uncertainty may explain cross-species differences regarding the impact of status on health outcomes.
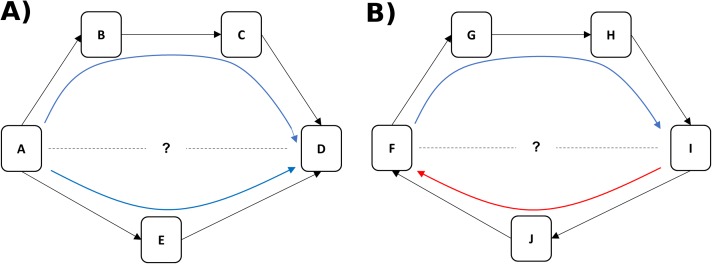
We use a nonhuman primate model to disentangle two components of status, dominance rank (linearly ordered status relative to other animals in the group) and dominance certainty (the probability that status is decided and stable), using a novel computational network-based approach called Percolation and Conductance (Fushing, McAssey & McCowan, 2011; K. Fujii et al., 2014, unpublished data). This method characterizes the flow of status (i.e. the overall direction of aggression and submission) through pathways in the network and each individual’s fit within that hierarchical flow to quantify both dominance rank and dominance certainty. Like most methods for measuring dominance rank, Percolation and Conductance uses direct aggression and/or submission data to create a dominance hierarchy. Unlike other methods, however, it uses information from indirect pathways through the network of aggressive interactions to modify the likely rank association between individuals and to measure the consistency of information flow through the network (Fushing, McAssey & McCowan, 2011). For example, if animal A directs aggression at animal E (and E submits) and E directs aggression at D (and D submits), we can infer that A likely outranks D even if they have never been observed to interact (see Fig. 1A). Greater consistency in the direction of dominance pathways from A to D results in higher certainty that A outranks D (Fig. 1A), whereas evidence of inconsistent direction (e.g. between F and I in Fig. 1B) reflects dominance ambiguity. Our method thus solves the problem of sparse or missing data in the win/loss matrix (e.g. the treatment of zeroes in the matrix is a non-trivial issue: (de Vries, 1995)) by using these dominance pathways as additional sources of information about each pairwise dominance relationship. Our method additionally provides a measure of how well the direction of each individual’s dominance interactions fit, on average, within the larger group-level pattern of aggression from dominants to subordinates, a measure we call dominance certainty.
Figure 1. Inference of dominance rank and certainty using a network.
(A) Although animals A and D do not directly interact, it can be inferred that A outranks D through the indirect pathways in the network. Certainty for this inference is increased when multiple pathways flow in the same direction (i.e. from A to D). (B) Although animals F and I do not interact, it can be inferred through the most direct pathway (through individual J) that I outranks F. Certainty for this inference, however, is lower due to the contradictory flow of dominance from F to I (through individuals G and H).
Our study aimed to examine the relative impacts of dominance rank and dominance certainty on biomarkers of inflammation and diarrhea, with particular interest in whether individual level dominance certainty either better explains variation in health or moderates the rank-health association. Similar to results reported in baboons and long-tailed macaques (Sapolsky & Share, 1994; Shively & Clarkson, 1994), we expected to find that low-ranked individuals exhibited poorer health than high-ranked individuals. We predicted, however, that reduced certainty in dominance relationships may be associated with poorer health outcomes either independent of rank or specifically among individuals that stand to lose rank, inasmuch as uncertainty in one’s relationships is likely to be stressful. We chose to include both biomarkers of immune function as well as a general health outcome because previous research suggests that effects of status are not always the same across health measures (Habig & Archie, 2015).
Materials and Methods
Subjects and housing
The subjects of this study were 252 rhesus macaques (71 males, 181 females; age range: 3–29 years, mean = 7.7 years) from three outdoor social groups (126–185 individuals per group) that were part of the breeding colony at the California National Primate Research Center (CNPRC) in Davis, CA. Animals were socially housed in 0.2 ha outdoor enclosures containing multiple A-frame structures, suspended barrels, swings, and perches and were free to interact as they chose. Animals were fed a standard diet of monkey chow twice per day at approximately 0700 h and between 1430 and 1530 h. Fresh fruit or vegetables were provided one time per week and seed mixture provided daily. Water was available ad libitum. Animals housed in outdoor enclosures were managed with a minimum level of disturbance. At the end of the study all subjects were relinquished to the CNPRC breeding colony. This research was approved by the University of California Davis Institutional Animal Care and Use Committee.
Rank and dominance certainty data collection
Each group was observed for 5–7 weeks, one group in spring (Group A: March–April 2013) and two in fall (Group B: September–October 2013, Group C: September–October 2014) as part of a larger study on social networks and health. Unexpected management events resulted in lengthening (e.g. Group A) or shortening (e.g. Group B) the six-week study period by a few days in certain groups. We used an event sampling design to collect all instances of aggressive and submissive interactions. Two observers collected data for 6 h on 4 days per week from 0900–1200 and 1300–1600 h. Aggressive and submissive events were recorded as a series of dyadic interactions. A total of 13,567 events were recorded during 444 h of observation. Aggression was categorized according to severity and included threat (open mouth stare, brow flash, ear flap), mild aggression (threat and follow, lunge, push, slap, chase < 6 meters), moderate aggression (grapple, wrestle, chase > 6 meters), and intense aggression (pin or bite). Submission categories included freeze/turn away, move away, run away < 6 meters, run away > 6 meters, and crouch. Data on all dyadic aggressive interactions were used for constructing aggression networks to calculate dominance rank and certainty.
Dominance rank and certainty were calculated using the Perc package in R (Fujii et al., 2015) which uses a new network-based approach that combines information from direct dominance interactions with information from multiple indirect dominance pathways (via common third parties) to quantify dyadic dominance relationships (Fushing et al., 2011; Fujii et al., 2015). Essentially, individuals with dominance pathways that run counter to the primary direction of aggression (or submission) in the hierarchy have more ambiguous status than individuals who do not. The method begins by using a modified random walk algorithm to exhaustively identify all directed pathways in the network (e.g., A→B←C is not a directed pathway, but A→B→C is). To determine how much to weight the imputed (i.e. indirect) ‘wins’ from these pathways, the transitivity of the network is calculated as the proportion of transitive triangles (as opposed to cyclic or nontransitive triangles). In networks with high transitivity such as ours (> 95% of triangles are transitive, such that A > B > C and A > C), a ‘win’ that is imputed from an indirect pathway is more likely to reflect the true direction of dominance, and thus given higher weight, than in a network with lower transitivity. Regardless, wins from direct interactions are always weighted more than imputed ‘wins’ from network pathways. Finally, given the number of direct wins and imputed ‘wins’ from pathways, we calculate the probability that the row animal is dominant to the column animal using a Beta distribution to incorporate a source of statistical probability that reflects the level of uncertainty expected given the current data on each pair (for a more detailed explanation, see: Fushing, McAssey & McCowan, 2011). The matrix of these dyadic dominance probability values (range: 0–1) thus represent the cumulative information from all network pathways between each pair of animals. A dyadic dominance probability of one reflects the highest possible certainty that the row animal outranks the column animal, whereas 0.5 means the dominance relationship is perfectly ambiguous. The matrix of dyadic dominance probabilities was then used to generate the lowest cost linear rank order (see Fushing et al., 2011 for details).
Sparse and missing data (i.e. pairs of animals that are either infrequently or never observed to interact) are a common problem in animal behavior, and most ranking methods (such as the IS&I method (de Vries, 1998), Elo-rating (Neumann et al., 2011; Viswanath, 2016) and the Bradley-Terry model: Boyd & Silk (1983) and Albers & de Vries (2001)) are vulnerable to sparse data. The Percolation and Conductance method addresses the problem of sparse and missing data by gathering dominance information from network pathways. For example, in our study group A only 42.3% of all possible dyads had at least one agonistic interaction in the network. The remaining dyads were never observed to interact, making estimation of their pairwise dominance relationships from direct interactions prone to error. Furthermore, of the 42.3% that did interact, they averaged less than two interactions per dyad (x = 1.89). Adding dominance pathways dramatically increased the information per dyad to an average of 207.4 dominance pathways across all pairs (using up to 3-step pathways: A→B→C→D). Given that transitivity is very high, the dominance information from pathways has a high probability of agreeing with the true relationship. Furthermore, it is likely that macaques have the cognitive capacity to use this dominance information because many social vertebrates are capable of transitive inference such as deducing A > C from A > B and B > C (McGonigle & Chalmers, 1977; Davis, 1992; Bond, Kamil & Balda, 2003; Grosenick, Clement & Fernald, 2007).
From these dyadic dominance probabilities (which contain both rank direction and certainty information), an average dominance probability was calculated for each subject to provide a metric of the overall certainty of each animal’s rank. Prior to averaging, we transformed the dyadic dominance probabilities (initially bounded by 0–1) to range between 0.5 (ambiguous) and 1.0 (certain), thereby focusing on the information about certainty and ignoring rank direction. We also transformed ordinal dominance ranks for each group (derived from permuting the rows and columns of the dominance probability matrix) into the proportion of animals outranked within their respective groups (i.e. 0 is the lowest ranked animal and 1 is the highest ranked animal) to account for differences in group size. For the purposes of graphical representation, individual-level average dominance certainty was categorized into high, moderate, and low certainty categories for each group. We examined empirical distributions of average dominance certainty values (performed on each group separately) using the segmented package in R to identify logical break-points for these categories (Muggeo, 2008). Groups differed in their break-points due to differences in distribution, and these group-specific categorizations of low vs. moderate vs. high dominance certainty are thus reflected in our plots.
Social network measures and independence
Statistical analysis of social network metrics requires some attention to the lack of independence in network data. The calculation of a network metric for one node (e.g., an individual) typically involves some of the same edges as the same calculation for other nodes (Croft, James & Krause, 2008). As a result, a change in the value of one node could potentially affect the values of other nodes. We experimentally verified that this lack of independence was not an issue in this study by recalculating network measures after removing single individuals from the network. We selected three individuals with differing betweenness centrality (i.e. highest, median, and lowest) and experimentally removed each individual from the aggression network, one-at-a-time, recalculating the values of the remaining individuals each time, for a total of three recalculations (one for each individual). We chose to examine betweenness centrality because it is defined by shortest paths between all pairs in the network, similar to the counting of all paths between pairs in our Percolation and Conductance method. Bivariate and Spearman rank order correlations between the original dominance certainty and the new values indicate very little change (bivariate pairwise correlations for (a), (b) and (c): 0.999 ≥ r ≥ 0.932; Spearman pairwise correlations: 0.998 ≥ r ≥ 0.941). Furthermore, our Percolation and Conductance method incorporates statistical probability/uncertainty into the calculation of each dyadic dominance probability value, which serves to dampen any effect of dependence in the data.
Blood sample collection
Blood samples were collected on a single day (between 0800–1200 h) during the fifth week of the 5–7 week observation period for each group using the CNPRC’s standard method for biannual physical examinations. On sampling days, all animals in the group were immobilized (10 mg/kg of ketamine) and given standard physical examinations by veterinary staff (e.g., checked for injuries, weighed, assessed for pregnancy). Blood samples (5 mL) were also obtained from the femoral vein and serum was aliquoted and stored at −80 °C for later assay. Due to the large number of animals, blood was collected in batches of ∼15 samples. Batches were labeled with collection start and end times and animal identity to track the order of sample processing and control for effects of the capture and sedation procedure for animals processed later in the morning.
General health assessments
Health indicators were recorded twice weekly by one observer between 0900–1200 and 1300–1600 h. The observer located and visually inspected each study subject in the group and scored presence/absence of liquid stool (i.e. observing defecation of liquid stool or observing fresh liquid fecal matter on the tail or rump). From these data, we counted the total frequency of bouts of diarrhea per subject across the 5–7 week observation period. A bout of diarrhea was defined as either a single observation day with liquid stool or multiple consecutive observation days with liquid stool with the end of a bout marked by at least one observation day with no evidence of liquid stool. Occasionally animals were temporarily removed from the cage for veterinary care and were not available for health observations—these absences were recorded to control for total observation days.
Pro-inflammatory proteins
We measured three pro-inflammatory proteins (interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP)) from frozen serum. These proteins were chosen because they are markers of general systemic inflammation and are demonstrated risk factors for multiple diseases (e.g. type 2 diabetes and atherosclerosis, Pradhan et al., 2001; Libby, Ridker & Maseri, 2002).
Cytokine assay
Serum levels of IL-6 and TNF-α were measured using commercially available, species specific Milliplex multi-analyte profiling (MAP) reagents purchased from EMD/Millipore (Billerica, MA, USA), and utilizing Luminex Xmap technology (Luminex, Austin, TX, USA). Color coded polystyrene microbeads coated with specific antibodies for IL-6 and TNF-α were incubated with the serum samples, washed, and then were further reacted with biotinylated detector antibodies followed by Streptavidin-PE to label the immune complexes on the beads. After a final washing to remove all unbound material, the beads were interrogated in a BioPlex dual laser (BioRad, Hercules, CA, USA). The median fluorescent index for each sample was compared to a standard curve to calculate the concentration. Samples were tested in duplicate and had an intra-assay coefficient of variability of 15.3%. Samples falling below the threshold sensitivity of the assay (1.6 pg/mL) were assigned a value of one.
C-reactive protein assay
Concentrations of CRP were determined using a latex particle immunoturbidmetric method on the Beckman Coulter AU480 clinical chemistry analyzer.
Data analysis
Data analysis proceeded through a two-step process. First, hypothesized models (see below and Table 1) were tested for four dependent variables including three pro-inflammatory proteins (CRP, TNF-α, and IL-6) and one general health outcome (frequency of diarrhea bouts). The sets of hypotheses that guided model-fitting were explored for the following reasons. Age and sex were included as main effects in all models because both have been previously found to influence health (Klein, 2000; Sansoni et al., 2008). The impact of rank on health is also known to vary by sex in some populations (Creel, MarushaCreel & Monfort, 1996; Abbott et al., 2003), and it is reasonable to expect that the impact of rank may manifest differently at different ages. Therefore, sex by rank and age by rank interaction terms were also explored. We also examined a sex by dominance certainty interaction because the inherent structural differences in male vs. female rank acquisition (i.e. individual-level features such as age and body size drive male rank: (Dittus, 1975; Sprague, 1992; Sprague, 1998; Sebastian, 2015); family-level features such as agonistic support drive female rank: (Sade, 1972; Datta, 1986)) suggest that ambiguity may arise more readily amongst males than females and the presence of ambiguous relationships may have greater costs for females than males.
Table 1. Hypotheses.
| Hypothesis | Question | Variables |
|---|---|---|
| H0 | Null model | Y = control variables |
| H1 | Does health differ by age or sex class? | Y = sex + age |
| H2 | Does rank influence health beyond effects of age and sex? | Y = sex + age + rank |
| H3 | Does dominance certainty influence health beyond effects of age and sex? | Y = sex + age + DC |
| H4 | What are the relative impacts of rank and dominance certainty on health? | Y = sex + age + rank + DC |
| H5 | Does the impact of rank on health depend upon dominance certainty? | Y = sex + age + rank + DC + rank*DC |
| H6 | Does the impact of rank differ for juveniles, adults and geriatric animals? | Y = sex + age + rank + rank*age |
| H7a | Does the impact of status (i.e. rank) differ for males and females? | Y = sex + age + rank + rank*sex |
| H7b | Does the impact of status (i.e. dominance certainty) differ for males and females? | Y = sex + age + rank + DC*sex |
| H8 | Due to sex differences in how status is attained, does the interaction of rank and DC affect males and females differently? | Y= sex + age + rank + DC + sex*rank + sex*DC + rank*DC + sex*DC*rank |
Notes:
All models include a random effect of cage.
DC, dominance certainty.
All data were analyzed using generalized linear mixed-effects regression models (McCullagh & Nelder, 1989; Raudenbush & Bryk, 2002) with group as a random effect. Models were run using a negative binomial distribution for IL-6, TNF-α and diarrhea bouts, and a gamma distribution for CRP. Appropriate distribution(s) for each outcome were chosen based on descriptive statistics, histograms, and Q-Q plots. We also evaluated the goodness of fit of these distributions using the Pearson chi-square statistic (SAS Institute Inc., 2009). We also included variables to control for known confounds: sample collection order for blood-based measures, and total health observation days for diarrhea. Due to the unavailability of blood samples for some animals, the cytokine and CRP analyses were run on 234 of the 252 subjects. Finally, ten of the study subjects showed CRP levels above 10 (a sign of possible infection), and these animals were included in analyses because they represent an important part of the health continuum. We note, however, that excluding these subjects from analyses did not change the magnitude or direction of the effects.
We used an Information Theoretic approach to determine which variables best explained each of our health outcomes. First, we ran all mixed-effects regression models to address our complete set of hypotheses outlined in Table 1. For each model, we present AICc scores, dAICc, model likelihoods (L = exp(−(1/2 * dAICc)), Akaike weights, and evidence ratios (ratio = weight of best model/weight of comparison model) as outlined in Burnham, Anderson & Huyvaert (2011). We then used model weights and dAICc to select a set of candidate models for each outcome variable. When the weight of the best model was < 0.90 (Burnham & Anderson, 2002; Symonds & Moussalli, 2011), we included in the candidate set those models with dAICc ≤ 5 and discuss the inferences based on all models in this candidate set. Analyses were performed in SAS 9.4 and R 3.3.1 (R Core Team, 2013). Plots were produced in Stata 14.1. Although plots of model results are typically generated from marginal effects tables, this was not appropriate for our data due to the presence of discontinuous regions. We have used the alternative method of generating model specific plots from predicted values (Hardin & Hilbe, 2007; Rabe-Hesketh & Skrondal, 2012).
Results
Rank and dominance certainty
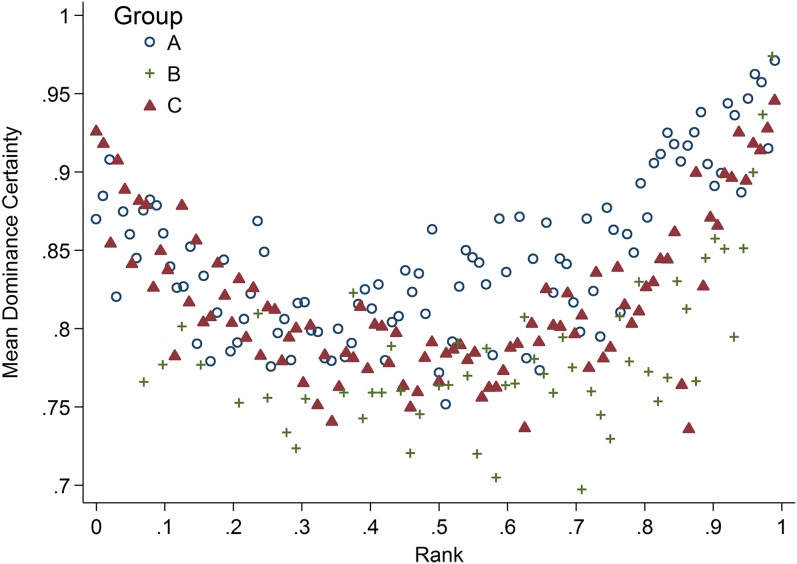
Rank and dominance certainty were associated in a nonlinear manner (Fig. 2). Specifically, animals of high- and low-rank exhibited higher dominance certainty than those in the middle of the rank distribution, a pattern that held true for all three study groups. In addition, there was variability in dominance certainty at each level of rank. Specifically, dominance certainty ranged from 0.70–0.97 and 0.72–0.93 among the highest and lowest ranked tertiles of the sample, respectively, which is very similar to the range for the sample as a whole (0.70–0.97).
Figure 2. Rank and dominance certainty.
Scatter plot of dominance rank and dominance certainty. Markers indicate group membership.
Pro-inflammatory measures
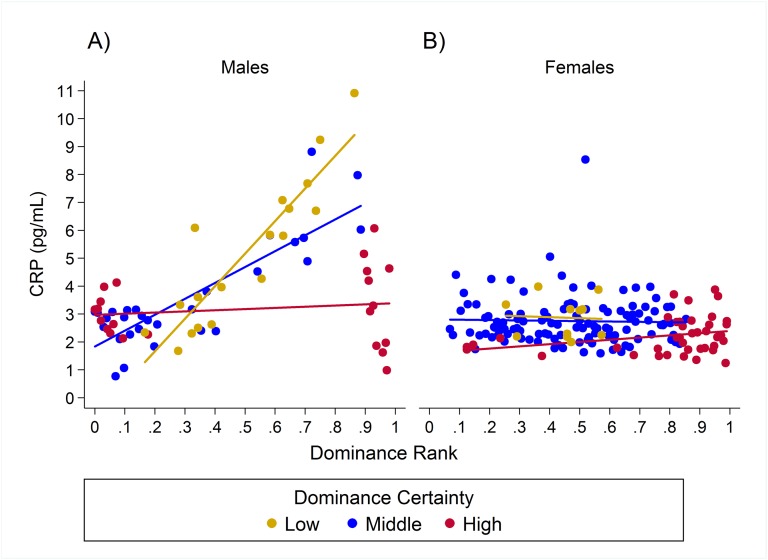
Results for CRP indicated a single model with the highest weight (w = 0.93, H8; Table 2) in which the relationship between rank and CRP was dependent on dominance certainty and sex. High-ranking males with low dominance certainty had higher CRP, whereas little to no effect of rank was found in males with high dominance certainty (Table 3; Fig. 3A). For males with highly certain dominance relationships, increasing rank by 0.25 (moving up a quartile in rank) was associated with a reduction in CRP levels by 1.17 times. In contrast, for males with low dominance certainty, increasing in rank by 0.25 was associated with a 1.80 times increase in CRP. In females there was no change in CRP with increasing rank at any level of dominance certainty (Table 3; Fig. 3B). Consistent with predictions, older animals also had higher levels of CRP than adults, subadults, or juveniles (Table 3).
Table 2. Model fitting for C-reactive protein.
| Model | AICc | ΔAICc | Model likelihood | Model weight | Evidence ratio | |
|---|---|---|---|---|---|---|
| H8 | Y = SO + IL-6 + sex + age + rank + DC + rank*DC + rank*sex + DC*sex + sex*rank*DC | 1,022.74 | 0.00 | 1.000 | 0.930 | 1.00 |
| H5 | Y = SO + IL-6 + sex + age + rank + DC + rank*DC | 1,029.58 | 6.84 | 0.033 | 0.030 | 30.55 |
| H7a | Y = SO + IL-6 + sex + age + rank + DC + rank*sex | 1,030.86 | 8.12 | 0.017 | 0.016 | 57.94 |
| H4 | Y = SO + IL-6 + sex + age + rank + DC | 1,031.87 | 9.13 | 0.010 | 0.010 | 95.83 |
| H3 | Y = SO + IL-6 + sex + age + DC | 1,032.62 | 9.88 | 0.007 | 0.007 | 139.82 |
| H7b | Y = SO + IL-6 + sex + age + rank + DC + DC*sex | 1,033.29 | 10.55 | 0.005 | 0.005 | 195.28 |
| H1 | Y = SO + IL-6 + sex + age | 1,036.40 | 13.66 | 0.001 | 0.001 | 923.19 |
| H6 | Y = SO + IL-6 + sex + age + rank + DC + rank*age | 1,037.00 | 14.26 | 0.001 | 0.001 | 1,246.43 |
| H2 | Y = SO + IL-6 + sex + age + rank | 1,037.85 | 15.11 | 0.001 | 0.000 | 1,910.99 |
| H0 | Y = SO + IL-6 | 1,044.31 | 21.57 | 0.000 | 0.000 | 48,356.29 |
Notes:
Random effect: Cage; N = 234; SO, Sampling order; DC, Dominance certainty.
Table 3. Model coefficients and SEs from the sets of candidate models for pro-inflammatory proteins.
| CRP (H8) | IL-6 (H5) | IL-6 (H8) | IL-6 (H3) | IL-6 (H1) | TNF-α (H5) | |
|---|---|---|---|---|---|---|
| coeff (SE) | coeff (SE) | coeff (SE) | coeff (SE) | coeff (SE) | coeff (SE) | |
| dAICc | 0 | 0 | 0.62 | 3.54 | 3.90 | 0 |
| Intercept | 2.48 (2.34) | −3.71 (3.15) | 0.65 (4.74) | 4.40 (1.23) | 2.46 (0.20) | −6.34 (3.87) |
| Rank | −0.08 (3.10) | 12.6 (4.48)* | 5.89 (6.23) | – | – | 22.0 (5.71)* |
| DC1 | −1.79 (2.86) | 7.35 (3.81)+ | 1.98 (5.79) | −2.46 (1.53)+ | – | 13.9 (4.70)* |
| Sex2 | −5.70 (3.12)+ | 0.034 (0.19) | −8.79 (6.58) | 0.18 (0.18) | 0.18 (0.18) | −0.4 (0.25) |
| Rank × DC | 0.11 (3.72) | −15.0 (5.30)* | −6.88 (7.44) | – | – | −26.2 (6.75)* |
| Rank × Sex2 | 13.2 (4.57)* | – | 19.3 (9.51)* | – | – | – |
| DC × Sex2 | 6.75 (3.77)+ | – | 10.7 (7.96) | – | – | – |
| Rank × DC × Sex2 | −14.9 (5.41)* | – | −23.5 (11.2)* | – | – | – |
| IL-6 | 0.004 (0.002) | – | – | – | – | – |
| SO3 | 0.026 (0.02)+ | 0.053 (0.03)+ | 0.054 (0.03)+ | 0.051 (0.03)+ | 0.041 (0.03) | 0.061 (0.03)+ |
| Age4-3 years | −0.004 (0.12) | −0.66 (0.25)* | −0.68 (0.25)* | −0.64 (0.23)* | 0.65 (0.23)* | −0.47 (0.34) |
| Age-4–5 years | −0.046 (0.11) | −0.88 (0.21)* | −0.88 (0.21)* | −0.82 (0.21)* | −0.82 (0.21)* | −0.83 (0.28)* |
| Age-13+ years | 0.27 (0.13)* | 0.24 (0.25) | 0.31 (0.25) | 0.16 (0.25) | 0.11 (0.26) | 0.33 (0.35) |
Notes:
DC, Dominance certainty.
Males were referent category.
Sampling order.
Adults (6–12 years) were the referent category.
p < 0.05.
p < 0.1.
Figure 3. Sex differences in the impact of dominance certainty and rank on CRP.
Predicted values of CRP. Panels A and B depict effects for CRP for males and females, respectively (based on model H8). Separate lines represent the interaction between dominance rank and dominance certainty.
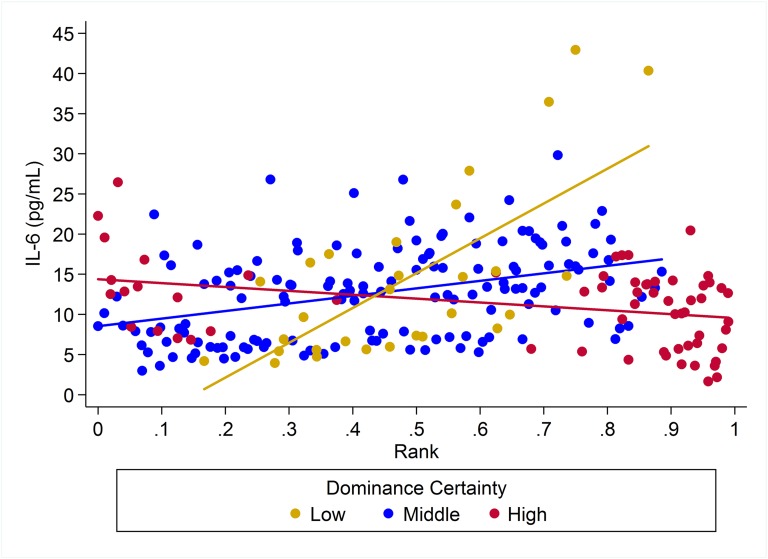
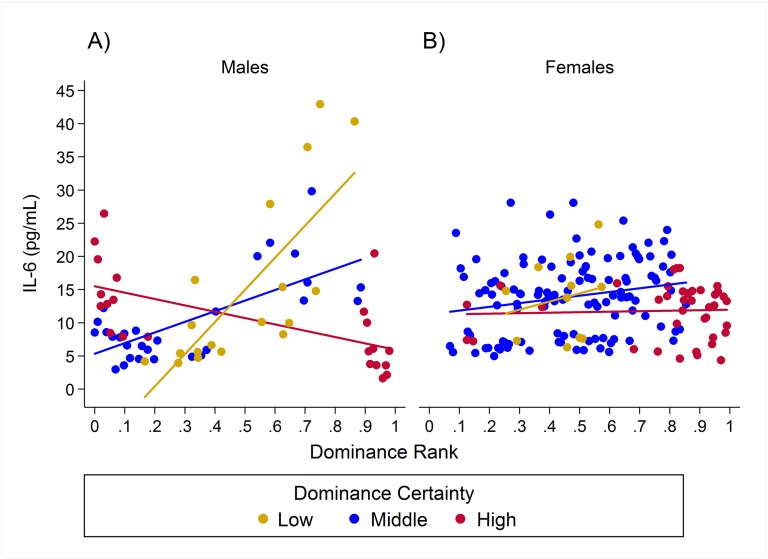
For IL-6 two models with similar weight accounted for nearly 80% of total weight (Table 4), and both models provided evidence that the effect of rank on IL-6 levels was dependent on dominance certainty (w = 0.45, H5; Fig. 4) and also potentially on sex (w = 0.33, H8; Fig. 5). Specifically, for animals with more certain dominance relationships, an increase in rank of 0.25 was associated with 1.41 times lower levels of IL-6 (H5), an effect that may be stronger in males than in females (H8; effect size: 2.16 vs. 1.14, respectively; Table 3; Fig. 5). Among animals with less certain relationships, an increase in rank of 0.25 was associated with a 1.50 times (H5) increase in IL-6 with effects possibly being stronger in males than in females (H8; effect size = 2.11 vs. 1.24, respectively). Taken together these results also indicate that high-rank animals with more ambiguous dominance relationships (i.e. low or moderate dominance certainty) exhibited higher levels of IL-6 than high-rank animals with more certain dominance relationships and that this effect may be specific to males.
Table 4. Model fitting for IL-6.
| Model | AICc | ΔAICc | Model likelihood | Model weight | Evidence ratio | |
|---|---|---|---|---|---|---|
| H5 | Y = SO + sex + age + rank + DC + rank*DC | 1,626.20 | 0.00 | 1.000 | 0.451 | 1.00 |
| H8 | Y = SO + sex + age + rank + DC + rank*DC + rank*sex + DC*sex + sex*rank*DC | 1,626.81 | 0.61 | 0.737 | 0.332 | 1.36 |
| H3 | Y = SO + sex + age + DC | 1,629.74 | 3.54 | 0.170 | 0.077 | 5.88 |
| H1 | Y = SO + sex + age | 1,630.10 | 3.90 | 0.143 | 0.064 | 7.02 |
| H4 | Y = SO + sex + age + rank + DC | 1,631.86 | 5.66 | 0.059 | 0.027 | 16.96 |
| H2 | Y = SO + sex + age + rank | 1,632.17 | 5.97 | 0.050 | 0.023 | 19.81 |
| H7a | Y = SO + sex + age + rank + DC + rank*sex | 1,633.32 | 7.12 | 0.028 | 0.013 | 35.16 |
| H7b | Y = SO + sex + age + rank + DC + DC*sex | 1,634.07 | 7.86 | 0.020 | 0.009 | 51.01 |
| H6 | Y = SO + sex + age + rank + DC + rank*age | 1,635.03 | 8.83 | 0.012 | 0.005 | 82.66 |
| H0 | Y = SO | 1,641.48 | 15.28 | 0.000 | 0.000 | 2,079.21 |
Notes:
Random effect: Cage; N = 234; SO, Sampling order; DC, Dominance certainty.
Figure 4. The impact of dominance certainty and rank on inflammation based on serum levels of interleukin-6 (IL-6).
Predicted values of IL-6 based on model H5. Separate lines represent the interaction between dominance rank and dominance certainty.
Figure 5. Sex differences in the impact of dominance certainty and rank on inflammation based on serum levels of interleukin-6 (IL-6).
Predicted values of IL-6 based on model H8. Panels A and B depict effects for IL-6 for males and females, respectively. Separate lines represent the interaction between dominance rank and dominance certainty.
Two simpler nested models were also part of the candidate set for IL-6 (Table 4). Model H3 (w = 0.08) included a main effect for dominance certainty plus age and sex terms, and indicated that an increase in dominance certainty of 0.10 was associated with 1.28 times lower levels of IL-6 (Table 3). Model H1 (w = 0.06) included only age and sex terms. For all four candidate models young animals (juveniles and subadults) had lower levels of IL-6 than adults (Table 3).
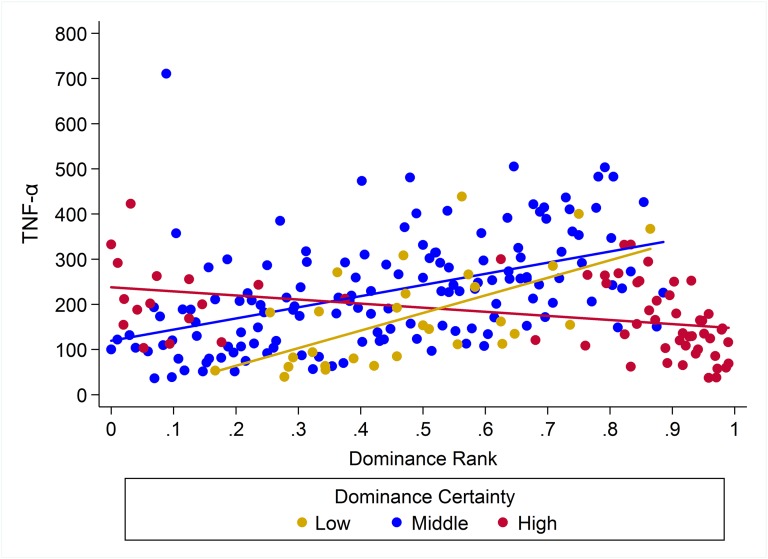
For the pro-inflammatory cytokine TNF-α, the model for H5 had the highest weight (w = 0.91; Table 5) indicating that the effect of rank on cytokine levels was dependent on dominance certainty for both males and females (Table 3; Fig. 6). For animals with more ambiguous dominance relationships (i.e. low dominance certainty) increasing in rank by 0.25 was associated with 2.07 times higher levels of TNF-α whereas increasing rank by 0.25 was associated with 1.78 times decrease in TNF-α for animals with highly certain dominance relationships (Fig. 6). Among low-ranked individuals, those with more certain dominance relationships had slightly higher levels of TNF-α compared to those with lower dominance certainty. Finally, juveniles and subadults had lower levels of TNF-α than adults (Table 3).
Table 5. Model fitting for TNF-α.
| Model | AICc | ΔAICc | Model likelihood | Model weight | Evidence ratio | |
|---|---|---|---|---|---|---|
| H5 | Y = SO + sex + age + rank + DC + rank*DC | 2,722.67 | 0.00 | 1.000 | 0.907 | 1 |
| H8 | Y = SO + sex + age + rank + DC + rank*DC + rank*sex + DC*sex + sex*rank*DC | 2,728.28 | 5.61 | 0.061 | 0.055 | 16.52 |
| H1 | Y = SO + sex + age | 2,731.06 | 8.39 | 0.015 | 0.014 | 66.39 |
| H0 | Y = SO | 2,732.14 | 9.47 | 0.009 | 0.008 | 113.64 |
| H3 | Y = SO + sex + age + DC | 2,732.42 | 9.76 | 0.008 | 0.007 | 131.30 |
| H2 | Y = SO + sex + age + rank | 2,733.22 | 10.56 | 0.005 | 0.005 | 195.88 |
| H4 | Y = SO + sex + age + rank + DC | 2,734.61 | 11.94 | 0.003 | 0.002 | 391.11 |
| H7b | Y = SO + sex + age + rank + DC + DC*sex | 2,735.81 | 13.14 | 0.001 | 0.001 | 713.37 |
| H7a | Y = SO + sex + age + rank + DC + rank*sex | 2,736.43 | 13.76 | 0.001 | 0.001 | 972.63 |
| H6 | Y = SO + sex + age + rank + DC + rank*age | 2,739.28 | 16.61 | 0.000 | 0.000 | 4,035.96 |
Notes:
Random effect: Cage; N = 234; SO, Sampling order; DC, Dominance certainty.
Figure 6. Dominance certainty moderates the effect of rank on TNF-α.
Predicted values for TNF-α based on model H5. Separate lines represent the interaction between rank and dominance certainty.
Plots of the raw data for CRP, IL-6, and TNF-α relative to rank and dominance certainty are presented in Figs. S1–S3.
Diarrhea bouts
Results for diarrhea bouts showed no single model with highest weight (Table 6). The set of candidate models included H4, H3, H7b, H2, H5 and H7a. Dominance certainty was included as a main effect only (i.e. not part of interaction terms) in models H4, H3 and H7a and was predictive of the incidence of diarrhea in each of these models (Table 7). According to these models, reducing the certainty of one’s dominance relationships by 0.10 (e.g., from 0.85 to 0.75) was associated with between 1.95 and 2.44 times greater incidence of diarrhea (Table 7), regardless of rank or sex. Models H5 and H7b were more complex, incorporating dominance certainty into interaction terms to determine whether its impact on health differed by rank or sex. The presence of these models in the candidate set suggest that the effect of dominance certainty might be dependent on rank or the effect of dominance certainty might be more pronounced in males (Table 7). However, addition of these interaction terms did not improve model fit, as AIC increased by 1.6 and 2.0 in H7b and H5, respectively (Table 6).
Table 6. Model fitting for diarrhea.
| Model | AICc | ΔAICc | Model likelihood | Model weight | Evidence ratio | |
|---|---|---|---|---|---|---|
| H4 | Y = sex + age + rank + DC | 394.55 | 0 | 1.00 | 0.257 | 1.00 |
| H3 | Y = sex + age + DC | 394.65 | 0.10 | 0.95 | 0.245 | 1.05 |
| H7b | Y = sex + age + rank + DC + DC*sex | 396.17 | 1.62 | 0.45 | 0.114 | 2.25 |
| H2 | Y = sex + age + rank | 396.49 | 1.94 | 0.38 | 0.097 | 2.64 |
| H5 | Y = sex + age + rank + DC + rank*DC | 396.60 | 2.04 | 0.36 | 0.092 | 2.78 |
| H7a | Y = sex + age + rank + DC + rank*sex | 396.64 | 2.09 | 0.35 | 0.090 | 2.84 |
| H0 | Y = | 397.25 | 2.70 | 0.26 | 0.067 | 3.86 |
| H6 | Y = sex + age + rank + DC + rank*age | 400.08 | 5.52 | 0.06 | 0.016 | 15.81 |
| H1 | Y = sex + age | 400.09 | 5.54 | 0.06 | 0.016 | 15.92 |
| H8 | Y = sex + age + rank + DC + rank*DC + rank*sex + DC*sex + sex*rank*DC | 402.43 | 7.88 | 0.02 | 0.005 | 51.31 |
Notes:
Random effect: Cage; N = 252; Offset variable: days in cage; DC, Dominance certainty.
Table 7. Model coefficients from the set of candidate models for diarrhea bouts.
| (H4) coeff | (H3) coeff | (H7b) coeff | (H2) coeff | (H5) coeff | (H7a) coeff | |
|---|---|---|---|---|---|---|
| (SE) | (SE) | (SE) | (SE) | (SE) | (SE) | |
| dAICc | 0 | 0.10 | 1.62 | 1.94 | 2.04 | 2.09 |
| Intercept | 0.16 (2.78) | 1.16 (2.73) | −1.30 (3.39) | −5.22 (0.50) | 2.19 (6.39) | −0.01 (2.84) |
| Rank | −0.96 (0.64) | – | −1.14 (0.69)+ | 1.42 (0.60) | −4.28 (9.37)* | −1.09 (0.80)+ |
| DC1 | −7.00 (3.54)* | −8.90 (3.36)* | −5.10 (4.34) | – | −9.46 (7.81) | −6.71 (3.68)+ |
| Sex2 | −0.31 (0.35) | −0.27 (0.35) | 3.60 (5.31) | −0.27 (0.35) | −0.29 (0.35) | −0.47 (0.66) |
| Rank × DC | – | – | – | – | 3.96 (11.2) | – |
| Rank × Sex2 | – | – | – | – | – | 0.36 (1.25) |
| DC × Sex2 | – | – | −4.85 (6.60) | – | – | – |
| Rank × DC × Sex2 | – | – | – | – | – | – |
| Age3-3 years | −1.09 (0.54)* | −0.90 (0.53)+ | −1.09 (0.55)* | −1.13 (0.55)* | −1.11 (0.55)* | −1.09 (0.55)* |
| Age–4–5 years | 0.067 (0.36) | 0.20 (0.35) | 0.09 (0.36) | −0.05 (0.36) | 0.07 (0.36) | 0.08 (0.36) |
| Age-13+ years | 0.65 (0.42) | 0.62 (0.42) | 0.69 (0.43) | 0.60 (0.43) | 0.63 (0.43) | 0.64 (0.42) |
Notes:
DC, Dominance certainty.
Males were referent category.
Adults (6–12 years) were the referent category.
p < 0.05.
p < 0.1.
Dominance rank was included as a main effect only in models H2, H4, and H7b (Table 6). According to these models, reducing dominance rank by 0.25 (e.g. from outranking 75% to outranking 50%) was associated with between 1.25 and 1.42 times greater incidence of diarrhea (Table 7). Models H5 and H7a incorporated dominance rank into interaction terms with dominance certainty (described above) and with sex. Addition of the dominance rank × sex interaction did not improve model fit and AIC increased by 2.09 (Table 6). As expected, older animals showed a higher incidence of diarrhea bouts than adults and juveniles across all models (Table 7).
Discussion
A wealth of literature highlights the fact that uncertainty is a potent stressor and can lead to a wide range of negative health outcomes (Baum & Fleming, 1993; Heaney, Israel & House, 1994) highlighting the potential importance of variation of dominance certainty in studies of social status. The results we present demonstrate that more complex conceptualizations of social status provide a possible explanation for seemingly contradictory findings in the status-health literature across species. The health risk for any given animal is dependent on a combination of both the individual’s absolute status and a metric of the certainty or stability of that status.
The measure of relationship uncertainty used here, an average of all dyadic level dominance certainty, can also potentially offer a metric of how well an individual “fits” within the dominance hierarchy overall. Low average dominance certainty could reflect an animal that is changing in position within the hierarchy (moving up or down), or, due to the use of direct and indirect pathways in a network, low dominance certainty could also arise for an individual because others in their local community are changing their position(s) in the hierarchy. For example in Fig. 1B, we can see that there is ambiguity in the likely rank relationship between animals F and I, not because they are in a direct contest with each other, but due to inconsistency in the direction of the flow of dominance in their local community (i.e. information inferred from the blue and red arrows is contradictory). A network approach can examine how instability in dyadic relationships might ripple throughout a local community and potentially impact the position of nearby neighbors in the network.
Rank and dominance certainty
Variation in social status, and thus its potential impact on health, includes not only whether one has high or low status but also the relative predictability or certainty of one’s status relationships. Dominance rank and dominance certainty showed a nonlinear (U-shaped) relationship where individuals of both high and low dominance rank had relatively greater dominance certainty than individuals in the middle of the hierarchy. However, variability in dominance certainty at all levels of dominance rank suggests that these two metrics may describe two complementary aspects of social status that can have independent and interacting effects on an animal’s health. The idea that dominance rank stability can have a critical impact on the status-health relationship has been suggested previously (Sapolsky, 1992; Sapolsky, 2005; Marmot & Sapolsky, 2014). The current paper, however, expands on previous research by using a more general measurement of each individual’s “fit” within the group hierarchy, as opposed to examining the status-health relationship in stable vs. unstable groups, or only in individuals that experienced dominance rank reversals.
Status and measures of inflammation
Our research suggests that whether individuals have high or low dominance rank in the hierarchy does not fully represent the complexity with which social status affects health. Instead, dominance certainty modified the impact of rank on biomarkers of inflammation. Under conditions of low dominance certainty, we found that high-ranking animals had higher levels of pro-inflammatory cytokines and CRP. This is consistent with previous findings in African wild dogs, baboons, chimpanzees, dwarf mongooses, and ringtail lemurs (Creel, MarushaCreel & Monfort, 1996; Cavigelli, 1999; Muller & Wrangham, 2004; Gesquiere et al., 2011), where high dominance rank individuals (or at least the alpha individual, as in Gesquiere et al. (2011)) had elevated GCs. Because dominance certainty is quantified as the consistency in the flow of dominance between pairs in the aggression network, having low certainty may be evidence of an individual-level tendency to protest or challenge others’ dominance rank or precursors of a rank change (within a hierarchy that is stable overall). Thus, high-ranking animals with ambiguous dominance relationships likely have reduced predictability that others will submit to them and greater potential risk of losing status (e.g., Crockford et al., 2008), and this type of uncertainty could represent a major psychosocial stressor. Our findings are consistent with previous research indicating that instability, whether due to dominance style (e.g. chimpanzees, Muller & Wrangham, 2004) or due to current social factors (e.g. baboon dominance rank reversals, Sapolsky, 1992), is particularly bad for high-ranking individuals, possibly due to potential for loss of status. For example, in an examination of rank reversal in male baboons, Sapolsky (1992) reported that despite similar levels of participation in aggressive interactions, only males about to lose their rank showed elevated GCs, not those about to rise in rank.
In contrast, for animals with a high degree of certainty in their dominance relationships, we found a small reversal of the effect with high-ranking animals having slightly lower levels of inflammatory markers than low-ranking animals. This effect is weak, but notably, more closely matches results from humans, baboons, macaques, meerkats, and spotted hyenas in which low status individuals exhibited risky health profiles (Sapolsky & Share, 1994; Shively & Clarkson, 1994; Goymann et al., 2001; Young et al., 2006; Ostner, Heistermann & Schülke, 2008; Gesquiere et al., 2011; Marmot & Sapolsky, 2014). In contrast to high status individuals, where uncertainty may have been associated with a potential loss in status, dominance uncertainty may not be a stressor for low-status individuals because they have little to lose; it may even be the case that low-ranking individuals with low dominance certainty may have an opportunity to increase their status—a possibility that needs to be explored further in future studies. Among low-ranking animals, being certain of one’s low dominance rank is likely to be stressful because such individuals can reliably expect to receive aggression, harassment, and/or intimidation from dominants, have little control over the occurrence of such interactions, and have fewer social outlets to cope with this harassment (Schino, 2001; Sapolsky, 2005). This is largely consistent with Shively & Clarkson’s findings that female macaques that were experimentally arranged to lose status (via group membership manipulations) increased atherosclerosis by 500%, whereas those that gained status increased atherosclerosis by a far smaller amount—44% (Shively & Clarkson, 1994). Thus, dominance rank changes may be stressful for both parties, but losing status is likely worse than gaining status.
Sex differences
Although sex differences in the status-health relationship are not often studied, sex differences have been noted previously (Kaplan & Manuck, 1999). Our results for the pro-inflammatory proteins, CRP and IL-6, suggest that there may be sex differences in the impact of rank on inflammation. Elevated levels of CRP in high-ranking individuals with lower dominance certainty was found only in males, not in females. The lack of an effect for females of this species is not surprising given the behavioral biology of rhesus macaques, specifically due to sex differences in how dominance rank is gained and maintained. Rhesus macaque males emigrate, and once they are established in a new group, they can increase their rank through both alliances and direct competition. Female macaques, on the other hand, remain in their natal group and inherit their dominance rank from their mothers with all the females of one family outranking all the females from another family (Lindburg, 1971; Sade, 1972; Melnick, Pearl & Richard, 1984). This process results in a male hierarchy that is more labile and changeable, but changes in dominance rank are rarer for females (Sade, 1972; Berard, 1990). For females, due to inherited rank and lifetime tenure in social groups, we would expect to see little dominance uncertainty, except during periods of larger scale group instability, such as when one matriline threatens to overthrow another matriline (Ehardt & Bernstein, 1986; Beisner et al., 2011).
Diarrhea
Dominance uncertainty was a better predictor of diarrhea than dominance rank with lower certainty being associated with greater risk of diarrhea. While animals with lower dominance certainty may be more vulnerable to diseases causing diarrhea, stress is also a known contributor to diarrhea. It may be the case that the increased stress of uncertainty in social relationships is contributing to the incidence of non-pathogenic diarrhea (e.g., Stasi et al., 2012; Buckley, O’Mahony & O’Malley, 2014). The small effect of dominance rank for diarrhea is consistent with other studies in baboons, macaques, meerkats, and spotted hyenas that demonstrate a wide range of negative biomarkers of health, as well as poor health outcomes in low-ranking individuals (Sapolsky & Share, 1994; Shively & Clarkson, 1994; Goymann et al., 2001; Young et al., 2006). Notably, unlike the biomarkers of inflammation discussed above, there was not strong evidence for an interaction between dominance rank and dominance certainty. This result suggests that the impact of social status on health is specific to the type of status measure being examined (e.g. certainty vs. rank) as well as the particular health or fitness outcome of interest (e.g. biomarkers of inflammation).
Conclusion
Our research demonstrates the importance of more complex representations of social status for understanding its impact on health. Our data show that the effect of social status on health is much better understood by accounting for status certainty. Indeed, the interaction between a linear measure of status and status certainty in our data reconciles the contradictory patterns in dominance rank and health found in the previous literature as a direct result of framing social status in terms of its certainty. Our work suggests that expanding the examination of the certainty of social relationships, or fit within one’s social class, may be a critical step toward understanding status effects on health outcomes. As such, the innovative methods leading to this more complex conceptualization of status, as presented here, promises to significantly enhance our ability to detect more effectively who may experience health related costs in society.
Our results also demonstrate that computational social network techniques have the capacity to advance our understanding of the impact of social status on health by disentangling the relative effects of linear measures of rank versus individual-level uncertainty of rank relationships. To the best of our knowledge, we are the first to empirically demonstrate that uncertainty in an individual’s dominance relationships, as measured by inconsistency in the direction of one’s dominance network pathways, are associated with multiple indicators of poorer physical well-being. In contrast, the impact of dominance rank on pro-inflammatory proteins was dependent upon dominance certainty, suggesting that the effects of social status on health are highly dependent on the context in which they occur.
Supplemental Information
Raw values of CRP. A and B depict effects for CRP for males and females, respectively. Separate lines represent different levels of dominance certainty.
Raw values of IL-6. A and B depict effects for CRP for males and females, respectively. Separate lines represent different levels of dominance certainty.
Raw values of TNF-α plotted against dominance rank. Separate lines represent different levels of dominance certainty.
Acknowledgments
We would like to thank our dedicated team that collected the behavioral and physiological data (A. Barnard, T. Boussina, A. Vitale, E. Cano, J. Greco, N. Sharpe, S. Seil, J. Carabez, R. Pisano, I. Wong, K. Balasubramaniam, H. Schwertscharf, C. Bonilla). We would also like to thank the reviewers of this manuscript for their valuable comments and feedback.
Funding Statement
This research was funded by an NIH grant awarded to Brenda McCowan (R01-HD068335) and the California National Primate Research Center base grant (P51-OD01107-53). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Jessica J. Vandeleest conceived and designed the experiments, performed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Brianne A. Beisner conceived and designed the experiments, performed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Darcy L. Hannibal conceived and designed the experiments, performed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Amy C. Nathman performed the experiments, wrote the paper, reviewed drafts of the paper.
John P. Capitanio conceived and designed the experiments, contributed reagents/materials/analysis tools, wrote the paper, reviewed drafts of the paper.
Fushing Hsieh conceived and designed the experiments, contributed reagents/materials/analysis tools, reviewed drafts of the paper.
Edward R. Atwill conceived and designed the experiments, contributed reagents/materials/analysis tools, reviewed drafts of the paper.
Brenda McCowan conceived and designed the experiments, contributed reagents/materials/analysis tools, wrote the paper, reviewed drafts of the paper.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
This research was approved by the University of California Davis Institutional Animal Care and Use Committee.
Data Deposition
The following information was supplied regarding data availability:
Dash (https://dash.cdlib.org/) DOI 10.15146/R3V883; http://n2t.net/ark:/c5146/r3v883.
References
- Abbott et al. (2003).Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, Jr, Sapolsky RM. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Hormones and Behavior. 2003;43(1):67–82. doi: 10.1016/S0018-506X(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Adler et al. (1994).Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, Syme SL. Socioeconomic status and health: the challenge of the gradient. American Psychologist. 1994;49(1):15–24. doi: 10.1037/0003-066X.49.1.15. [DOI] [PubMed] [Google Scholar]
- Albers & de Vries (2001).Albers PCH, de Vries H. Elo-rating as a tool in the sequential estimation of dominance strengths. Animal Behaviour. 2001;61(2):489–495. doi: 10.1006/anbe.2000.1571. [DOI] [Google Scholar]
- Archie, Altmann & Alberts (2012).Archie EA, Altmann J, Alberts SC. Social status predicts wound healing in wild baboons. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(23):9017–9022. doi: 10.1073/pnas.1206391109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold & Dittami (1997).Arnold W, Dittami J. Reproductive suppression in male alpine marmots. Animal Behaviour. 1997;53(1):53–66. doi: 10.1006/anbe.1996.0277. [DOI] [Google Scholar]
- Baum & Fleming (1993).Baum A, Fleming I. Implications of psychological research on stress and technological accidents. American Psychologist. 1993;48(6):665–672. doi: 10.1037/0003-066X.48.6.665. [DOI] [PubMed] [Google Scholar]
- Beisner et al. (2011).Beisner BA, Jackson ME, Cameron AN, McCowan B. Detecting instability in animal social networks: genetic fragmentation is associated with social instability in rhesus macaques. PLoS ONE. 2011;6(1):e2394. doi: 10.1371/journal.pone.0016365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berard (1990).Berard JD. Eugene: University of Oregon; 1990. Life history patterns of male Rhesus Macaques on Cayo Santiago. PhD thesis. [Google Scholar]
- Bond, Kamil & Balda (2003).Bond AB, Kamil AC, Balda RP. Social complexity and transitive inference in corvids. Animal Behaviour. 2003;65(3):479–487. doi: 10.1006/anbe.2003.2101. [DOI] [Google Scholar]
- Boyd & Silk (1983).Boyd R, Silk JB. A method for assigning cardinal dominance ranks. Animal Behaviour. 1983;31(1):45–58. doi: 10.1016/S0003-3472(83)80172-9. [DOI] [Google Scholar]
- Buckley, O’Mahony & O’Malley (2014).Buckley MM, O’Mahony SM, O’Malley D. Convergence of neuro-endocrine-immune pathways in the pathophysiology of irritable bowel syndrome. World Journal of Gastroenterology. 2014;20(27):8846–8858. doi: 10.3748/wjg.v20.i27.8846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham & Anderson (2002).Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. New York: Springer-Verlag; 2002. [Google Scholar]
- Burnham, Anderson & Huyvaert (2011).Burnham KP, Anderson DR, Huyvaert KP. AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behavioral Ecology and Sociobiology. 2011;65(1):23–35. doi: 10.1007/s00265-010-1029-6. [DOI] [Google Scholar]
- Cavigelli (1999).Cavigelli SA. Behavioural patterns associated with faecal cortisol levels in free-ranging female ring-tailed lemurs, Lemur catta. Animal Behaviour. 1999;57(4):935–944. doi: 10.1006/anbe.1998.1054. [DOI] [PubMed] [Google Scholar]
- Chetty et al. (2016).Chetty R, Stepner M, Abraham S, Lin S, Scuderi B, Turner N, Bergeron A, Cutler D. The association between income and life expectancy in the United States, 2001–2014. Journal of the American Medical Association. 2016;315(16):1750–1766. doi: 10.1001/jama.2016.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creel (2001).Creel S. Social dominance and stress hormones. Trends in Ecology & Evolution. 2001;16(9):491–497. doi: 10.1016/S0169-5347(01)02227-3. [DOI] [Google Scholar]
- Creel et al. (2013).Creel S, Dantzer B, Goymann W, Rubenstein DR. The ecology of stress: effects of the social environment. Functional Ecology. 2013;27(1):66–80. doi: 10.1111/j.1365-2435.2012.02029.x. [DOI] [Google Scholar]
- Creel, MarushaCreel & Monfort (1996).Creel S, MarushaCreel N, Monfort SL. Social stress and dominance. Nature. 1996;379(6562):212. doi: 10.1038/379212a0. [DOI] [Google Scholar]
- Crockford et al. (2008).Crockford C, Wittig RM, Whitten PL, Seyfarth RA, Cheney DL. Social stressors and coping mechanisms in wild female baboons (Papio hamadryas ursinus) Hormones and Behavior. 2008;53(1):254–265. doi: 10.1016/j.yhbeh.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Croft, James & Krause (2008).Croft DP, James R, Krause J. Exploring Animal Social Networks. Princeton: Princeton University Press; 2008. [Google Scholar]
- Datta (1986).Datta SB. The role of alliances in the acquisition of rank. In: Else JG, Lee PC, editors. Primate Ontogenty, Cognition and Social Behaviour. New York: Cambridge University Press; 1986. pp. 219–225. [Google Scholar]
- Davis (1992).Davis H. Transitive inference in rats (Rattus norvegicus) Journal of Comparative Psychology. 1992;106(4):342–349. doi: 10.1037/0735-7036.106.4.342. [DOI] [PubMed] [Google Scholar]
- de Vries (1995).de Vries H. An improved test of linearity in dominance hierarchies containing unknown or tied relationships. Animal Behaviour. 1995;50(5):1375–1389. doi: 10.1016/0003-3472(95)80053-0. [DOI] [Google Scholar]
- de Vries (1998).de Vries H. Finding a dominance order most consistent with a linear hierarchy: a new procedure and review. Animal Behaviour. 1998;55(4):827–843. doi: 10.1006/anbe.1997.0708. [DOI] [PubMed] [Google Scholar]
- Dittus (1975).Dittus W. Population dynamics of the toque monkey Macaca sinica. In: Tuttle RH, editor. Socioecology and Psychology of Primates. Den Haag: Mouton; 1975. pp. 125–151. [Google Scholar]
- Ehardt & Bernstein (1986).Ehardt CL, Bernstein IS. Matrilineal overthrows in rhesus monkey groups. International Journal of Primatology. 1986;7(2):157–181. doi: 10.1007/BF02692316. [DOI] [Google Scholar]
- Fujii et al. (2015).Fujii K, Jin J, Shev A, Beisner B, McCowan B, Fushing H. Perc: using percolation and conductance to find information flow certainty in a direct network. http://cran.r-project.org/package=Perc 2015
- Fushing et al. (2011).Fushing H, McAssey MP, Beisner B, McCowan B. Ranking network of a captive rhesus macaque society: a sophisticated corporative kingdom. PLoS ONE. 2011;6(3):e2394. doi: 10.1371/journal.pone.0017817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fushing, McAssey & McCowan (2011).Fushing H, McAssey MP, McCowan B. Computing a ranking network with confidence bounds from a graph-based beta random field. Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences. 2011;467(2136):3590–3612. doi: 10.1098/rspa.2011.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesquiere et al. (2011).Gesquiere LR, Learn NH, Simao MCM, Onyango PO, Alberts SC, Altmann J. Life at the top: rank and stress in wild male baboons. Science. 2011;333(6040):357–360. doi: 10.1126/science.1207120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goymann et al. (2001).Goymann W, East ML, Wachter B, Höner OP, Möstl E, Van’t Holf TJ, Hofer H. Social state-dependent and environmental modulation of faecal corticosteroid levels in free-ranging female spotted hyenas. Proceedings of the Royal Society B: Biological Sciences. 2001;268(1484):2453–2459. doi: 10.1098/rspb.2001.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosenick, Clement & Fernald (2007).Grosenick L, Clement TS, Fernald RD. Fish can infer social rank by observation alone. Nature. 2007;445(7126):429–432. doi: 10.1038/nature05511. [DOI] [PubMed] [Google Scholar]
- Habig & Archie (2015).Habig B, Archie EA. Social status, immune response and parasitism in males: a meta-analysis. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015;370(1669):20140109. doi: 10.1098/rstb.2014.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackländer, Möstl & Arnold (2003).Hackländer K, Möstl E, Arnold W. Reproductive suppression in female Alpine marmots, Marmota marmota. Animal Behaviour. 2003;65(6):1133–1140. doi: 10.1006/anbe.2003.2159. [DOI] [Google Scholar]
- Hardin & Hilbe (2007).Hardin JW, Hilbe J. Generalized Linear Models and Extensions. College Station: Stata Press; 2007. p. 387. [Google Scholar]
- Hawkley & Capitanio (2015).Hawkley LC, Capitanio JP. Perceived social isolation, evolutionary fitness and health outcomes: a lifespan approach. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015;370(1669):20140114. doi: 10.1098/rstb.2014.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney, Israel & House (1994).Heaney CA, Israel BA, House JS. Chronic job insecurity among automobile workers: effects on job satisfaction and health. Social Science & Medicine. 1994;38(10):1431–1437. doi: 10.1016/0277-9536(94)90281-X. [DOI] [PubMed] [Google Scholar]
- Hinde (1976).Hinde RA. Interactions, relationships and social structure. Man. 1976;11(1):1–17. doi: 10.2307/2800384. [DOI] [Google Scholar]
- Hostinar, Sullivan & Gunnar (2014).Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development. Psychological Bulletin. 2014;140(1):256–282. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan & Manuck (1999).Kaplan JR, Manuck SB. Status, stress, and atherosclerosis: the role of environment and individual behavior. Annals of the New York Academy of Sciences. 1999;896(1):145–161. doi: 10.1111/j.1749-6632.1999.tb08112.x. [DOI] [PubMed] [Google Scholar]
- Klein (2000).Klein SL. The effects of hormones on sex differences in infection: from genes to behavior. Neuroscience & Biobehavioral Reviews. 2000;24(6):627–638. doi: 10.1016/S0149-7634(00)00027-0. [DOI] [PubMed] [Google Scholar]
- Libby, Ridker & Maseri (2002).Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- Lindburg (1971).Lindburg DG. The rhesus monkey in north India: an ecological and behavioral study. In: Rosenblum LA, editor. Primate Behavior: Development in Field and Laboratory Research. New York: Academic Press; 1971. pp. 1–106. [Google Scholar]
- Lynch, Ziegler & Strier (2002).Lynch JW, Ziegler TE, Strier KB. Individual and seasonal variation in fecal testosterone and cortisol levels of wild male tufted capuchin monkeys, Cebus apella nigritus. Hormones and Behavior. 2002;41(3):275–287. doi: 10.1006/hbeh.2002.1772. [DOI] [PubMed] [Google Scholar]
- Marmot & Sapolsky (2014).Marmot M, Sapolsky RM. Of baboons and men: social circumstances, biology, and the social gradient in health. In: Weinstein M, Lane MA, editors. Sociality, Hierarchy, Health: Comparative Biodemography: A Collection of Papers. Washington, D.C.: The National Academies Press; 2014. pp. 365–388. [PubMed] [Google Scholar]
- Mays, Vleck & Dawson (1991).Mays NA, Vleck CM, Dawson J. Plasma luteinizing hormone, steroid hormones, behavioral role and nesting stage in the cooperatively breeding Harris’s hawk (Parabuteo unicinctus) Auk. 1991;108(3):619–637. doi: 10.2307/4088103. [DOI] [Google Scholar]
- McCowan et al. (2016).McCowan B, Beisner B, Bliss-Moreau E, Vandeleest J, Jin J, Hannibal D, Hsieh F. Connections matter: social networks and lifespan health in primate translational models. Frontiers in Psychology. 2016;7:433. doi: 10.3389/fpsyg.2016.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh & Nelder (1989).McCullagh P, Nelder JA. Generalized Linear Models. London: Chapman and Hall; 1989. [Google Scholar]
- McGonigle & Chalmers (1977).McGonigle BO, Chalmers M. Are monkeys logical? Nature. 1977;267(5613):694–696. doi: 10.1038/267694a0. [DOI] [PubMed] [Google Scholar]
- Melnick, Pearl & Richard (1984).Melnick DJ, Pearl MC, Richard AF. Male migration and inbreeding avoidance in wild rhesus monkeys. American Journal of Primatology. 1984;7(3):229–243. doi: 10.1002/(ISSN)1098-2345. [DOI] [PubMed] [Google Scholar]
- Muggeo (2008).Muggeo VMR. segmented: an R package to fit regression models with broken-line relationships. R News. 2008;8(1):20–25. [Google Scholar]
- Muller & Wrangham (2004).Muller MN, Wrangham RW. Dominance, cortisol and stress in wild chimpanzees (Pan troglodytes schweinfurthii) Behavioral Ecology and Sociobiology. 2004;55(4):332–340. doi: 10.1007/s00265-003-0713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann et al. (2011).Neumann C, Duboscq J, Dubuc C, Ginting A, Irwan AM, Agil M, Widdig A, Engelhardt A. Assessing dominance hierarchies: validation and advantages of progressive evaluation with Elo-rating. Animal Behaviour. 2011;82(4):911–921. doi: 10.1016/j.anbehav.2011.07.016. [DOI] [Google Scholar]
- Nunn et al. (2015).Nunn CL, Craft ME, Gillespie TR, Schaller M, Kappeler PM. The sociality–health–fitness nexus: synthesis, conclusions and future directions. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015;370(1669):20140115. doi: 10.1098/rstb.2014.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostner, Heistermann & Schülke (2008).Ostner J, Heistermann M, Schülke O. Dominance, aggression, and physiological stress in wild male Assamese macaques (Macaca assamensis) Hormones and Behavior. 2008;54(5):613–619. doi: 10.1016/j.yhbeh.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Pachucki et al. (2015).Pachucki MC, Ozer EJ, Barrat A, Cattuto C. Mental health and social networks in early adolescence: a dynamic study of objectively-measured social interaction behaviors. Social Science & Medicine. 2015;125:40–50. doi: 10.1016/j.socscimed.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Perkins, Subramanian & Christakis (2015).Perkins JM, Subramanian SV, Christakis NA. Social networks and health: a systematic review of sociocentric network studies in low- and middle-income countries. Social Science & Medicine. 2015;125:60–78. doi: 10.1016/j.socscimed.2014.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan et al. (2001).Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. Journal of the American Medical Association. 2001;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- R Core Team (2013).R Core Team . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2013. [Google Scholar]
- Rabe-Hesketh & Skrondal (2012).Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata: Continuous Responses. College Station: Stata Press Publication; 2012. p. 497. [Google Scholar]
- Raudenbush & Bryk (2002).Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. Thousand Oaks: Sage Publications; 2002. [Google Scholar]
- Sade (1972).Sade DS. A longitudinal study of social behavior of rhesus monkeys. In: Tuttle R, editor. The Functional and Evolutionary Biology of Primates. Chicago: Aldine-Atherton; 1972. pp. 378–398. [Google Scholar]
- Sansoni et al. (2008).Sansoni P, Vescovini R, Fagnoni F, Biasini C, Zanni F, Zanlari L, Telera A, Lucchini G, Passeri G, Monti D, Franceschi C, Passeri M. The immune system in extreme longevity. Experimental Gerontology. 2008;43(2):61–65. doi: 10.1016/j.exger.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Sapolsky (1992).Sapolsky RM. Cortisol concentrations and the social significance of rank instability among wild baboons. Psychoneuroendocrinology. 1992;17(6):701–709. doi: 10.1016/0306-4530(92)90029-7. [DOI] [PubMed] [Google Scholar]
- Sapolsky (2005).Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308(5722):648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Sapolsky & Mott (1987).Sapolsky RM, Mott GE. Social subordinance in wild baboons is associated with suppressed high density lipoprotein-cholesterol concentrations: the possible role of chronic social stress. Endocrinology. 1987;121(5):1605–1610. doi: 10.1210/endo-121-5-1605. [DOI] [PubMed] [Google Scholar]
- Sapolsky, Romero & Munck (2000).Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Sapolsky & Share (1994).Sapolsky RM, Share LJ. Rank-related differences in cardiovascular function among wild baboons: role of sensitivity to glucocorticoids. American Journal of Primatology. 1994;32(4):261–275. doi: 10.1002/ajp.1350320404. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. (2009).SAS Institute Inc. SAS/STAT® 9.2 User’s Guide. Cary: SAS Institute Inc.; 2009. [Google Scholar]
- Schino (2001).Schino G. Grooming, competition and social rank among female primates: a meta-analysis. Animal Behaviour. 2001;62(2):265–271. doi: 10.1006/anbe.2001.1750. [DOI] [Google Scholar]
- Schoech, Mumme & Moore (1991).Schoech SJ, Mumme RL, Moore MC. Reproductive endocrinology and mechanisms of breeding inhibition in cooperatively breeding Florida Scrub Jays (Aphelocoma c. coerulescens) Condor. 1991;93(2):354–364. doi: 10.2307/1368951. [DOI] [Google Scholar]
- Sebastian (2015).Sebastian SO. Using individual attributes to predict hierarchical position in a Macaca sylvanus group at ‘La forêt des singes’, Rocamadour. Behavioural Processes. 2015;111:109–117. doi: 10.1016/j.beproc.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Shively & Clarkson (1994).Shively CA, Clarkson TB. Social status and coronary artery atherosclerosis in female monkeys. Arteriosclerosis, Thrombosis, and Vascular Biology. 1994;14(5):721–726. doi: 10.1161/01.ATV.14.5.721. [DOI] [PubMed] [Google Scholar]
- Sprague (1992).Sprague DS. Life history and male intertroop mobility among Japanese macaques (Macaca fuscata) International Journal of Primatology. 1992;13(4):437–454. doi: 10.1007/BF02547827. [DOI] [Google Scholar]
- Sprague (1998).Sprague DS. Age, dominance rank, natal status, and tenure among male macaques. American Journal of Physical Anthropology. 1998;105(4):511–521. doi: 10.1002/(SICI)1096-8644(199804)105:4<511::AID-AJPA8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Stasi et al. (2012).Stasi C, Rosselli M, Bellini M, Laffi G, Milani S. Altered neuro-endocrine-immune pathways in the irritable bowel syndrome: the top-down and the bottom-up model. Journal of Gastroenterology. 2012;47(11):1177–1185. doi: 10.1007/s00535-012-0627-7. [DOI] [PubMed] [Google Scholar]
- Symonds & Moussalli (2011).Symonds MRE, Moussalli A. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behavioral Ecology and Sociobiology. 2011;65(1):13–21. doi: 10.1007/s00265-010-1037-6. [DOI] [Google Scholar]
- Thoits (2011).Thoits PA. Mechanisms linking social ties and support to physical and mental health. Journal of Health and Social Behavior. 2011;52(2):145–161. doi: 10.1177/0022146510395592. [DOI] [PubMed] [Google Scholar]
- Viswanath (2016).Viswanath G. Age and the Elo rating system, how underrated are the kids? 2016. https://en.chessbase.com/post/elo-rating-system-how-underrated-are-the-kids https://en.chessbase.com/post/elo-rating-system-how-underrated-are-the-kids
- Wingfield, Hegner & Lewis (1991).Wingfield JC, Hegner RE, Lewis DM. Circulating levels of luteinizing hormone and steroid hormones in relation to social status in the cooperatively breeding white-browed sparrow weaver, Plocepasser mahali. Journal of Zoology. 1991;225(1):43–58. doi: 10.1111/j.1469-7998.1991.tb03800.x. [DOI] [Google Scholar]
- Young et al. (2006).Young AJ, Carlson AA, Monfort SL, Russell AF, Bennett NC, Clutton-Brock T. Stress and the suppression of subordinate reproduction in cooperatively breeding meerkats. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(32):12005–12010. doi: 10.1073/pnas.0510038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Raw values of CRP. A and B depict effects for CRP for males and females, respectively. Separate lines represent different levels of dominance certainty.
Raw values of IL-6. A and B depict effects for CRP for males and females, respectively. Separate lines represent different levels of dominance certainty.
Raw values of TNF-α plotted against dominance rank. Separate lines represent different levels of dominance certainty.