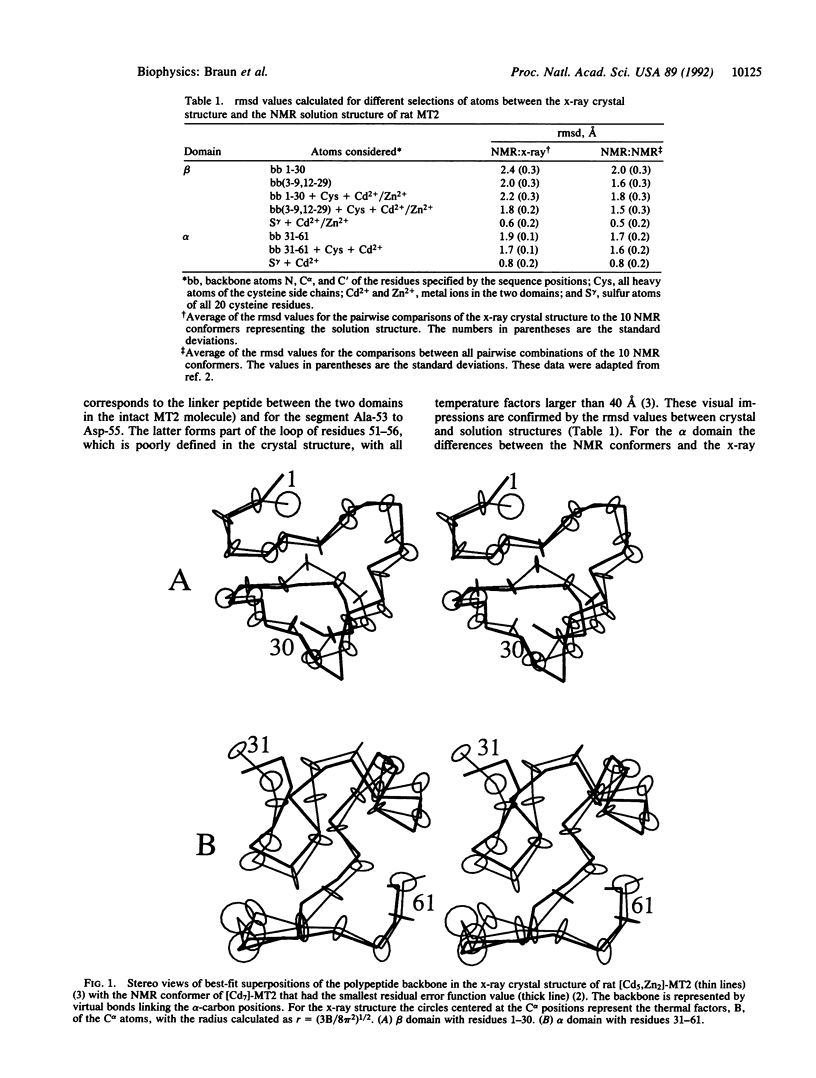
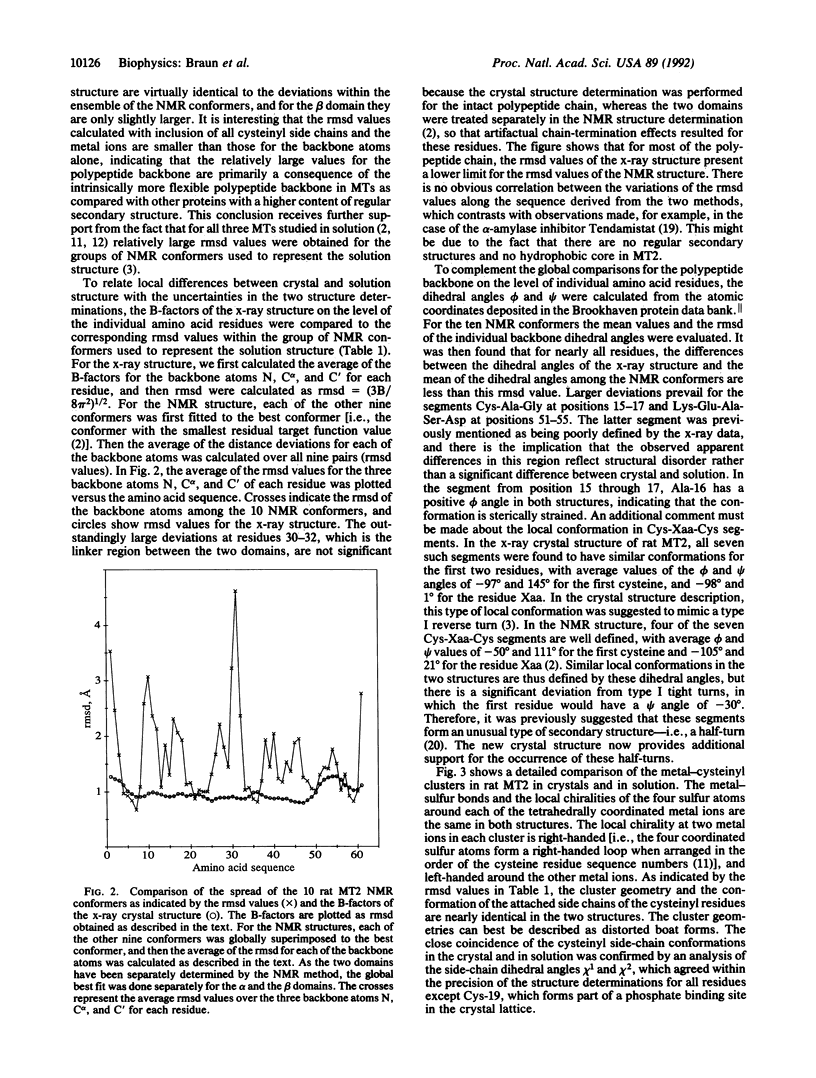
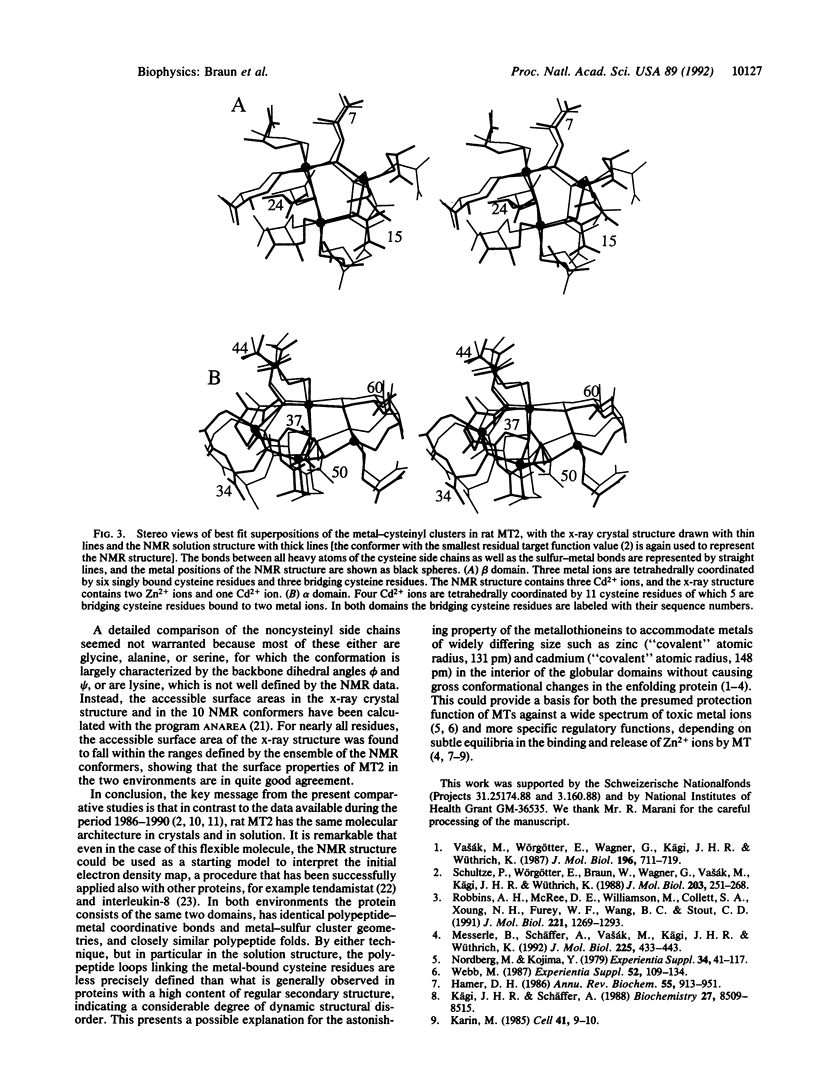
Abstract
Metallothioneins are small cysteine-rich proteins capable of binding heavy metal ions such as Zn2+ and Cd2+. They are ubiquitous tissue components in higher organisms, which tentatively have been attributed both unspecific protective functions against toxic metal ions and highly specific roles in fundamental zinc-regulated cellular processes. In this paper a detailed comparison of the NMR solution structure [Schultze, P., Wörgötter, E., Braun, W., Wagner, G., Vasák, M., Kägi, J. H. R. & Wüthrich, K. (1988) J. Mol. Biol. 203, 251-268] and a recent x-ray crystal structure [Robbins, A. H., McRee, D. E., Williamson, M., Collett, S. A., Xoung, N. H., Furey, W. F., Wang, B. C. & Stout, C. D. (1991) J. Mol. Biol. 221, 1269-1293] of rat metallothionein-2 shows that the metallothionein structures in crystals and in solution have identical molecular architectures. The structures obtained with both techniques now present a reliable basis for discussions on structure-function correlations in this class of metalloproteins.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arseniev A., Schultze P., Wörgötter E., Braun W., Wagner G., Vasák M., Kägi J. H., Wüthrich K. Three-dimensional structure of rabbit liver [Cd7]metallothionein-2a in aqueous solution determined by nuclear magnetic resonance. J Mol Biol. 1988 Jun 5;201(3):637–657. doi: 10.1016/0022-2836(88)90644-4. [DOI] [PubMed] [Google Scholar]
- Baldwin E. T., Weber I. T., St Charles R., Xuan J. C., Appella E., Yamada M., Matsushima K., Edwards B. F., Clore G. M., Gronenborn A. M. Crystal structure of interleukin 8: symbiosis of NMR and crystallography. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):502–506. doi: 10.1073/pnas.88.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter M., Kline A. D., Braun W., Huber R., Wüthrich K. Comparison of the high-resolution structures of the alpha-amylase inhibitor tendamistat determined by nuclear magnetic resonance in solution and by X-ray diffraction in single crystals. J Mol Biol. 1989 Apr 20;206(4):677–687. doi: 10.1016/0022-2836(89)90575-5. [DOI] [PubMed] [Google Scholar]
- Braun W. Distance geometry and related methods for protein structure determination from NMR data. Q Rev Biophys. 1987 May;19(3-4):115–157. doi: 10.1017/s0033583500004108. [DOI] [PubMed] [Google Scholar]
- Braun W., Epp O., Wüthrich K., Huber R. Solution of the phase problem in the X-ray diffraction method for proteins with the nuclear magnetic resonance solution structure as initial model. Patterson search and refinement for the alpha-amylase inhibitor tendamistat. J Mol Biol. 1989 Apr 20;206(4):669–676. doi: 10.1016/0022-2836(89)90574-3. [DOI] [PubMed] [Google Scholar]
- Braun W., Go N. Calculation of protein conformations by proton-proton distance constraints. A new efficient algorithm. J Mol Biol. 1985 Dec 5;186(3):611–626. doi: 10.1016/0022-2836(85)90134-2. [DOI] [PubMed] [Google Scholar]
- Furey W. F., Robbins A. H., Clancy L. L., Winge D. R., Wang B. C., Stout C. D. Crystal structure of Cd,Zn metallothionein. Science. 1986 Feb 14;231(4739):704–710. doi: 10.1126/science.3945804. [DOI] [PubMed] [Google Scholar]
- Hamer D. H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Karin M. Metallothioneins: proteins in search of function. Cell. 1985 May;41(1):9–10. doi: 10.1016/0092-8674(85)90051-0. [DOI] [PubMed] [Google Scholar]
- Kägi J. H., Schäffer A. Biochemistry of metallothionein. Biochemistry. 1988 Nov 15;27(23):8509–8515. doi: 10.1021/bi00423a001. [DOI] [PubMed] [Google Scholar]
- Messerle B. A., Schäffer A., Vasák M., Kägi J. H., Wüthrich K. Comparison of the solution conformations of human [Zn7]-metallothionein-2 and [Cd7]-metallothionein-2 using nuclear magnetic resonance spectroscopy. J Mol Biol. 1992 May 20;225(2):433–443. doi: 10.1016/0022-2836(92)90930-i. [DOI] [PubMed] [Google Scholar]
- Messerle B. A., Schäffer A., Vasák M., Kägi J. H., Wüthrich K. Three-dimensional structure of human [113Cd7]metallothionein-2 in solution determined by nuclear magnetic resonance spectroscopy. J Mol Biol. 1990 Aug 5;214(3):765–779. doi: 10.1016/0022-2836(90)90291-S. [DOI] [PubMed] [Google Scholar]
- Metallothionein and other low molecular weight metal-binding proteins. report from the "first international meeting on metallothionein and other low molecular weight metal-binding proteins". Experientia Suppl. 1979;34:41–135. [PubMed] [Google Scholar]
- Moore J. M., Lepre C. A., Gippert G. P., Chazin W. J., Case D. A., Wright P. E. High-resolution solution structure of reduced French bean plastocyanin and comparison with the crystal structure of poplar plastocyanin. J Mol Biol. 1991 Sep 20;221(2):533–555. doi: 10.1016/0022-2836(91)80071-2. [DOI] [PubMed] [Google Scholar]
- Richmond T. J. Solvent accessible surface area and excluded volume in proteins. Analytical equations for overlapping spheres and implications for the hydrophobic effect. J Mol Biol. 1984 Sep 5;178(1):63–89. doi: 10.1016/0022-2836(84)90231-6. [DOI] [PubMed] [Google Scholar]
- Robbins A. H., McRee D. E., Williamson M., Collett S. A., Xuong N. H., Furey W. F., Wang B. C., Stout C. D. Refined crystal structure of Cd, Zn metallothionein at 2.0 A resolution. J Mol Biol. 1991 Oct 20;221(4):1269–1293. [PubMed] [Google Scholar]
- Robbins A. H., Stout C. D. X-ray structure of metallothionein. Methods Enzymol. 1991;205:485–502. doi: 10.1016/0076-6879(91)05134-h. [DOI] [PubMed] [Google Scholar]
- Schultze P., Wörgötter E., Braun W., Wagner G., Vasák M., Kägi J. H., Wüthrich K. Conformation of [Cd7]-metallothionein-2 from rat liver in aqueous solution determined by nuclear magnetic resonance spectroscopy. J Mol Biol. 1988 Sep 5;203(1):251–268. doi: 10.1016/0022-2836(88)90106-4. [DOI] [PubMed] [Google Scholar]
- Vasák M., Wörgötter E., Wagner G., Kägi J. H., Wüthrich K. Metal co-ordination in rat liver metallothionein-2 prepared with or without reconstitution of the metal clusters, and comparison with rabbit liver metallothionein-2. J Mol Biol. 1987 Aug 5;196(3):711–719. doi: 10.1016/0022-2836(87)90042-8. [DOI] [PubMed] [Google Scholar]
- Wagner G., Neuhaus D., Wörgötter E., Vasák M., Kägi J. H., Wüthrich K. Nuclear magnetic resonance identification of "half-turn" and 3(10)-helix secondary structure in rabbit liver metallothionein-2. J Mol Biol. 1986 Jan 5;187(1):131–135. doi: 10.1016/0022-2836(86)90413-4. [DOI] [PubMed] [Google Scholar]
- Webb M. Toxicological significance of metallothionein. Experientia Suppl. 1987;52:109–134. doi: 10.1007/978-3-0348-6784-9_6. [DOI] [PubMed] [Google Scholar]
- Wüthrich K. Protein structure determination in solution by nuclear magnetic resonance spectroscopy. Science. 1989 Jan 6;243(4887):45–50. doi: 10.1126/science.2911719. [DOI] [PubMed] [Google Scholar]



