Abstract
Australia's tropical waters represent predicted ‘hotspots' for nitrogen (N2) fixation based on empirical and modelled data. However, the identity, activity and ecology of diazotrophs within this region are virtually unknown. By coupling DNA and cDNA sequencing of nitrogenase genes (nifH) with size-fractionated N2 fixation rate measurements, we elucidated diazotroph dynamics across the shelf region of the Arafura and Timor Seas (ATS) and oceanic Coral Sea during Austral spring and winter. During spring, Trichodesmium dominated ATS assemblages, comprising 60% of nifH DNA sequences, while Candidatus Atelocyanobacterium thalassa (UCYN-A) comprised 42% in the Coral Sea. In contrast, during winter the relative abundance of heterotrophic unicellular diazotrophs (δ-proteobacteria and γ-24774A11) increased in both regions, concomitant with a marked decline in UCYN-A sequences, whereby this clade effectively disappeared in the Coral Sea. Conservative estimates of N2 fixation rates ranged from <1 to 91 nmol l−1 day−1, and size fractionation indicated that unicellular organisms dominated N2 fixation during both spring and winter, but average unicellular rates were up to 10-fold higher in winter than in spring. Relative abundances of UCYN-A1 and γ-24774A11 nifH transcripts negatively correlated to silicate and phosphate, suggesting an affinity for oligotrophy. Our results indicate that Australia's tropical waters are indeed hotspots for N2 fixation and that regional physicochemical characteristics drive differential contributions of cyanobacterial and heterotrophic phylotypes to N2 fixation.
Introduction
Biological nitrogen (N2) fixation is a fundamental process within the ocean, helping to alleviate nitrogen limitation, thereby supporting primary production and the sequestration of carbon to the deep sea (Sohm et al., 2011; Karl et al., 2012). N2 fixation is mediated by a diverse range of microorganisms (Zehr et al., 2003), including the photoautotrophic cyanobacterium Trichodesmium, as well as unicellular cyanobacteria, diatom-associated cyanobacteria and heterotrophic bacteria, all of which are distributed across tropical and subtropical latitudes (Capone et al., 1997, 2005; Montoya et al., 2004; Moisander et al., 2008, 2010, 2014; Foster et al., 2009).
High rates of marine N2 fixation have been observed in Australia's tropical waters (Montoya et al., 2004), and N2 fixation rate models predict rates sometimes >100 μmol m−2 day−1 in this region (Luo et al., 2014). Additionally, ecosystem models predict cyanobacterial diazotrophs will be abundant in northern Australian waters (Monteiro et al., 2010). Indeed, Trichodesmium has long been recognised as an important member of the phytoplankton in this region (Hallegraeff and Jeffrey, 1984; Burford et al., 1995, 2009), and unicellular diazotrophs are assumed to be highly active here as well (Montoya et al., 2004). However, compared with the South Pacific Ocean, where unicellular diazotrophs, including Candidatus Atelocyanobacterium thalassa (UCYN-A), Crocosphaera watsonii and the γ-proteobacterial clade γ-24774A11, are known to be abundant (Moisander et al., 2010, 2014), we currently lack any detailed understanding of patterns in the diversity, activity and ecology of diazotrophs within tropical Australian waters.
We surveyed two distinct oceanographic provinces in northern Australia that have important roles in global climate and ocean circulation. These include the semi-enclosed Arafura and Timor shelf sea regions (ATS), which form part of the Indian Pacific Warm Pool (Alongi et al., 2011), and the open ocean Coral Sea, where the South Pacific western boundary current originates (Qu and Lindstrom, 2002). The ATS is considered autotrophic (McKinnon et al., 2011) and highly productive, particularly during the tropical dry season (Austral winter) (Alongi et al., 2011), despite relatively low surface nitrogen concentrations (<2 μm nitrate) and no deep-water reservoir of nutrients (Lyne and Hayes, 2005). Annual primary production in the Coral Sea is relatively low and nitrogen limitation is predicted (Condie and Dunn, 2006), with the upper 100 m of the water column being highly oligotrophic throughout the year (Lyne and Hayes, 2005).
N2 fixation has been found to be a significant biogeochemical feature of this important region of the ocean (Montoya et al., 2004; Luo et al., 2014), but we observed substantial physicochemical variability, manifest in differential temperature and salinity signatures and nutrient availability between the ATS and Coral Sea, which may influence the relative importance of diazotroph activity, particularly given the highly dynamic nature of diazotroph communities (Robidart et al., 2014). By combining nifH sequencing and size-fractionated 15N2 rate measurements, we assessed spatial and temporal patterns in the diversity and activity of N2-fixing bacteria across this region with the aim of characterising the dynamics of diazotrophy within this putative global N2 fixation hotspot.
Materials and methods
Sample collection
Sampling was performed during two voyages aboard the R/V Southern Surveyor consisting of a 2500 km transect from Darwin to Cairns conducted in the Austral spring (October 2012; ss2012_t07; Figure 1a), and a 5000 km transect from Broome to Brisbane during the Austral winter (July–August; ss2013_t03; Figure 1b). October marks the beginning of the tropical wet season, while the July–August period corresponds to the middle of the dry season.
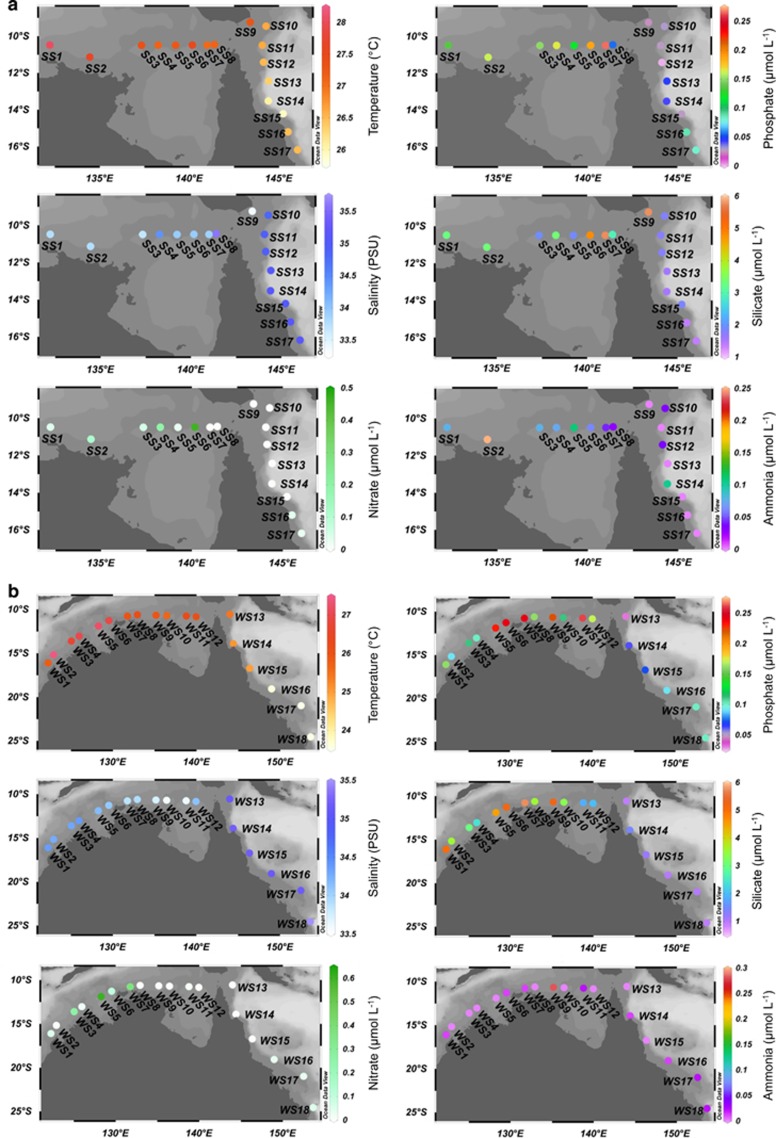
Figure 1.
Physical and chemical characteristics of the surface waters sampled within the ATS (stations SS1–8; WS1–12) and Coral Sea (stations SS9–17; WS13–18) during (a) spring and (b) winter. Note the different scales within and between panels (a) and (b).
Seawater was sampled daily at dawn during both transects for diazotroph diversity (DNA) and gene expression (cDNA) analyses and N2 fixation rate measurements, as well as mid-afternoon for analysis of diazotroph diversity (DNA) only, resulting in stations separated by 100–300 km. Samples were collected from the surface at all stations, and the chlorophyll maximum (cmax) when a fluorescence peak was discernible in the water column (see Supplementary Information).
N2 fixation rates
Net 15N2 assimilation (Montoya et al., 1996) was measured to obtain estimates of N2 fixation by the whole community (WC) and <10 μm unicellular size fraction (USF) of diazotrophs as previously described (Church et al., 2009; see Supplementary Information). Experiments conducted with surface and cmax samples were incubated at in situ temperature and light levels for 24 h and terminated by filtration (see Supplementary Information). Assimilation rates were calculated as previously described (Montoya et al., 1996), based on a theoretical enrichment of ca. 8 atom% 15N2, and are considered conservative estimates of N2 fixation owing to the known incomplete dissolution of the 15N2 gas bubble (Mohr et al., 2010).
Nucleic acid collection and extraction
At each station, 4–8 l of seawater was filtered through 0.22μm Sterivex filter units (EMD Millipore, Billerica, MA, USA). Filters were immediately frozen in liquid nitrogen and stored at −80 °C. Community DNA was extracted using the PowerWater DNA Extraction Kit (MoBio Laboratories, Carlsbad, CA, USA) according to the manufacturer's instructions, including an additional 10 min heating step with solution PW1 to ensure complete cell lysis. DNA yield was quantified using a Broad Range DNA Qubit Assay (Invitrogen, Carlsbad, CA, USA) with a Qubit 2.0 Fluorometer.
RNA samples were collected from all N2 fixation incubation stations. Seawater (1–2 l) was filtered through a 0.22μm Durapore membrane filter (Millipore) within 15 min of collection, after which RNAlater solution (300 μl; Ambion, Austin, TX, USA) was added, and filters were frozen in liquid nitrogen and stored at −80 °C. Total RNA was extracted as previously described (Frias-Lopez et al., 2008; Stewart et al., 2010; see Supplementary Information).
nifH PCR amplification and amplicon pyrosequencing
A nested PCR protocol was used to amplify an ~359-bp region of the nitrogenase gene, using the degenerate primers: nifH3, nifH4, nifH1, and nifH2 (Zani et al., 2000; Zehr and Turner, 2001). Equal volumes of DNA or cDNA were used as template (2 μl) in the first stage of the reaction, and 1 μl of PCR product was used as template in the second stage, using previously described reaction conditions (Messer et al., 2015; see Supplementary Information).
The nifH amplicons were sequenced using the 454 FLX Titanium pyrosequencing platform (Roche, Nutley, NJ, USA; Molecular Research LP, Shallowater, TX, USA) following an additional 10 PCR cycles with custom barcoded nifH1 and nifH2 primers under the same PCR reaction conditions (Dowd et al., 2008; Farnelid et al., 2011, 2013; Messer et al., 2015; Supplementary Information). Raw sequences were quality filtered, whereby sequences with a quality score <25 and reads <200-bp long were removed and clustered into operational taxonomic units (OTUs) at 95% sequence identity (Penton et al., 2013) using UCLUST (Edgar, 2010) and rarefied to the lowest number of sequences per sample (872 sequences) in QIIME (Caporaso et al., 2010). To assign putative taxonomy, representative sequences from nifH OTUs were aligned to the closest sequence in a custom nifH database (updated in April 2014) (Zehr et al., 2003; Heller et al., 2014) and placed in a phylogenetic tree using the maximum parsimony tool in ARB (Westram et al., 2011). Translated nifH sequences were compared with the Ribosomal Database Project's nifH protein database using FrameBot from the Fungene pipeline (Fish et al., 2013; Wang et al., 2013). Sequence data have been deposited in the National Centre for Biotechnology Information Sequence Read Archive under Biosample accession number SAMN04251458.
Statistical analyses
Rarefied sequence data were square-root transformed, and a resemblance matrix was generated using Bray–Curtis similarity. Environmental parameters (Supplementary Table S1) were normalised, and a resemblance matrix was generated using Euclidean distance (Clarke and Warwick, 2001). Statistical analyses, including analysis of similarities (ANOSIM; Clarke 1993), distance-based linear modelling (DistLM) and distance-based redundancy analysis (dbRDA) (Legendre and Anderson, 1999; McArdle and Anderson, 2001), were performed in the PRIMER+PERMANOVA software package (v6; Clarke and Warwick, 2001).
In order to identify associations (linear regression, p) between N2 fixation rates, expressed nifH OTUs and environmental parameters, we calculated the maximal information coefficient (MIC) between all variable (n=255) pairs from all samples (n=28) using the MINE statistics package (Reshef et al., 2011). Strongly co-linear variables (P>0.9 or >−0.9) were removed from the analyses. After correction for multiple testing (Benjamini and Hochberg, 1995), statistically significant co-occurrence relationships (P<0.01; MIC>0.473) between pairs of variables were input into Cytoscape v 2.8 (Smoot et al., 2011) and used to generate network diagrams for visualisation.
Results
Physicochemical characteristics of the ATS and Coral Sea
During both spring and winter, ATS waters were warmer, exhibited lower salinities and had higher nutrient concentrations and phytoplankton abundances than Coral Sea waters (Figure 1; Supplementary Table S1). Mean sea surface temperature (SST) in the ATS was 27.4 °C in spring and 26.4 °C in winter, compared with 26.5 °C and 24.3 °C in the Coral Sea. During both sampling periods, salinity increased from 33.5 to >35 practical salinity units at a longitude of approximately 143 °E, reflecting the transition from the shelf region (ATS) to the open ocean (Coral Sea) (Figure 1). Mean nitrate and phosphate concentrations were greatest during the spring, being 3.5 and 3.8 times greater in the ATS than in the Coral Sea respectively, with this discrepancy falling to 2.6 and 1.8 times in the winter. N:P ratios were always <16:1, indicating an excess of phosphate compared with nitrate and ammonium, particularly in surface waters (Supplementary Table S1). We also observed intra-region nutrient differences, for example, silicate concentrations were highest in the eastern region of the ATS in spring (5.97 μmol l−1; SS7), and the western region in winter (5.74 μmol l−1; WS1; Figure 1). Pigment analyses indicated that phytoplankton communities within the ATS were dominated by microphytoplankton such as diatoms, whereas picophytoplankton and nanophytoplankton were relatively more abundant in the Coral Sea (Supplementary Table S1).
N2 fixation rates
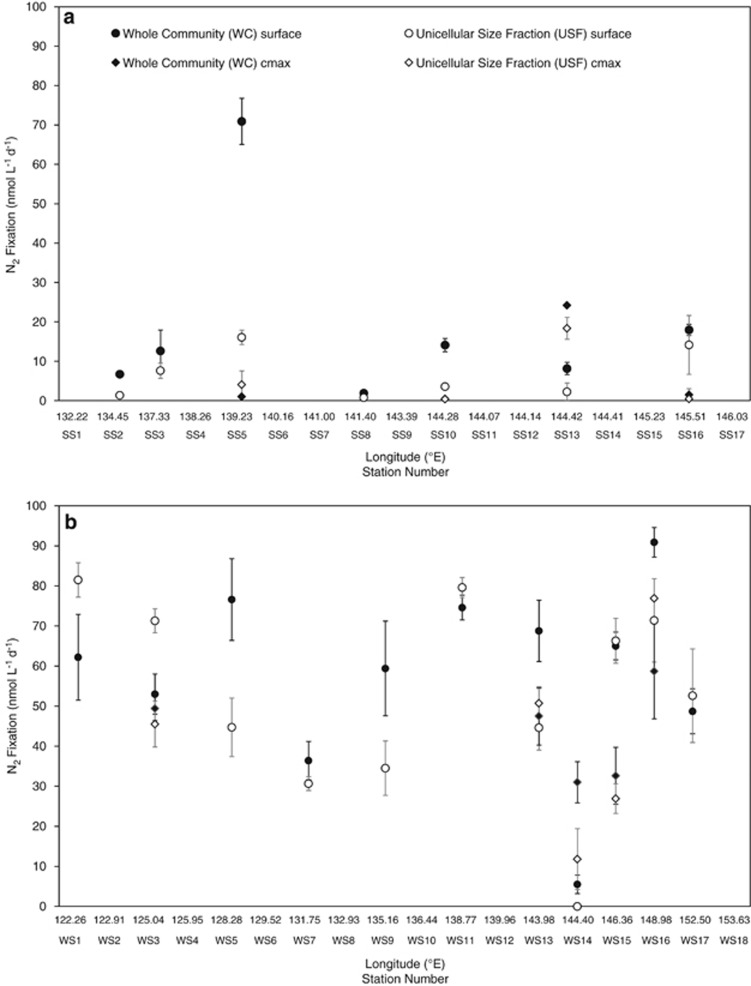
N2 fixation rates within the WC and USF ranged from <1 to 91 nmol l−1 day−1 and at times displayed substantial variability between the ATS and Coral Sea and between seasons (Figure 2). During the Austral spring, mean WC N2 fixation rates (±s.d.) were 23±32 nmol l−1 day−1 compared with 6±7 nmol l−1 day−1 in the USF. However, it is notable that the majority of N2 fixation occurred within the <10μm size class at three out of the four ATS sites (Figure 2a). Within ATS waters, there was a peak in N2 fixation at site SS5 where WC rates reached 71±10 nmol l−1 day−1, significantly greater than the USF rates of 16±3 nmol l−1 day−1 (one-way analysis of variance, Tukey's honestly significant difference, P<0.05). This was the only ATS site sampled for N2 fixation rate measurements where a cmax was observed, and rates within it were comparatively low at ⩽4 nmol l−1 day−1 for both size classes (Figure 2a).
Figure 2.
Mean N2 fixation rates (±s.e., n=3) performed by the WC and <10 μm USF at the surface and chlorophyll maxima during the spring (SS; (a)) and winter (WS; (b)) transects.
Spring N2 fixation rates in the Coral Sea were lower than in the ATS, with mean rates of 13±5 nmol l−1 day−1 (WC) and 7±6 nmol l−1 day−1 (USF). However, within this region the contribution made by unicellular organisms was higher, being ~50% of the total. Rates in Coral Sea surface waters reached a maximum of 18±2 nmol l−1 day−1 (WC) and 14±13 nmol l−1 day−1 (USF) at SS16 (Figure 2a). N2 fixation rates in the cmax were at times higher than in the surface waters, reaching up to 24±0.6 nmol l−1 day−1 (WC) and 18±5 nmol l−1 day−1 (USF) (Figure 2a).
A marked increase in N2 fixation was observed in both regions during the Austral winter (Figure 2b). Within the ATS surface waters, WC N2 fixation increased almost threefold in winter, with a mean of 60±15 nmol l−1 day−1 across the six sites. Furthermore, the majority of this activity was attributable to the USF (one-way analysis of variance, Tukey's honestly significant difference, P>0.05), such that mean USF rates increased almost 10-fold in winter to 57±23 nmol l−1 day−1. Maximum rates recorded in the ATS surface waters were similar to that of spring (Figure 2). Only one winter ATS site had a discernible cmax, and here, in contrast to the spring, N2 fixation was also relatively high (Figure 2b).
Mean winter N2 fixation rates in the Coral Sea were four and seven times higher than during the spring in the WC (56±32 nmol l−1 day−1) and USF (47±28 nmol l−1 day−1), respectively. Although mean N2 fixation rates were again lower in the Coral Sea than in the ATS, the maximum rates recorded in the Coral Sea surface waters were the highest observed, reaching 91±7 nmol l−1 day−1 (WC) and 71±18 nmol l−1 day−1 (USF) at station WS16, towards the southern end of the transect (Figure 2b). During winter, four Coral Sea sites had discernible cmax, where N2 fixation rates were also relatively high (Figure 2b).
Diazotroph population dynamics in the ATS and Coral Sea waters
A total of 174 nifH OTUs were resolved from our samples. Phylogenetic analysis revealed the presence of photoautotrophic, photoheterotrophic and heterotrophic diazotrophs during both transects, and these clustered with environmental nifH sequences originating from the Pacific and Atlantic Oceans and the South China Sea (Supplementary Figure S1).
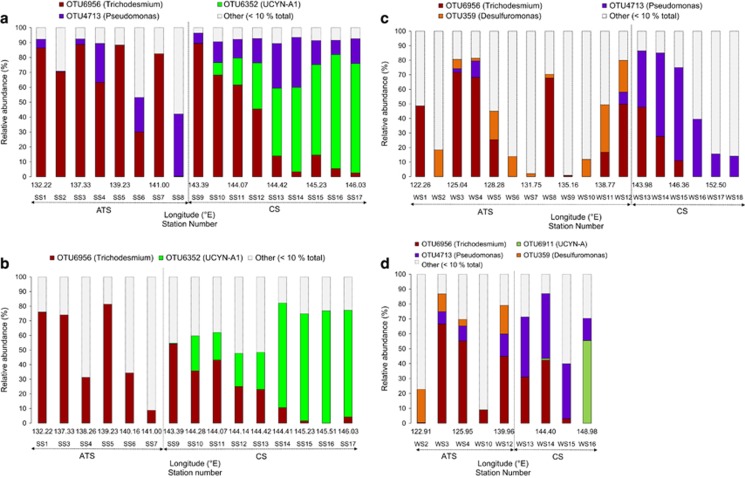
Three nifH OTUs were dominant across the data set, comprising 67% of total nifH DNA sequences retrieved. These included OTU6956, which was 100% identical to Trichodesmium erythraeum (IMS 101; hereafter Trichodesmium) in nifH amino acid (aa) composition, OTU6352, which was 100% identical in aa composition to UCYN-A and clustered with the UCYN-A1 ecotype (Supplementary Figure S1), and OTU4713, which shared 91% similarity in aa composition to the γ-proteobacteria Pseudomonas stutzeri, and clustered within the γ–24774A11 clade (Moisander et al., 2008, 2014). Despite the ubiquity of these dominant OTUs, significant partitioning of diazotroph population structure was observed between the ATS and Coral Sea (Figure 3; ANOSIM, Global R: 0.471, P<0.001) and between the Austral spring and winter sampling (ANOSIM, Global R: 0.399, P<0.001). No significant differences were observed between surface and cmax diazotroph communities.
Figure 3.
Relative abundance of nifH OTUs recovered from community DNA (percentage of sequences) at the surface and chlorophyll maxima during the spring (SS; a and b respectively) and winter (WS; c and d respectively) transects. In parentheses are the closest cultured representatives that share ⩾90% amino-acid identity with the detected sequences. ATS, Arafura and Timor Seas, CS, Coral Sea.
During the Austral spring, Trichodesmium comprised 60% of nifH sequences in the ATS and at some sites reached >80% of sequences in both the surface and cmax (SS3 and SS5, respectively; Figures 3a and b). γ-24774A11 was also detected throughout the ATS during spring, where it represented 14% of total sequences, and reached a maximum abundance of >40% of the diazotroph population at SS8 and SS6 (surface and cmax, respectively; Figure 3a; Supplementary Figure S2).
In contrast, the Coral Sea was dominated by the unicellular cyanobacterium UCYN-A during the spring. UCYN-A1 was conspicuously absent from the ATS samples but comprised 42% of the total nifH sequences in the Coral Sea, with a maximum abundance of 77% at SS16 (Figure 3a). Similar to the ATS, γ-24774A11 was also a significant feature of Coral Sea springtime diazotroph populations, where it constituted 21% of sequences, and reached up to 34% and 46% of diazotrophs at the surface and cmax at SS13 and SS14, respectively (Figure 3a; Supplementary Figure S2).
During the Austral winter, Trichodesmium still generally dominated throughout the ATS, representing 35% of total nifH sequences, but there was an increase in the relative number of heterotrophic diazotrophs compared with the spring (Figure 3). The putative heterotrophic δ-proteobacterial OTUs 359, 7075 and 811, which shared between 96% and 99% aa identity to Desulfuromonas acetoxidans, collectively comprised 19% of total sequences, and up to 55% of the diazotroph population in the ATS surface waters (Figure 3c; Supplementary Figure S2). Notably, these OTUs only accounted for 7% of diazotrophs in the ATS during spring (Supplementary Figure S2).
Sequences associated with heterotrophic diazotrophs also increased during winter in the Coral Sea, relative to spring (Figures 3c and d). However, here they were primarily associated with γ-24774A11, which represented 34% of wintertime Coral Sea nifH sequences, reaching a maximum of 64% of diazotrophs at the surface, and 43% at the cmax (Figures 3c and d). In contrast to the spring sampling where they dominated, UCYN-A sequences only comprised 3% of Coral Sea diazotrophs during winter and remained absent from the ATS (Supplementary Figure S2).
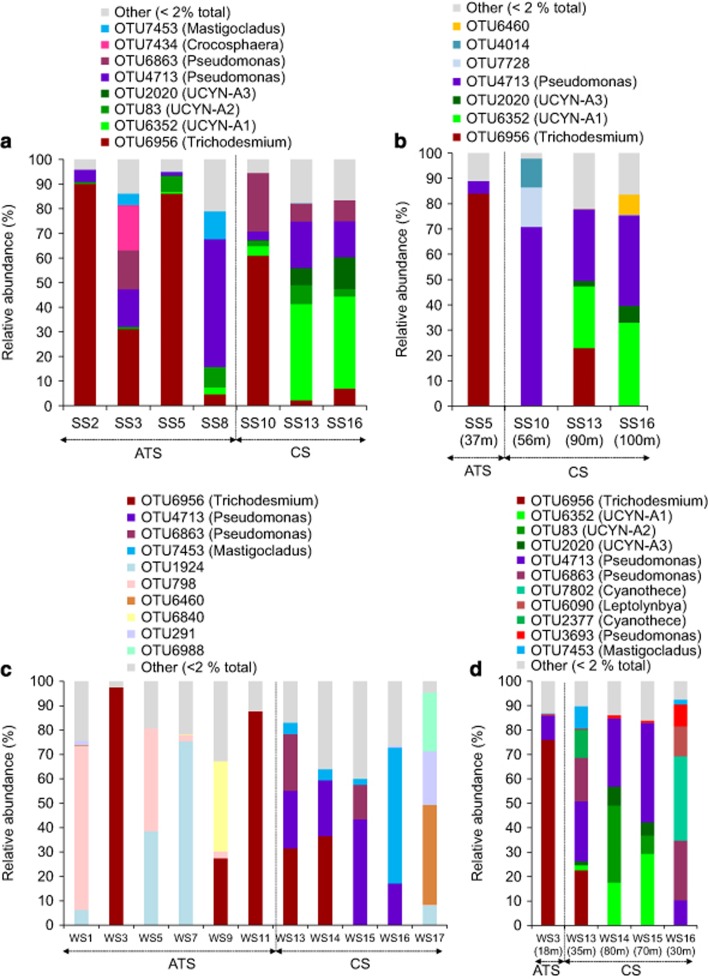
Patterns in nifH expression
Consistent with DNA profiles, Trichodesmium, UCYN-A1 and γ-24774A11 dominated nifH transcripts across the data set (Figure 4), and significant differences were observed between transcription profiles from the ATS and Coral Sea (ANOSIM, Global R: 0.595, P<0.01). In ATS surface waters, Trichodesmium (OTU6956) and γ-24774A11 (OTU4713) dominated nifH transcripts during spring. Specifically, Trichodesmium comprised up to 86% of transcripts at sites where high rates of N2 fixation attributable to the >10μm size class were recorded (SS5, Figure 4a). γ-24774A11 represented up to 52% of transcripts during the spring transect (SS8; Figure 4a), even though unicellular N2 fixation was recorded at relatively low levels (<1 nmol l−1 day−1; Figure 2a).
Figure 4.
Relative abundance (percentage of sequences) of nifH transcripts detected in the surface and chlorophyll maxima waters for spring (a and b, respectively) and winter (c and d, respectively) voyages.
Within Coral Sea surface waters in spring, nifH transcripts mainly consisted of Trichodesmium, UCYN-A and γ-24774A11 (Figure 4a). Although Trichodesmium transcripts decreased throughout the Coral Sea, UCYN-A1 transcripts increased from 4% to 39%, towards southern latitudes where the peak in Coral Sea springtime N2 fixation was observed (Figures 4a and 2a respectively). Transcripts associated with γ-24774A11 were most abundant in cmax samples, where they represented up to 71% of expressed nifH genes (Figure 4b), although cmax N2 fixation rates were relatively low (<1 nmol l−1 day−1; Figure 2a).
Similar to the spring sampling, Trichodesmium transcripts were often highly abundant in the ATS surface waters during winter, accounting for 98% of transcripts at station WS3 (Figure 4c). Two additional OTUs, OTU1924 and OTU798, also constituted a significant fraction (up to 76% and 67%) of transcripts in the ATS; however, these OTUs shared only 20% and 25% aa identity, respectively, with available nifH sequences, with closest matches to members of the Firmicutes. Interestingly, transcripts associated with γ-24774A11 and UCYN-A were not detected in the ATS during the winter (Figure 4c) despite high unicellular N2 fixation rates (Figure 2b).
In contrast to the spring, where cyanobacterial transcripts were dominant, nifH expression in Coral Sea surface waters was more variable during the winter. For example, both γ-24774A11 and OTU7453, which shared 98% aa identity with the cyanobacterium Mastigocladus laminosus, contributed up to ~56% of nifH transcripts at stations where unicellular N2 fixation rates were high (WS15 and WS16, respectively; Figures 4c and 2b respectively). Notably, no transcripts associated with UCYN-A ecotypes were detected in the Coral Sea surface waters during winter, but all three ecotypes were present in transcripts from the cmax, along with Trichodesmium, γ-24774A11 and some additional cyanobacterial diazotrophs (Figures 4c and d).
Potential drivers of diazotroph populations and activity
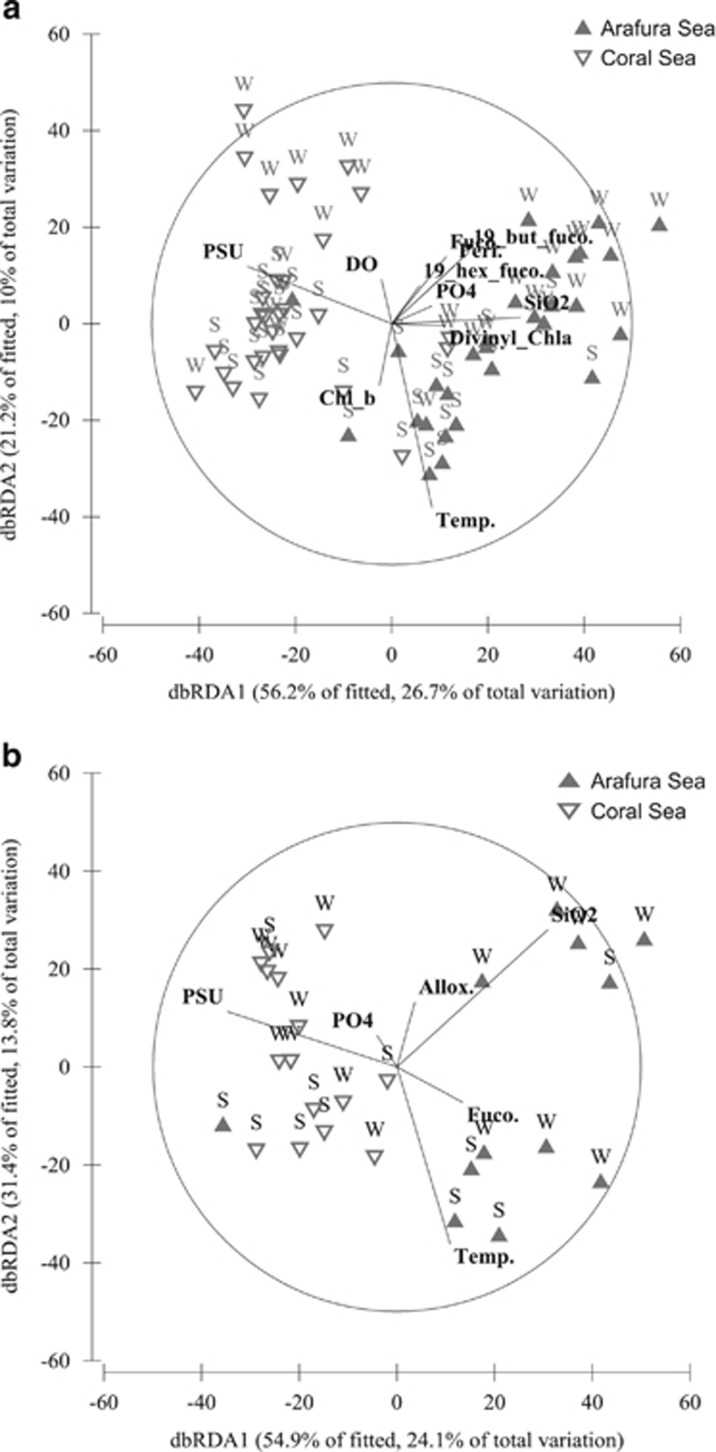
Analysis of the diazotroph populations using both nifH DNA (Figure 5a) and cDNA (Figure 5b) data indicated clear separation between ATS and Coral Sea regions. DistLM identified SST and salinity as significant explanatory variables (P<0.05), reflecting the increase in salinity and decrease in temperature associated with the Coral Sea region (Figures 1, 5a and b; Supplementary Table S2). Dissolved silicate and phosphate were also significant (P<0.05) explanatory variables in both the DNA and cDNA DistLM analysis, as was the photosynthetic carotenoid fucoxanthin (P<0.05), all of which exhibited higher relative concentrations in the ATS compared with the Coral Sea. Interestingly, dissolved oxygen and divinyl chlorophyll a were significant (P<0.05) explanatory variables within the nifH DNA model but not within the nifH cDNA model (Figures 5a and b). However, for both models, >50% of the total variation between diazotroph populations remained unexplained.
Figure 5.
Distance-based redundancy analysis constrained by the significant (P<0.05) explanatory environmental variables for the observed variation in nifH composition within (a) DNA and (b) cDNA profiles in spring (S) and winter (W).
Network analysis revealed that N2 fixation by the USF exhibited a significant, but only moderately strong, negative correlation to ammonium concentration (p=−0.43; MIC strength=0.66; P<0.01) and was also negatively correlated to the relative abundance of UCYN-A2 transcripts (p=−0.33; MIC strength=0.71; P<0.01), possibly reflecting the relatively low abundance of this ecotype (Supplementary Figure S2). N2 fixation was not significantly correlated to any other environmental parameters or nifH OTUs.
A number of nifH OTUs were negatively correlated with phosphate and silicate (Figures 6a and b), including two of the most abundant diazotrophs, OTU4713 from the γ-24774A11 clade and OTU6352 from the UCYN-A1 ecotype. These γ-24774A11 and UCYN-A1 OTUs were also positively correlated to salinity, to each other and to a range of other nifH OTUS, including the UCYN-A2 (OTU83) and UCYN-A3 (OTU2020) ecotypes, and other γ-24774A11 OTUs (OTU2710 and OTU454; Figures 6c and d; Supplementary Figure S1). No significant correlations were observed between nifH transcripts and the concentration of dissolved inorganic nitrogen species.
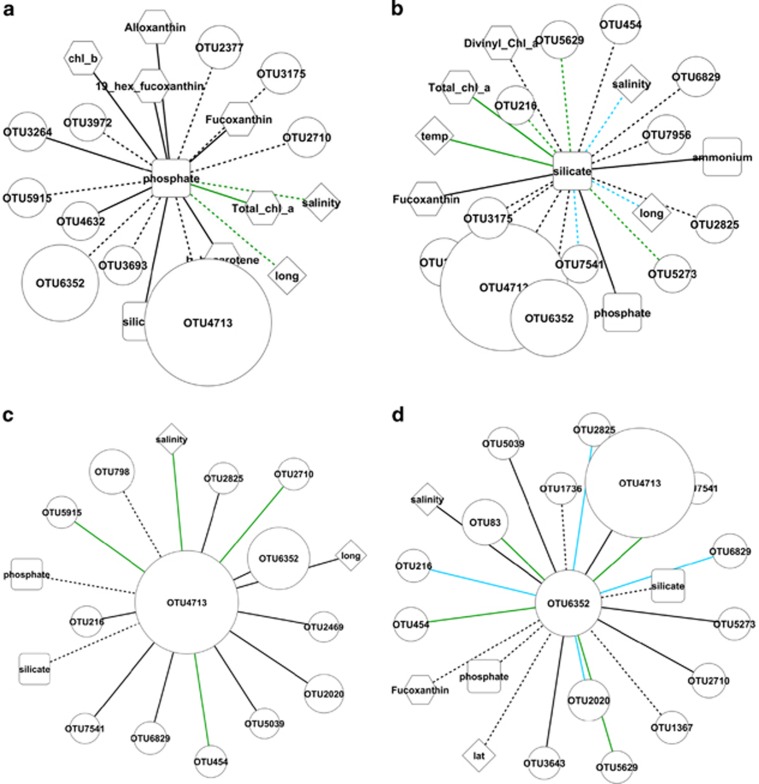
Figure 6.
Network analysis demonstrating significant (P<0.01) associations only between (a) phosphate concentrations and (b) silicate concentrations and other environmental variables, as well as the relative abundance of expressed nifH OTUs. In addition, significant (P<0.01) associations between (c) OTU4713 of the γ-24774A11 clade and (d) OTU6352 Candidatus Atelocyanobacterium thalassa (UCYN-A1), environmental data and the relative abundance of other expressed nifH OTUs are also shown. Circular nodes=nifH cDNA OTUs, the relative abundance is demonstrated by the relative size of the node; diamond nodes=physical variables; square nodes=dissolved inorganic nutrients; hexagon nodes=photosynthetic pigments. Positive linear regressions between nodes are denoted by solid lines; negative linear regressions are dashed. Line colour represents the strength of the test statistic, MIC: high>0.8<1=blue; medium>0.6<0.8=green; low>0.47<0.6=black.
Discussion
Identifying the factors that influence the composition and activity of diazotrophs is key to understanding the relative importance of N2 fixation on local and global scales (Zehr and Kudela, 2011; Robidart et al., 2014). N2 fixed by diverse diazotrophic taxa may have different fates within the marine environment (Glibert and Bronk 1994; Mulholland, 2007; Foster et al., 2011; Karl et al., 2012; Benavides et al., 2013), and therefore characterisation of the composition of active N2-fixing assemblages, combined with size-fractionated N2 fixation rates, is necessary to determine the differential contribution of newly fixed N to pelagic ecosystems. Here we report changes in the biogeographical distribution and activity of diazotrophs across a broad tropical region, which has been identified as a potential global ‘hotspot' for marine N2 fixation (Montoya et al., 2004; Monteiro et al., 2010; Luo et al., 2014).
Previously, Montoya et al. (2004) reported rates of USF N2 fixation up to 480 nmol l−1 day−1 in the ATS waters (25 m below surface) during the Austral spring. Herein we sampled during both spring and winter, at similar latitudes (within 0–1 °S) and longitudes (within 0.3–2 °E), yet observed maximum USF rates that were substantially less than those reported by Montoya et al. (2004). Recently, Raes et al. (2014) reported mean WC rates of ~36 nmol l−1 day−1 within the westerly region of the ATS during the Austral spring. Although not directly comparable owing to methodological differences (Wilson et al., 2012), these values are in line with those we measured in the same region during the Austral winter, suggesting that relatively high rates are maintained here across seasons. Based on methodological comparisons, there appears to be no clear trend in the level of N2 fixation rate underestimation using the method by Montoya et al. (1996), owing to multiple factors influencing the dissolution of the 15N2 bubble (Mohr et al., 2010; Großkopf et al., 2012). However, comparisons made in the North Pacific and Atlantic Oceans indicate that this method could lead to N2 fixation underestimates of 50% (Wilson et al., 2012; Benavides et al., 2013) or greater, depending on the composition of the diazotroph community (Großkopf et al., 2012).
Compared with the near surface waters of similar latitudes, including the tropical western South Pacific (<1 nmol l−1 d−1; Moisander et al., 2010), western equatorial Pacific (<40 nmol l−1 d−1; Bonnet et al., 2009), eastern tropical South Pacific (ca. ⩽1 nmol l−1 d−1; Dekaezemacker et al., 2013) and the tropical South Pacific Gyre (⩽3 nmol l−1 day−1; Raimbault and Garcia 2008), the maximum conservative rates of N2 fixation reported here during the Austral winter (91 nmol l−1 day−1) are relatively high. Indeed, our observations, along with those previously reported (Montoya et al., 2004; Raes et al., 2014), support the proposition that the tropical waters of northern Australia are a ‘hotspot' of diazotroph activity within the Southern Hemisphere. However, our data also demonstrate significant temporal and spatial variability in N2 fixation in this region, which we propose is driven by the highly dynamic and heterogeneous nature of the resident diazotroph populations.
Across our data set, the majority of N2 fixation activity was observed within the USF, and USF N2 fixation rates were greater in winter than in spring, with mean rates 10 times higher in the ATS and seven times greater in the Coral Sea. Although it must be noted that Trichodesmium is known to release some of the N2 it fixes as dissolved organic nitrogen (Glibert and Bronk, 1994), and therefore a proportion of 15N–N2 fixed could have been transferred to the USF during the incubation, these increased USF rates occurred in parallel to an increase in the relative abundance of δ- and γ-proteobacterial nifH sequences and γ-24774A11 nifH transcripts. This highlights the potential importance of heterotrophic diazotrophs to biogeochemical cycling during winter across these two quite different regions.
This study provides the first detailed characterisation of active diazotroph populations throughout northern Australia. It must be noted that amplicon sequencing approaches can only reconcile relative abundances and therefore do not allow for the absolute quantification of colonial versus single-celled diazotrophs and that it is difficult to directly equate diazotroph communities to N2 fixation activity. However, we identified a range of photoautotrophic, photoheterotrophic and heterotrophic bacteria that share high similarities in nifH sequences to those recovered from similar oceanic environments (for example, Langlois et al., 2005; Moisander et al., 2010; Bombar et al., 2013; Moisander et al., 2014; Thompson et al., 2014).
Despite its shelf sea nature, ATS diazotroph communities typically resembled those of similar latitudes, such as the tropical Atlantic Ocean, where Trichodesmium dominates (Langlois et al., 2005; Foster et al., 2009; Goebel et al., 2010). However, heterotrophic groups were also a feature of ATS communities, and we observed a shift from γ- to δ-proteobacterial phylotypes between spring and winter. Conversely, Coral Sea communities contained a greater diversity of cyanobacterial phylotypes, including ecotypes of UCYN-A, alongside a lower frequency of Trichodesmium sequences, as well as heterotrophic diazotrophs. The composition of Coral Sea diazotroph populations appears similar to those found within the wider tropical and subtropical South Pacific (Moisander et al., 2010, 2014; Halm et al., 2012). However, a seasonal shift in the composition of Coral Sea populations was also observed, such that the relative abundance of UCYN-A ecotypes decreased while γ-24774A11 increased between spring and winter, respectively. Previously, Moisander et al. (2014) reported the distribution of γ-24774A11 to be ubiquitous and relatively homogeneous in South Pacific surface waters during the Austral autumn. By examining spatial and temporal patterns in diazotroph community dynamics, we show that the distribution and relative abundance of γ-24774A11 is variable and that the significance of this group may increase during the Austral winter.
Surprisingly, no significant differences between surface and cmax diazotroph communities were observed in the present study, with Trichodesmium, UCYN-A1 and γ–24774A11 sequences all detected down to 120 m below surface. Previously, Trichodesmium and unicellular diazotrophs have been shown to have differential depth distributions in the western South Pacific Ocean, with Trichodesmium and γ−24774A11 most abundant in upper euphotic zone waters and UCYN-A more abundant deeper within the water column (Moisander et al., 2010, 2014). We found that all three of these groups were relatively abundant in both surface and cmax waters depending on the sampling region and season. We also detected nifH expression by Trichodesmium 90 m below surface, and UCYN-A1 and γ−24774A11 100 m below surface, indicating that all three groups were active at depth too. This suggests that the physicochemical variables identified to be potential drivers of diazotroph distribution in the present study were most relevant over horizontal rather than vertical scales, which could be due to similarities between surface and cmax physicochemical signatures (Supplementary Table S1).
The differences in diazotrophic taxa and N2 fixation activity observed between the ATS and Coral Sea during both seasons are likely attributable to the observed physicochemical conditions, including higher SST, lower salinities and higher nutrient concentrations, in the ATS compared with the Coral Sea, features which are characteristic of the study regions (Condie and Dunn, 2006; Alongi et al., 2011; Ceccarelli, 2011). In particular, SST was identified as a strong indicator of the dominant diazotrophic taxa in our study regions. We observed that Trichodesmium dominated communities in the warmer waters of the ATS, displaying maximum relative abundances when SST was >27 °C, while UCYN-A dominated communities in the cooler Coral Sea waters, although maximum relative abundances of UCYN-A1 transcripts occurred when SST was ~26 °C during spring. Previously, UCYN-A have been shown to occur at specific temperature optima between 24 °C and 26 °C in the western South Pacific (Moisander et al., 2010, 2014). In contrast, during the cooler winter sampling, when SST in the Coral Sea was closer to 24 °C, we observed an increase in relative abundances of γ-24774A11, while the relative abundance of UCYN-A decreased substantially. Maximum relative abundances of γ-24774A11 occurred where SST was ~25 °C, and maximum γ-24774A11 nifH expression occurred at 25.8 °C, suggesting a temperature optima around 25–26 °C for this group. Recently, the occurrence of the γ-24774A11 clade has been found to be positively, non-linearly correlated with temperature, with maximum abundances associated with surface waters >26 °C (Moisander et al., 2014). Overall, our data are consistent with the observed distributions of these organisms across a range of oceanic provinces (Capone et al., 1997; Mazard et al., 2004; Langlois et al., 2008; Moisander et al., 2010) and supports previous findings that temperature is an important determinant of diazotroph spatiotemporal dynamics.
Dissolved silicate and phosphate concentrations, and the concentration of the pigment fucoxanthin, were also identified as significant discriminating factors explaining some of the heterogeneity between ATS and Coral Sea diazotroph populations. Whether these correlations indicate a direct or indirect effect on diazotroph abundance and consequently N2 fixation is unclear. Despite high silicate concentrations and pigment indications of diatom-dominated phytoplankton communities in the ATS, only one OTU associated with the heterocystous cyanobacterial symbiont of diatoms, Richelia, was observed in our sequence data, and this represented a total of only six nifH sequences (data not shown). The significant negative correlation observed between silicate and UCYN-A1 and γ-24774A11 transcripts, and UCYN-A1 and fucoxanthin, could be indicative of shifting phytoplankton communities between the ATS and Coral Sea, given that UCYN-A is known to live in association with a prymnesiophyte host (Thompson et al., 2012; Hagino et al., 2013; Krupke et al., 2013). Currently, the lifestyle (for example, free-living, particle attached or symbiont) of γ-24774A11 remains unknown (Langlois et al., 2015); however, it has been speculated that it may depend upon phytoplankton-produced dissolved organic carbon (Moisander et al., 2012, 2014). Although strongly co-linear variables were removed from our analyses, silicate was inversely correlated to salinity and positively correlated to temperature, therefore it could also be indicative of a water mass tracer rather than a biological causation.
Conversely, phosphate availability is known to directly influence N2 fixation and nifH expression in natural populations of diazotrophs (Sañudo-Wilhelmy et al., 2001; Rees et al., 2006; Turk-Kubo et al., 2012), as well as the oceanic distribution of diazotrophs in general (Sohm et al., 2011). In the present study, phosphate concentrations were relatively high (for example, 0.27 μmol l−1 and 0.26 μmol l−1) in the ATS where Trichodesmium- and δ-proteobacterial-dominated communities were observed. In contrast, in the Coral Sea, where UCYN-A1 and γ-24774A11 dominated communities, phosphate concentrations were comparatively low (for example, 0.09 μmol l−1 and 0.07 μmol l−1, respectively). This implies a potential role of phosphate limitation in the shift in diazotroph community composition. UCYN-A1 and γ-24774A11 both appear to be broadly distributed throughout low-nutrient marine waters (Thompson et al., 2014; Langlois et al., 2015), and it has been hypothesised that these taxa thrive in oligotrophic conditions (Church et al., 2008; Krupke et al., 2014). In line with our findings in the ATS and Coral Sea, Moisander et al. (2014) recently demonstrated that γ-24774A11 was significantly negatively correlated to soluble reactive phosphorous in the western South Pacific. Taken together, our findings suggest that UCYN-A and γ-24774A11 increase in relative abundance and activity when oligotrophic conditions prevail.
Although no correlations were observed between the different diazotrophic groups and oxidised forms of N, USF N2 fixation rates exhibited a moderate negative correlation to ammonium concentration, although this is unlikely to be due to an inhibitory effect, because concentrations observed here were below those expected to inhibit N2 fixation (Knapp, 2012). It has been suggested that tropical Australian waters are constantly nitrogen limited (Condie and Dunn, 2006), and across all sites and depths measured, N:P ratios indicated an excess of phosphate relative to nitrate and ammonium (N:P ⩽6). This may confer a competitive advantage to diazotrophic taxa (Moutin et al., 2008; Knapp, 2012) and suggests that N2 fixation could have an important role in helping to alleviate nitrogen limitation within this region.
However, it is notable that much of the variability between ATS and Coral Sea nifH profiles remained unexplained in our analysis, and despite combined analyses and stringent standardisation across samples, some rare OTUs in the DNA appeared highly active in the cDNA, potentially indicating a disconnect between relative organismal abundance and N2 fixation activity at some locations. Therefore, other factors may have had a significant influence on diazotroph distribution and activity, for instance, dissolved iron (dFe) availability is known to limit marine N2 fixation (see Sohm et al., 2011) and dFe additions can stimulate diazotrophic activity (Moisander et al., 2012; Turk-Kubo et al., 2012). In the western South Pacific ocean, Moisander et al, (2012) demonstrated that the abundances of unicellular diazotrophs, including UCYN-A and γ-24774A11, not only increased substantially in response to dFe amendments but also exhibited signs of iron and phosphorous co-limitation. Although we did not measure dFe throughout the ATS and Coral Sea during the present study, previous evidence suggests that concentrations of particulate Fe are relatively high in the Timor region of the ATS (Waite et al., 1995), but dFe concentrations may be relatively low (Sohm et al., 2008), and Trichodesmium colonies have been shown to experience Fe limitation in this region (Kustka et al., 2003). Although this indicates that dFe availability could have influenced diazotroph distributions during our study, additional work exploring dFe dynamics, and the potential influence on diazotroph diversity and activity in the ATS and Coral Sea, is required to further understand the mechanisms structuring diazotroph populations and N2 fixation throughout these regions.
Despite the relatively low concentrations of dissolved inorganic nitrogen, primary production peaks in both the ATS and Coral Sea during the Austral winter (Lyne and Hayes, 2005; Brewer et al., 2007). It was during this season we observed a substantial increase in both unicellular N2 fixation and the relative abundance of heterotrophic nifH sequences. Primary production estimates from the winter sampling are detailed elsewhere (Robinson et al. (in preparation)); however, using this data we calculated that wintertime USF in the ATS and Coral Sea could supply up to 46% and 42%, respectively, of the N required to sustain biomass assimilation from measured primary production rates (assuming Redfield ratios for particulate matter). Thus we propose that primary production here may be enhanced by the influx of newly fixed N mediated by heterotrophic diazotrophs. Although it has been argued that the abundances of heterotrophic N2-fixing bacteria in oceanic environments are not considered sufficient to account for measured rates of N2 fixation (Turk-Kubo et al., 2013), a recent large-scale analysis of the abundance and distribution of marine γ-proteobacterial nifH indicates that this phylotype could play a significant role in oceanic N2 fixation (Langlois et al., 2015). It must be noted that the fate of N fixed by marine heterotrophic diazotrophs has not yet been determined, and as such direct evidence of production supported by heterotrophic N2 fixation is lacking. However, our limited data suggest that heterotrophic diazotrophs are an important component of N2-fixing populations throughout tropical northern Australia and that they may contribute to relatively high USF rates of N2 fixation. Given the apparent importance of heterotrophic diazotrophs in our study region, future research determining the ultimate fate of N fixed by these heterotrophs will be valuable for estimating the extent to which these populations ultimately influence pools of bioavailable N and support primary production.
Our study has shown that the composition and activity of diazotrophs in Australia's tropical waters are highly variable across shelf and open ocean environments. Overall, our rate measurements confirm that diazotroph activity is an important process here, but our molecular and statistical analyses suggest that the distinct physicochemical characteristics of these waters drive heterogeneity in populations of photoautotrophic, photoheterotrophic and heterotrophic N2-fixing bacteria. This heterogeneity in turn leads to substantial changes in rates of N2 fixation and the subsequent addition of newly fixed N to the ocean. As such, spatial (even within relatively localised boundaries) and seasonal shifts in diazotroph diversity and activity must be considered in future regional and global marine N-cycle budgets and modelling efforts. This can only be achieved by further attempts to more precisely map marine diazotrophic processes with increased spatiotemporal resolution.
Acknowledgments
We thank the scientific crew, captain and scientists on-board the R/V Southern Surveyor during ss2012_t07 and ss2013_t03 for assistance with sampling and nutrient sample analyses. This research was supported by the Australian Research Council Discovery Grant Scheme (DP120102764 to JS and MB, DP1092892 to MD, FT130100218 to JS and DP0988002 to MB).
Author contributions
LFM, CM, MVB and JRS conceived the study; LFM, CMR, MVB and JRS acquired data; TCJ, KGB, JBI, MO and MAD assisted with data acquisition; LFM analysed the data; LFM, CM, TCJ, MVB and JRS interpreted the data; LFM, MVB and JRS wrote the manuscript; and all authors revised the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Alongi D, Edyvane K, do Ceu Guterres M, Pranowo W, Wirasantosa S, Wasson R (eds). (2011) Biophysical Profile of the Arafura and Timor Seas. Arafura and Timor Seas Ecosystem Action (ATSEA) Program, Jakarta.
- Benavides M, Agawin N, Arístegui J, Peene J, Stal L. (2013). Dissolved organic nitrogen and carbon release by a marine unicellular diazotrophic cyanobacterium. Aquat Microb Ecol 69: 69–80. [Google Scholar]
- Benavides M, Bronk DA, Agawin NSR, Pérez-Hernández MD, Hernández-Guerra A, Arístegui J. (2013). Longitudinal variability of size-fractionated N2 fixation and DON release rates along 24.5°N in the subtropical North Atlantic. J Geophys Res Ocean 118: 3406–3415. [Google Scholar]
- Benjamini Y, Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 57: 289–300. [Google Scholar]
- Bombar D, Turk-Kubo KA, Robidart J, Carter BJ, Zehr JP. (2013). Non-cyanobacterial nifH phylotypes in the North Pacific Subtropical Gyre detected by flow- cytometry cell sorting. Environ Microbiol Rep 5: 705–715. [DOI] [PubMed] [Google Scholar]
- Bonnet S, Biegala IC, Dutrieux P, Slemons LO, Capone DG. (2009). Nitrogen fixation in the western equatorial Pacific: Rates, diazotrophic cyanobacterial size class distribution, and biogeochemical significance. Global Biogeochem Cycles 23: 1–13. [Google Scholar]
- Brewer D, Flynn A, Skewes T, Corfield J, Pearson B, Alawo J, Young J. (2007). Ecosystems of the East Marine Planning Region. CSIRO, Cleveland, OH, USA.
- Burford M, Rothlisberg P, Wang Y. (1995). Spatial and temporal distribution of tropical phytoplankton species and biomass in the Gulf of Carpentaria, Australia. Mar Ecol Prog Ser 118: 255–266. [Google Scholar]
- Burford MA, Rothlisberg PC, Revill AT. (2009). Sources of nutrients driving production in the Gulf of Carpentaria, Australia: a shallow tropical shelf system. Mar Freshw Res 60: 1044. [Google Scholar]
- Capone D, Zehr J, Paerl H, Bergman B, Carpenter E. (1997). Trichodesmium, a globally significant marine cyanobacterium. Science 276: 1221–1229. [Google Scholar]
- Capone DG, Burns J, Montoya JP, Subramaniam A, Mahaffey C, Gunderson T et al. (2005). Nitrogen fixation by Trichodesmium spp.: an important source of new nitrogen to the tropical and subtropical North Atlantic Ocean. Global Biogeochem Cycles 19: GB2024. [Google Scholar]
- Caporaso J, Kuczynski J, Stombaugh J, Bittinger K, Bushman F, Costello EK et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7: 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli D. (2011). Australia's Coral Sea: A Biophysical Profile. Report for the protect our Coral Sea coalition, Pew Environment Group, Australia.
- Church M, Bjorkman K, Karl D, Saito MA, Zehr J. (2008). Regional distributions of nitrogen-fixing bacteria in the Pacific Ocean. Limnol Oceanogr 53: 63–77. [Google Scholar]
- Church MJ, Mahaffey C, Letelier RM, Lukas R, Zehr JP, Karl DM. (2009). Physical forcing of nitrogen fixation and diazotroph community structure in the North Pacific subtropical gyre. Global Biogeochem Cycles 23: GB2020. [Google Scholar]
- Clarke K. (1993). Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18: 117–143. [Google Scholar]
- Clarke K, Warwick R. (2001) Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd edn. PRIMER-E Ltd: Plymouth, UK. [Google Scholar]
- Condie SA, Dunn JR. (2006). Seasonal characteristics of the surface mixed layer in the Australasian region: implications for primary production regimes and biogeography. Mar Freshw Res 57: 569. [Google Scholar]
- Dekaezemacker J, Bonnet S, Grosso O, Moutin T, Bressac M, Capone DG. (2013). Evidence of active dinitrogen fixation in surface waters of the eastern tropical South Pacific during El Niño and La Niña events and evaluation of its potential nutrient controls. Global Biogeochem Cycles 27: 768–779. [Google Scholar]
- Dowd SE, Callaway TR, Wolcott RD, Sun Y, McKeehan T, Hagevoort RG et al. (2008). Evaluation of the bacterial diversity in the feces of cattle using 16S rDNA bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP). BMC Microbiol 8: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26: 2460–2461. [DOI] [PubMed] [Google Scholar]
- Farnelid H, Andersson AF, Bertilsson S, Al-Soud WA, Hansen LH, Sørensen S et al. (2011). Nitrogenase gene amplicons from global marine surface waters are dominated by genes of non-cyanobacteria. PLoS One 6: e19223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnelid H, Bentzon-Tilia M, Andersson AF, Bertilsson S, Jost G, Labrenz M et al. (2013). Active nitrogen-fixing heterotrophic bacteria at and below the chemocline of the central Baltic Sea. ISME J 7: 1413–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish JA, Chai B, Wang Q, Sun Y, Brown CT, Tiedje JM et al. (2013). FunGene: the functional gene pipeline and repository. Front Microbiol 4: 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster RA, Subramaniam A, Zehr JP. (2009). Distribution and activity of diazotrophs in the Eastern Equatorial Atlantic. Environ Microbiol 11: 741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster RA, Kuypers MMM, Vagner T, Paerl RW, Musat N, Zehr JP. (2011). Nitrogen fixation and transfer in open ocean diatom-cyanobacterial symbioses. ISME J 5: 1484–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias-Lopez J, Shi Y, Tyson GW, Coleman ML, Schuster SC, Chisholm SW et al. (2008). Microbial community gene expression in ocean surface waters. Proc Natl Acad Sci USA 105: 3805–3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glibert P, Bronk D. (1994). Release of dissolved organic nitrogen by marine diazotrophic cyanobacteria, Trichodesmium spp. Appl Environ Microbiol 60: 3996–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel NL, Turk KA, Achilles KM, Paerl R, Hewson I, Morrison AE et al. (2010). Abundance and distribution of major groups of diazotrophic cyanobacteria and their potential contribution to N2 fixation in the tropical Atlantic Ocean. Environ Microbio. 12: 3272–3289. [DOI] [PubMed] [Google Scholar]
- Großkopf T, Mohr W, Baustian T, Schunck H, Gill D, Kuypers MMM et al. (2012). Doubling of marine dinitrogen-fixation rates based on direct measurements. Nature 488: 361–364. [DOI] [PubMed] [Google Scholar]
- Hagino K, Onuma R, Kawachi M, Horiguchi T. (2013). Discovery of an endosymbiotic nitrogen-fixing cyanobacterium UCYN-A in Braarudosphaera bigelowii (Prymnesiophyceae). PLoS One 8: e81749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallegraeff G, Jeffrey S. (1984). Tropical phytoplankton species and pigments of continental shelf waters of north and north-west Australia. Mar Ecol Prog Ser 20: 59–74. [Google Scholar]
- Halm H, Lam P, Ferdelman TG, Lavik G, Dittmar T, LaRoche J et al. (2012). Heterotrophic organisms dominate nitrogen fixation in the South Pacific Gyre. ISME J 6: 1238–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller P, Tripp HJ, Turk-Kubo K, Zehr JP. (2014). ARBitrator: a software pipeline for on-demand retrieval of auto-curated nifH sequences from GenBank. Bioinformatics 30: 1–8. [DOI] [PubMed] [Google Scholar]
- Karl DM, Church MJ, Dore JE, Letelier RM, Mahaffey C. (2012). Predictable and efficient carbon sequestration in the North Pacific Ocean supported by symbiotic nitrogen fixation. Proc Natl Acad Sci USA 109: 1842–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp AN. (2012). The sensitivity of marine N2 fixation to dissolved inorganic nitrogen. Front Microbiol 3: 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupke A, Lavik G, Halm H, Fuchs BM, Amann RI, Kuypers MMM. (2014). Distribution of a consortium between unicellular algae and the N2 fixing cyanobacterium UCYN-A in the North Atlantic Ocean. Environ Microbiol 16: 3153–3167. [DOI] [PubMed] [Google Scholar]
- Krupke A, Musat N, Laroche J, Mohr W, Fuchs BM, Amann RI et al. (2013). In situ identification and N2 and C fixation rates of uncultivated cyanobacteria populations. Syst Appl Microbiol 36: 259–271. [DOI] [PubMed] [Google Scholar]
- Kustka A, Sanudo-Wilhelmy S, Carpenter E, Capone D, Burns J, Sunda W. (2003). Iron requirements for dinitrogen-and ammonium-supported growth in cultures of Trichodesmium (IMS 101): Comparison with nitrogen fixation rates and iron: carbon ratios of field populations. Limnol Oceanogr 48: 1869–1884. [Google Scholar]
- Langlois R, Großkopf T, Mills M, Takeda S, LaRoche J. (2015). Widespread distribution and expression of gamma A (UMB), an uncultured, diazotrophic, γ- proteobacterial nifH phylotype. PLoS One 10: e0128912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois R, LaRoche J, Raab P. (2005). Diazotrophic diversity and distribution in the tropical and subtropical Atlantic Ocean. Appl Environ Microbiol 71: 7910–7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois RJ, Hümmer D, LaRoche J. (2008). Abundances and distributions of the dominant nifH phylotypes in the Northern Atlantic Ocean. Appl Environ Microbiol 74: 1922–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P, Anderson M. (1999). Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecol Monogr 69: 1–24. [Google Scholar]
- Luo Y-W, Lima ID, Karl DM, Deutsch CA, Doney SC. (2014). Data-based assessment of environmental controls on global marine nitrogen fixation. Biogeosciences 11: 691–708. [Google Scholar]
- Lyne V, Hayes D. (2005). Pelagic Regionalisation: National Marine Bioregionalisation Intgration Project. CSIRO Marine and Atmospheric Research, Hobart.
- Mazard S, Fuller N, Orcutt K, Bridle O, Scanlan D. (2004). PCR analysis of the distribution of unicellular cyanobacterial diazotrophs in the Arabian Sea. Appl Environ Microbiol 70: 7355–7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle B, Anderson M. (2001). Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology 82: 290–297. [Google Scholar]
- McKinnon A, Carleton J, Duggan S. (2011). Determinants of pelagic metabolism in the Timor Sea during the inter-monsoon period. Mar Freshw Res 62: 130–140. [Google Scholar]
- Messer LF, Doubell M, Jeffries TC, Brown MV, Seymour JR. (2015). Prokaryotic and diazotrophic population dynamics within a large oligotrophic inverse estuary. Aquat Microb Ecol 74: 1–15. [Google Scholar]
- Mohr W, Grosskopf T, Wallace DWR, LaRoche J. (2010). Methodological underestimation of oceanic nitrogen fixation rates. PLoS One 5: e12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisander PH, Beinart RA, Voss M, Zehr JP. (2008). Diversity and abundance of diazotrophic microorganisms in the South China Sea during intermonsoon. ISME J 2: 954–967. [DOI] [PubMed] [Google Scholar]
- Moisander PH, Beinart RA, Hewson I, White AE, Johnson KS, Carlson CA et al. (2010). Unicellular cyanobacterial distributions broaden the oceanic N2 fixation domain. Science 327: 1512–1514. [DOI] [PubMed] [Google Scholar]
- Moisander PH, Serros T, Paerl RW, Beinart RA, Zehr JP. (2014). Gammaproteobacterial diazotrophs and nifH gene expression in surface waters of the South Pacific Ocean. ISME J 8: 1962–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisander PH, Zhang R, Boyle EA, Hewson I, Montoya JP, Zehr JP. (2012). Analogous nutrient limitations in unicellular diazotrophs and Prochlorococcus in the South Pacific Ocean. ISME J 6: 733–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro FM, Follows MJ, Dutkiewicz S. (2010). Distribution of diverse nitrogen fixers in the global ocean. Global Biogeochem Cycles 24: 1–16. [Google Scholar]
- Montoya J, Holl C, Zehr J, Hansen A. (2004). High rates of N2 fixation by unicellular diazotrophs in the oligotrophic Pacific Ocean. Nature 430: 1027–1031. [DOI] [PubMed] [Google Scholar]
- Montoya JP, Voss M, Kahler P, Capone DG. (1996). A simple, high-precision, high-sensitivity tracer assay for n2 fixation. Appl Environ Microbiol 62: 986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutin T, Karl D, Duhamel S, Rimmelin P, Raimbault P, Van Mooy B et al. (2008). Phosphate availability and the ultimate control of new nitrogen input by nitrogen fixation in the tropical Pacific Ocean. Biogeosciences 5: 95–109. [Google Scholar]
- Mulholland MR. (2007). The fate of nitrogen fixed by diazotrophs in the ocean. Biogeosciences 4: 37–51. [Google Scholar]
- Penton CR, Johnson TA, Quensen JF, Iwai S, Cole JR, Tiedje JM. (2013). Functional genes to assess nitrogen cycling and aromatic hydrocarbon degradation: primers and processing matter. Front Microbiol 4: 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu T, Lindstrom EJ. (2002). A climatological interpretation of the circulation in the Western South Pacific. J Phys Oceanogr 32: 2492–2508. [Google Scholar]
- Raes E, Waite A, McInnes A, Olsen H, Nguyen H, Hardman-Mountford N et al. (2014). Changes in latitude and dominant diazotrophic community alter N2 fixation. Mar Ecol Prog Ser 516: 85–102. [Google Scholar]
- Raimbault P, Garcia N. (2008). Evidence for efficient regenerated production and dinitrogen fixation in nitrogen-deficient waters of the South Pacific Ocean: impact on new and export production estimates. Biogeosciences 5: 323–338. [Google Scholar]
- Rees AP, Law CS, Woodward EMS. (2006). High rates of nitrogen fixation during an in-situ phosphate release experiment in the Eastern Mediterranean Sea. Geophys Res Lett 33: L10607. [Google Scholar]
- Reshef D, Reshef Y, Finucane H. (2011). Detecting novel associations in large data sets. Science 334: 1518–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robidart JC, Church MJ, Ryan JP, Ascani F, Wilson ST, Bombar D et al. (2014). Ecogenomic sensor reveals controls on N2-fixing microorganisms in the North Pacific Ocean. ISME J 8: 1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sañudo-Wilhelmy SA, Kustka A B, Gobler CJ, Hutchins D A, Yang M, Lwiza K et al. (2001). Phosphorus limitation of nitrogen fixation by Trichodesmium in the central Atlantic Ocean. Nature 411: 66–69. [DOI] [PubMed] [Google Scholar]
- Smoot ME, Ono K, Ruscheinski J, Wang P-L, Ideker T. (2011). Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics 27: 431–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohm JA, Mahaffey C, Capone DG. (2008). Assessment of relative phosphorus limitation of Trichodesmium spp. in the North Pacific, North Atlantic, and the north coast of Australia. Limnol Oceanogr 53: 2495–2502. [Google Scholar]
- Sohm JA, Webb EA, Capone DG. (2011). Emerging patterns of marine nitrogen fixation. Nat Rev Microbiol 9: 499–508. [DOI] [PubMed] [Google Scholar]
- Stewart FJ, Ottesen EA, DeLong EF. (2010). Development and quantitative analyses of a universal rRNA-subtraction protocol for microbial metatranscriptomics. ISME J 4: 896–907. [DOI] [PubMed] [Google Scholar]
- Thompson A, Carter BJ, Turk-Kubo K, Malfatti F, Azam F, Zehr JP. (2014). Genetic diversity of the unicellular nitrogen-fixing cyanobacteria UCYN-A and its prymnesiophyte host. Environ Microbiol 16: 3238–3249. [DOI] [PubMed] [Google Scholar]
- Thompson AW, Foster RA, Krupke A, Carter BJ, Musat N, Vaulot D et al. (2012). Unicellular cyanobacterium symbiotic with a single-celled eukaryotic alga. Science 337: 1546–1550. [DOI] [PubMed] [Google Scholar]
- Turk-Kubo KA, Karamchandani M, Capone DG, Zehr JP. (2013). The paradox of marine heterotrophic nitrogen fixation: abundances of heterotrophic diazotrophs do not account for nitrogen fixation rtes in the Eastern Tropical South Pacific. Environ Microbiol 16: 30095–3114. [DOI] [PubMed] [Google Scholar]
- Turk-Kubo KA, Achilles KM, Serros TRC, Ochiai M, Montoya JP, Zehr JP. (2012). Nitrogenase (nifH) gene expression in diazotrophic cyanobacteria in the Tropical North Atlantic in response to nutrient amendments. Front Microbiol 3: 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite TD, Szymczak R, Espey QI, Furnas MJ. (1995). Diel variations in iron speciation in northern Australian shelf waters. Mar Chem 50: 79–91. [Google Scholar]
- Wang Q, Quensen J, Fish J, Lee T, Sun Y, Tiedje J, Cole JR. (2013). Ecological patterns of nifH genes in four terrestrial climatic zones explored with targeted metagenomics using FrameBot, a new informatics tool. MBio 4: e00592–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westram R, Baider K, Prusse E, Kuhmar Y, Meirer H, Glockner FO et al. (2011). ARB: a software environment for sequence data. In: Bruijn FJ (ed). Handbook of Molecular Microbial Ecology I: Metagenomics and Complementary Approaches. John Wiley & Sons, Inc., Hoboken, NJ, USA, pp 399–406.
- Wilson ST, Böttjer D, Church MJ, Karl DM. (2012). Comparative assessment of nitrogen fixation methodologies, conducted in the oligotrophic North Pacific Ocean. Appl Environ Microbiol 78: 6516–6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zani S, Mellon M, Collier J, Zehr J. (2000). Expression of nifH genes in natural microbial assemblages in Lake George, New York, detected by reverse transcriptase PCR. Appl Environ Microbiol 66: 3119–3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr J, Jenkins B, Short S, Steward G. (2003). Nitrogenase gene diversity and microbial community structure: a cross system comparison. Environ Microbiol 5: 539–554. [DOI] [PubMed] [Google Scholar]
- Zehr J, Turner P. (2001). Nitrogen fixation: nitrogenase genes and gene expression. Methods Microbiol 30: 271–285. [Google Scholar]
- Zehr JP, Kudela RM. (2011). Nitrogen cycle of the Open Ocean: from genes to ecosystems. Ann Rev Mar Sci 3: 197–225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.