Abstract
Evidence to date indicates that elevated seawater temperatures increase the occurrence of coral disease, which is frequently microbial in origin. Microbial behaviors such as motility and chemotaxis are often implicated in coral colonization and infection, yet little is known about the effect of warming temperatures on these behaviors. Here we present data demonstrating that increasing water temperatures induce two behavioral switches in the coral pathogen Vibrio coralliilyticus that considerably augment the bacterium's performance in tracking the chemical signals of its coral host, Pocillopora damicornis. Coupling field-based heat-stress manipulations with laboratory-based observations in microfluidic devices, we recorded the swimming behavior of thousands of individual pathogen cells at different temperatures, associated with current and future climate scenarios. When temperature reached ⩾23 °C, we found that the pathogen's chemotactic ability toward coral mucus increased by >60%, denoting an enhanced capability to track host-derived chemical cues. Raising the temperature further, to 30 °C, increased the pathogen's chemokinetic ability by >57%, denoting an enhanced capability of cells to accelerate in favorable, mucus-rich chemical conditions. This work demonstrates that increasing temperature can have strong, multifarious effects that enhance the motile behaviors and host-seeking efficiency of a marine bacterial pathogen.
Introduction
Although disease is a natural process in any ecosystem, the influence of a changing climate on host–pathogen interactions has the potential to markedly shift the incidence of disease and thus alter ecosystem structure and functions in unpredictable ways (Burge et al., 2014). Within coral reef ecosystems there is growing evidence that coral diseases are most prominent during the warmest months of the year and that corals are losing their seasonal reprieve from disease advancement as mean winter temperatures rise (Weil et al., 2009; Heron et al., 2010; Case et al., 2011; Burge et al., 2014). However, it remains unclear whether this pattern occurs as a consequence of increased coral susceptibility above specific thermal thresholds, shifts in the behavior or virulence of the responsible pathogens, or a combination of both factors.
Coral reefs around the globe are declining due to a confluence of factors, including increased disease and rising ocean temperatures (Hoegh-Guldberg 2004; Pandolfi et al., 2003; Ruiz-Morenol et al., 2012). Although we know that elevated seawater temperatures destabilize the coral–algal symbiosis that is critical for coral survival (Baker et al., 2008), we know far less about the linkages between warming waters and coral disease. Understanding how pathogen navigation and host-detection are influenced by changing environmental conditions is important to allow these processes to be accounted for in understanding coral heath and in forecasting areas of high risk for disease outbreaks (Maynard et al., 2015).
Several bacterial diseases of corals have been linked to increases in seawater temperatures, yet the mechanisms by which warmer temperatures favor disease processes remain elusive. For example, the bacterium Vibrio shiloi causes only slow partial bleaching at 23 °C, but rapid and severe bleaching at 29 °C in the Mediterranean coral Oculina patagonica (Toren et al., 1998; Banin et al., 2001). Similarly, the globally distributed bacterial pathogen Vibrio coralliilyticus (Pollock et al., 2010) causes rapid tissue lysis of its coral host, Pocillopora damicornis, when temperatures exceed 26 °C (Ben-Haim and Rosenberg 2002; Ben-Haim et al., 2003) and is more abundant in heat-stressed corals (Tout et al., 2015b), whereas increases in Caribbean Yellow Band Disease between 1998 and 2010 have been associated with elevated winter seawater temperatures (Burge et al., 2014; Weil et al., 2009).
Recently, we demonstrated that V. coralliilyticus uses two specific behaviors to navigate toward its host: the ability to swim up chemical gradients (chemotaxis) and the ability to increase swimming speed in the presence of coral mucus (chemokinesis). Furthermore, we found that the concentration of an important chemical signal in that process, the sulfur compound dimethylsulfoniopropionate, is higher when the coral hosts are heat-stressed (Garren et al., 2014). This evidence suggests that elevated temperatures may improve the pathogen's capacity to locate and colonize its host owing to increased signal production. However, the effects of temperature directly on the pathogen's host-sensing behaviors are unknown. Although warmer temperatures might increase the chance of infection in several ways, such as increasing bacterial growth rates or virulence (Kimes et al., 2012), the effect of temperature on pathogen motility appears particularly important because all putative bacterial pathogens of corals that have been identified thus far are motile (Garren et al., 2014), with both motility and increased seawater temperatures independently implicated in the infection process (Banin et al., 2001; Meron et al., 2009).
Temperature has been shown to influence motility and chemotaxis in enteric and marine bacteria. Escherichia coli increases its average swimming speed almost linearly with temperature, from 15 μm s−1 at 20 °C to 55 μm s−1 at 40 °C, yet faster swimming does not correspond to stronger chemotaxis (Maeda et al., 1976). Among marine bacteria, two temperate Vibrio species increase their swimming speed with temperature: Vibrio anguillarum increases its speed from 25 μm s−1 at 5 °C to 40 μm s−1 at 25 °C (Larsen et al., 2004) and exhibits stronger chemotaxis at the warmer temperatures within this range, whereas Vibrio alginolyticus increases its speed from 77 μm s−1 at 25 °C to 116 μm s−1 at 35 °C (Magariyama et al., 1995). Albeit based on relatively low-resolution quantification of motility (tracking of small cell numbers) and chemotaxis (via the capillary assay), these observations highlight the important role that temperature can have on bacterial motility and chemotaxis. It is currently unknown how the changing environmental conditions in coral reef ecosystems will influence the behavior and ecology of bacterial pathogens that affect regional and global-scale coral health. Here, we quantify the effect of temperature on V. coralliilyticus' navigational performance toward mucus from its coral host as an important step in predicting the effects of warming seawater temperature on coral disease outbreaks.
Materials and methods
Two sets of experiments were conducted to (i) determine the effect of temperature on pathogen behavior and to (ii) test the simultaneous and combined effect of temperature on host and pathogen. The first set of experiments was conducted by growing the coral pathogen, V. coralliilyticus, at four temperatures (20 °C, 23 °C, 27 °C and 30 °C) spanning the range of seasonal mean temperatures experienced by this host–pathogen pair and include two temperatures (23 °C and 27 °C) that straddle the previously determined 26 °C trigger point for increased virulence (Ben-Haim et al., 2003; Kimes et al., 2012). We explored the bacterium's chemotactic and chemokinetic responses at these four temperatures by using a single, homogenized pool of coral mucus collected from laboratory-cultured corals growing at a moderate temperature (25 °C). The second set of experiments coupled pathogen growth at ambient and high temperatures (22 °C and 30 °C) with mucus from field-collected corals before (22 °C) and after a heat-stress (31 °C) treatment to test the simultaneous effect of temperature on the host–pathogen interactions.
Organism growth conditions
Small colonies of the coral P. damicornis were cultured in the laboratory at 25 °C±1 °C in artificial seawater (Instant Ocean, Spectrum Brands Company, Cincinnati, OH, USA) on a 12 h light–dark cycle. All experiments were conducted using the bacterium V. coralliilyticus, strain BAA-450, acquired from the American Type Culture Collection (www.atcc.org, Manassas, VA, USA). In the first set of experiments, cells were grown for 16–18 h, shaking (300 rpm), in 0.2 μm-filtered, autoclaved seawater with 1% marine broth (2216; BD Difco, Franklin Lakes, NJ, USA) at each of four temperatures: 20 °C, 23 °C, 27 °C and 30 °C. For growth below room temperature (20 °C and 23 °C), a chilling incubator (Multi-Therm, Denville Scientific, South Plainfield, NJ, USA) was used. Given the availability of only one chilling incubator, experiments were run back to back on subsequent days with 30 °C treatments repeated on each day as a standard: all replicates for 20 °C, 27 °C and 30 °C were performed on day 1 and all replicates for 23 °C and 30 °C were performed on day 2. Quantifications of the strength of chemotactic accumulation, measured by a normalized chemotactic index IC (see below) for each temperature, were normalized to the 30 °C experiment from each given day and are consistently reported as the mean of all replicates (three technical replicates for each of three biological replicates). Three replicate cultures were grown at each temperature each day, all from the same three starting colonies streaked from glycerol stocks onto marine broth agar plates. The same starting colonies for a given experiment were used on both days to ensure that every temperature had identical biological replicates. In the second set of experiments using mucus collected in the field (see below), bacteria were grown at temperatures corresponding to the ambient (22 °C) and heat-stressed (30 °C) coral conditions, again using the same three starting colonies to initiate the biological replicates for both temperatures.
Mucus collection
For the first set of experiments, designed to isolate the effect of temperature on pathogen behavior, mucus was collected in the laboratory from coral colonies cultured at 25 °C by exposing them to air for 3 min over a sterile 50 ml tube (Falcon, Corning Life Sciences, Tewksbury, MA, USA). Owing to the volume requirements for completing all microfluidic experiments with a homogeneous pool of mucus, collection was carried out on each of four colonies once per day for 5 days, and mucus was stored at −80 °C. Directly before the experiment, a single homogenized pool of mucus was created by thawing all of the frozen mucus (all colonies, all days), pooling samples in a single 50 ml tube, vortexing for 2 min and subsequently using the pooled sample in microfluidic experiments.
In the second set of experiments, designed to test the simultaneous and combined effect of temperature on host and pathogen, we assayed chemotaxis (see below) of pathogen cells grown at one of two temperatures (22 °C and 30 °C) to mucus from coral fragments before (T0=22 °C) and after (Tf=31 °C) high-temperature stress conditions. A field experiment carried out on Heron Island, Great Barrier Reef, Australia (23° 26' 37" S/151° 54' 44" E) to obtain these mucus samples was described previously (Garren et al., 2014). In brief, clonally replicated, 1-week duration heat-stress experiments were performed on Heron Island using P. damicornis. Mucus was collected from each fragment at the beginning (temperature T0) and end (temperature Tf) of the experiment by air exposure, as described above. Samples were immediately frozen at −80 °C, shipped to MIT on dry ice and subsequently used in microfluidic experiments.
Microfluidic chemotaxis experiments
To test the chemotactic response of V. coralliilyticus, we used a 600 μm-wide, 100 μm-deep microfluidic channel with three inlets (Figure 1a) to establish a transient chemical gradient driven solely by molecular diffusion. The device was fabricated using soft lithography techniques described previously (Weibel et al., 2007; Seymour et al., 2008), bonded to a microscope slide, and mounted onto a Nikon Ti microscope (Tokyo, Japan). All experiments were conducted at the same temperature at which the cells were grown, using a temperature controlled stage insert (Warner Instruments, Hamden, CT, USA). Cell filtrate was used both as the control attractant and as the buffer in the center inlet, to create a band separating the cells and the mucus in the microchannel (see below). Cell filtrate was obtained by passing 1.5 ml of each cell culture through a 0.2 μm syringe filter into a sterile 1.5 ml tube (Eppendorf, Hamburg, Germany). The filtrate from each of three cultures (biological replicates) at each temperature was prepared, and then the three filtrates for a given temperature were pooled to create one homogenous filtrate for each temperature.
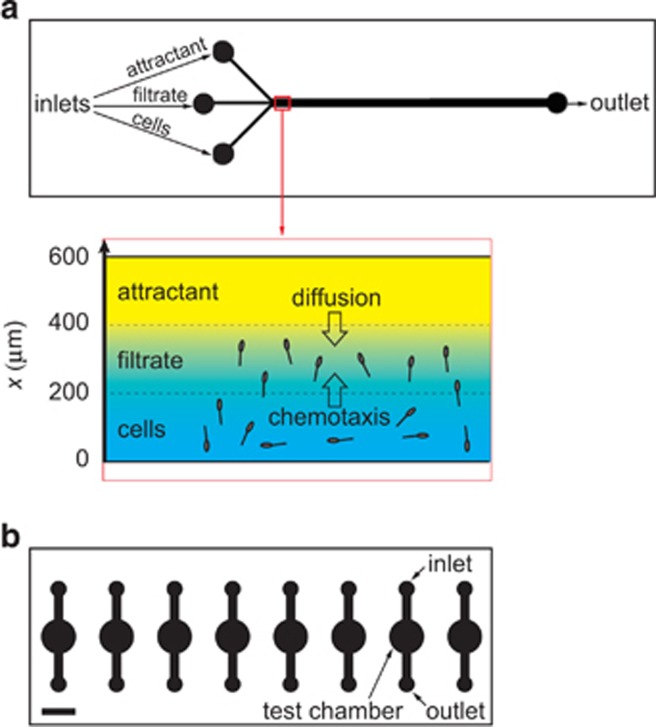
Figure 1.
Schematics of the microfluidic devices used to study the temperature dependence of motility behaviors of the coral pathogen Vibrio coralliilyticus. Both panels presented from the microscopist's perspective, where the objective is directly below the image shown and the devices are mounted onto a standard 1 × 3 inch microscope slide. (a) Planar view of the three-inlet channel used for the chemotaxis experiments. Three inlets converge into a 600 μm-wide, 100 μm-deep channel, creating three, 200 μm wide streams of cells, filtrate and attractant, respectively. The imaging window is denoted by the red box. (Inset) Diffusion of the attractant across the channel (x direction) creates chemical gradients to which bacteria can respond by chemotaxis. (b) Parallel holding chambers, 100 μm in depth and 5 mm in diameter, used for the chemokinesis experiments. Bacteria injected through the inlet were observed in the test chamber under quiescent conditions. The eight chambers on a chip allowed for parallel experiments in conjunction with the automated, programmable microscope stage that can image successive chambers efficiently and return to the precise imagine position each time. Scale bar=5 mm.
The microfluidic devices were loaded using 23-gauge blunt-end needles (Grainger, Lake Forest, IL, USA) attached to 1 ml gastight luer tip syringes (Hamilton, Reno, NV, USA) and infused from a syringe pump (PHD 2000, Harvard Apparatus, Holliston, MA, USA). Three syringes were filled with cells, filtrate and attractant, respectively, and connected by Tygon tubing (0.02” ID, 0.06” OD; Cole-Parmer, Vernon Hills, IL, USA) to the three inlets of the channel. Syringe contents were flowed into the device in this order to form three adjacent, 200 μm-wide bands (Figure 1a). The flow was then stopped, resulting in the mucus chemicals diffusing across the width of the microchannel and forming a gradient in the x direction (Figure 1a). The pathogens' behavior in response to this gradient was recorded for 165 s with video microscopy directly downstream of the convergence point of the three inlets (Figure 1a). Negative control experiments were performed by replacing the coral mucus with filtrate. Syringes were washed with 70% ethanol and deionised water between experiments.
Three technical replicates, using the same microfluidic channel, were carried out for each biological replicate. The microchannel was freshly loaded with the three bands from the three syringe reservoirs in between each technical replicate, by flushing for 1 min at 50 μl min−1 and then slowing the flow to 2 μl min–1 before beginning a new replicate run.
Microfluidic chemokinesis experiments
Chemokinesis is defined as a change in swimming speed induced by a change in the chemical concentration in the surrounding medium, and is different from chemotaxis because it does not relate to a bias in the swimming direction, but rather to a temporal change in speed. To isolate and quantify the temperature dependence of chemokinesis in V. coralliilyticus in response to mucus, we used a second microfluidic device, equipped with a small (5 mm diameter, 100 μm height) holding chamber for visualization (Figure 1b). A single device contained eight individual chambers in parallel, so that multiple samples could be imaged in rapid sequence, repeatedly over time, using computer-controlled motion of the microscope stage. The mucus treatment consisted of a 1:1 mix of cells and the same mucus pools used in the chemotaxis experiments. To allow the cells enough time to respond to the chemical environment before imaging, all treatments were incubated for 8 min in 1.5 ml sterile tubes with or without mucus at their appropriate temperature (matching cell growth temperature). Thereafter, for each biological replicate at a given temperature we loaded 20 μl of the mucus-added treatment in one chamber and 20 μl of the control in the neighboring chamber, and immediately began acquisition of a 30 s long video of each treatment at 30 frames s−1. We repeated the process to image a total of three technical replicates, using fresh cells each time, for each treatment, at each temperature, for all three biological replicates. The videos were processed as described below to obtain swimming speeds from cell trajectories.
Microscopy, image analysis, swimming speed quantification and statistics
All images were acquired using phase-contrast video microscopy with a 20 × objective (0.45 NA) and an Andor Neo camera (6.5 μm pixel−1; Andor, Belfast, Northern Ireland). The focal plane was always at channel mid-depth to avoid wall effects on motility. All videos were acquired at 30 frames s–1 to robustly capture sharp directional changes in the swimming trajectories and detect reorientations. Videos were exported in tagged image file format for analysis in MATLAB (MathWorks, Natick, MA, USA) using in-house, automated image segmentation and trajectory reconstruction software. Background subtraction and cross-correlation routines were used to detect any residual flow and to exclude non-motile cells and mucus particulates from the analysis. Individual trajectories were reconstructed from identified cell positions through subsequent frames using a particle tracking routine. Cells tracked for less than three consecutive frames were excluded from the analysis, as were trajectories with average velocity <10 μm s−1 (considered non-motile). From the reconstructed trajectories, we calculated the average swimming speed of each cell by averaging the instantaneous speed over the duration of that cell's trajectory. The mean speed of the population, V, was quantified by averaging over all trajectories at each temperature. We tested for significant differences among swimming speeds in the chemokinesis experiments using a two-tailed t-test to compare the mean swimming of cells at a given temperature under the two mucus conditions (with and without). The difference in speeds between those two conditions at a given temperature were also compared with the difference in speeds observed between with and without mucus conditions in the 20 °C experiments as a baseline. The significance of temperature's influence on chemotactic accumulation was tested using a one-way analysis of variance comparing the mean maximum value of cell accumulation in the mucus region (B(x)Max; x>500 μm) for each temperature treatment of the chemotaxis experiments. When we found a significant effect of temperature, we removed the coolest temperature and repeated the analysis of variance to test for significant differences among the three warmer temperatures.
Results
We previously reported that V. corallilyticus grown at a single temperature (30 °C) employs a combination of strong chemotactic and chemokinetic behaviors in response to coral mucus (Garren et al., 2014). Here, we focused on the influence of temperature on the bacterium's behavioral responses to whole coral mucus and found that chemotaxis and chemokinesis are both positively, but differentially, impacted by elevated seawater temperatures.
Temperature effect on pathogen chemotaxis
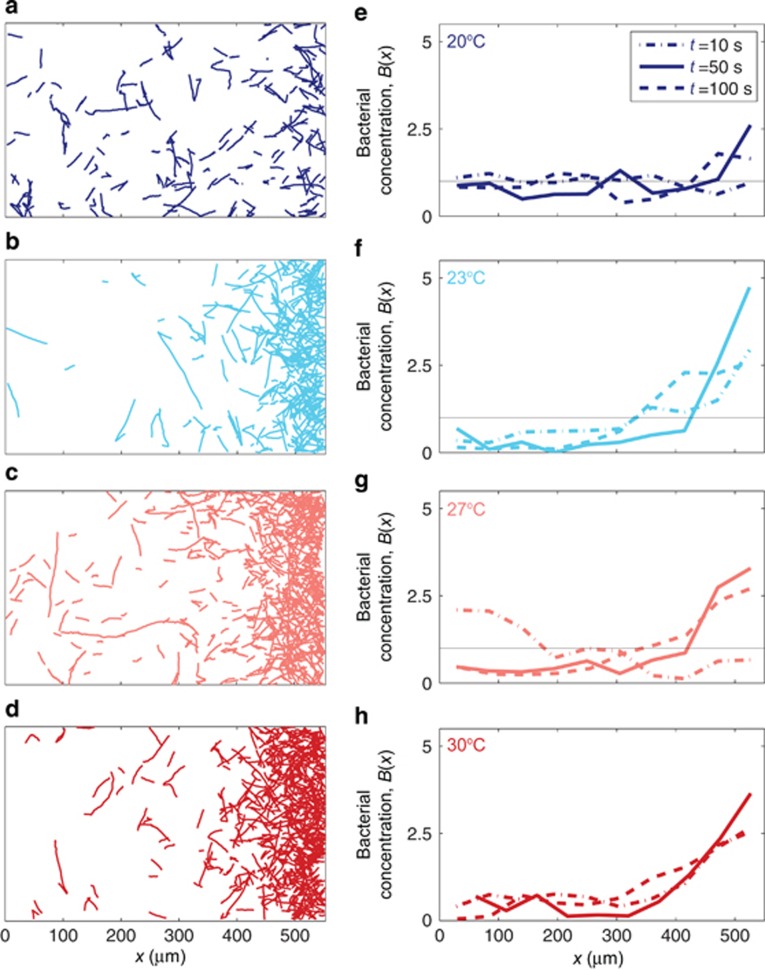
The chemotaxis experiments demonstrated that the ability of V. coralliilyticus cells to migrate up a gradient of coral mucus was substantially impaired at the lowest temperature (20 °C; Figures 2a and e; Supplementary Figures S1 and S2, blue; one-way analysis of variance with P<0.01). Only a weak accumulation of cells in the regions of highest mucus concentration occurred at 20 °C. In contrast, at all temperatures ⩾23 °C, cell trajectories (Figures 2b–d) and spatial distributions (Figures 2f–h) were indicative of a significant migration toward higher coral mucus concentrations, and the maximum levels of accumulation reached were not significantly different from each other (Supplementary Figure S1 and S2; one-way analysis of variance with P>0.01). For example, the bacterial concentration in the high mucus concentration region (x=512±12 μm) at t=50 s was 4.7-fold larger than the mean at 23 °C (Figure 2f), compared with only 2.3-fold larger than the mean at 20 °C (Figure 2e). Furthermore, a strong accumulation of bacteria in the high-mucus region persisted for considerably longer times at temperatures ⩾23 °C (Figures 2f–h) compared with the accumulation observed at 20 °C (Figure 2e). The weaker accumulation at 20 °C was not associated with a reduction in swimming speed, which was nearly constant over the temperatures tested (Figure 3c), suggesting that the bacterium's sensing system, rather than its propulsion system, was impaired under the coolest conditions tested.
Figure 2.
The strength of chemotaxis toward coral mucus by the pathogen V. coralliilyticus is temperature dependent. (a–d) Individual cell trajectories across the width of the three-inlet microchannel (Figure 1a), showing accumulation toward the side where mucus was initially injected (400 μm<x<600 μm; see Figure 1a). The trajectories shown here were acquired from 43 s to 50 s after bacteria were exposed to the mucus layer. (e–h) Concentration of bacteria across the channel, B(x), at different times after cells were exposed to the mucus layer under diffusive gradient conditions: 10 s (dash-dotted line), 50 s (solid line) and 100 s (dashed line). The cell concentration was normalized to have a mean of one in all cases (black solid line). In all panels (a–h) accumulation toward the right denotes positive chemotaxis to coral mucus. Panels correspond to different temperatures at which cells were grown and assayed: 20 °C (a, e; blue), 23 °C (b, f; cyan), 27 °C (c, g; pink) and 30 °C (d, h; red). Accumulation at 20 °C is significantly weaker (P<0.01) than the levels of accumulation observed in all other temperature treatments (see also Supplementary Figure S1).
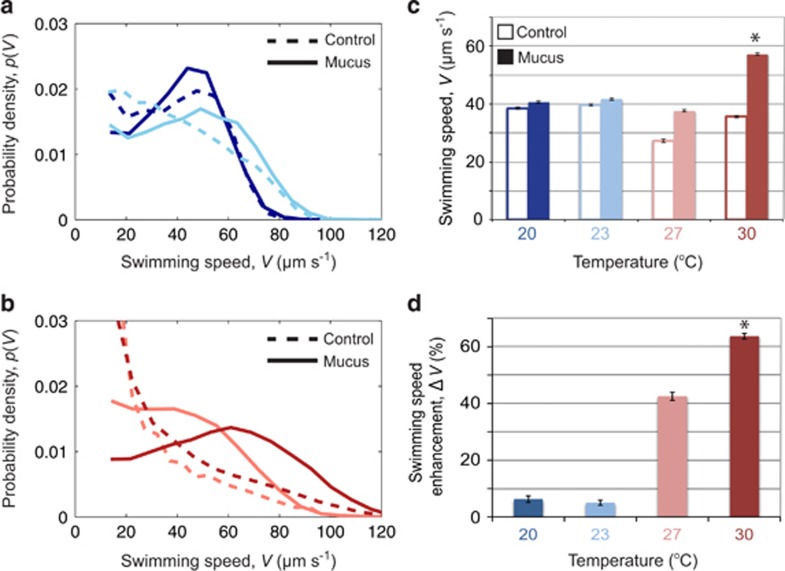
Figure 3.
The strength of chemokinesis of V. coralliilyticus in coral mucus of uniform concentration is strongly temperature dependent. (a and b) The distribution of swimming speeds within the pathogen population for all replicates in each experiment. Curves correspond to different temperatures at which cells were grown and assayed: 20 °C (a, blue), 23 °C (a, cyan), 27 °C (b, pink), 30 °C (b, red). Solid lines are treatments with mucus and dashed lines are controls with cell filtrate in lieu of mucus. (c) The mean swimming speed, V, for cells with (solid bars) and without (open bars, controls) the addition of a uniform concentration of mucus. Cells were grown and assayed at four temperatures, indicated on the axis and by the color-coding. * denotes P-value <0.05 comparing the with and without mucus conditions within a given temperature. (d) The enhancement in the mean swimming speed of a population in the presence of coral mucus over the no-mucus control. * denotes P-value <0.05 when the mean is compared with the 20 °C case. Data in c and d are means of >2000 individual cell tracks for each case and the error bars denote standard errors. Experiments were performed in the circular microfluidic-holding chambers (Figure 1b).
The magnitude of the chemotactic response and its temporal evolution were quantified and compared using a normalized chemotactic index, IC (Seymour et al., 2010), which measures the relative difference in cell concentration between the 200 μm-thick layer initially occupied by mucus and the 400 μm-wide region outside of that layer (Figure 1a). Absence of chemotaxis corresponds to IC=0. Some variability in the magnitude of the response among biological replicates notwithstanding, the chemotactic accumulation of cells at ⩾23 °C consistently reached >40% higher IC values than the maximum accumulation observed in any replicate from cells at 20 °C (Supplementary Figure S2). The chemotactic response of cells at and above 23 °C was both significantly stronger (P<0.01) and the maximum levels of accumulation were reached more rapidly than the 20 °C treatment (P<0.01). For example, 20 °C cells reached their maximum state of accumulation (IC,MAX=0.2–0.4) in 60–70 s (Supplementary Figure S2a, b), in contrast to cells at warmer temperatures that surpassed that level of accumulation in <25 s (Supplementary Figure S2). Together these data demonstrate that, at temperatures ⩾23 °C, the chemotactic response of V. coralliilyticus increases by ⩾60% when measured as the average increase in IC,MAX at 23 °C, 27 °C and 30 °C over the value of IC,MAX at 20 °C.
Temperature effect on pathogen chemokinesis
To differentiate chemokinesis from chemotaxis at each temperature, we quantified the pathogen's response to a uniform mucus addition in the absence of a gradient. We used this approach because chemotaxis and chemokinesis can occur simultaneously, the former allowing cells to climb the gradient, the latter resulting in increases in swimming speed when substrate concentrations exceed a threshold, and both behaviors are thus folded into the quantification of chemotaxis presented above. Experiments in uniform chemical environments, without gradients, allow for the measurement of chemokinesis alone: here we quantified the absolute change in the mean swimming speed, ΔV, and the percentage change, ΔV%, after the uniform addition of mucus. These experiments revealed that the swimming speed of V. coralliilyticus was not appreciably affected (P>0.05) by the addition of mucus for cells grown at 20 °C (ΔV=2±4 μm s–1; ΔV%=6%) or 23 °C (ΔV=2±9 μm s−1; ΔV%=5%) (Figures 3a, c and d). In contrast, swimming speeds appeared elevated by mucus addition in cells grown at 27 °C (ΔV=10±15 μm s−1; ΔV%=42%) and were significantly enhanced at 30 °C (ΔV=23±7 μm s−1; ΔV%=64% P<0.05) (Figures 3b–d).
We emphasize that here we report not a change in swimming speed with temperature per se, as done previously for E. coli (Maeda et al., 1976) and other Vibrios (Larsen et al., 2004; Magariyama et al., 1995), but rather an increase in swimming speed elicited by mucus addition, as a function of temperature. In fact, all swimming speed enhancements occurred against the backdrop of an otherwise temperature-independent swimming speed, which in the absence of mucus, was consistently in the range V=30–40 μm s−1 at the four temperatures tested (Figure 3c). This is in contrast to previous reports for E. coli (Maeda et al., 1976) and two Vibrio species (Larsen et al., 2004; Magariyama et al., 1995), where speed increased monotonically with temperature over the ranges tested. Acknowledging that our current methods allow for much higher throughput analysis of individual cell behaviors than previous methods have provided, we note that temperature may have a strain-specific impact on motility, potentially with stark differences even among Vibrios. Our experiments demonstrate that, in addition to the advantage in gradient-sensing (chemotaxis) that the pathogen experiences at and above 23 °C, there is a second mechanism of motility enhancement in the form of increased swimming speeds (chemokinesis) as sea temperatures warm further above 30 °C.
Pathogen-intrinsic and host-derived factors combine to influence pathogen behavior
The experiments described thus far focused on the effect of temperature on the bacterial pathogen, and the mucus we used came from a uniform pool collected from coral colonies grown at 25 °C. In the natural environment, however, bacterial pathogens and corals will experience the same temperature conditions. In previous work, we have shown that the composition of coral mucus is altered when corals are subjected to elevated temperatures (Garren et al., 2014). In particular, an increase in temperature from 22 °C to 31 °C resulted in a fivefold increase in the concentration of dimethylsulfoniopropionate in the coral mucus, which in turn triggered a twofold stronger chemotactic response of the pathogen (as measured by the chemotactic index) when compared with mucus from non-stressed corals (Garren et al., 2014). When taken together with the results presented here, these previous findings suggest a compounding effect of temperature, which increases not only the production of the chemical signals in the mucus that attract the pathogens, but also the motility performance of the pathogen per se. To specifically test this combined effect of temperature on the pathogen behavior, we carried out a further set of chemokinesis (Figure 4) and chemotaxis (Figure 5) experiments where the pathogen was grown at a temperature identical or very close to that in which the corals were living when their mucus was collected. We focused on two temperatures: 22 °C, the ambient seawater temperature at the field site on Heron Island, and 30–31 °C, representative of elevated summer temperatures, for the heat-stressed mucus.
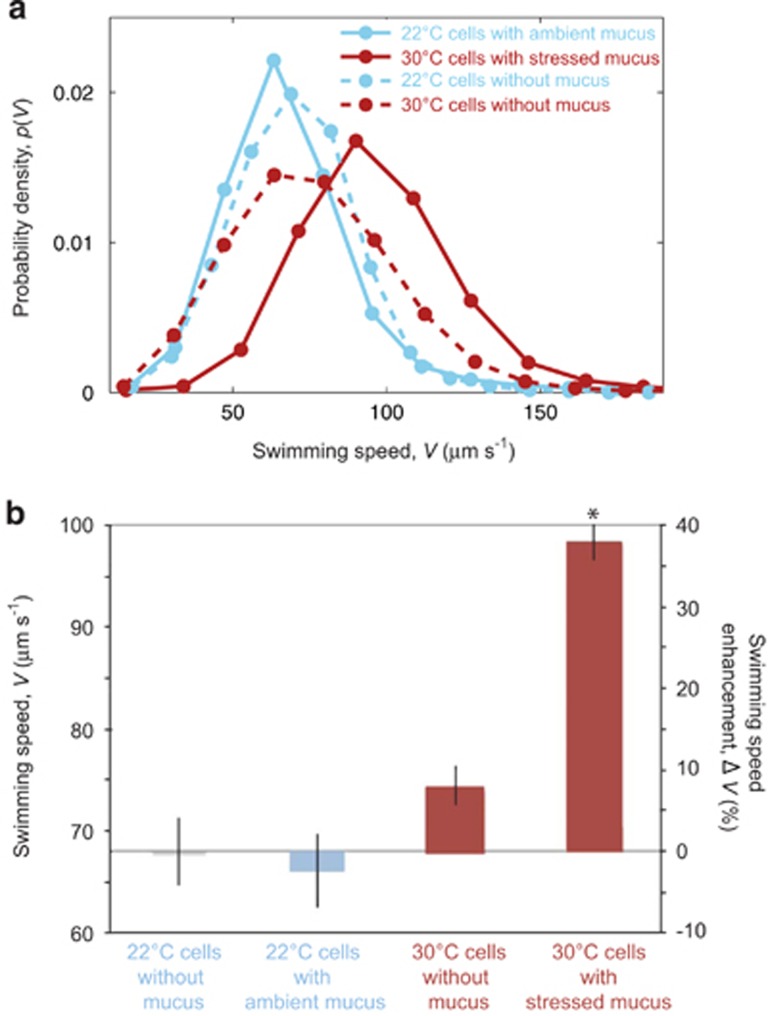
Figure 4.
Chemokinetic response of V. coralliilyticus to mucus collected from control and heat-stressed corals. (a) The distribution of swimming speeds within the pathogen population showing the complete data set from all replicates of a given treatment. Pathogens grown at 22 °C were presented with a uniform concentration of mucus from healthy corals grown at ambient temperature (solid blue line), whereas pathogens grown at 30 °C were presented with a uniform concentration of mucus from heat-stressed corals (solid red line). Dashed lines represent no-mucus controls for each case. (b) The mean swimming speed (left axis) of all replicates for each of the four cases in panel a. As a second metric of the same data, values are also expressed in terms of the percent speed enhancement over the 22 °C experiment with no mucus (right axis). Error bars denote standard errors and * denotes values significantly different from both the 22 °C and 30 °C without mucus cases (P-value <0.01). Experiments were performed in the circular microfluidic-holding chambers (Figure 1b).
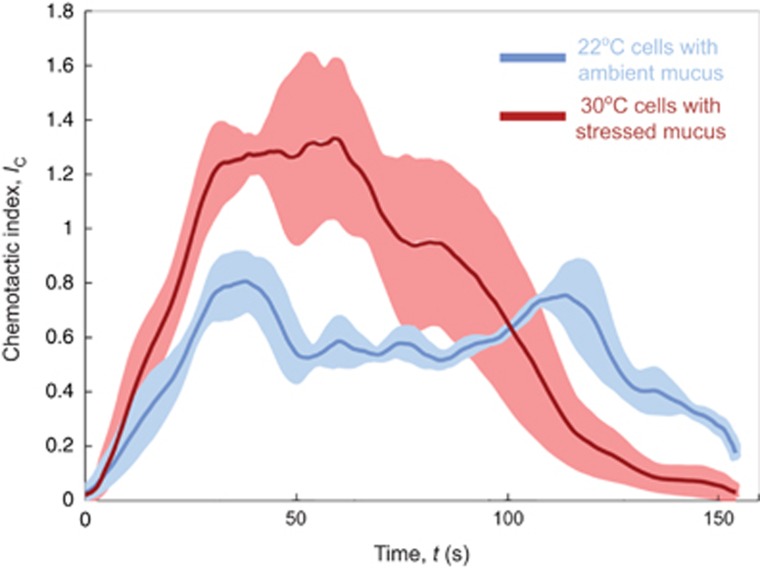
Figure 5.
Chemotactic response of V. coralliilyticus demonstrating the combined effect of temperature on pathogen motility and host physiology. Temporal evolution of the chemotactic index, IC, for experiments in which the pathogen was grown at the same temperatures that corals experienced when their mucus was collected: 22 °C (blue) and 30 °C (red). The chemotactic index was defined as in Supplementary Figure S2, except that here normalization occurred relative to the maximum value in the 22 °C experiments. Solid lines are means of three biological and three technical replicates. Shadings correspond to the standard error. Experiments were performed in the three-inlet channel (Figure 1a).
The temperature-paired pathogen-mucus experiments confirmed that chemokinesis only occurs at elevated temperatures. When ‘ambient mucus' (that is, mucus from corals living at 22 °C when mucus was harvested) was added to ‘ambient cells' (that is, cells grown at 22 °C), the swimming speed of the cells remained nearly unchanged (ΔV=2±3 μm s−1; Figure 4). In contrast, when ‘heat-stressed mucus' (that is, mucus collected from heat-stressed corals) was added to ‘heat-grown cells' (that is, cells grown at 30 °C), the swimming speed increased significantly by >30% (P<0.01, ΔV=21±2 μm s–1; Figure 4). In the absence of mucus, the speeds of ambient cells and heat-grown cells were within 10% of each other (Figure 4b). These results are consistent with the previous set of experiments testing the effect of temperature on the pathogen alone, which also showed a ΔV~20 μm s−1 increase in the swimming speed of 30 °C cells upon addition of mucus (Figures 3c and d). This comparison suggests that the chemical composition of heat-stressed mucus only influences chemotaxis (Garren et al., 2014) and does not influence the magnitude of chemokinesis.
The most remarkable feature of V. coralliilyticus' host-seeking behavior in the presence of heat-stressed mucus was the marked increase in its chemotactic performance, induced by the cumulative benefit that warmer waters confer to the pathogen by intrinsically improving its motility via chemokinesis (Figure 4, Supplementary Figure S3) and by enhancing the chemical signals it follows by influencing the composition of coral mucus (Garren et al., 2014). This is clearly evidenced by the higher chemotactic indices reached by heat-grown cells exposed to heat-stressed mucus, compared with ambient cells exposed to ambient mucus (Figure 5). Although the pathogen does not exhibit chemokinesis at either 22 °C (Figure 4b) or 23 °C (Figure 3d), relatively strong chemotaxis occurs at both of these temperatures (with IC,MAX values of 0.8 and 0.9, respectively; Figure 5, Supplementary Figure S1–S3). However, unlike the nearly identical chemotactic responses observed in cells grown at 23 °C (IC,MAX=0.9) and 30 °C (IC,MAX=1.0) in response to 25 °C mucus (Supplementary Figure S2), cells grown at 30 °C accumulated more strongly (IC,MAX=1.3; Figure 5) when responding to heat-stressed mucus than cells grown at 22 °C and exposed to ambient (22 °C) mucus (IC,MAX=0.8; Figure 5). Not only will this improved chemotactic ability potentially give the bacterium an advantage in finding its host, but it could also improve cell growth by lengthening residence times in nutrient-rich (that is, mucus-rich) zones.
Discussion
Our findings suggest that under warming seawater conditions, V. coralliilyticus will exhibit a marked improvement in gradient-sensing abilities owing to increases in chemotactic and chemokinetic responses. These temperature-induced alterations in behavior combined with previously described changes in chemical ecology elicited in corals by heat stress (Garren et al., 2014) will together strongly favor the pathogen in reaching its targeted host. The cellular mechanisms underlying the switch from poor to acute chemotactic sensing and the induction of chemokinesis remain an open question. The answers will likely require a combined approach that couples genetic and transcriptomic techniques with direct observation and quantification of pathogen behaviors.
Our observations paint a distinctly different picture of the effects of increased temperature on the motility behaviors of V. coralliilyticus, compared with previous results using different methods for E. coli (Larsen et al., 2004), V. alginolyticus (Magariyama et al., 1995) and V. anguillarum (Maeda et al., 1976). For those species, increased temperature per se augmented swimming speed, whereas we observed that temperature alone has little effect on the swimming speed of V. coralliilyticus. Instead, we found that the change in speed upon a change in the chemical environment (chemokinesis) is strongly temperature dependent, a behavioral adaptation that to the best of our knowledge has not been reported to date for any other species.
As new tools continually improve our ability to observe bacterial behaviors, it is becoming evermore clear that bacterial motility and chemotaxis are widespread in many marine habitats (Stocker and Seymour, 2012; Son et al., 2015). The marine environment is a heterogeneous landscape at the microscale and a bacterium's ability to accurately navigate gradients of nutrients and infochemicals may provide it with a competitive advantage (Taylor and Stocker 2012). Chemotaxis is found governing bacteria−phytoplankton interactions (Bell and Mitchell 1972; Blackburn et al., 1998; Stocker and Seymour 2012), facilitating symbioses such as that between Vibrio fischeri and squid (Mandel et al., 2012), implicated in the onset of disease (Otoole et al., 1996; Banin et al., 2001; Rosenberg and Falkovitz 2004; Larsen et al., 2004; Meron et al., 2009) and common throughout coral reefs (Tout et al., 2015b). Within this framework, reef-building corals are becoming a focal system for studying the role of motility in bacteria–host interactions in the ocean. Corals also provide an advantage in the context of facilitating in situ visualization because they host an important portion of their microbiome on their external surface (Garren and Azam 2012). It is also a timely choice of from an ecological perspective because disease has recently been identified as an under-appreciated driver of the future state of reefs (Maynard et al., 2015).
Our observations are pertinent within the framework of changing environmental conditions that currently threaten coral reefs at a global scale. Maynard et al. (2015) present climate model projections for the onset of increased pathogen virulence, which was defined as a doubling in the number of months at which sea temperatures are likely to induce heightened host-seeking behaviors (that is, >historical mean monthly maximum, found experimentally to be ~27 °C). Under business as usual forecasts for greenhouse gas emissions, it is projected that pathogen virulence will increase for >40% of reefs worldwide by 2030 (Maynard et al., 2015). Given that all currently known putative coral pathogens are motile (Garren et al., 2014) and that chemotaxis is both a common feature in the reef environment (Tout et al., 2015a) and implicated in the success of bacterial pathogens of corals (Banin et al., 2001; Meron et al., 2009), our observations that both chemotactic and chemokinetic behaviors of V. coralliilyticus are influenced by temperature add to our current understanding of coral disease to suggest that motility adaptations under future climate scenarios have the potential to favor bacterial pathogens over their coral hosts (Garren et al., 2014; Maynard et al., 2015).
The goal of high-resolution observations of microbial pathogen behavior such as those reported here is to ultimately gain a better understanding of bacterial processes that drive coral disease and incorporate these into predictive models that can aid management decisions and facilitate community planning where people are dependent on reef resources. Pathogen motility is one element that may affect the outcomes of host–pathogen interactions, and disease remains an important stressor actively deteriorating reefs globally. Although the overarching picture remains complex, the ever improving techniques available to observe bacterial behaviors and elucidate the mechanistic underpinnings of disease processes at the most pertinent scales are opening the door to understand the microscale mechanisms driving ecosystem-wide patterns. In this respect, the results presented here contribute to our understanding of the potential impacts of warming seawater temperatures on bacterial behaviors that influence coral disease.
Acknowledgments
We thank DG Bourne, JB Raina, PJ Ralph, V Fernandez, J Guasto, J Lindholm, T Santiano-McHatton, R Schilling and the staff at the Heron Island Research Station. This work was supported by the Human Frontiers in Science Program award no. RGY0089 to RS and JRS, by a Gordon and Betty Moore Foundation Investigator Grant (GBMF3783) to RS, by Australian Research Council Grants DP110103091 and FT130100218 to JRS, and by NSF awards CBET-1066566 and CBET-0966000 to RS. We are grateful to the Great Barrier Reef Marine Park Authority for coral collection permits G09/31733.1 (PJ Ralph, University of Technology Sydney) and G12/35236.1 (Australian Institute of Marine Science).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Baker AC, Glynn PW, Riegl B. (2008). Climate change and coral reef bleaching: An ecological assessment of long-term impacts, recovery trends and future outlook. Estuar Coast Shelf Science 80: 435–471. [Google Scholar]
- Banin E, Israely T, Fine M, Loya Y, Rosenberg E. (2001). Role of endosymbiotic zooxanthellae and coral mucus in the adhesion of the coral-bleaching pathogen Vibrio shiloi to its host. Fems Microbiol Lett 199: 33–37. [DOI] [PubMed] [Google Scholar]
- Bell W, Mitchell R. (1972). Chemotactic and growth responses of marine bacteria to algal extracellular products. Biol Bull 143: 265. [Google Scholar]
- Ben-Haim Y, Rosenberg E. (2002). A novel Vibrio sp pathogen of the coral Pocillopora damicornis. Marine Biol 141: 47–55. [Google Scholar]
- Ben-Haim Y, Zicherman-Keren M, Rosenberg E. (2003). Temperature-regulated bleaching and lysis of the coral Pocillopora damicornis by the novel pathogen Vibrio coralliilyticus. Appl Environ Microbiol 69: 4236–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn N, Fenchel T, Mitchell J. (1998). Microscale nutrient patches in planktonic habitats shown by chemotactic bacteria. Science 282: 2254–2256. [DOI] [PubMed] [Google Scholar]
- Burge CA, Eakin CM, Friedman CS, Froelich B, Hershberger PK, Hofmann EE et al. (2014). Climate change influences on marine infectious diseases: Implications for management and society. Annu Rev Marine Sci 6: 249–277. [DOI] [PubMed] [Google Scholar]
- Case RJ, Longford SR, Campbell AH, Low A, Tujula N, Steinberg PD et al. (2011). Temperature induced bacterial virulence and bleaching disease in a chemically defended marine macroalga. Environ Microbiol 13: 529–537. [DOI] [PubMed] [Google Scholar]
- Garren M, Azam F. (2012). Corals shed bacteria as a potential mechanism of resilience to organic matter enrichment. ISME J 6: 1159–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garren M, Son K, Raina JB, Rusconi R, Menolascina F, Shapiro OH et al. (2014). A bacterial pathogen uses dimethylsulfoniopropionate as a cue to target heat-stressed corals. ISME J 8: 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron SF, Willis BL, Skirving WJ, Eakin CM, Page CA, Miller IR. (2010). Summer hot snaps and winter conditions: Modelling White Syndrome outbreaks on Great Barrier Reef corals. Plos One 5: e12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoegh-Guldberg O. (2004). Coral reefs in a century of rapid environmental change. Symbiosis 37: 1–31. [Google Scholar]
- Kimes NE, Grim CJ, Johnson WR, Hasan NA, Tall BD, Kothary MH et al. (2012). Temperature regulation of virulence factors in the pathogen Vibrio coralliilyticus. ISME J 6: 835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen MH, Blackburn N, Larsen JL, Olsen JE. (2004). Influences of temperature, salinity and starvation on the motility and chemotactic response of Vibrio anguillarum. Microbiology 150: 1283–1290. [DOI] [PubMed] [Google Scholar]
- Maeda K, Imae Y, Shioi JI, Oosawa F. (1976). Effect of temperature on motility and chemotaxis of Escherichia coli. J Bacteriol 127: 1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magariyama Y, Sugiyama S, Muramoto K, Kawagishi I, Imae Y, Kudo S. (1995). Simultaneous measurement of bacterial flagellar rotation rate and swimming speed. Biophys J 69: 2154–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel MJ, Schaefer AL, Brennan CA, Heath-Heckman EAC, DeLoney-Marino CR, McFall-Ngai MJ et al. (2012). Squid-derived chitin oligosaccharides are a chemotactic signal during colonization by Vibrio fischeri. Appl Environ Microbiol 78: 4620–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard J, van Hooidonk R, Eakin CM, Puotinen M, Heron SF, Garren M et al. (2015). Climate projections of conditions that increase coral disease susceptibility and pathogen virulence. Nat Clim Change doi: 10.1038/nclimate2625.
- Meron D, Efrony R, Johnson WR, Schaefer AL, Morris PJ, Rosenberg E et al. (2009). Role of flagella in virulence of the coral pathogen Vibrio coralliilyticus. Appl Environ Microbiol 75: 5704–5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otoole R, Milton DL, Wolf WH. (1996). Chemotactic motility is required for invasion of the host by the fish pathogen Vibrio anguillarum. Mol Microbiol 19: 625–637. [DOI] [PubMed] [Google Scholar]
- Pandolfi JM, Bradbury RH, Sala E, Hughes TP, Bjorndal KA, Cooke RG et al. (2003). Global trajectories of the long-term decline of coral reef ecosystems. Science 301: 955–958. [DOI] [PubMed] [Google Scholar]
- Pollock FJ, Wilson B, Johnson WR, Morris PJ, Willis BL, Bourne DG. (2010). Phylogeny of the coral pathogen Vibrio coralliilyticus. Environ Microbiol Rep 2: 172–178. [DOI] [PubMed] [Google Scholar]
- Rosenberg E, Falkovitz L. (2004). The Vibrio shiloi/Oculina patagonica model system of coral bleaching. Annu Rev Microbiol 58: 143–159. [DOI] [PubMed] [Google Scholar]
- Ruiz-Morenol D, Willis BL, Page AC, Weil E, Croquer A, Vargas-Angel B et al. (2012). Global coral disease prevalence associated with sea temperature anomalies and local factors. Dis Aquat Organ 100: 249–261. [DOI] [PubMed] [Google Scholar]
- Seymour JR, Ahmed T, Marcos, Stocker R. (2008). A microfluidic chemotaxis assay to study microbial behavior in diffusing nutrient patches. Limnol Oceanogr Methods 6: 477–488. [Google Scholar]
- Seymour JR, Simó R, Ahmed T, Stocker R. (2010). Chemoattraction to Dimethylsulfoniopropionate throughout the marine microbial food web. Science 329: 342–345. [DOI] [PubMed] [Google Scholar]
- Stocker R, Seymour JR. (2012). Ecology and physics of bacterial chemotaxis in the ocean. Microbiol Mol Biol Rev 76: 792–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son K, Brumley DR, Stocker R. (2015). Live from under the lens: exploring microbial motility with dynamic imaging and microfluidics. Nat Rev Microbiol 13: 761–775. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Stocker R. (2012). Trade-offs of chemotactic foraging in turbulent water. Science 338: 675–679. [DOI] [PubMed] [Google Scholar]
- Toren A, Landau L, Kushmaro A, Loya Y, Rosenberg E. (1998). Effect of temperature on adhesion of Vibrio strain AK-1 to Oculina patagonica and on coral bleaching. Appl Environ Microbiol 64: 1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tout J, Jeffries TC, Petrou K, Tyson GW, Webster NS, Garren M et al. (2015. a). Chemotaxis by natural populations of coral reef bacteria. ISME J 9: 1764–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tout J, Siboni N, Messer LF, Garren M, Stocker R, Webster NS et al. (2015. b). Increased seawater temperature increases the abundance and alters the structure of natural Vibrio populations associated with the coral Pocillopora damicornis. Front Microbiol 6: 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel DB, DiLuzio WR, Whitesides GM. (2007). Microfabrication meets microbiology. Nat Rev Microbiol 5: 209–218. [DOI] [PubMed] [Google Scholar]
- Weil E, Croquer A, Urreiztieta I. (2009). Temporal variability and impact of coral diseases and bleaching in La Parguera, Puerto Rico from 2003–2007. Caribbean J Sci 45: 221–246. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.